Abstract
Carbohydrate restriction as a strategy for control of obesity is based on two effects: a behavioral effect, spontaneous reduction in caloric intake and a metabolic effect, an apparent reduction in energy efficiency, greater weight loss per calorie consumed. Variable energy efficiency is established in many contexts (hormonal imbalance, weight regain and knock-out experiments in animal models), but in the area of the effect of macronutrient composition on weight loss, controversy remains. Resistance to the idea comes from a perception that variable weight loss on isocaloric diets would somehow violate the laws of thermodynamics, that is, only caloric intake is important ("a calorie is a calorie"). Previous explanations of how the phenomenon occurs, based on equilibrium thermodynamics, emphasized the inefficiencies introduced by substrate cycling and requirements for increased gluconeogenesis. Living systems, however, are maintained far from equilibrium, and metabolism is controlled by the regulation of the rates of enzymatic reactions. The principles of nonequilibrium thermodynamics which emphasize kinetic fluxes as well as thermodynamic forces should therefore also be considered.
Here we review the principles of nonequilibrium thermodynamics and provide an approach to the problem of maintenance and change in body mass by recasting the problem of TAG accumulation and breakdown in the adipocyte in the language of nonequilibrium thermodynamics. We describe adipocyte physiology in terms of cycling between an efficient storage mode and a dissipative mode. Experimentally, this is measured in the rate of fatty acid flux and fatty acid oxidation. Hormonal levels controlled by changes in dietary carbohydrate regulate the relative contributions of the efficient and dissipative parts of the cycle. While no experiment exists that measures all relevant variables, the model is supported by evidence in the literature that 1) dietary carbohydrate, via its effect on hormone levels controls fatty acid flux and oxidation, 2) the rate of lipolysis is a primary target of insulin, postprandial, and 3) chronic carbohydrate-restricted diets reduce the levels of plasma TAG in response to a single meal.
In summary, we propose that, in isocaloric diets of different macronutrient composition, there is variable flux of stored TAG controlled by the kinetic effects of insulin and other hormones. Because the fatty acid-TAG cycle never comes to equilibrium, net gain or loss is possible. The greater weight loss on carbohydrate restricted diets, popularly referred to as metabolic advantage can thus be understood in terms of the principles of nonequilibrium thermodynamics and is a consequence of the dynamic nature of bioenergetics where it is important to consider kinetic as well as thermodynamic variables.
Background
Dietary carbohydrate provides both an energy source and, through its effects on insulin and other hormones, regulatory control of metabolism. In the context of obesity, diabetes and related pathologic states, it is argued by many researchers that the level of carbohydrate, by its hormonal effects, controls the disposition of nutrient intake beyond simple caloric balance [1-11]. From this point of view, fat plays a relatively passive role and the deleterious effects of high dietary fat are expected only if there is sufficient dietary carbohydrate to provide the hormonal state in which the fat will be stored rather than oxidized. In its practical application, the principle has given rise to several forms of popular diet strategies which have in common some degree of carbohydrate restriction [12-14] or effective glycemic level [3,15]. Experimentally, protocols based on carbohydrate restriction do as well or better than fat reduction for weight loss (reviews: [16-18]), but because they are somewhat iconoclastic with respect to official dietary recommendations and because they derive from the popular diets where discourse is heated, they remain controversial. The extent to which carbohydrate restriction is successful as a strategy for control of obesity or diabetes can be attributed to two effects. The strategy frequently leads to a behavioral effect, a spontaneous reduction in caloric intake as seen in ad lib comparisons. There is also a metabolic effect, an apparent reduction in energy efficiency seen in isocaloric comparisons, popularly referred to as metabolic advantage. The two are not necessarily independent: an association between thermogenesis, a reflection of inefficiency, and satiety has been established by Westerterp, et al., for example [19].
Experimental demonstrations of energy inefficiency in humans have recently been summarized [16,17,20] and the phenomenon has been demonstrated in animal models (e.g., ref. [21] and, most dramatically ref. [22]). This metabolic effect, however, is not universally accepted as a major component in human experiments, oddly even by investigators who have provided experimental support [23-26]. Variable energy efficiency, however, is known in many contexts: hormonal imbalance [27,28], intensive insulin therapy [29], studies of weight regain [30,31] and particularly knock-out experiments in animals [32-34]. Experiments demonstrating variable energy efficiency in the context of weight loss, however, remain controversial because of the difficulty in validating compliance in dietary interventions and because of a resistance to what is perceived as a violation of thermodynamics, that is, an intuitive feeling that, in the end, everything must even out. Thus, progress in this field still depends on a proper understanding of caloric efficiency and a description of how energy balance can account for differences in weight loss in isocaloric comparisons.
We have previously described how different isocaloric diets are actually expected to have different effects on metabolism and therefore on body mass [16,35,36]. Our previous arguments were largely based on equilibrium thermodynamics because this is most familiar. However, living systems, and in particular, TAG stores in adipocytes, are maintained far from equilibrium and the rates of breakdown of such high energy compounds are regulated by the kinetics of the enzymes that catalyze hydrolysis and re-synthesis. Because the system is maintained far from equilibrium, energy measurements provide values of (∂G/∂ξ)T,P where ξ is the reaction progress coordinate and the path-independence of state variables, that is, ΔG values measured in a calorimeter do not necessarily apply [37]. In essence, then, the problem is as much one of rates as of free energy. Much progress has been made in the development of nonequilibrium thermodynamics for the study of metabolism although there is no universally accepted approach ([38-40] and references therein) and the current work is intended to provide a first step towards developing the problem of energy efficiency in response to dietary macronutrients.
Here we review the basic ideas of nonequilibrium thermodynamics and provide an approach to the problem of maintenance and change in body mass following these ideas. The emphasis is on flux of metabolites in adipose tissue since, in the end, this is the major reflection of energy balance and obesity. The work has several goals:
1. To recast the problem of TAG accumulation and breakdown in the adipocyte in the language of nonequilibrium thermodynamics. In particular, we want to describe adipocyte physiology in terms of cycling between an efficient storage mode and a dissipative mode. Experimentally, this is reflected in the rate of fatty acid flux and fatty acid oxidation.
2. To provide a plausible mechanism for how different efficiencies of isocaloric diets can be accounted for by changes in kinetics. To show that hormonal levels controlled by changes in carbohydrate intake determine the relative contributions of the efficient and dissipative parts of the TAG-FA cycle.
Overall, the model is intended to provide a conceptual framework for energy efficiency in nutrition and to point the way to future research. We feel that the approach has general implications as well and is tied to the philosophical position espoused by Prigogine and followers in emphasizing the dynamic nature of physical processes, that is, the need to consider kinetics as well as thermodynamics [39,41-44].
We emphasize that metabolic efficiency is not always seen in diet comparisons. A thermodynamic analysis, however, shows that inefficiency is to be expected and it is the cases where "a calorie is a calorie" that need to be explained: it is the unique characteristics of living systems – maintenance of a steady-state through tightly controlled feed-back systems – not general physical laws that accounts for energy balance when it is found. Practically speaking, the importance of obesity and other metabolic disorders makes it important to see what the requirements are to break out of these stable states.
