Abstract
Vertebrate c-myb encodes a transcription factor thought to play an important role in the cell cycle. To gain further insight into myb function, we have been studying a related gene in Drosophila. We found that Drosophila myb is abundantly expressed throughout development in all mitotically active tissues, at lower levels in some postmitotic tissues, but not at detectable levels in polyploid larval tissues. We performed genetic screens to isolate recessive lethal mutations in the chromosomal region that includes the myb gene. We obtained two temperature-sensitive alleles of myb, demonstrating that the gene provides an essential function. Examination of the mutant phenotype revealed that Drosophila myb is important for both embryonic and imaginal development and that myb serves a role in the development of many tissues and during oogenesis.
Keywords: transcription factor, protooncogene, myb mutant, developmental mutant
Protooncogenes are those genes that when mutated have been implicated in contributing to the genesis of cancer. Activated oncogenes have profound effects on cellular differentiation and proliferation, and the normal versions of these genes have now been shown to play central roles in regulating these same processes (1, 2). Since most protooncogenes have been highly conserved during evolution (3, 4), a powerful approach for gaining further insights into their normal functions is to study their homologs in a genetically tractable model system, such as Drosophila melanogaster.
myb was first identified as an oncogene by virtue of its transduction into two independent avian retroviruses which cause myeloblastosis in chickens and transform myeloid cells in culture (for review, see refs. 5 and 6). Subsequent research has implicated mutant c-myb genes in the genesis of neoplastic disease in mice and humans (5, 6). The protooncogene c-myb is now known to represent a small gene family in vertebrates which has two other closely related members, A-myb and B-myb (7). All three myb genes encode nuclear, sequence-specific DNA-binding proteins that can regulate transcription.
myb genes have been implicated in regulating the cell cycle, specifically during the G1/S transition (8, 9, 10, 11). However, the signal transduction pathway(s) in which myb participates remain elusive: little is known about either gene products that act upstream of myb, in concert with myb, or downstream genes that are targets of myb proteins. Although a number of genes have now been identified that appear to participate in some of the same biochemical pathways as myb, the function of these genes has been associated more with cellular differentiation than proliferation (12, 13, 14, 15, 16).
The myb DNA binding domain has been well conserved during evolution. Several genes encoding proteins containing myb-related DNA binding domains have been isolated from organisms as phylogenetically distant as yeast and plants (17, 18, 19). Previously, we identified a single myb-related gene in the Drosophila genome (20). The Drosophila gene shares a considerably higher level of homology with vertebrate c-myb within the DNA binding domain than the other nonvertebrate genes, and unlike those genes, homology is not restricted to this domain; three additional regions of conservation are distributed throughout the protein (21). The same four regions of homology are shared with A-myb and B-myb. Previously, we referred to the Drosophila gene as Drosophila c-myb (20). Since it is now unclear whether this gene represents a true counterpart of c-myb or of one of the other vertebrate genes (or is the progenitor of all three), we will henceforth refer to this gene as Drosophila myb.
We have chosen to study the function of myb in D. melanogaster because it provides a powerful genetic and developmental system in which to dissect cellular and biochemical processes. Here we report that Drosophila myb is expressed in a fashion consistent with a role in cell proliferation. We have isolated two recessive lethal alleles with lesions in the myb gene. For both alleles, the penetrance of lethality is temperature dependent. Mutant phenotypes indicate that myb serves a role in many Drosophila tissues and that its activity is required during most stages of development.
MATERIALS AND METHODS
Transcriptional Analysis.
In situ hybridization using 35S-labeled probes prepared from a 2.65-kb cDNA fragment representing the bulk of the Drosophila myb transcription unit, autoradiography, staining, and photomicroscopy were performed as described (22). To control for nonspecific binding of the probe, parallel experiments were undertaken in which sections were either pretreated with RNase A or hybridized with a probe prepared with the pUC8 vector.
Drosophila stocks were generously provided as follows: shi mutations and the Dp(1:Y)shi+Y#1 were from Cliff Poodry (University of California, Santa Cruz); Df(1)sd72b26 was from Barry Ganetzky (University of Wisconsin, Madison); Df(1)l9,f and two of the Dp(1;4)r+f+ chromosomes were from Hermann Steller (Massachusetts Institute of Technology); a sd mutation was from Bob Levis (Fred Hutchinson Cancer Center, Seattle); the third Dp(1;4)r+f+ chromosome was from the California Institute of Technology stock center; and the remaining stocks were from T. Kornberg (University of California, San Francisco). (Df and Dp stand for deficiency and duplication, respectively.) The complete names of loci for which abbreviations are used are as follows: B, Bar; Bs, Bar-stone; exd, extradenticle; f, forked; r, rudimentary; ry, rosy; sd, scalloped; shi, shibire; w, white; wa, white apricot (23).
