Abstract
Persons with chronic disease experience multiple symptoms. Understanding the association between these symptoms and health outcomes would facilitate a targeted approach to symptom assessment and treatment. Our objectives were to determine the association of a range of symptoms with quality of life, self-rated health, and functional status among chronically ill adults and to assess methods for evaluating the independent associations of symptoms that may be inter-related. We consecutively enrolled 226 cognitively intact, community-dwelling adults, age 60 years or older with chronic obstructive pulmonary disease, heart failure or cancer. Seven symptoms (physical discomfort, pain, fatigue, problems with appetite, feelings of depression, anxiety, shortness of breath) assessed using the Edmonton Symptom Assessment Scale were examined for their association with self-rated quality of life, self-rated health, and functional status. Principal component analysis and logistic regression revealed similar results. The latter demonstrated that physical discomfort was associated with lower self-rated health (Adjusted odds ratio 1.9; 95% confidence interval 1.2-2.9), and functional disability (Adjusted odds ratio 1.8; 95% confidence interval 1.2-2.7). Feelings of depression were associated with poorer quality of life (Adjusted odds ratio 1.7; 95% confidence interval 1.1-2.6), and shortness of breath was associated with lower self-rated health (Adjusted odds ratio 1.5; 95% confidence interval 1.1-2.0). The association between a range of symptoms and quality of life, self-rated health, and functional status differed across outcomes, but only three symptoms—physical discomfort, feelings of depression and shortness of breath—maintained their associations when multiple symptoms were examined concurrently. These findings suggest that interventions targeting these symptoms could improve several health-related outcomes.
Keywords: Symptoms, Chronic Disease, Quality of life, Self-rated health, Functional status
Introduction
A primary objective in the care of persons with chronic disease is the alleviation of symptoms (1). One challenge to achieving this objective is the growing recognition that persons with chronic disease experience a large number of symptoms (2). Developing a targeted approach to symptom management is a promising means of addressing this challenge. If symptoms are considered as potentially modifiable risk factors for a range of health-related outcomes, symptom management strategies could be directed to the symptoms highly associated with the clinical outcome of interest. An improved understanding of the association between multiple symptoms and health outcomes is needed to develop this targeted approach to symptom management.
Most prior studies of symptoms in persons with chronic disease have examined the association of a limited number of symptoms, including pain, fatigue, and dyspnea, with a single health outcome (3-5). This research has demonstrated that these symptoms are associated with lower quality of life (3), poorer functional status (4), and higher mortality (5). Only one prior investigation examined the association between a range of symptoms and the outcomes of both quality of life and self-rated health (6). However, this study was limited to patients with HIV and examined predominantly disease-specific symptoms. In contrast, there is considerable overlap in the symptoms experienced by older persons with various chronic diseases (2), but the relationship between multiple non-specific symptoms and health outcomes in this population has not yet been examined. Moreover, all of the prior studies examined symptoms as independent factors. Because many symptoms may be highly inter-related, the most appropriate method for studying the relationship between multiple concurrent symptoms and health outcomes remains unclear (7). The techniques of factor and principal component analysis have been utilized to cluster symptoms. However, the studies utilizing these techniques have been limited to examining highly disease-specific symptoms experienced by persons with multiple sclerosis (8), HIV disease (9), or premenstrual syndrome (10), and they did not examine the relationship between symptom clusters and health outcomes.
Our primary objective was to determine which symptoms among a range of those experienced by persons with chronic obstructive pulmonary disease (COPD), heart failure (HF) or cancer are most strongly associated with three health outcomes of importance to older persons with chronic disease, namely, quality of life, self-rated health and functional status. A secondary objective was to examine the most appropriate methods to study the independent associations of potentially inter-related symptoms.
