Abstract
TRPA1 is an excitatory ion channel expressed by a subpopulation of primary afferent somatosensory neurons that contain substance P and calcitonin gene-related peptide. Environmental irritants such as mustard oil, allicin, and acrolein activate TRPA1, causing acute pain, neuropeptide release, and neurogenic inflammation. Genetic studies indicate that TRPA1 is also activated downstream of one or more proalgesic agents that stimulate phospholipase C signaling pathways, thereby implicating this channel in peripheral mechanisms controlling pain hypersensitivity. However, it is not known whether tissue injury also produces endogenous proalgesic factors that activate TRPA1 directly to augment inflammatory pain. Here, we report that recombinant or native TRPA1 channels are activated by 4-hydroxy-2-nonenal (HNE), an endogenous α,β-unsaturated aldehyde that is produced when reactive oxygen species peroxidate membrane phospholipids in response to tissue injury, inflammation, and oxidative stress. HNE provokes release of substance P and calcitonin gene-related peptide from central (spinal cord) and peripheral (esophagus) nerve endings, resulting in neurogenic plasma protein extravasation in peripheral tissues. Moreover, injection of HNE into the rodent hind paw elicits pain-related behaviors that are inhibited by TRPA1 antagonists and absent in animals lacking functional TRPA1 channels. These findings demonstrate that HNE activates TRPA1 on nociceptive neurons to promote acute pain, neuropeptide release, and neurogenic inflammation. Our results also provide a mechanism-based rationale for developing novel analgesic or anti-inflammatory agents that target HNE production or TRPA1 activation.
Keywords: oxidative stress, sensory signaling, TRP channel, nociception
Primary sensory neurons of the pain pathway (nociceptors) express one or more members of the transient receptor potential (TRP) family of ion channels, activation of which can produce acute pain and neurogenic inflammation through the release of neuropeptides, including calcitonin gene related peptide (CGRP), substance P (SP) and neurokinin A (NKA) (1, 2). To better understand the cellular mechanisms by which injury promotes these physiological responses, it is important to identify factors that activate or sensitize TRP channels in the setting of tissue damage and inflammation. For example, a variety of endogenous proalgesic agents, such as extracellular protons and bioactive lipids, have been shown to serve as positive allosteric modulators of the capsaicin receptor, TRPV1, thereby enhancing its sensitivity to heat and promoting thermal hyperalgesia. In addition to illuminating basic mechanisms underlying pain hypersensitivity, these findings provide insight into the types of pathophysiological conditions under which TRP channel antagonists may exert analgesic or antiinflammatory actions (3, 4).
Recent studies have shown that the wasabi receptor, TRPA1, also plays an important role in modulating nociceptor excitability and neurogenic inflammation in the setting of tissue injury (5, 6). This channel is activated by isothiocyanate, thiosulfinate, or cinnamaldehyde compounds that constitute the pungent ingredients in mustard, garlic, and cinnamon, respectively (7–10). TRPA1 is also targeted by environmental irritants such as acrolein, that elicit the nociceptive and inflammatory actions of smog, cigarette smoke, and metabolic byproducts of chemotherapeutic agents (5). TRPA1, together with TRPV1, mediates neurogenic inflammatory responses of bradykinin (and possibly other proalgesic agents) that gate the channel indirectly through intracellular calcium release or other consequences of phospholipase C activation (5, 7, 9, 11, 12). However, it is not yet known whether tissue injury also produces endogenous substances that directly activate or sensitize TRPA1 to cause pain and inflammation.
Here, we show that the α,β-unsaturated aldehyde, 4-hydroxy-2-nonenal (HNE), fulfills the role of an endogenous agonist for TRPA1, acting directly on the ion channel to effect gating and neuronal depolarization. HNE is the most abundant and reactive carbonyl species, generated through peroxidation of omega 6-polyunsaturated fatty acids, such as linoleic acid and arachidonic acid (13, 14). HNE is produced at high concentrations (1 μM to 5 mM) in response to oxidative insults and has been proposed to mediate many of the toxic effects of reactive oxygen species (ROS) in vivo (14), where its highly diffusible nature may account for the actions of free radical formation far from the site of injury (14, 15).