Nonequilibrium thermodynamics
It is traditional to separate thermodynamics and kinetics but such a division applies strictly only to equilibrium systems [41,45]. Systems that are far from equilibrium may undergo chemical reactions that never attain equilibrium and are characterized by the flux of material as well as energy. In a dietary intervention, the flux of material must be integrated over time to determine the total change in weight or fat loss. Thus, accumulated changes may be controlled by the presence of a catalyst or other factors that affect the rate of reaction.
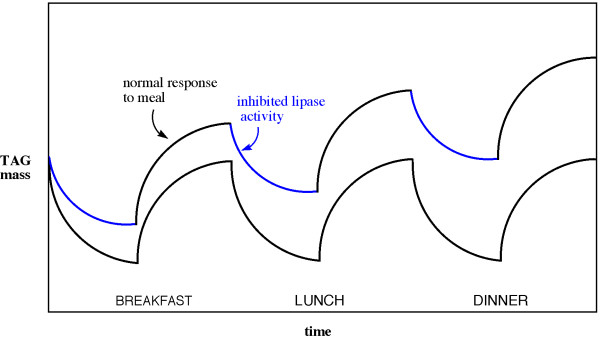
In the case at hand, adipocytes cycle between states of greater or lower net breakdown of fat (lipolysis and reesterification) depending on the hormonal state which, in turn, is dependent on the macronutrient composition of the diet. A hypothetical scheme for changes in adipocyte TAG and a proposal for how TAG gain or loss could be different for isocaloric diets with different levels of insulin is shown in Figure 1. Under normal control conditions of weight maintenance, the breakdown and utilization of TAG by lipolysis and oxidation is balanced by the re-synthesis from food intake. Assuming, for simplicity. an instantaneous spike in food at meals, the curves represent the net flow of material (possibly through several TAG-FA cycles) within the adipocyte. In a coarse-grained analysis, the integral over time of the fluctuations between different states, measures the change in stored TAG in the time of a dietary experiment. The average is stable, that is, appears as weight maintenance. If now each meal is maintained at constant calories but there is an increase in the percentage of carbohydrate leading to higher insulin levels, the lipases may be reduced in activity (blue line in Figure 1). The rate of re-synthesis of TAG is less perturbed by the elevated insulin [46] and indeed may go the other way. The system may cycle between states, which, while they never come to equilibrium, have the net affect of producing changes in the direction of accumulation of TAG.
Figure 1.
Hypothetical kinetics of fat storage and hydrolysis. Model for the effect of insulin on efficiency of storage. Black line indicates response under conditions of weight maintenance. Blue line shows the effect of added insulin on hormone sensitive lipase activity.
In carbohydrate restriction, the decrease in carbohydrate may be accompanied by an increase in dietary fat and the relative effect on rate of TAG accumulation due to disinhibition of lipolysis vs the effect of increased substrate will determine the efficiency. As noted below, experiments in the literature [47] show that after chronic exposure to a low carbohydrate diet (higher dietary TAG), the plasma levels of TAG following a high fat meal are reduced compared to controls. Of course, replacing dietary carbohydrate with dietary protein at constant lipid will be consistent with the model in the absence of compensating effects.
In these cases, the integrated change in TAG over the course of a day (or several days) will no longer be zero. In this way, two diets may lead to different weight gain (as indicated by accretion of fat), even though they have the same number of calories, simply because they affect hormonal levels differently. An analysis based on rates suggests further that a new steady state may be obtained in which TAG may be maintained at a higher or lower level even if the hormonal state returns to one that does not lead to further change. The cell may then relax from one steady state to another, the observed macroscopic weight gain or loss. The goal here is to ask what would it take to produce behavior like that in Figure 1.
For minor perturbations, there will be compensating effects of competing pathways (increase in insulin secretion due to fatty acid production [48,49], for example) and one can expect, insofar as the model corresponds to reality, there may be a threshold effect. This is reflected in the emphasis on extreme carbohydrate reduction in the early phases of popular weight loss diets [12-14]. We emphasize that all of the potential sources of metabolic inefficiency – increased reliance on gluconeogenesis and consequent increased protein turnover, up-regulation of uncoupling proteins – described previously [16,36] may still be operative but the net change in fat stores must be the final common output if body mass is to undergo change.
Formalism of nonequilibrium thermodynamics
For systems that are not at equilibrium, changes in entropy will drive the system towards equilibrium. If the system is close to equilibrium or, as in the case here, there is a small change in the total free energy – only a small fraction of TAG is actually hydrolyzed in the course of a day – then the change in entropy will be due to dSe, the flux of entropy that is exchanged with the environment and dSi, that due to the irreversible effect of the chemical reaction [41,50,51]. We are then interested in the rate of entropy production, Φ, due to chemical reactions at constant T and P:
| Φ = dSi/dt = - (1/T) ΣN μk dnk/dt |
In nonequilibrium thermodynamics, overall flux of entropy is considered as a product of forces (derivative of the potential), Xk and flows Jk, all forces and flows vanishing at equilibrium. In a chemical system, the force Xk is defined as the negative of the chemical potential of the kth reaction, sometimes referred to as the affinity A = - (∂G/∂ξ)T,P where ξ is the extent of chemical reaction. In other words, a positive sign of × indicates spontaneous forward driving force. The force, then, depends on the concentration of reactants and products, the standard free energy and the extent of reaction. It is worth noting that for the systems like the adipocyte that are maintained far from equilibrium the distinction between ΔG values and (∂G/∂ξ)T,P noted by other authors [37] is important, that is, the simple additivity of state variables that underlies the idea that all calories are equivalent, is not valid.
The flows, Jk, are identified with the flux of the kth reaction. The flux of fatty acid in an adipocyte, for example, J1 = vlipolysis + vsynthesis, the sum of breakdown and synthesis rates for TAG. In the phenomenologic approach of nonequilibrium thermodynamics, the forces and flows may be the sum of several individual processes.
In applying the principles of nonequilibrium thermodynamics, the analysis will be simplified if we make the assumption that the fluxes are linear functions of the forces, in analogy with similar linear equations such as Fick's law of diffusion (diffusion is a linear function of the concentration gradient), or Ohm's law (current is a linear function of the potential). The proportionality constant Lkj is called the phenomenological coefficient.
| Jk = Σn LkjXj |
Although the general requirement that condition (2) hold is that the system be close to equilibrium, the linear approximation is often observed to be appropriate for systems very far from equilibrium, subject to stabilizing feedback and in enzymatic systems operating in the range of substrate concentrations that are close to KM [52,53]. Further discussion is found in references [54-56]. Whereas the assumption of linearity is reasonable for the current model where small perturbations far from equilibrium occur in a region of high substrate, in the end, it is a working assumption and experimental tests of the model will ultimately determine if the assumption is justified.
Qualitative features of the adipocyte model and comparison to glycolysis
F igure 2 shows a simple model that is proposed for adipose tissue metabolism under conditions bearing on changes in body mass. The flux of TAG (1) represents the net accumulation or output with respect to the cell itself. This process driven by (2) the input of glycerol-3-phosphate from glycolysis or glyceroneogenesis and (3) fatty acid (FA) from plasma FA. The high energy form of the cycle, TAG, is stored. From the point of view of the organism, it is the FA output that provides fuel for oxidation and cell metabolism. This output may be taken as analogous to the system load as it is usually described in nonequilibrium thermodynamics. Oxidation and FA uptake are largely controlled independently, that is, the adipocyte system has high output conductance and low input conductance, that is, by analogy with an electronic system, is an ideal amplifier. Because there is effectively no load on the system and overall metabolic effect is simply to reduce the affinity of fatty acid, the analysis is greatly simplified. The flux of FA, J3 is of general physiologic importance and is the most experimentally accessible of the relevant parameters.
