Abstract
Tetracyclines have recently been shown to have “chondroprotective” effects in inflammatory arthritides in animal models. Since nitric oxide (NO) is spontaneously released from human cartilage affected by osteoarthritis (OA) or rheumatoid arthritis in quantities sufficient to cause cartilage damage, we evaluated the effect of tetracyclines on the expression and function of human OA-affected nitric oxide synthase (OA-NOS) and rodent inducible NOS (iNOS). Among the tetracycline group of compounds, doxycycline > minocycline blocked and reversed both spontaneous and interleukin 1β-induced OA-NOS activity in ex vivo conditions. Similarly, minocycline ≥ doxycycline inhibited both lipopolysaccharide- and interferon-γ-stimulated iNOS in RAW 264.7 cells in vitro, as assessed by nitrite accumulation. Although both these enzyme isoforms could be inhibited by doxycycline and minocycline, their susceptibility to each of these drugs was distinct. Unlike acetylating agents or competitive inhibitors of l-arginine that directly inhibit the specific activity of NOS, doxycycline or minocycline has no significant effect on the specific activity of iNOS in cell-free extracts. The mechanism of action of these drugs on murine iNOS expression was found to be, at least in part, at the level of RNA expression and translation of the enzyme, which would account for the decreased iNOS protein and activity of the enzyme. Tetracyclines had no significant effect on the levels of mRNA for β-actin and glyceraldehyde-3-phosphate dehydrogenase nor on levels of protein of β-actin and cyclooxygenase 2 expression. These studies indicate that a novel mechanism of action of tetracyclines is to inhibit the expression of NOS. Since the overproduction of NO has been implicated in the pathogenesis of arthritis, as well as other inflammatory diseases, these observations suggest that tetracyclines should be evaluated as potential therapeutic modulators of NO for various pathological conditions.
Nitric oxide (NO), a multifunctional mediator produced by and acting on various cells, participates in inflammatory and autoimmune-mediated tissue destruction. NO is produced by a family of ubiquitous enzymes, nitric oxide synthases (NOSs). The overexpression of NOS in a variety of inflammatory tissues has led many to conclude that the modulation of NO synthesis and action could represent a new approach to the treatment of inflammatory and autoimmune conditions (1, 2). Where examined, NO formation is found to be increased in autoimmune diseases [rheumatoid arthritis (RA), systemic lupus erythematosus, ulcerative colitis, and Crohn disease], and several classic inflammatory symptoms (erythema and vascular leakiness) are reversed by NOS inhibitors (2, 3, 4). The most compelling evidence for NO as a mediator of tissue injury has been in arthritis, based on studies carried out in animal models (5, 6), human osteoarthritis (OA) (7), and RA (8).
We have recently observed that human OA-affected cartilage can spontaneously release NO under ex vivo conditions in quantities sufficient to cause cartilage damage (7). The human OA-affected NOS (OA-NOS) is overexpressed in OA-affected cartilage and not detectable in normal cartilage. The inducible OA-NOS has properties similar to neuronal NOS (based on its molecular weight and antibody cross-reactivity among anti-NOS antibodies) and the 133-kDa inducible NOS [iNOS; sensitive to NF-κB and cycloheximide, up-regulated by interleukin (IL) 1β, tumor necrosis factor α, and lipopolysaccharide (LPS)]. NO is known to potentiate matrix degradation, which includes inhibition of proteoglycan and collagen type II synthesis (9, ∥) and up-regulation of metalloprotease activity (10).
Doxycycline and minocycline are members of the tetracycline family of broad-spectrum antibiotics. During recent years, it has been established that tetracyclines, which are rapidly absorbed and have a prolonged half-life, exert biological effects independent of their antimicrobial activity (11, 12, 13). Such effects include inhibition of matrix metalloproteases (MMPs) [including collagenase (MMP-1), gelatinase (MMP-2), and stromelysin (MMP-3) activity] and prevention of pathogenic tissue destruction (11). Furthermore, recent studies have also suggested that tetracyclines and inhibitors of metalloproteases inhibit tumor progression (14), bone resorption (15), and angiogenesis (16) and may have antiinflammatory properties (17).
In RA, these matrix metalloproteases have been identified in the synovial tissue, synovial fluids, and the proliferative pannus (18). These metalloproteases are also known to be up-regulated in OA-affected joints (19, 20). Interestingly, Yu et al. (21) have also shown that prophylactic administration of doxycycline markedly reduced the severity of OA in dog models. In humans, minocycline (a semisynthetic tetracycline) has recently been demonstrated to be superior to placebo in the treatment of RA (22).
Since NO is a putative mediator of inflammation that exerts catabolic effects on cartilage, including the activation of metalloproteases (10), we evaluated the action of tetracycline compounds on the spontaneous release of NO from OA-affected human cartilage in ex vivo conditions (7) and on iNOS in LPS-stimulated murine macrophages. Both these enzyme isoforms show distinct susceptibility to pharmacological intervention by hydrocortisone and transforming growth factor β (TGF-β) in vitro (7). In the present study we report that (i) doxycycline and minocycline inhibit the activity of murine macrophage iNOS (minocycline ≥ doxycycline) and human inducible OA-NOS (doxycycline > minocycline), (ii) doxycycline and minocycline inhibit iNOS expression at the level of iNOS mRNA and protein expression, thereby down-regulating its specific activity, and (iii) unlike acetylating agents or competitive inhibitors of l-arginine, doxycycline and minocycline do not directly inhibit the catalytic activity of iNOS in vitro in the l-arginine → l-citrulline conversion assay.
MATERIALS AND METHODS
Cell Lines and Reagents.