A number of known TRPA1 agonists, including acrolein and other α,β-unsaturated aldehydes, possess an electrophilic carbon or sulfur atom that is subject to nucleophilic attack by cysteine or lysine side chains. Such reactivity promotes channel gating through an unusual mechanism involving covalent modification of these amino acids within the cytoplasmic N-terminal domain of the channel (16, 17). Likewise, HNE undergoes Michael-addition reactions at its C C double bond through nucleophilic attack by cysteine (sulfhydryl group), lysine (ε-amino group), or histidine (imidazole group) side chains, thereby forming advanced lipoxidation end-products (18). In light of these similarities in chemical structure and reactivity, we hypothesized that HNE also excites nociceptors by targeting TRPA1 channels. We now show that HNE robustly activates native or recombinant TRPA1 channels and that the nocifensive and neurogenic inflammatory actions of HNE are eliminated by pharmacologic or genetic inhibition of TRPA1 function. Furthermore, key cysteine and lysine residues within the TRPA1 N-terminal domain that are required for activation by mustard oil or acrolein are also essential for activation by HNE. Thus we conclude that TRPA1 is targeted by HNE, and possibly other endogenous reactive carbonyl species, to elicit pain and inflammation in response to cellular and oxidative stress.
C double bond through nucleophilic attack by cysteine (sulfhydryl group), lysine (ε-amino group), or histidine (imidazole group) side chains, thereby forming advanced lipoxidation end-products (18). In light of these similarities in chemical structure and reactivity, we hypothesized that HNE also excites nociceptors by targeting TRPA1 channels. We now show that HNE robustly activates native or recombinant TRPA1 channels and that the nocifensive and neurogenic inflammatory actions of HNE are eliminated by pharmacologic or genetic inhibition of TRPA1 function. Furthermore, key cysteine and lysine residues within the TRPA1 N-terminal domain that are required for activation by mustard oil or acrolein are also essential for activation by HNE. Thus we conclude that TRPA1 is targeted by HNE, and possibly other endogenous reactive carbonyl species, to elicit pain and inflammation in response to cellular and oxidative stress.
Results
HNE Activates the Cloned TRPA1 Channel.
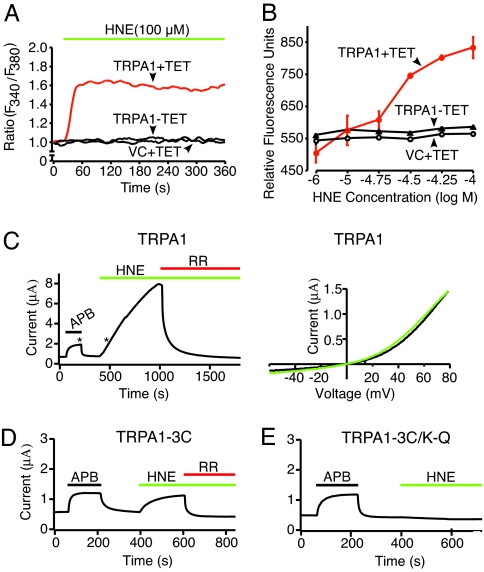
To determine whether HNE can serve as a TRPA1 agonist, we used live-cell calcium imaging to measure the effects of this compound on transfected HEK293 cells expressing the rat TRPA1 cDNA under control of a tetracycline-inducible promoter. Expression of a functional TRPA1 channel was verified by Western blotting, immunofluorescence, and calcium imaging in response to allicin [see supporting information (SI) Fig. 6]. Indeed, HNE elicited rapid, sustained, and concentration-dependent (10–100 μM) increase in intracellular calcium ([Ca2+]i) in HEK-TRPA1 cells treated with tetracycline (half-maximal effective concentration; EC50 = 27 μM) (Fig. 1 A and B). There was no detectable response to HNE in uninduced HEK-TRPA1 cells, or in cells expressing control vector (Fig. 1 A and B).
Fig. 1.
HNE activates the cloned TRPA1 channel. (A) HEK293 cells expressing rat TRPA1 after tetracycline induction (TRPA1+TET; red trace) were challenged with HNE (100 μM), and responses was assessed by calcium imaging. Uninduced (TRPA1-TET; black trace) or vector-transfected control cells (VC+TET; black trace) showed no significant response. (B) Dose–response analysis of HNE-evoked calcium responses in tetracycline-induced (TRPA1+TET; red), uninduced (TRPA1-TET) or vector-transfected control (VC+TET) HEK293 cells. Analysis was performed by using a multiwell fluorescence plate reader. Each well contained 30,000 cells; n = 4 wells per agonist concentration. (C) Representative voltage-clamp recording (+70 mV holding potential; Left) from Xenopus oocytes expressing human TRPA1 channel, showing robust activation by HNE and block by ruthenium red (RR). Asterisks indicate times at which voltage ramps were acquired to generate current–voltage relationships (Right) for APB (black) and HNE (green). (D and E) Oocytes expressing TRPA1–3C or TRPA1–3C/K-Q mutant channels were challenged with 2-aminoethyl diphenylborinate (APB; 200 μM; black), HNE (200 μM; green), or ruthenium red (RR; 10 μM; red), as indicated.
