Abstract
Recent evidence indicates that newly synthesized major histocompatibility complex (MHC) class I proteins interact with calnexin, a transmembrane endoplasmic reticulum protein specific for certain glycoproteins bearing monoglucosylated glycans. Here, we studied the association of newly synthesized class I proteins with calreticulin, a soluble calnexin-related ER protein, in murine T cells. We found that, unlike calnexin–class I interactions, calreticulin assembly with class I proteins was markedly decreased in the absence of β2 microglobulin expression and that calreticulin associated with a subset of class I glycoforms distinct from those assembled with calnexin but similar to those bound to TAP (transporter associated with antigen processing) proteins. Finally, these studies show that deglucosylation of N-linked glycans is important for dissociation of class I proteins from both calreticulin and TAP and that the vast majority of newly synthesized class I proteins associated with calreticulin are simultaneously assembled with TAP. The data demonstrate that calnexin and calreticulin chaperones assemble with distinct MHC class I assembly intermediates in the ER and show that glycan processing is functionally coupled to release of MHC class I proteins from peptide transport molecules.
Most major histocompatibility complex (MHC) class I proteins are expressed on the cell surface in association with β2 microglobulin (β2m) molecules and processed peptides (1, 2). Assembly of MHC class I protein complexes occurs in the endoplasmic reticulum (ER) and is proposed to be initiated by association of newly translated MHC class I heavy chains with calnexin (3, 4, 5, 6, 7), a lectin-like chaperone molecule (8, 9, 10). In the murine system, β2m proteins associate with calnexin–HC to form calnexin–HC–β2m complexes, followed by addition of peptides generated by proteosome processing of cytosolic proteins and transported into the ER lumen by TAP 1/2 (transporter associated with antigen presentation) heterodimers (11); addition of peptide to HC–β2m complexes has been suggested to trigger their dissociation from calnexin and facilitate their egress from the ER (7, 12, 13, 14, 15).
Immature glycan chains on nascent polypeptides have the structure Glc3Man9GlcNAc2 (Glc, glucose; Man, mannose; GlcNAc, N-acetyl glucosamine) and are initially processed by the sequential action of two ER enzymes, glucosidase I and glucosidase II (16). Within the past few years, it has been realized that processing of Glc residues, or Glc trimming, is an important step in the association of many glycoproteins with the molecular chaperone calnexin (8, 17, 18) and, more recently, calreticulin (19, 20, 21). Calnexin and calreticulin are transmembrane and lumenal ER proteins, respectively, that are two known members of a family of endogenous lectin-like proteins that recognize partially trimmed, monoglucosylated (Glc1Man9GlcNAc2) glycan chains on newly synthesized glycoproteins (19, 20, 22). Significant evidence exists that newly synthesized murine and human MHC class I proteins associate with calnexin chaperones (7, 12, 13, 14, 15, 23); assembly of class I proteins with calreticulin has only recently been evaluated (24). Interestingly, these studies show that calreticulin binds human class I–β2m dimers before peptide loading and that calreticulin remains associated with at least a subset of human class I proteins when they bind to TAP (24).
To further our understanding of the role of molecular chaperones in the assembly and expression of immune receptor proteins, we studied the association of calnexin and calreticulin molecules with newly synthesized H-2b class I proteins in murine T cells. The current report demonstrates that calnexin and calreticulin associate with specific class I assembly intermediates in the ER and documents that deglucosylation is an important step in the disassembly of MHC class I proteins from both calreticulin and TAP peptide transport molecules.
MATERIALS AND METHODS
Animals, Cell Preparation, and Reagents.
C57BL/6 (B6) mice (H-2b haplotype) were obtained from The Jackson Laboratory. β2m-deficient (β2m −/−) mice (H-2b haplotype; ref. 25) were kindly provided by Tak Mak (Ontario Cancer Institute, Toronto) and Dinah Singer (National Institutes of Health, Bethesda, MD); TAP 1-deficient (TAP 1 −/−) mice (H-2b haplotype; ref. 26) were kindly provided by Izumi Negishi and Dennis Loh (Nippon Roche Center, Kanagawa, Japan) and Dinah Singer. Splenic T cells were purified by incubating single-cell suspensions of spleen cells on rabbit anti-mouse immunoglobulin-coated (Organon Technika–Cappel) tissue culture plates for 60 min at 37°C, followed by isolation of nonadherent cells. The resultant cell populations were typically >85% CD3ɛ+ as determined by surface staining with monoclonal antibody to CD3ɛ (data not shown). Castanospermine (cas) was purchased from Calbiochem and was used at 100 μg/ml.