Murine macrophage cells (RAW 264.7) were obtained from the American Type Culture Collection. Anti-murine iNOS and anti-cyclooxygenase (COX-2) antibodies were obtained from Transduction Laboratories (Lexington, KY). OA-affected cartilage was obtained from OA patients who underwent knee replacement surgery and were free of steroidal/nonsteroidal antiinflammatory drugs for at least 2 weeks before surgery. Doxycycline, minocycline, hydrocortisone, and LPS were obtained from Sigma, and murine interferon (IFN)-γ and human IL-1β were from Promega.
Assay of OA-NOS in Organ Cultures.
This assay was basically carried out as described (7). Briefly, OA-affected cartilage was cut into 3-mm discs; four to six discs (100–200 mg) were placed in organ culture in 2 ml of medium (F-12 with 0.1% BSA) for 24–72 h in the incubator. The medium was analyzed for nitrite accumulation by modified Griess reaction (23).
Western Blot Analysis.
Equal amounts of protein (25–50 μg) estimated by BCA reagent (Pierce) were loaded onto SDS/PAGE gels and examined by Western blot analysis with a specific anti-iNOS or anti-cyclooxygenase 2 (COX-2) murine mAb as specified by Transduction Laboratories. Membranes with bound antibodies (e.g., iNOS) were stripped by submersion in stripping buffer (100 mM 2-mercaptoethanol/2% SDS/62.5 mM Tris·HCl, pH 6.7) and incubating at 50°C for 30 min with occasional agitation. Membranes were then washed twice for 10 min at room temperature using large volumes of wash buffer. This filter could then be reprobed with an anti-actin antibody provided by James L. Lessard (Children’s Hospital Medical Center, Cincinnati). Blots were developed using the ECL Western blot system (Amersham). Quantitation of the bands was performed using a densitometer from Molecular Dynamics.
Northern Blot Analysis.
Total RNA was isolated using TRI reagent (MRC, Cincinnati). Northern blot analysis was carried out as described (24, 25). Briefly, 30 μg of RNA was subjected to electrophoresis and transferred via capillary action onto a nylon membrane (Zeta-Probe, Bio-Rad). The membrane was hybridized with [32P]dCTP-labeled iNOS cDNA (4-kb SmaI fragment), a gift from James Cunningham (Harvard Medical School), and the blot was exposed to Kodak x-ray film for 24–48 h with intensifying screens at −70°C. The β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes were purchased from CLONTECH and probed as described above. Quantitation was performed using a PhosphorImager or Personal Densitometer (SI) (Molecular Dynamics).
Reverse Transcription-Coupled PCR (RT–PCR) Analysis.
The presence of iNOS and β-actin mRNA in cells was analyzed by RT of total RNA followed by PCR amplification of the cDNA as described (26, 27). The sense and antisense oligonucleotides for iNOS were, respectively, 5′-ACGGAGAAGCTTAGATCTGGAGCAGAAGTG-3′ (nt 142–171) and 5′-CTGCAGGTTGGACCACTGGATCCTGCCGAT-3′ (nt 767–796). The sense and antisense primers for β-actin were 5′-TCCTTCGTTGCCGGTCCACA-3′ (nt 44–63) and 5′-CGTCTCCGGAGTCCATCACA-3′ (nt 534–552), respectively. The predicted PCR product of the iNOS cDNA was 654 bp; that of the β-actin cDNA was 508 bp. The cDNA was prepared from equal amounts (1 μg) of total RNA using SuperScript RNase H reverse transcriptase (GIBCO/BRL). An equal amount of the cDNA was used to amplify the mRNA by PCR. PCR amplification was performed in 50 μl of a solution containing 1.5 mM MgCl2, 500 ng of iNOS primer, 100 ng of β-actin primer, all four dNTPs (each at 0.2 mM), 2.5 units of Taq DNA polymerase (GIBCO/BRL). The cycle conditions for amplification of cDNA were 1 min at 94°C, 1–2 min at 55°C, and 3 min at 72°C for 30 cycles for both iNOS and β-actin.
Assays for iNOS in Cell-Free Extracts.
Specific activity of iNOS was determined in cell-free extracts by monitoring the conversion of l-[3H]arginine to l-[3H]citrulline as described (25, 28). RAW 264.7 cells were induced with LPS (100 ng/ml) in the presence and absence of tetracyclines or hydrocortisone for 14–20 h. The cell-free extracts were prepared in Tris buffer (10 mM, pH 7.4) containing chymostatin at 10 μg/ml, antipain at 10 μg/ml, leupeptin at 10 μg/ml, pepstatin at 10 μg/ml, 1 mM DTT, and 1 mM phenylmethylsulfonyl fluoride (25). The protein was measured by BCA assay reagent using BSA as standard (29). The reaction mixture for iNOS assay was as described (25, 28). After 20 min, the assays were terminated by heating the reaction mixture at 90°C for 5 min; 10 μl (≈100,000 cpm) of the supernatant was spotted on activated Avicel TLC plates (Analtech Associates). The TLC plates were developed in a solvent system consisting of ethanol/water/ammonia, 80:16:4 (vol/vol). Quantitation of the spot for l-[3H]citrulline was performed by a Bioscan System 200 imaging scanner.
RESULTS AND DISCUSSION
Effect of Doxycycline and Minocycline on Inducible OA-NOS Activity in Human OA-Affected Cartilage.