We also used two-electrode voltage-clamp recording methods to measure HNE-evoked responses in frog oocytes expressing the human TRPA1 channel. Consistent with the known properties of TRPA1-mediated responses (5), we observed large sustained, outwardly rectifying membrane currents that were blocked by ruthenium red, an antagonist of several TRP channels, including TRPA1 (Fig. 1C). Recent studies have shown that environmental irritants, such as isothiocyanates and acrolein, activate TRPA1 through covalent modification of key cysteine and lysine residues within the amino-terminal cytoplasmic domain of the channel (16, 17). A TRPA1 mutant (TRPA1–3C) lacking three key cysteine residues within this region shows substantially reduced sensitivity to these compounds, whereas a mutant that also lacks a neighboring lysine residue (TRPA1–3C/K-Q) is completely insensitive (16). We therefore asked whether these mutants also show altered sensitivity to HNE. As expected, TRPA1–3C showed greatly diminished steady-state currents (80% reduction compared with wild-type TRPA1 at +70 mV), whereas the quadruple TRPA1–3C/K-Q mutant was completely insensitive to HNE (Fig. 1 D and E). As a positive control for expression of wild-type or mutant channels, we elicited responses by bath applying 2-aminoethyl diphenylborinate (2-APB), which activates TRPA1 through a distinct, but unknown mechanism (16). Taken together, these results strongly suggest that HNE activates TRPA1 through covalent cysteine or lysine modification, thereby resembling the action of environmental irritants such as acrolein and mustard oil.
HNE Activates Native TRPA1 Channels on Sensory Neurons.
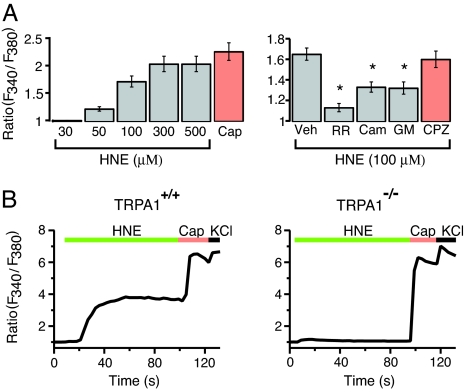
We next asked whether HNE is capable of exciting a subset of primary sensory neurons and, if so, whether this occurs through activation of TRPA1. Using calcium imaging, we examined the effects of bath applied HNE on cultured neurons prepared from dorsal root or trigeminal ganglia of rats or mice. HNE evoked a rapid and concentration-dependent (30–500 μM) increase in [Ca2+]i in a subset of rat DRG neurons with an EC50 value of 77 μM; threshold and maximal responses were observed at 50 and 300 μM agonist, respectively (Fig. 2A Left). Consistent with the known pattern of TRPA1 expression (9, 19), all HNE-sensitive neurons were responsive to capsaicin. HNE-evoked (100 μM) responses were inhibited by ruthenium red, as well as by camphor (1 mM) and gentamycin (100 μM), which also antagonize TRPA1 (20, 21), (Fig. 2A Right). In contrast, these agents had no effect on capsaicin-evoked (0.1 μM) responses (SI Fig. 7). Moreover, the TRPV1 antagonist, capsazepine (10 μM), had no effect on HNE-stimulated responses (Fig. 2A Right). Together, these results suggest that the excitatory actions of HNE on rat sensory neurons results from the selective activation of TRPA1.
Fig. 2.
TRPA1 mediates dose-dependent HNE-evoked responses in cultured sensory neurons. (A) Dose–response analysis (Left) for HNE-evoked responses in cultured rat DRG neurons as assessed by calcium imaging. Response to capsaicin (Cap) is shown for comparison. Effects of several antagonists, including ruthenium red (RR, 1 μM), (+) camphor (Cam, 1 mM), and gentamycin (GM, 100 μM) on HNE-evoked responses were assessed (Right). The TRPV1 antagonist, capsezapine (CPZ, 10 μM), had no effect on HNE-evoked responses. Error bars represent SEM; n ≥ 22 cells; *, P < 0.05, Bonferroni's test vs. vehicle (Veh). (B) Trigeminal neurons from TRPA1+/+ (Left) and TRPA1−/− (Right) mice were exposed to HNE (100 μM), followed by capsaicin (Cap, 1 μM) and then high potassium (KCl; 100 mM), and responses were assessed by calcium imaging. Among Cap-sensitive neurons from wild-type animals, a subpopulation of HNE-responsive cells was observed. No HNE-sensitive cells were detected from TRPA1-deficient mice. Each trace represents an average of 15 responsive cells; n ≥ 399 neurons examined in ≥3 independent cultures per genotype.
