Abstract
Background
Conflicting results have been reported regarding the association of gene polymorphisms in the renin-angiotensin system (RAS) with different aspects of coronary artery disease (CAD), such as myocardial infarction, neointimal hyperplasia or coronary artery vasomotion. Since previous studies have linked angiotensin II to aneurysmal disease, our study hypothesis was that RAS gene polymorphisms may be associated with aneurysm remodeling in response to CAD.
Methods
The study population was selected from a series of 3862 consecutive patients who underwent coronary angiography in our institution. One hundred and thirteen consecutive patients with at least one coronary aneurysm (CA) were compared to 226 randomized control patients without CA. DNA was extracted from white blood cells. The angiotensin-converting enzyme (ACE) I/D and angiotensin type 1 receptor (AT1-R) A/C polymorphisms were detected using previously published techniques.
Results
The distributions of the three ACE genotypes were similar in both groups: CA: 13%, 46%, and 41% for II, ID, and DD respectively; controls: 18%, 41%, and 41% for II, ID, and DD respectively, p = 0.45. The distributions of the three AT1-R genotypes were also similar in both groups: CA: 54%, 41%, and 5% for AA, AC, and CC respectively; controls: 55%, 33%, and 12%, for AA, AC, and CC respectively, p = 0.08.
Conclusion
Our results provide further information on the role of RAS polymorphisms on specific mechanisms implicated in CAD. Although an activated RAS may theoretically promote aneurysm formation, the 2 RAS polymorphisms analyzed in this study are not associated with this process in coronary arteries.
Background
Activation of the renin angiotensin system (RAS) plays an important role in the pathogenesis of coronary artery disease (CAD).
In humans, levels of angiotensin-converting enzyme (ACE) are partly under genetic control [1]; circulating levels of ACE are correlated with an insertion (I) / deletion (D) polymorphism [2]: DD genotypes have higher levels of ACE than either ID or II genotypes [3]. Although the concept that genetic factors may increase the risk of CAD via activation of the RAS is an attractive one, discordant results have been published regarding the association of the ACE I/D polymorphism with myocardial infarction. While the initial study by Cambien et al reported an increased frequency of the DD genotype in patients with myocardial infarction [4], a large prospective study by Lindpaintner et al did not confirm this association [5].
The effects of angiotensin II are exerted through the activation of specific high activity receptors [6]. A polymorphism located in the 3' untranslated region of the type 1 receptor (AT1-R) gene (corresponding to an adenine/guanine substitution at the 1166 nucleotid position of the mRNA) has been described [7]. Although it has been less studied than the ACE I/D polymorphism, discordant results have also been reported regarding the association of the AT1-R polymorphism with myocardial infarction. While Tiret et al reported synergistic effects of ACE and AT1-R gene polymorphisms on the risk of myocardial infarction [8], this was not confirmed in a recent study by Steeds et al [9].
A possible explanation for these discrepancies is that myocardial infarction is the results of a multifactorial process implicating different mechanisms such as plaque progression, vessel remodeling, plaque rupture, thrombosis or vasomotion [10,11]; each of these mechanisms may be affected in one way or another by a given genotype but a clear demonstration of the effect may be difficult if the sole endpoint studied is the occurrence of myocardial infarction. Smaller studies with carefully chosen intermediate end-points may be useful to elucidate the potential impact of RAS polymorphisms on CAD. Previously, we reported the relationships between RAS polymorphisms and several specific mechanisms implicated in the pathogenesis of CAD: the in vivo response to methylergonovine was used to study coronary artery vasomotion [12]; restenosis after stent implantation or balloon angioplasty were used as models of neointimal hyperplasia or constrictive remodeling [13,14]. In the present study, we sought to investigate another form of abnormal vessel remodeling in response to CAD: aneurysm formation.
