Abstract
Background
The binding of ligands to clusters of complement-type repeat (CR)-domains in proteins of the low-density lipoprotein receptor (LDLR) family is dependent on Ca2+ ions. One reason for this cation requirement was identified from the crystal structure data for a CR-domain from the prototypic LDLR, which showed the burial of a Ca2+ ion as a necessity for correct folding and stabilization of this protein module. Additional Ca2+ binding data to other CR-domains from both LDLR and the LDLR-related protein (LRP) have suggested the presence of a conserved Ca2+ cage within CR-domains from this family of receptors that function in endocytosis and signalling.
Results
We have previously described the binding of several ligands to a fragment comprising the fifth and the sixth CR-domain (CR56) from LRP, as well as qualitatively described the binding of Ca2+ ions to this CR-domain pair. In the present study we have applied the rate dialysis method to measure the affinity for Ca2+, and show that CR56 binds 2 Ca2+ ions with an average affinity of KD = 10.6 microM, and there is no indication of additional Ca2+ binding sites within this receptor fragment.
Conclusions
Both CR-domains of CR56 bind a single Ca2+ ion with an affinity of 10.6 microM within the range of affinities demonstrated for several other CR-domains.
Background
The ligand-binding domains of the entire LDLR family of cell surface receptors are comprised of imperfect repeats of about 40 amino acids, the CR-domains [1-3]. Each repeat contains six cysteine residues which are disulphide linked in the pattern one to three, two to five, and four to six, and each repeat harbors a Ca2+ binding site [4,5]. Furthermore, the modular domain architecture of LRP comprises several epidermal growth factor (EGF)-precursor homology domains, a segment spanning the plasma membrane and a cytoplasmic domain. The entire LRP molecule might contain as many as 39 Ca2+ binding sites, one located in each of the 31 CR-domains [5-7], and two binding sites present in each of 4 EGF-domain pairs [7-11] (see legend to Figure 1A).
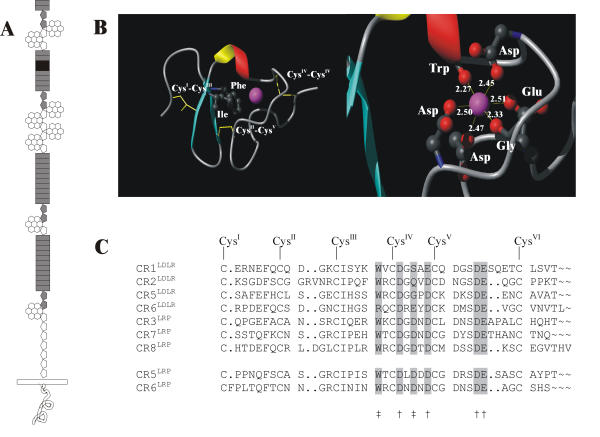
Figure 1.
(A) Schematic representation of the domain architecture of the LRP, with domains suggested to contain a Ca2+ binding site as shown in gray. For estimation of the stoichiometric binding of Ca2+ to the entire LRP molecule, each CR-domain is a potential cation carrier based on the fact that all CR-domain structures described so far include a bound Ca2+ ion [5-7]. The crystal structure of the LDLR YWTD-repeated β-propeller does not show any cations and we assume similar lack of Ca2+ binding to these fragments within LRP. In contrast, some EGF-domains do bind Ca2+ and some do not. For LDLR the EGF-domain pair amino-terminal to the β-propeller binds 2 Ca2+ ions, and accordingly, it is assumed that the four EGF-domain pairs within LRP are also Ca2+ binding. The carboxy-terminal EGF-domain is not chelating a Ca2+ ion [7-11]. By these assumptions, one LRP molecules could bind a total of at least 39 Ca2+ ions. Rectangles ( ) represent CR-domains, pentagons (
) represent CR-domains, pentagons ( ) represent EGF-domains, and hexagonals (
) represent EGF-domains, and hexagonals ( ) for each blade of the β-propellers. (B) Ribbon representation of the canonical CR-domain folding and Ca2+ binding site. Left, the backbone folding of CR5LDLR (Protein Data Bank accession code 1ajj [5]) showing the location of the Ca2+ ion indicated as the sphere. Right, zoom of the Ca2+ cage showing the octahedral cation coordination. (C) Alignment of the primary structures of Ca2+-binding CR-domains with a known 3D-structure, the first, the second, the fifth, and the sixth CR-domain from LDLR, and the third, the seventh, and the eighth CR-domain from LRP. The symbols below the alignment indicate residues involved in Ca2+ chelation (‡, coordination by backbone carbonyl; †, coordination by side chain carboxyl), and the six conserved cysteines are indicated above the sequences with roman numbers.
