Abstract
Aiming to improve understanding of the mechanisms behind specific anion effects in biological systems we have studied the effects of sodium salts of simple monovalent anions belonging to the Hofmeister series on the bilayers of the zwitterionic lipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine using small-angle x-ray scattering and the osmotic stress technique. NaCl, NaBr, NaNO3, NaI, and NaSCN were used in this investigation. The electrolytes were found to swell the bilayers and to increase the area per lipid headgroup at each value of the osmotic pressure, suggesting the association of anions with the bilayer-lipid interfaces. The effects follow the Hofmeister series with SCN− inducing the most pronounced changes. “Ion competition” experiments with mixed NaI/NaCl solutions at total salinity 0.1 and 0.5 M revealed that the effect of ions on the lipid equation-of-state is roughly linear at low concentrations, but strongly nonlinear at high concentrations. The experimental results are fitted in a companion article to provide “binding” or “partitioning” constants of anions in the lipid bilayers.
INTRODUCTION
Ion specificity and the Hofmeister series
The influence of aqueous electrolytes on various physicochemical and biological phenomena has been widely studied since the 19th century. More than 100 years ago, Hofmeister (1) published experimental results showing the effect of various salts on the aqueous solubility of proteins. Since then, numerous experimental studies have shown the importance of specific ion effects in a multitude of biological and physicochemical phenomena (2,3). On the basis of the magnitude of their effects, ions have been ordered into sequences (one for anions and one for cations), which are called the Hofmeister series. For anions, based on increasing salting-in potency for proteins from left to right the series is as follows:
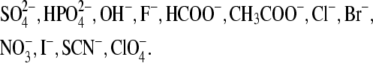 |
Despite the fact that the Hofmeister series plays a significant role in a broad range of phenomena, the precise origin of action of the ions in the series has not yet been clarified and no generally accepted explanation exists at the molecular level (2–4). Several different ideas about the nature of specific salt effects have been proposed to date. A favorite explanation of specific salt effects for a long time was that ions modify the structure and properties of water. The character of water as a solvent for biomolecules would thus change in specific ways in the presence of electrolytes. It has become standard practice to call ions on the left of Cl− in the Hofmeister series “cosmotropes” or “structure makers”, whereas ions on the right of Cl− are called “chaotropes” or “structure breakers”. Cavity models for the salting-out effect on biomolecules are based on such ideas (5,6). Biologists often refer to the presence of low-density and high-density water close to biological interfaces and the way that ions affect the two water regions (7–9). Consistent with this idea is the proposition by Chandler (10), that an extended hydrophobic surface is “dry” to a certain extent, in the sense of a reduced water density found in its proximity. This dryness would be enhanced by hydrophilic ions and reduced by hydrophobic ions. A related model was put forward by Collins and Washabaugh (2), who postulated that water at the interface between a hydrophobic surface and an electrolyte solution can be divided into three layers, according to the extent that the structure of water is affected by the ions. The disadvantage of such models is their complexity and their relative lack of predictive capability. In addition, considerable recent evidence supports the idea that the structure of water is not heavily perturbed by monovalent ions, and that there is no direct correlation between a solute's impact on water structure and its effect on biomolecule stability (11–13).
An entirely different approach, which has a long history in colloid science, assumes that ion interactions with specific groups on surfaces can explain the Hofmeister series (6,14). Although this approach may be successful for a variety of phenomena to a certain extent, it cannot be the only explanation of specific salt effects, since these are also observed in the absence of specific surface groups, as in the case of the surface tension of electrolyte solutions (3,15) and in the salting-out of small organic solutes or gases (3,6,16,17).
According to a proposition by Ninham and Yaminsky (18) the origin of ionic specificity could be due to the usually neglected dispersion interactions between ions and surfaces. It was proposed that an ionic dispersion potential acting between ions (or ions and water or ions and interfaces) must be included in the usual electrostatic theory to explain specific salt effects (18–21). However, the dispersion-forces-based calculation of the surface tension of electrolyte solutions by Boström et al. (22–24) predicts a layer free of ions close to the water surface, in qualitative disagreement with recent experiments and molecular simulations of electrolyte solution surfaces. These show that ions like Cl− have a higher affinity for the free water surface than Na+ ions, and that large polarizable anions, such as I−, prefer interfacial solvation sites and have significant concentration peaks at the air/water interface (25–37).
An alternative qualitative model argues that ion specificity arises as a result of the fine balance between ion-water and water-water interactions (38). Recent computer simulation studies on clusters giving emphasis to hydration interactions support this idea (39–41). Kjellander et al. (42) and Marcelja (43) have demonstrated that a way to account for ion specificity in statistical mechanical models of double layers is to use effective ion-ion and ion-surface potentials that indirectly account for ion-water interactions and the water structure. The related concept of an “active interface” was put forward recently by Aroti et al. (44,45). The presence of an active interface in an ion-containing system allows the reduction of the system free energy by “expelling” those ions that are easily dehydrated to the interfacial region and liberating water molecules that are reincorporated into the bulk water network. Two requirements must be fulfilled by an active interface: a), it must have a considerable degree of disorder, and b), it must be capable of accommodating ions, at a free-energy gain.
It can be concluded from the above broad spectrum of models that no general consensus exists today for the mechanism of specific ion effects, and it remains largely unclear whether ions act through precisely defined, specific, local interactions, or through more delocalized collective interactions. Elucidation of the mechanism of specific ion effects in a particular experimental situation will provide valuable insights for a multitude of ion-specific phenomena, and will have a strong impact on biology and chemistry.
Lipid model systems for the investigation of specific salt effects
Advances in the understanding of specific salt effects can be achieved by choosing appropriate model systems that allow discrimination between the many possible modes of ion-interface interaction. Phospholipids offer significant advantages as model systems: a), They are major constituents of biological membranes. b), Some are charged and others uncharged (e.g., serine versus choline headgroups), and some may become charged as the pH changes (e.g., lipids with ethanolamine headgroups). Zwitterionic phospholipids in particular do not interact strongly with the ions and thus may potentially render “visible” weaker specific interactions. c), They can be examined as bilayers (in the form of vesicles or lamellar phases), as monolayers at the air-water interface, and even as micelles in the case of single-tail phospholipids.
Point c above was explored by Aroti et al., who have used lipids with phosphatidylcholine headgroups in all possible geometries (micelles, monolayers, and bilayers), examining the effects of anions on all these systems with a goal to combine information and methods ((44–46); E. Leontidis, L. Belloni, and A. Aroti, unpublished data). The work on 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayers at the air-water interface yielded two useful results. First, it was found that lipids affect the disordered liquid-expanded phase of the DPPC monolayer, but not the ordered liquid-condensed phase (46). Second, it was found that classical electrostatic binding models cannot explain ion-monolayer interactions, but a model of ions partitioning within the monolayer was much more fruitful, supporting the active-interface idea ((45); E. Leontidis, L. Belloni, and A. Aroti, unpublished data).
Besides monolayers, phospholipid bilayer systems offer a range of experimental parameters that can be measured to quantify their interactions with anions such as: a), Structural changes like the maximum swelling, the bilayer thickness, and the area per headgroup of the lipid. b), Equation of state (osmotic pressure versus interbilayer distance) curves, which provide information about many important interactions mediated by the ions. c), Surface potential values, affected by conformational changes of the phospholipids, changes in the average tilt of the headgroup dipole, and the formation of an electrostatic double-layer in the presence of ions.
In what follows we will concentrate on bilayers of zwitterionic lipids. The interaction of zwitterionic phospholipid bilayers (either vesicles or bilayer stacks) with ions has been extensively studied in the past (47–76). Many studies originally concentrated on cations, given the biological function and importance of Na+, K+, Ca2+, and Mg2+ (47–57). Anion effects on the structural properties of lipid bilayers have been examined in a rather limited and nonsystematic way. Methods such as 1H-NMR, 2H-NMR, or 31P NMR, Raman spectroscopy, electron paramagnetic resonance (EPR) spectroscopy, x-ray diffraction and neutron diffraction, ζ-potential measurements, and differential scanning calorimetry have been used to study specific anion effects on the structural properties of lipid bilayers (58–76).