Methods
Participants
Participants for this study were enrolled in an investigation of the attitudes and health care preferences of adults with advanced disease (11). Inclusion criteria for the parent study included: 1) age 60 years old or greater, 2) community-dwelling, 3) primary diagnosis of advanced COPD, HF or cancer as determined by chart review to identify Connecticut Hospice (12) or Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment (SUPPORT) criteria (13), and 4) requiring assistance with at least one instrumental activity of daily living (IADL) (14), a criterion selected to improve the identification of persons with advanced disease (15). Exclusion criteria included: 1) cognitive impairment according to performance on the Short Portable Mental Status questionnaire (16) and/or the EXIT interview (17) and 2) part- time Connecticut residence. Enrollment was stratified by primary diagnosis. The human investigations committee of each site approved the study protocol. All participants provided written informed consent.
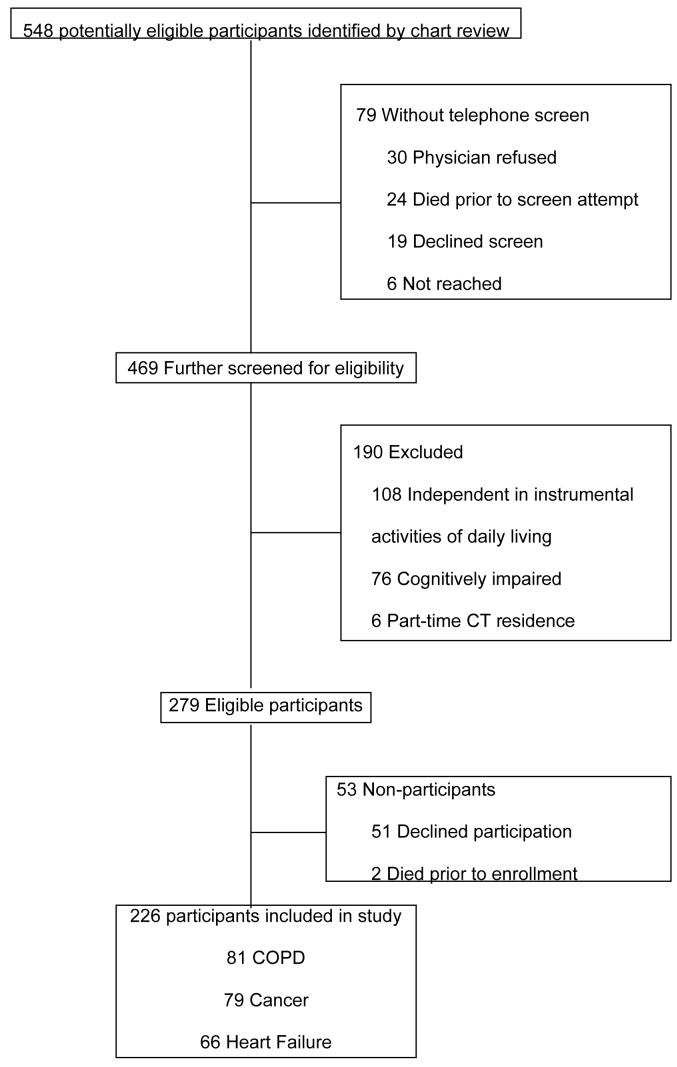
Potentially eligible participants for the parent study were identified by sequential review of charts from outpatient pulmonary, cardiology, or oncology practices in southern Connecticut, Veteran Affairs clinics, and from three hospitals—a university teaching hospital, a community hospital, and a Veterans Affairs hospital. The chart review process identified 548 potential participants and 469 persons received a telephone screen to determine their IADL status and whether they met any exclusion criteria. Telephone screens were not conducted for 30 persons because of physician refusal; 24 persons who died before telephone screening; 6 persons who could not be reached; and 19 persons who declined screening. Of the 469 potential participants who received a telephone screen, we excluded 108 persons who did not require assistance with at least one IADL, 76 persons with cognitive impairment, and 6 persons who were part-time Connecticut residents. Of the 279 eligible participants, 2 died before enrollment and 51 declined participation. Thus, the final sample consisted of 226 persons. Participant identification and enrollment is illustrated via flow chart in the Appendix. Participants and non-participants did not differ according to age or gender. A greater proportion of eligible COPD participants (25%) declined participation compared with eligible HF participants (8%) or eligible cancer participants (19%, p=0.02).