Studies in our laboratory have shown (7) that OA-affected cartilage spontaneously releases NO in ex vivo conditions sufficient to cause cartilage damage. NO has dual effects on matrix metabolism: not only does it potentiate activity of matrix-degrading metalloproteases (10) but also it inhibits synthesis of matrix components such as proteoglycans and collagen (9, ∥) . Therefore, we first examined whether doxycycline or minocycline could block human OA-NOS activity in ex vivo conditions. Generally accepted pharmacologically relevant concentrations were selected for this study based on previous reports (19, 30, 31, 32). OA-affected cartilage was obtained from patients with advanced OA undergoing knee replacement surgery. OA-affected cartilage slices (Fig. 1A) were incubated in 0.1% BSA/endotoxin-free medium with doxycycline or minocycline at 5–80 μg/ml for 24, 48, and 72 h in ex vivo conditions. Activity of NOS was monitored at different time intervals by estimating nitrite, the stable end-product, as described (7). The amount of NO spontaneously released (as measured by nitrite accumulation) at 0, 24, 48, and 72 h was 0, 4.8 ± 0.38, 16.4 ± 0.7, and 17.8 ± 0.9 μM, respectively. The results showed that doxycycline and minocycline significantly inhibited NO production in OA-affected cartilage in a dose-dependent manner (Fig. 1). These data also indicate that doxycycline was more potent in its ability to inhibit OA-NOS activity. For example, at 72 h, the IC50 for doxycycline was 32 μg/ml compared with 54 μg/ml for minocycline. These experiments further indicate that doxycycline and minocycline not only blocked the ongoing production of NO by OA-NOS ex vivo but also caused a decline in nitrite accumulation in cartilage organ culture for at least 72 h under conditions in which nitrite continues to accumulate in control cultures (Fig. 1). In a separate experiment, OA-affected cartilage was also exposed to IL-1β at 5 ng/ml, which augmented the release of nitrite from 3.6 ± 1.7 to 16.7 ± 2.5 μM (P < 0.0001) at 72 h. Addition of doxycycline at 20 and 40 μg/ml at the time of the IL-1β stimulation decreased the nitrite levels to 13.9 ± 2.0 μM (P < 0.06) and 8.5 ± 1.3 μM (P < 0.0006), respectively, when compared with the IL-1β-stimulated cartilage slices at the same time interval. Similarly, addition of minocycline (at 20 and 40 μg/ml) showed 16.6 ± 3.2 (P < 0.47) and 10.0 ± 2.0 (P < 0.0024) μM of nitrite accumulation when compared with the IL-1β-stimulated cartilage slices. These experiments also demonstrated that the IC50 levels for tetracyclines to inhibit NOS activity augmented by IL-1β were similar to the spontaneous release of NO. The concentrations of doxycycline that inhibited NO production in our studies are comparable to those required for the inhibition of matrix metalloproteases (19, 30, 31, 32, 33). In those studies, doxycycline at 20–50 μg/ml inhibited the activity of proteolytic enzymes such as collagenase and gelatinase, blocked proteoglycan degradation, reduced the cell death associated with proteoglycan loss, and augmented cartilage growth (31).
Figure 1.
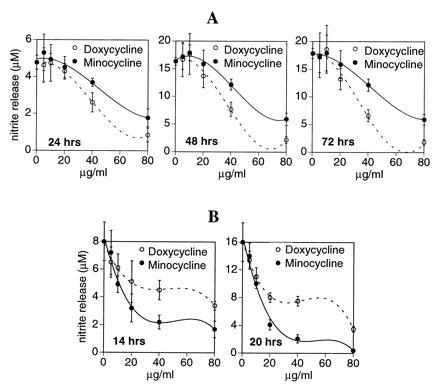
(A) Effect of doxycycline and minocycline on OA-NOS expression in human OA-affected cartilage. OA-affected knee articular cartilage from one OA-affected patient was placed in organ culture in 2 ml of medium in the presence or absence (control) of doxycycline and minocycline at 5–80 μg/ml. The spontaneous release of nitrite was monitored at different time intervals. Data are expressed as micromolar nitrite released (mean ± SD, n = 3–4). Zero time indicates spontaneous release of NO at 24, 48, and 72 h, which was 4.8 ± 0.38, 16.4 ± 0.7, and 17.8 ± 0.9, respectively. Statistics were derived using unpaired Student’s t test. Data represent one of three identical experiments with samples from different patients. (B) Effect of doxycycline and minocycline on nitrite accumulation in RAW 264.7 cells stimulated with LPS. Murine macrophage cells (RAW 264.7) were incubated with doxycycline or minocycline (5–80 μg/ml) for 1–2 h followed by addition of LPS at 100 ng/ml. After 14–20 h of incubation, the medium was used to estimate nitrite accumulation by the modified Griess reaction (23). Data are expressed as micromolar of nitrite accumulated at a given time interval (n = 3). Statistics were derived using unpaired Student’s t test. Data represent one of three similar experiments. Graphs were plotted using the delta graph curve-fitting program: polynomial of degree 3.
Effects of Doxycycline and Minocycline on iNOS in Murine Macrophages.
Our recent studies have indicated that human inducible OA-NOS is distinct from murine and human iNOS, based upon its size, immunoreactivity, and susceptibility to TGF-β and hydrocortisone (7). Therefore, we also evaluated the effect of tetracyclines on the expression of iNOS in murine macrophages. RAW 264.7 cells were activated with LPS (100 ng/ml) to induce iNOS (34) with and without doxycycline and minocycline at 5–80 μg/ml. Fig. 1B shows a concentration-dependent inhibition of nitrite accumulation in cells stimulated with LPS in the presence of doxycycline at 5–80 μg/ml at 14 and 20 h of incubation. The IC50 of doxycycline in this experiment was 72 μg/ml at 14 h and 22 μg/ml at 20 h of incubation in these cells. In the same set of experiments, minocycline was also administered at concentrations ranging from 5 to 80 μg/ml. The IC50 for minocycline was 17 μg/ml at 14 h and 12 μg/ml at 20 h of incubation in RAW 264.7 cells stimulated with LPS. Although a marginal difference in the potency of doxycycline and minocycline was seen at 20 h of incubation (based on IC50), significantly higher concentrations of doxycycline, as compared with minocycline, were generally required to inhibit iNOS by >50% at both time intervals. We also examined the effect of minocycline or doxycycline at 20 μg/ml on murine iNOS expression when stimulated with IFN-γ at 100 units/ml for 16 h (which induced 16.4 ± 0.5 μM nitrite). Addition of minocycline and doxycycline significantly decreased IFN-γ-induced nitrite production to 9.7 ± 0.3 μM (P < 0.0001) and 13.1 ± 1.1 μM (P < 0.005), respectively.
