Abstract
Centrosomes provide docking sites for regulatory molecules involved in the control of the cell division cycle. The centrosomal matrix contains several proteins, which anchor kinases and phosphatases. The large A-Kinase Anchoring Protein AKAP450 is acting as a scaffolding protein for other components of the cell signaling machinery. We selectively perturbed the centrosome by modifying the cellular localization of AKAP450. We report that the expression in HeLa cells of the C terminus of AKAP450, which contains the centrosome-targeting domain of AKAP450 but not its coiled-coil domains or binding sites for signaling molecules, leads to the displacement of the endogenous centrosomal AKAP450 without removing centriolar or pericentrosomal components such as centrin, γ-tubulin, or pericentrin. The centrosomal protein kinase A type II α was delocalized. We further show that this expression impairs cytokinesis and increases ploidy in HeLa cells, whereas it arrests diploid RPE1 fibroblasts in G1, thus further establishing a role of the centrosome in the regulation of the cell division cycle. Moreover, centriole duplication is interrupted. Our data show that the association between centrioles and the centrosomal matrix protein AKAP450 is critical for the integrity of the centrosome and for its reproduction.
INTRODUCTION
Over the past century, the centrosome has been proposed to play a key role in the cell cycle, but it is only recently that requirement of centrosomal activities for progression through G1 into S phase has been documented by laser ablation of the centrosome (Khodjakov and Rieder, 2001) or by microsurgical removal (Hinchcliffe et al., 2001). Although these approaches have been powerful to reveal the requirement of the centrosome, they have intrinsic limitations: they can be applied only to a very limited number of cells, precluding a quantitative analysis of the cell cycle progression; they affect hundreds of proteins and thus cannot be used to identify the molecular pathways involved.
The identification of a number of signal transduction components associated with the centrosome has led to the idea that the centrosome could fulfill multiple cell functions (Doxsey, 2001b; Rieder et al., 2001; Lange, 2002). The pericentriolar matrix contains very large coiled-coil proteins able to anchor and cluster components of the signaling pathways as well as components of the γ-tubulin complexes (Doxsey, 2001a; Bornens, 2002; Takahashi et al., 2002). These proteins might therefore have a key role in connecting centrosome activity with signaling pathways. The centrosome has been shown to concentrate several kinases and phosphatases such as cAMP-dependent protein kinase (PKA) type II (Nigg et al., 1985; DeCamilli, 1986), the mitotic cdc2 kinase (Bailly et al., 1989, 1992), the Nek2 NIMA-related kinase (Fry et al., 1998), the polo-like kinase Plk (Golsteyn et al., 1995), and phosphatases type 1 and 4 (Andreassen et al., 1998; Helps et al., 1998). However, little is known of the substrates and anchoring proteins for these enzymes. The coiled-coil protein C-Nap1 found at the proximal ends of centrioles has been shown to interact with a complex containing a NIMA-related kinase (Nek2) and protein phosphatase type 1 (PP1) (Helps et al., 2000).
We previously characterized a 453-kDa A-kinase anchoring protein (AKAP450) with coiled-coil structure located in human centrosomes (Keryer et al., 1993) and its cDNA containing an 11.7-kb open reading frame (Witczak et al., 1999). AKAP450 anchors not only PKA but also multiple signaling components such as PP1, PP2a, protein kinase C, and phosphodiesterase 4D (Takahashi et al., 1999, 2000, 2001). Interestingly, this protein also interacts with the transforming acidic coiled-coil containing protein 4 (Steadman et al., 2002). Another human centrosomal protein, kendrin, has been shown to anchor PKA (Diviani et al., 2000) and shares homologies with AKAP450.
To address centrosomal functions of AKAP450, we attempted to specifically delocalize this matrix protein from the centrosome by expressing the AKAP450 centrosomal targeting domain in the absence of the coiled-coil and signal molecule binding domains of the protein.
MATERIALS AND METHODS
Expression Constructs
Fragments of human AKAP450 were amplified by polymerase chain reaction (PCR) by using primers that incorporated restriction sites for cloning into green fluorescent protein (GFP) expression vector, the Advantage polymerase mix (BD Biosciences Clontech, Palo Alto, CA), and AKAP450 cDNA as template. The PCR products were subcloned into the corresponding sites of pEGFP-C3 (BD Biosciences Clontech) to yield vectors that direct expression of AKAP450 fragments fused to enhanced GFP (EGFP). All constructs were sequenced to verify intact reading frames. A construct covering the C terminus of AKAP450 (aa 3707–3908) and a series of shorter deletion constructs in the C terminus were generated. In addition, wild type and mutated C-ter (aa 3699–3796) were cloned into a Myc-tagged expression vector derived from the CVM vector pEGFP where the N-terminal GFP sequence was replaced by six Myc tags. The C terminus of human kendrin was isolated from the plasmid of EST R94777 by digestion with PvuII and ligated into pEGFP-C3 (ScaI) to yield a construct encoding amino acids 3110–3325 (numbering according to KIAA0402; Li et al., 2001; GenBank accession no. U52962).
Mutation of the Calmodulin Binding Site of Human AKAP450
The amino acids at positions 3766, 3767, 3773, 3778, and 3779 of human AKAP450 were all converted to alanine by a three-step PCR-based protocol (Stratagene, La Jolla, CA) in the C-ter construct covering amino acids 3699–3796.
Transfection of HeLa Cells
HeLa cells and Chinese hamster ovary (CHO) cells were grown in DMEM medium supplemented with 10% fetal calf serum and human RPE1 fibroblasts (BD Biosciences Clontech) were grown in DMEM/F-12 medium supplemented with 10% fetal calf serum. Exponentially growing HeLa cells, CHO, RPE1 cells, or HeLa cells stably expressing GFP-centrin 1 construct (Piel et al., 2000) were transfected by electroporation. Specifically, HeLa cells (5 × 106) were detached with trypsin, washed, and resuspended in 200 μl of DMEM medium containing 10% fetal calf serum and 15 mM HEPES, pH 7.5. Plasmid (40 μg) and carrier DNA (20 μg, salmon sperm DNA) were diluted in 50 μl of 210 mM NaCl solution and mixed with the cell suspension in a 4-mm electroporation cuvette. Cells were submitted to an electric pulse of 290 V, 960 μF, and unlimited resistance in the electroporator (Bio-Rad, Hercules, CA). Cells were then washed in 5 ml of medium containing 10% fetal calf serum and 15 mM HEPES, pH 7.5, and seeded on collagen-fibronectin–coated coverslips for immunofluorescence analyses. Four hours after transfection CHO cells were incubated with 2 mM hydoxyurea (HU) for 24–48 h (Balczon et al., 1995).
Antibodies
Polyclonal anti-γ-tubulin (Tassin et al., 1998) was used at 1:500 dilution. Anti-Myc antibody was from Santa Cruz Biotechnology (Santa Cruz, CA; clone 9E10) used at 1:1000 dilution. Monoclonal anti-bromodeoxyuridine (BrdU) was from ImmunologicalDirect Oxford Biotechnology (Oxford, United Kingdom) and used at 1:200 dilution for immunochemistry and 1:100 dilution for flow cytometry. Monoclonal (mAb) CTR453 that has been largely used as a pericentriolar marker (Bailly et al., 1989) recognizes exon 29 of human AKAP450 and does not cross-react with human kendrin/pericentrin (Kemmner et al., in preparation). Affinity-purified polyclonal antibody against human RIIα was previously described and used at a concentration of 100–500 ng/ml for immunofluorescence studies (Keryer et al., 1999). mAb GT335 was used to specifically decorate centrioles (Bobinnec et al., 1998).