Symptom assessment
We utilized the Edmonton Symptom Assessment Scale (ESAS) (18), a validated instrument for the assessment of symptoms in seriously ill persons (19) that was specifically designed to measure multiple symptoms (20). The ESAS asks participants to rate the intensity of nine symptoms—pain, fatigue, problems with appetite, feelings of depression, anxiety, shortness of breath, nausea, limited activity, and lack of well-being—without providing a definition or description of any of the symptoms. Participants completed the ESAS during an in-home interview.
Consistent with a prior investigation (21), we made two modifications to the ESAS. First, we added physical discomfort as a tenth symptom. Second, we modified the original visual analogue ESAS to a four-point scale (not present, mild, moderate, severe), thereby allowing participants to provide verbal rather than written responses. We solicited verbal responses because a substantial percentage of older participants in a prior study were unable to complete a visual analog scale, (22) and verbal symptom measurements have been shown to correlate well with visual analog scale measurements (23).
Quality of life, self-rated health and functional disability
We selected three outcomes that represent important and distinct domains of health assessment among older persons and that are applicable across specific diseases. First, we assessed participant's quality of life with a single item that asked them to rate their overall quality of life using a 5-point rating scale (best possible, good, fair, poor, worst possible). The use of a single item recognizes that subjective assessments of quality of life can remain high even when patients' objective health is poor (24), and it provides a measure of the unique values and preferences of individual patients (25). Second, we asked participants to rate their health using a 5-point response scale (excellent, very good, good, fair, poor). Self-rated health is a subjective measure of health that has been shown to be associated with mortality, even after adjustment for multiple potential confounders (26). We dichotomized quality of life as best possible/good versus fair/poor/worst possible and self-rated health as excellent/very good/good versus fair/poor in accordance with prior studies (27). Third, we assessed activities of daily living (ADLs) as an objective health measure. Participants reported their ability to complete seven ADLs, namely, bathing, grooming, dressing, eating, toileting, transferring from a bed to chair, and walking across a room (28), on a 3-point scale (requiring no help, requiring help, or unable to do). We defined functional disability as being dependent in (e.g. either requiring help or unable to do) at least one ADL.
Statistical analysis
We limited our analyses to the smallest number of potentially modifiable symptoms that retained the same information as the full set of symptoms. We excluded limited activity and lack of well-being for two reasons: 1) they may not be modifiable, and 2) they are more complex constructs that are the result of symptoms rather than symptoms in and of themselves. We also excluded nausea because 83% of the participants who reported nausea also reported problems with appetite but only 15% of participants who reported problems with appetite reported nausea. Thus, our analyses are based upon the 7 remaining symptoms.
We used logistic regression analysis to assess the bivariate associations between individual symptoms and quality of life, self-rated health, and functional disability. Multivariable associations were assessed using two separate methods. In the first method, we examined a multivariable model with the inclusion of all seven symptoms. In the second method, we combined symptoms using principal component analysis (PCA), which allowed us to take into account the inter-relatedness of symptoms. Symptoms were analyzed as a four-point scale, with the resulting odds ratios expressing the likelihood of lower quality of life or self-rated health or the likelihood of functional disability with each one point increase in symptom severity.
The number of principal components selected was determined by the magnitude of the eigenvalues, where eigenvalues greater than one suggest that the entire symptom component accounts for more of the variance than any one of the individual symptoms in the component. We selected three components based upon the magnitude of the eigenvalues. The first two eigenvalues were greater than one (2.33 and 1.17, respectively). The third eigenvalue was approximately one (0.96), thereby indicating a third component was acceptable but should include only one symptom. Component variables were ascertained by examination of the final rotated factor pattern matrix (29-30). Because several of the variables had high loading onto more than one component, clinical judgment in combination with a factor loading of greater than 0.40 was used to determine the composition of the components. Components were standardized by a T-score transformation before they were used in subsequent analyses because the number of symptoms in each component differed.