1H-NMR studies have shown that the strength of interaction of anions with zwitterionic lipids follows the Hofmeister series. I− and SCN− were found to interact strongly enough with phosphocholines, but the interaction could not be characterized as localized binding (58). Chaotropic anions caused splitting of the choline 1H resonances of EPC bilayers, whereas no changes could be detected in the glycerol and phosphate headgroup region, indicating that the ions do not bind to or affect the phosphate moiety of the lipid molecule (59). Using Raman spectroscopy Loschilova and Karvaly also concluded that the interaction of anions with PC lipids follows a Hofmeister series, and that electrostatics alone cannot explain the spectral changes observed in the presence of anions (60). Considerable insight was obtained from 2H-NMR experiments, since the deuterium quadrupolar splitting of deuterated cholines can be used to quantitate the degree of “binding” of ions to the lipid headgroups. An investigation of the influence of anions on 1-palmitoyl-2-oleoyl-phosphatidylcholine bilayers has shown that the chaotropic anions produce the most significant changes of the deuterium quadrupolar splittings (61,62). Assuming that the ions are adsorbed on the bilayers and using the DLVO theory, it was possible to estimate the surface potential of the bilayers and the binding constants of the ions to the lipids.
The influence of anions on the surface potential of lipid bilayers (PE and PC vesicles or PC bilayers) has been measured by McLaughlin et al. (64), Tatulian (65), and Clarke et al. (67) using either electrophoretic mobility or fluorescence spectroscopy with fluorescent dyes. They have observed that the lipid membrane potential becomes more negative through adsorption of the anions following the Hofmeister series. Tatulian (65) used the DLVO theory to calculate surface potentials and binding constants of anions to the lipids, whereas Clarke et al. (67) has used the fluorescence shift of specific dyes to obtain values of the intrinsic binding constant of 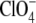 on dimyristoylphosphatidylcholine. A collection of binding constants of anions on phospholipids unfortunately shows significant variability between different experimental methods, or even between experiments of different groups using similar methods (see companion article).
on dimyristoylphosphatidylcholine. A collection of binding constants of anions on phospholipids unfortunately shows significant variability between different experimental methods, or even between experiments of different groups using similar methods (see companion article).
The effect of anions on the phase transition temperatures of lipids has also been studied repeatedly with the general result being that the chaotropic ions have pronounced effects on the main phase transition of lipids (Lβ → Lα) (63,68–73).
Structural information for zwitterionic lipid bilayers in the presence of electrolytes with emphasis on the anions has been obtained using x-ray diffraction or neutron diffraction measurements, but is surprisingly scarce (66,69,70,73,74). The influence of monovalent anions on the structural properties of DPPC bilayers has shown that the DPPC bilayers swell continuously in 1 M potassium salt solutions until a limiting bilayer repeat distance is obtained. In the presence of SCN− ions, the existence of an interdigitated structure was postulated (69,70,73). EPR measurements also suggested that the chaotropic anions I− and SCN− may induce an interdigitation of the DPPC hydrocarbon chains (75,76). Tatulian (66) performed neutron diffraction on bilayers and observed that addition of NaCl does not affect the DPPC lamellar structure whereas NaClO4 drastically influences the lamellar repeat spacing.
In recent years useful information at the atomic level on local interactions of anions with lipid bilayers has been obtained by molecular dynamic simulations (77–84). Sodium was found to create complexes with more than one lipid molecules (80–84) whereas anion penetration into zwitterionic lipid bilayers was found mostly for large anions (chaotropic anions) that can penetrate deeply into the bilayers (78,79). None of these studies was based on polarizable models for water and ions.
Where we stand
From the previously published work it can be concluded that a very visible interaction between anions and zwitterionic lipids exists, but it has not been studied very systematically to date, and the actual interaction was only semiquantified using chemical binding constants. In this work we reevaluate the effects of anions on DPPC bilayers in the fluid phase by applying the osmotic stress method in combination with small-angle x-ray scattering (SAXS) using a range of sodium salts to obtain an extensive experimental database for the application of models. The experimental results (equation of state curves of osmotic pressure versus interbilayer distance and area per lipid headgroup) are fitted in the companion article, using different theoretical models, especially regarding the electrostatic repulsion due to ion adsorption. In spirit, this work is related to the recent article by Petrache et al., who investigated the effects of KCl and KBr on dilauroylphosphatidylcholine (DLPC) bilayers (74). However, in this work we are using anions known to interact more strongly with the lipids and consequently observe effects not observed in previous investigations.
MATERIALS
DPPC was obtained either from Sigma-Aldrich (St. Louis, MO) or from Avanti Polar Lipids (Alabaster, AL), and used without further purification. All sodium salts were purchased from Sigma-Aldrich with purity >99%, with the exception of NaSCN, the purity of which was >98%. Salt solutions were prepared using ultrapure water (specific resistance of 18.2 MΩ cm) produced by a three-stage Millipore (Billerica, MA) Milli-Q Plus 185 purification system. Polyethyleneglycol (PEG) 20,000 was purchased from Fluka (Milwaukee, WI) and used without further purification. The pH value of the aqueous solution of PEG 20,000 was equal to 6.2.
METHODS
Small angle x-ray scattering
The x-ray diffraction method was used to determine the lipid bilayer structural parameters in the presence of sodium salt solutions of concentrations 0.05, 0.1, and 0.5 M. Absolute scaled SAXS experiments have been performed using a laboratory built High Flux camera with pinhole geometry and high sensitivity at the Service de Chimie Moléculaire, CEA-Saclay (France) (85,86). A two-dimensional gas-filled detector with a diameter equal to 0.3 m was used to record the experimental spectra. The effective q-range of this detector is from 0.02 to 0.4 Å−1 where q = 4π/λ sinθ. The exposure time for each sample was 30 min. All experiments have been performed at a controlled temperature T = 50 ± 1°C. The lamellar repeat spacing D was obtained experimentally using Bragg's diffraction law, and can be divided into the bilayer thickness, bL, and the water bilayer separation, dw. The bilayer thickness and water bilayer separation were calculated using the following expressions:
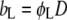 |
(1) |
 |
(2) |
ϕL is the volume concentration of the lipid in the sample, which is related to the weight fraction, c, of lipid in the sample as follows (87):
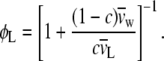 |
(3) |
The weight fraction, c, was determined by the Karl Fischer titration method.  and
and  are the partial specific volumes of water and phospholipid, respectively.
are the partial specific volumes of water and phospholipid, respectively. 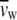 was taken as 1.00 ml/g and
was taken as 1.00 ml/g and 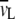 as 1.0091 ml/g for DPPC with melted hydrocarbon chains (88,89). In addition, the headgroup area, A, of the DPPC molecules can be estimated through the lipid bilayer thickness, bL, using the following geometric relationship:
as 1.0091 ml/g for DPPC with melted hydrocarbon chains (88,89). In addition, the headgroup area, A, of the DPPC molecules can be estimated through the lipid bilayer thickness, bL, using the following geometric relationship:
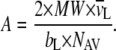 |
(4) |
MW in Eq. 4 is the lipid molecular weight and NAV is Avogadro's number. There is an alternative way to partition the lamellar repeat distance into bL and dw by assigning the phospholipid headgroups to the water layer (88–90). We have tried this method as well in the theoretical analysis of the data that we report in the companion article. Because we did not observe any significant qualitative differences between the two methods regarding the fitting results, we only report dw values based on Eqs. 1–3 above.
Osmotic stress
The osmotic stress technique for measuring interbilayer forces has been reviewed in detail by Rand and Parsegian (91). Briefly, the water between the bilayers is allowed to come to thermodynamic equilibrium with an excess solution of a high molecular weight polymer (PEG) of known osmotic pressure, which is in contact with, and competes for, the available water with the lipid multilamellar system. The osmotic pressure applied by the PEG solution is related to its concentration and temperature as follows:
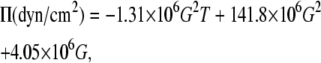 |
(5) |
where G = w/(100 − w) and w is the weight percent of the polymer in solution, and T = 50 ± 1°C in these experiments. The above equation was modified accordingly for Π in Pa.