Immunocytochemistry, Videorecording, Image Acquisition, and Data Processing
Twenty-four hours after transfection, cells grown on 22-mm glass coverslips were fixed with methanol at -20°C for 3 min, and then immunostained as described previously (Keryer et al., 1993). Finally, the cells were mounted in Mowiol and imaged on a DMRXA microscope (Leica, Wetzlar, Germany) controlled by MetaMorph software (Universal Imaging, Downingtown, PA) by using the 63× immersion PlanApo objective. After focusing on centrioles, the intensity of GFP associated with each centriole was measured on a constant area in the same focal plane. The intensity of GFP-C-ter AKAP450 expression was measured in cytoplasm as the mean of three areas of identical size to those used for centrioles, from which background from untransfected cells was subtracted. More than 200 individual cells were analyzed. Similar number of cells was used for measuring the staining of centrosomal markers (mAb CTR453, γ-tubulin) by using the MetaMorph program. For RII-immunostaining and centriole duplication, six to 10 sequential Z-axis images were collected in 0.2-μm steps and MetaMorph performed reconstructing images automatically in maximal-intensity projections.
To document cytokinesis defects, mitotic cells recording was performed on cells plated on cellocate coverslips (Eppendorf, Hamburg, Germany), allowing us to relocate dividing cells after recording. The coverslips were coated with fibronectin and mounted in sealed chambers containing culture medium equilibrated with 5% CO2 and maintained at 37°C. Phase contrast images were taken every 10 min by using a 20× 0.4 plan objective and a cooled charge-coupled device camera (Princeton Scientific Instruments, Monmouth Junction, NJ). At the end the cells were fixed and either observed for GFP or immunostained with anti-Myc antibody.
Calmodulin Overlay
The inserts from the mammalian expression plasmids C-ter E and C-ter E-Mut were subcloned into pGEX-2T (Amersham Biosciences, Piscataway, NJ) and expressed in Escherichia coli (called GST-WT, GST-Mut). Expression of glutathione S-transferase fusion proteins was induced with 1 mM isopropyl β-d-thiogalactoside for 3 h. The fusion proteins were separated on 12.5% SDS-PAGE directly from bacterial lysates. The calmodulin overlay assay was performed with biotinylated bovine brain calmodulin (Calbiochem, San Diego, CA) with modifications according to the protocol from the manufacturer (http://www.calbiochem.com/protocols/208697.pdf). Calmodulin overlay was performed after separation on 12.5% SDS-PAGE, eletrophoretic transfer to nitrocellulose filters; by incubation of the filters with biotinylated calmodulin (100 ng/ml) either with 1 mM CaCl2 (left) or with 5 mM EGTA (right) and detection with horseradish peroxidase-conjugated streptavidin and enhanced chemiluminescence. Calmodulin binding was achieved using biotinylated calmodulin (100 ng/ml) either with 1 mM CaCl2 or with 5 mM EGTA.
Flow Cytometry Analysis
Twenty-four hours after transfection with GFP-C-ter AKAP450, cells were incubated with 30 μM BrdU during 10 min and washed. They were then trypsinized, kept in the medium and sorted in the R1 window. They were then collected: parts of them were fixed with 70% ethanol for DNA content analysis after propidium iodide incubation. Another part was fixed in 70% cold ethanol for 3-h minimum for BrdU labeling. Analyses were carried out on a flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Windows for cell sorting were calibrated on the GFP-fluorescence intensity of HeLa cells expressing stably GFP-centrin 1 as a centriolar marker. BrdU staining was performed according to Taddei et al. (1999).
RESULTS
Expression of the Centrosome Targeting Domain of AKAP450 Dissociates Endogenous AKAP450 and PKA from Centrosomes
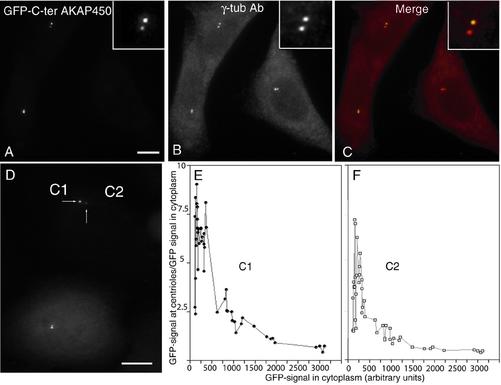
Expression of 201 (aa 3707–3908; our unpublished data) or 97 (aa 3699–3796) amino acid fragments of the C terminus of AKAP450 (C-ter-AKAP450) fused to GFP led to a majority of cells with one or two tiny GFP-labeled dots corresponding to centrosomes as identified by double immunofluorescence staining with anti-γ-tubulin antibody (Figure 1, A–C). GFP labeling of centrosomes was observed within 2 h after transfection. We most frequently observed an asymmetrical GFP staining (Fig. 1, compare insets in A and B), suggesting a smaller number of available binding sites on one of the two centrioles in the centrosome. The centrosome targeting was apparently a saturating process as the centrosome/cytoplasm ratio of GFP/signal decreased when the overexpression level of GFP-C-ter-AKAP450 increased and was observed on both of the centrioles (C1 and C2) present in one centrosome (Fig. 1, D and E, F for quantification). The C-ter-AKAP450 domains used for displacement encompass a putative calmodulin-binding site (Gillingham and Munro, 2000). To address the functional significance of this site, we transfected a stably expressing GFP-centrin 1 (GFP-Cen 1) cell line with a myc-tagged construct of the short 97 amino acid-fragment hereafter called C-ter WT. Centriole staining, visualized by anti-myc antibody or their GFP-Cen 1 content perfectly colocalized (Figure 2, A and C). Introduction of five alanine mutations in the putative calmodulin-binding site (see MATERIALS AND METHODS) abolished the centriolar targeting of GFP-C-ter-AKAP450 (Figure 2B) as well as the in vitro calmodulin-binding of GST-fragments of C-ter-AKAP450 (Figure 2E). Although no nuclear localization signal was found in the C-terminal of AKAP450, the k-NN predictions (Psort II prediction; http://psort.nibb.ac.jp/) for nuclear localization was 39% for the wild type and 70% for the mutated C-terminal AKAP450. This could explain a stronger nuclear localization of the mutated GFP-C-ter AKAP450 in the nucleus (Figures 2B and 3D) compared with the wild-type (WT) GFP-C-ter AKAP450, which showed nuclear localization only at high levels of expression.
Figure 1.
The C-terminal part of AKAP450 targets the protein to centrosomes in a saturable manner. HeLa cells were transiently transfected with a construct expressing the C terminus of AKAP450 (aa 3699–3796) fused to GFP and observed 24 h later for GFP fluorescence (A, C, and D), and centrosomal γ-tubulin staining (B and C). Merge in C. (D) Difference in accumulation of GFP-C-ter AKAP450 signal from centriole pairs (C1 and C2; centriole with high accumulation, C1; low accumulation, C2). (E and F) GFP-signal intensity at each centriole (respectively, C1 and C2) in >500 cells with different levels of expression. Centriolar GFP signal is expressed as a ratio to average GFP signal from three cytoplasm areas of similar size. Bars, 10 μm (A–D).
