Abstract
Background
Estimates of excess risk of valvular heart disease among prior users of fenfluramine and dexfenfluramine have varied widely. Two major forms of bias appear to contribute to this variability and also result in a systematic under-estimation of risk. The first, a form of nondifferential misclassification, is the result of including background, prevalent cases among both exposed and unexposed persons in calculations of risk. The second bias results from not considering the relatively short duration of exposure to drugs.
Methods
We examined data from all available echocardiographic studies reporting the prevalence of aortic regurgitation (AR) and mitral regurgitation (MR) among persons exposed to fenfluramine or dexfenfluramine and a suitable control group. We also included one study in which previously existing AR or MR had been excluded. We corrected for background prevalent cases, estimated incidence rates in unexposed persons, and performed a person-years analysis of apparent incidence rates based on exposure time to provide an unbiased estimate of relative risk.
Results
Appearance of new AR was strongly related to duration of exposure (R2 = 0.75, p < 0.0001). The summary relative risk for mild or greater AR was 19.6 (95% CI 16.3 – 23.5, p < 0.00001); for moderate or greater MR it was 5.9 (95% CI 4.0 – 8.6, p < 0.00001).
Conclusion
These findings provide strong support for the view that fenfluramine and dexfenfluramine are potent causal factors in the development of both aortic and mitral valvular heart disease.
Keywords: fenfluramine, dexfenfluramine, aortic regurgitation, mitral regurgitation, risk
Background
Since the initial report by Connolly, et al [1] of valvulopathy in women taking the combination of fenfluramine and phentermine (fen-phen), controversy has surrounded the issue of associated risk. Initial reports of remarkably high prevalence of aortic and mitral regurgitation (AR and MR) as assessed by echocardiography in five separate populations [2] were subsequently supported by most [3-5] but not all investigators [6]. These uncontrolled reports were soon followed by a series of studies in which prevalence of echocardiographically-defined AR and MR were assessed in persons who had taken fenfluramine, dexfenfluramine, phentermine, or combinations of these medications and compared to unexposed, matched control populations [7-15]. The most recent of these studies found 30% of persons currently taking fenfluramine or dexfenfluramine had mild or greater AR or moderate or greater MR, similar to the very high prevalence reported in older studies [15]. The only prospective study found a significant, exposure-related increase in the risk of clinically diagnosed valve disease among users of fenfluramine and dexfenfluramine, but not phentermine [16].
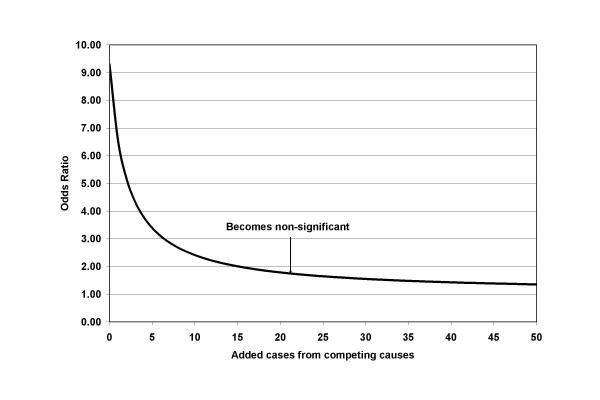
Some recent reviews conclude that the risks associated with fenfluramine and dexfenfluramine are similar, real but modest for aortic valve disease, and barely detectable for mitral valve disease; phentermine appears not to be associated with increased risk [17-20]. Nevertheless, interpretation of the results of these studies has been so varied that some authors have even questioned any causal relationship for fenfluramine exposure [21]. However, analyses to date have not formally considered major biases that would act to deflate the actual risk associated with fenfluramine use. Most investigators have calculated only prevalence ratios or prevalence odds ratios, dividing the prevalence or odds of valve disease in exposed persons by prevalence or odds in an unexposed control group. This approach contrasts with a prospective design wherein relative risk is calculated using only newly arising (incident) cases and with conventional case-control studies in which an estimate of relative risk (the odds ratio) can be made. If persons with prior valve disease are included in both the exposed and the unexposed groups, a gross underestimate of actual risk may occur. The dilution in estimated risk is likely to be particularly important if the background prevalence among unexposed persons is substantial but the incidence of new cases during the observation period is small, as illustrated in figure 1. While other investigators have noted this potentially large bias [22], no attempts at appropriate correction have been made.
Figure 1.
Effect of adding cases from competing causes on the odds ratio in a case control study. In this hypothetical example of 500 exposed and 500 unexposed persons, when no extra cases were added (far left) there were 18 cases of disease in the exposed and 2 cases among the unexposed yielding an odds ratio of 9.30 (p value = 0.0007). Adding an equal number of cases due to unrelated causes to both the exposed and unexposed groups results in dilution of the odds ratio. The odds ratio drops below 2 after 16 cases are added and becomes non-significant after 21 cases are added (representing just 4.2% of exposed or unexposed groups).
A further source of under-estimated risk in prior studies is the lack of consideration given to the time element. In a typical prospective study, both the exposed and unexposed groups are generally followed for an equal period of time. However, no prior echocardiographic study examining anorexic drug-associated valve disease has considered the fact that measurable differences in prevalence have arisen among persons exposed to these drugs over a remarkably short period of time (usually a few months) whereas the prevalent valve disease in the comparison groups likely arose over a number of years or even a lifetime.
In this meta-analysis, we estimated incidence rates based on duration of drug exposure and compared these to expected incidence rates in order to obtain unbiased estimates of relative risk for the development of AR and MR.
Methods
Study Search, Selection, and Data Extraction
We first sought to identify all published studies with echocardiographic assessment of cardiac valve regurgitation in persons exposed to fenfluramine or dexfenfluramine and in suitable controls. The search for studies included examination of prior meta-analyses and reviews, MEDLINE search (using the terms fenfluramine or dexfenfluramine, diet drugs, echocardiogram or echocardiographic, and valvular heart disease), and review of references of identified articles. For this analysis we included only studies that reported duration of medication use. In some studies, results were reported separately by duration of exposure. Each duration group was considered as a separate observation if number of persons exposed, number of cases observed, and duration of exposure was reported.
Data Synthesis
We first evaluated studies reporting echocardiographic prevalence of FDA-positive valvulopathy (mild or greater AR and moderate or greater MR) [2] among subjects who had taken either fenfluramine or dexfenfluramine (alone or in combination with phentermine) and in matched controls [7-14]. In most of these studies, echocardiograms were performed after the drugs had been discontinued and were read without knowledge of prior drug exposure by multiple readers (table 1). In some studies, control subjects were derived from patients assigned to placebo in a formal randomized trial of fenfluramine or dexfenfluramine. In others, controls matched for age, gender and BMI were recruited and examined.