We next sought to corroborate these findings by comparing the effects of HNE on DRG neurons prepared from normal and TRPA1-deficient mice (5). A subpopulation (24.5%) of sensory neurons from wild-type animals responded to HNE (100 μM) with a robust and sustained increase in [Ca2+]i (Fig. 2B Left). All of the HNE-sensitive neurons responded to capsaicin (1 μM), whereas only a subset (45.0%) of capsaicin-sensitive neurons responded to HNE. This is entirely consistent with the fact that TRPA1-expressing, mustard oil-sensitive neurons represent a subset (≈50%) of TRPV1-expressing, capsaicin responsive cells (7, 9). In striking contrast, the number of HNE-sensitive neurons was greatly diminished in DRG cultures from TRPA1-deficient mice (4.7% compared with 24.5% in wild-type cultures). Moreover, HNE elicited significantly smaller responses in this residual population, averaging <10% of that observed in wild-type neurons (change in F340/F380 of 0.2 ± 0.3 versus 2.5 ± 0.4). Both TRPA1-deficient and wild-type cultures showed normal responses to capsaicin (Fig. 2B Right). Neurons from mice lacking both TRPA1 and TRPV1 behaved similarly to those lacking TRPA1 alone, indicating that TRPV1 does not contribute to the residual HNE sensitivity observed in the absence of TRPA1 (data not shown). Thus, HNE excites a subpopulation of capsaicin-sensitive primary afferent nociceptors in a manner that requires the presence of functional TRPA1 channels, consistent with the notion that TRPA1 constitutes the primary molecular site of HNE action.
HNE Stimulates Neuropeptide Release and Plasma Extravasation.
The transmission of nociceptive signals is associated with the release of SP and CGRP from the central endings of peptidergic nociceptors that terminate within lamina I and II of the dorsal horn of spinal cord (22). These neurons also release peptides from peripheral nerve endings to elicit neurogenic inflammation in a number of target tissues, which is characterized by hyperemia, edema, and granulocyte infiltration (23). TRPA1 is found exclusively within a subpopulation of peptidergic, capsaicin-sensitive neurons, and the TRPA1 agonists, allyl isothiocyanate, and allicin, elicit vascular relaxation through the release of CGRP from primary afferent nerve terminals (8). Thus, we hypothesized that HNE should also excite these same cells to cause release of CGRP and SP from both central and peripheral nerve endings.
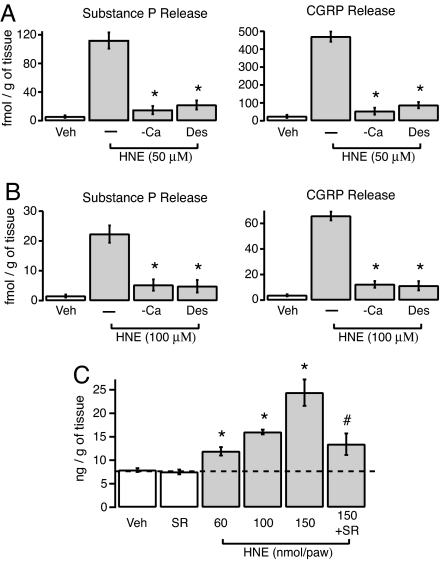
To test this prediction, we examined the ability of HNE and the known TRPA1 agonist cinnamaldehyde to release neuropeptides from superfused segments of rat dorsal spinal cord and esophagus (24). HNE (50 or 100 μM) or cinnamaldehyde (10 or 50 μM), but not vehicle, stimulated release of both CGRP and SP from spinal cord and esophagus (Fig. 3 A and B and Table 1). Desensitization of sensory nerve terminals with capsaicin, or removal of extracellular Ca2+ ions from the bath solution, significantly attenuated neuropeptide release (≥80% inhibition) in response to HNE and cinnamaldehyde. Thus, each agonist evokes neurosecretion of proinflammatory and proalgesic peptides from both the central and peripheral endings of capsaicin-sensitive neurons.
Fig. 3.
HNE mediates neurogenic inflammation by triggering release of neuropeptides and promoting plasma extravasation. (A and B) Release of substance P or CGRP peptides from rat dorsal spinal cord (A) or esophagus (B) was detected after exposure of tissue slices to HNE (50 or 100 μM, as indicated) for 20 min. Desensitization (Des) by pretreatment with capsaicin (10 μM; 20 min before HNE application), or chelation of extracellular Ca2+ (−Ca) by addition of 2 mM EGTA to the bath abolished HNE-evoked neuropeptide release. Error bars represent ±SEM; n ≥ 4 slices per condition. *, P < 0.05, Bonferroni's test versus HNE alone. (C) Injection of HNE into the rat hind paw leads to dose-dependent plasma extravasation as determined by measuring accumulation of circulating Evan's blue dye. The tachykinin NK1 receptor antagonist SR140333 reduced HNE-evoked plasma extravasation. Error bars represent SEM; n ≥ 4 measurements per condition. *, P < 0.05 versus vehicle (Veh) control; #, P < 0.05 versus HNE (150 nmol/30 μl/paw) alone, Bonferroni's test.