Antibodies.
The following antibodies were used in this study: SPA-860 anti-calnexin (Stressgen Biotechnologies, Victoria, BC); PA3-900 anti-calreticulin (Affinity BioReagents, Neshanic Station, NJ); anti-H-2b, which recognizes both H-2Kb and H-2Db proteins (27), kindly provided by Ettore Appella (National Institutes of Health); anti-p8, directed against the exon 8-encoded region of the cytoplasmic tail of H-2Kb (28, 29), kindly provided by Hidde Ploegh and Rob Machold (Massachusetts Institute of Technology, Cambridge, MA); anti-TAP 1 (30), kindly provided by Ted Hansen (Washington University, St. Louis); and anti-HLA A2, specific for human HLA A2 heavy chain proteins, kindly provided by Mike Shields and John Coligan (National Institutes of Health).
Metabolic Labeling, Immunoprecipitation, Gel Electrophoresis, and Glycosidase Digestion.
Metabolic pulse labeling was performed as previously described (23). Cells were lysed by solubilization in 1% digitonin (Wako Biochemicals, Kyoto, Japan) lysis buffer (20 mM Tris/150 mM NaCl, plus protease inhibitors) at 1 × 108 cells/ml for 25 min at 4°C; lysates were clarified by centrifugation to remove insoluble material. Sequential immunoprecipitation and release/recapture procedures and glycosidase digestion experiments were performed as described (31). One- and two-dimensional SDS/PAGE were done according to previous methods (23).
RESULTS AND DISCUSSION
Newly Synthesized Murine Class I Proteins Interact with Calnexin, Calreticulin, and TAP Molecules.
Initially, we examined the assembly of H-2Kb proteins synthesized in splenic T cells with calnexin, calreticulin, and TAP (transporter associated with antigen processing) molecules. For these studies, splenic T cells were metabolically labeled with [35S]methionine for 5 min and chased in medium containing excess unlabeled methionine for various time periods, and digitonin lysates were immunoprecipitated with anti-calnexin, anti-calreticulin, anti-TAP, or anti-p8 (Kb) antibodies; precipitates were boiled in 1% SDS to release bound proteins and H-2Kb proteins isolated by secondary precipitation with anti-p8. Assembly of newly synthesized H-2Kb proteins with calnexin occurred very rapidly and was quite transient, with most calnexin–class I protein complexes that had assembled during the short 5-min pulse period dissociating within 10 min of chase (Fig. 1A). Association of newly synthesized class I proteins with calreticulin was somewhat delayed compared with calnexin interaction and occurred coincident with TAP assembly (Fig. 1A). Interestingly, H-2Kb proteins were associated with calreticulin and TAP for similar time periods, remaining assembled for at least 40 min after their synthesis (Fig. 1A). By 80 min of chase, most newly synthesized H-2Kb proteins had egressed from the ER and reached the medial Golgi compartment, as evidenced by their acquisition of resistance to digestion with endoglycosidase H (Endo H), specific for immature, high-mannose glycans (Fig. 1A). Densitometric analysis of the data shown in Fig. 1A indicated that ≈7% and 15% of total radiolabeled H-2Kb proteins coprecipitated with calnexin and calreticulin chaperones in splenic T-cell lysates (Fig. 1B), with intermediate amounts associated with TAP (≈11%; Fig. 1B). Taken together, these data demonstrate that H-2Kb proteins interact with calnexin, calreticulin, and TAP molecules shortly after their synthesis in splenic T cells. Note that calreticulin–class I interactions did not occur post-lysis, as determined in mixing experiments using unlabeled H-2Kb expressing T cells and unlabeled, non-H-2Kb-expressing (H-2Kk) T cells (data not shown).