) for each blade of the β-propellers. (B) Ribbon representation of the canonical CR-domain folding and Ca2+ binding site. Left, the backbone folding of CR5LDLR (Protein Data Bank accession code 1ajj [5]) showing the location of the Ca2+ ion indicated as the sphere. Right, zoom of the Ca2+ cage showing the octahedral cation coordination. (C) Alignment of the primary structures of Ca2+-binding CR-domains with a known 3D-structure, the first, the second, the fifth, and the sixth CR-domain from LDLR, and the third, the seventh, and the eighth CR-domain from LRP. The symbols below the alignment indicate residues involved in Ca2+ chelation (‡, coordination by backbone carbonyl; †, coordination by side chain carboxyl), and the six conserved cysteines are indicated above the sequences with roman numbers.
The understanding of how LRP binds Ca2+ ions is important. The binding of all ligands is dependent on the presence of Ca2+ [12-14] and ligand dissociation within the endocytic pathway has been suggested to occur as a possible consequence of the decrease in pH and the accompanying loss of affinity for Ca2+ in the acidic endosomes [8].
Ligand recognition requires key residues in the CR-domains of the LDLR-like proteins as well as the presence of Ca2+ ions. One important residue for this interaction is located at the center position between the fourth and the fifth cysteine residue, where an acidic side chain is important for the efficient recognition of multiple protein ligands [15,16]. The backbone carbonyl group of residues located at identical positions in several domains homologous to CR5 and CR6 is involved in the coordination of a Ca2+ ion [5,6]. The coordination sphere of the bound Ca2+ ion is well defined in octahedral geometry, with four carboxylate oxygen atoms from the acidic motif in one plan and two carbonyl oxygen atoms completing the coordination sphere at the apices (Figure 1B).
We have previously demonstrated the specific binding of LRP ligands to a minimum receptor fragment comprising only 2 CR-domains [15], and focused on the ligand interaction with the tandem domain CR56 fragment [17,18]. The affinity for a bound Ca2+ ion has been reported for several CR-domains, and in order to better understand the Ca2+-dependent ligand binding properties of CR56 we have determined the stoichiometry and affinity for Ca2+ binding to this CR-domain pair. Furthermore, we undertook a stringent/direct method of affinity determination using the microchamber rate dialysis method [19] independent of the local molecular environment at the Ca2+ localization site, and could compare the determined affinity with data obtained by less direct methods, e.g. fluorescence analysis. We conclude that in general the affinities do not vary significantly among the CR-domains investigated.
Results and Discussion
The demonstration of Ca2+ binding to the ubiquitin-fused CR56 protein [15] strongly suggested that CR56 contains at least one efficient Ca2+ binding site similar to other CR-domains [1,5]. However, from the previously adopted approach we could not determine the stoichiometry and affinity between Ca2+ and CR56. This was the main objective of the current study. Furthermore, we wanted to test whether the coupling of a CR-domain to a neighboring domain would influence the affinity of the single CR-domain, compared to the increasing pool of data describing the binding of Ca2+ to isolated CR-domains [6,20-23]. After affinity purification of ubiquitin-fused CR56 containing the authentic amino acid sequence, we liberated the CR56 domain pair from its fusion partner by factor Xa cleavage (as described in ref. [15]). 2D gel analysis showed a high degree of purity, since the recombinant CR56 protein migrated as a single major spot (not shown). Ca2+ binding was measured using the microchamber rate dialysis technique [19,24].