Table 1.
Release of SP or CGRP-like immunoreactivity (LI) (fmol/gr/20 min) from slices of rat dorsal spinal cord or esophagus
| Peptide immunoreactivity | Control | Agonist | Ca2+-free | Capsaicin des |
|---|---|---|---|---|
| Spinal cord (cinnamaldehyde, 10 μM) | ||||
| SP-LI | 8.3 ± 1.7 | 160.5 ± 16.7* | 34.5 ± 9.7† | 23.7 ± 7.2† |
| CGRP-LI | 38.5 ± 7.4 | 720.4 ± 43.7* | 118.5 ± 25.8† | 63.8 ± 10.8† |
| Esophagus (cinnamaldehyde, 50 μM) | ||||
| SP-LI | 2.4 ± 0.9 | 31.5 ± 8.2* | 6.3 ± 2.1† | 5.8 ± 1.9† |
| CGRP-LI | 4.8 ± 1.3 | 93.2 ± 21.4* | 19.2 ± 4.6† | 17.8 ± 5.1† |
*, P < 0.05 versus control (vehicle);
†, P < 0.05 versus cinnamaldehyde, alone.
Each entry represents the mean ± SEM of at least four experiments.
Release of SP from the peripheral endings of primary afferents activates the tachykinin NK1 receptor on endothelial cells of postcapillary venules to cause extravasation of plasma proteins and inflammatory edema (1). Consistent with this mechanism, we found that intraplantar administration of HNE (60–150 nmol per paw in 30 μl) produced a dose-dependent increase in plasma extravasation in the hind paws of treated rats (Fig. 3C). Moreover, pretreatment with the NK1 receptor antagonist SR140333 markedly attenuated this response (Fig. 3C), demonstrating that HNE causes plasma extravasation in a manner that depends, in part, on release of tachykinins and activation of NK1 receptors.
HNE Activates TRPA1 to Cause Pain-Related Behavior and Allodynia.
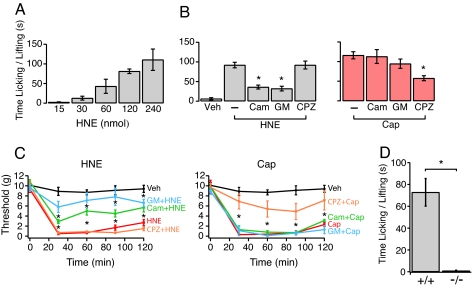
We next asked whether HNE can elicit pain-related (nocifensive) behavior in vivo. Injection of HNE into hind paws of mice (15–240 nmol per paw in 20 μl) induced paw licking and lifting in a dose-dependent manner (Fig. 4A). The response to HNE was unaffected by the TRPV1 antagonist, capsazepine (1.0 nmol per paw, 20 μl), suggesting that TRPV1 plays no significant role in HNE-evoked behavior (Fig. 4B Left). In contrast, the TRPA1 antagonists, camphor (125 nmol per paw, 20 μl) and gentamycin (250 nmol per paw, 20 μl), significantly inhibited the response to HNE (Fig. 4B Left). Neither camphor nor gentamycin inhibited capsaicin-evoked (0.1 nmol per paw, 20 μl) responses, which were attenuated by capsazepine (Fig. 4B Right).
Fig. 4.
TRPA1 is both necessary and sufficient to mediate nocifensive behavior in rodents. (A) Injection of HNE (15–240 nmol/20 μl) into the hind paw of Swiss mice induced pain-related behavior (licking and lifting of affected paw) in a dose-dependent manner. (B Left) (+) camphor (Cam, 125 nmol/20 μl), gentamycin (GM, 250 nmol/20 μl), but not capsazepine (CZP, 1.0 nmol/20 μl) reduced effect of HNE (60 nmol/20 μl). In contrast, these agents had no effect on nociceptive behavior induced by capsaicin (Cap, 0.1 nmol/20 μl), which was blocked by capsazepine (B Right). (C Left) Injection of HNE (150 nmol/50 μl; red trace) into the rat hind paw induced tactile allodynia. Coinjection with (+) camphor (250 nmol/50 μl; green trace) or gentamycin (50 nmol/50 μl; blue trace) reduced the effect of HNE. In contrast, coinjection with capsazepine (0.5 nmol/50 μl; yellow trace) had no blocking effect. No effect was observed upon injection of vehicle alone (Veh, 0.9% saline, 20 μl; black trace). (C Right) Injection of capsaicin (Cap, 20 nmol/50 μl; red trace) also produced tactile allodynia that was unaffected by camphor or gentamycin (green and blue trace, respectively) but attenuated by capsazepine (orange trace). Error bars represent mean ± SEM; n ≥ 6 animals per condition. *, P < 0.05 versus HNE or capsaicin alone. (D) Hind paws of wild-type (+/+) or TRPA1-deficient (−/−) littermate mice were injected with HNE (120 nmol in 20 μl of 0.9% saline). Total time spent licking or lifting the injected paw was recorded over a period of 5 min. Error bars indicate average responses ± SEM (n = 6 animals per trial; *, P < 0.0002, ANOVA single-factor analysis). All measurements were carried out blind to genotype.