Figure 1.
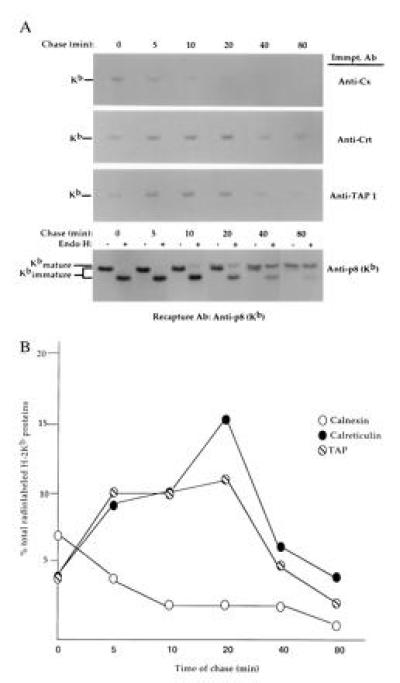
Assembly of newly synthesized H-2b proteins with calnexin and calreticulin chaperones. (A) Splenic T cells were metabolically labeled with [35S]methionine for 5 min and chased for the time period indicated. Digitonin lysates were precipitated with anti-calnexin, anti-calreticulin, anti-TAP, or anti-p8 with sufficient antibody to capture >95% of their respective antigens (data not shown). Precipitates were boiled in 1% SDS to release bound material, Nonidet P-40 detergent was added, and H-2Kb proteins were recaptured by precipitation with anti-p8. Anti-p8 precipitates of total H-2Kb proteins were mock-treated or digested with Endo H to evaluate processing of N-linked glycan chains. Kimmatureb = Endo H-sensitive H-2Kb proteins; Kmatureb = Endo H-resistant H-2Kb proteins. (B) The relative amounts of H-2Kb proteins coprecipitating with calnexin, calreticulin, and TAP in A were determined by densitometric scanning and are expressed as the percentage of total labeled H-2Kb proteins in splenic T-cell lysates. Multiple exposures of autoradiographs were scanned to ensure linearity.
Molecular Requirements for Assembly of Newly Synthesized Murine Class I Proteins with Calreticulin.
Next, we examined the molecular requirements for assembly of newly synthesized class I proteins with calreticulin using splenic T cells from mice genetically deficient in expression of β2m (β2m −/−) or TAP (TAP −/−) molecules. As shown in Fig. 2A, newly synthesized class I proteins were assembled with calnexin in both wild type (WT) and β2m −/− T-cell lysates (Fig. 2A Upper, indicated with arrow). In contrast, calreticulin association with newly synthesized class I proteins was markedly decreased in β2m −/− T cells relative to WT T cells (Fig. 2A Lower, indicated with arrow), which was verified by release/recapture experiments and immunoblotting studies (data not shown). In contrast, assembly of newly synthesized H-2Kb proteins with calreticulin was similar in WT and TAP −/− T cells (Fig. 2B Upper). Taken together, these results indicate that most H-2Kb proteins associate with calreticulin subsequent to β2m assembly, but before addition of processed peptide. The role of TAP expression in the dissociation of class I molecules from calreticulin and their egress from the ER was evaluated in pulse-chase studies. As shown in Fig. 2B, unlike H-2Kb proteins made in WT T cells, which showed decreased mobility in chase groups relative to pulse groups due to processing of N-linked glycans by Golgi maturation enzymes (verified by Endo H digestion; data not shown), H-2Kb proteins in TAP 1 −/− T cells showed no evidence of Golgi processing during the chase period (Fig. 2B Lower, left side). Most importantly, it can be seen that interaction of H-2Kb proteins with calreticulin was prolonged in TAP 1 −/− T cells relative to WT T cells (Fig. 2B Lower, right side), indicating that release of H-2Kb proteins from calreticulin is facilitated by TAP 1-dependent peptide addition.
Figure 2.