Previous studies have linked angiotensin II to aneurysm formation. In apo E -/- mice, angiotensin II infusion dramatically increased the extent of atherosclerosis and was associated with the formation of large abdominal aortic aneurysms [15]. Different mechanisms may be involved: angiotensin II may promote development of aneurysms by contributing to the inflammatory response [15,16], altering smooth muscle cell migration [17], or inducing matrix metalloproteinases production [18,19]. Aneurysmal coronary artery disease is characterized by abnormal dilation of a localized or diffuse segment of the coronary arterial tree. Approximately 1 to 5% of the patients with coronary atherosclerosis have coronary aneurysm(s) (CA) [20-23]. In the present study, we investigated whether the ACE and AT1-R gene polymorphisms may be associated with CA. Alleles and genotypes frequencies were compared between 113 patients with atherosclerotic CA and 226 control patients.
Methods
Study Population
The study population was selected from a series of 3862 consecutive patients who underwent coronary angiography in our institution. CA were defined as localized or diffuse coronary dilations that exceeded the diameter of normal adjacent segments by 1.5 times [21]. One hundred and thirteen consecutive patients with evidence of coronary atherosclerosis and at least one coronary aneurysm were selected and constitute the CA group. During the same period, we randomly selected 226 patients (2 :1 ratio) who presented with coronary atherosclerosis but without CA to form the control group. Patients gave informed consent for the study. We previously reported in the same population the association between functional polymorphisms in matrix metalloproteinase genes and coronary aneurysms [24].
Clinical and Angiographic Data
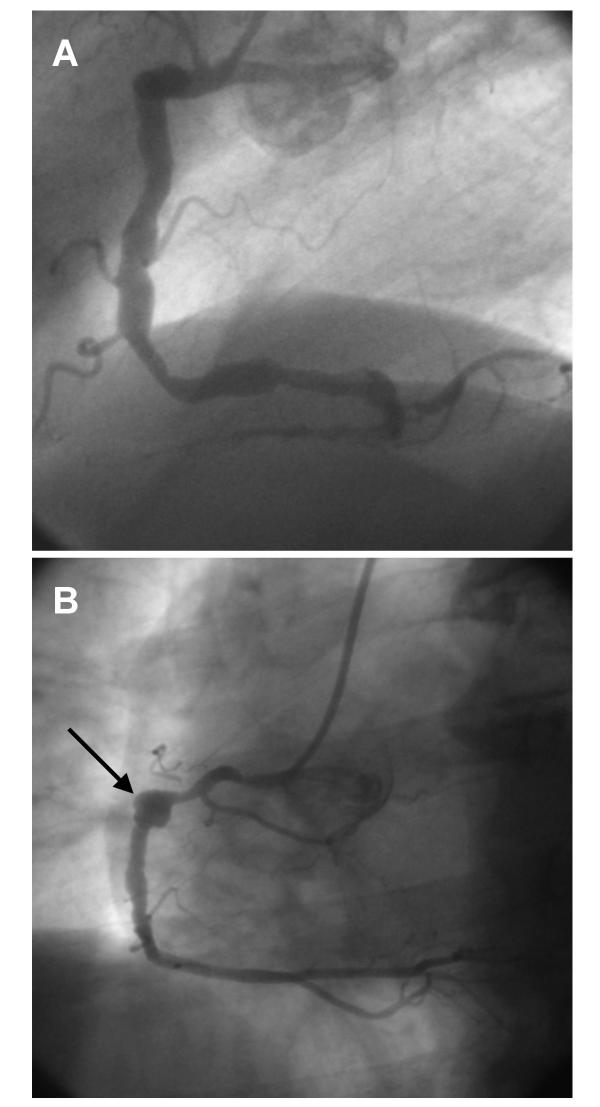
Epidemiological and clinical characteristics were collected by trained physicians. Coronary angiograms were analysed by two experienced interventionnal cardiologists. The number of non significant (≤ 50%) or significant (>50%) stenoses was recorded for each patient. The extent of aneurysmal disease (number of segments and vessels diseased per patient) was also noted as was the type (diffuse or focal) of CA. Aneurysmal segment was classified as focal when it involved a discrete portion of a segment with adjacent normal segment within the same segment and diffuse when the entire segment was dilated with no normal vessel within the segment [25]. Representative examples of diffuse and focal CA are shown in Figure 1.