All analyses were conducted using the statistical software package SAS, version 8.2 (SAS Institute, Inc., Cary, North Carolina).
Results
Description of participants
The 226 participants had a mean age (± standard deviation) of 73 ± 7 years, as shown in Table 1. Over 90% of the participants were Caucasian and 43% were female. There were 81 participants with a primary diagnosis of COPD, 79 participants with a primary diagnosis of cancer and 66 participants with a primary diagnosis of HF. Almost 75% of the participants had been hospitalized at least once in the prior 12 months. The mean (± SD) number of symptoms experienced was 5.5 ± 1.9 (range 1 –10). The median number of symptoms was 5.8. Physical discomfort, fatigue, problems with appetite and shortness of breath were each reported by more than 50% of the participants. Pain, feelings of depression, and anxiety were each reported by at least 30% of the participants. Approximately one-third of participants rated their quality of life to be fair, poor or worst possible. More than 60% of participants considered their health to be fair or poor.
Table 1.
Participant characteristics (N=226)
| Mean Age, years | 73 ± 7 |
| Female, % | 43 |
| Caucasian, % | 91 |
| Diagnosis | |
| COPD, n | 81 |
| HF, n | 66 |
| Cancer, n | 79 |
| ≥ 1 Hospitalizations in prior 12 months, % | 73 |
| Physical discomfort, % | 76 |
| Fatigue, % | 72 |
| Problems with appetite, % | 60 |
| Pain, % | 49 |
| Feelings of depression, % | 37 |
| Anxiety, % | 34 |
| Shortness of breath, % | 61 |
| Fair/poor/worst possible QOL, % | 36 |
| Fair/poor SRH, % | 64 |
| ≥ 1 ADL dependency, % | 38 |
COPD = chronic obstructive pulmonary disease, HF = heart failure, QOL = quality of life, SRH = self-rated health, ADL = activities of daily living
Bivariate associations
Individual symptoms
As shown in Table 2, five of the seven symptoms were significantly associated (P < 0.05) with at least one of the health outcomes. Physical discomfort was the symptom most strongly associated with lower self-rated health (odds ratio [OR] 2.3; 95% confidence interval [CI] 1.58, 3.19) and functional disability (OR 2.0; CI 1.42, 2.72). Feelings of depression was the symptom most strongly associated with poorer quality of life (OR 1.9; CI 1.35, 2.72). Shortness of breath and fatigue were associated with each of the outcomes, with ORs ranging from 1.4 to 1.7. Pain was associated only with lower self-rated health (OR 1.4; CI 1.06, 1.82). Problems with appetite and anxiety were not associated with any of the outcomes.
Table 2.
Bivariate associations between individual symptoms and the odds of poorer quality of life, lower self-rated health, or functional disability
| Symptom | Quality of Life | Self-Rated Health | Functional disability |
|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Physical Discomfort | 1.7 (1.20, 2.26) | 2.3 (1.58, 3.19) | 2.0 (1.42, 2.72) |
| Fatigue | 1.6 (1.19, 2.02) | 1.3 (1.03, 1.73) | 1.4 (1.11, 1.87) |
| Problems with appetite | 1.1 (0.79, 1.65) | 1.5 (0.99, 2.18) | 1.3 (0.91, 1.88) |
| Pain | 1.2 (0.94, 1.55) | 1.4 (1.06, 1.82) | 1.2 (0.92,1.51) |
| Feelings of depression | 1.9 (1.35, 2.72) | 1.6 (1.06, 2.36) | 1.6 (1.15, 2.24) |
| Anxiety | 1.3 (0.96, 1.75) | 1.4 (0.98, 1.90) | 1.4 (1.00, 1.82) |
| Shortness of Breath | 1.4 (1.11, 1.85) | 1.7 (1.28, 2.25) | 1.4 (1.08, 1.79) |
Principal components
The factor loadings and definitions of components are presented in Table 3. Physical discomfort and fatigue had high loadings onto two factors, the first of which included pain and problems with appetite; the second of which included shortness of breath. We elected to include fatigue and physical discomfort with the component that included pain in accordance with recognized symptom clusters (31). Therefore, we combined physical discomfort, fatigue, problems with appetite, and pain into a “Physical” component. Feelings of depression and anxiety comprised the second component, which we called “Affective.” Shortness of breath comprised the third component.
