Abstract
We show that the diverse ecoregions of Madagascar share one distinctive climatic feature: unpredictable intra- or interannual precipitation compared with other regions with comparable rainfall. Climatic unpredictability is associated with unpredictable patterns of fruiting and flowering. It is argued that these features have shaped the evolution of distinctive characteristics in the mammalian fauna of the island. Endemic Herpestidae and Tenrecidae and members of five endemic primate families differ from closely related species elsewhere, exhibiting extremes of “fastness” and “slowness” in their life histories. Climatic features may also account for the dearth of frugivorous birds and mammals in Madagascar, and for the evolutionary prevalence of species with large body mass.
Keywords: climate, life history, unpredictability, mammals
Recent field research on Madagascar has revealed related vertebrates with both the fastest and slowest life histories. How can such differences evolve under similar environmental conditions? The unique natural communities of Madagascar are famous, but efforts to explain their evolution are unsatisfactory. We show here that the climates of Madagascar are distinctive, with highly unpredictable rainfall, and argue that some of the natural communities of the island represent evolutionary responses to this unusually variable climatic regime.
The communities of Madagascar are characterized by high levels of endemicity, great species diversity in some taxonomic groups, and a complete absence of others. These three features are dramatically evident in the native nonflying mammals. The four orders native to and widespread within Madagascar (Carnivora, Insectivora, Primata, and Rodentia) are all represented by endemic genera or families. The only other Recent mammals are the African bush pig (Potamochoerus larvatus), the extinct pygmy hippopotamus species (Hippopotamus spp.), and the poorly known and extinct Plesiorycteropus. Groups widely distributed elsewhere, such as the canids, felids, cervids, bovids, and anthropoid primates, are absent. Successful colonization by mammals has been rare (1). The high level of endemicity has been attributed to the long isolation of the island from other continents. This isolation predates the evolution of most recent families of mammals, and the limited suite of Malagasy mammals has been attributed to chance dispersals across the Mozambique Canal over the past 70 million years (1, 2).
The Malagasy fauna exhibit other distinctive features not readily explained by isolation. For example, the biological peculiarities of the primates of Madagascar have been widely noted (3). The extreme seasonality and unpredictability and frequent tropical cyclones of Madagascar have been invoked to explain these peculiarities and relate them to a special need to conserve energy (3, 4). However, Madagascar does not exhibit an unusual degree of seasonality (5), and, lacking comparative evidence until now, there has been no assessment of the unpredictability of the climate of Madagascar compared with other landmasses.
Several authors (6, 7) also have remarked on the low number of frugivorous species of birds and mammals in Madagascar. In other tropical communities, the dominant arboreal frugivores are primates; however, in Madagascar, there are very few medium- to large-sized frugivorous lemurs, and the proportion of fruit in the diets of extant Malagasy lemurs is low compared with other primate communities (8). With decreasing body mass, primate species tend to include more fruit and less foliage in their diet, but medium-sized lemurs (down to 1 kg body mass) tend to be folivorous rather than frugivorous (5).
Madagascar: Environmental Variation
Year-to-Year Variability.
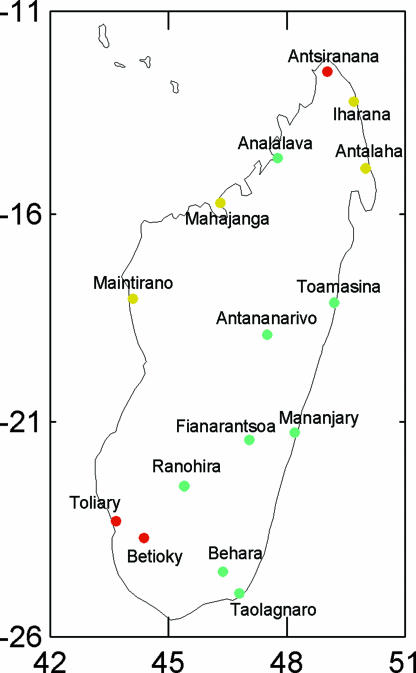
Dewar and Wallis (9) examined interannual variation in rainfall on tropical landmasses. Of a global sample of 1,492 stations, only two stations were in Madagascar. To explore patterns of interannual rainfall variability in Madagascar, we sought additional monthly rainfall data (Global Historical Climatological Network version 2; www.ncdc.noaa.gov/oa/climate/research/ghcn/ghcngrid.html) (Table 1). Fig. 1 shows the geographical distribution of interannual rainfall variability at 15 Malagasy stations. In the north and the southwest were regions with unusually high interannual variation. All of the other regions fell within the global midrange spread identified by Dewar and Wallis (9), but most western stations showed more interannual variation than the median, and eastern regions somewhat less. Thus, some regions of Madagascar differ markedly from global distributions, but unusual interannual variability in rainfall is not a general characteristic of the island.