Genetic Screen and Mutagenesis Procedure.
Mutagenesis with ethyl methanesulfonate (EMS) was carried out using standard procedures (24). Mutations that were unable to complement the Df(1)sd72b26 chromosome were rescued by allowing surviving females (l(1)/FM7c) to mate with sibling males [FM7c/Dp(1:Y)shi+Y#1], thereby creating balanced stocks. The myb1 (em20) chromosome was allowed to recombine with the parental white chromosome to remove any other mutations that may have occurred during the mutagenesis treatment.
For the second F2 genetic screen, the wild-type myb transgene described below was used to rescue mutations in males instead of Dp(1:Y)shi+Y#1. Since the myb transgenic construct also included the white gene, the markers in the screen had to be adjusted appropriately. A total of 17,000 mutagenized chromosomes were tested in this screen, which produced one new mutant allele of myb, designated myb2.
P-Element-Mediated Germ-Line Transformation.
DNA fragments described in the text were subcloned into the pW8 vector, which uses the white gene under control of the hsp70 promoter as a marker for chromosomal integration (25). Each construct was coinjected with a helper P-element plasmid, pπ25.7wcΔ2-3 (26, 27) into an appropriate recipient strain, Df(1)w67c23y (provided by C. Chen, University of California, San Francisco). Transformants were obtained using standard protocols (28). Several transgenic lines were tested for their ability to rescue (complement) mutations representing each of the various complementation groups: males carrying one copy of the transgene on an autosome, Df(1)w,y/Y; P(w+,myb+ or mybFS)/+, were mated at 25°C to females heterozygous for a recessive lethal chromosome, w,em#/FM7c, and the progeny were examined for the presence of red eyed males.
Testing Viability During Development.
For all experiments to test the viability of the myb mutants, flies were maintained in plastic bottles upside down with food and fresh yeast being provided in small Petri dishes. Food was changed frequently before and during an experiment to ensure optimal conditions. Embryos were collected and gently transferred to fresh agar plates where hatching was monitored. Hatched larvae in groups of 30–50 were transferred to fresh vials containing food.
Temperature shift experiments were conducted in three different ways. Homozygous myb1 embryos were either (i) collected at 18°C and shifted to 25°C at various times during development, (ii) collected at 25°C and shifted to 18°C at various times during development, or (iii) collected at 18°C, shifted to 25°C for a period of time, and then returned to 18°C.
RESULTS
Expression of Drosophila myb Messenger RNA.
Analysis of polyadenylylated RNA extracted from whole Drosophila showed that myb was expressed as a single-sized mRNA of ≈3.2 kb throughout development (29) [note that this corrects transcript size and number from our previous report (20)]. To examine the spatial distribution of the Drosophila myb message, in situ hybridization was performed to RNA in frozen sections of developing embryos, larvae, pupae, and adults. Drosophila myb transcripts were abundant and evenly distributed in preblastoderm embryos, where they were presumably of maternal origin (not shown, but see Fig. 2C). Levels of message continued to be relatively uniform during cellular blastoderm formation and germ band extension (Fig. 1A). Differential expression became evident in germ-band-shortened embryos where message levels were relatively low in developing salivary glands and undetectable in the amnioserosa (not shown); in later stages of embryogenesis, myb message continued to be present at high levels only in the central nervous system (Fig. 1B).
Figure 2.
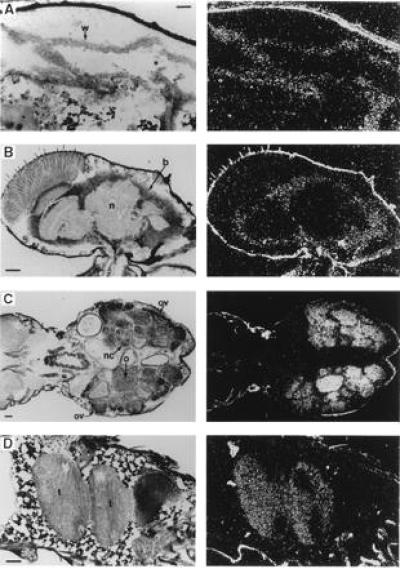
Expression of Drosophila myb in pupae and adults. Each panel includes a bright-field (Left) and dark-field (Right) photomicrograph taken after autoradiography. (Bars = 0.05 mm.) Abbreviations: b, brain; n, neuropile; nc, nurse cells; o, oocytes; ov, ovary; t, testes; w, wing. (A) Section (anterior left) of a very young pupa showing part of a newly everted wing disc. (B) Parasagittal section (anterior up, dorsal right) showing the adult head, including the brain. (C) Horizontal section (anterior left) showing the abdomen and part of the thorax of a mature adult female, including the ovaries. (D) A parasagittal section (anterior left, dorsal up) showing the abdomen of a young male adult, including the coiled region of one testis.