Table 1.
Controlled prevalence studies. Each of the studies compared the prevalence of aortic (mild or greater) and mitral (moderate or greater) regurgitation in a series of patients exposed to either fenfluramine or dexfenfluramine. Only studies reporting duration of exposure to these drugs are included here. A single study excluded prior valve disease by baseline echocardiograms and is also included. Controls were generally recruited matching for age, gender, height and weight.
| First author, year | Study design, echocardiography, comments |
| Khan, 1998 | Exposed patients participated in one of 3 studies at a medical center. One study used 30–60 mg fenfluramine with phentermine 30 mg/day (exposure duration 26.5 months). The other two studies used dexfenfluramine 30 mg/day (one without phentermine, exposure duration 4.9 months; the other allowed phentermine 30 mg/day, exposure duration 9 months). Controls were recruited later, matched to sex, age, height, BMI. Echocardiographers were apparently unblinded with regard to patient status. The first reader was blinded for all but potentially 60 exposed cases. All films were read by a second reader. A third reader was used if the 1st and 2nd reader did not agree. Good concordance reported |
| Weissman, 1998 | Participants were in a randomized, placebo-controlled trial comparing dexfenfluramine, sustained-release dexfenfluramine, and placebo. Original protocol was for 16 weeks but was stopped prematurely due to withdrawal of fenfluramine and dexfenfluramine from market. Blinding maintained and echocardiography scheduled soon after stopped. Investigators and patients remained blinded to treatment. Standardized protocol. Echocardiographers were unaware of patient status. Echocardiograms read at an independent reading center. Scans were-re-read if any valve abnormality seen. 5% of scans read by other readers to judge reproducibility. Excellent reproducibility. |
| Shively, 1999 | 26 sites were to supply 5 or more cases who had taken dexfenfluramine (no other anorexic) for at least 3 months and 5 or more controls (matched by age, gender, and BMI) who had used no anorexics for 5 or more years. Echocardiographers (at centers near each site) were blinded to subject status. There were three blinded readers (2 of 3 readers had to agree or re-reading required). Good inter-reader agreement reported. |
| Hensrud, 1999 | Participants of a small, double-blind, randomized trial assigned to either fen-phen or placebo. Echocardiograms read by a blinded reviewer. |
| Ryan, 1999 | Patients were enrolled in a long-term research study with fenfluramine (and some with dexfenfluramine) with baseline echocardiograms. 86 patients were re-scanned after fenfluramine and dexfenfluramine were withdrawn. 7 of these (8.1%) had pre-existing regurgitation and are eliminated in this analysis. Good inter- and intra-reader concordance was demonstrated for baseline and follow-up echocardiograms. Readings were blinded as to order (baseline versus follow-up) of echocardiograms. |
| Gardin, 2000 | Investigators at 25 centers who had frequently prescribed dexfenfluramine or fen-phen and could enroll subjects were invited to participate. Initially, controls were matched on 4 criteria (age, sex, BMI, and geographical area), then less stringent geographic criteria were implemented. Standardized protocol. Echocardiographers were blinded with regard to patient treatment status. Echocardiograms were read blind at a core lab. Good inter- and intra-reader concordance reported. |
| Jollis, 2000 | 33 practices with large numbers of prescriptions were invited to participate. Required 3 months+ treatment with fen-phen. Matched controls from same centers. Dyspnea on exertion more frequent in drug group. SSRI use was not associated with AR or MR. Standardized protocol. Readers blinded to treatment status. Good agreement in 350 scans read by 2 or more cardiologists. |
| Davidoff, 2001 | Female smokers who had participated in a double-blind placebo-controlled trial of fenfluramine for smoking cessation were contacted approximately 4.5 years after the study was completed to undergo echocardiography. Standardized protocol. Echocardiographers were blinded to patient history. Tapes read at central lab. Re-reading performed when abnormalities present. Good inter-reader agreement reported. |
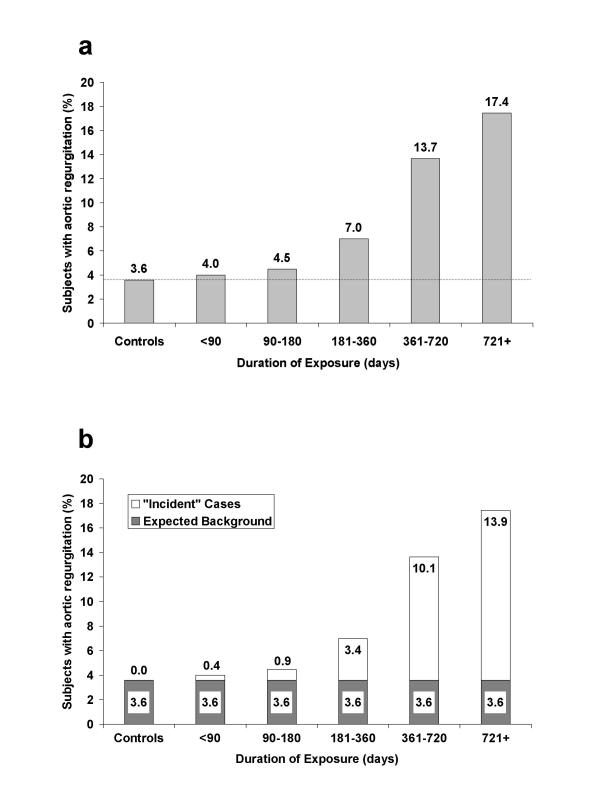
In a true prospective study, persons with valvulopathy at baseline would be excluded from follow-up or calculation of incidence rates. Since only one study had performed echocardiography on a substantial number of subjects prior to initiation of the diet drugs [11], some means of estimating and correcting for the baseline prevalence in the other studies was necessary. For this analysis we have used the unexposed control groups for each study to provide such an estimate. Thus, the prevalence rate in the control (unexposed) group of each study was used to estimate the number of background cases expected in the exposed group(s). Any cases observed beyond this background number were considered "new" or incident cases. This approach is mathematically equivalent to modelling the rate of change in prevalence in the exposed group with the control group providing the initial estimate of prevalence. The concept is illustrated in figure 2 using data from Jollis, et al [13]. Duration of drug use provided the time factor to estimate incidence in the exposed groups. Two studies which did not report duration of drug exposure were therefore excluded from this meta-analysis [15,23].