Figure 1.
Representative examples of coronary aneurysms: A. Diffuse aneurysmal disease of the right coronary artery. B. A focal coronary aneurysm of the mid-portion of the right coronary artery (arrow)
The largest diameter of each aneurysmal vessel was measured by quantitative coronary angiography with use of the CMS system as previously described [26].
Genetic Study
Genomic DNA was extracted from white blood cells by a « salting out » procedure as previously described [27]. The ACE fragment [13] containing the I/D sequence and AT1 receptor fragment containing the A/C -1166 substitution [12] were amplified by Polymerase Chain Reaction (PCR). The ACE and AT1 receptor A/C polymorphisms were detected as previously described [12].
Statistical Analysis
Statistical analysis was conducted with SAS software, version 6.12 (SAS Institute Inc., Cary, NC). Mean values ± SD were calculated for quantitative data. The quantitative variables were compared between groups with use of unpaired Student t tests. Qualitative variables were compared by the Pearson's χ2-test, or the Fisher's exact test when necessary.
Results
The baseline characteristics are presented in Tables I and II. Except for male gender (CA group: 90%, control group: 80%; p = 0.02), previous myocardial infarction (CA group: 68%, control group: 52%; p = 0.005) and an history of aortic aneurysm (CA group: 7%, control group: 0.4%; p = 0.0003) which were more frequent in the CA group, the baseline characteristics were similarly distributed in both groups. Forty eight percent of the patients had aneurysmal involvement of more than one coronary vessel. The most frequent location of CA was the right coronary artery. In most cases, this involvement was classified as diffuse. The maximal diameter of the aneurysmal segments was 6.6 ± 1.2 mm.
Table 1.
Clinical and angiographic characteristics in CA and control patients as a function of the ACE I/D polymorphism.
| CA (n = 113) | Controls (n = 226) | |||||
| II (n = 15) | ID (n = 52) | DD (n = 46) | II (n = 41) | ID (n = 92) | DD (n = 93) | |
| Age, years | 63 ± 13 | 64 ± 10 | 60 ± 13 | 64 ± 11 | 61 ± 11 | 62 ± 11 |
| Body Mass Index, kg/m2 | 28.4 ± 5 | 27.3 ± 4 | 27.6 ± 4 | 27.3 ± 4 | 27.8 ± 12 | 26.6 ± 4 |
| Male gender, % | 93 | 92 | 87 | 73 | 83 | 81 |
| Smoking, % | 80 | 77 | 80 | 63 | 76 | 67 |
| Hypercholesterolemia, % | 87 | 79 | 80 | 88 | 71 | 75 |
| Hypertension, % | 40 | 40 | 48 | 46 | 53 | 47 |
| Diabetes, % | 13 | 13 | 20 | 24 | 27 | 23 |
| Previous myocardial infarction, % | 73 | 67 | 67 | 56 | 59 | 44 |
| History of aortic aneurysm, % | 7 | 4 | 11 | 2 | 0 | 0 |
| Number of stenotic lesions, % | ||||||
| - Non significant | 0 | 6 | 7 | 15 | 9 | 7 |
| - Single-vessel | 13 | 30 | 28 | 24 | 35 | 37 |
| - Two-vessel | 60 | 42 | 43 | 37 | 32 | 31 |
| - Three-vessel | 27 | 22 | 22 | 24 | 25 | 25 |
| Coronary aneurysm(s) location, % | ||||||
| - Left Main | 0 | 4 | 13 | |||
| - Left Anterior Descending Artery | 60 | 52 | 50 | NA | NA | NA |
| - Circumflex | 67 | 34 | 35 | NA | NA | NA |
| - Right Coronary Artery | 67 | 74 | 72 | NA | NA | NA |
| Number of vessel(s) with CA, % | ||||||
| - 1 | 33 | 56 | 52 | NA | NA | NA |
| - 2 | 40 | 28 | 35 | NA | NA | NA |
| - 3 | 27 | 16 | 13 | NA | NA | NA |
| Type of aneurysm(s), % | ||||||
| - Diffuse | 93 | 79 | 78 | NA | NA | NA |
| - Focal | 7 | 21 | 22 | NA | NA | NA |
Table 2.