Figure 1.
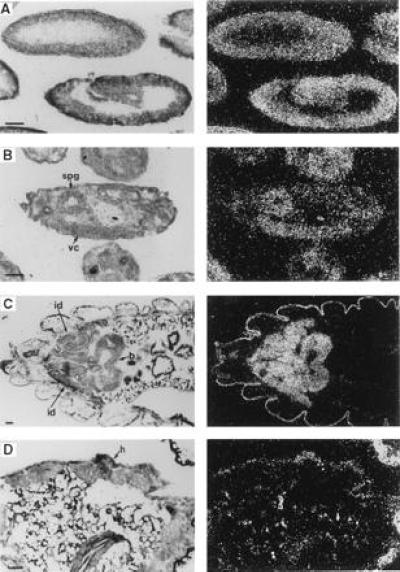
Expression of Drosophila myb in embryos and larvae. Each panel includes a bright-field (Left) and dark-field (Right) photomicrograph taken after autoradiography. Sections are oriented with anterior to the left. Stages referred to for embryos are those described by Campos-Ortega and Hartenstein (30). (Bars = 0.05 mm.) Abbreviations: b, brain; h, abdominal histoblasts; id, imaginal discs; spg, supraoesophageal ganglia; vc, ventral nerve cord. (A) Parasagittal sections of a cellular blastoderm embryo (Top), stage 5; and a germ-band-extended embryo (Bottom, dorsal up), stage 9. (B) Parasagittal section (dorsal up) of an older embryo, stage 16. (C) A slightly oblique horizontal section showing the anterior half of a third instar larva including several imaginal discs and the developing brain. (D) An oblique section of a prepupa, showing proliferating abdominal histoblasts.
In climbing stage third instar larvae, Drosophila myb transcripts were abundant in all tissues that contribute to the adult organism: imaginal discs, brain, ventral ganglia, testes, ovaries, and nests of imaginal cells within the gut (first two, Fig. 1C; latter four, not shown). myb transcripts were not detectable in any nonproliferative polyploid larval tissues (Fig. 1C). These expression patterns were maintained in prepupae. In addition, transcripts were present (albeit at lower levels than in imaginal disc cells) in the abdominal histoblasts, which undergo a period of rapid division immediately after puparium formation (Fig. 1D) and in epithelial cells of the developing imaginal gut (not shown). After cephalic eversion, levels of myb message began to decline in the everted imaginal discs (Fig. 2A) and transcripts were not detectable during the latter half of pupal development. In contrast, abundant levels of message were observed throughout pupal development in neural tissue, ovaries, and testes (not shown).
In both newly emerged and mature adults (3–5 days after eclosion), Drosophila myb was expressed at relatively low, but detectable levels in the brain (Fig. 2B) and thoracic ganglia (not shown). Abundant and comparable levels of Drosophila myb message were detected in ovaries (Fig. 2C) and testes (Fig. 2D). In ovaries, the message was localized to nurse cells and developing oocytes, confirming that the transcripts present in preblastoderm embryos were of maternal origin. In testes, specific cell types were not identified, but expression extended well beyond the germinal proliferation center (located at the distal tip). In summary, Drosophila myb message was generally and abundantly distributed in developing embryonic and imaginal tissues; transcripts were present in all mitotically active tissue, but were also present in some nonproliferating tissues. For example, myb transcripts were present at roughly equal levels in most tissues of germ-band-shortened embryos (9–11 hr of development), even though virtually all embryonic cell divisions are completed by 6.5 hr after fertilization (30). myb message was also detected, albeit at relatively low levels, in the postmitotic adult nervous system.
Mutagenesis Strategy and Isolation of Mutations in Drosophila myb.