Figure 2.
a. Prevalence of mild or greater aortic regurgitation by duration of exposure compared with controls. Adapted from data presented in Jollis, et al (reference [13]). b. Rationale for present study using data from figure 2a. The controls are considered to provide an estimate of the background or baseline risk of AR prior to exposure (shown in dark grey). Prevalent cases beyond this estimated background rate were considered to have arisen during the period of drug exposure, thus providing an estimate of incidence (white portion of bars).
Of major importance in this analysis is the estimation of the expected incidence rate of FDA-positive AR and MR (for estimation of relative risk). The most straightforward estimate of incidence would require a sample of persons from the general population having no AR or MR at baseline and who had a repeat echocardiogram some time in the future. While two studies performed echocardiograms one year apart in sizable populations, both showed either no net progression or a net decrease in regurgitation in the unexposed, control groups [24,25]. These findings underscore the infrequency of new onset, FDA-positive AR or MR in the unexposed population.
As an alternative estimate of expected incidence we modelled change in prevalence in two populations. In the first, data from the Framingham study were utilized [26]. In that study, the prevalence of FDA-positive AR or MR in persons under age 40 was zero. The prevalent cases in the following decade (ages 40–49) were therefore assumed to have arisen since age 40. For each subsequent decade, the increase in prevalence was assumed to have arisen since the prior decade. Additional assumptions were that incident cases did not drop out (that is, there was no differential mortality and change in prevalence was the result of cumulative incidence), that incidence rates were constant within decades, and that there was no cohort effects (i.e., that incidence rates in older individuals when they were young were similar to the incidence rates in currently young individuals). Given theses assumptions, the change in prevalence is given by the expression
1 - (1 - i)t
were i is the incidence rate per year and t is the number of years between prevalence data points (10 for full decades). The equation was solved for i given the observed change in prevalence between decades and time elapsed (using the end of each time period). Incidence rates were calculated for FDA-positive AR and MR separately for each decade in men and women. These estimated incidence rates are shown in table 2. As a conservative estimate for relative risk calculations, all the rates were pooled (as the unweighted mean of the calculated rates in table 2 for men and women), recognizing that this will yield a higher "unexposed" rate than was likely present for the younger and predominantly female populations exposed to the anorexic drugs. The average estimated Framingham incidence rates for age 40 and over calculated in this manner were 0.357% per year for mild or greater AR and 0.143% per year for moderate or greater MR.
Table 2.
2a. Aortic regurgitation incidence (percent per year) estimated from Framingham Study prevalence (Singh, et al, 1999). Prevalence of mild or greater aortic regurgitation utilized.
| Men | Women | |||||
| Age | N | Prevalence (%) | Incidence | N | Prevalence (%) | Incidence |
| 26–39 | 91 | 0 | - | 93 | 0 | - |
| 40–49 | 352 | 1.7 | 0.171 | 451 | 0.7 | 0.070 |
| 50–59 | 433 | 4.2 | 0.253 | 515 | 2.1 | 0.141 |
| 60–69 | 359 | 12.7 | 0.884 | 390 | 6.8 | 0.480 |
| 70–83 | 91 | 14.4 | 0.132 | 90 | 16.9 | 0.816 |
| 2b. Mitral regurgitation incidence estimated from Framingham Study prevalence rates (Singh, et al, 1999). Prevalence of moderate or greater mitral regurgitation utilized. | ||||||
| Men | Women | |||||
| Age | N | Prevalence (%) | Incidence | N | Prevalence (%) | Incidence |
| 26–39 | 91 | 0 | - | 93 | 0 | - |
| 40–49 | 351 | 0.3 | 0.030 | 452 | 0.9 | 0.090 |
| 50–59 | 432 | 1.6 | 0.131 | 515 | 1.0 | 0.010 |
| 60–69 | 372 | 2.4 | 0.080 | 395 | 2.3 | 0.131 |
| 70–83 | 90 | 11.2 | 0.706 | 90 | 0.0 | 0.000 |
The second data set used to estimate unexposed incidence rates was the control groups of the studies listed in table 1. Cases of FDA-positive AR and MR reported in the control groups were assumed to have arisen between age 40 and the average age of the control group in each study. A mean of the estimated incidence from each study was calculated and weighted by the number of controls per study. Since FDA-positive AR and MR are found in other populations under age 40 [27], the assumption that all the observed AR and MR occurred only after age 40 will result in conservatively high estimates of incidence (resulting in lower estimates of relative risk).
The observed number of "incident" cases of AR or MR in the exposed group was calculated as the total number of cases minus the number expected at baseline (estimated from the prevalence in the unexposed control group in each study). Any negative numbers were set to 0 (one instance of AR with under 3 months exposure). The expected number of cases in the exposed population (for Poisson analyses) was calculated as the incidence rate (per year) determined from the pooled rate in controls (obtained as described above) multiplied by the duration of exposure (in years). The probability of observing the number of "incident" cases in the exposed group of each individual study given the number expected from the pooled estimate was calculated using a Poisson distribution. Confidence intervals for the percent of incident cases during each study (figures 3 and 4) were calculated as suggested by Fleiss for observed versus expected proportions [28]. Effect of exposure duration and other factors on percent of incident cases during each study was tested in a weighted analysis of covariance using the GLM procedure in SAS (SAS Institute, Carey, North Carolina).
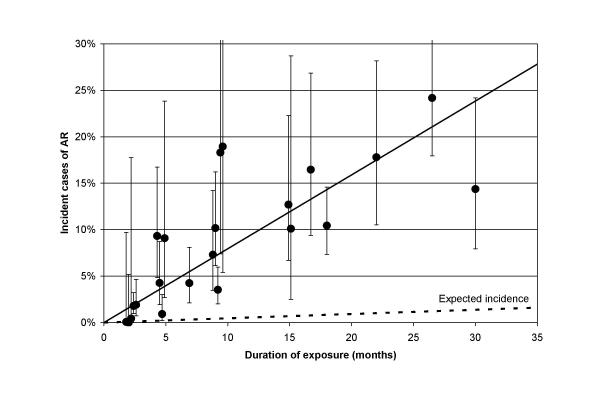
Figure 3.
Estimated percent of incident cases with mild or greater AR arising during the exposure period (± 95% confidence intervals) versus duration of exposure to fenfluramine or dexfenfluramine. Some studies provided multiple point estimates based on different exposure times. The midpoint of the duration interval was used to plot these observations. Expected incident cases were calculated using the pooled estimate described in the text.
