Abstract
Most studies examining continental-to-global patterns of species richness rely on the overlaying of extent-of-occurrence range maps. Because a species does not occur at all locations within its geographic range, range-map-derived data represent actual distributional patterns only at some relatively coarse and undefined resolution. With the increasing availability of high-resolution climate and land-cover data, broad-scale studies are increasingly likely to estimate richness at high resolutions. Because of the scale dependence of most ecological phenomena, a significant mismatch between the presumed and actual scale of ecological data may arise. This may affect conclusions regarding basic drivers of diversity and may lead to errors in the identification of diversity hotspots. Here, we examine avian range maps of 834 bird species in conjunction with geographically extensive survey data sets on two continents to determine the spatial resolutions at which range-map data actually characterize species occurrences and patterns of species richness. At resolutions less than 2° (≈200 km), range maps overestimate the area of occupancy of individual species and mischaracterize spatial patterns of species richness, resulting in up to two-thirds of biodiversity hotspots being misidentified. The scale dependence of range-map accuracy poses clear limitations on broad-scale ecological analyses and conservation assessments. We suggest that range-map data contain less information than is generally assumed and provide guidance about the appropriate scale of their use.
Keywords: biodiversity, birds, geographic range, range occupancy, species richness pattern
A growing number of studies is concerned with continental-to-global scale patterns of biodiversity (1–4) or summary patterns of species' ecological traits such as body size (5) and range size (6, 7). At similarly broad scales, many conservation studies are examining the richness of rare or threatened taxa (8–11) and their distribution in relation to existing reserve networks (12–14). Over such broad geographical extents, expert-drawn range maps (extent of occurrence maps) are typically the primary source of data on species distributions, and thus taxon richness is estimated by counting the number of species ranges that overlap each cell of an underlying grid system. Of course, it has been appreciated for decades that species do not occur at all locations throughout their range (7), and recent work (15–17) has shown that discrepancies may exist between richness patterns inferred from range maps and those inferred from survey data. This mismatch has been attributed to an inherent difference in the spatial scale at which range maps and surveys capture distributional information (15). However, although the spatial scale of surveys is typically explicit in the sampling methodology (e.g., 0.1-hectare Gentry forest plots, 40-km North American Breeding Bird Survey routes, and 0.25° × 0.25° South African Bird Atlas cells), the actual scale at which range maps characterize the distribution of species is unclear (18). Despite this uncertainty, ecologists must choose a scale (or occasionally, multiple scales) at which to carry out range-map-based analyses. With the increasing availability of high-resolution climate and land-cover data, more and more studies using range-map data are being carried out at extremely fine resolutions (Table 1).
Table 1.
Number of studies of broad-scale species richness patterns utilizing range maps (extent > 800 km) by time period and the proportion of those studies examining richness at a resolution of 1° or finer
| Time period | Number of studies | Proportion ≤ 1° |
|---|---|---|
| 1960–1989 | 7 | 0.14 |
| 1990–1994 | 5 | 0.20 |
| 1995–1999 | 12 | 0.42 |
| 2000–2004 | 23 | 0.57 |
| 2004–2007 | 26 | 0.96 |
Studies were identified from Table 1 of Hawkins et al. (4) by searching the terms ″richness pattern*″ or ″diversity pattern*″ in the ISI Web of Science database and from a handful of other studies with which we were familiar. A complete list of studies is available in supporting information (SI) Table 3.
Understanding the true ecological resolution of range-map data is critical for a number of reasons. Richness patterns are scale-dependent, with some variables being better predictors of richness at one scale and other variables better predictors at other scales (19–21). If the true resolution of range-map data differs substantially from that of the chosen grid, then attempts to explain richness using environmental data extracted at that grid resolution will suffer from a mismatch in scale. If range maps are only coarse-scale characterizations of species distribution, then their use to test hypotheses related to fine-scale mechanisms of coexistence is clearly problematic (18). In addition, a scale mismatch in spatial autocorrelation structure between predictor and response can exacerbate these effects (16). In conservation studies, analysis of range-map data at inappropriately fine resolutions may lead to the identification of erroneous “biodiversity hotspots,” overly optimistic estimates of species representation in reserves, and potentially invalid complementarity sets for identifying conservation priorities. Approaches for refining range maps to better represent actual area of occupancy exist but have only recently started to be explored (22, 23). Consequently, an understanding of the scale dependence of range-map accuracy is crucial for any analysis based on range-map data.