To determine the function of myb during Drosophila development, we designed an F2 genetic screen (Fig. 3A) to isolate recessive lethal mutations in the chromosomal region that includes Drosophila myb [based on the approach used by Judd et al. (31)]. Drosophila myb was previously mapped to position 13E-F on the X chromosome (20). To test existing specialized chromosomes for their usefulness in a mutagenesis strategy, we exploited a naturally occurring restriction enzyme polymorphism, an EcoRI site present in some Drosophila strains (20). This polymorphism made it possible to determine that Drosophila myb was in the region deleted by Df(1)sd72b26 (13F1-14B1; ref. 35) and duplicated in Dp(1:Y)shi+Y#1 (a specialized Y-chromosome that carries an unknown amount of the X chromosome proximal to 13F1-4; ref. 36). These results place Drosophila myb in region 13F.
Figure 3.
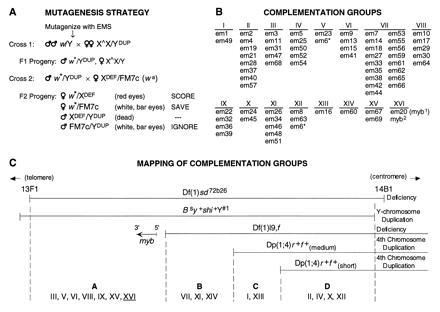
Genetic analysis of the region of the X chromosome including Drosophila myb. (A) Mutagenesis scheme. The strategy for isolating recessive lethal mutations in the vicinity of Drosophila myb (13F/14A) is schematically represented. All crosses were performed at 25°C. Adult white-eyed (w) males were aged 2 to 3 days after eclosion and then were subjected to EMS mutagenesis (25, 30, 35, or 50 mM) before mating. To ensure the independence of isolated mutations, males were removed after 3 days of mating. Specialized chromosomes are as follows: ∗, mutagenized chromosome; XDef, deficiency chromosome deleted for myb, Df(1)sd72b26 (w+); YDup, Y chromosome with small duplication from X chromosome including myb, Dp(1:Y)shi+Y#1 (note that this chromosome is also marked with Bs and y+ and does not rescue Df(1)sd72b26); X∧X, attached X chromosome, ywf:=; FM7c, balancer X chromosome carrying many markers including B and wa. Approximately 10,000 EMS-mutagenized chromosomes were tested in cross 2 (single pair matings) for their inability to complement the Df(1)sd72b26 chromosome. When the mutagenized X chromosome carries a lethal mutation uncovered by Df(1)sd72b26, white+ (red-eyed) females are not viable and will not be represented in the F2 progeny. (B) Complementation groups. Mutations isolated using the strategy in A fell into 16 lethal complementation groups. The asterisk (∗) indicates that the em6 chromosome appeared to contain two lesions since it failed to complement members of groups V and XII. Five groups correspond to previously or subsequently identified loci: group III mutations are lethal alleles of scalloped (sd) (32); group IV mutations are alleles of extradenticle (exd) (33); group VI alleles represent the first mutations isolated in the clathrin heavy chain gene (Chc) (34); group XV mutations are alleles of stunted (sun) (T. Kidd and A.L.K., unpublished); and group VII mutations are alleles of shibire (shi). Complementation analysis of this last group revealed genetic complexity not previously recognized (29). (C) Mapping of complementation groups. Deficiency [Df(1)sd72b26] and duplication [Dp(1:Y)shi+Y#1] chromosomes used in the genetic screen are schematically represented at the top of the panel. Shown below is another deficiency chromosome and two duplication chromosomes that subdivide the mutagenized region into four subregions, designated A through D (most distal to most proximal, respectively). Also depicted is a schematic representation of the Drosophila myb gene, based on molecular analysis of the Df(1)l9,f chromosome, which showed that myb is located within region A, but immediately adjacent to region B [≈7.5–8.5 kbp from the distal breakpoint of Df(1)l9,f], and that it is oriented on the X chromosome such that transcription occurs in a proximal to distal direction. Dp(1;4)r+f+is a specialized fourth chromosome that is supposed to contain an insert of the X chromosome extending from 13F to 16A2. We analyzed three versions of this chromosome from different stocks, found that two of them have lost part of the duplications, and designated them “short” and “medium.”
Sixty-seven mutant alleles were isolated and designated with the acronym em (e = EMS, m = myb region) and a number. They were found to represent 16 lethal complementation groups in which the number of members ranged from 1 to 17 (Fig. 3B). Three groups contained a single member, indicating that this screen may not have identified all of the loci that can be mutated to lethality in this region. Five complementation groups correspond to previously or subsequently identified loci: scalloped, extradenticle, shibire, stunted, and the newly identified gene encoding the clathrin heavy chain (see Fig. 3B legend). After the genetic screen was initiated, other potentially useful deficiency and duplication chromosomes were obtained. Each was examined for the presence or absence of the myb gene and tested for its ability to complement the isolated mutants. This analysis subdivided the mutagenized region into four smaller regions, and myb was localized to region A (Fig. 3C).