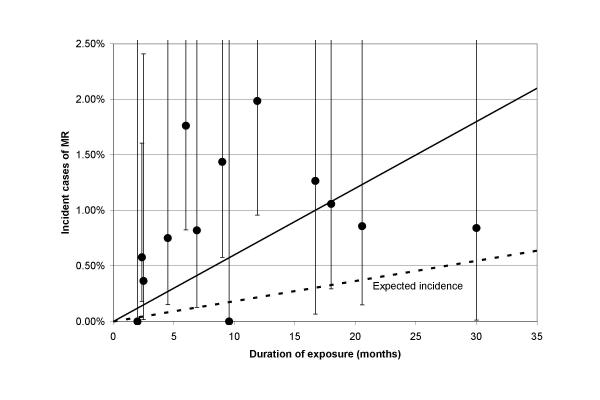
Figure 4.
Estimated percent of incident cases with moderate or greater MR arising during the exposure period (± 95% confidence intervals) versus duration of exposure to fenfluramine or dexfenfluramine.
Relative risks were calculated as (incident cases / person-years in exposed) / (cases in controls / person-years in controls). Person-years in the exposed group were calculated as: (total number exposed – cases expected at baseline) × (mean duration of drug use reported for each study). Person-years in unexposed controls were calculated as: (total number in the control group) × (the number of years from age 40 to the average age of the group). Note that pooled incidence rates for the unexposed were not used in this analysis (so as not to inflate power); rather, each study provided its own estimates. Significance and confidence intervals for the relative risks were calculated as suggested by Kleinbaum, et al [29] for a density type follow-up study. This approach tests the difference in incidence between two groups given that follow-up time may be different in the exposed and unexposed populations.
Results
Raw data from each study is given for mild or greater AR in table 3 and for moderate or greater MR in table 4. Estimates of unexposed incidence of AR and MR utilizing data from the control groups in these studies yielded values approximately 50% higher than estimates from the Framingham study; 0.555% per year for mild or greater AR and 0.219% per year for moderate or greater MR. To provide conservative estimates of relative risk, these rates were used to calculate the expected number affected (given the duration of exposure in each study) for Poisson analyses in tables 5 and 6. Estimates of "incident" cases and relative risks based on person-years are also given in tables 5 and 6.
Table 3.
Raw data for aortic regurgitation (mild or greater). When duration of exposure was reported as a range, the midpoint of the range is given here. "Time off" refers to the time between the last dose of anorexic and the echocardiogram.
| Author, year | Mean Age | Fen dose (mg/day) | Dexfen dose (mg/day) | Duration (months) | Time off (months) | Controls (N) | Affected in Controls | Exposed (N) | Affected in Exposed |
| Khan, 1998 | 46.0 | 90 | 0 | 26.5 | 3.9 | 233 | 3 | 163 | 41 |
| 43.0 | 0 | 30 | 4.9 | 3.9 | 39 | 4 | |||
| 44.7 | 0 | 30 | 9.0 | 3.9 | 31 | 6 | |||
| Weissman, 1998 | 45.1 | 0 | 30 | 2.4 | 1.3 | 330 | 12 | 671 | 36 |
| Shively, 1999 | 50.0 | 0 | 30 | 6.9 | 8.5 | 189 | 4 | 223 | 14 |
| Hensrud, 1999 | 42.0 | 40 | 0 | 9.6 | 1.5 | 11 | 1 | 19 | 5 |
| Ryan, 1999 | 49.1 | 60 | 30 | 16.7 | 12.2 | 0 | 0 | 79 | 13 |
| Gardin, 2000 | 46.9 | 46 | 0 | 2.0 | 6.8 | 536 | 22 | 48 | 2 |
| 46.9 | 46 | 0 | 4.5 | 6.8 | 115 | 15 | |||
| 46.9 | 46 | 0 | 9.0 | 6.8 | 117 | 13 | |||
| 46.9 | 46 | 0 | 15.0 | 6.8 | 86 | 14 | |||
| 46.9 | 46 | 0 | 22.0 | 6.8 | 85 | 18 | |||
| 48.4 | 0 | 29 | 2.0 | 5.3 | 92 | 0 | |||
| 48.4 | 0 | 29 | 4.5 | 5.3 | 183 | 15 | |||
| 48.4 | 0 | 29 | 9.0 | 5.3 | 166 | 23 | |||
| 48.4 | 0 | 29 | 15.0 | 5.3 | 29 | 4 | |||
| Jollis, 2000 | 46.1 | 60 | 0 | 2.0 | 15 | 669 | 24 | 25 | 1 |
| 46.1 | 60 | 0 | 4.5 | 15 | 313 | 14 | |||
| 46.1 | 60 | 0 | 9.0 | 15 | 415 | 29 | |||
| 46.1 | 60 | 0 | 18.0 | 15 | 315 | 43 | |||
| 46.1 | 60 | 0 | 30.0 | 15 | 86 | 15 | |||
| Davidoff, 2001 | 48.8 | 60 | 0 | 2.5 | 53 | 254 | 11 | 276 | 17 |
Table 4.
Raw data for mitral regurgitation (moderate or greater). When duration of exposure was reported as a range, the midpoint of the range is given here. "Time off" refers to the time between the last dose of anorexic and the echocardiogram.
| Author, year | Mean Age | Fen dose (mg/day) | Dexfen dose (mg/day) | Duration (months) | Time off (months) | Controls (N) | Affected in Controls | Exposed (N) | Affected in Exposed |
| Khan, 1998 | 45.3 | 90 | 30 | 20.6 | 3.9 | 233 | 0 | 233 | 2 |
| Weissman, 1998 | 45.1 | 0 | 30 | 2.4 | 1.3 | 333 | 4 | 677 | 12 |
| Shively, 1999 | 50.0 | 0 | 30 | 6.9 | 8.5 | 189 | 1 | 223 | 3 |
| Hensrud, 1999 | 42.0 | 40 | 0 | 9.6 | 1.5 | 11 | 0 | 19 | 0 |
| Ryan, 1999 | 49.1 | 60 | 30 | 16.7 | 12.2 | 0 | 0 | 79 | 1 |
| Gardin, 2000 | 46.9 | 46 | 0 | 11.9 | 6.8 | 537 | 17 | 452 | 23 |
| Gardin, 2000 | 48.4 | 0 | 29 | 6 | 5.3 | 472 | 23 | ||
| Jollis, 2000 | 46.1 | 60 | 0 | 2 | 15 | 668 | 10 | 25 | 0 |
| 46.1 | 60 | 0 | 4.5 | 15 | 313 | 7 | |||
| 46.1 | 60 | 0 | 9 | 15 | 412 | 12 | |||
| 46.1 | 60 | 0 | 18 | 15 | 315 | 8 | |||
| 46.1 | 60 | 0 | 30 | 15 | 86 | 2 | |||
| Davidoff, 2001 | 48.8 | 60 | 0 | 2.5 | 53 | 254 | 12 | 276 | 14 |
Table 5.