This calibration expression has been established by Michel (92,93) for PEG with an average molecular weight of 8000 and is strictly valid in the range 5°C ≤ T ≤ 40°C and up to G = 0.8. We have used this expression with PEG 20,000 because the osmotic pressure is known to be roughly independent of MW in the range of 8000–20,000 for concentrations such that the solution is in the semidilute regime (94). Moreover, Eq. 5 has been used at temperatures exceeding 40°C without further verification because, according to Dubois et al. (95), PEG is not subject to hydrolysis or fragmentation that might modify the applied osmotic pressure under these conditions.
Experimentally, a known amount of PEG was mixed with NaA salt solutions of various concentrations and then added to dry DPPC in weighing bottles. A semipermeable membrane was not used to separate the polymer from the lipid solution, since the PEG 20,000 mixes very poorly with the sample solution. The samples were allowed to equilibrate at room temperature for 48–72 h and then were transferred to an oven that was thermostated at T = 50 ± 1°C for 18–20 h before using the samples. After reaching equilibration the samples were transferred to aluminum x-ray sample holders, sealed with Kapton, and mounted immediately to a thermostated cell at T = 50 ± 1°C. The time allowed to the samples to equilibrate at T = 50 ± 1°C was established by reference experiments using DPPC in pure water, which fully reproduced literature results. For DPPC in the presence of electrolytes a number of samples have been allowed to equilibrate for 20 h as well as for 48–72 h before their use. In all cases we obtained the same results for the lamellar spacings as functions of the applied osmotic pressure, indicating that equilibration is already reached at a time of 20 h even in the presence of salts. Repeat spacings were determined by x-ray diffraction as described before.
Equilibration of the samples
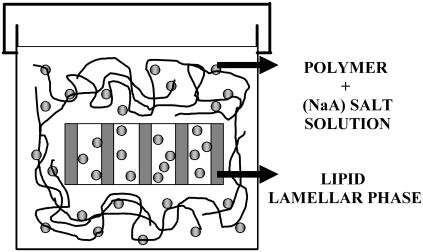
Various methods exist for the preparation of the samples to achieve equilibration (96) such as: a), dropping dry DPPC into salt water; b), mixing DPPC with limited amounts of salt water then allowing further equilibration with excess salt solutions; and c), cycling through the main phase transition in excess salt solution. In our experiments, we have used only the first preparation method because it is the most common and also the most often used in the literature (see Fig. 1). Usually the problem of equilibration is confronted by letting the sample overnight at a temperature higher than the chain melting temperature. We followed this procedure without observing any problem to the equilibration of the samples. In addition many researchers have used this method in the past with success for the equilibration of their samples.
FIGURE 1.
Equilibrium of the samples.
Effect of salt on the osmotic pressure
The osmotic pressure of the PEG polymer can be affected by the presence of electrolyte, which may influence the Flory-Huggins interaction parameter as shown in Eq. 6. The osmotic pressure, πmix, due to the mixing of polymer and solvent molecules (e.g., salt solution) can be expressed by the Flory-Huggins equation (97):
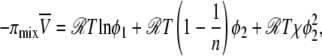 |
(6) |
where ϕ1 is the volume fraction of the solvent, ϕ2 is the volume fraction of the polymer, n is the number of segments in the polymer chains (proportional to the molecular weight), and χ is the Flory-Huggins interaction parameter, which characterizes the interaction between the polymer segments and the solvent molecules.
However, according to Parsegian et al. (91), the effect of an electrolyte solution on the osmotic pressure of a PEG solution is small, and does not depend strongly on the ionic strength for small electrolyte concentrations. The strongest effect of a salt solution on the osmotic pressure of PEG was reported for the chaotropic salt NaClO4, which lowers the osmotic pressure exerted by PEG 20,000 in 1 M solutions by up to 40% (91).
If we accept that the effect of any salt solution on the osmotic pressure of PEG is the maximum found for NaClO4 (40%), then according to Eq. 5 the change in the osmotic pressure of PEG for two limiting concentrations, e.g., G = 0.015 and G = 0.79 is as shown in Table 1. From Table 1 we see that even if the effect of a salt solution on the osmotic pressure is considerable, the error in the logΠ scale used in the representation of the experimental results is significantly compressed. In our case, we accepted that the salt solution affects the osmotic pressure of the polymer by ∼20% and we took this into consideration for calculating the exerted osmotic pressure of PEG 20,000.
TABLE 1.
Osmotic pressure variation of PEG solutions in the presence of salt
| G | Π (dyn/cm2) | Π (40%) (dyn/cm2) | logΠ (dyn/cm2) |
|---|---|---|---|
| 0.015 | 77,918 | 77,918 ± 31,167 | 4.89 ± 0.2 |
| 0.79 | 50,300,335 | 50,300,335 ± 20,120,134 | 7.70 ± 0.2 |
Maximum swelling
To determine the maximum swelling of DPPC in pure water and in the presence of sodium salts solutions, a known amount of lipid was added to varied amounts of solution and allowed to equilibrate for 48–72 h at room temperature. Then the samples were transferred to an oven thermostated at T = 50 ± 1°C for 18–20 h before x-ray measurements.
Karl Fischer
The water content of the samples was determined by Karl Fischer (KF) titrations using a 684 KF Coulometer (Brinkmann Metrohm, Westbury, NY). I2 was generated electrolytically. The KF reagent (Hydranal coulomat) was purchased from Riedel-De Haen (Seelze, Germany).
EXPERIMENTAL RESULTS
In this work we have carried out the following experimental measurements:
Determination of the maximum swelling of DPPC in pure water (no salt) and in different sodium salt solutions of concentration 0.05, 0.1, and 0.5 M by small angle x-ray scattering.
Determination of the bilayer thickness of DPPC in pure water (no salt) and in different sodium salt solutions of concentration 0.05, 0.1, and 0.5 M using small angle x-ray scattering and Karl Fischer titrations.
Construction of osmotic pressure–interbilayer distance curves using the osmotic stress technique in combination with small angle x-ray scattering experiments to obtain an equation of state (EOS) of the zwitterionic (DPPC) bilayer system in the fluid state (T = 50°C) in the presence of different sodium salt solutions.
Ion “competition” experiments, using NaI/NaCl mixtures at constant total salinity of 0.1 and 0.5 M, and varying the relative amount of the two anions. In these experiments the interbilayer distance was measured at constant osmotic pressure.
Maximum swelling
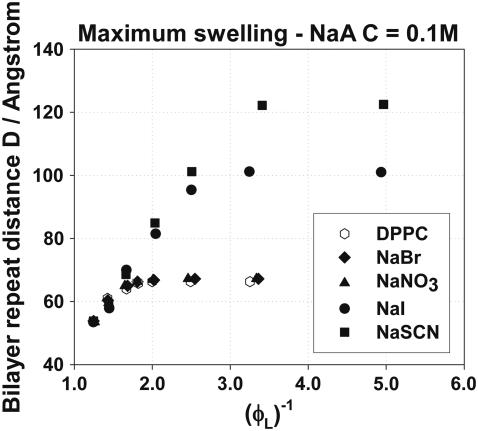
The maximum swelling curves for DPPC bilayers in NaA salt solutions of concentration 0.1 M are presented in Fig. 2 as plots of the repeat distance D versus the inverse of the lipid volume fraction. This way of plotting is suggested by Eq. 1, according to which D should be inversely proportional to ϕL for constant bilayer thickness. From Fig. 2 we can see that the maximum swelling is influenced by the anion type in a significant way. Similar maximum swelling curves were obtained with NaA concentrations of 0.05 and 0.5 M and are not shown here (45). The anions used influence the maximum swelling in the order Br− < 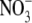 < I− < SCN−, which is a direct Hofmeister series with the SCN− having the largest effect on the equilibrium separation. The influence of the concentration is more complex; it depends on the type of the anion used and will be discussed below. Table 2 summarizes the maximum swelling results for DPPC in the presence of the sodium salt solutions of various concentrations whereas in Table 3 the maximum swelling parameters of DPPC in pure water found in this work are compared with those found in the literature, and a good agreement is observed (87,98).
< I− < SCN−, which is a direct Hofmeister series with the SCN− having the largest effect on the equilibrium separation. The influence of the concentration is more complex; it depends on the type of the anion used and will be discussed below. Table 2 summarizes the maximum swelling results for DPPC in the presence of the sodium salt solutions of various concentrations whereas in Table 3 the maximum swelling parameters of DPPC in pure water found in this work are compared with those found in the literature, and a good agreement is observed (87,98).