To determine which, if any, of the complementation groups represented mutations in Drosophila myb, a 6.7-kbp KpnI–BclI chromosomal restriction fragment encompassing the entire myb transcription unit was inserted into the polylinker of the P-element vector, pW8 (25). A negative control construct was prepared by introducing a frameshift into the coding domain. Several independent experimental and control transgenic lines were generated and tested for complementation with mutations representing each of the various complementation groups. The wild-type transgene rescued a single complementation group, XVI, which was represented by one allele, em20. The frameshift construct did not rescue em20, confirming that an intact Drosophila myb coding domain was required for complementation. We designate the em20 mutation myb1.
A second F2 genetic screen was performed in which the wild-type myb transgene described above was used to rescue mutations in males instead of Dp(1:Y)shi+Y#1. A total of 17,000 chromosomes were tested and one new mutant allele of myb, designated myb2, was isolated.
myb1 and myb2 Are Hypomorphic Alleles with Severity of Phenotype Being Temperature-Dependent.
The myb1 and myb2 mutant alleles were isolated because females hemizygous for these mutations (myb1 or myb2/Df(1)sd72b26) were inviable. Further analysis revealed that for both alleles, survival of hemizygous males and homozygous females was temperature-dependent (Fig. 4). myb1/Df(1)sd72b26 females, however, were not viable at any temperature; myb2/Df(1)sd72b26 females survived occasionally at 18°C but not at higher temperatures. Both the temperature dependence of lethality and the finding that the lethal phenotype was tighter when either mutation was over a deficiency than in homozygotes indicate that both alleles are hypomorphic rather than null. For both mutations, the phenotype is likely to be the consequence of an altered myb protein since the size and steady-state levels of myb mRNA were unaltered in mutant animals (not shown).
Figure 4.
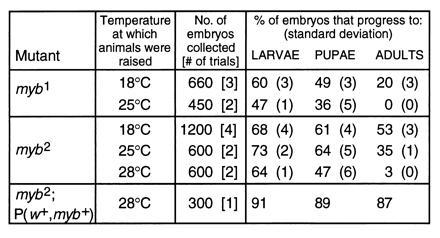
Viability studies of myb mutants at different temperatures. myb1, myb2, and myb2;P(w+,myb+) animals were raised at 18°C and kept at this temperature for 5 days after the adults emerged from their pupal cases. They were then divided into groups and incubated at the indicated temperatures. Batches of embryos were collected over the next 2 days and the percentage of animals that hatched into larvae, pupated, and emerged as adults was monitored. Number of batches tested for each genotype and temperature is indicated in brackets. Standard deviations are shown in parentheses.
myb1 and myb2 homozygotes raised at 18°C were fertile and could be maintained as homozygous stocks; adult homozygotes transferred to restrictive temperatures were viable. Homozygous stocks could therefore be used to determine the developmental stage of inviability at restrictive temperature and the degree of viability at permissive temperature (Fig. 4). Homozygous females and hemizygous males (raised at 18°C) were divided into groups which were then maintained at the specified temperatures. Embryos were collected from each population and counted, and the number of animals progressing through each stage of development was monitored. myb2 mutants rescued by the wild-type myb transgene were used as a control. myb1 animals showed a biphasic distribution of reduced viability at both 18 and 25°C, with the first period of low viability occurring during embryogenesis and the second during pupal development. The principal difference in viability between the two temperatures was during the latter period. At both temperatures, embryos died throughout embryogenesis, although many appeared to be fully formed, albeit unhatched, larvae. No specific defects were observed. Pupae also died at different developmental stages, with many appearing to be virtually fully formed (pharate) adults. myb2 animals also displayed reduced viability during embryogenesis at all temperatures and during pupal development at 25°C and 28°C.