Table 1.
Rainfall stations of Madagascar and Africa
| Station | Location | Latitude | Longitude | Elevation, m | MAR, mm | P | C | M | n |
|---|---|---|---|---|---|---|---|---|---|
| Toliary | Madagascar | −23.34 | 43.67 | 5 | 386 | 0.281 | 0.074 | 0.206 | 53 |
| Behara | Madagascar | −24.95 | 46.38 | 244 | 532 | 0.270 | 0.154 | 0.116 | 41 |
| Betioky | Madagascar | −23.71 | 44.38 | 263 | 666 | 0.320 | 0.088 | 0.233 | 48 |
| Ranohira | Madagascar | −22.55 | 45.41 | 765 | 966 | 0.340 | 0.083 | 0.257 | 49 |
| Antsiranana | Madagascar | −12.27 | 49.28 | 33 | 1034 | 0.296 | 0.047 | 0.249 | 49 |
| Maintirano | Madagascar | −18.07 | 44.03 | 13 | 1043 | 0.356 | 0.080 | 0.275 | 47 |
| Fianarantsoa | Madagascar | −21.43 | 47.08 | 1162 | 1179 | 0.421 | 0.162 | 0.259 | 60 |
| Iharana | Madagascar | −13.24 | 50.03 | 2 | 1301 | 0.405 | 0.294 | 0.111 | 31 |
| Antananarivo | Madagascar | −18.92 | 47.52 | 1400 | 1368 | 0.352 | 0.073 | 0.279 | 108 |
| Mahajanga | Madagascar | −15.72 | 46.32 | 36 | 1539 | 0.424 | 0.104 | 0.320 | 42 |
| Taolagnaro | Madagascar | −25.03 | 47.00 | 14 | 1620 | 0.401 | 0.333 | 0.068 | 47 |
| Analalava | Madagascar | −14.63 | 47.75 | 23 | 1824 | 0.438 | 0.113 | 0.325 | 34 |
| Antalaha | Madagascar | −14.88 | 50.28 | 4 | 2244 | 0.370 | 0.239 | 0.131 | 36 |
| Mananjary | Madagascar | −21.22 | 48.33 | 13 | 2726 | 0.411 | 0.292 | 0.119 | 32 |
| Toamasina | Madagascar | −18.14 | 49.38 | 7 | 3338 | 0.437 | 0.277 | 0.163 | 47 |
| En Nahud | Sudan | 12.70 | 28.43 | 610 | 388 | 0.613 | 0.302 | 0.310 | 77 |
| Mourdiah | Mali | 14.40 | −7.40 | 314 | 532 | 0.581 | 0.240 | 0.341 | 56 |
| Nkayi | Zimbabwe | −19.00 | 28.9 | 1130 | 666 | 0.441 | 0.149 | 0.292 | 49 |
| Koutiala | Mali | 12.38 | −5.47 | 365 | 964 | 0.563 | 0.190 | 0.372 | 64 |
| Ngara | Tanzania | −2.40 | 30.60 | 1463 | 1031 | 0.473 | 0.242 | 0.232 | 52 |
| Kelo | Chad | 9.30 | 15.80 | 378 | 1043 | 0.652 | 0.252 | 0.400 | 33 |
| Dabakala | Ivory Coast | 8.30 | −4.40 | 244 | 1180 | 0.434 | 0.213 | 0.221 | 52 |
| Minna | Nigeria | 9.62 | 6.53 | 262 | 1303 | 0.579 | 0.238 | 0.341 | 64 |
| Yei | Sudan | 4.00 | 30.60 | 1036 | 1363 | 0.527 | 0.251 | 0.276 | 29 |
| Sassandra | Ivory Coast | 4.95 | 6.08 | 62 | 1539 | 0.371 | 0.167 | 0.204 | 60 |
| Meiganga | Cameroon | 6.53 | 14.37 | 1027 | 1618 | 0.592 | 0.243 | 0.350 | 36 |
| Lagos | Nigeria | 6.60 | 3.40 | 19 | 1813 | 0.387 | 0.152 | 0.235 | 82 |
| Lauderdale | Malawi | −12.00 | 33.80 | 930 | 2166 | 0.399 | 0.189 | 0.210 | 65 |
| Edea | Cameroon | 3.80 | 10.10 | 31 | 2522 | 0.612 | 0.346 | 0.266 | 37 |
| Harbel | Liberia | 6.20 | −10.20 | 53 | 3396 | 0.559 | 0.228 | 0.332 | 41 |
Elevation is given as meters above sea level. MAR, mean annual rainfall; P, C, and M are as defined in ref. 11; n, number of years of records used.