FIGURE 2.
Maximum swelling of DPPC in the presence of NaA salt solutions of concentration C = 0.1 M.
TABLE 2.
Maximum swelling of DPPC in NaA salt solutions of different concentrations
| Concentration | Parameters | NaBr | NaNO3 | NaI | NaSCN |
|---|---|---|---|---|---|
| C = 0.05M | Dmax (Å) | – | – | 74.8 | 148.0 |
| ϕL(max)% | – | – | 48.8 | 26.3 | |
| dw(max) (Å) | – | – | 38.3 | 109.05 | |
| C = 0.1M | NaBr | NaNO3 | NaI | NaSCN | |
| Dmax (Å) | 67.2 | 67.2 | 101 | 122.5 | |
| ϕL(max)% | 52.2 | 52.2 | 39.2 | 33.3 | |
| dw(max) (Å) | 32.1 | 32.1 | 61.5 | 81.7 | |
| C = 0.5M | NaBr | NaNO3 | NaI | NaSCN | |
| Dmax (Å) | 68.0 | 66.8 | 77.5 | 85.6 | |
| ϕL(max)% | 52.5 | 52.1 | 46.5 | 42.7 | |
| dw(max) (Å) | 32.3 | 32.0 | 41.5 | 49.0 |
TABLE 3.
Comparison of the maximum swelling of DPPC in pure water with values found in the literature
Bilayer thickness
The bilayer thickness was determined using Eq. 1. Fig. 3 shows the dependence of the bilayer thickness on the osmotic pressure exerted on the DPPC bilayers for NaA solutions of concentration 0.1 M. Similar experimental results were obtained with NaA concentrations of 0.05 and 0.5 M and are presented as supporting information. In general, the bilayer thickness of DPPC is affected by the type and concentration of anions used. The chaotropic anions I− and SCN− are those that have the strongest effect on the bilayer thickness of DPPC whereas Br− and 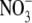 do not seem to have a significant influence. In Fig. 3 it is shown that the bilayer thickness decreases at small osmotic pressures indicating that the presence of anions induces significant membrane thinning. This considerable thinning may be evidence of lipid interdigitation.
do not seem to have a significant influence. In Fig. 3 it is shown that the bilayer thickness decreases at small osmotic pressures indicating that the presence of anions induces significant membrane thinning. This considerable thinning may be evidence of lipid interdigitation.
FIGURE 3.
Bilayer thickness, bL, versus the osmotic pressure, Π, exerted on DPPC bilayers in the presence of NaA solutions of concentration 0.1 M.
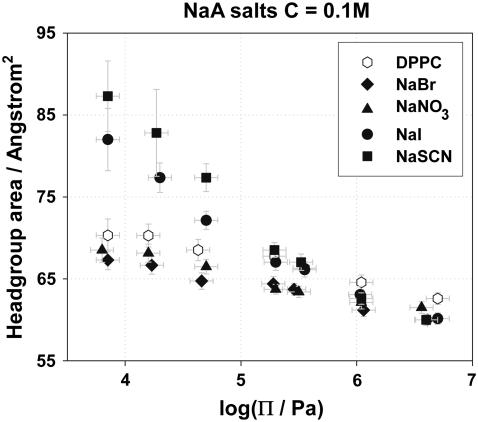
Area per headgroup
The headgroup area, A, of a DPPC molecule in the absence and presence of various NaA salt solutions was calculated using Eq. 4. Fig. 4 shows the dependence of the headgroup area on the osmotic pressure exerted on the lipid bilayers in the presence of 0.1 M NaA salt solutions, and proves that the headgroup area is affected by the type of anions used. At small osmotic pressures, the chaotropic anions I− and SCN− have the strongest effect on the headgroup area, an effect that may be attributed to ion binding, although partial lipid interdigitation may not be ruled out. Br− and 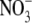 dehydrate the DPPC headgroup to some extent, and it appears that the dehydration effect is stronger than ion binding for these less chaotropic ions. In addition, as the osmotic pressure increases, the headgroup area in the presence of NaI and NaSCN decreases in a faster way, and at high pressures it becomes roughly independent of the salt present, indicating that the dehydration of the headgroups with pressure plays a more important role than ion binding. Similar experimental results were obtained with NaA concentrations of 0.05 and 0.5 M and are provided as supporting information.
dehydrate the DPPC headgroup to some extent, and it appears that the dehydration effect is stronger than ion binding for these less chaotropic ions. In addition, as the osmotic pressure increases, the headgroup area in the presence of NaI and NaSCN decreases in a faster way, and at high pressures it becomes roughly independent of the salt present, indicating that the dehydration of the headgroups with pressure plays a more important role than ion binding. Similar experimental results were obtained with NaA concentrations of 0.05 and 0.5 M and are provided as supporting information.
FIGURE 4.
Headgroup area, A, versus the osmotic pressure, Π, exerted on DPPC bilayers in the presence of NaA solutions of concentration 0.1 M.
Pressure-distance isotherms (equation-of-state curves)
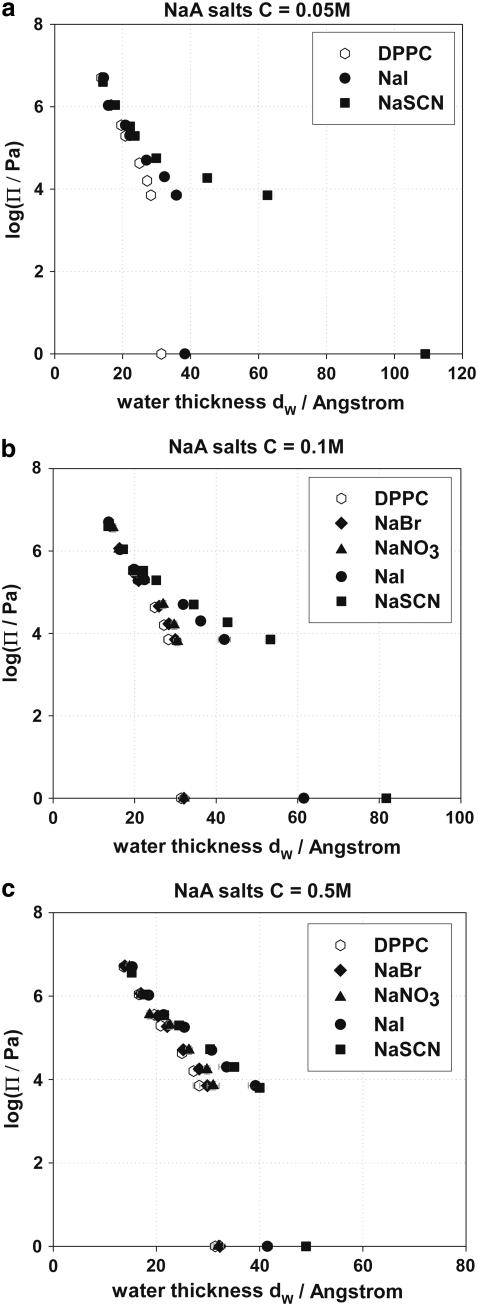
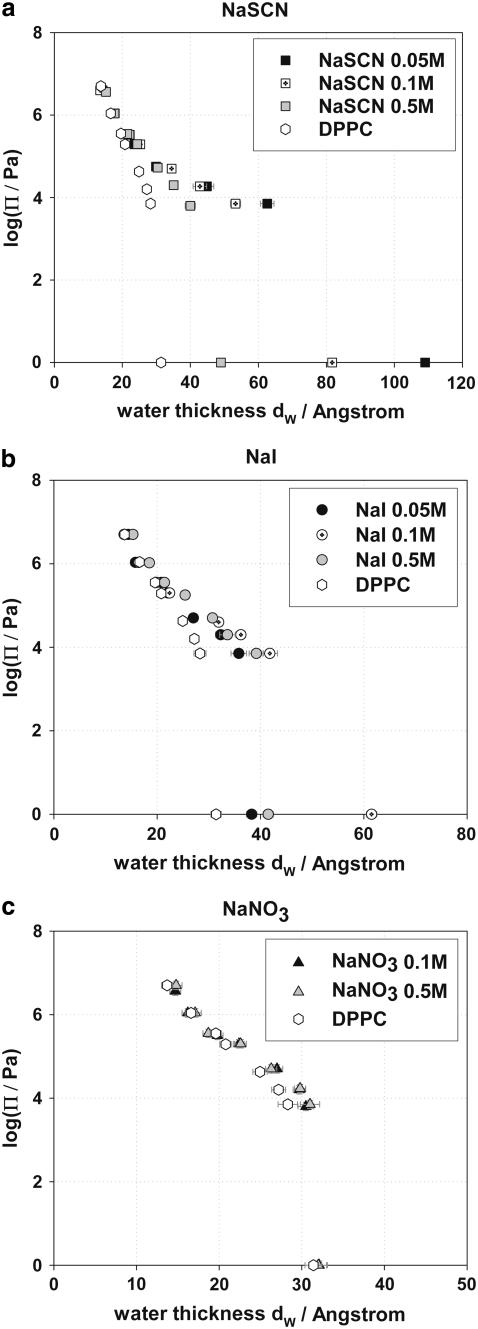
Osmotic pressure versus interbilayer distance curves (logΠ − dw) have been used by many investigators to provide information on forces acting between lipid bilayers (49,50,53,66,69,70,73,74,87,91,98). The logΠ − dw curves for DPPC in pure water and in NaBr, NaNO3, NaI, and NaSCN salt solutions of concentrations 0.05, 0.1, and 0.5 M are presented in Fig. 5, a–c, respectively. The experimental points at logΠ = 0 are the equilibrium separations at maximum swelling of DPPC bilayers in water and in the presence of salt solutions. Assigning maximum swelling values to logΠ = 0 is an assumption regularly made in the literature, but we conclude in the companion article that it generates some serious problems for the theoretical modeling of the interaction curves.