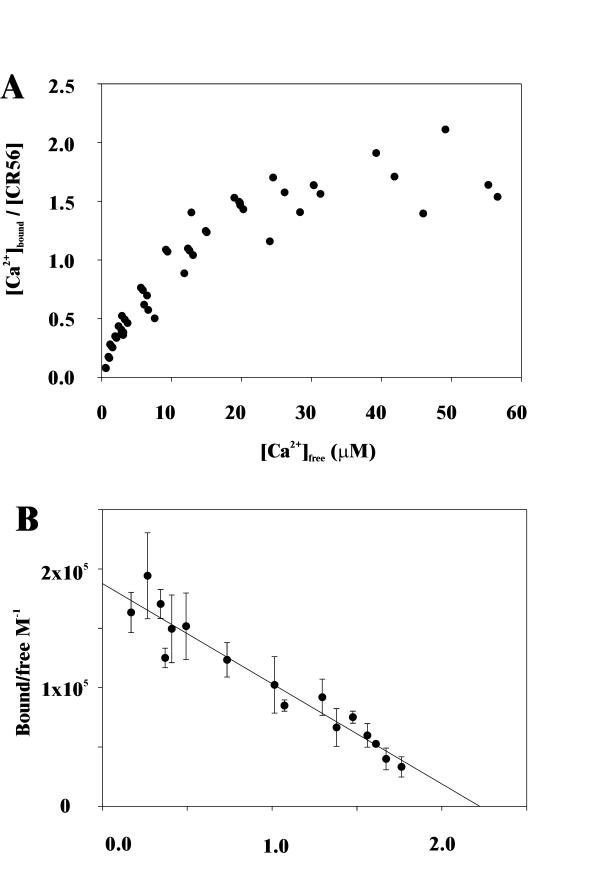
The Ca2+ binding data is shown in Figure 2A, and from Scatchard analysis of the data (Figure 2B), we conclude that the folded CR56 domain pair binds two Ca2+ ions with an average affinity of KD = 10.6 μM assuming two identical binding sites. Even though there is a small derivation from the exact 1:2 stoichiometry (n = 2.0 sites), the observed n = 2.2 is within experimental error not significantly different from 2.0. Secondly, the number is in accord with other data determining Ca2+ affinity to tandem CR-domain pair [25]. Bieri et al. found a similar stoichiometry (n = 2.2) when they measured the Ca2+ binding to a concatemer of CR1LDLR-CR2LDLR, and neither solution nor crystal structure indicate that there is more than two Ca2+ binding sites within this fragment [7,26]. Furthermore, the recently solved crystal structure of the LDLR luminal domain shows a single bound cation for each CR-domain without any additional interdomain binding sites [7]. The determined affinity is very close to the reported Ca2+ affinities for homologous LRP CR-domains, CR3LRP, CR7LRP, CR8LRP and for the two LDLR CR-domains CR1LDLR and CR2LDLR (listed in Table 1), which indicates that the binding sites might be similar. Apparently, most CR-domains bind Ca2+ with an approximate affinity KD~10–20 μM, except CR5LDLR and CR6LDLR which show a higher affinity. Furthermore, our data suggests that the various methods used to measure the affinities are reliable.
Figure 2.
(A) The Ca2+-affinity of CR56 was determined by rate dialysis [Ca2+]bound/[CR56] versus the free Ca2+ concentration. The concentration of CR56 was 15 μM throughout the analysis. (B) Scatchard analysis of the data shown in panel A. The solid line represents the best fit by least-squares analysis as described in the materials and methods section. The fact that the experimental points are located on a straight line indicates absence of cooperativity between the two Ca2+ binding sites. Strong positive cooperativity would result in upward curvature, while strong negative cooperativity would result in downward curvature of the Scatchard plot. The average affinity is determined to, KD = 10.6 μM assuming two identical sites (n = 2.2).