These studies indicate that both doxycycline and minocycline inhibit NO production in murine macrophages stimulated with either LPS or IFN-γ. Furthermore, these experiments together with our prior observations (7) show that the iNOS and inducible OA-NOS have distinct susceptibility to doxycycline, minocycline, TGF-β, and hydrocortisone. This is not surprising since it has been reported that two different forms of collagenase, MMP-8 (IC50, 7–15 μg/ml) and MMP-1 (IC50, 140 μg/ml), in two different cell types (i.e., neutrophils and fibroblasts), show distinct susceptibility to inhibition by tetracyclines (35). Furthermore, it should be noted that the same enzyme expressed in two closely related cell lines can have differential susceptibility to tetracyclines. For example, two osteoblastic cell lines, UMR 106–01 (IC50, >200 μg/ml) and ROS 17/2.8 (IC50, 20–30 μg/ml), showed differential susceptibility to doxycycline when evaluated for gelatinase activity (36). Finally, another factor that may contribute to the differential IC50 values of tetracyclines on NOS activity in cartilage slices compared with macrophage cells is the ability of each drug to penetrate the cartilage matrix and act on chondrocytes (37).
In view of the recent observation that in vivo administration of minocycline in rats augments the percentage of splenocytes exhibiting a rise in intracellular Ca2+ under ex vivo conditions upon stimulation with concanavalin A, depending on the concentration of Ca2+ in the medium, we examined the effect of extracellular Ca2+ on the influence of iNOS expression upon stimulation with LPS (38). RAW 264.7 cells were supplemented (−1 h) with an additional 0, 0.34, and 0.68 mM Ca2+ above that present in the medium (1.3 mM) and stimulated with LPS. The nitrite levels at 24 h were 24.5 ± 4.9, 26.6 ± 1.8, and 27.3 ± 0.3 μM in medium supplemented with 0, 0.34 mM, and 0.68 mM Ca2+, respectively. Addition of LPS and minocycline at 40 μg/ml at the time of the addition of Ca2+ (−1 h) reduced the nitrite levels to 8.8 ± 1.04 (P < 0.0029), 12.7 ± 0.7 (P < 0.007), and 12.1 ± 0.9 (P < 0.006) μM, for the 0, 0.34 mM, and 0.68 mM Ca2+-supplemented cultures, respectively. Thus, there was no significant difference between the Ca2+-supplemented and nonsupplemented controls, indicating that supplemental Ca2+ does not have a significant influence on the tetracycline-dependent NOS inhibition in the murine macrophages tested in vitro. It should be noted that tetracyclines also inhibit IL-1β- and IFN-γ-induced NOS expression; unlike LPS, these cytokines do not flux extracellular Ca2+ into the cells upon activation (39, 40). These experiments indicate that tetracyclines do not exert their effects on NOS via the chelation of extracellular Ca2+. However, it should be noted that intracellular Ca2+ is critical for the enzyme activity of NOS, which usually appears 3–4 h after LPS stimulation (3).
Based on the above studies, we sought to evaluate the mechanism of action of tetracyclines on NOS expression in the murine macrophage model where the biochemistry, enzymology, and molecular biology of iNOS are well-characterized (3, 34, 41, 42). We did not perform similar studies of OA-NOS since it was not possible to precisely and reproducibly quantitate the expression of OA-NOS directly from the OA-affected cartilage without disturbing the architecture of the cartilage, which plays a significant role in chondrocyte function.
Using LPS-induced RAW 264.7 cells, we examined the following hypotheses. Tetracyclines may (i) decrease only the catalytic activity of iNOS without influencing the expression of iNOS protein; (ii) decrease both the catalytic activity of iNOS and the expression of iNOS protein, which in turn cumulatively leads to decrease in the accumulation of nitrite in the medium; or (iii) decrease the expression of iNOS protein and, therefore, the production of nitrite.
Effect of Doxycycline and Minocycline on the Enzyme Activity of iNOS in Whole Cells.
RAW 264.7 cells were exposed to doxycycline or minocycline in the presence of LPS for 16–18 h; cell-free extracts prepared at the end of each time period were evaluated for iNOS enzyme activity using the l-arginine → l-citrulline conversion assay in total cell extracts. As shown in Fig. 2, preexposure of cells to either doxycycline or minocycline inhibits the conversion of arginine to citrulline in cell lysates in a dose-dependent manner when compared with the control LPS-stimulated activity. Doxycycline at 20, 40, and 80 μg/ml significantly reduced citrulline accumulation by 57%, 72%, and 85%, respectively; similar inhibition was also observed for minocycline (45%, 69%, and 69%, respectively). As expected, pretreatment of cells with 10 μM hydrocortisone and 75 μM l-N-monomethyl arginine (l-NMMA) blocked iNOS activity by 60% and 64%, respectively.
Figure 2.