HNE also induced a dose-dependent, robust, and sustained mechanical hypersensitivity lasting for >120 min in response to the highest dose (150 nmol per paw, 50 μl) (Fig. 4C Left). Consistent with the acute behaviors described above, mechanical allodynia was unaffected by capsazepine (0.5 nmol per paw, 50 μl), but markedly inhibited by camphor (250 nmol per paw, 50 μl) and gentamycin (50 nmol per paw, 50 μl) (Fig. 4C Left). In contrast, neither camphor nor gentamycin affected capsaicin-evoked (20 nmol per paw, 50 μl) sensitization, which was reduced by capsazepine (Fig. 4C Right). Thus, we conclude that HNE produces acute nocifensive behavior and subsequent mechanical allodynia through its actions at TRPA1.
To corroborate these findings using genetics, we examined HNE-induced nociception in TRPA1-deficient mice (5). Intraplantar injection of 4-HNE (120 nmol per paw, 20 μl) into wild-type mice produced a robust and sustained paw licking and lifting behavior (Fig. 4D). Strikingly, this response was completely absent in TRPA1-deficient littermates (Fig. 4D). Taken together, our results demonstrate that TRPA1 is both necessary and sufficient to mediate the nociceptive, inflammatory, and pain-sensitizing actions of HNE.
Discussion
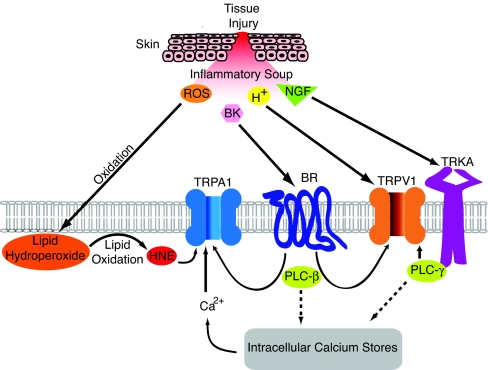
TRP channels are remarkably versatile proteins that contribute to a wide range of physiological processes through their capacity to detect a broad spectrum of chemical and physical stimuli (2, 25). This ability to function as a polymodal signal detector represents a mechanism whereby TRP channels can integrate information from environmental and endogenous stimuli. This scenario is perhaps best exemplified by the capsaicin receptor, TRPV1, whose ability to detect thermal and chemical stimuli enables the nociceptor to survey the local tissue environment for evidence of injury and inflammation, adjusting its heat sensitivity accordingly (26). Chemical sensitization of TRPV1 is mediated by two general mechanisms: some factors, such as extracellular protons or bioactive lipids, interact with the channel directly, thereby serving as positive allosteric regulators; other components of the “inflammatory soup,” such as bradykinin or neurotrophins, mediate their effects indirectly by binding to their cognate receptors on primary afferent neurons and potentiating TRPV1 through one or more downstream signaling pathways (4). We now extend these concepts to TRPA1 by showing that this channel also exhibits two modes of regulation by endogenous inflammatory agents (Fig. 5). Thus, HNE can activate TRPA1 directly via a mechanism resembling the actions of environmental irritants, such as mustard oil and acrolein. As in the case of TRPV1, this may be accompanied by the actions of other inflammatory factors, such as bradykinin, which activate TRPA1 indirectly downstream of PLC-evoked signaling (5–7, 9).
Fig. 5.
Inflammatory agents regulate TRPA1 and TRPV1 through direct and indirect mechanisms. Tissue injury, ischemia, or cellular stress generates an array of proalgesic and proinflammatory agents, collectively referred to as the “inflammatory soup.” This includes extracellular protons (H+), bradykinin (BK), and nerve growth factor (NGF), as well as reactive oxygen species (ROS) that convert polyunsaturated fatty acids into reactive carbonyl species, such as 4-hydroxy-2-nonenal (HNE). Some factors, such as HNE and protons, activate TRPA1 or TRPV1 directly, whereas others, such as BK and NGF, modulate channel gating indirectly by binding to cognate receptors (BR and TRKA, respectively) to activate cellular signaling cascades, most notably those downstream of phospholipase C (PLC). Thus, TRPA1 and TRPV1 function as polymodal signal integrators capable of detecting chemically diverse products of cell and tissue injury. In doing so, these channels promote pain hypersensitivity by depolarizing the primary afferent nerve fiber and/or lowering thermal or mechanical activation thresholds.