Table 1.
Ca2+ binding properties of various CR-domains
| CR-domain | Ca2+ affinity (KD) μM | References | Method a) | Conditions |
| CR1LDLR | 7 | [22] | Fluorescence | 25°C |
| 10 | [30] | |||
| CR2LDLR | 14 | [25] | ||
| CR5LDLR | 0.040 | [27] | Fluorescence | pH 7.0 |
| 0.070 | [20,27] | Fluorescence | pH 7.0 | |
| 0.500 | [6] | ITC | pH 7.4, 30°C | |
| 13 | [6] | ITC | pH 5.0, 30°C | |
| CR6LDLR | 0.200 | [27] | Fluorescence | pH 7.0 |
| CR3LRP | 8 | [6] | ITC | pH 7.4, 30°C |
| 24 | [22] | Fluorescence | 25°C | |
| CR5LRP | 11 | The present data | Rate dialysis | pH 7.4, 37°C |
| CR6LRP | 11 | The present data | Rate dialysis | pH 7.4, 37°C |
| CR7LRP | 13 | [6] | ITC | pH 7.4, 30°C |
| CR8LRP | 6 | [6] | ITC | pH 7.4, 30°C |
| 13 | [22] | Fluorescence | 25°C | |
| CRTva | 40 | [23] | ITC | pH 7.4, 30°C |
a) ITC; Isothermal titration calorimetry CRXLDLR, CRXLRP, and CRTva denote the Xth CR-domain counting from the amino-terminal end of LDLR, LRP and the Tva receptor of avian leucosis and sarcoma virus type A, respectively.
The Ca2+ ions in the hitherto solved domain structures are located in identical Ca2+ cages as for CR5LDLR described previously by Fass and colleagues [5] (Figure 1B). From a high level of sequence conservation for the sequences of CR5LRP and CR6LRP compared to the sequences of the CR-domains with a solved domain structure (Figure 1C), we suggest that the binding site for Ca2+ within both CR5 and CR6 are very similar to these. This is very important information for the assignment of nuclear magnetic resonances for the solution structure determination of CR56 (ongoing project).
The demonstration of independent folding of each CR-domain in tandem CR-domain pairs is substantiated by the reports of a total lack of interdomain interactions, and that Ca2+ coordination does not involve chelates from adjacent CR-domains [27,28]. In line with this our data suggest that two and only two Ca2+ are bound per tandem fragment as also reported for domain pairs from LDLR comprising CR1LDLR-CR2LDLR and CR5LDLR-CR6LDLR [25,27]. Since the Ca2+ affinity for CR5 and CR6 is similar to other known binding sites, it is tempting to believe that the Ca2+ ion is located in a similar Ca2+cage as for these domains [1,5,6], and therefore the chelating residues located at identical positions within the primary structure is also cation coordinating in these domains. If this indeed is the case, we have recently demonstrated that the two residues speculated to provide electrons for Ca2+coordination via their backbone functional group (Trp-953/Asp-958 in CR5 and Trp-994/Asp-999 in CR6) both contribute significantly to ligand binding [17,18]. The possibility that Ca2+ most likely are intimately linked to these residues suggest that Ca2+ binding exerts influence on ligand binding to CR-domains, because of a lack of dynamic and flexibility of residues at this particular position. In addition, especially the acidic residues is also involved in the intramolecular binding of CR-domains to the EGF-precursor homology domain at low pH, speculated to result in structural rearrangement and ligand release within endosomes, underscoring the importance of understanding the local environment around the Ca2+ binding site [7].
Conclusions
Both CR-domains of the CR56-domain pair bind a single Ca2+ ion with an average affinity, KD~10.6 μM.