Fig. 1.
Residuals from a global nonlinear regression of interannual rainfall variation against mean annual rainfall at 15 stations in Madagascar, as in ref. 9: stations with variation, adjusted for mean, that is >75% of global stations are shown in red, stations with variation that falls between the median and the 75th percentile are shown in yellow, and those with variation between the 25th percentile and the median are shown as green.
Predictability of Monthly Rainfall.
Colwell (10) proposed a method for examining the predictability (P) of periodic phenomena and used monthly rainfall patterns as an illustration. P falls on a scale of 0 to 1, with higher values representing greater predictability. P is the sum of C and M, where C is a measure of constancy (in our case, the extent to which rainfall is constant and thereby predictable) from month to month, and M is a measure of contingency (the extent to which rains fall in similar amounts in each month from year to year).
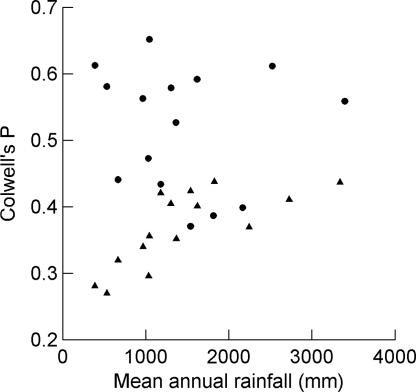
P was calculated for the 15 sites in Madagascar. We selected a matching sample of stations from the nearly 600 continental African stations used by Dewar and Wallis (9). For each Malagasy station, we selected the African station with the mean annual rainfall closest to it (Table 1). P did not covary positively with the length of the climate record in the total sample (r = 0.108, P = 0.571) nor in either the continental (r = 0.403, P = 0.136) or Malagasy (r = 0.198, P = 0.479) subsets. P was, however, significantly different for the African and Malagasy samples (F1,28 = 28.68, P < 0.0001): continental stations were, as a group, much more predictable than those in Madagascar. In Madagascar, P covaried positively with mean annual rainfall (r = 0.727, P = 0.002), but P did not covary with annual precipitation in continental Africa (r = 0.092, P = 0.743).
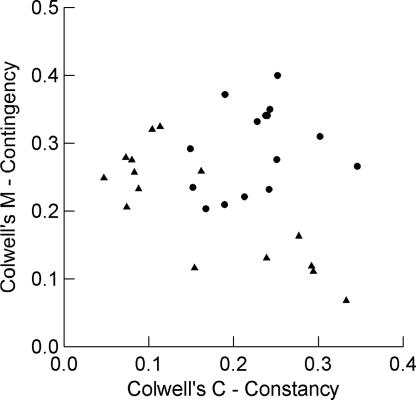
Although some stations in Africa fall within the range of unpredictability exhibited by the Malagasy stations, all of the Malagasy stations are strikingly unpredictable (Fig. 2). Their low P values result from two patterns: either markedly low contingency with unexceptional constancy or low constancy with unexceptional, even high contingency in relation to the African data set (Fig. 3).
Fig. 2.
Colwell's P in relation to mean rainfall for 15 Madagascar stations (triangles) and 15 matching stations from continental Africa (circles).
Fig. 3.
Colwell's C (constancy) vs. Colwell's M (contingency) for 15 Madagascar stations (triangles) and 15 matching stations from continental Africa (circles).
The five stations from the east coast of Madagascar (Iharana, Antalaha, Toamasina, Mananjary, and Taolagnaro) form a cluster on the lower right of Fig. 3. With some rain in every month, they have relatively high scores for constancy; however, with a poorly predictable seasonal pattern, they score low on contingency. Stations in the center, north, west, and south form another cluster in the upper left of Fig. 3, reflecting higher contingency and lower constancy: more predictable patterning of rainfall seasons but low constancy as the result of the long dry season.