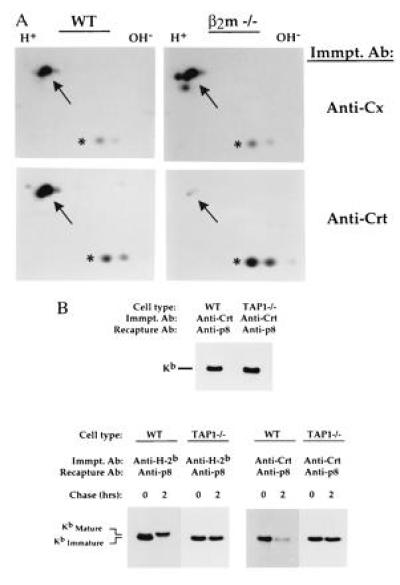
Calreticulin–class I protein associations in β2m- and TAP 1-deficient T cells. (A) Splenic T cells were purified from wild-type (WT) and β2m-deficient (β2m −/−) mice and metabolically labeled with [35S]methionine for 30 min. Digitonin lysates were immunoprecipitated with anti-calnexin or anti-calreticulin; precipitates were analyzed on two-dimensional NEPHGE–SDS/PAGE gels under reducing conditions. Arrow indicates the position of H-2b proteins, determined by parallel precipitations with anti-H-2b and mixing experiments (data not shown). As our splenic T-cell populations contained small amounts of B cells (≈10-15% of total cells), invariant chain proteins were also present in these lysates, which are readily visualized in such [35S]methionine-labeling experiments because of their high methionine content (32); note that similar amounts of invariant chain proteins were present in anti-calreticulin precipitates of WT and β2m −/− lysates (denoted by asterisk), demonstrating that precipitation of β2m −/− lysates with anti-calreticulin was effective in these experiments. (B Upper) Splenic T cells from wild-type (WT) and TAP 1-deficient (TAP 1 −/−) mice were metabolically labeled with [35S]methionine for 30 min and solubilized in digitonin, and lysates were immunoprecipitated with anti-calreticulin; precipitates were boiled in SDS and H-2Kb proteins recaptured with anti-p8. (B Lower) Splenic T cells from WT and TAP 1 −/− mice were metabolically labeled with [35S]methionine for 30 min and chased for 2 hr; digitonin lysates were precipitated with anti-H-2b and anti-calreticulin; and bound material was released by boiling in SDS and H-2Kb proteins recaptured with anti-p8. Kimmatureb = Endo H-sensitive H-2Kb proteins; Kmatureb = Endo H-resistant H-2Kb proteins.
Calnexin and Calreticulin Associate with Distinct H-2Kb Glycoforms.
Because the above results suggested that calnexin and calreticulin interact with distinct class I assembly intermediates in the ER, we examined the glycan processing (Glc trimming) status of newly synthesized H-2Kb proteins associated with calnexin and calreticulin chaperones. For these studies, jack bean mannosidase (JB) was used, which removes eight mannose residues from fully trimmed oligosaccharide chains devoid of Glc residues (Man9GlcNAc2 glycans) but only five mannoses from incompletely trimmed oligosaccharide chains (Glc1–3Man9GlcNAc2 glycans; refs. 10 and 31). Because H-2Kb proteins are modified by addition of two N-linked glycans, three potential JB glycoforms may exist, containing 0, 1, or 2 incompletely trimmed oligosaccharide chains. As shown in Fig. 3, JB digestion of total H-2Kb proteins radiolabeled during a 30-min pulse period in splenic T cells revealed the existence of three major H-2Kb glycoforms, denoted A, B, and C (Fig. 3). Most importantly, these data show that H-2Kb glycoforms associated with calreticulin were distinct from those assembled with calnexin but were similar to those coprecipitating with TAP molecules (Fig. 3). Note that the anti-TAP 1 antibody used in these experiments does not cross-react with calreticulin, as determined by precipitation and immunoblotting studies using WT and TAP-1 deficient T cells (data not shown). These data show that calnexin and calreticulin associate with distinct H-2Kb glycoforms in the ER and that H-2Kb glycoforms associated with calreticulin were similar to those bound to TAP.
Figure 3.