TABLE 3.
Factor Loadings of symptoms from Principal Component Analysis
| Symptom | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Physical Discomfort | 0.6508 | 0.2298 | 0.4191 |
| Fatigue | 0.4640 | 0.1286 | 0.5353 |
| Problems with appetite | 0.5438 | −0.1384 | 0.0643 |
| Pain | 0.7914 | 0.2099 | −0.0940 |
| Feelings of depression | 0.1777 | 0.8206 | 0.0761 |
| Anxiety | −0.0551 | 0.8448 | 0.1347 |
| Shortness of Breath | −0.0382 | 0.0989 | 0.9094 |
Multivariable associations
Logistic regression with individual symptoms
Of all the symptoms associated with the outcomes in bivariate analysis, only physical discomfort, feelings of depression and shortness of breath retained their significance in multivariable analyses (Table 4). Feelings of depression were independently associated with poorer quality of life (OR 1.7; CI 1.13- 2.57). Physical discomfort (OR 1.9; CI 1.21- 2.86) and shortness of breath (OR 1.5; CI 1.08- 2.00) were independently associated with lower self-rated health. Physical discomfort was also independently associated with functional disability (OR 1.8; CI 1.19- 2.67). In addition to these associations, there was a trend toward an association between the symptoms physical discomfort (OR 1.3; CI 0.85, 1.91), fatigue (OR 1.3; CI 0.92, 1.68), and shortness of breath (OR 1.2; CI 0.93, 1.64) and quality of life.
Table 4.
Multivariable associations between individual symptoms and the odds of poorer quality of life, lower self-rated health, or functional disability
| Symptom | Quality of Life | Self-Rated Health | Functional disability |
|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Physical Discomfort | 1.3 (0.85, 1.91) | 1.9 (1.21, 2.86) | 1.8 (1.19, 2.67) |
| Fatigue | 1.3 (0.92, 1.68) | 0.9 (0.67, 1.26) | 1.1 (0.84, 1.52) |
| Problems with appetite | 1.0 (0.67, 1.47) | 1.4 (0.88, 2.12) | 1.2 (0.78, 1.71) |
| Pain | 1.0 (0.72, 1.32) | 1.1 (0.76, 1.48) | 0.9 (0.64, 1.17) |
| Feelings of depression | 1.7 (1.13, 2.57) | 1.3 (0.79, 2.13) | 1.3 (0.88, 1.97) |
| Anxiety | 0.9 (0.65, 1.35) | 1.0 (0.68, 1.51) | 1.1 (0.75, 1.52) |
| Shortness of Breath | 1.2 (0.93, 1.64) | 1.5 (1.08, 2.00) | 1.1 (0.85, 1.50) |
Logistic regression with symptom components
As seen in Table 5, the Physical component (OR 1.4; CI 1.03, 1.89) and the Affective component (OR 1.4; CI 1.02, 1.83) were associated with poorer quality of life. The Physical component (OR 1.6; CI 1.18, 2.29) and Shortness of breath component (OR 1.5; CI 1.12, 2.14) were associated with lower self-rated health. The Physical component was the only component associated with functional disability (OR 1.5; CI 1.10, 2.01).
Table 5.