Figure 2.
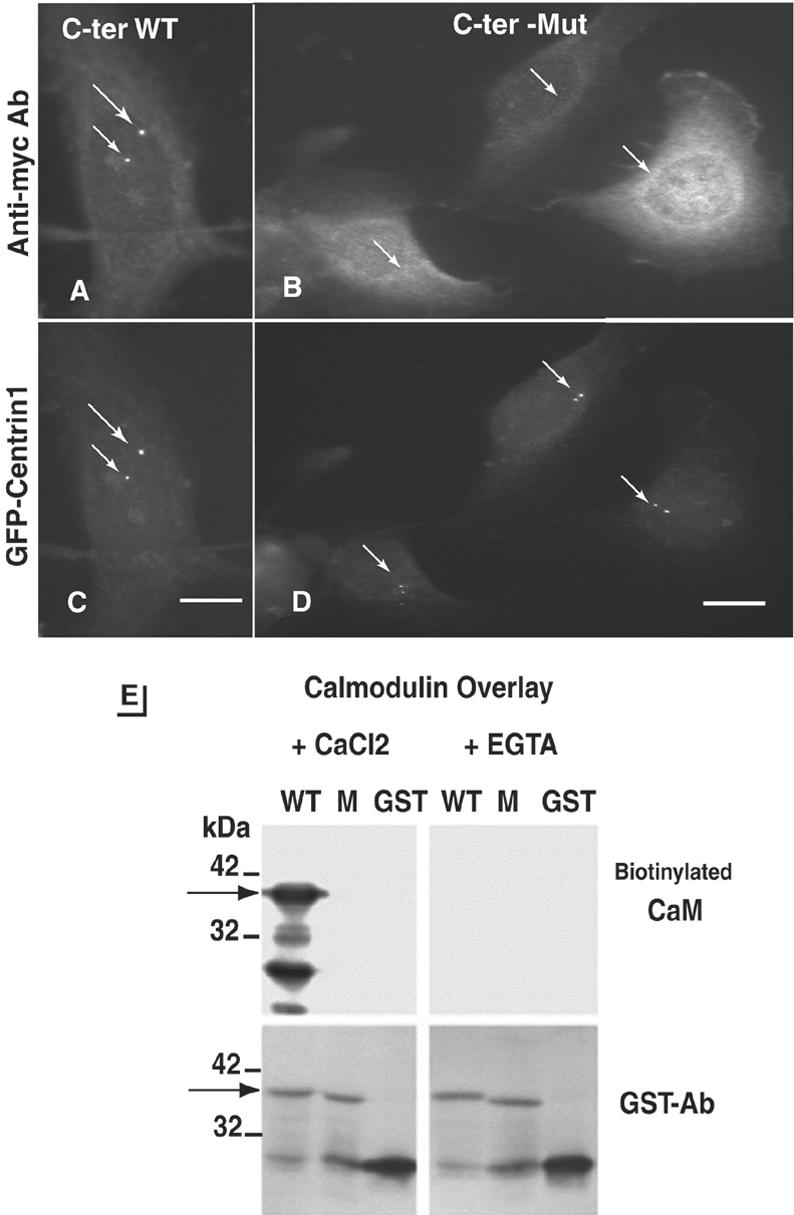
Mutation of a calmodulin-binding domain in C-ter-AKAP450 abolishes AKAP450 targeting to the centrosome. (A–D) GFP-Cen 1 expressing cells were transiently transfected with the Myc-tagged C-ter-AKAP450 wild-type (A and C) and the mutant (B and D) constructs and were observed for anti-myc (A and B) and GFP-centrin 1 (C and D) signals. Position of centrioles is indicated with arrows. Note that the mutated C-terminal fragment of AKAP450 was never found at centrioles (white arrows in B) but observed in the nucleus. Bars, 9 μm. (E) Calmodulin-binding to GST-fused WT and mutant (M) forms of C-ter AKAP450 and to GST without fusion partner (GST). Calmodulin binding was performed using biotinylated calmodulin (100 ng/ml) either with 1 mM CaCl2 or with 5 mM EGTA. The arrow indicates the position of the 39-kDa GST-fusion proteins. The amount of recombinant protein is checked by anti-GST antibody staining (bottom panels).
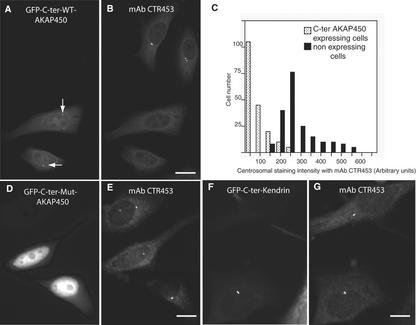
Figure 3.
Targeting of the C-ter-AKAP450 to centrioles displaces the endogenous centrosomal AKAP450. HeLa cells were observed for GFP targeting 24 h after transfection with the C terminus of AKAP450 (A), and the same cells were immunostained with mAb CTR453 that specifically recognizes centrosomal AKAP450 (B). Arrows indicate the position of centrosome in expressing cells. Bar, 10 μm. (C) Quantitative analysis of the CTR453 staining in >250 nonexpressing and C-ter AKAP450-expressing cells. (D and E) HeLa cells were transfected with mutated GFP-C-ter AKAP450 (D and E) or GFP-C-ter kendrin (F and G) and costained with mAb CTR453. Bars, 7 μm (D and E); 5 μm (F and G).
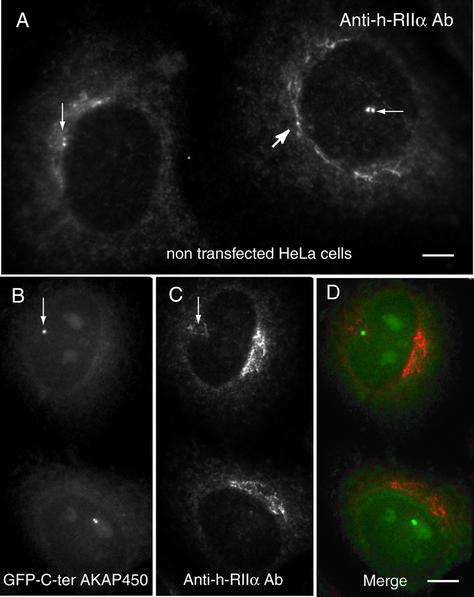
The effect of expressing the C-terminal constructs of AKAP450 on the subcellular localization of the endogenous AKAP450 protein and of γ-tubulin was next monitored by immunofluorescence experiments. Twenty-four hours after transfection, we observed that the centrosomal staining of AKAP450, obtained with the mAb CTR453 (Bailly et al., 1989) was greatly reduced or absent (Figure 3, A and B). A quantitative analysis of the displacement of centrosomal AKAP450 showed that even at low level of expression of the AKAP450 C terminus, a decrease in the amount of the endogenous centrosomal AKAP450 could be measured (Figure 3C). This effect was not observed for γ-tubulin (Figure 1B), which was not delocalized from centrioles when endogenous centrosomal AKAP450 was completely removed (Gillingham and Munro, 2000). In agreement with the observations by these authors, the endogenous centrosomal pericentrin was not displaced from centrioles in low-expressing C-ter AKAP450 HeLa cells. Moreover, the expression of the mutated C-ter AKAP450 did not modify the endogenous centrosomal AKAP450 (Figure 3, D and E). The expression of the C terminus of human kendrin (aa 3110–3325) that shares a high degree of homology in the sequence (Li et al., 2001) did not displace the endogenous centrosomal AKAP450 (Figure 3, F and G). PKA type IIα associates with AKAP450 via its regulatory subunit RIIα at the centrosome (Figure 4 A, thin arrows) and in the Golgi apparatus (Figure 4A, thick arrow; Nigg et al., 1985; DeCamilli, 1986; Keryer et al., 1999). The centrosomal PKA was affected by the displacement of endogenous AKAP450 from centrosome (Figure 4, B and C). Similar quantification profiles as those observed for centrosomal AKAP450 were obtained for the displacement of RII from centrosomes (our unpublished data). However, the PKA present in the Golgi apparatus was not removed (compare A and C).