Methods
Proteins
Production and RAP-affinity purification of the ubiquitin-fused CR-domain pair comprising the fifth and the sixth CR-domain from LRP (see Figure 1) was described previously [15]. Purity was verified by two-dimensional gel electrophoresis showing the sample to be highly homogeneous (not shown).
Calcium binding analysis
Qualitative 45Ca blotting analysis to ubiquitin-fused CR56 has been described [15]. The quantitative rate dialysis method [19] was applied to determine the Ca2+ binding constants for CR56. The binding experiments were performed in a medium containing 10 mM HEPES pH. 7.0, 150 mM NaCl and a final CR56 concentration at 15 μM. Buffers and protein solutions were passed through a Chelex 100 column (BioRAD) in order to obtain cation free solutions before use. The resin was pre-equilibrated with HEPES binding buffer before use. We have previously shown that this procedure is able to bring the Ca2+ content of the solutions to negligible levels as determined by atomic absorption spectrometry [29]. The dialysis membrane was of cellulose, Type 14.10, molecular cut-off 5000, from Diachema (Munich, Germany). The following equation was used to calculate the free Ca2+ concentration from the total Ca2+ concentration:
[Ca2+]free = - [Ca2+]total [k*(t+t0)]-1 ln [(Aleft - Aright)/(Aleft + Aright)]
where k is a pre-determined rate constant, t is time of dialysis and t0 is an experimentally determined value which is dependent upon the procedure of filling, withdrawal, and rinsing of chambers and varies with the substance dialysed [19]. Aleft and Aright denote the radioactivity in the left and right solution, respectively, after dialysis measured by liquid scintillation counting in an LKB Wallac 1218 Rackbeta scintillation counter. The values of k and t0 for the Ca2+ ligand used were 0.04650 min-1 and 0.18 min. They were determined in separate experiments with no protein present and using varying dialysis times as described in detail [19]. In short, values of ln [(Aleft - Aright)/(Aleft + Aright)] are plotted versus the dialysis time. The rate constant k is then determined from the slope of the curve while t0 is determined as the numerical value of the intercept with the time-axis (x-axis). The average number of Ca2+ ions bound per protein molecule, r, was calculated from
r = ([Ca2+]total - [Ca2+]free)/ [CR56]
Under the presumption that CR56 contains a number, n, of identical and independent Ca2+-binding sites the binding isotherm was fitted to the Scatchard equation by linear regression:
r/ [Ca2+]free = - r/KD + n/KD,
where KD is the dissociation constant.
Abbreviations
CR-domain, complement-type repeat domain; EGF, epidermal growth factor; LDLR, low-density lipoprotein receptor; LRP, LDLR-related protein
Authors' contributions
OMA produced and purified recombinant protein, prepared figures and drafted the manuscript. HV and BH performed the quantitative rate dialysis measurements and participated in drafting the manuscript. HCT conceived the study as well as participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Kirsten Peterslund for excellent technically assistance. This work was supported by Grant 9901730 from the Danish National Science Research Council (HCT), the Danish Medical Research Council and the Novo Nordisk Foundation.
Contributor Information
Olav M Andersen, Email: o.andersen@mdc-berlin.de.
Henrik Vorum, Email: hv@biokemi.au.dk.
Bent Honoré, Email: bh@biokemi.au.dk.
Hans C Thøgersen, Email: hct@biobase.dk.