Clinical and angiographic characteristics in CA and control patients as a function of the AT1 receptor A/C polymorphism.
| CA (n = 113) | Controls (n = 226) | |||||
| AA (n = 61) | AC (n = 46) | CC (n = 6) | AA (n = 123) | AC (n = 75) | CC (n = 28) | |
| Age, years | 61 ± 13 | 64 ± 11 | 63 ± 7 | 61 ± 11 | 61 ± 12 | 64 ± 9 |
| Body Mass Index, kg/m2 | 27.2 ± 4 | 28.4 ± 4 | 26 ± 6 | 27.7 ± 10 | 26.4 ± 5 | 27.3 ± 5 |
| Male gender, % | 90 | 91 | 83 | 80 | 81 | 75 |
| Smoking, % | 80 | 78 | 67 | 67 | 75 | 71 |
| Hypercholesterolemia, % | 80 | 83 | 67 | 75 | 73 | 86 |
| Hypertension, % | 41 | 43 | 67 | 54 | 41 | 54 |
| Diabetes, % | 16 | 13 | 33 | 26 | 19 | 36 |
| Previous myocardial infarction, % | 70 | 72 | 17 | 54 | 53 | 39 |
| History of aortic aneurysm, % | 10 | 4 | 0 | 0 | 0 | 4 |
| Number of stenotic lesions, % | ||||||
| - Non significant | 6 | 4 | 0 | 6 | 11 | 22 |
| - Single-vessel | 22 | 29 | 67 | 32 | 37 | 32 |
| - Two-vessel | 45 | 51 | 0 | 33 | 31 | 32 |
| - Three-vessel | 27 | 16 | 33 | 29 | 21 | 14 |
| Coronary aneurysm(s) location, % | ||||||
| - Left Main | 7 | 7 | 17 | NA | NA | NA |
| - Left Anterior Descending Artery | 47 | 60 | 50 | NA | NA | NA |
| - Circumflex | 33 | 49 | 17 | NA | NA | NA |
| - Right Coronary Artery | 72 | 73 | 67 | NA | NA | NA |
| Number of vessel(s) with CA, % | ||||||
| - 1 | 60 | 40 | 0 | NA | NA | NA |
| - 2 | 27 | 38 | 50 | NA | NA | NA |
| - 3 | 13 | 22 | 50 | NA | NA | NA |
| Type of aneurysm(s), % | ||||||
| - Diffuse | 80 | 80 | 83 | NA | NA | NA |
| - Focal | 20 | 20 | 17 | NA | NA | NA |
Regarding ACE I/D genotypes, there was no statistically significant difference in the baseline characteristics both in the CA group and in the control group (Table I). The distributions of the three genotypes were similar in both groups: CA: 13%, 46%, and 41% for II, ID, and DD respectively; controls: 18%, 41%, and 41% for II, ID, and DD respectively; p = 0.45. The proportions of D allele were 64% in the CA group and 62% in the control group; p = 0.58.
Regarding AT1 receptor A/C genotypes, there was no statistically significant difference in the baseline characteristics both in the CA group and in the control group (Table II). The distributions of the three genotypes were similar in both groups: CA: 54%, 41%, and 5% for AA, AC, and CC respectively; controls: 55%, 33%, and 12%, for AA, AC, and CC respectively; p = 0.08. The proportions of C allele were 26% in the CA group and 29% in the control group; p = 0.36.