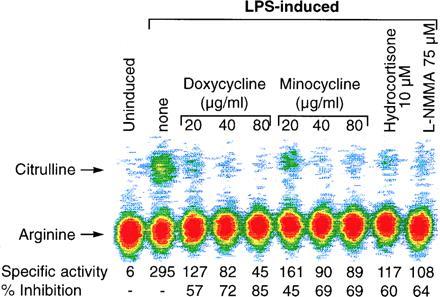
Effect of doxycycline and minocycline on iNOS enzyme activity in RAW 264.7 cells stimulated with LPS. Murine macrophage cells (RAW 264.7) were incubated with doxycycline or minocycline (20–80 μg/ml), hydrocortisone (10 μM), or l-NMMA (75 μM) for 1 h, followed by addition of LPS at 100 ng/ml for 16–18 h. The cell-free extracts were prepared and the l-arginine → l-citrulline conversion assay was carried out in cell-free extracts. The data have been represented as specific activity of the enzyme, which was defined as pmol of citrulline per min per mg of protein. Percent inhibition represents comparison with the LPS-stimulated cells in the absence of any modulator. Data represent one of three similar experiments.
Direct Effect of Doxycycline and Minocycline on iNOS Enzyme Activity in Cell-Free Extracts.
Recent studies have indicated that tetracyclines inhibit collagenase activity via direct effects on the enzyme (30, 43), which could be partially reversed by addition of Ca2+ to the reaction mixture (44). Another mechanism proposed for this phenomenon is that procollagenase is reduced to inactive fragments upon activation in the presence of doxycycline (43). We have recently shown that acetylating agents, such as aspirin and N-acetylimidazole (25), as well as competitive inhibitors of l-arginine (2, 3, 4), inhibit iNOS catalytic activity in vitro. To evaluate the direct effect of doxycycline and minocycline on iNOS enzyme activity, we induced RAW 264.7 cells with LPS for 16 h in the absence of these agents and prepared cell-free extracts as a source of iNOS enzyme in l-arginine → l-citrulline conversion assay. Separate aliquots of equal amounts of enzyme (in total cell extracts) were preincubated for 15 min with doxycycline at 20–80 μg/ml, minocycline at 20–80 μg/ml, 1 mM N-acetylimidazole, and 200 μM l-NMMA, respectively, before the enzyme reaction was initiated by adding the cofactors. The experiment showed that, unlike N-acetylimidazole or l-NMMA, doxycycline/minocycline had no significant effect directly on the units of enzyme activity or specific activity of iNOS in cell-free extracts (Fig. 3). These experiments indicate that the action of these drugs on iNOS seems to be distinct from that reported for metalloproteases such as procollagenases (30, 43, 45). Minocycline and doxycycline could not block an ongoing l-arginine → l-citrulline reaction catalyzed by iNOS in cell-free extracts. Furthermore, these experiments also indicate that, unlike the inhibition of collagenase activity, which can be reversed by addition of 10–50 μM Ca2+ in the enzyme assay (44), the tetracyclines exert no significant effect on the iNOS catalytic activity in cell-free extracts, and therefore, action via their Ca2+-chelating properties can be excluded.
Figure 3.
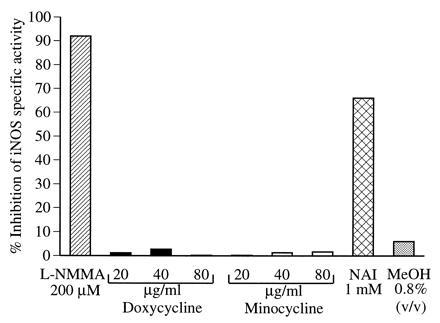
Effect of doxycycline and minocycline on the specific activity of iNOS in vitro. RAW 264.7 cells were stimulated with LPS for 16 h and cell-free extracts were prepared as a source of iNOS. Various modulators including methanol (MeOH), the carrier for N-acetylimidazole (NAI), were added 15 min prior to the addition of the cofactors to initiate the iNOS reaction. The specific activity was calculated and percent inhibition compared with the original LPS-stimulated extract; 100% specific activity was 200 pmol of citrulline per min per mg of protein. Data represent one of two similar experiments.
Effect of Doxycycline and Minocycline on iNOS Protein Expression in Murine Macrophages.
Based on the above data, which indicated that tetracyclines did not directly affect the specific activity of NOS, we assumed that the decrease in specific activity in the cells preincubated with tetracyclines might be due to a decrease specifically in the iNOS protein in the total cell extracts. Therefore, we examined iNOS protein expression. RAW 264.7 cells stimulated with LPS in the presence and absence of various concentrations of doxycycline and minocycline were incubated for 16 h; cell-free extracts were prepared. The extracts were examined for 133-kDa iNOS by Western blot analysis, using specific antibodies. Fig. 4 shows a dose-dependent inhibition of iNOS protein expression (IC50, <20 μg/ml) in the presence of both doxycycline and minocycline. There was no significant total effect on the levels of β-actin synthesis (data not shown) and COX-2 expression (that is also induced with iNOS by LPS) in the same samples treated with doxycycline or minocycline at 20–40 μg/ml, thus indicating that the effects of doxycycline and minocycline on iNOS are not nonspecific. It should be noted that the IC50 values for the inhibition of nitrite accumulation and iNOS protein expression for each tetracycline derivative were similar, consistent with the thesis that the inhibition of protein expression accounted for the inhibition of NOS-specific activity. Indeed, since both drugs inhibited iNOS protein expression but failed to block l-arginine → l-citrulline conversion in cell-free extracts, we conclude that decrease in unit activity of iNOS is principally due to inhibition of iNOS protein expression, which accounts for the decreased accumulation of nitrite in cells treated with doxycycline and minocycline.
Figure 4.
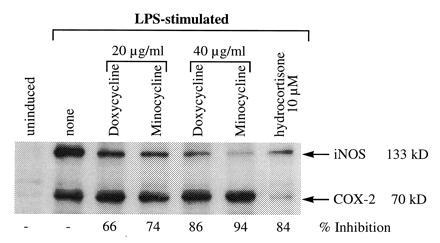
Western blot analysis of iNOS in RAW 264.7 cells exposed to doxycycline or minocycline in the presence of LPS. RAW 264.7 cells activated with LPS (100 ng/ml) for 16–18 h, with and without doxycycline or minocycline (20–40 μg/ml) and hydrocortisone (10 μM) were analyzed for total iNOS and COX-2 protein. Briefly, 30 μg of cell extract was loaded onto SDS/PAGE gels and the filter probed simultaneously with anti-iNOS and anti-COX-2 mouse mAb followed with an anti-mouse serum conjugated to horseradish peroxidase on the same filters. Bands were quantitated on a densitometer and the percent inhibition was compared with the LPS-stimulated cells. Data represent one of four similar experiments.