References
- Brown MS, Herz J, Goldstein JL. LDL-receptor structure. Calcium cages, acid baths and recycling receptors. Nature. 1997;388:629–630. doi: 10.1038/41672. [DOI] [PubMed] [Google Scholar]
- Neels JG, Horn IR, van den Berg BMM, Pannekoek H, van Zonneveld A-J. Ligand-receptor interactions of the low density lipoprotein receptor-related protein, a multi-ligand endocytic receptor. Fibrinolysis & Proteolysis. 1998;12:219–240. [Google Scholar]
- Herz J. Deconstructing the LDL receptor – a rhapsody in pieces. Nat Struct Biol. 2001;8:476–478. doi: 10.1038/88519. [DOI] [PubMed] [Google Scholar]
- Bieri S, Djordjevic JT, Daly NL, Smith R, Kroon PA. Disulfide bridges of a cysteine-rich repeat of the LDL receptor ligand-binding domain. Biochemistry. 1995;34:13059–13065. doi: 10.1021/bi00040a017. [DOI] [PubMed] [Google Scholar]
- Fass D, Blacklow S, Kim PS, Berger JM. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- Simonovic M, Dolmer K, Huang W, Strickland DK, Volz K, Gettins PGW. Calcium coordination and pH dependence of the calcium affinity of ligand-binding repeat CR7 from the LRP. Comparison with related domains from the LRP and the LDL receptor. Biochemistry. 2001;40:15127–15134. doi: 10.1021/bi015688m. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Henry L, Henderson K, Ichtchenko K, Brown MS, Goldstein JL, Deisenhofer J. Structure of the LDL receptor extracellular domain at endosomal pH. Science. 2002;298:2353–8. doi: 10.1126/science.1078124. [DOI] [PubMed] [Google Scholar]
- Malby S, Pickering R, Saha S, Smallridge R, Linse S, Downing AK. The first epidermal growth factor-like domain of the low-density lipoprotein receptor contains a noncanonical calcium binding site. Biochemistry. 2001;40:2555–2563. doi: 10.1021/bi002322l. [DOI] [PubMed] [Google Scholar]
- Kurniawan ND, Aliabadizadeh K, Brereton IM, Kroon PA, Smith R. NMR structure and backbone dynamics of a concatemer of epidermal growth factor homology modules of the human low-density lipoprotein receptor. J Mol Biol. 2001;311:341–356. doi: 10.1006/jmbi.2001.4867. [DOI] [PubMed] [Google Scholar]
- Saha S, Boyd J, Werner JM, Knott V, Handford PA, Campbell ID, Downing AK. Solution structure of the LDL Receptor EGF-AB pair: A paradigm for the assembly of tandem calcium binding EGF domains. Structure. 2001;9:451–456. doi: 10.1016/S0969-2126(01)00606-2. [DOI] [PubMed] [Google Scholar]
- Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 2001;8:499–504. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Nykjær A, Olivecrona-Bengtsson G, Lookene A, Moestrup SK, Petersen CM, Weber W, Beisiegel U, Gliemann J. The α2-macroglobulin receptor/low density lipoprotein receptor-related protein binds lipoprotein lipase and beta-migrating very low density lipoprotein associated with the lipase. J Biol Chem. 1993;268:15048–15055. [PubMed] [Google Scholar]
- Andersen OM, Christensen LL, Christensen PA, Sørensen ES, Jacobsen C, Moestrup SK, Etzerodt M, Thøgersen HC. Identification of the Minimal Functional Unit in the Low Density Lipoprotein Receptor-related Protein for Binding the Receptor-associated Protein (RAP) J Biol Chem. 2000;275:21017–21024. doi: 10.1074/jbc.M000507200. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Christensen PA, Christensen LL, Jacobsen C, Moestrup SK, Etzerodt M, Thøgersen HC. Specific Binding of α-Macroglobulin to Complement-type Repeat CR4 of the Low Density Lipoprotein Receptor-related Protein. Biochemistry. 2000;39:10627–10633. doi: 10.1021/bi000498h. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Petersen HH, Jacobsen C, Moestrup SK, Etzerodt M, Andreasen PA, Thøgersen HC. Analysis of a two-domain binding site for the urokinase-type plasminogen activator – plasminogen activator inhibitor-1 complex in low density lipoprotein receptor-related protein. Biochem J. 2001:289–296. doi: 10.1042/0264-6021:3570289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OM, Schwarz FP, Eisenstein E, Jacobsen C, Moestrup SK, Etzerodt M, Thøgersen HC. Dominant thermodynamic role of the third independent receptor binding site in the receptor-associated protein RAP. Biochemistry. 2001;40:15408–15417. doi: 10.1021/bi0110692. [DOI] [PubMed] [Google Scholar]