FIGURE 5.
(a) logΠ − dw curves of DPPC in the presence of NaBr, NaNO3, NaI, and NaSCN at concentration C = 0.05 M. (b) logΠ − dw curves of DPPC in the presence of NaBr, NaNO3, NaI, and NaSCN at concentration C = 0.1 M. (c) logΠ − dw curves of DPPC in the presence of NaBr, NaNO3, NaI, and NaSCN at concentration C = 0.5 M.
Generally, we observe that the water bilayer separation dw for the same osmotic pressure increases when salts are present (at all salt concentrations). The change of dw is more pronounced at small osmotic pressures and diminishes as the osmotic pressure applied to the bilayers increases. Most experimental curves appear to converge at high osmotic pressures (low dw), implying that the hydration forces that dominate the interactions at these distances do not depend strongly on salt presence. The increase of the water bilayer separation depends on the type of anion in the sodium salts, with SCN− having the strongest effect on dw, and Br− having the smallest. The effect of the anions on dw follows the Hofmeister series. NaCl and NaBr appear to have a much smaller effect on DPPC at 50°C, than that of KCl and KBr observed by Petrache et al. on DLPC bilayers at lower temperatures (74).
The increase of the water bilayer separation depends on the concentration of the sodium salt solutions. Fig. 6 a shows the influence of NaSCN on dw whereas Fig. 6 b shows the influence of NaI on dw at three concentrations, 0.05, 0.1, and 0.5 M. It is observed that dw decreases continuously at fixed osmotic pressure upon increasing the concentration of NaSCN salt from 0.05 to 0.5 M. On the contrary, NaI is more effective at a concentration of 0.1 M whereas 0.05 M provides the smallest effect and 0.5 M gives intermediate results. Similar behavior for DPPC in the presence of Ca2+ ions has been observed by Lis et al. (49). This complex behavior may be explained by two contrasting phenomena that determine the electrostatic repulsive force between the bilayers; these are ion binding and electrostatic screening. It can be assumed that the influence of NaI on dw at a concentration of 0.05 M is weaker because the adsorption of I− on the DPPC headgroups and hence interfacial charging is still relatively small. In contrast, the influence of NaI on dw at a concentration of 0.5 M is weak due to high electrostatic screening. As a result, there will be an intermediate concentration, for which the repulsion between the DPPC bilayers due to the adsorbed anions is highest. NaSCN behaves differently; the electrostatic repulsion decreases consistently with increasing salt concentration. This behavior may be explained by assuming a very strong binding of SCN− on the DPPC headgroups. Even at low concentrations, e.g., 0.05 M, SCN− binds more strongly to the DPPC molecules than I−, and thus the electrostatic repulsion that is generated is stronger than that observed in the presence of an equal concentration of I−. As NaSCN concentration increases more SCN− ions bind to the DPPC headgroups but apparently the increased binding is more than counterbalanced by the double-layer screening. It is possible that a maximum repulsive force between the bilayers might also have been observed at NaSCN concentrations lower than 0.05 M, but such concentrations were not examined in our experiments.
FIGURE 6.
(a) logΠ − dw of DPPC in the presence of NaSCN at concentration 0.05, 0.1, and 0.5 M. (b) logΠ − dw of DPPC in the presence of NaI at concentration 0.05, 0.1, and 0.5 M. (c) logΠ − dw of DPPC in the presence of NaNO3 at concentration 0.05 and 0.1 M.
On the contrary, the presence of NaBr and NaNO3 salt solutions does not appear to affect significantly the water bilayer separation, dw, by varying the salt concentration as observed in Fig. 6 c for NaNO3. The KCl and KBr experiments of Petrache et al. demonstrated the opposite ionic effect (74). In those experiments, dw continuously increased as the salt concentration increased, which must be attributed to the relatively weak binding of these ions, which require high bulk concentrations to reach interfacial saturation.
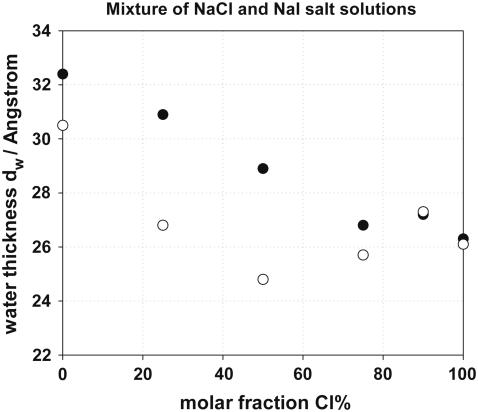
Cl−/I− ion competition experiments
To compare the effect of a relatively hydrophilic (Cl−) and a chaotropic (I−) ion on the structure of DPPC bilayers, the equilibrium spacing was measured in the presence of mixtures of NaCl and NaI salt solutions, as shown in Fig. 7. Two series of experiments were performed at a constant total [NaCl] + [NaI] concentration, changing the percentage of NaI from 0% to 100%. The total salt concentration used in the two cases was 0.1 M and 0.5 M. All spacings were measured at an osmotic pressure of log[Π / Pa] = 4.6. Starting at 100% NaCl and adding NaI we observe significant changes in the spacing as soon as NaI becomes >20% of the total salt in the solution. The increase of dw upon addition of iodide can be explained by the stronger interfacial iodide adsorption, which creates a surface charge on the bilayers; dw does not change linearly with the percentage of NaI in the mixture, especially at a total salt concentration of 0.5 M, at which it appears to have a minimum value. Nonlinear behavior was also observed by Petrache et al. (74) in their Cl−/Br− exchange experiments. In Fig. 7, we see that the effect on dw strongly depends on the total salt concentration used.
FIGURE 7.
Water thickness of DPPC in a mixture of NaCl and NaI salt solution at concentration 0.1 and 0.5 M. Total [NaCl] + [NaI] = 0.1 M (•) and [NaCl] + [NaI] = 0.5 M (○).