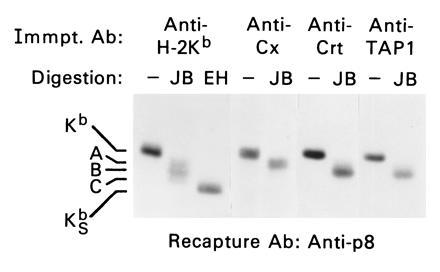
Calnexin and calreticulin interact with distinct H-2Kb glycoforms. Digitonin lysates of metabolically labeled splenic T cells were immunoprecipitated with anti-H-2Kb (p8), anti-calnexin, anti-calreticulin, and anti-TAP 1; precipitated material was released and H-2Kb proteins recaptured as in Fig. 1. Isolated H-2Kb proteins were either mock-treated or digested with JB or Endo H (EH), as indicated. A, B, and C denote H-2Kb glycoforms thought to contain 2, 1, and 0 incompletely trimmed glycan chains, respectively. KSb = H-2Kb proteins containing immature, Endo H-sensitive glycan chains. Note that H-2Kb proteins assembled with calnexin exist in the A glycoform, whereas those assembled with both calreticulin and TAP exist in the B glycoform.
Deglucosylation Is Important for Dissociation of Class I Proteins from Both Calreticulin and TAP.
Initial assembly of glycoproteins with calnexin/calreticulin chaperones requires removal of Glc residues from immature glycan chains by ER glucosidase enzymes (refs. 7, 19, and 23 and unpublished observations). To determine the importance of deglucosylation in the disassembly of class I proteins from calreticulin, experiments were performed in which the glucosidase inhibitor cas (33) was present only during the chase period (subsequent to formation of chaperone–class I protein complexes), as inclusion of cas during the pulse precludes assembly of murine class I proteins with lectin-like chaperones (7, 19, 23). As shown in Fig. 4, dissociation of H-2Kb proteins from calnexin was not affected by cas treatment, with few H-2Kb proteins remaining associated with calnexin in both medium and cas-treated chase groups (Fig. 4). In contrast, disassembly of H-2Kb proteins from calreticulin was significantly impaired in cas-treated groups relative to control groups (Fig. 4), showing that removal of Glc residues from N-linked glycans is an important step in the dissociation of H-2Kb proteins from calreticulin. Surprisingly, we found that dissociation of H-2Kb proteins from TAP was also impeded by cas treatment (Fig. 4), indicating that deglucosylation is also required for effective disassembly of H-2Kb proteins from TAP molecules. Similar results were obtained in the H-2b T cell tumor line EL4 (data not shown). Thus, we conclude from these studies that deglucosylation is an important step in the dissociation of H-2Kb proteins from both calreticulin and TAP peptide transport molecules.
Figure 4.
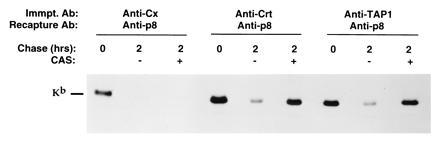
Deglucosylation is important for disassembly of H-2Kb proteins from both calreticulin and TAP. Splenic T cells were metabolically pulse-labeled for 30 min with [35S]methionine (in the absence of cas) and chased in the presence or absence of cas for the time period indicated. Cells were solubilized in 1% digitonin, and lysates were precipitated with anti-calnexin (Cx), anti-calreticulin (Crt), and anti-TAP 1; bound material was released by boiling in 1% SDS and H-2Kb proteins were recaptured with anti-p8. The position of H-2Kb proteins is indicated.
Most Class I Proteins That Are Assembled with Calreticulin Are Simultaneously Associated with TAP.