Estimated incidence rates and relative risks for mild or greater AR. The number of expected cases were based on estimated incidence rates utilizing the pooled control (unexposed) groups of these studies. Relative risks and z statistics were calculated within each study.
| Author, year | Duration (months) | "Incident" cases | Expected cases | p-value by Poisson | Relative Risk (RR) | Lower 95% CI of RR | Upper 95% CI of RR | z | p-value |
| Khan, 1998 | 26.5 | 38.9 | 1.939 | <0.00001 | 59.5 | 31.6 | 112.1 | 12.7 | <0.00001 |
| 4.9 | 3.5 | 0.086 | <0.00001 | 69.1 | 31.1 | 153.4 | 10.4 | <0.00001 | |
| 9.0 | 5.6 | 0.125 | <0.00001 | 108.0 | 57.1 | 204.4 | 14.4 | <0.00001 | |
| Weissman, 1998 | 2.4 | 11.6 | 0.696 | <0.00001 | 15.3 | 8.4 | 27.9 | 8.9 | <0.00001 |
| Shively, 1999 | 6.9 | 9.3 | 0.685 | <0.00001 | 38.4 | 18.9 | 78.0 | 10.1 | <0.00001 |
| Hensrud, 1999 | 9.6 | 3.3 | 0.075 | <0.00001 | 7.8 | 1.2 | 51.8 | 2.1 | 0.016 |
| Ryan, 1999 | 16.7 | 13 | 0.600 | <0.00001 | - | - | - | - | - |
| Gardin, 2000 | 2.0 | 0.03 | 0.042 | 0.96 | 0.7 | 0.0 | 61295 | -0.1 | 0.52 |
| 4.5 | 10.3 | 0.226 | <0.00001 | 47.8 | 31.3 | 73.0 | 17.9 | <0.00001 | |
| 9.0 | 8.2 | 0.459 | <0.00001 | 18.8 | 10.6 | 33.3 | 10.0 | <0.00001 | |
| 15.0 | 10.5 | 0.563 | <0.00001 | 19.5 | 11.6 | 32.9 | 11.2 | <0.00001 | |
| 22.0 | 14.5 | 0.816 | <0.00001 | 18.7 | 11.6 | 30.0 | 12.1 | <0.00001 | |
| 2.0 | 0 | 0.080 | 0.92 | 0.0 | 0.0 | - | -0.3 | 0.60 | |
| 4.5 | 7.5 | 0.359 | <0.00001 | 26.1 | 15.0 | 45.2 | 11.6 | <0.00001 | |
| 9.0 | 16.2 | 0.652 | <0.00001 | 31.0 | 20.6 | 46.7 | 16.5 | <0.00001 | |
| 15.0 | 2.8 | 0.190 | 0.0010 | 18.5 | 7.6 | 45.1 | 6.4 | <0.00001 | |
| Jollis, 2000 | 2.0 | 0.10 | 0.022 | 0.022 | 5.1 | 0.0 | 1233 | 0.6 | 0.28 |
| 4.5 | 2.8 | 0.618 | 0.026 | 4.8 | 1.6 | 14.9 | 2.8 | 0.0029 | |
| 9.0 | 14.1 | 1.638 | <0.00001 | 9.3 | 5.4 | 15.9 | 8.1 | <0.00001 | |
| 18.0 | 31.7 | 2.486 | <0.00001 | 13.8 | 9.2 | 20.6 | 12.7 | <0.00001 | |
| 30.0 | 11.9 | 1.131 | <0.00001 | 11.4 | 6.6 | 19.7 | 8.7 | <0.00001 | |
| Davidoff, 2001 | 2.5 | 5.0 | 0.300 | <0.00001 | 20.8 | 9.9 | 43.4 | 8.1 | <0.00001 |
| Summary, 2002 | 8.78 | 220.8 | 14.01 | <0.00001 | 19.6 | 16.3 | 23.5 | 31.7 | <0.00001 |
Table 6.
Estimated incidence rates and relative risks for moderate or greater MR. The number of expected cases were based on estimated incidence rates utilizing the pooled control (unexposed) groups of these studies. Relative risks and z statistics were calculated within each study. No cases of MR were observed in unexposed groups of the Khan and Hensrud studies making relative risks inestimable. These studies did contribute to the summary estimates.
| Author, year | Duration (months) | "Incident" MR cases | Expected cases | p-value by Poisson | Relative Risk (RR) | Lower 95% CI of RR | Upper 95% CI of RR | z | p-value |
| Khan, 1998 | 20.6 | 2.00 | 0.873 | 0.059 | - | - | - | 2.7 | 0.0033 |
| Weissman, 1998 | 2.4 | 3.87 | 0.289 | 0.00023 | 14.9 | 5.2 | 42.5 | 5.0 | <0.00001 |
| Shively, 1999 | 6.9 | 1.82 | 0.279 | 0.032 | 29.7 | 6.2 | 142.8 | 4.2 | 0.000012 |
| Hensrud, 1999 | 9.6 | 0.00 | 0.033 | 0.97 | - | - | - | - | - |
| Ryan, 1999 | 16.7 | 1.00 | 0.241 | 0.025 | - | - | - | - | - |
| Gardin, 2000 | 11.9 | 8.69 | 0.950 | <0.00001 | 5.0 | 2.4 | 10.4 | 4.3 | <0.00001 |
| 6 | 8.06 | 0.500 | <0.00001 | 10.5 | 5.3 | 20.5 | 6.8 | <0.00001 | |
| Jollis, 2000 | 2 | 0.00 | 0.009 | 0.99 | 0.0 | 0.0 | - | -0.1 | 0.54 |
| 4.5 | 2.31 | 0.253 | 0.0022 | 9.5 | 3.0 | 30.5 | 3.8 | 0.000079 | |
| 9 | 5.83 | 0.666 | 0.000069 | 9.1 | 3.9 | 21.1 | 5.1 | <0.00001 | |
| 18 | 3.28 | 1.018 | 0.020 | 3.3 | 1.0 | 10.8 | 2.0 | 0.022 | |
| 30 | 0.71 | 0.463 | 0.37 | 1.6 | 0.1 | 17.3 | 0.4 | 0.35 | |
| Davidoff, 2001 | 2.5 | 0.96 | 0.120 | 0.11 | 3.6 | 0.5 | 25.3 | 1.3 | 0.096 |
| Summary, 2002 | 8.89 | 38.5 | 5.7 | <0.00001 | 5.9 | 4.0 | 8.6 | 9.1 | <0.00001 |
The percent of incident cases of AR during each study (calculated without dividing by duration of exposure for this analysis) was found to be clearly related to duration of exposure. This is illustrated in figure 3. In a weighted analysis of covariance including all the studies in table 5, R2 was 0.75, suggesting that 75% of the variance between the studies of the percent of incident cases was explained by duration of exposure. The coefficient relating estimated incidence to duration (in months) was 0.00720 (p < 0.0001). In contrast, time since stopping drug and dose were not related to estimated incidence in this analysis. All but three of the 95% confidence intervals from these 22 point estimates included the regression line, suggesting a fairly homogeneous estimate of risk. Remarkably, the predicted cumulative incidence after 1 year exposure was 9.6%. The weighted estimate of incidence from all studies combined (ignoring duration of exposure) was 6.29% (95% CI 5.51% – 7.16%, z = 14.2, p < 0.00001) during a mean exposure time of 8.78 months. This contrasts with an expected incidence of 0.42% in 9 months at the rate estimated from the pooled control groups of these studies. The summary relative risk for AR was 19.6 (95% CI 16.3 – 23.5, p < 0.00001).