The distinctive and unusually high variability in precipitation in Madagascar is likely to be the product of several determinants, all of them linked to the global configuration of continents and oceans. These determinants, and the resulting climatic patterns affecting Madagascar today, were probably established when the continents bordering the Indian Ocean achieved their current positions, and the Indian Ocean monsoon system developed. A recent review argues that climatic conditions were essentially modern throughout Madagascar by ≈5 million years ago (11).
Patterns of Fruiting and Flowering
Terborgh and van Schaik (12) have proposed that the underrepresentation of frugivores in Madagascar is due to a distinctive phenology of the forests. The authors predicted that fruiting in Madagascar would be confined to a very narrow season of the year, thus requiring consumers to rely heavily on resources other than fruit at other times of year. Subsequent phenological studies at three sites along the east coast (13–18) instead reveal considerable year-to-year variability in the timing and quantities of available fruit overall and, in many cases, of fruit available from individual species.
Evolutionary Responses to Environmental Unpredictability
Harvey et al. (19) argue that the variation in life histories of placental mammals is best explained as a product of differences in fertility and mortality schedules, memorably as “living fast and dying young” (20). Moreover, models of stochastic demography show that high environmental variance in survivorship or fertility can reverse evolutionary patterns predicted under fixed vital rates and stable age structures (21, 22). Depending on initial life-history parameters and the level of environmental stochasticity, selection favors a shift toward either more iteroparity or more-concentrated breeding over a shorter life span. When adult survivorship is highly variable, reproduction should occur earlier in the life cycle; when the variance is most important in fertility and juvenile survivorship, selection will be for a longer reproductive span or “bet hedging.”
Madagascar's variable climate appears to have been an important evolutionary determinant across the array of native mammals and probably the bird community as well. Malagasy mammalian life histories, and the array of recently extinct species have characteristics that are best understood as responses to environmental variability.
Carnivora.
The native carnivorous mammals of Madagascar are assigned to seven endemic genera, members of a monophyletic radiation of herpestids (1). This radiation is ecologically diverse (23) but displays a shared distinctiveness in life-history traits that sets its members apart from other herpestids.
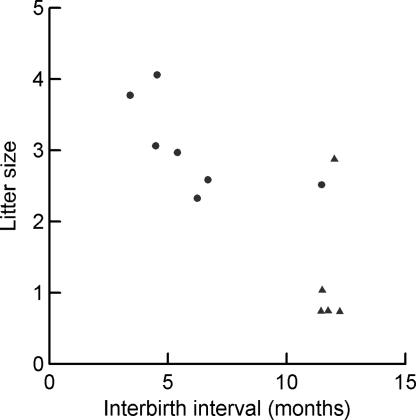
Without exception, Malagasy herpestids give birth only once a year in a species-specific birth season, and most give birth to a single offspring each year. Both of these traits are highly unusual in herpestids elsewhere and cannot be attributed to differences in female body mass (23, 24) (Fig. 4). The median mass of Malagasy species is 1.60 kg (range, 0.8 to 9.5 kg; n = 5) compared with 1.24 kg (range, 0.27 to 3.5 kg; n = 16) in species from Asia and Africa. Analysis of variance of log (interbirth interval) reveals a highly significant difference between Malagasy (median, 12 months) and non-Malagasy species (median, 6 months) (F1,10 = 19.04; P < 0.001). An ANOVA of litter size makes a similar distinction (median of Malagasy species, 1; median of other herpestids, 3; F1,19 = 26.97; P < 0.0001).
Fig. 4.
Interbirth interval and litter size for Malagasy herpestids (triangles) and continental African and Asian herpestids (circles). The slight random jitter has been added for clarity.
Primates.
The extant lemurs, the primate radiation of Madagascar, are assigned to five endemic families (25). Many lemurs share features unusual compared with other primates, including low basal metabolic rate and life-history patterns exhibiting extremes of “slowness” and “fastness” (3, 26, 27).