Finally, because our results showed that calreticulin and TAP were associated with a similar subset of H-2Kb glycoforms in the ER (Fig. 3) and that disassembly of class I proteins from both calreticulin and TAP was prevented by cas treatment (Fig. 4), we explored the idea that class I proteins were assembled together with calreticulin and TAP into a multisubunit complex. As shown in Fig. 5A, the amount of H-2Kb proteins coprecipitating with TAP was dramatically reduced by prior precipitation with anti-calreticulin (Fig. 5A). Preclearing was specific in that no significant reduction of TAP-associated class I proteins was seen when anti-HLA A2 (specific for human HLA class I molecules) or anti-calnexin was used (Fig. 5A). Similarly, preclearing of lysates with anti-TAP specifically reduced the amount of class I proteins coprecipitating with calreticulin molecules (Fig. 5B). Taken together, these data indicate that the vast majority of H-2Kb proteins associated with calreticulin were simultaneously assembled with TAP molecules. To rule out the possibility that formation of calreticulin–class I–TAP complexes occurred postlysis, mixing experiments were performed using radiolabeled WT T cells and TAP −/− T cells as our previous results showed that calreticulin assembled effectively with H-2Kb proteins in TAP −/− T cells (Fig. 2B). As demonstrated, radiolabeled H-2Kb proteins coprecipitated with both calreticulin and TAP in control groups (radiolabeled WT cells plus unlabeled WT cells) but were specifically associated with calreticulin in experimental groups (radiolabeled TAP −/− cells plus unlabeled WT cells; Fig. 5C). Thus, calreticulin–class I complexes present in TAP −/− lysates did not associate postsolubilization with TAP molecules in WT lysates.
Figure 5.
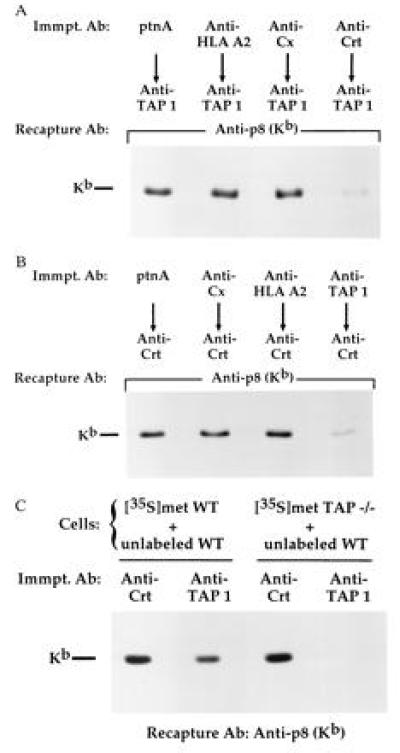
Most H-2Kb proteins associated with calreticulin are simultaneously assembled with TAP. (A) Digitonin lysates of metabolically labeled splenic T cells were precipitated with two rounds each of protein A Sepharose (no antibody control), anti-calnexin (Cx), anti-HLA A2, or anti-calreticulin (Crt). Precleared lysates were then sequentially precipitated with anti-TAP. TAP precipitates were boiled in 1% SDS and H-2Kb proteins were recaptured with anti-p8. (B) Same as in A except that lysates were precleared with the indicated antibodies, then sequentially precipitated with anti-calreticulin (Crt). (C) Metabolically labeled WT and TAP −/− splenic T cells were mixed with an equivalent amount of unlabeled WT T cells as indicated; digitonin lysates were precipitated with anti-calreticulin (Crt) or anti-TAP and H-2Kb proteins were recaptured from precipitates as in A.
The current study demonstrates that calnexin and calreticulin associate with distinct class I assembly intermediates in the ER and significantly extends previous results on the role of glycan processing in the initial assembly of MHC class I proteins with lectin-like chaperones (7, 23), by demonstrating that deglucosylation is an important quality control step in subsequent (terminal) stages of class I assembly.
The precise function of calnexin and calreticulin chaperones in the assembly of MHC class I protein complexes is unclear. It was originally noted by Moore and Spiro (34) that blockade of Glc trimming by cas treatment resulted in rapid degradation of unassembled class I molecules in the CMT-cKd1 cell line. These findings were extended by Balow et al. (23), who showed that calnexin association and assembly of MHC class I protein complexes in BW thymoma cells was greatly reduced by cas treatment (23); interestingly, however, normal class I assembly was observed in the glucosidase II-deficient BW variant, BW PHAR2.7, in which calnexin association (17) and calreticulin association (35) is severely impaired. Thus, it appears that alternative pathways exist for the assembly of class I proteins that do not require glucosidase activity and calnexin/calreticulin associations that are variably utilized, depending on the cell type. The molecular basis for normal MHC class I assembly in glucosidase-deficient cells is unknown but has been suggested to involve expression of other chaperones that are up-regulated under ER stress conditions (23).