The percent of incidence cases of moderate or greater MR was lower than AR in these studies. There was no significant correlation with duration of exposure (R2 = 0.126, regression coefficient = 0.00037, p = 0.39) or with dose or time since stopping drug (by weighted analysis of covariance using continuous variables). The percent of incidence cases was marginally greater in those exposed for 3 months or more (1.30% ± 0.19, mean ± SE) compared to those with lower exposure times (0.54% ± 0.31, p = 0.09). Overall, estimated incidence was markedly greater than expected in most studies as shown in figure 4 and table 6. The weighted estimate of incidence of MR including all studies was 1.09% (95% CI 0.78% – 1.50%, z = 5.2, p < 0.00001) during an average exposure time of 8.89 months with an expected incidence of 0.16% in 9 months based on the pooled control groups. The summary relative risk for MR was 5.9 (95% CI 4.0 – 8.6, p < 0.00001).
Discussion
Prior summary estimates of risk for developing FDA-positive AR and MR have been based on simple comparisons of prevalence in persons exposed and unexposed to anorexic drugs. For example, without making any adjustments to observed prevalence rates, Loke, et al [18] reported a summary "relative risk" of 2.82 (95% CI 2.20 – 3.61, z = 8.21, p < 0.0001) for FDA-positive AR and 1.55 (95% CI 1.06 – 2.25, z = 2.28, p = 0.02) for MR utilizing most of the same studies included in the present report. Our findings suggest that these estimates are strongly biased toward the null because of counting background, prevalent cases as incident cases in both exposed and unexposed groups (a form of nondifferential misclassification) and not considering the remarkably short period of exposure during which most observed differences in prevalence arose. In contrast, we found much higher relative risks using methods to correct for these biases; 19.6 for AR and 5.9 for MR (both p < 0.00001). Furthermore, our estimates of percent incident cases of AR among exposed were highly correlated with duration of exposure and appeared homogeneous, suggesting a biologically relevant, strong, causal relationship.
Are these high estimates of risk unprecedented? In the only true prospective study comparing risk in exposed and unexposed persons, Jick, et al [16] identified clinically diagnosed valvular disease using the large UK General Practice Research Database. Using the data provided in the paper, a valve-specific analysis may be performed comparing incidence of aortic and mitral disease in those exposed for 4 months or longer versus all others. The results reveal a relative risk of 17.1 for AR and 34.3 for MR, both with p < 0.0001 by chi-square. Since the few cases that arose within 3 months of exposure are included in the "unexposed" group in this analysis, even these high risks represent an underestimate. Nevertheless, the close similarity in the estimate of AR relative risk to the present study is remarkable. The much higher risk for MR compared to our estimate may represent fundamental biological differences between clinically apparent valve disease and echocardiographically assessed valve disease.
A single study among those reviewed in our meta-analysis excluded persons with valve disease using echocardiograms taken prior to starting drug [11]. That study yielded estimates of AR and MR incidence that were similar to our summary estimates, further supporting our findings. The fact that so many studies have observed statistically significant differences in prevalence after relatively short exposure to drug suggest that underlying incidence rates of new valvulopathy must be extraordinarily high compared to the expected low unexposed rate.
Data provided in this study can be used to estimate the relative contribution of exposure to fenfluramines to overall incidence of echocardiographically defined, FDA-positive AR and MR. Given that approximately 2.5% of the population of the United States had used anorexic prescription drugs (mostly fenfluramine and dexfenfluramine) between 1996 and 1998 [30], approximately 32% and 11% of all FDA-positive AR and MR respectively could be attributed to fenfluramine or dexfenfluramine use. Among those who had actually taken the drugs, the attributable fraction rises to 95% and 83% for AR and MR respectively.
Why was there no apparent effect of exposure duration on MR incidence? This may well be due to low power. If only 0.25 of an incident case had been seen in the exposed group of Hensrud, et al [10] and 1 extra case had been seen in the group exposed 30 months in Jollis, et al [13] then a significant logarithmic relationship between duration and percent incident cases would have become apparent, illustrating the precarious nature of correlations when events are few. While we cannot claim such a relationship does exist, the relatively low incidence seen generally for moderate or greater MR makes establishing strong correlations difficult. Importantly, we cannot conclude that a positive association between exposure duration and incident MR does not exist, only that the current studies are inconclusive regarding this relationship. We can conclude, however that the current studies provide sufficient power in aggregate to clearly demonstrate an overall excess risk for developing MR.
Another approach to expressing excess risk is the calculation of "number needed to harm" (NNH). NNH may be calculated as the inverse of the difference between risk in exposed and risk in unexposed persons. For example, the cumulative prevalence of AR was 95.6 per 1000 in those exposed to fenfluramines without regard to exposure time (342 cases among 3576 exposed, from table 3) compared to 34.7 per thousand among unexposed (77 cases among 2222 unexposed) for a NNH of 16.4. For MR, the cumulative prevalence was 29.9 per 1000 in exposed (107 of 3582 exposed, from table 4) versus 19.8 per thousand (34 of 2225 exposed) for a NNH of 99. A risk difference, as used to calculate NNH, is less subject to nondifferential misclassification than is a risk ratio. However, demonstrating association with graded exposures and statistical methods to determine significance or confidence intervals are well defined for relative risk but not for NNH. We have therefore emphasized estimates of relative risk. Importantly, estimates of NNH as calculated by differences between exposed and unexposed prevalence rates, shown above, were very similar to NNH determined using differences between our estimated 9-month incidence rates in exposed and unexposed (NNH was 17 for AR and 108 for MR by this approach), further illustrating the internal consistency of our analysis.