Multivariable associations between component groups and health outcomes
| Quality of Life | Self-Rated Health | Functional disability | |
|---|---|---|---|
| Component | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Physical | 1.4 (1.03, 1.89) | 1.6 (1.18, 2.29) | 1.5 (1.10, 2.01) |
| Affective | 1.4 (1.02, 1.83) | 1.2 (0.85, 1.66) | 1.3 (0.96, 1.71) |
| Shortness of Breath |
1.3 (0.97, 1.73) | 1.5 (1.12, 2.14) | 1.2 (0.93, 1.66) |
Logistic regression with individual symptoms compared with symptom components
For two of the three outcomes, self-rated health and functional disability, examining symptom components did not provide additional information to that provided by examining individual symptoms. For these outcomes, the independent associations observed with the symptom components were also observed with the individual symptom within the component that, according to the results of bivariate analysis, was most strongly associated with the outcome. For example, the Physical component was independently associated with the outcome of self-rated health (OR 1.6; CI 1.18, 2.29). This association was also seen in the analysis of individual symptoms. Physical discomfort, the symptom within the Physical component most strongly associated with self-rated health in bivariate analysis, was independently associated with self-rated health in the multivariable analysis (OR 1.9; CI 1.21, 2.86).
For the quality of life outcome, the examination of symptom components did provide additional information compared to the examination of individual symptoms. The Physical component was independently associated with quality of life (OR 1.4; CI 1.03, 1.89) whereas none of the individual symptoms belonging to this component retained an independent association in the multivariable analysis of individual symptoms. Of note, however, several of these individual symptoms (physical discomfort and fatigue) demonstrated a trend toward association, suggesting that their association with quality of life depends upon their combined effect, as can be accounted for when they are analyzed as a Physical symptom component.
Discussion
The association between a range of symptoms and quality of life, self-rated health, and functional status differed across health outcomes, but only three symptoms—physical discomfort, feelings of depression and shortness of breath—maintained their associations when multiple symptoms were examined concurrently. Despite the inter-related nature of symptoms, combining them into components provided little additional information compared with an examination of individual symptoms as independent factors.
The concept that symptoms are inter-related in multiple and complex ways (32) is supported by the results of our principal component analysis, in which multiple symptoms loaded onto several factors. Despite this inter-relatedness, logistic regression using multiple individual symptoms to examine the relationship between symptoms and health outcomes provides similar information to that generated by principal component analysis and therefore appears to be an appropriate approach to determining independent associations. However, when multiple symptoms are associated with a given outcome but with a small magnitude, methods to group these symptoms, such as principal component analysis, may be necessary to reveal their additive effects (7).
Prior investigators examined the association between symptoms as independent factors and health outcomes in HIV positive patients. They demonstrated that disease-specific symptoms, e.g. white patches in mouth, and non-specific symptoms, e.g. headache, were associated with poorer quality of life, lower self-rated health or both (6). Our investigation generalizes these findings by demonstrating that a common set of symptoms is associated with self-report of not only subjective, but also an objective health outcome (functional status) across a variety of chronic illnesses.
Pain, which has been the focus of much investigation, has been shown to be associated with poor quality of life, low self-rated health and disability (33-35). We found that, compared with pain, physical discomfort had a higher prevalence and stronger associations with each of the outcomes examined in this study. Although physical discomfort has received less attention, several prior investigations demonstrate that patients, nurses and physicians all differentiate physical discomfort from pain (21, 36-37). Physical discomfort has been found to be more prevalent than pain according to patient self-report (21, 36-37) and nurse assessment (37). In addition, in one investigation, physicians were more likely to honor requests for a hastened death from patients who experienced physical discomfort compared with patients who experienced pain (37). This finding suggests that physicians differentiate the two symptoms and, moreover, perceive physical discomfort to be less tolerable than pain. However, it remains unclear how pain clinically differs from physical discomfort. Qualitative studies that focus on the meaning of pain and physical discomfort are needed to elucidate how patients define these symptoms. Nevertheless, our findings combined with those of prior investigators emphasize the importance of assessing physical discomfort.