In the comparison of different diets, an additional component is (4) input of fatty acid from TAG-containing lipoproteins. Our treatment of the problem is to consider fluxes in the absence of this input since that is how it is usually described in the literature and then to consider the effect of input from lipoproteins as a perturbation. Focusing on the reaction in the absence of lipoprotein input, the overall relations of fluxes and flows:
| J1 = L11X1 + L12X2 |
| J2 = L21X1 + L22X2 |
| J3 = L13X1 + L33X3 |
As an example of the application of these principles, Aledo, et al. addressed the negative correlation between glycolytic flux and intracellular ATP concentration in yeast, the so-called ATP paradox [54,57,58]. The paradox was resolved by showing that if ATP-consuming pathways are more sensitive to glucose than the glycolytic pathway, the cell can switch from an efficient (ATP-conserving) to a dissipative (ATP-utilizing) regime [54,58]. The dissipative regime offers higher output at high glucose cost, whereas the efficient regime has higher accumulation of ATP but lower glycolytic flux.
In the adipocyte model, periodic switching between dissipative and conservative regimes is meant to describe the dynamic cycling of TAG. The goal in development of the model is to show the constraints on the system for conservation of fat mass, and conversely, how isocaloric dietary inputs of different composition might plausibly bring about weight gain or loss, that is, how efficiency is regulated in the TAG-FA cycle and the activity of the reactants. In essence, we want to know what it would take for the blue line in Figure 1 to occur.
The major controlling variables will be the Lij, the phenomenologic constants which depend on hormonal levels, and the thermodynamic activity of plasma triglyceride (supplying fatty acid). Looking ahead, the simplest application will be the effect of replacing dietary carbohydrate with dietary protein at constant lipid where a semi-quantitative prediction can be made. In the most general case, however, we also want to know the relative impact of insulin reduction on the Lij (reduced lipolysis rate) compared to the increase in thermodynamic activity (X4) due to increased dietary fat.
The variables as they apply to the adipocyte model are as follows:
X1 = the output force is the affinity of the lipolysis-TAG synthesis cycle. The analysis can be simplified by the assumption that lipolysis of available TAG (and possibly re-synthesis) in an adipocyte occurs at a heterogeneous interface. We can therefore take the thermodynamic activity of TAG as 1, that is, although other concentrations may influence X1, the amount of TAG will not. (The contribution of TAG activity is unlikely to change in any case since perturbations in TAG concentrations are extremely small compared to the total stored TAG).
| X1 = -RT (ln (Keq)FA-TAG - ln ([FA]3 [glyc-3-P]/[TAG]) = - 3 RT ln (([FA] [glyc-3-P]/K') |
X2 = the driving force for supply of glycerol-3-phosphate whose major term is normally the availability of carbohydrate. Under conditions of carbohydrate restriction, however, there is also an increase in glyceroneogenesis from protein [59,60].
X3 = = the driving force for supply of fatty acid from cellular TAG.
X4 = the force due to the supply of fatty acid from lipoproteins (chylomicrons and VLDL).
In the approach taken here,
L11, L12 are the sensitivities of the flux of TAG to the levels of TAG and the levels of substrate (glycerol-3-phosphate) which depend primarily on the hormonal levels (via phosphorylation of the lipases and other enzymes). It is generally assumed on theoretical grounds (Onsager relation) that L12 = L21 although this has to actually be established for systems that are not close to equilibrium.
L22 is the sensitivity of the glycerol-3-phosphate flux to the availability of carbohydrate (or other sources) which may also be controlled by hormonal levels.
Although somewhat beyond the level of analysis presented here, it is worth noting some of the derived parameter that are traditional in a NET analysis. The degree of coupling, q = L12 /√L11L22 is a dimensionless parameter that indicates how tightly the output process is coupled to the driver process [55] and takes on values from 0 to 1 in the forward direction. In the model in Figure 2, q will vary with different subjects and different metabolic states, in particular, is strongly under the control of insulin.
Figure 2.
Model for adipocyte metabolism. See text for details.
The phenomenological stoichiometry is defined as Z = (√L11/L22)
It should be noted that L11, L12 and L22 and the derived parameters, q and Z, in general, are where the enzymatic activity and the effect of hormones reside. It is important to emphasize that many important variables, such as coenzyme levels are hidden in the phenomenologic constants. For the adipocyte Z = 1, that is, TAG synthesis is tightly coupled to glycerol-3-P production. These parameters hold the promise to quantify insulin resistance, at least in the adipocyte.
The experimental parameters that are most frequently determined in the literature are the rates of appearance in the blood of fatty acid and glycerol, traditionally written Ra (FA) and Ra (glycerol), and the total rate of TAG oxidation (largely oxidation in non-adipocyte), denoted here as ROX. For the simple two compartment model considered here, there are four species, adipocyte TAG, plasma TAG, FA and CO2 (from oxidation).
The goal is to re-cast the problem of metabolism and regulation of body mass in the formalism of nonequilibrium thermodynamics, or more simply, in a way that emphasizes rates in addition to energetics. Applying the traditional measurements above leads to particularly simple form. From conservation of carbon mass of fatty acid species, we can write for the mass fluxes:
| 0 = d(FA)/dt + d(TAG)/dt + d(CO2)/dt = Ra (FA) + J1 + ROX + J4 |
| or J1 = - (ROX + Ra (FA) + J4) |
| J3 = Ra (FA) |
Although no experiment in the literature has been done that would allow for a complete quantitative test of the model, further analysis can support the value of a nonequilibrium approach in understanding variable efficiency in weight loss experiments. Experiments comparing the effect of different macronutrient composition, for example, can allow us to look at the effects on TAG accumulation (J1) without explicit analysis of the individual reactions. Results from the literature that support the underlying thesis show that 1) fatty acid flux and oxidation (ROX + Ra (FA), eq. (6)), follow the levels of dietary carbohydrate, 2) the effect of carbohydrate is expressed in the regulation of insulin levels, 3) lipolysis is the primary target of insulin, 4) the availability of substrate affects efficiency, 5) insulin increases J4 and finally, 6) chronic diet can affect the force X4 and thereby the response to dietary input in a single experiment. In the following sections, we consider these in turn. The net effect is that accumulated time-dependent changes due to carbohydrate intake control the efficiency of fat storage and we consider that a nonequilibrium thermodynamic approach allows clear justification as to how variable weight gain can be expected on isocaloric diets.