Previous studies (15, 24) have quantified the degree to which species are observed throughout their range by using measures of “range occupancy.” These studies found that species typically occur at 40–65% of the sites over which they are expected based on their geographic ranges, suggesting that at the resolution of these surveys, range maps are often a fairly poor characterization of distribution. Here, we perform a comprehensive analysis of the effective scale of range maps across 834 species of birds in Australia and southern Africa. We assess the congruence of range map and gridded survey (atlas) data based on species' range occupancy values and species richness patterns over six spatial resolutions ranging from 0.25° to 8° (≈25–800 km). We also examine how well range-map and atlas data agree with respect to the identification of diversity hotspots and in the perceived efficiency of the existing reserve network for protecting those hotspots.
Results and Discussion
Fine-scale atlas data illustrate the patchy and nonuniform distribution of species within their geographic range (Fig. 1A). As demonstrated by Eremomela gregalis in Fig. 1, at coarser spatial resolutions, range maps more accurately (but less precisely) characterize species distributions and are less prone to false absences. On average, species occurred in only 53% of the 0.25° atlas grid cells in which they were expected according to their range map in Australia and in 64% of the expected grid cells in southern Africa. This figure is comparable to the mean value of range occupancy found in North American birds (15). Thus, even for birds, a taxonomic group for which we have arguably the most complete distributional information globally, our study highlights the fact that fine-scale distributions are much patchier than suggested by even the most detailed expert-drawn range maps.
Fig. 1.
The range map of E. gregalis (gray) in southern Africa, atlas cells in which the species was observed (red), and well surveyed atlas cells in which it was not observed (blue) at 0.25° (A), 0.5° (B), 1° (C), and 2° (D). Values reflect the range occupancy of the species (atlas cell occurrences/total number of well surveyed atlas cells falling within the species' geographic range) at each scale.
Across species, the entire distribution of these range occupancy values shifted asymptotically toward one with coarsening resolution (Fig. 2Upper). Very few species in either region have distributions that are well characterized by range maps (range occupancy ≥ 0.95) at 0.25° or 0.5°, but at scales of 2° or coarser, the majority of species in both regions have range occupancy values of at least 0.95 (Fig. 2 Lower). This suggests that any spatial discontinuities, or “holes,” in species ranges are typically smaller than ≈200 km in diameter. Further investigation might identify whether such discontinuities are related to particular topographical or land-cover features. Combining expert knowledge of species' habitat preferences with coarse-scale distribution data has much potential for examining fine-scale patterns at the species level (23, 25).
Fig. 2.
Boxplots illustrating the distribution of range occupancy values (Upper) and the cumulative proportion of species with range occupancy values ≥0.95 (Lower) in southern Africa (n = 435 species) and Australia (n = 399 species).
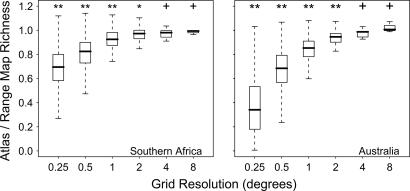
Because species do not occur everywhere within their geographic range, estimates of species richness based on fine-scale atlas data are typically lower than richness estimates based on overlaying range maps (Figs. 3 and 4). At 0.25° resolution, atlas richness was typically just over one-third of range-map richness in Australia and just over two-thirds in southern Africa. As expected from the scale dependence of range occupancy, richness overestimation by range maps decreases at coarser spatial resolutions. At a resolution of ≥4°, range-map and atlas richness values are statistically indistinguishable in both regions (Wilcoxon signed rank test, P > 0.10), and at 2°, atlas richness was within 3–5% of range-map richness for the majority of grid cells (Fig. 4).
Fig. 3.
Spatial patterns of species richness in southern Africa (left two columns) and Australia (right two columns) across six levels of spatial resolution, from ≈0.25° grid cells to 8° grid cells. For each region, the column on the left reflects species richness based on atlas data, whereas the column on the right reflects richness based on the overlaying of range maps. Only grid cells with sufficient sampling effort are shown.
Fig. 4.
Boxplots reflecting the ratio between atlas- and range-map-based richness in southern Africa and Australia over six levels of spatial resolution. Asterisks denote where the distribution of atlas richness values differed significantly from the corresponding range-map richness values based on Wilcoxon signed-rank tests. **, P < 0.0001; *, P < 0.001; +, P > 0.1.