Effect of Doxycycline and Minocycline on Expression of iNOS mRNA.
Doxycycline and minocycline may suppress iNOS before synthesis of the enzyme, leading to inhibition of iNOS protein expression and specific activity and accumulation of nitrite. This assumption is based on the observation that, in macrophages, TGF-β1, cyclosporin, hydrocortisone, NF-κB inhibitors, and to some extent Fe2+ suppress iNOS expression by decreasing mRNA expression and subsequently the rate of translation of iNOS protein (3, 46). In addition, recent studies by Pfeilschifter et al. (47) have shown that dexamethasone acts at multiple levels (including transcription of iNOS) to suppress IL-1β-induced iNOS expression in mesangial cells. Therefore, to determine the level at which tetracycline inhibited iNOS protein expression, RAW 264.7 cells treated with LPS for 16 h in the presence or absence of doxycycline and minocycline were analyzed for iNOS mRNA accumulation by RT–PCR after normalizing the concentrations of the total RNA. Doxycycline and minocycline, at concentrations (20–40 μg/ml) that also inhibited iNOS protein expression, consistently and significantly decreased (>50%) iNOS mRNA accumulation (Fig. 5). As expected in both experiments, hydrocortisone-treated cells showed decreased iNOS mRNA (50–100%), as compared with LPS-stimulated cells. The iNOS PCR signals were normalized with β-actin as shown in Fig. 5 A and B.
Figure 5.
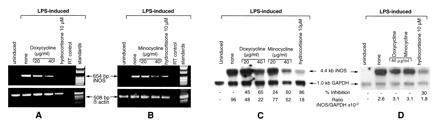
Analysis of iNOS mRNA expression by RT–PCR and Northern blot in the presence and absence of tetracyclines. (A and B) RT–PCR analysis of iNOS and β-actin mRNA expression in RAW 264.7 cells was carried out after stimulation with LPS ± doxycycline, minocycline, or hydrocortisone. Equal amounts of RNA were analyzed for iNOS and β-actin expression. “RT control” designates preparation of RT–PCRs in the absence of reverse transcriptase using the LPS-stimulated RNA as the template from RAW 264.7 cells. Data represent one of two similar and separate experiments. (C and D) Northern blot analysis of iNOS and GAPDH mRNA expression in RAW 264.7 cells was carried out after stimulation with LPS ± doxycycline, minocycline, or hydrocortisone at 16 h (C) or 4 h (D) after stimulation. The iNOS/GAPDH signal was determined and quantitated using both a PhosphoImager and a densitometer. The percent inhibition of iNOS expression was normalized with the GAPDH signal and compared with the values of the LPS-stimulated cells. Data represent one of three similar experiments.
In a separate set of experiments (Fig. 5C), Northern blot analysis of iNOS and GAPDH mRNA was also carried out to evaluate precisely the effect of iNOS expression in the presence of doxycycline and minocycline. The cells were preincubated with tetracyclines, stimulated with LPS for 16 h, and analyzed for iNOS mRNA expression. These experiments also showed that doxycycline (20–40 μg/ml) and minocycline (20–40 μg/ml) significantly blocked iNOS mRNA accumulation in a dose-dependent manner with no effects on the GAPDH mRNA accumulation. These experiments indicate that the action of doxycycline and minocycline on iNOS is also at the level of iNOS mRNA expression, which contributes to decreased NOS proteins and specific activity of the enzyme in whole cell extracts.
We further examined whether tetracyclines inhibit iNOS transcription or render the iNOS mRNA more susceptible to degradation—or both. It is quite possible that due to the broad spectrum of effects of doxycycline and minocycline on various enzymes and cellular functions, a common target (such as NF-κB) cannot be ruled out. Inhibitors of transcription factor NF-κB (e.g., pyrrolidine dithiocarbamate or hydrocortisone) showed a decrease in the accumulation of iNOS mRNA in RAW 264.7 cells within 4 h after LPS stimulation (3). Cells preincubated (−1 h) with doxycycline or minocycline at 40 μg/ml were stimulated with LPS. The total RNA was extracted after 4 h and analyzed for iNOS expression by RT–PCR (data not shown) and Northern blot analysis (Fig. 5D). Tetracyclines (40 μg/ml) had no significant effect on the iNOS mRNA expression when compared with the LPS-stimulated cells. However, hydrocortisone-treated cells showed ≈30% inhibition in iNOS mRNA accumulation when compared with LPS-stimulated cells after normalizing the values with GAPDH. It should be noted that the iNOS/GAPDH ratio at 4 h was high due to the preferential accumulation of iNOS mRNA after LPS stimulation; the ratio had decreased at 16 h due to the low steady state of iNOS mRNA production when compared with 4 h (3, 46). These experiments indicate that tetracyclines may not interfere in the early events of iNOS transcription as observed with hydrocortisone but may, like TGF-β (46), render the iNOS mRNA susceptible to degradation (seen at ≈12 h), which is evident by the decrease in iNOS mRNA accumulation at 16 h after stimulation with LPS in the presence of tetracyclines (Fig. 5D).