Fatty acid flux and oxidation follow the levels of dietary carbohydrate
Similarity of starvation and carbohydrate restriction
Over the years, several investigators have made the observation that the metabolic response to carbohydrate restriction resembles the response to starvation, in particular, for the current model, increased fatty acid mobilization and oxidation [61-65]. Perhaps the best example is an elegant study by Klein & Wolfe [65] comparing responses of subjects on an 84 hour fast to the same subjects on a similar fast in which lipids were infused at a level equal to resting energy requirements. Table 1 shows that the levels of glucose, insulin, the rates of appearance of fatty acid and fat oxidation, ROX + Ra (FA), were similar in the two groups. For comparison to equation (6), the molar fluxes would have to be converted to mass and the role of J4 would have to be considered explicitly. In fact, as measured here, J4 is subsumed in the rate of fatty acid appearance and appears to have little effect despite large differences in X4. These rather dramatic results were summarized by the authors as demonstrating that "carbohydrate restriction, not the presence of a negative energy balance, is responsible for initiating the metabolic response to fasting." It might be said that this was the fundamental observation for understanding the role of carbohydrates in energy balance and the need for a kinetic rather than equilibrium thermodynamic analysis. The controlling variables are presumed to be carbohydrate itself which provides substrate for glycerol-3-P synthesis and insulin which will affect the phenomenologic constants. Bisschop, et al. [62] showed a similar increase in FA rate of appearance and oxidation in a low carbohydrate, high fat diet (CHO:Lipid:Protein = 2:83:15) compared to either a high carbohydrate (85:0:15) or control (44:41:15) diets, and there is agreement with Klein & Wolfe's data (Table 1). Considering the difference in protocol, the similarity of the response to carbohydrate restriction, fasting and fasting + lipid is very good. Although the subjects in Klein & Wolfe's study lost comparable amounts of weight in the two procedures, the short duration and the substantial changes in body water make it difficult to accurately determine whether TAG storage follows the calculated value of J1 [65]. It is important to point out that in Bisschop's experiment, fatty acid oxidation does not keep up with the increase in dietary TAG but according to equations (6) and (7), the flux of TAG is increasing in the direction of breakdown of TAG and, again, explicit inclusion of J4 would further bias the results in that direction.
Table 1.
Similarity of the effects of starvation and carbohydrate restriction on fatty acid flux and oxidation
| Study/subjects | Glucose | FA | Rox | Ra FA | - (ROX + RaFA) |
| (mg/dl) | (μmol/l) | (μmol/kg/min) | |||
| Ref: [62] High CHO | 91.8 | 0.38 | 0.90 | 1.52 | 2.42 |
| control | 91.8 | 0.42 | 1.25 | 1.76 | 3.01 |
| High Fat | 77.4 | 0.75 | 1.83 | 2.98 | 4.81 |
| Ref: [65] Fast 84 h | 68 | 0.92 | 1.94 | 0.97 | 2.91 |
| Fast + Lipid | 66 | 1.02 | 1.67 | 1.00 | 2.67 |
Data from references [62] and [65].
Although it would obviously be difficult to carry out experiments for long periods of time in humans, studies by Tomé's group have shown that rats fed a high fat diet without carbohydrate ate less and also gained less weight per calorie consumed than rats fed a high fat diet that included carbohydrate [21]. Similar results have recently been published by Kennedy, et al. have shown that a high fat/ketogenic diet could reverse the obesity induced by an isocaloric high fat diet that also contained sucrose [22]. The principle that the level of dietary TAG plays a passive role and that carbohydrate restriction is controlling suggests that evidence from the older literature showing weight loss on very high fat diets [66] might be worth re-examining. These were presumably not followed up because they were so counter-intuitive.
Glucose flux regulates TAG flux
Wolfe and Peters [67] measured the response to infusions of glucose in humans. The data shown in Table 2 indicate that the flux of glucose regulates the rate of TAG synthesis largely through the inhibition of lipolysis. The effect of glucose, in turn, is presumed to rest primarily with the effect of insulin.
Table 2.
The effect of glucose flux on calculated TAG flux
| Ra(glucose) (μmol/kg/min) | fat oxidation (μmol/kg/min) | Ra FA (μmol/kg/min) | - SUM | Δ SUM |
| Basal | 3.74 | 5.78 | 9.52 | - |
| 5.6 | 3.14 | 4.96 | 8.10 | 1.42 |
| 22.2 | 3.48 | 3.25 | 6.73 | 2.79 |
| 44.4 | 1.99 | 2.43 | 4.42 | 5.10 |
Data from reference [67].
The effect of carbohydrate is expressed in the regulation of insulin levels
Lipolysis is the primary target of insulin
It is well established that the primary effect of insulin, both kinetically and in terms of physiologic effect is on the inhibition of lipolysis and there is a large literature studying this effect (Review: [68]). In the language of nonequilibrium thermodynamics, this is expressed in the phenomenologic constant, L11. Campbell, for example, studied fatty acid metabolism in humans infused intravenously with insulin [46]. Figure 3 shows the decline in fatty acid flux as the plasma insulin is increased. Oxidation of fatty acid was also inhibited but by a much smaller amount, from 2.7 to 0.9 μmol/kg lean body mass/min. The total rate of primary reesterification (from fatty acid that is not released to the plasma after lipolysis) was similarly increased. Insulin levels further increase the uptake of plasma TAG due to increase lipoprotein lipase (LPL) activity. Frayn and coworkers[69,70] have shown how the combination of LPL and lipolysis leads to increase in flux towards TAG storage. Again, the relative hormonal reduction in lipolysis and any increase in esterification due to mass action if plasma TAG is increased will determine if net TAG accumulation will occur. The importance of insulin can be seen in studies in which insulin secretion is indirectly inhibited via administration of a somatostatin antagonist octreotide. This intervention leads to a reduction in fat mass [6]. Conversely, it has long been known that chronic insulin therapy for diabetes leads to weight gain and decreased flux of fatty acids compared to isocaloric controls.
Figure 3.
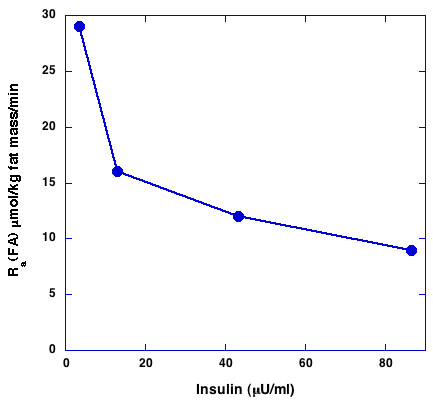
Effect of insulin on fatty acid flux. Free fatty acid appearance in plasma R(a) were examined in healthy humans infused intravenously with insulin. Data from reference [46]. Units converted for comparison to figure 5.
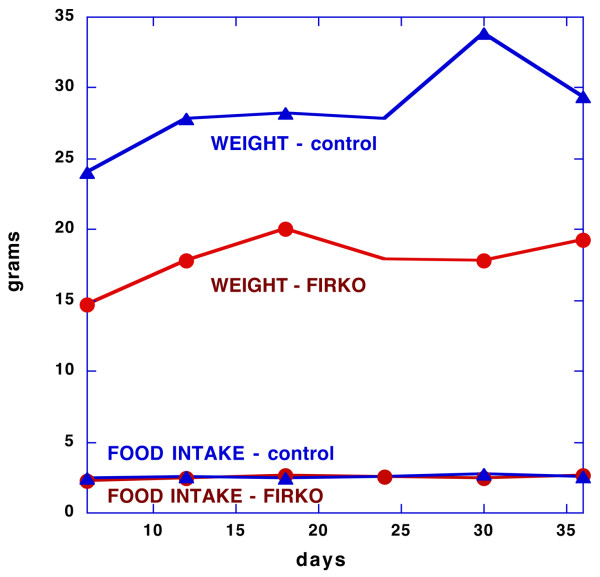
The most dramatic if abstract demonstration of the potential effect of carbohydrate restriction on insulin stimulation of fat cells comes from the study of the adipose-specific insulin receptor knockout mice FIRKO mouse of Bluher & Kahn [32,71]. These animals have a knockout of the insulin receptor specific to the adipocyte. Widely discussed because of their increased longevity they also show greatly reduced efficiency in the storage of lipid and are significantly thinner than the wild type even though both groups consumed the same amount of food (Figure 4).