To determine whether myb1 was particularly sensitive to the restrictive temperature at any specific period of development, several types of temperature shift experiments were performed (see Materials and Methods). Reductions in viability were greatest when middle to late third instar larvae and very early prepupae were incubated at 25°C (not shown). However, exposure to the restrictive temperature during other periods of development produced nonlethal phenotypes: incubation of first, second, and early third instar larvae at 25°C produced infertile adults, whereas incubation of prepupae and very early pupae at 25°C produced adults with more severe cuticular defects than usually observed at 18°C (see below). myb1 mutant embryos collected from myb1 homozygous females were delayed in hatching by 2 to 3 hr and myb1 adults emerged 1 to 2 days later than either parental white flies or mutant animals rescued by the wild-type myb transgene. myb1 larvae and adults were normal in size and did not appear to be grossly malformed. However, closer inspection of adults revealed defects in wings, abdominal cuticle, and flight ability (Table 1 and unpublished data).
Table 1.
Gross abnormalities displayed by myb mutants raised at permissive temperature
| Structure or process | Phenotypic defect |
|---|---|
| Wing | Reduced number of hairs; hair and bristle orientation disturbed |
| Abdomen | Missing bristles and hairs; hair and bristle orientation disturbed; patches of undifferentiated cuticle |
| Flight | myb1 flight ability impaired; myb2 flight ability unaffected |
| Oogenesis | Viability of embryos derived from mutant females greatly reduced when oogenesis occurs at restrictive temperature |
We also found that when embryos were collected from mutant myb1 or myb2 females that had been subjected to restrictive temperatures for more than 2 days, embryonic viability (as judged by hatching percentage) decreased dramatically from the levels shown in Fig. 4 (Table 1 and data not shown). When the females were returned to 18°C, embryonic viability remained low initially and recovered over time. The delayed effect of changes in temperature on hatching of mutant embryos indicates that a deficiency of myb activity during oogenesis resulted in decreased embryonic viability. After 9 days at 25°C, there was also a marked decrease in the number of embryos deposited by myb1 females during a given time period. This decrease was much slower than the effect on embryonic viability, and recovered slowly after the flies were returned to 18°C. Therefore, the changes in the number of mutant embryos collected at 25°C appeared to be the result of maternal effects exerted by the myb1 mutation early in oogenesis at the restrictive temperature. These results indicated that the Drosophila myb gene served a role in many of the tissues in which it was expressed, and that its activity was required throughout Drosophila development.
DISCUSSION
The Drosophila myb Expression Pattern Is Consistent with the Gene Playing a Role in Cell Proliferation.
Drosophila myb is generally and abundantly expressed during embryonic and imaginal development. myb transcripts are present, usually at high levels in all proliferating cells. myb message is also present in some nondividing cells, but generally decreases over time until it is no longer detectable. For example, myb mRNA is present in most tissues of germ-band-shortened embryos (9–11 hr of development), even though virtually all embryonic cell divisions in nonneural cells are completed by 6.5 hr after fertilization (30). Later in embryogenesis, the levels of message decline in nonneural tissues. The only example of continuous expression in postmitotic tissue is the presence of Drosophila myb message in the adult nervous system, albeit at relatively low levels. myb transcripts are not present at detectable levels in any nonproliferative polyploid larval tissues, indicating that myb is not required for endoreplication. The expression pattern of Drosophila myb is consistent with it playing a role in regulating cell proliferation, but the continuous neural expression suggests that it may also participate in other cellular processes.
Since the original experiments analyzing vertebrate c-myb expression suggested that it was exclusively expressed in hemopoietic tissues (37), it seemed likely that Drosophila myb would be expressed in a highly specialized fashion, a hypothesis that turned out to be incorrect. More recent studies have shown that vertebrate c-myb is expressed in non-hemopoietic tissues as well (8, 38, 39) and the two closely related vertebrate genes, A-myb and B-myb, are more broadly expressed than c-myb (7, 40, 41). When we searched for and identified the Drosophila myb gene, we were unable to find any other related genes in the Drosophila genome (20), and none have been reported by others. Since Drosophila myb is equally similar to each of the vertebrate myb genes, it seems likely that it represents the progenitor of all three genes and may encompass their combined functions.
Drosophila myb Is an Essential Gene That Is Functionally Important Throughout Development.
The isolation of recessive lethal alleles of Drosophila myb demonstrates that this gene provides an essential function to the organism. Though neither genetic screen was designed to specifically isolate temperature sensitive mutations, both mutant myb alleles behave in a temperature-dependent fashion. The improved viability of myb1 at 18°C could be a consequence of the rate of growth at 18°C being approximately half of that at 25°C. Only a fraction (≈20%) of the homozygous mutant embryos develop into viable adults at 18°C (and these adults exhibit a variety of phenotypic defects), demonstrating that the myb1 product is not fully functional at the “permissive temperature;” the finding that some mutants do not die until late in pupal development at 25°C suggests that the gene product remains partially functional at the “restrictive temperature.” In contrast, the phenotypic behavior of myb2 indicates that it may represent a more classical temperature sensitive allele, with the activity of the gene product being affected by temperature. The temperature range that determines whether this allele is viable or not is much tighter (25°C, viable; 28°C, inviable) than for myb1, making it less likely that the rate of development is the basis of the difference in viability. In addition, myb2 mutants raised at 18°C exhibit very few phenotypic abnormalities, indicating that the myb2 product may be almost fully functional at this temperature.