It is unknown if calnexin and calreticulin function redundantly in the ER quality control system or if they perform distinct molecular functions in the folding/assembly of newly synthesized glycoproteins. Peterson et al. (19) recently demonstrated that the population of cellular proteins bound to calreticulin partially overlaps those bound to calnexin; and, at least for one protein, the influenza virus hemagglutinin protein, assembly with calnexin and calreticulin was indistinguishable, as measured by disulfide bond formation and conformation analysis. In the current study, we demonstrate by several criteria that calnexin and calreticulin associate with distinct MHC class assembly intermediates in the ER, suggesting that calnexin and calreticulin may perform specific functions in the formation of class I heavy chain–β2m–peptide complexes. Whether or not newly synthesized class I proteins interact successively with calnexin and calreticulin chaperones remains to be determined.
The data in the current study show that unlike calnexin, calreticulin interacts primarily with class I–β2m heterodimers, and, importantly, that the vast majority of class I proteins associated with calreticulin in splenic T cells are simultaneously assembled with TAP. These results are in agreement with recently reported findings by Cresswell and coworkers (24) studying human class I–calreticulin–TAP interactions (24). Importantly, the current study documents that deglucosylation of N-linked glycans is an important step in the disassembly of MHC class I proteins from both calreticulin and TAP molecules. Previous studies have shown that glucosidase activity is important for release of various molecules from calreticulin (19, 35); the finding that calreticulin, class I, and TAP assemble together into a multisubunit complex (ref. 24 and this study) provides a molecular basis for the requirement of glucosidase activity in the release of MHC class I proteins from TAP molecules. It is unclear why disassembly of calnexin–class I protein complexes was not blocked by cas treatment in our studies, as dissociation of other proteins from calnexin, for example, the influenza virus hemagglutinin protein, is blocked by cas (19). It is possible that cas addition does not act fast enough to prevent glucosidase-driven release of class I proteins from calnexin, as these complexes are more short-lived than those with calreticulin; alternatively, it is possible that deglucosylation of class I proteins is finalized subsequent to their dissociation from calnexin.
Finally, recent data by Sadasivan et al. (24) suggests that a novel glycoprotein, tapasin, mediates calreticulin–class I interaction with TAP. Thus, it will be interesting to determine if Glc trimming of glycan chains on class I molecules, tapasin, or both is important for disassembly of calreticulin–class I–TAP protein complexes.
Acknowledgments
We wish to thank Drs. Juan Bonifacino, Ted Hansen, Randy Ribaudo, Paul Roche, Dinah Singer, and Alfred Singer for critical reading of the manuscript. We are also extremely grateful to the many investigators who provided material for our studies, specifically, Drs. Tak Mak and Dinah Singer for making β2m −/− mice available to us; Drs. Izumi Negishi, Dennis Loh, and Dinah Singer for making TAP-1 −/− mice available; Dr. Ettore Appella for the gift of anti-H-2b; Dr. Ted Hansen for the gift of anti-TAP-1 antisera; Dr. Ron Germain for the gift of anti-Invariant chain antibody; Drs. Mike Shields and John Coligan for the gift of anti-HLA A2; and especially Drs. Hidde Ploegh and Rob Machold for supplying the anti-p8 antibodies for use in our studies.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: MHC, major histocompatibility complex; β2m, β2 microglobulin; ER, endoplasmic reticulum; Glc, glucose; GlcNAc, N-acetyl glucosamine; Man, mannose; cas, castanospermine; Endo H, endoglycosidase H; JB, jack bean mannosidase.