Figure 4.
Weight change and food intake of the FIRKO mouse. Data from reference [71, 88] Adipose-specific insulin receptor knockout (FIRKO) mice have normal or increased food intake but are protected from obesity.
Insulin Flux
The flux of insulin for diabetic patients under two dietary conditions is shown in Figure 5. A consistently lower level of insulin throughout the day is seen under conditions of lower carbohydrate intake. In addition, Such behavior has been measured frequently in the literature. Chronic carbohydrate restriction means that this reduced insulin never catches up with control. The study from Gannon & Nuttal [72] was carried out under conditions of weight maintenance so that there is presumably a compensating fatty acid oxidation but it is clear that insulin flux is controlled by dietary carbohydrate which, in turn, reduces the flux of fatty acid.
Figure 5.
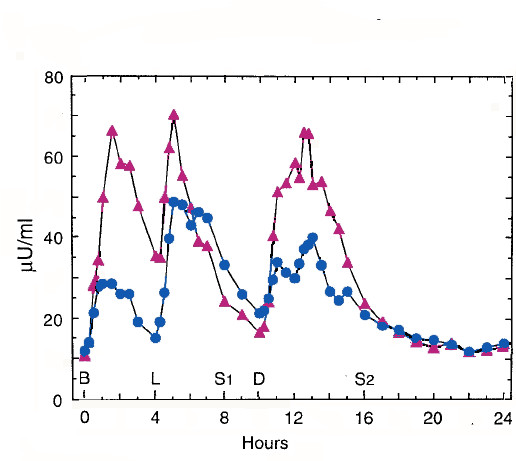
Effect of diet on serum insulin concentration. Mean serum insulin concentration before (red) and after (blue) 5 weeks on a reduced carbohydrate diet (CHO:Lipid:Protein = 20:50:30) using a randomized crossover design with a 5-week washout period. Data from reference [72]. The control diet was (55:30:15). As noted in the text, the insulin values are in the linear range of the dependence of fatty acid flux on insulin and the pattern roughly proportional to the flux of fatty acid.
Availability of substrate affects efficiency
Glycerol-3-phosphate: PEPCK overexpression
The key substrate for TAG synthesis is glycerol-3-phosphate. Because adipocytes normally have very low levels of glycerol kinase, the flux of TAG is dependent on processes (J2) that supply glycerol-3-phosphate: glycolysis or, under conditions of starvation or glucose deprivation, glyceroneogenesis, a truncated form of gluconeogenesis [59]. These processes are dependent on the composition of the diet and the hormonal state of the organism. One approach to separating the effect of glucose from the effect of glucose-induced insulin, is the genetic manipulation of the level of enzymes under conditions of low glucose. Such a strategy allows one to isolate the driving force from the effects of hormone on the phenomenologic constants, L22 and L21. Franckhauser [73] overexpressed phosphoenolpyruvate carboxykinase (PEPCK) in mice adipocytes. Under conditions of starvation, transgenic mice showed increased glyceroneogenesis which was accompanied by increased reesterification of free fatty acids (FAs), and a corresponding decrease in circulating FAs, both reflecting an increase in stored TAG (Table 3). In fact, the transgenic mice showed increased adipocyte size and fat mass, and higher body weight. Insulin sensitivity was preserved. When fed, nutrient consumption was the same for the experimental animals and the wild type. Thus, the change in the enzymatic activity of PEPCK affects the accretion of fat in the absence of any change in caloric intake or change in hormonal level that normally triggers changes in PEPCK levels. An overall change in the efficiency of food utilization is 2-fold for the heterozygotes and almost 4-fold for the homozygotes.
Table 3.
Effect of overexpression of PEPCK of starved mice on feeding
| Starved mice | PEPCK activity (%) | (J2) pyruvate -> glycerol (cpm/mg prot/2 hr) | (J1) FA reesterification (mmol/mg prot/2 hr) | J1/J2 (mmol/cpm) | FAT PAD Wt. (mg) | Food consumed mg ± SE |
| control | 100 | 300 | 300 | 1.0 | 400 | 3.3 ± 0.1 |
| hetero-PEPCK | 400 | 650 | 550 | 1.18 | 800 | 3.2 ± 0.1 |
| homo-PEPCK | 1300 | 750 | 620 | 1.21 | 1500 | 3.4 ± 0.1 |
Data from reference [73].
In similar experiments, Shepherd, et al. [74] overexpressed adipocyte GLUT4 in transgenic mice. Body lipid was increased 2–3 fold in these mice compared to wild-type and the mutants had increased insulin sensitivity. Direct comparison to the simple model in Figure 2 is complicated by the fact that the transgenic mice showed fat cell hyperplasia rather than a simple increase in size.
Dietary fat and the effect of chronic carbohydrate restriction
The key question in the application of the model is the extent to which lipolysis and other catabolic processes that are increased by reductions in insulin are compensated for by the increased availability of dietary TAG (X4) if carbohydrate in the diet is replaced by fat. At this point, we can consider the process indicated by J4, the influx of plasma FA from plasma TAG, as a perturbation on overall TAG storage. The activity of lipoprotein lipase (J4) is increased by higher insulin and will be reduced by chronic carbohydrate restriction [75]. The effect of chronic diet on the response to dietary fat challenge can provide further data on this point. Sharman, et al. [47] showed that six weeks on a low carbohydrate ketogenic diet led to a substantially reduced postprandial serum triacylglycerol (TAG) response in normal-weight men (Figure 6). The low carbohydrate group, in distinction to controls, showed drastically reduced (-34 %) insulin levels. Thus, despite the higher fat intake, the rate of lipolysis increased and the contribution of activity of TAG (X4) went down.
Figure 6.
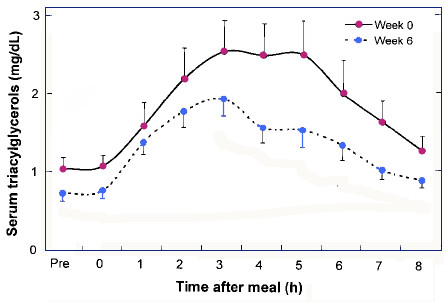
Effect of chronic diets on postprandial response to high fat meal. Responses to high fat meal before and after 6 weeks on low carbohydrate (< 10% energy) ketogenic diet in overweight men. Data from reference [47].
The bottom line: efficient and dissipative modes
While no experiment in the literature measures all the relevant variables, comparisons of Figures 3, 5 and 6 give a sense of the difference in time dependent responses on low carbohydrate and high carbohydrate (high insulin) diets. The individual components that contribute are as follows:
1. Rate of lipolysis. Insulin represses lipolysis as shown in Figure 3. This is true even in insulin-resistant states such as diabetes. Carbohydrate restriction reduces insulin fluxes as indicated in Figure 5.
2. Figure 6 shows that the effect of chronic carbohydrate restriction compared to controls is to reduce plasma triglycerides (X4) in response to a fat challenge, reducing the activity of FA in the carbohydrate-restricted state compared to the higher carbohydrate state.
3. Lipoprotein lipase is known to be up-regulated by higher insulin increasing the flux of FA into the adipocyte (J4) under conditions of high carbohydrate.