In viability studies, mutant animals died both as embryos and pupae, indicating that Drosophila myb serves a role during both embryonic and imaginal development, results that are in accordance with the expression pattern. Data from temperature shift experiments revealed that Drosophila myb serves an essential function during middle to late third instar larvae and very early in pupal development and participates in other aspects of development as well, including adult fertility, oogenesis, flight ability, and wing and abdominal epidermal development. Though these findings are very informative, they do not necessarily mean that other developmental stages and processes do not require myb function as well. myb messenger RNA is abundant and may be present in large excess in many tissues during development (Figs. 1 and 2). If so, the level of activity retained in myb1 and myb2 mutants might be sufficient for most developmental processes to occur normally, even at restrictive temperature. In this case, the effects of the mutations would only be evident in tissues where the levels of myb transcript are close to the required levels.
Final determination of all of the tissues and developmental stages in which myb function is required will have to await the isolation of stronger (or null) alleles. However, the two conditional mutant alleles currently in hand, myb1 and myb2, have allowed us to determine that myb function is required for proper maturation of a variety of tissues throughout development. We have pursued experiments to determine the underlying cellular basis of the wing defect and found that the mutant wing cells fail to complete their final cell division, and are most likely blocked during the G2/M transition (unpublished data). myb1 and myb2 alleles are also ideally suited for genetic screens designed to isolate suppressors and/or enhancers of the mutant phenotype. These studies will allow us to begin the dissection of the biochemical pathways in which Drosophila myb participates.
Acknowledgments
We thank Chun-Nan Chen for instruction on the preparation of transgenic flies and Suzanne Eaton for instruction on wing dissection; Tom Kornberg, Barry Drees, Barry Ganetzky, Larry Kauvar, Bob Levis, Cliff Poodry, and Hermann Steller for providing materials; Jean Jackson for help with maintaining Drosophila stocks; and Tom Kornberg, Gary Ramsay, Michael Simon, Chun-Nan Chen, Shelagh Campbell, Robert Paulson, Jean Jackson, and Sabine Schirm for useful discussion. Work reported here was supported by Grant CA44338 from the National Institutes of Health and by funds from the G. W. Hooper Research Foundation. A.L.K. was supported in part by a National Science Foundation Graduate Fellowship, a President’s Dissertation Year Fellowship from the University of California, and by National Institutes of Health Postdoctoral Training Grant T32-CA 09043.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: EMS, ethyl methanesulfonate; Df, deficiency; Dp, duplication.
References
- 1.Bishop J M. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- 2.Cantley L C, Auger K R, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann F M. Curr Top Microbiol Immunol. 1989;147:1–29. doi: 10.1007/978-3-642-74697-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Perrimon N. Curr Opin Cell Biol. 1994;6:260–266. doi: 10.1016/0955-0674(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 5.Lyon J, Robinson C, Watson R. Crit Rev Oncog. 1994;5:373–388. doi: 10.1615/critrevoncog.v5.i4.30. [DOI] [PubMed] [Google Scholar]
- 6.Thompson M A, Ramsay R G. BioEssays. 1995;17:341–350. doi: 10.1002/bies.950170410. [DOI] [PubMed] [Google Scholar]
- 7.Nomura N, Takahashi M, Matsui M, Ishii S, Date T, Sasamoto S, Ishizaki R. Nucleic Acids Res. 1988;16:11075–11089. doi: 10.1093/nar/16.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson C B, Challoner P B, Neiman P E, Groudine M. Nature (London) 1986;319:374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay R G, Ikeda K, Rifkind R A, Marks P A. Proc Natl Acad Sci USA. 1986;83:6849–6853. doi: 10.1073/pnas.83.18.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gewirtz A M, Anfossi G, Venturelli D, Valpreda S, Sims R, Calabretta B. Science. 1989;245:180–183. doi: 10.1126/science.2665077. [DOI] [PubMed] [Google Scholar]