Figure 4.
Targeting of the C ter-AKAP450 to centrioles displaced PKA type II associated with centrosomes but not that present in the Golgi apparatus. HeLa cells were immunostained with anti-human RIIα antibodies (A–D). Arrows indicate RIIα concentrated around the two centrioles (A, thin arrows) and the Golgi apparatus (thick arrow). (B–D) HeLa cells were transfected with GFP-C-ter AKAP450 and examined for GFP fluorescence (B and D) and RIIα immunostaining (C and D). Thin arrows in B and C point out the position of centrioles in cells expressing the C-ter-AKAP450. Bars, 4 μm (A); 6 μm (B–D).
Expression of the C Terminus of AKAP450 in HeLa Cells Induces Cytokinesis Defects and Increases Polyploid Cells
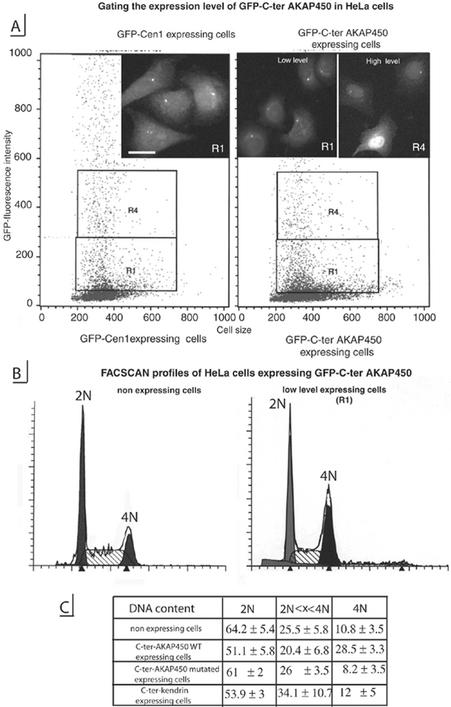
Twenty-four hours after transfection, 70–90% of cells expressed GFP-C-ter-AKAP450 at centrioles, allowing us to use flow cytometry and cell sorting to monitor the effects of AKAP450 displacement on the cell cycle. HeLa cells that stably express GFP-centrin 1 (GFP-Cen 1) were used to calibrate windows for cell sorting (Figure 5A, left). It has been shown that GFP-Cen1 does not affect the cell cycle and that it can be used to monitor in vivo dynamics of centrioles in several cell types (Piel et al., 2000). In this way, we could discriminate between cells in which the GFP-C-ter-AKAP450-domain was expressed at low levels and essentially was targeted to centrioles (Figure 5A, R1, right) and cells with high level of expression where the GFP-C-ter-AKAP450 was in large excess, being also accumulated in the cytoplasm and the nucleus (Figure 5A, R4). All cells sorted from the R1 window contained GFP-labeled centrioles, which seemed similar to those observed for GFP-centrin1 and the following analyses were done on such cells.
Figure 5.
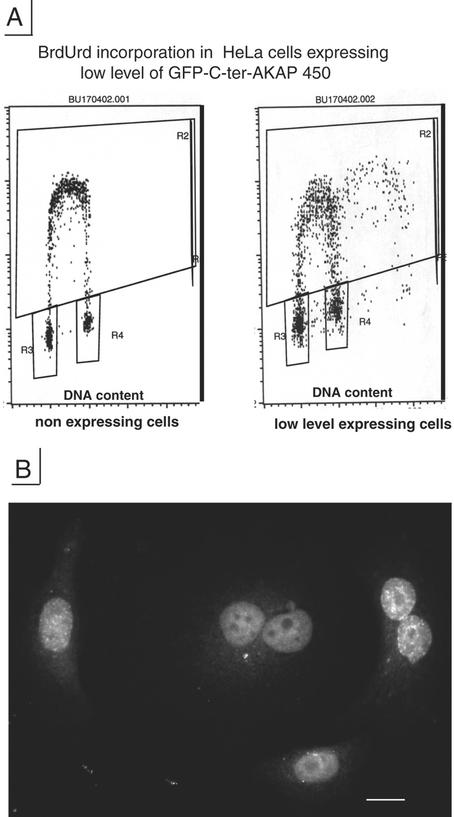
Expression of C-ter-AKAP450 in HeLa cells affects the cell cycle. (A) Stably expressing centrin 1 HeLa cells (GFP-Cen1-expressing cells, left) were analyzed by FACScan for level of GFP expression and used for calibrating windows for cell sorting of low (R1) and high level (R4) of GFP-C-ter AKAP450 expression (right). Micrograph insets, cells corresponding to the windows (R1, left inset, GFP-Cen1–expressing cells; R1, right inset, low level GFP-C-ter AKAP450-expressing cells; R4, right inset, high level GFP-C-ter AKAP450-expressing cells). Bar, 10 μm. (B) Cell cycle profiles by fluorescence-activated cell sorting analysis of DNA content of nonexpressing cells (left) and low level expressing cells (right) of C-ter AKAP450 construct. Shaded and solid peaks correspond to 2N- and 4N-containing cells, respectively. Hatched area indicate cells in S phase as defined by ModFit software from BD Biosciences. (C) Distribution of DNA content of nonexpressing cells and low expressing cells transfected with C-ter AKAP450, mutated C-ter AKAP450 and C-ter kendrin.