4. Carbohydrate represses per cent fat oxidation.
Thus, all of the differences in high and low insulin states are in the direction of efficient modes in the former and toward more dissipative modes in the latter.
As a guide to future research, then, the continuous monitoring of FA flux and oxidation, or other variables that allow determination of TAG flux can be done with current technology and it is possible to test the role of kinetic regulation in different weight loss strategies and to rationalize variable efficiency. It is important to point out that a thermodynamic analysis explains the potential for the metabolic advantage for particular diets but can, as well, point the way to identifying other factors that maintain homeostasis. In other words, isocaloric diets do not always show differences in efficiency and the thermodynamic analysis suggests that it is as important to explain cases where metabolic advantage does not occur as those where it does.
Conductance matching
A metabolic scheme of the type considered here is traditionally evaluated in terms of the effect of the demand on the output by the load, or conductance matching by analogy with electronic systems [54,58]. The assumptions of the model in Figure 2 is that there is effectively no load on the adipocyte: output of fatty acid and its subsequent utilization by other tissues, are independently regulated and the adipocyte, in effect, has very high output conductance, that is, supplies whatever fatty acid is required. Wolfe and coworkers [76-78] have emphasized the extent to which glucose controls fatty acid metabolism rather than the other way around as originally suggested in the Randle cycle [79,80]. From the perspective of further metabolic analysis, the adipocyte may be considered a discrete modular element and could be patched into a larger network.
Discussion
Variable metabolic efficiency due to the macronutrient composition of the diet is plausibly explained in terms of nonequilibrium thermodynamics by a shift in the cycling between dissipative lipolytic modes and efficient storage modes. Such a mechanism is consistent with experimental data on the effect of diet on metabolism. The nonequilibrium thermodynamic approach and the application to the FA-TAG cycle may raise general questions about metabolism.
Fatty acid flux, insulin resistance
There is an increasing perception that circulating fatty acids are critical in metabolic responses and, in particular, in the development of insulin resistance and type 2 diabetes [81-83]. The effect of insulin resistance on the disinhibition of lipolysis and an increase in fatty acid flux may be as important for the adipocyte as the effect on glucose uptake. In combination, the two effects may reduce TAG storage and may represent a down-regulation in response to excess insulin. As such, it may be thought of as beneficial for obesity and, at the same time, suggests that reduction in insulin directly or via carbohydrate restriction will improve insulin resistance.
The increase in circulating fatty acid remains problematical in that, whereas it does indicate that less TAG is stored, it is generally considered deleterious and may lead to peripheral insulin resistance. In addition, fatty acids are known to stimulate insulin secretion. On the other hand, the effects of high plasma FA may be different under conditions of low carbohydrate: FA-induced insulin secretion, for example, is strongly dependent on carbohydrate levels [48] and is probably not a factor at all if plasma glucose is low. In practice, carbohydrate restriction improves insulin resistance and the increased fatty acids may be considered a reflection of a more general paradox: it is observed that fatty acid levels are increased in obesity[68] and references therein), diabetes and insulin resistance but are also elevated by those conditions that mediate against these conditions: exercise, starvation and carbohydrate restriction. It is also paradoxical that the TZD's increase insulin sensitivity but also pre-dispose to obesity. The latter effect has been shown to be due at least partly to the increase in glyceroneogenesis (X2) [59,84]. It could also be argued that the high levels indicate that FA is not being taken up by peripheral tissues as happens in insulin-resistant states. A recent review by Westman argues similarly that a so-called glycolytic pressure controls the disposition of fatty acid as fuel in muscles [85].
General perspective
Animal models provide very clear-cut demonstrations of inefficiency as a function of macronutrient composition and therefore it seems there is no theoretical barrier to accepting demonstrations in humans where ideal control is not possible. The driving force for TAG flux in the proposed model is the availability of carbohydrate and the key regulating phenomenologic constant depends on insulin and other hormones. Of course, the system is going to be subject to other cells and processes. De novo fatty acid synthesis is a significant effect. Moreover, this simple model makes no attempt to account for compensatory processes and the nonlinear effects that are ultimately expected in complex biological systems. For example, hepatic production of β-hydroxybutyrate, which increases twenty-fold during very low carbohydrate diets, inhibits lipolysis [86], likely blunting the effects of reduced insulin concentrations. The increased fatty acid flux under carbohydrate restriction will lead to increased insulin secretion and, at some point, these process would have to be added back into the model.
Relation to previous arguments on reduced energy efficiency
We previously pointed out a number of errors in the idea that weight regulation is necessarily independent of diet composition (and therefore insulin levels) [16,35,36]. We proposed several mechanisms and, in a practical sense, all of these – increased gluconeogenesis and associated increased protein turnover, increased mitochondrial uncoupling and increased substrate cycling – must be reflected in the flux of TAG if fat loss is to be effected. We have also pointed out that in a dietary intervention it is important to be specific about changes in fat mass not simply weight loss [87]. The mechanism is ultimately through fatty acid oxidation which, again, will be under separate control of glucose and hormones.
From a theoretical standpoint, the simplest objection to the idea that calorimeter values are sufficient to understand processing of food is that it assumes that no process other than complete oxidation takes place, that is, that metabolic reactions are the same as calorimeter reactions. This is obviously not generally true since living organisms use other reactants and make all kinds of products, proteins, ATP, etc. In comparing two diets of different macronutrient composition each diet itself must conform to the first law, but because they may be carrying out different overall chemical reactions, there is no requirement that the energy changes are the same in the two biological reactions just because the reference calorimeter values are the same. In addition, it is expected that different pathways will have different efficiencies as dictated by the second law. Thus, it is not thermodynamics, but the special characteristics of living systems that explain why energy balance is usually observed. Under most conditions, a steady state can be attained in which oxidation of food to CO2 and water is the major process, and the differences between the diets in the other reactions are small.
Finally, as noted above, application of thermodynamic laws is limited in systems that do not come to equilibrium. This has been described in the literature as the inappropriate use of ΔG values [37] when what is really measured under conditions where equilibrium is not attained is (∂G/∂ξ)T,P where ξ is the reaction progress coordinate. In the end, a thorough going analysis of the potential for inefficiency must consider nonequilibrium conditions.
Conclusion
Emphasis on kinetics and nonequilibrium thermodynamics provides a conceptual framework for understanding the effect of macronutrient composition on maintenance and change of body mass and possibly for analysis of adipocyte metabolism in general. The simple model presented is intended to be consistent with a general shift away from equilibrium thermodynamics and towards a more dynamic analysis of cellular processes.
Abbreviations
FA: fatty acid
FIRKO: Adipose-specific insulin receptor knockout mice
LPL: lipoprotein lipase
PEPCK: phosphoenolpyruvate carboxykinase
Ra : rate of appearance
TAG: triacylglycerol (triglycerides)
TZD: thiazolidinedione
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
The authors contributed equally to the preparation of this work and have read and approved the final manuscript.
Contributor Information
Richard D Feinman, Email: rfeinman@downstate.edu.
Eugene J Fine, Email: efine@downstate.edu.