Recent studies have shown that isothiocyanates and α,β-unsaturated aldehydes activate TRPA1 through an unusual process involving covalent modification of cysteine and lysine residues located within the amino-terminal cytoplasmic domain of the channel (16, 17). Our analysis of TRPA1 point mutants at these sites provides strong evidence that HNE provokes channel gating by the same mechanism, consistent with its resemblance to acrolein in regard to chemical structure and reactivity. Unlike isothiocyanates, protein carbonylation by reactive aldehydes shows little, if any, reversibility (27), and thus it is not surprising that HNE elicits a long-lasting and essentially irreversible effect on TRPA1 gating. This suggests that HNE may produce both short- and long-term actions on nociceptor excitability and pain hypersensitivity in response to tissue injury and cellular stress.
Reactive oxygen species are generated at the site of inflammation by different mechanisms, and one major action of ROS is the production of HNE and other reactive carbonyl species, which ultimately promote protein carbonylation. Elevated levels of cabonylated proteins are found in the blood of patients suffering from pain and inflammatory diseases, such as rheumatoid arthritis, diabetes or pancreatitis (27–29), or in the lungs of patients with chronic obstructive pulmonary disease (30). Consistent with this, levels of HNE may increase by severalfold after tissue insult or injury, such as that associated with ischemia and reperfusion (31), achieving concentrations as high as 5 mM in affected areas (14). Thus many forms of injury, particularly those leading to oxidative stress, can generate HNE in amounts that greatly exceed its EC50 for TRPA1 activation (≈50 μM). As such, this novel nociceptive and inflammatory pathway represents a potentially important target for the development of analgesic and anti-inflammatory drugs with which to treat a variety of tissue injury and degenerative conditions that promote inflammation and chronic pain.
Materials and Methods
Reagents.
Chemicals and drugs were obtained from the following sources: 4-hydroxy-2-nonelal (Alexis, Lausen, Switzerland), cinnamaldehyde, capsaicin, capsazepine, ruthenium red, (+) camphor, gentamycin, 2-APB (Sigma, St. Louis, MO). SR140333 was a gift of X. Emonds-Alt (Sanofi-Aventis, Montpellier, France).
Animals.
Experiments were approved by Institutional Animal Care and Use Committees of the University of Florence and University of California, San Francisco. Male Sprague–Dawley rats (250 g), male Swiss mice, or male TRPA1-deficient (5) and wild-type littermate controls (30 g on average) were housed in a temperature- and humidity-controlled vivarium (12 h dark/light cycle, free access to food and water). All experiments with TRPA1-deficient and wild-type littermates were performed while blind to the genotype.
Cellular Analysis.
HA11 epitope was added to the C terminus of the rat TRPA1 cDNA and introduced into the pcDNA5/FRT/TO expression vector. Stable cell lines expressing TRPA1 or vector control were generated by using HEK293 FLPTREX cells and the Flp-In system according to the manufacturer's guidelines (Invitrogen, Carlsbad, CA). Cells were grown in DMEM, 10% HIFBS, 100 μg/ml hygromycin B, and 5 μg/ml blasticidin (95% air, 5% CO2, 37°C). TRPA1 expression was induced by addition of tetracycline (0.1 μg/ml) to the medium 16 h before experiments, and confirmed by indirect immunofluorescence and Western blotting using an anti-HA11 antibody (32). [Ca2+]i was measured in these cells with Fura2/AM by using a Flex Station (Molecular Devices, Sunnyvale, CA). DRG neurons were cultured from rats or mice and analyzed by Fura2/AM ratiometric calcium imaging by using Metafluor software (Molecular Devices) or Laboratory Automation 2.0 (RCS, Florence, Italy), as described (8, 9, 24).
Neuropeptide Release.
Slices (≈0.4 mm) of the lumbar enlargements of the dorsal spinal cord or esophagus from rats were stabilized for 60 min in Krebs' solution at 37°C. Fractions (4 ml) were collected at 10-min intervals into ethanoic acid (final concentration 2 N) before, during and after administration of HNE or cinnamaldehyde. Fractions were freeze-dried, reconstituted with assay buffer, and analyzed for CGRP and SP immunoreactivities as described (33). Neither HNE nor cinnamaldehyde (100 μM) show cross-reactivity with SP or CGRP antisera. Exposure to capsaicin (10 μM) for 20 min produced a specific and complete desensitization of TRPV1-expressing sensory nerve terminals, rendering them unresponsive to any stimulus, including TRPA1 agonists.
Plasma Protein Extravasation.