Cell cycle analysis of nonexpressing Hela cells, and of cells transiently expressing GFP-C-ter-AKAP450 construct during 24 h, showed a different cell cycle distribution with a higher number of transfected cells with 4N DNA content among which 39–50% were binucleated. Although the transfection efficiency was high, we observed only a small number of mitotic transfected cells. Mitotic index varied from 6 to 18% in nonexpressing cells, whereas it was only 1.7–3% in cells expressing GFP-C-ter-AKAP450 (as judged by GFP-labeling at the poles, by γ-tubulin and DAPI staining). To identify cells in G2 and mitosis, GFP-Cen1 stable expressers and cells transiently expressing GFP-C-ter-AKAP450 were immunostained with an antibody against the phosphorylated form of histone H3 (Cheung et al., 2000). Although G2 and mitotic cells could be observed in GFP-Cen1–expressing cells as expected (these cells displayed four centrioles labeled by GFP, either as two nonseparated pairs in G2 or as individual pairs at each pole during metaphase), no cells in G2 phase or in mitosis could be observed in GFP-C-ter-AKAP450–expressing cells (our unpublished data). Thus, the high number of cells with 4N DNA content in low expressing cells (Figure 5C, right column) could correspond to tetraploid cells. Cell sorting using the same window as in Figure 5B showed that contrary to the wild-type fragment of AKAP450, the mutated fragment did not significantly modify the cell cycle (Figure 5C). Furthermore, the expression of the C terminus of kendrin/pericentrin (aa 3110–3325) that shares a high degree of homology with AKAP450 did not significantly alter the cell cycle. In agreement with the FACSCAN analysis some BrdU incorporation was observed in tetraploid cells expressing low level of GFP-C-ter-AKAP450 (Figure 6) and binucleated cells were incorporating BrdU (Figure 6B).
Figure 6.
Low-level C-ter AKAP450-expressing cells show a high number of binucleated cells. A, cell cycle profile by fluorescence-activated cell sorting analysis analysis after BrdU incorporation of nonexpressing HeLa cells and cells expressing low levels of C-ter-AKAP450. R2, S-phase cells; R3, G1 phase cells; R4, G2/M phase cells or G1 tetraploid arrested cells. (B) immunofluorescence analysis with anti-BrdU antibody of low-level C-ter AKAP450 expressing cells. Bar: 20 μm.
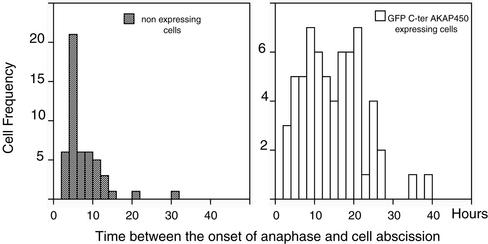
To document cytokinesis defects in HeLa cells expressing the C-ter AKAP450, time-lapse recording was done on cells electroporated alone or transfected either with GFP or Myc-tagged C-ter AKAP450 constructs. The time between the onset of anaphase and cell bridge abscission was measured in >75 mitotic cells (Fig. 7). Abscission occurred from 5 to 7 h after the onset of anaphase in nontransfected cells treatment (Piel et al., 2000, 2001). In cells expressing the GFP-C-ter AKAP450, the time in cytokinesis was largely increased up to 24 h, a time where nonexpressing cells were reentering mitosis. A similar result was obtained in cells expressing the Myc-C-ter AKAP450. Thus, the low number of mitotic cells is explained by a dramatic increase in the duration of cytokinesis, which is likely how binucleated cells are generated.
Figure 7.
Cytokinesis defects in C-ter AKAP450-expressing cells analyzed by time-lapse videomicroscopy. Nonexpressing and GFP or Myc C-ter AKAP450-expressing HeLa cells plated on cellocate coverslips (see MATERIALS AND METHODS) were videorecorded at 6 frames/h in phase contrast microscopy for 24–48 h. Then they were fixed and observed for GFP or immunostained with anti-Myc antibody. The time between the onset of anaphase and cell abscission was measured in >75 mitotic cells for each condition: nonexpressing cells, GFP or Myc C-ter AKAP450-expressing cells.
Expression of the C Terminus of AKAP450 in RPE1 Cells Induces a G1 Arrest
Because HeLa cells are p53-deregulated cells, we used infinity telomerase-immortalized human fibroblasts with a functional p53 (RPE1 cell line; Morales et al., 1999) to address the effect of AKAP450 displacement also in the presence of p53. To be able to monitor BrdU incorporation in individual cells correlated to the expression level of C-ter-AKAP450, a Myc-tagged construct was used. Nonincorporating cells, and low C-ter AKAP450-expressing cells, were counted after double immunostaining of cells with anti-BrdU and anti-Myc antibodies (Table 1). Twenty-four hours posttransfection, RPE1 cells transfected with the C-ter Myc AKAP450 construct were also analyzed by FASCAN for their DNA content. In RPE1 cells expressing C-ter AKAP450 at centrioles, cells that did not incorporate BrdU corresponded mainly to G1 cells as no mitotic cells and a few G2 cells were observed. Therefore, the 4N-containing cells corresponded most probably to nonexpressing G2 or mitotic cells (the population of C-ter Myc AKAP450-transfected cells contained roughly 30% of nonexpressing cells).
Table 1.
BrdU incorporation and FACSCAN analysis of human diploid RPE1 fibroblasts in the absence or presence of Myc-C-ter AKAP450 expression (low level)
|
BrdU incorporation (%)
|
DNA content (%)
|
||||
|---|---|---|---|---|---|
| BrdU- | BrdU+ | 2N | 2N < X < 4N | 4N | |
| Nonexpressing RPE1 cells | 55 | 45 | 58 | 30 | 12 |
| Low-expressing Myc-C-ter AKAP450 RPE1 cells | 71 | 28 | 69 | 20 | 11 |
Twenty-four hours after transfection with the Myc-C-ter AKAP450, RPE1 cells were either incubated with BrdU, fixed, and double immunostained with anti-Myc and anti-BrdU antibodies or analyzed by FACSCAN. Only low expressing RPE1 cells were scored for BrdU staining (>500 cells). DNA contents of nontransfected and Myc-C-ter AKAP450 expressing RPE1 cells are the mean of two separate experiments
Expression of the C Terminus of AKAP450 Impairs Centriole Duplication
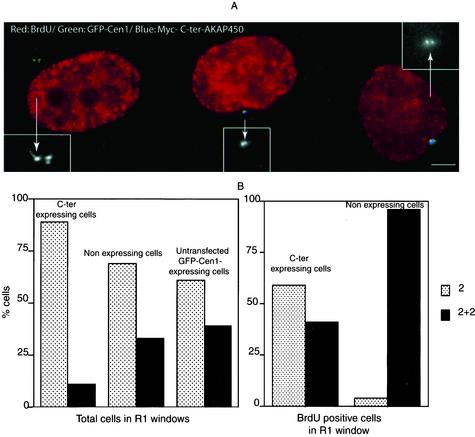
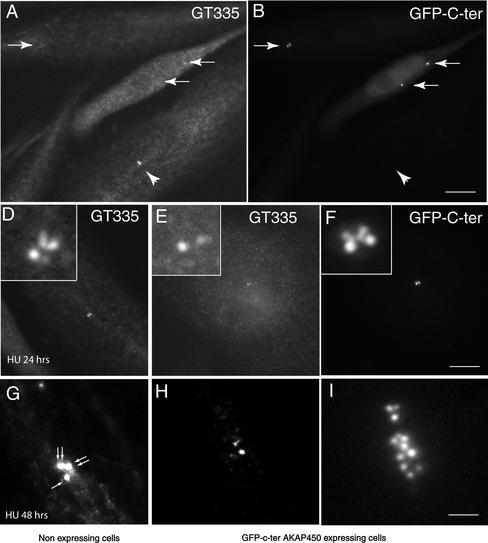