References
- Feinman RD, Makowske M. Metabolic Syndrome and Low-Carbohydrate Ketogenic Diets in the Medical School Biochemistry Curriculum. Metabolic Syndrome and Related Disorders. 2003;1:189–198. doi: 10.1089/154041903322716660. [DOI] [PubMed] [Google Scholar]
- Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:261S–267S. doi: 10.1093/jn/133.1.261S. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. Dietary glycemic index and obesity. J Nutr. 2000;130:280S–283S.. doi: 10.1093/jn/130.2.280S. [DOI] [PubMed] [Google Scholar]
- Phinney SD, Bistrian BR, Evans WJ, Gervino E, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32:769–776. doi: 10.1016/0026-0495(83)90106-3. [DOI] [PubMed] [Google Scholar]
- Rabast U, Schonborn J, Kasper H. Dietetic treatment of obesity with low and high-carbohydrate diets: Comparative studies and clinical results. Int J Obes. 1979;3:201–211. [PubMed] [Google Scholar]
- Velasquez-Mieyer PA, Cowan PA, Arheart KL, Buffington CK, Spencer KA, Connelly BE, Cowan GW, Lustig RH. Suppression of insulin secretion is associated with weight loss and altered macronutrient intake and preference in a subset of obese adults. Int J Obes Relat Metab Disord. 2003;27:219–226. doi: 10.1038/sj.ijo.802227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon M, Mavropoulos J, Transue M, Yancy WS, Jr, Westman EC. Clinical Experience of a Carbohydrate-Restricted Diet: Effect on Diabetes Mellitus. Metabolic Syndrome and Related Disorders. 2003;1:233–237. doi: 10.1089/154041903322716714. [DOI] [PubMed] [Google Scholar]
- Volek JS, Sharman MJ, Gomez AL, DiPasquale C, Roti M, Pumerantz A, Kraemer WJ. Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. J Am Coll Nutr. 2004;23:177–184. doi: 10.1080/07315724.2004.10719359. [DOI] [PubMed] [Google Scholar]
- Volek JS, Sharman MJ, Gomez AL, Judelson DA, Rubin MR, Watson G, Sokmen B, Silvestre R, French DN, Kraemer WJ. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Metab (Lond) 2004;1:13. doi: 10.1186/1743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med. 2002;113:30–36. doi: 10.1016/S0002-9343(02)01129-4. [DOI] [PubMed] [Google Scholar]
- Yancy WS, Jr., Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- Atkins RC. Dr. Atkins' New Diet Revolution. New York , Avon Books; 2002. [Google Scholar]
- Eades MR, Eades MD. Protein Power. New York , Bantam Books; 1996. [Google Scholar]
- Agatston A. The South Beach Diet. New York , Random House; 2003. [Google Scholar]
- Wolever TM, Gibbs AL, Spolar M, Hitchner EV, Heimowitz C. Equivalent glycemic load (EGL): A method for quantifying the glycemic responses elicited by low carbohydrate foods. Nutr Metab (Lond) 2006;3:33. doi: 10.1186/1743-7075-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman RD, Fine EJ. Thermodynamics and metabolic advantage of weight loss diets. Metabolic Syndrome and Related Disorders. 2003;1:209–219. doi: 10.1089/154041903322716688. [DOI] [PubMed] [Google Scholar]
- Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- Westman EC, Mavropoulos J, Yancy WS, Volek JS. A Review of Low-carbohydrate Ketogenic Diets. Curr Atheroscler Rep. 2003;5:476–483. doi: 10.1007/s11883-003-0038-6. [DOI] [PubMed] [Google Scholar]
- Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1:5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EJ, Feinman R, Isasi C, Wylie-Rosett J. A new systematic review of low carbohydrate diets. Obesity Research. 2004;12 (Suppl):223P. [Google Scholar]
- Marsset-Baglieri A, Fromentin G, Tome D, Bensaid A, Makkarios L, Even PC. Increasing the Protein Content in a Carbohydrate-Free Diet Enhances Fat Loss during 35% but Not 75% Energy Restriction in Rats. J Nutr. 2004;134:2646–2652. doi: 10.1093/jn/134.10.2646. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Pissios P, Otu HH, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A High Fat, Ketogenic Diet, Induces a Unique Metabolic State in Mice. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- Brehm BJ, Spang SE, Lattin BL, Seeley RJ, Daniels SR, D'Alessio DA. The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J Clin Endocrinol Metab. 2004 doi: 10.1210/jc.2004-1540. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW, Mann JI. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med. 2003;139:205–213. [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes. 1993;42:1700–1707. doi: 10.2337/diabetes.42.12.1700. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Hudgins LC, Leibel RL, Rosenbaum M. Diet composition and energy balance in humans. Am J Clin Nutr. 1998;67:551S–555S. doi: 10.1093/ajcn/67.3.551S. [DOI] [PubMed] [Google Scholar]
- MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1306–15. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/S1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen HC, Jensen DR, Myers HM, Eckel RH, Farese RV., Jr. Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2003;111:1715–1722. doi: 10.1172/JCI200315859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ. Hormone-sensitive lipase knockouts. Nutr Metab (Lond) 2006;3:12. doi: 10.1186/1743-7075-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman RD, Fine EJ. "A Calorie is a calorie" violates the second law of thermodynamics. Nutrition Journal. 2004;3 doi: 10.1186/1475-2891-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EJ, Feinman RD. Thermodynamics of weight loss diets. Nutr Metab (Lond) 2004;1:15. doi: 10.1186/1743-7075-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch GR. Some problems in the usage of Gibbs free energy in biochemistry. J Theor Biol. 1985;114:433–446. doi: 10.1016/s0022-5193(85)80177-6. [DOI] [PubMed] [Google Scholar]
- Qian H, Beard DA. Thermodynamics of stoichiometric biochemical networks in living systems far from equilibrium. Biophys Chem. 2005;114:213–220. doi: 10.1016/j.bpc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Qian H, Reluga TC. Nonequilibrium thermodynamics and nonlinear kinetics in a cellular signaling switch. Phys Rev Lett. 2005;94:28101. doi: 10.1103/PhysRevLett.94.028101. [DOI] [PubMed] [Google Scholar]
- Yugi K, Nakayama Y, Kinoshita A, Tomita M. Hybrid dynamic/static method for large-scale simulation of metabolism. Theor Biol Med Model. 2005;2:42. doi: 10.1186/1742-4682-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondepudi D, Prigogine I. Modern Thermodynamics. From Heat Engines to Dissipative Structures. Chichester , John Wiley & Sons; 1998. [Google Scholar]
- Nicolis G, Prigogine I. Exploring Complexity. An Introduction . New York , W. H. Freeman and Company; 1989. [Google Scholar]
- Prigogine I, Stengers I. Order out of Chaos. New York , Bantam Books; 1984. [Google Scholar]
- Ho MW. the Rainbow and the Worm. The Physics of Organisms. New Jersey , World Scientific; 1998. [Google Scholar]
- Aledo JC, Del Valle AE. Glycolysis in Wonderland: The importance of energy dissipation in metabolic pathways. J Chem Educ. 2004;79:1336–1339. [Google Scholar]
- Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. Am J Physiol. 1992;263:E1063–9. doi: 10.1152/ajpendo.2006.263.6.E1063. [DOI] [PubMed] [Google Scholar]