Evans Blue dye (30 mg/kg) was administered into the rat tail vein 1 min before intraplantar application of vehicle or HNE. Rats were euthanized by exsanguination 30 min after application of the stimulus. The skin of the hind paws was removed and the extravasated dye extracted in formamide for 48 h at room temperature in the dark and measured by using a spectrophotometer (620 nm). The amount of extracted dye was expressed as nanogram of dye per gram of wet tissue, as described (24). The tachykinin NK1 receptor antagonist, SR140333, was administered i.v. (1.6 μmol/kg) 15 min before injection of vehicle or HNE into the hind paw.
Nocifensive Response.
Intraplantar (i.pl.) injection of capsaicin (0.1 nmol/20 μl per paw) or HNE (120 nmol/20 μl per paw) was used to induce nociceptive responses as described (34, 35). Immediately after injection, mice were placed inside a Plexiglas chamber. Total time spent licking and lifting of the injected hind paw was recorded for 5 min. Camphor, gentamycin, or capsazepine were administered i.pl. together with capsaicin or HNE. Control mice received equivalent volumes of the relevant vehicle (0.9% saline for HNE or 4% dimethyl sulfoxide for capsaicin). Administration of camphor, gentamycin, capsazepine, or vehicle produced minimal behavior lasting <10 seconds.
Tactile Allodynia.
Animals were placed on a wire mesh platform in a transparent Plexiglas chamber and allowed to habituate for 30 min before the test. After a stabilization period, i.pl. injection of capsaicin (20 nmol/50 μl per paw) or HNE (150 nmol/50 μl per paw) were performed and the allodynic state determined with calibrated von Frey filaments (Biological Instruments, Varese, Italy) by using the up-and-down paradigm (36). Camphor, gentamycin, or capsazepine were administered i.pl., together with HNE or capsaicin. Control animals received an equal volume of vehicles (0.9% saline or 4% dimethyl sulfoxide, respectively).
Statistical Analysis.
All data were expressed as mean ± SEM. Statistical significance was determined by using one- or two-way ANOVA, followed by Bonferroni's post hoc analysis. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by Ente Cassa di Risparmio di Firenze (Florence), the Italian Ministry of Research (FIRB, PRIN) (Rome, Italy), grants from the National Institutes of Health (to D.J., A.I.B., and N.W.B.), a Burroughs Welcome Fund Career Award in Biomedical Sciences (to D.M.B.), and a postdoctoral fellowship from the International Human Frontier Science Program Organization (to J.S.).
Abbreviations
- TRP
transient receptor potential
- SP
substance P
- CGRP
calcitonin gene-related peptide
- NKA
neurokinin A
- ROS
reactive oxygen species.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705923104/DC1.
References
- 1.Geppetti P, Holzer P. Boca Raton, FL: CRC; 1996. pp. 1–338. [Google Scholar]
- 2.Julius D, Basbaum AI. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, et al. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julius D, McCleskey E. In: Textbook of Pain. McMahon S, Koltzenburg M, editors. Philadelphia: Elsevier; 2006. pp. 35–48. [Google Scholar]
- 5.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 10.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 12.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 13.Benedetti A, Comporti M, Esterbauer H. Biochim Biophys Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 14.Esterbauer H, Schaur RJ, Zollner H. Free Radical Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Szweda LI, Chae HZ, Stadtman ER. Proc Natl Acad Sci USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinman A, Chuang HH, Bautista DM, Julius D. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 18.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 20.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Blair NT, Clapham DE. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 23.McMahon S, Bennett D, Bevan S. In: Textbook of Pain. McMahon S, Koltzenburg M, editors. Philadelphia: Elsevier; 2006. pp. 49–72. [Google Scholar]
- 24.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, et al. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 25.Ramsey IS, Delling M, Clapham DE. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 27.Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Med Res Rev. 2006 doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- 28.Renke J, Popadiuk S, Korzon M, Bugajczyk B, Wozniak M. Free Radical Biol Med. 2000;29:101–104. doi: 10.1016/s0891-5849(00)00288-4. [DOI] [PubMed] [Google Scholar]
- 29.Winterbourn CC, Bonham MJ, Buss H, Abu-Zidan FM, Windsor JA. Pancreatology. 2003;3:375–382. doi: 10.1159/000073652. [DOI] [PubMed] [Google Scholar]
- 30.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 31.Siems WG, Grune T, Esterbauer H. Life Sci. 1995;57:785–789. doi: 10.1016/0024-3205(95)02006-5. [DOI] [PubMed] [Google Scholar]
- 32.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. J Biol Chem. 2007;282:12260–12271. doi: 10.1074/jbc.M606338200. [DOI] [PubMed] [Google Scholar]
- 33.Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, Matera M, Geppetti P, Bianchi A. J Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 35.Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 36.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.