Our investigation has several limitations. The cross-sectional design of our study precludes a definitive determination of the direction of the relationships we observed. However, it is unlikely that the health outcomes we examined were responsible for patients' symptoms. Furthermore, when patients were asked to rate their quality of life and self-rated health, it is likely that their perceptions are based upon the symptoms they are experiencing at the time they make their ratings and not on prior symptoms. Therefore, it is not clear that a longitudinal study would provide additional information. In addition, because of the limitations in our sample, we cannot know whether our results are generalizable to minorities, persons who do not reside in the community, and/or persons with diseases other than COPD, HF or cancer.
Our investigation demonstrates that, despite the presence of multiple symptoms, a limited number are independently associated with lower quality of life, self-rated health and functional status in older adults with COPD, HF or cancer. These findings suggest that a targeted approach to symptom assessment is possible, but additional research is necessary to determine whether alleviation of physical discomfort, feelings of depression and shortness of breath can lead to improvements in the health outcomes of community-dwelling chronically ill older adults.
Acknowledgments
Financial support: The research reported in this manuscript was supported by grants PCC 98-070-1 from VA Health Services Research & Development, R01 AG19769 from the National Institute on Aging, and P30 AG21342 from the Claude D. Pepper Older Americans Independence Center at Yale University and by a Paul Beeson Physician Faculty Scholars Award. Dr. Walke was supported by a Diversity Supplement to grant R01 AG19769 during the period of this research. Dr. Byers is supported by grant T32 MH19132 from the National Institute of Mental Health. Dr. Gallo is supported by grant K01 AG 21983 from the National Institute on Aging. Dr. Fried is supported by grant K02 AG20113 from the National Institute on Aging.
Appendix
Flow of Participants Through the Study
Footnotes
Portions of the manuscript were presented at the American Geriatrics Society National Convention in Baltimore, MD on May 16, 2003
References
- 1.NIH State-of-the-Science Conference on Improving End-of-life Care. Available at http://www.consensus.nih.gov/2004/2004EndOfLifeCareSOS024PDF.pdf.. Accessed December 8, 2005.
- 2.Walke LM, Gallo WT, Tinetti ME, Fried TR. The burden of symptoms among community-dwelling older persons with advanced chronic disease. Arch Intern Med. 2004;164:2321–4. doi: 10.1001/archinte.164.21.2321. [DOI] [PubMed] [Google Scholar]
- 3.Owen JE, Klapow JC, Casebeer L. Evaluating the relationship between pain perception and health-related quality of life in outpatients with metastatic or recurrent neoplastic disease. Qual Life Res. 2000;9:855–863. doi: 10.1023/a:1008944211294. [DOI] [PubMed] [Google Scholar]
- 4.Brown DJF, McMilan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103:377–82. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt J, Smeeth L, Bulpitt CJ, Tulloch AJ, Fletcher AE. Respiratory symptoms in older people and their association with mortality. Thorax. 2005;60:331–334. doi: 10.1136/thx.2004.029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenz KA, Shapiro MF, Asch SM, Bozzette SA, Hays RD. Associations of symptoms and health-related quality of life: findings from a national study of persons with HIV infection. Ann Intern Med. 2001;134:854–60. doi: 10.7326/0003-4819-134-9_part_2-200105011-00009. [DOI] [PubMed] [Google Scholar]
- 7.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: Conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Gulick EE. Model confirmation of the MS-Related Symptom Checklist. Nurs Res. 1989;38:147–153. [PubMed] [Google Scholar]