CONCLUSIONS
The experimental results described above show that anions strongly influence the properties of the DPPC bilayers. The swelling effects observed always follow the Hofmeister series and depend on salt concentration. Swelling of the interbilayer distance is linked to an increase of headgroup area (Fig. 4), which can be attributed to lateral electrostatic interactions arising from charging through ionic adsorption. The experimental logΠ − dw curves of DPPC bilayers in the presence of salt solutions show that the water bilayer separation, dw, (for the same osmotic pressure) increases when salts are present, a fact observed at all salt concentrations. Most of the difference between the force curves (logΠ − dw) of DPPC in water and in the presence of NaA solutions is believed to arise from the existence of an electrostatic repulsive force created by the adsorption of the anions to the DPPC headgroups. A lowering of the Hamaker constant between the bilayers is expected in the presence of electrolytes (74,96,99,100) but, as will be discussed in the companion article and in contrast to the conclusion of Petrache et al. (74), it does not suffice to explain the strong effects of chaotropic anions or the many different structural changes observed in these experiments as functions of salt concentration. The effects of electrolyte concentration on many structural parameters may be explained by two phenomena that determine the electrostatic repulsive force between the lipid bilayers. These are ion binding and ion screening. Interdigitation of the lipids may also occur at low osmotic pressures for the strongly chaotropic SCN− and I− ions.
The maximum swelling experiments in the presence of different NaA electrolytes have been performed for the first time in such a systematic way for DPPC bilayers in the fluid state. The experimental results show that the maximum water uptake by the bilayers is influenced both by the type of the anion and the concentration of the sodium salt solutions with SCN− inducing the greatest effect.
The headgroup area, A, of DPPC molecules at the bilayer surfaces was computed and was found to be affected by the type and concentration of anions used. The DPPC headgroup area in pure water agrees with those reported in the literature determined by the Gravimetric x-ray method with or without a compressibility correction (87,98). The chaotropic anions I− and SCN− have the strongest effect on the DPPC headgroup area, increasing it considerably, especially at small osmotic pressures. This increase of A supports the notion that these chaotropic anions strongly associate with the lipid headgroups.
As stated in the introduction, the goal of this work was to improve understanding of the mechanism of action of Hofmeister anions. The experimental results presented here provide considerable insights, but a more quantitative analysis of the data yielding ion-lipid association parameters is necessary to obtain deeper understanding. The experimental database created in this article allows the application of two completely different theoretical formalisms. One concerns the equation-of-state data and uses a summation of forces between bilayers as is usually done in osmotic stress experiments; it is a “perpendicular pressure” model. The second formalism aims to reproduce the lipid headgroup area as a function of salinity, and is a “lateral pressure” model. The great challenge is to fit both types of data with the same ionic “binding constants” or related association parameters. This extensive theoretical modeling is attempted in the companion article.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the French-German Network, Commissariat de l' Energie Atomique (CEA) Saclay, France, and Max Planck Institute of Colloids and Interfaces Golm/Potsdam, Germany.
Editor: Antoinette Killian.
References
- 1.Kunz, W., J. Henle, and B. W. Ninham. 2004. About the science of the effect of salts: Franz Hofmeister's historical papers. Curr. Opin. Colloid Int. Sci. 9:19–37. [Google Scholar]
- 2.Collins, K. D., and M. W. Washabaugh. 1985. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 18:323–422. [DOI] [PubMed] [Google Scholar]
- 3.Cacace, M. G., E. M. Landau, and J. J. Ramsden. 1997. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 30:241–277. [DOI] [PubMed] [Google Scholar]
- 4.Curr. Op. Colloid Int. Sci. 2004. 9:1–197.
- 5.Melander, W., and C. Horvath. 1977. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins: an interpretation of the lyotropic series. Arch. Biochem. Biophys. 183:200–215. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin, R. L. 1996. How Hofmeister ion interactions affect protein stability. Biophys. J. 71:2056–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsegian, V. A. 2002. Protein-water interactions. Int. Rev. Cytol. 215:1–31. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn, L. A., M. A. Siani, M. E. Pique, C. L. Fisher, E. D. Getzoff, and J. A. Tainer. 1992. The interdependence of protein surface topography and bound water molecules revealed by surface accessibility and fractal density measures. J. Mol. Biol. 228:13–22. [DOI] [PubMed] [Google Scholar]
- 9.Thanki, N., J. M. Thornton, and J. M. Goodfellow. 1988. Distributions of water around amino acid residues in proteins. J. Mol. Biol. 202:637–657. [DOI] [PubMed] [Google Scholar]
- 10.Chandler, D. 2005. Interfaces and the driving force of hydrophobic assembly. Nature. 437:640–647. [DOI] [PubMed] [Google Scholar]
- 11.Omta, A. W., M. F. Kropman, S. Woutersen, and H. J. Bakker. 2003. Influence of ions on the hydrogen-bond structure in liquid water. J. Chem. Phys. 119:12457–12461. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor, J. D., A. Olteanu, A. Tripathy, and G. J. Pielak. 2004. Impact of protein denaturants and stabilizers on water structure. J. Am. Chem. Soc. 126:1958–1961. [DOI] [PubMed] [Google Scholar]
- 13.Cappa, C. D., J. D. Smith, K. R. Wilson, B. M. Messer, M. K. Gilles, R. C. Cohen, and R. J. Saykally. 2005. Effects of alkali metal halide salts on the hydrogen bond network of liquid water. J. Phys. Chem. B. 109:7046–7052. [DOI] [PubMed] [Google Scholar]
- 14.Lyklema, J. 2003. Lyotropic sequences in colloid stability revisited. Adv. Colloid Int. Sci. 100–102:1–12. [Google Scholar]
- 15.Weissenborn, P. K., and R. J. Pugh. 1996. Surface tension of aqueous solutions of electrolytes: relationship with ion hydration, oxygen solubility, and bubble coalescence. J. Colloid Int. Sci. 184:550–563. [DOI] [PubMed] [Google Scholar]
- 16.Long, F. A., and W. F. McDevit. 1952. Activity coefficients of nonelectrolyte solutes in aqueous salt solutions. Chem. Rev. 51:119–169. [Google Scholar]
- 17.Grover, P. K., and R. L. Ryall. 2005. Critical appraisal of salting-out and its implications for chemical and biological sciences. Chem. Rev. 105:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Ninham, B. W., and V. Yaminsky. 1997. Ion binding and ion specificity: the Hofmeister effect and Onsager and Lifshitz theories. Langmuir. 13:2097–2108. [Google Scholar]
- 19.Boström, M., D. R. M. Williams, and B. W. Ninham. 2001. Specific ion effects: why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 87:168103. [DOI] [PubMed] [Google Scholar]
- 20.Boström, M., D. R. M. Williams, P. R. Stewart, and B. W. Ninham. 2003. Hofmeister effects in membrane biology: the role of ionic dispersion potentials. Phys. Rev. E. 68:041902. [DOI] [PubMed] [Google Scholar]
- 21.Boström, M., and B. W. Ninham. 2004. Contributions from dispersion and Born self-free energies to the solvation energies of salt solutions. J. Phys. Chem. B. 108:12593–12595. [Google Scholar]
- 22.Boström, M., D. R. M. Williams, and B. W. Ninham. 2001. Surface tension of electrolytes: specific ion effects explained by dispersion forces. Langmuir. 17:4475–4478. [Google Scholar]