Our study has several limitations. There is no direct measure of true incidence of AR or MR in the unexposed population. The only studies to perform repeated echocardiograms (separated by one year in both) in large numbers of unexposed persons found either no net progression or net regression in their control groups [24,25]. Because these studies did not report usable direct measures of incidence, we made several assumptions to estimate unexposed incidence using raw data from the Framingham study and from the studies reviewed here. Our observation that incidence rates estimated from both populations were of a similar magnitude supports the feasibility of this approach. Further, our estimate is conservative. For example, using the Framingham estimates would have yielded 50% higher relative risks. Had we subtracted the prevalence of FDA-positive AR (1.2%) and MR (1.0%) seen in the CARDIA study [27] and considered the time course of new valve disease development to commence at age 35 (the ending age of the CARDIA study), the unexposed incidence would have been approximately half the rate we used and the relative risks doubled.
Our estimate of apparent incidence of AR and MR among the exposed groups is also based on several assumptions. However, the strong, consistent relationship between the percent of incident cases of AR in each study and duration of exposure suggests that our estimates were reasonable and biologically relevant. We acknowledge that there were a number of differences between the study groups included in our analysis. There were differences in study design and methods (though most were quite similar), fenfluramine or dexfenfluramine doses (though in most studies, doses were similar), additional drugs used, patient characteristics, interval between cessation of therapy and echocardiography, and completeness of follow-up. However, these differences would likely add random error and therefore decrease any observed correlation. Estimates of incidence among unexposed controls from each study were more variable, resulting in greater differences between studies in estimates of relative risk. This is not surprising considering the relatively rare occurrence of new onset AR or MR in unexposed middle-aged men and women.
We have not formally considered other potential sources of bias in our analyses. These include measurement bias (related to acquisition and reading of echocardiograms) [22], selection bias (particularly in studies with incomplete follow-up) [31], and potential reversibility of valvulopathy [10,24,25,32]. If a reversible component occurred rather quickly after stopping drugs, then one might expect a stable, lower prevalence in studies performed well after drugs were stopped, with no relationship between prevalence of valve dysfunction and time off medication. Indeed, the most recently published study (which did not suffer from measurement bias) showed a high prevalence (30%) of AR in persons taking fenfluramine or dexfenfluramine at the time of the echocardiogram, similar to the older prevalence studies [15]. Regarding the potential reversibility of valve dysfunction, great caution must be exercised in assuming any substantial reversal of valvular plaques, as recent pathological studies suggest a progressive nature to these lesions [33]. Furthermore, limited, early apparent echocardiographic improvement of acute rheumatic carditis has also been recently demonstrated [34]. The early improvement seen in this setting certainly does not preclude later progression of rheumatic valvular heart disease.
In conclusion, we have estimated risks associated with use of fenfluramine or dexfenfluramine for developing FDA-positive AR and MR after correcting for two major sources of bias. Accordingly, our estimates of relative risk associated with fenfluramine or dexfenfluramine use, 19.6 for AR and 5.9 for MR, are higher than in prior reviews. These findings lend strong support to the view that fenfluramine and dexfenfluramine are potent causal factors in the development of both aortic and mitral valvular heart disease.
Competing Interests
Dr. Hopkins and Dr. Polukoff have been retained as consultants for plaintiff attorneys representing persons who have taken fenfluramine and dexfenfluramine.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Paul N Hopkins, Email: paul@ucvg.med.utah.edu.
Gerald I Polukoff, Email: Gerald.Polukoff@hsc.utah.edu.
References
- Connolly HM, Crary JL, McGoon M, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Bowen R, Glicklich A, Khan M, Rasmussen S, Wadden T, Bilstad J, Graham D, Green L, Lumpkin M, O’Neill R, Sobel S, Hubbard VS, Yanovski S, Sopko G. Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services Interim Public Health Recommendations. MMWR. 1997;46:1061–1066. [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Silvestry F, Vogt RA, St John Sutton MG, Stunkard AJ, Foster GD, Aber JL. The fen-phen finale: a study of weight loss and valvular heart disease. Obes Res. 1998;6:278–284. doi: 10.1002/j.1550-8528.1998.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Lepor NE, Gross SB, Daley WL, Samuels BA, Rizzo MJ, Luko SP, Hickey A, Buchbinder NA, Naqvi TZ. Dose and duration of fenfluramine-phentermine therapy impacts the risk of significant valvular heart disease. Am J Cardiol. 2000;86:107–110. doi: 10.1016/S0002-9149(00)00840-7. [DOI] [PubMed] [Google Scholar]
- Teramae CY, Connolly HM, Grogan M, Miller F. A., Jr. Diet drug-related cardiac valve disease: the Mayo Clinic echocardiographic laboratory experience. Mayo Clin Proc. 2000;75:456–461. doi: 10.4065/75.5.456. [DOI] [PubMed] [Google Scholar]
- Burger AJ, Sherman HB, Charlamb MJ, Kim J, Asinas LA, Flickner SR, Blackburn GL. Low prevalence of valvular heart disease in 226 phentermine-fenfluramine protocol subjects prospectively followed for up to 30 months. J Am Coll Cardiol. 1999;34:1153–1158. doi: 10.1016/S0735-1097(99)00321-6. [DOI] [PubMed] [Google Scholar]
- Khan MA, Herzog CA, St Peter JV, Hartley GG, Madlon-Kay R, Dick CD, Asinger RW, Vessey JT. The prevalence of cardiac valvular insufficiency assessed by transthoracic echocardiography in obese patients treated with appetite-suppressant drugs. N Engl J Med. 1998;339:713–718. doi: 10.1056/NEJM199809103391101. [DOI] [PubMed] [Google Scholar]