- 9.Holzemer WL, Henry SB, Nokes KM, et al. Validation of the Sign and Symptom Check-List for persons with HIV disease. J Adv Nurs. 1999;30:1041–1049. doi: 10.1046/j.1365-2648.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 10.Woods NF, Mitchell ES, Lentz M. Prementstrual symptoms: delineating symptom clusters. J Women's Health. 1999;8:1053–1062. doi: 10.1089/jwh.1.1999.8.1053. [DOI] [PubMed] [Google Scholar]
- 11.Fried TR, Bradley EH, Towle VR, Allore E. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 12.Summary Guidelines for Initiation of Advanced Care. Connecticut Hospice; Branford: 1996. [Google Scholar]
- 13.Murphy D, Knaus W, Lynn J. Study population in SUPPORT: patients (as defined by disease categories and mortality projections), surrogates, and physicians. J Clin epidemiol. 1990;43:11S–28S. doi: 10.1016/0895-4356(90)90213-9. [DOI] [PubMed] [Google Scholar]
- 14.Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 15.Inouye S, Peduzzi P, Robison J, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 17.Royall D, Mahurin R, Gray K. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruera E, Kuehn N, Miller MJ, Selmser P, MacMillian K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 19.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–71. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Paice JA. Assessment of symptom clusters in people with cancer. J Natl Cancer Inst Monographs. 2004;32:98–102. doi: 10.1093/jncimonographs/lgh009. [DOI] [PubMed] [Google Scholar]
- 21.Nelson JE, Meier DE, Oei EJ, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277–82. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Littman GS, Walker BR, Schneider BE. Reassessment of verbal and visual analog ratings in analgesic studies. Clin Pharmacol Ther. 1985;38:16–23. doi: 10.1038/clpt.1985.127. [DOI] [PubMed] [Google Scholar]
- 23.Downie WW, Leatham PA, Rind VM, et al. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr AJ, Higginson IJ. Are quality of life measures patient centred? BMJ. 2001;322:1357–1360. doi: 10.1136/bmj.322.7298.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. [PubMed] [Google Scholar]
- 26.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 27.Farquhar M. Elderly people's definitions of quality of life. Soc Sci Med. 1995;41:1439–1446. doi: 10.1016/0277-9536(95)00117-p. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. 2nd edition Duxbury Press; New York, NY: 1987. [Google Scholar]
- 30.Hatcher L. A step-by-step approach to using the SAS System for factor analysis and structural equation modeling. SAS Institute Inc.; Cary, NC: 1994. [Google Scholar]
- 31.Fleishman SB. Treatment of symptom clusters: pain, depression, and fatigue. J Natl Cancer Inst Monogr. 2004;32:119–123. doi: 10.1093/jncimonographs/lgh028. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28:270–282. doi: 10.1097/00002820-200507000-00005. quiz 283-284. [DOI] [PubMed] [Google Scholar]
- 33.Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6:353–363. doi: 10.1016/j.jpain.2005.01.359. [DOI] [PubMed] [Google Scholar]
- 34.Al Snih S, Raji MA, Peek MK, Ottenbacher KJ. Pain, lower-extremity muscle strength, and physical function among older Mexican Americans. Arch Phys Med Rehab. 2005;86:1394–1400. doi: 10.1016/j.apmr.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 35.Perruccio AV, Power JD, Badley EM. Arthritis onset and worsening self-rated health: a longitudinal evaluation of the role of pain and activity limitations. Arthritis & Rheumatism. 2005;53:571–577. doi: 10.1002/art.21317. [DOI] [PubMed] [Google Scholar]
- 36.Hall-Lord ML, larsson G, Steen B. Pain and distress among elderly intensive care unit patients: comparison of patients' experiences and nurses' assessments. Heart Lung. 1998;27:123–132. doi: 10.1016/s0147-9563(98)90020-6. [DOI] [PubMed] [Google Scholar]
- 37.Meier DE, Emmons CA, Litke A, Wallenstein S, Morrison RS. Characteristics of patients requesting and receiving physician-assisted death. Arch Inter Med. 2003;163:1537–1542. doi: 10.1001/archinte.163.13.1537. [DOI] [PubMed] [Google Scholar]