- 23.Boström, M., and B. W. Ninham. 2004. Dispersion self-free energies and interaction free energies of finite-sized ions in salt solutions. Langmuir. 20:7569–7574. [DOI] [PubMed] [Google Scholar]
- 24.Boström, M., W. Kunz, and B. W. Ninham. 2005. Hofmeister effects in surface tension of aqueous electrolyte solution. Langmuir. 21:2619–2623. [DOI] [PubMed] [Google Scholar]
- 25.Knipping, E. M., M. J. Lakin, K. L. Foster, P. Jungwirth, D. J. Tobias, R. B. Gerber, D. Dabdub, and B. J. Finlayson-Pitts. 2000. Experiments and simulations of ion-enhanced interfacial chemistry on aqueous NaCl aerosols. Science. 288:301–306. [DOI] [PubMed] [Google Scholar]
- 26.Jungwirth, P., and D. J. Tobias. 2001. Molecular structure of salt solutions: a new view of the interface with implications for heterogeneous atmospheric chemistry. J. Phys. Chem. B. 105:10468–10472. [Google Scholar]
- 27.Jungwirth, P., and D. J. Tobias. 2002. Ions at the air/water interface. J. Phys. Chem. B. 106:6361–6373. [Google Scholar]
- 28.Jungwirth, P., and D. J. Tobias. 2006. Specific ion effects at the air/water interface. Chem. Rev. 106:1259–1281. [DOI] [PubMed] [Google Scholar]
- 29.Dang, L. X., and T.-M. Chang. 2002. Molecular mechanism of ion binding to the liquid/vapor interface of water. J. Phys. Chem. B. 106:235–238. [Google Scholar]
- 30.Dang, L. X. 2002. Computational study of ion binding to the liquid interface of water. J. Phys. Chem. B. 106:10388–10394. [Google Scholar]
- 31.Chang, T. M., and L. X. Dang. 2006. Recent advances in molecular simulations of ion solvation at liquid interfaces. Chem. Rev. 106:1305–1322. [DOI] [PubMed] [Google Scholar]
- 32.Archontis, G., E. Leontidis, and G. Andreou. 2005. Attraction of iodide ions by the free water surface, revealed by simulations with a polarizable force field based on Drude oscillators. J. Phys. Chem. B. 109:17957–17966. [DOI] [PubMed] [Google Scholar]
- 33.Archontis, G., and E. Leontidis. 2006. Dissecting the stabilization of iodide at the air-water interface into components: a free energy analysis. Chem. Phys. Lett. 420:199–203. [Google Scholar]
- 34.Petersen, P. B., and R. J. Saykally. 2004. Confirmation of enhanced anion concentration at the liquid water surface. Chem. Phys. Lett. 397:51–55. [Google Scholar]
- 35.Liu, D., G. Ma, L. M. Levering, and H. C. Allen. 2004. Vibrational spectroscopy of aqueous sodium halide solutions and air-liquid interfaces. Observation of Increased Interfacial Depth. J. Phys. Chem. B. 108:2252–2260. [Google Scholar]
- 36.Gopalakrishnan, S., D. Liu, H. C. Allen, M. Kuo, and M. J. Shultz. 2006. Vibrational spectroscopic studies of aqueous interfaces: salts, acids, bases, and nanodrops. Chem. Rev. 106:1155–1175. [DOI] [PubMed] [Google Scholar]
- 37.Ghosal, S., J. C. Hemminger, H. Bluhm, B. S. Mun, E. L. D. Hebenstreit, G. Ketteler, D. F. Ogletree, F. G. Requejo, and M. Salmeron. 2005. Electron spectroscopy of aqueous solution interfaces reveals surface enhancement of halides. Science. 307:563–566. [DOI] [PubMed] [Google Scholar]
- 38.Collins, K. D. 1997. Charge density-dependent strength of hydration and biological structure. Biophys. J. 72:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlström, G., and D. Hagberg. 2002. Toward an understanding of the Hofmeister effect: a computer game with dipoles and an ion. J. Phys. Chem. B. 106:11585–11592. [Google Scholar]
- 40.Hagberg, D., S. Brdarski, and G. Karlström. 2005. On the solvation of ions in small water droplets. J. Phys. Chem. B. 109:4111–4117. [DOI] [PubMed] [Google Scholar]
- 41.Hribar, B., N. T. Southall, V. Vlachy, and K. A. Dill. 2002. How ions affect the structure of water. J. Am. Chem. Soc. 124:12302–12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kjellander, R., A. P. Lyubartsev, and S. Marcelja, S. 2001. McMillan-Mayer theory for solvent effects in inhomogeneous systems: calculation of interaction pressure in aqueous electrical double layers. J. Chem. Phys. 114:9565–9577. [Google Scholar]
- 43.Marcelja, S. 2004. Short-range forces in surface and bubble interaction. Curr. Opin. Colloid Int. Sci. 9:165–167. [Google Scholar]
- 44.Zemb, T., L. Belloni, M. Dubois, A. Aroti, and E. Leontidis. 2004. Can we use area per surfactant as a quantitative test model of specific ion effects? Curr. Opin. Colloid Int. Sci. 9:74–80. [Google Scholar]
- 45.Aroti, A. 2005. Study of the effect of Hofmeister anions on monolayer, bilayer and micelle lipid model systems through experiments and theory. PhD thesis. University of Cyprus, Nicosia, Cyprus.
- 46.Aroti, A., E. Leontidis, E. Maltseva, and G. Bresezinski. 2004. Effects of Hofmeister anions on DPPC Langmuir monolayers at the air-water interface. J. Phys. Chem. B. 108:15238–15245. [Google Scholar]
- 47.Simon, S. A., L. J. Lis, J. W. Kauffmann, and R. C. Macdonald. 1975. A calorimetric and monolayer investigation of the influence of ions on the thermodynamic properties of phosphatidylcholine. Biochim. Biophys. Acta. 375:317–326. [DOI] [PubMed] [Google Scholar]
- 48.Hauser, H., C. C. Hinckley, J. Krebbs, B. A. Levine, M. C. Phillips, and R. J. P. Williams. 1977. The interaction of ions with phosphatidylcholine bilayers. Biochim. Biophys. Acta. 468:364–377. [DOI] [PubMed] [Google Scholar]
- 49.Lis, L. J., V. A. Parsegian, and R. P. Rand. 1981. Binding of divalent cations to dipalmitoylphosphatidylcholine bilayers and its effect on bilayer interaction. Biochemistry. 20:1761–1770. [DOI] [PubMed] [Google Scholar]
- 50.Lis, L. J., W. T. Lis, V. A. Parsegian, and R. P. Rand. 1981. Adsorption of divalent cations to a variety of phosphatidylcholine bilayers. Biochemistry. 20:1771–1777. [DOI] [PubMed] [Google Scholar]
- 51.Akutsu, H., and J. Seelig. 1981. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 20:7366–7373. [DOI] [PubMed] [Google Scholar]
- 52.Altenbach, C., and J. Seelig. 1984. Calcium binding to phosphatidylcholine bilayers as studied by deuterium magnetic resonance. Evidence for the formation of a calcium complex with two phospholipid molecules. Biochemistry. 23:3913–3920. [DOI] [PubMed] [Google Scholar]
- 53.Ohshima, H., Y. Inoko, and T. Mitsui. 1982. Hamaker constant and binding constants of Ca2+ and Mg2+ in dipalmitoyl phosphatidylcholine/water system. J. Colloid Int. Sci. 86:57–72. [Google Scholar]
- 54.Afzal, S., W. J. Tesler, S. K. Blessing, J. M. Collins, and L. J. Lis. 1983. Hydration force between phosphatidylcholine surfaces in aqueous electrolyte solutions. J. Colloid Int. Sci. 97:303–307. [Google Scholar]
- 55.Roux, M., and M. Bloom. 1990. Calcium, magnesium, lithium, sodium, and potassium distributions in the headgroup region of binary membranes of phosphatidylcholine and phosphatidylserine as seen by deuterium NMR. Biochemistry. 29:7077–7089. [DOI] [PubMed] [Google Scholar]
- 56.Rappolt, M., G. Pabst, H. Amenitsch, and P. Laggner. 2001. Salt-induced phase separation in the liquid crystalline phase of phosphatidylcholines. Colloids and Surfaces A: Phys. Eng. Asp. 183–185:171–181. [Google Scholar]
- 57.Scarpa, M. V., F. A. Maximiano, H. Chaimovich, and I. M. Cuccovia. 2002. Interfacial concentrations of chloride and bromide and selectivity for ion exchange in vesicles prepared with dioctadecyldimethylammonium halides, lipids, and their mixtures. Langmuir. 18:8817–8823. [Google Scholar]
- 58.Jendrasiak, G. L. 1972. Halide interaction with phospholipids: proton magnetic resonance studies. Chem. Phys. Lipids. 9:133–146. [DOI] [PubMed] [Google Scholar]
- 59.Jendrasiak, G. L., R. Smith, and A. A. Ribeiro. 1993. Chaotropic anion-phosphatidylcholine membrane interactions: an ultra high field NMR study. Biochim. Biophys. Acta. 1145:25–32. [DOI] [PubMed] [Google Scholar]
- 60.Loshchilova, E., and B. Karvaly. 1978. Laser Raman studies of molecular interactions with phosphatidylcholine multilayers. II. Effects of mono- and divalent ions on bilayer structure. Biochim. Biophys. Acta. 514:274–285. [DOI] [PubMed] [Google Scholar]