- Sharman MJ, Gomez AL, Kraemer WJ, Volek JS. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J Nutr. 2004;134:880–885. doi: 10.1093/jn/134.4.880. [DOI] [PubMed] [Google Scholar]
- Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DT, Stevenson BE, Chester MW, Basit M, Daniels MB, Turley SD, McGarry JD. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest. 1997;100:398–403. doi: 10.1172/JCI119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan SR, Essig A. Bioenergetics and linear nonequilibrium thermodynamics. The steady state. Cambridge, MA , Harvard University Press; 1983. [Google Scholar]
- Prigogine I. Thermodynamics of irreversible processes. Second revised. New York , Interscience Publishers; 1961. [Google Scholar]
- Rottenberg H. The thermodynamic description of enzyme-catalyzed reactions. The linear relation between the reaction rate and the affinity. Biophys J. 1973;13:503–511. doi: 10.1016/S0006-3495(73)86004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. Non-equilibrium thermodynamics of energy conversion in bioenergetics. Biochim Biophys Acta. 1979;549:225–253. doi: 10.1016/0304-4173(79)90001-6. [DOI] [PubMed] [Google Scholar]
- Aledo JC, Del Valle AE. The ATP paradox is the expression of an economizing fuel mechanism. J Biol Chem. 2004;279:55372–55375. doi: 10.1074/jbc.M410479200. [DOI] [PubMed] [Google Scholar]
- Caplan SR. The degree of coupling and its relation to efficiency of energy conversion in multiple-flow systems. J Theor Biol. 1966;11:346–347. doi: 10.1016/0022-5193(66)90172-X. [DOI] [PubMed] [Google Scholar]
- Cukrowski AS, Kolbus A. On validity of linear phenomenological nonequilibrium thermodynamics equations in chemical kinetics. Acta Physica Polonica B. 2005;36:1485–1507. [Google Scholar]
- Somsen OJ, Hoeben MA, Esgalhado E, Snoep JL, Visser D, van der Heijden RT, Heijnen JJ, Westerhoff HV. Glucose and the ATP paradox in yeast. Biochem J. 2000;352 Pt 2:593–599. doi: 10.1042/0264-6021:3520593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle AE, Aledo JC. What process is glycolytic stoichiometry optimal for? J Mol Evol. 2006;62:488–495. doi: 10.1007/s00239-005-0135-y. [DOI] [PubMed] [Google Scholar]
- Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- Brito MN, Brito NA, Brito SR, Moura MA, Kawashita NH, Kettelhut IC, Migliorini RH. Brown adipose tissue triacylglycerol synthesis in rats adapted to a high-protein, carbohydrate-free diet. Am J Physiol. 1999;276:R1003–9. doi: 10.1152/ajpregu.1999.276.4.R1003. [DOI] [PubMed] [Google Scholar]
- Azar GJ, Bloom WL. Similarities Of carbohydrate deficiency and fasting. Ii. Ketones, nonesterified fatty acids and nitrogen excretion. Arch Intern Med. 1963;112:338–343. doi: 10.1001/archinte.1963.03860030092007. [DOI] [PubMed] [Google Scholar]
- Bisschop PH, Ackermans MT, Endert E, Ruiter AF, Meijer AJ, Kuipers F, Sauerwein HP, Romijn JA. The effect of carbohydrate and fat variation in euenergetic diets on postabsorptive free fatty acid release. Br J Nutr. 2002;87:555–559. doi: 10.1079/BJNBJN2002578. [DOI] [PubMed] [Google Scholar]
- Bloom WL, Azar GJ. Similarities of carbohydrate deficiency and fasting. I. weight loss, electrolyte excretion, and fatigue. Arch Intern Med. 1963;112:333–337. doi: 10.1001/archinte.1963.03860030087006. [DOI] [PubMed] [Google Scholar]
- Fery F, Bourdoux P, Christophe J, Balasse EO. Hormonal and metabolic changes induced by an isocaloric isoproteinic ketogenic diet in healthy subjects. Diabete Metab. 1982;8:299–305. [PubMed] [Google Scholar]
- Klein S, Wolfe RR. Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol. 1992;262:E631–6. doi: 10.1152/ajpendo.1992.262.5.E631. [DOI] [PubMed] [Google Scholar]
- Kekwick A, Pawan GL. Metabolic study in human obesity with isocaloric diets high in fat, protein or carbohydrate. Metabolism. 1957;6:447–460. [PubMed] [Google Scholar]
- Wolfe RR, Peters EJ. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987;252:E218–23. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35:177–193. [PubMed] [Google Scholar]
- Bulow J, Simonsen L, Wiggins D, Humphreys SM, Frayn KN, Powell D, Gibbons GF. Co-ordination of hepatic and adipose tissue lipid metabolism after oral glucose. J Lipid Res. 1999;40:2034–2043. [PubMed] [Google Scholar]
- Frayn KN, Shadid S, Hamlani R, Humphreys SM, Clark ML, Fielding BA, Boland O, Coppack SW. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol. 1994;266:E308–17. doi: 10.1152/ajpendo.1994.266.3.E308. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53:2375–2382. doi: 10.2337/diabetes.53.9.2375. [DOI] [PubMed] [Google Scholar]
- Franckhauser S, Munoz S, Pujol A, Casellas A, Riu E, Otaegui P, Su B, Bosch F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51:624–630. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- Farese RV, Jr., Yost TJ, Eckel RH. Tissue-specific regulation of lipoprotein lipase activity by insulin/glucose in normal-weight humans. Metabolism. 1991;40:214–216. doi: 10.1016/0026-0495(91)90178-Y. [DOI] [PubMed] [Google Scholar]
- Sidossis LS. The role of glucose in the regulation of substrate interaction during exercise. Can J Appl Physiol. 1998;23:558–569. doi: 10.1139/h98-031. [DOI] [PubMed] [Google Scholar]
- Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- Garland PB, Randle PJ, Newsholme EA. Citrate as an intermediary in the inhibition of Phosphofructokinase in rat heart muscle by fatty acids, ketone bodies, pyruvate, diabetes, and starvation. Nature. 1963;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/S0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Boden G. Free fatty acids and insulin secretion in humans. Curr Diab Rep. 2005;5:167–170. doi: 10.1007/s11892-005-0004-5. [DOI] [PubMed] [Google Scholar]
- Boden G, Laakso M. Lipids and glucose in type 2 diabetes: what is the cause and effect? Diabetes Care. 2004;27:2253–2259. doi: 10.2337/diacare.27.9.2253. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Dobbins RL, Stein DT. Fatty acids, insulin resistance and pancreatic beta cell function. Journ Annu Diabetol Hotel Dieu. 1998:1–10. [PubMed] [Google Scholar]
- Hanson RW, Reshef L. Glyceroneogenesis revisited. Biochimie. 2003;85:1199–1205. doi: 10.1016/j.biochi.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Westman EC, Yancy Jr. WS, Haub MD, Volek JS. Insulin Resistance from a Low-Carbohydrate, High Fat Diet Perspective. Metabolic Syndrome and Related Disorders. 2005;3:3–7. doi: 10.1089/met.2005.3.14. [DOI] [PubMed] [Google Scholar]
- Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta -hydroxybutyrate Inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- Feinman RD. To: McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW, Mann JI (2005) Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia 48:8-16. Diabetologia. 2005;48:1420–1421. doi: 10.1007/s00125-005-1777-4. [DOI] [PubMed] [Google Scholar]
- Bluher M. Transgenic animal models for the study of adipose tissue biology. Best Pract Res Clin Endocrinol Metab. 2005;19:605–623. doi: 10.1016/j.beem.2005.07.006. [DOI] [PubMed] [Google Scholar]