Tetracyclines at pharmacological concentrations did achieve >50% inhibition of NOS activity in our studies. These compounds therefore differ from competitive inhibitors of the enzyme (e.g., l-NMMA), which can inhibit NOS activity >95%. This is important because even modest effects (10–50%) of NOS inhibitors in vivo can have profound attenuating effects on inflammatory events (e.g., paw swelling), as shown in animal models of arthritis (6).
Our data and previous findings by others indicate that tetracyclines exert pleiotropic functions independent of their antimicrobial activity, which include inhibition of MMPs, NOS expression, NO production, tumor progression, bone resorption (14, 15), angiogenesis (16), and inflammation (17). We speculate that the pleiotropic properties of tetracyclines (48) may be partially attributed to their ability to target another multifunctional signaling molecule, NO, that is known to exert similar effects on many of the pathological conditions and manifestations listed above (1, 2, 10, 49, 50, 51, 52). Our studies also indicate that tetracyclines, at similar IC50 values, exert dual effects on MMPs: (i) direct inhibition of the specific activity of MMPs at the enzyme level (30, 43); (ii) inhibition of NO production, which has been reported to up-regulate MMP activity (10). In addition, since NO is known to mediate several catabolic activities of IL-1β on cartilage (9, ‖, 10), the inhibition of NOS activity by tetracyclines may, therefore, exert additional protective effects on cartilage degradation in arthritis.
Our studies indicate that tetracyclines inhibit iNOS activity not via a direct inhibition at the enzyme level (as reported for the metalloproteases) but through an inhibition of NOS mRNA expression, which leads to the decrease in protein expression and NOS activity. This unique property of tetracyclines makes them promising candidates as safe and acceptable modulators of NO for various pathological conditions.
Acknowledgments
We thank Dr. James Cunningham for his gift of an iNOS cDNA probe, Dr. James Lessard for anti-actin antibody, and Ms. Ann Rupel for preparation of the manuscript.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: NOS, nitric oxide synthase; iNOS, inducible NOS; l-NMMA, l-N-monomethyl arginine; OA, osteoarthritis; RA, rheumatoid arthritis; IL-1β, interleukin 1β; LPS, lipopolysaccharide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; COX-2, cyclooxygenase 2; MMP, matrix metalloprotease; TGF-β, transforming growth factor β; RT–PCR, reverse transcription-coupled PCR; IFN-γ, interferon γ.
Cao, M., Westerhausen-Larson, A., Niyibizi, C., Kavalkovich, K., Georgescu, H. I., Rizzo, C. F., Stefanovic-Racic, M. & Evans, C. H. (1996) 42nd Annual Meeting of the Orthopedic Research Society, p. 533 (abstr.).
References
- 1.Vane J R, Mitchell J A, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby D A. Proc Natl Acad Sci USA. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt H H H W, Walter U. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Xie Q. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 4.Marletta M A. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 5.McCartney-Francis N, Allen J B, Mizel D E, Albina J E, Xie Q W, Nathan C F, Wahl S M. J Exp Med. 1993;178:749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanovic-Racic M, Meyers K, Meschter C, Coffey J W, Hoffman R A, Evans C H. Arthritis Rheum. 1994;37:1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- 7.Amin A R, Di Cesare P, Vyas P, Attur M, Tzeng E, Billiar T R, Stuchin S A, Abramson S B. J Exp Med. 1995;182:2097–2102. doi: 10.1084/jem.182.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai H, Kohsaka H, Liu M F, Higashiyama H, Hirata Y, Kanno K, Saito I, Miyasaka N. J Clin Invest. 1995;96:2357–2363. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C E. Biochem Biophys Res Commun. 1994;200:142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 10.Murrell G A C, Jang D, Williams R J. Biochem Biophys Res Commun. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 11.Golub L M, Ramamurthy N S, McNamara T F. Crit Rev Oral Biol Med. 1991;2:297–322. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 12.Golub L M, Sorsa T, Suomalainen K. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- 13.Uitto V J, Firth J D, Nip L, Golub L M. Ann NY Acad Sci. 1994;732:140–151. doi: 10.1111/j.1749-6632.1994.tb24731.x. [DOI] [PubMed] [Google Scholar]
- 14.DeClerck Y A, Shimada H, Taylor S M, Langley K E. Ann NY Acad Sci. 1994;732:222–232. doi: 10.1111/j.1749-6632.1994.tb24738.x. [DOI] [PubMed] [Google Scholar]
- 15.Rifkin B R, Vernillo A T, Golub L M, Ramamurthy N S. Ann NY Acad Sci. 1994;732:165–180. doi: 10.1111/j.1749-6632.1994.tb24733.x. [DOI] [PubMed] [Google Scholar]
- 16.Maragoudakis M E, Peristeris P, Missirlis E, Aletras A, Andriopoulou P, Haralabopoulos G. Ann NY Acad Sci. 1994;732:280–293. doi: 10.1111/j.1749-6632.1994.tb24743.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramamurthy N, Greenwald R, Moak S, Scuibba J, Goren A, Turner G, Rifkin B, Golub L. Ann NY Acad Sci. 1994;732:427–430. doi: 10.1111/j.1749-6632.1994.tb24775.x. [DOI] [PubMed] [Google Scholar]