- 61.McDonald, P. M., and J. Seelig. 1988. Anion binding to neutral and positively charged lipid membranes. Biochemistry. 27:6769–6775. [DOI] [PubMed] [Google Scholar]
- 62.Rydall, J. R., and P. M. McDonald. 1992. Investigation of anion binding to neutral lipid membranes using deuterium NMR. Biochemistry. 31:1092–1099. [DOI] [PubMed] [Google Scholar]
- 63.Epand, R. M., and M. Bryszewska. 1988. Modulation of the bilayer to hexagonal phase transition and solvation of phosphatidylethanolamines in aqueous salt solutions. Biochemistry. 27:8776–8779. [DOI] [PubMed] [Google Scholar]
- 64.McLaughlin, S., A. Bruder, S. Chen, and C. Moser. 1975. Chaotropic anions and the surface potential of bilayer membranes. Biochim. Biophys. Acta. 394:304–313. [DOI] [PubMed] [Google Scholar]
- 65.Tatulian, S. A. 1983. Effect of lipid phase transition on the binding of anions to dimyristoylphosphatidylcholine liposomes. Biochim. Biophys. Acta. 736:189–195. [DOI] [PubMed] [Google Scholar]
- 66.Tatulian, S. A., V. I. Gordeliy, A. E. Sokolova, and A. G. Syrykh. 1991. A neutron diffraction study of the influence of ions on phospholipid membrane interactions. Biochim. Biophys. Acta. 1070:143–151. [DOI] [PubMed] [Google Scholar]
- 67.Clarke, R. J., and C. Lüpfert. 1999. Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: a basis for the Hofmeister effect. Biophys. J. 76:2614–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chapman, D., W. E. Peel, B. Kingston, and T. H. Lilley. 1977. Lipid phase transitions in model biomembranes: the effect of ions on phosphatidylcholine bilayers. Biochim. Biophys. Acta. 464:260–275. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham, B. A., and L. J. Lis. 1986. Thiocyanate and bromide ions influence the bilayer structural parameters of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 861:237–242. [DOI] [PubMed] [Google Scholar]
- 70.Cunningham, B. A., L. J. Lis, and P. J. Quinn. 1986. The influence of monovalent anions on dipalmitoylphosphatidylcholine bilayer phase transitions: a time resolved X-Ray diffraction study. Mol. Cryst. Liq. Cryst. 141:361–367. [Google Scholar]
- 71.Sanderson, P. W., L. J. Lis, P. J. Quinn, and W. P. Williams. 1991. The Hofmeister effect in relation to membrane lipid phase stability. Biochim. Biophys. Acta. 1067:43–50. [DOI] [PubMed] [Google Scholar]
- 72.Pryzyczyna, A. B., Rózycka-Roszk, and M. Langer. 2002. The effect of selected anions on dipalmitoylphosphatidylcholine phase transitions. Z. Naturforsch. 57:712–716. [DOI] [PubMed] [Google Scholar]
- 73.Cunningham, B. A., and L. J. Lis. 1989. Interactive forces between phosphatidylcholine bilayers in monovalent salt solutions. J. Colloid Int. Sci. 128:15–25. [Google Scholar]
- 74.Petrache, H. I., T. Zemb, L. Belloni, and V. A. Parsegian. 2006. Salt screening and specific ion adsorption determine neutral-lipid membrane interactions. Proc. Natl. Acad. Sci. USA. 103:7982–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartucci, R., and L. Sportelli. 1994. Spin label EPR study of the effects of monovalent cations, anions, and chaotropics on DPPC multilayers. Biochim. Biophys. Acta. 1195:229–236. [DOI] [PubMed] [Google Scholar]
- 76.Bartucci, R., S. Belsito, and L. Sportelli. 1996. Neutral lipid bilayers interacting with chaotropic anions. Chem. Phys. Lipids. 79:171–180. [Google Scholar]
- 77.Kaznessis, Y. N., S. Kim, and R. G. Larson. 2002. Simulations of zwitterionic and anionic phospholipid monolayers. Biophys. J. 82:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sachs, J. N., and T. B. Woolf. 2003. Understanding the Hofmeister effect in interactions between chaotropic anions and lipid bilayers. Molecular dynamics simulations. J. Am. Chem. Soc. 125:8742–8743. [DOI] [PubMed] [Google Scholar]
- 79.Sachs, J. N., H. Nanda, H. I. Petrache, and T. B. Woolf. 2004. Changes in phosphatidylcholine headgroup tilt and water order induced by monovalent salts. Molecular Dynamics Simulations. Biophys. J. 86:3772–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Böckmann, R. A., A. Hac, T. Heimburg, and H. Grubmüller. 2003. Effect of sodium chloride on a lipid bilayer. Biophys. J. 85:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Böckmann, R. A., and H. Grubmüller. 2004. Multistep binding of divalent cations to phospholipid bilayers: a molecular dynamics study. Angew. Chem. Int. Ed. Engl. 43:1021–1024. [DOI] [PubMed] [Google Scholar]
- 82.Pandit, S. A., D. Bostick, and M. L. Berkowitz. 2003. Mixed bilayer containing dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylserine: lipid complexation, ion binding, and electrostatics. Biophys. J. 84:3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandit, S. A., D. Bostick, and M. L. Berkowitz. 2003. Molecular dynamics simulation of a dipalmitoyl-phosphatidylcholine bilayer with NaCl. Biophys. J. 84:3743–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gurtovenko, A. A. 2005. Asymmetry of lipid bilayers induced by monovalent salt: atomistic molecular-dynamics study. J. Chem. Phys. 122:244902. [DOI] [PubMed] [Google Scholar]
- 85.Zemb, T., O. Taché, F. Né, and O. Spalla. 2003. A high-sensitivity pinhole camera for soft condensed matter. J. Appl. Crystallogr. 36:800–805. [Google Scholar]
- 86.Zemb, T., O. Taché, F. Né, and O. Spalla. 2003. Improving sensitivity of a small angle x-ray scattering camera with pinhole collimation using separated optical elements. Rev. Sci. Inst. 74:2456–2462. [Google Scholar]
- 87.Lis, L. J., M. McAlister, N. Fuller, R. P. Rand, and V. A. Parsegian. 1982. Interactions between neutral phospholipid bilayer membranes. Biophys. J. 37:657–665. [PMC free article] [PubMed] [Google Scholar]
- 88.Nagle, J. F., and S. Tristram-Nagle. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta. 1469:159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tristram-Nagle, S., and J. F. Nagle. 2004. Lipid bilayers: thermodynamics, structure, fluctuations, and interactions. Chem. Phys. Lipids. 127:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McIntosh, T. J., and S. A. Simon. 1986. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 25:4058–4066. [DOI] [PubMed] [Google Scholar]
- 91.Parsegian, V. A., R. P. Rand, N. L. Fuller, and D. C. Rau. 1986. Osmotic stress for the direct measurement of intermolecular forces. Meth. Enzym. 127:400–416. [DOI] [PubMed] [Google Scholar]
- 92.Michel, B. E., and M. R. Kaufmann. 1973. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 51:914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michel, B. E. 1983. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 72:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Gennes, P. G. 1979. Scaling Concepts in Polymer Physics. Cornell University Press, Ithaca, NY
- 95.Dubois, M., T. Zemb, N. Fuller, R. P. Rand, and V. A. Parsegian. 1998. Equation of state of a charged bilayer system: measure of the entropy of the lamellar–lamellar transition in DDABr. J. Chem. Phys. 108:7855–7869. [Google Scholar]
- 96.Petrache, H. I., S. Tristram-Nagle, D. Harries, N. Kucerka, J. F. Nagle, and V. A. Parsegian. 2006. Swelling of phospholipids by monovalent salt. J. Lipid Res. 47:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hiemenz, P. C., and R. Rajagopalan. 1997. Principles of Colloid and Surface Chemistry. Marcel Dekker, New York.
- 98.Rand, R. P., and V. A. Parsegian. 1989. Hydration forces between phospholipid bilayers. Bioch. Biophys. Acta. 988:351–376. [Google Scholar]
- 99.Ninham, B. W., and V. A. Parsegian. 1970. Van der Waals interactions in multilayer systems. J. Chem. Phys. 53:3398–3402. [Google Scholar]
- 100.Parsegian, V. A. 2005. Van der Waals Forces: A Handbook for Biologists, Chemists, Engineers and Physicists. Cambridge University Press, Cambridge, UK.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.