For example, Propithecus verreauxi is a widely distributed, diurnal, folivorous member of the family Indriidae. An 18-year study of a population of P. verreauxi at Beza Mahafaly in southwest Madagascar has demonstrated high adult survivorship, highly irregular fertility, and irregular and low first-year survivorship. P. verreauxi is “slower” than other mammals for which comparable data are available: females delay reproduction later and continue reproduction much longer than expected for a 3-kg mammal and have life expectancies at birth that are much longer than expected (27). We interpret these results as a manifestation of bet-hedging, evolved in response to environmental unpredictability and its impact on infant and juvenile survival.
A population of Lemur catta (adult mass, 2.2 kg) at Beza Mahafaly exhibits similarly irregular and low first-year survivorship but, in combination with lower adult survivorship, much earlier age at first reproduction and higher fertility (28). This has been interpreted as an alternative “response…to unusual climate and environmental conditions” (28).
Tilden and Oftedal (29, 30) has shown that, compared with African bushbabies (Otolemur spp.), several species of Malagasy lemurs have low rates of maternal reproductive investment during gestation and lactation. In relation to maternal body mass, for example, energy transfer by lactating Eulemur fulvus is very low compared with other mammals. This is consistent with a risk-spreading strategy, where variance in survival is greatest at weaning and where survivorship is environmentally controlled.
In contrast, Martin (31) reported that one of the smallest lemurs, the 60-g Microcebus murinus, may give birth for the first time at the age of 12 months, producing litters of two to three infants twice during a single year when environmental conditions are good, thus establishing Microcebus as the “fastest” of primates, with the highest potential rate of reproduction in the order (32). High, adult-biased predation rates on Microcebus are reported in some areas (33). In this instance, high adult predation rates and environmental unpredictability may together drive this species toward a different “solution,” involving an unusually speeded-up life-history strategy.
Other Mammals.
The Tenrecidae comprise 28 species, ranging in body mass from 3 g to ≈2 kg. Little is known about patterns of mortality in the wild, but the metabolic and reproductive patterns of tenrecs are well known and unusual (34, 35). Unlike most mammalian radiations, there is no correlation between maternal body mass and gestation length among tenrecs (36); there is no positive correlation between body mass and reported longevity in zoos (37); and litter size covaries positively with body mass, with the largest tenrec (Tenrec ecaudatus) having the largest known litters of any mammal, up to 32 neonates. Tenrec approaches a semelparous pattern, with evidence that adult survivorship is very low, and that females that attempt a second year of breeding have much reduced success.
Of the 22 species of endemic rodents (38), the only well studied species, Hypogeomys antimena, is a 1.1-kg, hopping herbivore living in burrows and often described as the “rabbit” of Madagascar (39). However, the practice of Hypogeomys antimena to give birth to a single offspring once a year is decidedly not rabbit-like.
Extinct Species.
The recent wave of size-biased extinctions obscures the evolutionary prevalence of species with large body mass among the native species of Madagascar. This is evident in at least three major radiations on the island. First, the preponderance of large-bodied, folivorous primates before the Holocene extinctions exceeds that of primate radiations elsewhere (5, 40). Second, Madagascar experienced the recent extinction of a diverse radiation of ratite birds of the family Aepyornithidae, including Aepyornis maximus, which, at ≈450 kg, was the heaviest of all birds. Third, among the most common extinct species are two giant tortoises. Large body mass thus evolved repeatedly among the vertebrates of Madagascar, and by mid-Holocene times, the herbivore community strongly reflected this pattern. The causes of gigantism in the Malagasy fauna are unknown, but the ubiquity of gigantism suggests a general environmental feature, and increase in body mass is associated with delays in age of maturation (41).
Discussion
Evolutionary Consequences of Hypervariability.
The climate of Madagascar is characterized by very high unpredictability of rainfall. The eastern region experiences this as high intraannual variability in the patterning of rainfall. In the north and the south, it takes the form of high interannual variability in total precipitation. We hypothesize that this unpredictability is primarily responsible for the unusual phenology of the forests of Madagascar and that the unpredictability of fruit has limited the evolution of flower- and fruit-eating species. Of equal importance is that the unpredictability of resources must contribute to variance in vital rates among many of its vertebrates. A high level of reproductive variance has been demonstrated for the best-studied species, and there are congruent reports for other, less well known species.
The life-history patterns of Malagasy mammals studied thus far contrast with those of closely related taxa in other regions. In some cases, like certain lemurs and the Carnivora, selection appears to have favored extension of the reproductive span or reduced litter size. In other cases, like T. ecaudatus or M. murinus, selection has favored concentrated reproduction unusually early in life. The result is an island whose native mammals differ strikingly from those in tropical regions with more predictable climates. The distinctive features of the climate of Madagascar may also be linked to the gigantism characteristic of the fauna of the island through the effect of these features on the evolution of life-history patterns.