Discussion
CA is a relatively rare manifestation of CAD. In the present study, 2.9% of patients undergoing coronary angiography had CA; previous studies have reported rates ranging between 1 and 5% [20-23]. The exact mechanisms by which CA may occur in response to atherosclerosis are unknown but histological and clinical studies suggest that processes similar to those implicated in abdominal aortic aneurysms (AAA) may be involved [22]. Experimental studies have linked angiotensin II to aneurysm formation. Nishijo et al observed that angiotensin II stimulated aneurysm formation in hypertensive transgenic mice that overproduce angiotensin II [28]. Recently, Daugherty et al found that continuous perfusion of angiotensin II was associated with the formation of large AAA in atherosclerotic apoE-/- mice with a dose-response effect [15]. Moreover, angiotensin II-induced AAA development was significantly reduced by doxycycline, a broad spectrum MMP inhibitor [19]. Furthermore, Huang et al showed that ACE inhibition reduced the rate of spontaneous rupture of the internal elastic lamina of the abdominal aorta [29] and Liao et al reported total inhibition of elastase-induced AAA in a rat model by treatment with 3 different ACE inhibitors [30].
In humans, limited data are available regarding the implication of the RAS in aneurysm formation; however, genetic association studies may provide interesting information. Discordant results have been reported regarding the ACE I/D polymorphism and abdominal aortic aneurysm. Although Hamano et al failed to show any positive correlation between this polymorphism and the occurrence of AAA [31], a recent study by Pola et al suggested that the ACE DD genotype may be a risk factor for AAA in normotensive patients [32]. In the present study, the distribution of the ACE I/D genotypes were similar in the CA and the control groups, suggesting that the ACE I/D polymorphism is not an important determinant of CA formation. To the best of our knowledge, the association of the AT1-R genotype with AAA has never been specifically analyzed. In the present study, the distribution of the AT1-R genotypes were similar in the CA and the control groups, suggesting that the AT1-R polymorphism is not an important determinant of CA formation.
Study Limitations
The interpretation of negative results may be limited by a relatively small sample size. With a statistical power of 80% and a significant value of 0.05, the sample size of this study allowed us to detect a 11% difference in the frequency of the ACE D allele and a 10% difference in the frequency of the AT1-R C allele. However, the 113 patients with CA were prospectively recruited by a systematic analysis of 3862 consecutive angiography procedures; the design of larger studies would imply a multicentric recruitment. Finally, this was an angiographic study; as shown by Maehara et al, the use of intravascular ultrasound may allow a better analysis of aneurysmal segments [33].
Conclusions
Epidemiological studies have suggested a link between RAS polymorphisms and CAD [4-8]. Our results provide further information on the role of RAS polymorphisms on specific mechanisms implicated in CAD. We [14] and others [34] previously reported that the ACE I/D polymorphism was associated with neointimal hyperplasia in response to coronary stent implantation. We also showed that the AT1-R polymorphism (but not the ACE I/D polymorphism) was associated with coronary artery vasoconstriction in response to methylergonovine [12]. By contrast, the ACE I/D and AT1-R polymorphisms are not associated with restenosis of coronary arteries after balloon angioplasty [13], a process which is mainly related to constrictive vessel remodeling [35]; in the present study, we focused on another form of vessel remodeling, aneurysm formation, which again was not associated with either the ACE I/D or the AT1-R polymorphisms. Although an activated RAS may theoretically promote aneurysm formation via mechanisms such as an induction of metalloproteinases [18] or an increased inflammation [15], the 2 RAS polymorphisms analyzed in this study are not associated with this process in coronary arteries.
Competing interests
We have no competing interests to disclose for this paper
Authors' contributions
NL, CB and PA participated in the design of the study and performed the statistical analysis. NL, XH and NH carried out the molecular genetic studies. NL, JML and CB participated in the selection of patients and in angiographic analyses. NL and CB drafted the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Nicolas Lamblin, Email: nicolas.lamblin@pasteur-lille.fr.
Xavier Hermant, Email: xavier.hermant@pasteur-lille.fr.
Jean-Marc Lablanche, Email: jmlablanche@chru-lille.fr.
Nicole Helbecque, Email: nicole.helbecque@pasteur-lille.fr.
Philippe Amouyel, Email: philippe.amouyel@pasteur-lille.fr.
Christophe Bauters, Email: cbauters@chru-lille.fr.