References
- 1.Jackson M R, Peterson P A. Annu Rev Cell Biol. 1993;9:207–235. doi: 10.1146/annurev.cb.09.110193.001231. [DOI] [PubMed] [Google Scholar]
- 2.Yewdell J W, Bennink J R. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- 3.Hochstenbach F, David V, Watkins S, Brenner M B. Proc Natl Acad Sci USA. 1992;89:4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degen E, Williams D B. J Cell Biol. 1991;112:1099–1115. doi: 10.1083/jcb.112.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahluwalia N, Bergeron J J M, Wada I, Degen E, Williams D B. J Biol Chem. 1992;167:10914–10918. [PubMed] [Google Scholar]
- 6.Galvin K, Krishna S, Ponchel F, Frolich M, Cummings D E, Carlson R, Wands J R, Isselbacher K J, Pillai S, Ozturk M. Proc Natl Acad Sci USA. 1992;89:8452–8456. doi: 10.1073/pnas.89.18.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassilakos A, Cohen-Doyle M F, Peterson P A, Jackson M R, Williams D B. EMBO J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond C, Braakman I, Helenius A. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware F E, Vassilakos A, Peterson P A, Jackson M A, Lehrman M A, Williams D B. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- 10.Hebert D N, Foellmer B, Helenius A. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- 11.Heemels M T, Ploegh H. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 12.Degen E, Cohen-Doyle M F, Williams D B. J Exp Med. 1992;175:1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh W K, Cohen-Doyle M F, Fruh K, Wang K, Peterson P A, Williams D B. Science. 1994;264:1322–1326. doi: 10.1126/science.8191286. [DOI] [PubMed] [Google Scholar]
- 14.Jackson M R, Cohen-Doyle M F, Peterson P A, Williams D B. Science. 1994;263:384–387. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 16.Moreman K W, Trimble R B, Herscovics A. Glycobiology. 1994;4:113–125. doi: 10.1093/glycob/4.2.113. [DOI] [PubMed] [Google Scholar]
- 17.Kearse K P, Williams D B, Singer A. EMBO J. 1994;13:3678–3686. doi: 10.1002/j.1460-2075.1994.tb06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ora A, Helenius A. J Biol Chem. 1995;270:26060–26062. doi: 10.1074/jbc.270.44.26060. [DOI] [PubMed] [Google Scholar]
- 19.Peterson J R, Ori A, Van P N, Helenius A. Mol Biol Cell. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otteken A, Moss B. J Biol Chem. 1996;271:97–103. doi: 10.1074/jbc.271.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Spiro R G, Zin Q, Bhoyroo V, Soling V. J Biol Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- 22.Nauseef W M, McCormick S J, Clark R A. J Biol Chem. 1995;270:4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- 23.Balow J P, Weissman J D, Kearse K P. J Biol Chem. 1995;270:29025–29029. doi: 10.1074/jbc.270.48.29025. [DOI] [PubMed] [Google Scholar]
- 24.Sadasivan B, Lehner P J, Ortmann B, Spies T, Cresswell P. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 25.Koller B H, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 26.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 27.Rogers M J, Robinson E A, Appella E. J Biol Chem. 1979;254:11126–11133. [PubMed] [Google Scholar]
- 28.Smith M J, Parker J M R, Hodges R S, Barber B H. Mol Immunol. 1986;23:1077–1092. doi: 10.1016/0161-5890(86)90006-4. [DOI] [PubMed] [Google Scholar]
- 29.Machold R P, Andree S, Van Kaer L, Ljunggren H G, Ploegh H L. J Exp Med. 1995;181:1111–1122. doi: 10.1084/jem.181.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carreno B M, Solheim J C, Harris M, Stroynowski I, Connolly J M, Hansen T H. J Immunol. 1995;155:4726–4733. [PubMed] [Google Scholar]
- 31.Van Leeuwen J E M, Kearse K P. J Biol Chem. 1996;271:9660–9665. doi: 10.1074/jbc.271.16.9660. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Jones P P. Nucleic Acids Res. 1989;17:447–448. doi: 10.1093/nar/17.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaushal G P, Elbein A D. Methods Enzymol. 1994;230:316–329. doi: 10.1016/0076-6879(94)30021-6. [DOI] [PubMed] [Google Scholar]
- 34.Moore S E H, Spiro R G. J Biol Chem. 1993;268:3809–3812. [PubMed] [Google Scholar]
- 35.Van Leeuwen, J. E. M. & Kearse, K. P. (1996) J. Biol. Chem., in press. [DOI] [PubMed]