Although atlas richness is typically only a fraction of range-map richness, if these two richness measures are strongly correlated, one might expect the relative spatial patterns of richness to be nearly identical. Although atlas and range-map richness patterns clearly show a positive association, atlas richness varies widely for a given level of range-map richness at the finest spatial resolution (Fig. 5). This is particularly true for Australia, with data points occurring throughout the entire region below the 1:1 line (Fig. 5B). At coarser spatial resolutions, the spatial richness patterns generated by range-map and atlas data become increasingly congruent (Figs. 3–5), with the correlation coefficient between the two spatial patterns ≥0.97 for both regions at 2° resolution. These results parallel the findings of Graham and Hijmans (17), who found a higher correlation between richness maps created using different methodologies at coarser resolutions.
Fig. 5.
The relationship between species richness based on range maps and species richness based on atlas data at 0.25° (Upper) and 2° (Lower) resolution. The solid line indicates y = x; the red shaded area indicates values ≥95th percentile for range-map richness (range-map hotspots); blue shaded areas indicate values in the ≥95th percentile for atlas richness (atlas hotspots). Green indicates where the identification of hotspots coincides. Southern Africa, n = 1,384 0.25° grid cells (A) and n = 45 2° grid cells (C). Australia, n = 2,021 0.25° grid cells (B) and n = 57 2° grid cells (D). The outlier in D represents a grid cell on Tasmania that also includes two pixels on the mainland.
As a result of these mismatches between geographic richness patterns, diversity hotspots identified by range maps often do not coincide with those identified by “on-the-ground” atlas data at fine (0.25°) resolutions (Fig. 6). Of the grid cells identified as being in the richest 5% based on range maps (red and green shaded areas in Figs. 5 and 6), only one-third to one-half were also identified as being among the richest 5% based on atlas data (blue and green shaded areas; Table 2). Visual inspection suggests that range maps also yielded more spatially autocorrelated estimates of hotspot locations compared with atlas data (Fig. 6). As such, the overall richness encompassed within the set of range-map hotspots (361 species) was less than the overall richness of atlas hotspots (381 species). Distribution data are commonly used to evaluate the effectiveness of the existing network of protected areas in encompassing regions of high diversity (26, 27). We found that the protected area network designated by the International Union for Conservation of Nature and Natural Resources (IUCN) had substantial overlap (≥25% of the 0.25° grid cell area) with only 11.6% (8 of 69) of range-map hotspots, whereas >20% of atlas hotspots (14 of 69) overlapped with these protected areas. At coarser resolutions, there is greater correspondence in the identification of hotspots between the two data sets (Table 2). A number of studies have commented on the scale dependence of conservation planning efforts with fine- and coarse-grained analyses often leading to different results (e.g., refs. 28 and 29). Given that 80–85% of the 102,102 protected areas recorded in the 2003 United Nations List of Protected Areas are <100 km2 in size, and nearly 60% are smaller than 10 km2 (30), range-map data alone may be unsuitable for identifying local hotspots or complementary sets of fine-scale areas for protection (8, 9).
Fig. 6.
Lack of congruence between atlas (blue) and range-map (red) hotspots (the 5% most species-rich grid cells) in southern Africa using 0.25° grid cells. Gray areas indicate IUCN-designated protected areas, and green grid cells represent hotspots identified by both types of data. At this resolution, only 31% of the range-map-based hotspots are also identified as hotspots by atlas data. No data were available for countries in white.
Table 2.
Percent of biodiversity hotspots (the 5% richest grid cells) identified by both atlas and range-map data at four spatial resolutions for two regions
| Spatial resolution | Region |
|
|---|---|---|
| Australia | Southern Africa | |
| 0.25° | 52.2 (n = 249) | 31.4 (n = 70) |
| 0.5° | 44.4 (n = 45) | 48.4 (n = 31) |
| 1° | 60.0 (n = 15) | 77.8 (n = 9) |
| 2° | 66.7 (n = 3) | 100.0 (n = 3) |
The total number of hotspots at each resolution is given in parentheses.