To monitor centriole duplication, we transfected GFP-Cen1 stably expressing HeLa cells with a Myc-C-ter AKAP450 construct. Cells were isolated in the R1 window and incubated with BrdU, fixed, and immunostained with anti-Myc antibody. GFP-labeled centrioles and centriolar buds were counted in nonexpressing and in C-ter AKAP450-expressing cells, either in the whole population or in cells having incorporated BrdU. In BrdU-positive cells that did not express the C-ter-AKAP450 construct, two centrioles + two buds were observed (Figure 8 A, left cell). Cells expressing the C-ter-AKAP450 frequently showed two centrioles without distinct buds (Figure 8A, middle and right cell). In the total population, the number of cells with only two centrioles (which should correspond to cells in G1) was higher in C-ter AKAP450 expressing cells than in nonexpressing ones (Figure 8B, left). Cells with either two centrioles + two buds or with four fully elongated centrioles was higher in the nonexpressing cells. When only BrdU-positive cells were counted in R1 window (Figure 8B, right), the majority of nonexpressing cells had two centrioles + two buds, whereas more than half of the cells expressing the C-ter AKAP450 showed only two centrioles. In an attempt to check whether this corresponded to a block in the initiation of procentriole budding or in the elongation of procentrioles, we used the established centrosome duplication assay in CHO cells. Four hours after transfection with C-ter AKAP450, CHO cells were cultivated in the presence of hydroxyurea for 24–48 h. Centrioles were observed and counted with the centriolar marker mAb GT335 that recognized polyglutamylated tubulins (Bobinnec et al., 1998). After 24 h, the mean value of centrioles in nonexpressing cells was 3 (n = 250 cells) (Figure 9, A, B, and D). In expressing cells unexpectedly, centrioles were poorly decorated by mAb GT335 and were often in reduced number (Figure 9, A, B, and I). However, GFP C-ter AKAP450 was concentrated on them but also on additional dots in their vicinity (Figure 9, E and F). At 48 h, the mean value of centrioles in nonexpressing cells was 5.3 (n = >250 cells; Figure 9G). In expressing cells, centrioles were barely detected with GT335 but were surrounded by a cloud of GFP dots (Figure 9, H and I) (mean number 10, n > 250 cells). Together, these results strongly suggested that overexpression of C-ter AKAP450 impairs centriole biogenesis and stability.
Figure 8.
Expression of C-ter-AKAP450 impairs centriole duplication. GFP-centrin 1-expressing cells were transfected with Myc-C-ter AKAP450, sorted with the R1 window 24 h later, and subsequently incubated with BrdU and fixed. Sequential Z-axis images (6–10) were collected in a 0.2-μm step and image reconstruction was made using MetaMorph in maximal intensity projections. (A) Merged image of anti-BrdU antibody (red) and Myc-antibody (blue) double staining together with GFP fluorescence (green). GFP fluorescence is also shown in each case in insets (twice magnified). The cell on the left is not expressing Myc-C-ter AKAP450. Bar, 5 μm. (B) The fractions of non-budding (two centrioles, gray bars) and budding/fully duplicated (2 + 2 centrioles, black bars) were quantified in C-ter–expressing and nonexpressing cells as well as in untransfected GFP-Cen1–expressing cells, either in the total cell population in the R1 window from cell sorting (left) or in the population of BrdU-positive sorted cells (right). More than 200 cells were scored for centrioles profile in each case.
Figure 9.
Expression of C-ter AKAP450 in CHO cells treated with HU impairs centriole amplification. CHO cells were transfected with the GFP C-ter AKAP450 construct. Four hours later, 2 mM hydoxyurea was added. Cells were then cultivated for 24–48 h. They were fixed and immunostained with mAb GT335 that recognizes the centrioles. (A and B) mAb GT335 staining and GFP-C-ter AKAP450 localization in CHO cells treated for 24 h with HU. Whereas centrioles were strongly immunolabeled by GT335 in nonexpressing cells (arrowhead), they were almost not detectable in cells expressing the C-ter AKAP450 protein but clearly identified in the GFP channel (arrows). (D–F) Higher magnification of a nonexpressing (D) or C-ter AKAP450-expressing cell (E and F). Note that four centrioles can be detected by mAb GT335 in D. Although only two centrioles are visible in expressing cells (E), four dots were observed in the GFP channel (F). Insets are a 5× magnification of the centrosome area. (G–I) CHO cells treated for 48 h with HU. A cluster of three centrosomes containing six centrioles (arrows) is the most often observed pattern in nonexpressing cells (G). In C-ter AKAP450-expressing cells, centrioles are poorly decorated by mAb GT335 (H). However, numerous dots were observed in the GFP channel (I). Bars, 5 μm (A and B); 4 μm (D–F); 1.2 μm (G–I).
DISCUSSION
To address the role of the centrosome and individual centrosomal proteins in the cell cycle control, we looked for an alternative approach to laser ablation or microsurgical removal of the whole centrosome organelle (Hinchcliffe et al., 2001; Khodjakov and Rieder, 2001), which could be used on whole cell populations. We attempted to selectively modify the activity of the centrosome by expressing a C-terminal region of AKAP450 reported to contain the centrosomal targeting domain of this protein (Gillingham and Munro, 2000). We first demonstrated that the C terminus of AKAP450 were targeted to centrioles and that this targeting was able to compete for endogenous AKAP450 binding to centrioles and, as a consequence, to displace PKA type II. We next showed that displacement of endogenous AKAP450 impaired centriole duplication and blocked cell cycle progression.
The C-terminal domain of AKAP450 is directly targeted to centrosomes in a microtubule-independent manner and binds directly to centrioles: 1) The labeling observed with a GFP-tagged version of this domain was very discrete and quite similar to the centrin-labeling that corresponds to intraluminal accumulation of centrin (Paoletti et al., 1996). 2) The docking of the C-terminal domain of AKAP450 was rapidly saturated (within 1–2 h), suggesting a limited number of binding sites. 3) The same results were observed when the AKAP C-ter domain was expressed in the presence of a low dose of nocodazole or paclitaxel from the beginning of expression (our unpublished data). Centriole walls are the most obvious candidates for docking AKAP450, and an appealing possibility would be that AKAP450 binds directly to centriole triplet microtubules, a possibility that would be consistent with the microtubule-binding properties of the full-length protein (our unpublished data). Strikingly, one centriole was more labeled than the other throughout the cell cycle, suggesting a constitutive asymmetry of the centriole pair. It is likely that such a feature reflects the generation process and corresponds to a differentiation between the two centrioles. The centriole with higher level of GFP-C-ter-AKAP450 behaves as the mother-centriole during cytokinesis or during mild nocodazole treatment (Piel et al., 2000, 2001; our unpublished data). A saturable binding to centrosomes was previously described for WD40-containing p80 subunit of katanin, a protein involved in severing of microtubules (Hartman et al., 1998).