References
- Cambien F, Alhenc-Gelas F, Herbeth B, Andre JL, Rakotovao R, Gonzales MF, Allegrini J, Bloch C. Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet. 1988;43:774–80. [PMC free article] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D, Luc G, Bard JM, Bara L, Ricard S, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–4. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, Buring J, Hennekens CH. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–11. doi: 10.1056/NEJM199503163321103. [DOI] [PubMed] [Google Scholar]
- Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;165:196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Bonnardeaux A, Davies E, Jeunemaitre X, Fery I, Charru A, Clauser E, Tiret L, Cambien F, Corvol P, Soubrier F. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–9. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- Tiret L, Bonnardeaux A, Poirier O, Ricard S, Marques-Vidal P, Evans A, Arveiler D, Luc G, Kee F, Ducimetiere P, et al. Synergistic effects of angiotensin-converting enzyme and angiotensin-II type 1 receptor gene polymorphisms on risk of myocardial infarction. Lancet. 1994;344:910–3. doi: 10.1016/S0140-6736(94)92268-3. [DOI] [PubMed] [Google Scholar]
- Steeds RP, Wardle A, Smith PD, Martin D, Channer KS, Samani NJ. Analysis of the postulated interaction between the angiotensin II sub-type 1 receptor gene A1166C polymorphism and the insertion/deletion polymorphism of the angiotensin converting enzyme gene on risk of myocardial infarction. Atherosclerosis. 2001;154:123–8. doi: 10.1016/S0021-9150(00)00438-X. [DOI] [PubMed] [Google Scholar]
- Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture. Circulation. 1995;94:2013–20. doi: 10.1161/01.cir.94.8.2013. [DOI] [PubMed] [Google Scholar]
- Amant C, Hamon M, Bauters C, Richard F, Helbecque N, McFadden EP, Escudero X, Lablanche JM, Amouyel P, Bertrand ME. The angiotensin II type 1 receptor gene polymorphism is associated with coronary artery vasoconstriction. J Am Coll Cardiol. 1997;29:486–90. doi: 10.1016/S0735-1097(96)00535-9. [DOI] [PubMed] [Google Scholar]
- Hamon M, Bauters C, Amant C, McFadden EP, Helbecque N, Lablanche JM, Bertrand ME, Amouyel P. Relation between the deletion polymorphism of the angiotensin-converting enzyme gene and late luminal narrowing after coronary angioplasty. Circulation. 1995;92:296–9. doi: 10.1161/01.cir.92.3.296. [DOI] [PubMed] [Google Scholar]
- Amant C, Bauters C, Bodart JC, Lablanche JM, Grollier G, Danchin N, Hamon M, Richard F, Helbecque N, McFadden EP, Amouyel P, Bertrand ME. D allele of the angiotensin I-converting enzyme is a major risk factor for restenosis after coronary stenting. Circulation. 1997;96:56–60. doi: 10.1161/01.cir.96.1.56. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–12. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning MW, Cassi LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med. 2002;7:45–54. doi: 10.1191/1358863x02vm413ra. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Luscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotids and angiotensin 1 receptors. J Clin Invest. 1995;96:141–9. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Laouar A, Huberman E. Autocrine regulation of macrophage differentiation and 92-kDa gelatinase production by tumor necrosis factor-alpha via alpha5 beta1 integrin in HL-60 cells. J Biol Chem. 1998;273:11583–8. doi: 10.1074/jbc.273.19.11583. [DOI] [PubMed] [Google Scholar]
- Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–8. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- Befeler B, Aranda MJ, Embi A, Mullin FL, El-Sherif N, Lazzara R. Coronary artery aneurysms: study of the etiology, clinical course and effect on left ventricular function and prognosis. Am J Med. 1977;62:597–607. doi: 10.1016/0002-9343(77)90423-5. [DOI] [PubMed] [Google Scholar]
- Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, Mudd JG, Gosselin AJ. Aneurysmal coronary artery disease. Circulation. 1983;67:134–8. doi: 10.1161/01.cir.67.1.134. [DOI] [PubMed] [Google Scholar]
- Virmani R, Robinowitz M, Atkinson JB, Forman MB, Silver MD, McAllister HA. Acquired coronary arterial aneurysms: an autopsy study of 52 patients. Hum Pathol. 1986;17:575–83. doi: 10.1016/s0046-8177(86)80129-0. [DOI] [PubMed] [Google Scholar]
- Demopoulos VP, Olympios CD, Fakiolas CN, Pissimissis EG, Economides NM, Adamopoulou E, Foussas SG, Cokkinos DV. The natural history of aneurysmal coronary artery disease. Heart. 1997;78:136–41. doi: 10.1136/hrt.78.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamblin N, Bauters C, Hermant X, Lablanche JM, Helbecque N, Amouyel P. Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. J Am Coll Cardiol. 2002;40:43–8. doi: 10.1016/S0735-1097(02)01909-5. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Ports TA, Amidon TM, Goldberger JJ, Bhushan V, Kane JP, Yock P, Malloy MJ. Increased prevalence of coronary ectasia in heterozygous familial hypercholesterolemia. Circulation. 1995;91:1375–80. doi: 10.1161/01.cir.91.5.1375. [DOI] [PubMed] [Google Scholar]
- Reiber JH, van der Zwet PM, Koning G, von Land CD, van Meurs B, Gerbrands JJ, Buis B, van Voorthuisen AE. Accuracy and precision of quantitative digital coronary arteriography: observer-, short-, and medium-term variabilities. Cathet Cardiovasc Diagn. 1993;28:187–98. doi: 10.1002/ccd.1810280301. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo N, Sugiyama F, Kimoto K, Taniguchi K, Murakami K, Suzuki S, Fukamizu A, Yagami K. Salt-sensitive aortic aneurysm and rupture in hypertensive transgenic mice that overproduce angiotensin II. Lab Invest. 1998;78:1059–66. [PubMed] [Google Scholar]
- Huang W, Alhenc Gelas F, Osborne-Pellegrin MJ. Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ Res. 1998;82:879–90. doi: 10.1161/01.res.82.8.879. [DOI] [PubMed] [Google Scholar]
- Liao S, Miralles M, Kelley BJ, Curci JA, Borhani M, Thompson RW. Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J Vasc Surg. 2001;33:1057–64. doi: 10.1067/mva.2001.112810. [DOI] [PubMed] [Google Scholar]
- Hamano K, Ohishi M, Ueda M, Fujioka K, Katoh T, Zempo N, Fujimura Y, Okamura A, Rakugi H, Higaki J, Ogihara T, Esato K. Deletion polymorphism in the gene for angiotensin-converting enzyme is not a risk factor predisposing to abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;18:158–61. doi: 10.1053/ejvs.1999.0873. [DOI] [PubMed] [Google Scholar]
- Pola R, Gaetani E, Santoliquido A, Gerardino L, Cattani P, Serricchio M, Tondi P, Flore R, Grande M, Carbonin P, Fadda G, Pola P. Abdominal aortic aneurysm in normotensive patients: association with angiotensin-converting enzyme gene polymorphism. Eur J Vasc Endovasc Surg. 2001;21:445–9. doi: 10.1053/ejvs.2001.1339. [DOI] [PubMed] [Google Scholar]
- Maehara A, Mintz GS, Ahmed JM, Fuchs S, Castagna MT, Pichard AD, Satler LF, Waksman R, Suddath WO, Kent KM, Weissman NJ. An intravascular ultrasound classification of angiographic coronary artery aneurysms. Am J Cardiol. 2001;88:365–70. doi: 10.1016/S0002-9149(01)01680-0. [DOI] [PubMed] [Google Scholar]
- Ribichini F, Steffenino G, Dellavalle A, Matullo G, Colajanni E, Camilla T, Vado A, Benetton G, Uslenghi E, Piazza A. Plasma activity and insertion/deletion polymorphism of angiotensin I-converting enzyme: a major risk factor and a marker of risk for coronary stent restenosis. Circulation. 1998;97:147–54. doi: 10.1161/01.cir.97.2.147. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong C, Hong MK, Kovach JA, Leon MB. Arterial remodeling after coronary angioplasty: a serial intravascular untrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]