- 18.Brinckerhoff C E. Arthritis Rheum. 1991;34:1073–1075. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald R A. Ann NY Acad Sci. 1994;732:181–198. doi: 10.1111/j.1749-6632.1994.tb24734.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohtai M, Smith R L, Schurman D J, Taub Y, Torti F M, Hutchinson N I, Stetler-Stevenson W G, Goldberg G I. J Clin Invest. 1993;92:179–185. doi: 10.1172/JCI116547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu L P, Jr, Smith G N, Jr, Brandt K D, Myers S L, O’Connor B L, Brandt D A. Arthritis Rheum. 1992;35:1150–1159. doi: 10.1002/art.1780351007. [DOI] [PubMed] [Google Scholar]
- 22.Tilley B C, Alarcon G S, Heyse S P, Trentham D E, Neuner R, Kaplan D A, Clegg D O, Leisen J C C, Buckley L, Cooper S M, Duncan H, Pillemer S R, Tuttleman M, Fowler S E. Ann Intern Med. 1995;122:81–89. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- 23.Gilliam M B, Sherman M P, Griscavage J M, Ignarro L J. Anal Biochem. 1993;212:359–365. doi: 10.1006/abio.1993.1341. [DOI] [PubMed] [Google Scholar]
- 24.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin A R, Vyas P, Attur M, Leszczynska-Piziak J, Patel I R, Weissman G, Abramson S B. Proc Natl Acad Sci USA. 1995;92:7926–2930. doi: 10.1073/pnas.92.17.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svetic A, Finkelman F D, Jian Y C, Dieffenbach C W, Scott D E, McCarthy K F, Steinberg A D, Gause W C. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- 27.Ehlers S, Smith K A. J Exp Med. 1991;173:25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyas P, Attur M, Ou G M, Haines K A, Abramson S B, Amin A R. In: The Biology of Nitric Oxide. Moncada S, Stamler J, Gross S, Higgs E A, editors. London: Portland Press Proceedings; 1996. Part 544. [Google Scholar]
- 29.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D B. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Yu L P, Jr, Smith G N, Jr, Hasty K A, Brandt K D. J Rheumatol. 1991;18:1450–1452. [PubMed] [Google Scholar]
- 31.Cole A A, Chubinskaya S, Chlebek K, Orth M W, Luchene L L, Schmid T M. Ann NY Acad Sci. 1994;732:414–415. doi: 10.1111/j.1749-6632.1994.tb24770.x. [DOI] [PubMed] [Google Scholar]
- 32.Mallya S K, Hall J E, Lee H M, Roemer E J, Simon S R, Golub L M. Ann NY Acad Sci. 1994;732:303–314. doi: 10.1111/j.1749-6632.1994.tb24745.x. [DOI] [PubMed] [Google Scholar]
- 33.Cole A A, Chubinskaya S, Luchene L J, Chlebek K, Orth M W, Greenwald R A, Kuettner K E, Schmid T M. Arthritis Rheum. 1994;12:1727–1734. doi: 10.1002/art.1780371204. [DOI] [PubMed] [Google Scholar]
- 34.Stuehr J D, Cho H J, Kwon N S, Weiss M S, Nathan C F. Proc Natl Acad Sci USA. 1991;88:7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suomalainen K, Sorsa T, Golub L M, Ramamurthy N, Lee H M, Uitto V J, Saari H, Konttinen Y T. Antimicrob Agents Chemother. 1992;36:227–229. doi: 10.1128/aac.36.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vernillo A T, Ramamurthy N S, Lee H M, Mallya S, Auszmann J, Golub L M, Rifkin B R. J Dent Res. 1993;73:367A. [Google Scholar]
- 37.Gilman A G, Rall T W, Nies A S, Taylor P, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 8th Ed. New York: McGraw–Hill; 1993. [Google Scholar]
- 38.Sewell K L, Breedveld F, Furrie E, O’Brien J, Brinckerhoff C, Dynesius-Trentham R, Nosaka Y, Trentham D E. Cell Immunol. 1996;167:195–204. doi: 10.1006/cimm.1996.0027. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill L A J, Bird T A, Saklatvala J. Immunol Today. 1990;11:392–394. doi: 10.1016/0167-5699(90)90155-3. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt H H H W, Warner T D, Nakane M, Förstermann U, Murad F. Mol Pharmacol. 1992;41:615–624. [PubMed] [Google Scholar]
- 41.Salvemini D, Misko T P, Masferrer J L, Seibert K, Currie M G, Needleman P. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Q W, Kashiwabara Y, Nathan C. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 43.Smith G N, Jr, Brandt K D, Hasty K A. Ann NY Acad Sci. 1994;732:436–438. doi: 10.1111/j.1749-6632.1994.tb24778.x. [DOI] [PubMed] [Google Scholar]
- 44.Golub L M, Lee H M, Lehrer G, Nemiroff A, McNamara T F, Kaplan R, Ramamurthy N S. J Periodont Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 45.Golub L M, Goodson J M, Lee H M, Vidal A M, McNamara T F, Ramamurthy N S. J Periodontol. 1985;56:93–97. doi: 10.1902/jop.1985.56.11s.93. [DOI] [PubMed] [Google Scholar]
- 46.Vodovotz Y, Bogdan C, Paik J, Xie Q W, Nathan C. J Exp Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeilschifter, J., Walker, G., Eberhardt, W. & Kunz, D. (1995) Endothelium 3, Suppl., S51 (abstr.).
- 48.Greenwald R A, Golub L M. Ann NY Acad Sci. 1994;732:1–507. doi: 10.1111/j.1749-6632.1994.tb24772.x. [DOI] [PubMed] [Google Scholar]
- 49.Farias-Eisner R, Sherman M P, Aeberhard E, Chaudhuri G. Proc Natl Acad Sci USA. 1994;91:9407–0411. doi: 10.1073/pnas.91.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasten T P, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko T P, Settle S L, Currie M G, Nickols G A. Proc Natl Acad Sci USA. 1994;91:3569–3573. doi: 10.1073/pnas.91.9.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pipili-Synetos E, Sakkoula E, Maragoudakis M E. Br J Pharmacol. 1993;108:855–857. doi: 10.1111/j.1476-5381.1993.tb13476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pipili-Synetos E, Sakkoula E, Haralabopoulos G, Andriopoulou P, Peristeris P, Maragoudakis M E. Br J Pharmacol. 1994;111:894–902. doi: 10.1111/j.1476-5381.1994.tb14822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