Dissecting the C terminus of AKAP450 by deletion mapping allowed us to restrict the centriole-targeting domain to a stretch of 97 amino acids. This stretch corresponds to the PACT domain described by others (Gillingham and Munro, 2000), which is shared by the large coiled-coil centrosomal proteins kendrin/pericentrin and by AKAP450. We observed that specific mutations of the five amino acids necessary for calmodulin-binding completely abolished the centriolar targeting of the C-terminal AKAP450 fragment. Our result contrasts those of Gillingham and Munro (2000) showing that a mutated 226 amino acids fragment with a deletion of the 27 amino acids calmodulin-binding site localized efficiently to centrosomes. We also observed that partial deletion in the middle of the calmodulin-binding site (3699–3778 amino acid fragment) in part impairs centrosomal targeting (our unpublished data). A possible explanation for these discrepancies between mutation and deletion approaches can be found in the predicted secondary structures of the C terminus of AKAP450 when calmodulin-binding is abolished by mutation or by partial deletion (using 3D-PSSM software): alanine replacement of five specific amino acids not only disrupts the amphiphatic helix necessary for calmodulin binding but also alters secondary structures in upstream domains contrary to the deletion of the whole calmodulin-binding stretch. These domains were shown by Gillingham and Munro (2000) to be probably important for centrosome binding. Our results support this conclusion. We stress however that our results do not demonstrate that calmodulin is actually required for AKAP450 binding to centrioles. As a matter of fact, calmodulin does not concentrate on the interphase centrosome (Moisoi et al., 2002), although it does on the spindle pole body of budding and fission yeasts (Spang et al., 1996; Moser et al., 1997). Search for centriolar proteins able to interact with this PACT domain is under investigation.
The experimental approach taken in this study leads to the delocalization of AKAP450 but not centriolar components such as centrin or other components of the centrosomal matrix such as γ-tubulin or pericentrin. As a consequence, a displacement of one signaling molecule bound to AKAP450, PKA type II from the centrosome was observed possibly together with other members of the cAMP regulatory pathway such as phosphodiesterase 4D (Tasken et al., 2001). The expression of the kendrin-C terminus did not modify the presence of PKA type II at the centrosome. Our strategy should not affect localization of other pools of PKA bound to AKAPs elsewhere in the cell, because the C terminus of AKAP450 does not contain the RII binding motifs and should not disrupt anchored PKA activities at other loci in the cell. It is noteworthy that PKA association with the Golgi apparatus was not modified in these conditions (Figure 4), contrary to what was observed when we expressed one or the other of the two RII-binding domains of AKAP450, which displaced PKA from both the centrosome and the Golgi apparatus (our unpublished data). Others have shown that deleting the RII-binding site of AKAP75 precludes localization of PKAII in the membranes and leads to an early decrease in p27 and to a shorter G1 phase (Feliciello et al., 2000). Thus, disrupting PKA activity targeted via another AKAP promotes a very different effect on the cell cycle progression from what we obtained by delocalizing centrosomal AKAP450, where cells expressing C-ter-AKAP450, in which the centrosome is principally targeted, entered mitosis but did not complete cytokinesis. Interestingly, expression of the mutated form of C-terminal AKAP450, which does not bind to centrioles or to calmodulin, has no significant effect on cell cycle progression. Furthermore, expression of the C terminus of kendrin/pericentrin with the same length (200 aa), the same PACT domain, which also targets GFP to centrioles, and which carries an intact calmodulin binding site did not significantly affect cell cycle progression as the mutated C-ter. Thus, our results suggest a specific requirement for a pool of PKA type II activity at the centrosome targeted via AKAP450. Clustering several regulatory molecules at the centrosome as an AKAP450-orchestrated signaling complex could push their activity above a threshold in a time-dependent manner. Alternatively, a concerted action of several cross-talking signaling events could come into play. However, given the capability of AKAP450 to anchor several signaling molecules acting as cell cycle effectors (Lange, 2002), other factors could explain cell cycle arrest. At low levels of expression, HeLa cells showed a large number of cells with 4N DNA content. We could demonstrate that these cells were neither G2 nor mitotic cells and that cytokinesis was dramatically lengthened. Moreover, a high proportion of tetraploid cells corresponded to binucleated cells. A p53-dependent arrest in tetraploid G1 state induced by impairing cytokinesis has been recently reported (Borel et al., 2002; Meraldi et al., 2002). We however observed that binucleated HeLa cells could incorporate BrdU (Figure 6). This is likely to be linked to the inactivation of p53 in HeLa cells (Thomas et al., 1999). Diploid cells such as RPE1 Infinity arrested in G1 upon expression of the C terminus of AKAP450 without any detectable increase in ploidy. We assumed that RPE1 could be blocked in cytokinesis at a stage earlier to that of HeLa cells and thus would not be detectable by FACScan analysis, because the intercellular bridge between daughter cells would be disrupted.
Finally, we documented an effect on centrosome reproduction, which is not a consequence of G1 arrest because it is observed in BrdU-incorporating cells, but that suggests the interesting possibility that the centrosome matrix, which is known to depend on centrioles for its cohesion (Bobinnec et al., 1998), is in turn having a role on the formation of procentrioles. This conclusion was further supported in the centrosome reduplicating CHO cell line. Centrioles identified by mAb GT335 were no longer increasing in number during HU treatment. Moreover, GT335 staining decreased significantly on the remaining centrioles, suggesting that these were destabilized. This assay also revealed an unexpected result: an increasing number of GFP dots were observed during HU treatment in the vicinity of the centrosome. Although this has to be further investigated in more detail, we interpret these dots as accumulations of C-ter AKAP450 on abortive buds unable to recruit tubulins dimers and to elongate procentrioles. The ability of AKAP450 to act as a scaffold for many regulatory proteins could be important not only for the activity of the centrosome as a microtubule organizing center but also for its stability and biogenesis.
In conclusion, by using an approach that disrupts centrosome activity by dissociating the scaffolding centrosomal matrix protein AKAP450 from the centriole pair, we demonstrate the role of the centrosome and AKAP450 in the regulation of cell cycle progression at the level of the whole cell population, as well as on centriole duplication. We speculate that AKAP450 function could be to organize local pools of signaling molecules acting as cell cycle regulators into close proximity of centrioles to regulate both centrosome activity and centriole biogenesis.
Acknowledgments
This work was supported by the Centre National dela Recherche Scientifique and by the Association pour la Recherche sur le Cancer (no. 5521, to G.K.), by the Max-Planck Society, and by grants from the Norwegian Research Council, the Norwegian Cancer Society, Anders Jahre's Foundation, and Novo Nordic Research Committee.