Range maps represent a fundamental tool for macroecological studies and conservation planning (29, 31, 32). As a result, the number of studies using these maps to examine broad-scale richness patterns has been steadily increasing (Table 1). However, the utility of this data type is contingent on the accuracy with which it represents species distributions over the spatial scale of interest. Many continental to global-richness studies examine patterns at 1° resolution (8, 9, 33), although others have used 0.25° grid cells (34–36) or even point estimates (37). Our results confirm the suggestions of others (18, 21) that representing range-map-based richness patterns at such fine resolutions can result in the distortion of pattern, and that such estimates have a much higher degree of uncertainty relative to estimates at coarser resolutions. Furthermore, range-map data are inherently more spatially autocorrelated than survey or atlas data (15, 16), and therefore analyzing such data at a fine resolution increases the sample size of the analysis without increasing the concomitant information (21). With the increasing availability of high-resolution predictor variables such as climatic and land-cover data sets, it is tempting to analyze range-map-derived ecological patterns at finer resolutions than appropriate. Our results suggest that the characterization of richness and other macroecological patterns based on range-map data at a resolution less than 2°, and certainly less than 1°, may be misleading. As such, unless refined or appropriately down-scaled to more accurately represent area of occupancy, range-map data are highly limited for addressing hypotheses that invoke local scale processes such as biotic interactions or disturbance regime. Their utility for identifying local (e.g., 100-km2) hotspots for conservation is similarly problematic. Although the results of the many recent studies conducted at 1° are likely to be qualitatively similar to the results that would be obtained at 2°, we suggest that future range-map-based studies should carry out analyses only at scales appropriate to the data. Currently, especially for less well known taxa such as amphibians or insects, this scale appears to be 2° or coarser. Our study outlines a general approach for validating range data that will aid conservation assessments as well as the pursuit of basic research in geographical ecology. It confirms the crucial need for standardized spatially extensive survey data to adequately address some of the most general and pressing questions in these fields.
Data and Methods
Range-Map Data.
We extracted expert-opinion maps of bird ranges from regional sources. Only breeding ranges were included, and all species that feed predominantly in open water habitat were excluded from the analysis. Additionally, the following groups are known to have different detection probabilities in surveys and were therefore excluded: Nightjars and Allies (Caprimulgiformes), Owls (Strigiformes), birds of prey (Falconides), and shorebirds (Charadriides). Range maps for nonpasserine species in both regions were taken from the Handbook of the Birds of the World (38–44). Range maps for southern African passerines were taken from The Birds of Africa (45–47). Original digital files used in these volumes were kindly provided by the respective publishers. Maps were subsequently georegistered and converted to Geographic Information Systems format. Maps for Australian passerine species were digitized by hand from Simpson and Day (48) to a projected map. The different sources for passerine and nonpasserine birds had no noticeable effects on range occupancy (effect of passerine/nonpasserine membership on range occupancy at the fine scale; southern Africa, F1,435 = 0.17, P > 0.9; Australia, F1,392 = 0.10, P > 0.9).
Survey Data.
We use two broad-scale regional bird atlases, one for the Australian continent and one for southern Africa. The advantage of survey data collected over a standardized atlas grid is that the data are easily rescaled to coarser resolutions by aggregating grid cells. Survey data for the spring and summer months (October through March) in Australia were provided by the Australian Atlas team across 25 × 25 km (625 km2) equal-area grid cells (49). Atlas data for the spring and summer in southern Africa were extracted from the recent bird atlas conducted across 0.25° latitude–longitude squares in Mozambique, South Africa, Swaziland, Lesotho, Namibia, and Zimbabwe (50). The area of southern African 0.25° atlas grid cells used in the analysis varied from 636 km2 for the southernmost to 742 km2 for the northernmost grid cells. This degree of variation is negligible in broad-scale analyses of species richness (51). Furthermore, species with ranges restricted to the larger-grid cells in the northern half of the survey area did not have higher values of range occupancy than species with ranges restricted to the southern half of the region, as would be predicted under a grid cell area bias (mean range occupancy of northern species = 0.47; mean range occupancy of southern species = 0.57, t = 1.55, df = 48.72, P = 0.13). The difference in grid-cell area of up to 19% between Australia and southern Africa is trivial relative to the 4-fold variation in area across levels of scale that we examine, and thus we treat the two data sets as being of essentially equal scale and for simplicity refer to scale using the measure in degrees (0.25°, 0.5°, 1°, etc.).
We excluded a number of species from analyses (n = 5 in Australia, n = 18 in southern Africa) for which there was a major mismatch in distribution between the range maps and the atlas data. Mismatches could be due to errors in the range maps themselves, errors in the process of digitization, or inclusion of atlas occurrences outside of the breeding season. We also excluded species (n = 12 in Australia, n = 11 in southern Africa) with geographic ranges spanning <40 atlas grid cells (≈25,000 km2), because range-map uncertainty will have a greater effect on range occupancy values for narrowly ranged species. In total, we analyzed 399 species from Australia and 435 species from southern Africa.
Analysis.