- 11.Badiani P, Corbella P, Kioussis D, Marvel J, Weston K. Genes Dev. 1994;8:770–782. doi: 10.1101/gad.8.7.770. [DOI] [PubMed] [Google Scholar]
- 12.Ness S A, Marknell A, Graf T. Cell. 1989;59:1115–1125. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- 13.Siu G, Wurster A L, Lipsick J S, Hedrick S M. Mol Cell Biol. 1992;12:1592–1604. doi: 10.1128/mcb.12.4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek H, Tantravahi R V, Rao V N, Reddy E S, Reddy E P. Proc Natl Acad Sci USA. 1992;89:1291–1295. doi: 10.1073/pnas.89.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burk O, Mink S, Ringwald M, Klempnauer K H. EMBO J. 1993;12:2027–2038. doi: 10.1002/j.1460-2075.1993.tb05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Munain C, Krangel M S. Mol Cell Biol. 1995;1555:3090–3099. doi: 10.1128/mcb.15.6.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paz-Ares J, Ghosal D, Wienand U, Peterson P A, Saedler H. EMBO J. 1987;6:3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tice-Baldwin K, Fink G R, Arndt K T. Science. 1989;246:931–935. doi: 10.1126/science.2683089. [DOI] [PubMed] [Google Scholar]
- 19.Ohi R, McCollum D, Hirani B, Den Haese G J, Zhang X, Burke J D, Turner K, Gould K L. EMBO J. 1994;13:471–483. doi: 10.1002/j.1460-2075.1994.tb06282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzen A L, Kornberg T B, Bishop J M. Cell. 1985;41:449–456. doi: 10.1016/s0092-8674(85)80018-0. [DOI] [PubMed] [Google Scholar]
- 21.Bishop J M, Eilers M, Katzen A L, Kornberg T, Ramsay G, Schirm S. Cold Spring Harbor Symp Quant Biol. 1991;56:99–107. doi: 10.1101/sqb.1991.056.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Katzen A L, Kornberg T, Bishop J M. Development (Cambridge, UK) 1990;110:1169–1183. doi: 10.1242/dev.110.4.1169. [DOI] [PubMed] [Google Scholar]
- 23.Lindsley D L, Zimm G G. The Genome of Drosophila melanogaster. San Diego: Academic; 1992. [Google Scholar]
- 24.Ashburner M. In: Drosophila: A Laboratory Handbook. Ashburner M, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 299–418. [Google Scholar]
- 25.Klemenz R, Weber U, Gehring W J. Nucleic Acids Res. 1987;15:3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karess R E, Rubin G M. Cell. 1984;38:135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- 27.Laski F A, Rio D C, Rubin G M. Cell. 1986;44:7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- 28.Rubin G M, Spradling A C. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 29.Katzen A L. Proto-oncogenes in Drosophila: Molecular and Genetic Analysis. San Francisco: Univ. of California Press; 1990. [Google Scholar]
- 30.Campos-Ortega J A, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin: Springer; 1985. [Google Scholar]
- 31.Judd B H, Shen M W, Kaufman T C. Genetics. 1972;71:139–156. doi: 10.1093/genetics/71.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell S D, Duttaroy A, Katzen A L, Chovnick A. Genetics. 1991;127:367–380. doi: 10.1093/genetics/127.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peifer M, Wieschaus E. Genes Dev. 1990;4:1209–1223. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
- 34.Bazinet C, Katzen A L, Morgan M, Mahowald A P, Lemmon S K. Genetics. 1993;134:1119–1134. doi: 10.1093/genetics/134.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craymer L, Roy E. Drosoph Inf Serv. 1980;55:200–204. [Google Scholar]
- 36.Poodry C. Drosoph Inf Ser. 1980;55:210. [Google Scholar]
- 37.Gonda T J, Sheiness D K, Bishop J M. Mol Cell Biol. 1982;2:617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiele C J, Cohen P S, Israel M A. Mol Cell Biol. 1988;8:1677–1683. doi: 10.1128/mcb.8.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyson P J, Poirier F, Watson R J. Differentiation. 1989;42:24–27. doi: 10.1111/j.1432-0436.1989.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 40.Reiss K, Travali S, Calabretta B, Baserga R. J Cell Physiol. 1991;148:338–343. doi: 10.1002/jcp.1041480303. [DOI] [PubMed] [Google Scholar]
- 41.Trauth K, Mutschler B, Jenkins N A, Gilbert D J, Copeland N G, Klempnauer K H. EMBO J. 1994;13:5994–6005. doi: 10.1002/j.1460-2075.1994.tb06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