References
- Andreassen, P.R., Lacroix, F.B., Villa-Moruzzi, E., and Margolis, R.L. (1998). Differential subcellular localization of protein phosphatase-1α, γ1, and δ isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 141, 1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly, E., Dorée, M., Nurse, P., and Bornens, M. (1989). p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 8, 3985-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly, E., Pines, J., Hunter, T., and Bornens, M. (1992). Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J. Cell Sci. 101, 529-545. [DOI] [PubMed] [Google Scholar]
- Balczon, R., Bao, L., Zimmer, W.E., Brown, K., Zinkowski, R.P., and Brinkley, B.R. (1995). Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 130, 105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec, Y., Khodjakov, A., Mir, L.M., Rieder, C.L., Edde, B., and Bornens, M. (1998). Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel, F., Lohez, D.O., Lacroix, F.B., and Margolis, R.L. (2002). Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein compromised cells. Proc. Natl. Acad. Sci. USA 99, 9819-9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens, M. (2002). Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25-34. [DOI] [PubMed] [Google Scholar]
- Cheung, P., Allis, C.D., and Sassone-Corsi, P. (2000). Signaling to chromatin through histone modifications. Cell 103, 263-271. [DOI] [PubMed] [Google Scholar]
- De Camilli, P., Moretti, M., Denis-Donini, S., Walter, U., and Lohmann, S. (1986). Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers and in the area of the Golgi complex. J. Cell Biol. 103, 189-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani, D., Langeberg, L.K., Doxsey, S.J., and Scott, J.D. (2000). Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr. Biol. 10, 417-420. [DOI] [PubMed] [Google Scholar]
- Doxsey, S. (2001a). Re-evaluating centrosome function. Nat. Rev. Mol. Cell. Biol. 2, 688-698. [DOI] [PubMed] [Google Scholar]
- Doxsey, S.J. (2001b). Centrosomes as command centres for cellular control. Nat. Cell Biol. 3, E105-E108. [DOI] [PubMed] [Google Scholar]
- Feliciello, A., Gallo, A., Mele, E., Porcellini, A., Troncone, G., Garbi, C., Gottesman, M.E., and Avvedimento, E.V. (2000). The localization and activity of cAMP-dependent protein kinase affect cell cycle progression in thyroid cells. J. Biol. Chem. 275, 303-311. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., Meraldi, P., and Nigg, E.A. (1998). A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 17, 470-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham, A.K., and Munro, S. (2000). The Pact domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep 1, 524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn, R.M., Mundt, K.E., Fry, A.M., and Nigg, E.A. (1995). Cell cycle regulation of the activity and subcellular localization of PLK1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129, 1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, J.J., Mahr, J., McNally, K., Okawa, K., Iwamatsu, A., Thomas, S., Cheesman, S., Heuser, J., Vale, R.D., and McNally, F.J. (1998). Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93, 277-287. [DOI] [PubMed] [Google Scholar]
- Helps, N.R., Brewis, N.D., Lineruth, K., Davis, T., Kaiser, K., and Cohen, P.T. (1998). Protein phosphatase 4 is an essential enzyme required for organisation of microtubules at centrosomes in Drosophila embryos. J. Cell Sci. 111, 1331-1340. [DOI] [PubMed] [Google Scholar]
- Helps, N.R., Luo, X., Barker, H.M., and Cohen, P.T. (2000). NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem. J. 349, 509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., Miller, F.J., Cham, M., Khodjakov, A., and Sluder, G. (2001). Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291, 1547-1550. [DOI] [PubMed] [Google Scholar]
- Keryer, G., Rios, R.M., Landmark, B., Skahlegg, B., Lohmann, S.M., and Bornens, M. (1993). A high affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II in the centrosome of human cells. Exp. Cell. Res. 204, 230-240. [DOI] [PubMed] [Google Scholar]
- Keryer, G., Skalhegg, B.S., Landmark, B.F., Hansson, V., Jahnsen, T., and Tasken, K. (1999). Differential localization of protein kinase A type II isozymes in the Golgi-centrosomal area. Exp. Cell Res. 249, 131-146. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., and Rieder, C.L. (2001). Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 153, 237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, B.M. (2002). Integration of the centrosome in cell cycle control, stress response and signal transduction pathways. Curr. Opin. Cell Biol. 14, 35-43. [DOI] [PubMed] [Google Scholar]
- Li, Q., Hansen, D., Killilea, A., Joshi, H.C., Palazzo, R.E., and Balczon, R. (2001). Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1. J. Cell Sci. 114, 797-809. [DOI] [PubMed] [Google Scholar]
- Meraldi, P., Honda, R., and Nigg, E.A. (2002). Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 21, 483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisoi, N., Erent, M., Whyte, S., Martin, S., and Bayley, P.M. (2002). Calmodulin-containing substructures of the centrosomal matrix released by microtubule perturbation. J. Cell Sci. 115, 2367-2379. [DOI] [PubMed] [Google Scholar]
- Morales, C.P., Holt, S.E., Ouellette, M., Kaur, K.J., Yan, Y., Wilson, K.S., White, M.A., Wright, W.E., and Shay, J.W. (1999). Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21, 115-118. [DOI] [PubMed] [Google Scholar]
- Moser, M.J., Flory, M.R., and Davis, T.N. (1997). Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J. Cell Sci. 110, 1805-1812. [DOI] [PubMed] [Google Scholar]
- Nigg, E.A., Schäfer, G., Hiltz, H., and Eppenberger, H.M. (1985). Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell 41, 1039-1051. [DOI] [PubMed] [Google Scholar]
- Paoletti, A., Moudjou, M., Paintrand, M., Salisbury, J.L., and Bornens, M. (1996). Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 109, 3089-3102. [DOI] [PubMed] [Google Scholar]
- Piel, M., Meyer, P., Khodjakov, A., Rieder, C.L., and Bornens, M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel, M., Nordberg, J., Euteneuer, U., and Bornens, M. (2001). Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550-1553. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., Faruki, S., and Khodjakov, A. (2001). The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11, 413-419. [DOI] [PubMed] [Google Scholar]
- Spang, A., Grein, K., and Schiebel, E. (1996). The spacer protein Spc110p targets calmodulin to the central plaque of the yeast spindle pole body. J. Cell Sci. 109, 2229-2237. [DOI] [PubMed] [Google Scholar]
- Steadman, B.T., Schmidt, P.H., Shanks, R.A., Lapierre, L.A., and Goldenring, J.R. (2002). Transforming acidic coiled-coil-containing protein 4 interacts with centrosomal AKAP350 and the mitotic spindle apparatus. J. Biol. Chem. 277, 30165-30176. [DOI] [PubMed] [Google Scholar]
- Taddei, A., Roche, D., Sibarita, J.B., Turner, B.M., and Almouzni, G. (1999). Duplication and maintenance of heterochromatin domains. J. Cell Biol. 147, 1153-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., Mukai, H., Oishi, K., Isagawa, T., and Ono, Y. (2000). Association of immature hypo-phosphorylated protein kinase C epsilon with an anchoring protein CG-NAP. J. Biol. Chem. 275, 34592-34596. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Shibata, H., Shimakawa, M., Miyamoto, M., Mukai, H., and Ono, Y. (1999). Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J. Biol. Chem. 274, 17267-17274. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Yamagiwa, A., Nishimura, T., Mukai, H., and Ono, Y. (2002). Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring γ-tubulin ring complex. Mol. Biol. Cell 13, 3235-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasken, K.A., Collas, P., Kemmner, W.A., Witczak, O., Conti, M., and Tasken, K. (2001). Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J. Biol. Chem. 2, 21999-22002. [DOI] [PubMed] [Google Scholar]
- Tassin, A.M., Celati, C., Moudjou, M., and Bornens, M. (1998). Characterization of the human homologue of the yeast spc98p and its association with γ-tubulin. J. Cell Biol. 141, 689-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, M., Pim, D., and Banks, L. (1999). The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene 18, 7690-7700. [DOI] [PubMed] [Google Scholar]
- Witczak, O., Skalhegg, B.S., Keryer, G., Bornens, M., Tasken, K., Jahnsen, T., and Orstavik, S. (1999). Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J. 18, 1858-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]