On the few tropical landmasses where rainfall variability is as high as it is on Madagascar, unusual life histories have been reported, and it has been suggested that they evolved in response to unpredictability. For example, the odd life-history characteristics of the Galapagos cactus finch have been attributed to environmental variation (42). Very unusual life histories in Australia have likewise been ascribed to unpredictability (43). Further research in regions of comparable variability would provide additional tests of our ideas, as would studies of the life histories and demography of other mammal populations in Madagascar itself.
Biogeographers have long explained the rarity of mammalian groups on Madagascar as the result of infrequent “sweepstakes dispersal” across the canal of Mozambique. We suggest that two different filters may have winnowed the vertebrates of Madagascar: isolation by the surrounding sea and the emergence of a regime of environmental hypervariability during the late Cenozoic that posed strong challenges for both some residents and immigrants.
Discussions of the distinctiveness of the fauna of Madagascar have a tendency to trail off, in need of a yet-to-be-identified deus ex machina. The unpredictability of the climate documented in this paper may be a critical environmental feature for the vertebrates of Madagascar. We suggest some of the ways in which this unusual environmental context may have shaped the evolution of species and communities on the island, recognizing that these arguments are but a starting point for a broader reconsideration of the evolutionary biology of one of the world's most intriguing landmasses. Future field studies of the natural communities of Madagascar may provide opportunities for confirmation and modification of the role of environmental variability in the shaping of those communities.
Acknowledgments
We thank the staff of the Direction de Météorologie et Hydrologie (Antananarivo, Madagascar) and John F. Griffiths (Texas A & M University, College Station, TX) for assistance in acquiring rainfall data and R. Colwell, J. Diamond, R. D. Martin, M. Nicoll, M. Schwartz, S. Stearns, J. Wallis, A. Yoder, and S. Zack for insights and helpful discussion.
Footnotes
The authors declare no conflict of interest.
References
- 1.Yoder AD, Burns MM, Zehr M, Delefosse T, Veron G, Goodman SM, Flynn JJ. Nature. 2003;421:734–737. doi: 10.1038/nature01303. [DOI] [PubMed] [Google Scholar]
- 2.Krause DW, Hartman JH, Wells NA. In: Natural Change and Human Impact in Madagascar. Goodman SM, Patterson BD, editors. Washington, DC: Smithsonian Institution Press; 1997. pp. 3–43. [Google Scholar]
- 3.Wright PC. Yrbk Phys Anthropol. 1999;42:31–72. doi: 10.1002/(sici)1096-8644(1999)110:29+<31::aid-ajpa3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Ganzhorn JU. Ambio. 1995;24:124–125. [Google Scholar]
- 5.Richard AF, Dewar RE. Annu Rev Ecol Syst. 1991;22:145–175. [Google Scholar]
- 6.Fleming TR, Breitwisch R, Whitesides GH. Annu Rev Ecol Syst. 1987;18:91–109. [Google Scholar]
- 7.Goodman SM, Ganzhorn JU. Rev Ecol (Terre Vie) 1997;52:321–329. [Google Scholar]
- 8.Fleagle JG, Reed KE. J Hum Evol. 1996;30:489–510. [Google Scholar]
- 9.Dewar RE, Wallis JR. J Climate. 1999;12:3457–3466. [Google Scholar]
- 10.Colwell RK. Ecology. 1974;55:1148–1153. [Google Scholar]
- 11.Wells NA. In: The Natural History of Madagascar. Goodman SM, Benstead JP, editors. Chicago: Univ Chicago Press; 2003. pp. 16–34. [Google Scholar]
- 12.Terborgh J, van Schaik CP. In: Organization of Communities, Past and Present. Gee HR, Giller PS, editors. Oxford: Blackwell; 1987. pp. 205–226. [Google Scholar]