- Honoré B. Equilibrium dialysis and rate dialysis. In: Harding SE, Chowdhry BZ, editor. Protein-Ligand Interactions: Hydrodynamics and Calorimetry. A Practical Approach. Oxford: Oxford University Press; 2001. pp. 19–46. [Google Scholar]
- Blacklow SC, Kim PS. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nat Struct Biol. 1996;3:758–762. doi: 10.1038/nsb0996-758. [DOI] [PubMed] [Google Scholar]
- Atkins AR, Brereton IM, Kroon PA, Lee HT, Smith R. Calcium is essential for the structural integrity of the cysteine-rich, ligand-binding repeat of the low-density lipoprotein receptor. Biochemistry. 1998;37:1662–1670. doi: 10.1021/bi972529n. [DOI] [PubMed] [Google Scholar]
- Dolmer K, Huang W, Gettins PGW. Characterization of the calcium site in two complement-like domains from the low-density lipoprotein receptor-related protein (LRP) and comparison with a repeat from the low-density lipoprotein receptor. Biochemistry. 1998;37:17016–17023. doi: 10.1021/bi982022s. [DOI] [PubMed] [Google Scholar]
- Wang QY, Dolmer K, Huang W, Gettins PG, Rong L. Role of calcium in protein folding and function of Tva, the receptor of subgroup A avian sarcoma and leukosis virus. J Virol. 2001;75:2051–2058. doi: 10.1128/JVI.75.5.2051-2058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorum H, Liu X, Madsen P, Rasmussen HH, Honoré B. Molecular cloning of a cDNA encoding human calumenin, expression in Escherichia coli and analysis of its Ca2+-binding activity. Biochim Biophys Acta. 1998;1386:121–131. doi: 10.1016/S0167-4838(98)00089-2. [DOI] [PubMed] [Google Scholar]
- Bieri S, Atkins AR, Lee HT, Winzor DJ, Smith R, Kroon PA. Folding, calcium binding, and structural characterization of a concatemer of the first and second ligand-binding modules of the low-density lipoprotein receptor. Biochemistry. 1998;37:10994–11002. doi: 10.1021/bi980452c. [DOI] [PubMed] [Google Scholar]
- Kurniawan ND, Atkins AR, Bieri S, Brown CJ, Brereton IM, Kroon PA, Smith R. NMR structure of a concatemer of the first and second ligand-binding modules of the human low-density lipoprotein receptor. Protein Sci. 2000;9:1282–1293. doi: 10.1110/ps.9.7.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North CL, Blacklow SC. Structural independence of ligand-binding modules five and six of the LDL receptor. Biochemistry. 1999;38:3926–3935. doi: 10.1021/bi9821622. [DOI] [PubMed] [Google Scholar]
- North CL, Blacklow SC. Evidence that familial hypercholesterolemia mutations of the LDL receptor cause limited local misfolding in an LDL-A module pair. Biochemistry. 2000;39:13127–13135. doi: 10.1021/bi0015156. [DOI] [PubMed] [Google Scholar]
- Vorum H, Madsen P, Rasmussen HH, Etzerodt M, Svendsen I, Celis JE, Honoré B. Expression and divalent cation binding properties of the novel chemotactic inflammatory protein psoriasin. Electrophoresis. 1996;17:1787–1796. doi: 10.1002/elps.1150171118. [DOI] [PubMed] [Google Scholar]
- van Driel IR, Goldstein JL, Südhof TC, Brown MS. First cysteine-rich repeat in ligand-binding domain of low density lipoprotein receptor binds Ca2+ and monoclonal antibodies, but not lipoproteins. J Biol Chem. 1987;262:17443–17449. [PubMed] [Google Scholar]