- Weissman NJ, Tighe J. F., Jr., Gottdiener JS, Gwynne JT. An assessment of heart-valve abnormalities in obese patients taking dexfenfluramine, sustained-release dexfenfluramine, or placebo. Sustained-Release Dexfenfluramine Study Group. N Engl J Med. 1998;339:725–732. doi: 10.1056/NEJM199809103391103. [DOI] [PubMed] [Google Scholar]
- Shively BK, Roldan CA, Gill EA, Najarian T, Loar SB. Prevalence and determinants of valvulopathy in patients treated with dexfenfluramine. Circulation. 1999;100:2161–2167. doi: 10.1161/01.cir.100.21.2161. [DOI] [PubMed] [Google Scholar]
- Hensrud DD, Connolly HM, Grogan M, Miller FA, Bailey KR, Jensen MD. Echocardiographic improvement over time after cessation of use of fenfluramine and phentermine. Mayo Clin Proc. 1999;74:1191–1197. doi: 10.4065/74.12.1191. [DOI] [PubMed] [Google Scholar]
- Ryan DH, Bray GA, Helmcke F, Sander G, Volaufova J, Greenway F, Subramaniam P, Glancy DL. Serial echocardiographic and clinical evaluation of valvular regurgitation before, during, and after treatment with fenfluramine or dexfenfluramine and mazindol or phentermine. Obes Res. 1999;7:313–322. doi: 10.1002/j.1550-8528.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA. 2000;283:1703–1709. doi: 10.1001/jama.283.13.1703. [DOI] [PubMed] [Google Scholar]
- Jollis JG, Landolfo CK, Kisslo J, Constantine GD, Davis KD, Ryan T. Fenfluramine and phentermine and cardiovascular findings: effect of treatment duration on prevalence of valve abnormalities. Circulation. 2000;101:2071–2077. doi: 10.1161/01.cir.101.17.2071. [DOI] [PubMed] [Google Scholar]
- Davidoff R, McTiernan A, Constantine G, Davis KD, Balady GJ, Mendes LA, Rudolph RE, Bowen DJ. Echocardiographic examination of women previously treated with fenfluramine: long-term follow-up of a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2001;161:1429–1436. doi: 10.1001/archinte.161.11.1429. [DOI] [PubMed] [Google Scholar]
- Palmieri V, Arnett DK, Roman MJ, Liu JE, Bella JN, Oberman A, Kitzman DW, Hopkins PN, Morgan D, de Simone G, Devereux RB. Appetite suppressants and valvular heart disease in a population-based sample: the HyperGEN study. Am J Med. 2002;112:710–715. doi: 10.1016/S0002-9343(02)01123-3. [DOI] [PubMed] [Google Scholar]
- Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339:719–724. doi: 10.1056/NEJM199809103391102. [DOI] [PubMed] [Google Scholar]
- Weissman NJ. Appetite suppressants and valvular heart disease. Am J Med Sci. 2001;321:285–291. doi: 10.1097/00000441-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Loke YK, Derry S, Pritchard-Copley A. Appetite suppressants and valvular heart disease - a systematic review. BMC Clin Pharmacol. 2002;2:6. doi: 10.1186/1472-6904-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghatol FF, Rigolin VH. Appetite suppressants and valvular heart disease. Curr Opin Cardiol. 2002;17:486–492. doi: 10.1097/00001573-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. Am Heart J. 2002;144:1065–1073. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- Schiller NB. Fen/phen and valvular heart disease: if it sounds too bad to be true, perhaps it isn't. J Am Coll Cardiol. 1999;34:1159–1162. doi: 10.1016/S0735-1097(99)00315-0. [DOI] [PubMed] [Google Scholar]
- Khan MA, St. Peter JV, Herzog CA. Appetite-suppressant drugs and valvular heart disease [letter] N Engl J Med. 1999;340:478. doi: 10.1056/NEJM199902113400613. [DOI] [PubMed] [Google Scholar]
- Wee CC, Phillips RS, Aurigemma G, Erban S, Kriegel G, Riley M, Douglas PS. Risk for valvular heart disease among users of fenfluramine and dexfenfluramine who underwent echocardiography before use of medication. Ann Intern Med. 1998;129:870–874. doi: 10.7326/0003-4819-129-11_part_1-199812010-00005. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Weissman NJ, Leung C, Panza JA, Fernicola D, Davis KD, Constantine GD, Reid CL. Clinical and echocardiographic follow-up of patients previously treated with dexfenfluramine or phentermine/fenfluramine. JAMA. 2001;286:2011–2014. doi: 10.1001/jama.286.16.2011. [DOI] [PubMed] [Google Scholar]
- Weissman NJ, Panza JA, Tighe JF, Gwynne JT. Natural history of valvular regurgitation 1 year after discontinuation of dexfenfluramine therapy. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:267–273. doi: 10.7326/0003-4819-134-4-200102200-00009. [DOI] [PubMed] [Google Scholar]
- Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/S0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- Reid CL, Gardin JM, Yunis C, T. Kurosaki, Flack JM. Prevalence and clinical correlates of aortic and mitral regurgitation in a young adult population: The CARDIA Study [abstract] Circulation. 1994;90 (suppl I):I–282. [Google Scholar]
- Fleiss JL. Statistical Methods for Rates and Proportions. Second. New York, John Wiley & Sons; 1981. [Google Scholar]
- Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research. Principles and Quantitative Methods. New York, Van Nostrand Reinhold Company, Inc.; 1982. [Google Scholar]
- Khan LK, Serdula MK, Bowman BA, Williamson DF. Use of prescription weight loss pills among U.S. adults in 1996-1998. Ann Intern Med. 2001;134:282–286. doi: 10.7326/0003-4819-134-4-200102200-00011. [DOI] [PubMed] [Google Scholar]
- Moye LA, Annegers AF. Underestimation of the valvulopathy effect of fenfluramine. J Am Coll Cardiol. 2000;36:1434–1436. doi: 10.1016/S0735-1097(00)00835-4. [DOI] [PubMed] [Google Scholar]
- Mast ST, Jollis JG, Ryan T, Anstrom KJ, Crary JL. The progression of fenfluramine-associated valvular heart disease assessed by echocardiography. Ann Intern Med. 2001;134:261–266. doi: 10.7326/0003-4819-134-4-200102200-00008. [DOI] [PubMed] [Google Scholar]
- Volmar KE, Hutchins GM. Aortic and mitral fenfluramine-phentermine valvulopathy in 64 patients treated with anorectic agents. Arch Pathol Lab Med. 2001;125:1555–1561. doi: 10.5858/2001-125-1555-AAMFPV. [DOI] [PubMed] [Google Scholar]
- Voss LM, Wilson NJ, Neutze JM, Whitlock RM, Ameratunga RV, Cairns LM, Lennon DR. Intravenous immunoglobulin in acute rheumatic fever: a randomized controlled trial. Circulation. 2001;103:401–406. doi: 10.1161/01.cir.103.3.401. [DOI] [PubMed] [Google Scholar]