- 13.Morland HS. New Haven, CT: Yale Univ; 1991. PhD thesis. [Google Scholar]
- 14.Sterling EJ. New Haven, CT: Yale Univ; 1993. PhD thesis. [Google Scholar]
- 15.Meyers DM, Wright PC. In: Lemur Social Systems and Their Ecological Basis. Kappeler PM, Ganzhorn JU, editors. New York: Plenum; 1993. pp. 179–192. [Google Scholar]
- 16.Overdorff DJ. Durham, NC: Duke Univ; 1991. PhD thesis. [Google Scholar]
- 17.Hemingway CA. Durham, NC: Duke Univ; 1995. PhD thesis. [Google Scholar]
- 18.Hemingway CA. Int J Primatol. 1996;17:637–660. [Google Scholar]
- 19.Harvey PH, Read AF, Promislow DEL. Oxford Surv Evol Biol. 1989;6:13–32. [Google Scholar]
- 20.Promislow DEL, Harvey PH. J Zool. 1990;220:417–437. [Google Scholar]
- 21.Tuljapurkar S. Population Dynamics in Variable Environments. New York: Springer; 1990. [Google Scholar]
- 22.Orzack SH, Tuljapurkar S. Ecology. 2001;82:2659–2665. [Google Scholar]
- 23.Albignac R. Faune de Madagascar, Mammifères carnivores. Vol 36. Paris: Office de la Recherche Scientifique et Technique Outre-Mer/Centre National Recherche Scientifique; 1973. [Google Scholar]
- 24.Gittleman JL. Am Nat. 1986;127:744–771. [Google Scholar]
- 25.Yoder AD. Evol Anthropol. 1997;6:11–22. [Google Scholar]
- 26.Martin RD. Primate Origins and Evolution. Princeton: Princeton Univ Press; 1990. [Google Scholar]
- 27.Richard AF, Dewar RE, Schwartz M, Ratsirarson J. J Zool. 2002;256:421–436. [Google Scholar]
- 28.Gould L, Sussman RW, Sauther ML. Am J Phys Anthropol. 2003;120:182–194. doi: 10.1002/ajpa.10151. [DOI] [PubMed] [Google Scholar]
- 29.Tilden CR, Oftedal OT. In: Creatures of the Dark: The Nocturnal Prosimians. Alterman L, Doyle GA, Izard MK, editors. New York: Plenum; 1995. pp. 119–132. [Google Scholar]
- 30.Tilden CR, Oftedal OT. Am J Primatol. 1997;41:195–211. doi: 10.1002/(SICI)1098-2345(1997)41:3<195::AID-AJP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Martin RD. Z Tierpsychol. 1972;9(Suppl):43–89. [Google Scholar]
- 32.Ross C. J Zool. 1988;214:199–220. [Google Scholar]
- 33.Goodman SM, Langrand O, Raxworthy CJ. Ostrich. 1993;64:160–171. [Google Scholar]
- 34.Stephenson PJ, Racey PA. Comp Biochem Physiol. 1997;112A:215–223. [Google Scholar]
- 35.Eisenberg JF, Gould E. The Tenrecs: A Study in Mammalian Behavior and Evolution. Washington, DC: Smithsonian Contrib Zool; 1970. [Google Scholar]
- 36.Eisenberg JF. The Mammalian Radiations: An Analysis of Trends in Evolution. Chicago: Univ of Chicago Press; 1981. [Google Scholar]
- 37.Eisenberg JF. In: Evolution of Life Histories of Mammals. Boyce MS, editor. New Haven, CT: Yale Univ Press; 1988. pp. 291–310. [Google Scholar]
- 38.Goodman SM, Ganzhorn JU, Rakotondravony D. In: The Natural History of Madagascar. Goodman SM, Benstead JP, editors. Chicago: Univ of Chicago Press; 2003. pp. 1159–1186. [Google Scholar]
- 39.Sommer ML. J Zool. 1997;241:301–314. [Google Scholar]
- 40.Godfrey L, Jungers W, Reed KE, Simons EL, Chatrath PS. In: Natural Change and Human Impact in Madagascar. Goodman SM, Patterson BD, editors. Washington, DC: Smithsonian Institution Press; 1997. pp. 218–256. [Google Scholar]
- 41.Charnov EL. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford Univ Press; 1993. [Google Scholar]
- 42.Grant BR, Grant PR. Evolutionary Dynamics of a Natural Population: The Large Cactus Finch of the Galapagos. Chicago: Univ of Chicago Press; 1989. [DOI] [PubMed] [Google Scholar]
- 43.Flannery T. The Future Eaters: An Ecological History of the Australasian Lands and People. New York: Grove; 1994. [Google Scholar]