For analysis, range maps and atlas grid cells were geographically overlaid, resulting in a list of survey and range-map presence records for each 0.25° atlas grid cell. Range occupancy measures the extent to which species are reliably observed throughout their range (15). For any given spatial scale, range occupancy was calculated by dividing the number of atlas cell occurrences of a species by the total number of surveyed atlas cells falling within the species' geographic range. In all atlas assessments of this type, survey effort varies from region to region, which may potentially introduce bias; grid cells that are poorly surveyed are more likely to register false absences. Thus, for a particular species, all atlas cells identifying it as present were used regardless of survey effort, whereas only grid cells with at least 20 surveys were used to characterize a species' absence. This measure is more conservative with respect to false absences than one including only observed presences in well surveyed cells. Using this survey threshold, there was no relationship between the average number of surveys per grid cell across a species' geographic range and the estimate of range occupancy for that species (southern Africa, r = 0.04, P = 0.48; Australia, r = −0.03, P = 0.49). Range occupancy values based on other survey level thresholds (from 10 to 50 surveys per pixel) yielded qualitatively similar results.
Range-map and atlas data at the finest resolution (0.25° pixels) were scaled out to coarser resolutions of 0.5, 1, 2, 4, and 8°, and a species was recorded as present in a coarse grid cell if it was present in any constituent pixel. In scaling out atlas data, a species was considered absent from a coarse grid cell if it was not observed in any surveys within the cell, and at least half of the constituent pixels had been surveyed with an average of at least 20 surveys per pixel.
For any given spatial resolution, the range-map richness of a grid cell was calculated as the total number of species to occur on at least one pixel within that cell. Calculating the atlas-based richness of coarse-resolution grid cells is complicated by the fact that grid cells vary in the total number of surveyed pixels as well as the mean number of surveys per pixel. We used the same survey constraints as for the calculation of range occupancy, including only grid cells that had at least one-half of their constituent pixels surveyed with an average of at least 20 surveys per pixel. Nevertheless, variation in the number of surveys can still affect estimates of atlas-based species richness per grid cell, and thus in addition to examining atlas richness, we also considered the residuals of atlas richness after controlling for the total number of surveys conducted in the grid cell [see supporting information (SI) Table 4 and SI Appendix for a more detailed description of these results].
Survey Threshold.
The results presented in this study hinge on the correct identification of absences in species' distributions. As a result, the threshold number of surveys used to determine which grid cells were adequately surveyed can affect the distribution of range occupancy values as well as estimates of atlas richness, and a tradeoff exists between the survey-effort threshold and the number of grid cells that can be used in analyses. We chose a threshold that we felt represented a reasonable balance between the thoroughness of grid-cell sampling and the spatial coverage of qualifying grid cells, and our results were robust to a broad range of potential survey effort thresholds (see SI Appendix). Our results are also consistent with analyses of North American birds (15) that found values of range occupancy similar to those described here, even when an absence was characterized over a smaller area (and therefore easier to census) and over 10 years' worth of surveys. In addition, interspecific variation in range occupancy can be explained by ecological traits such as niche breadth (24), confirming a theoretical expectation for why some ranges are “holier” than others.
Supplementary Material
Acknowledgments
We thank Lauren Buckley, Jonathan Davies, Frank LaSorte, Daniel Kissling, and especially Ethan White for providing comments on an earlier draft of this manuscript. We also thank the researchers who have contributed to the collection and dissemination of the bird atlas data used here. A.H.H. was supported as a Postdoctoral Associate at the National Center for Ecological Analysis and Synthesis, a center funded by National Science Foundation Grant DEB-0072909, and the University of California, Santa Barbara. This material is partly based on work supported by National Science Foundation Grant BCS-0648733 (to W.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704469104/DC1.
References
- 1.Buckley LB, Jetz W. Proc R Soc London Ser B. 2007;274:1167–1173. doi: 10.1098/rspb.2006.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis AP, Currie DJ. Am Nat. 2003;161:523–536. doi: 10.1086/368223. [DOI] [PubMed] [Google Scholar]
- 3.Bellwood DR, Hughes TP, Connolly SR, Tanner J. Ecol Lett. 2005;8:643–651. [Google Scholar]
- 4.Hawkins BA, Field R, Cornell HV, Currie DJ, Guegan JF, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O'Brien EM, et al. Ecology. 2003;84:3105–3117. [Google Scholar]
- 5.Olalla-Tarraga MA, Rodriguez MA, Hawkins BA. Global Ecol Biogeogr. 2006;33:781–793. [Google Scholar]
- 6.Graves GR, Rahbek C. Proc Natl Acad Sci USA. 2005;102:7871–7876. doi: 10.1073/pnas.0500424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapoport E. Areography: Geographical Strategies of Species. New York: Fundación Bariloche by Pergamon Press; 1982. [Google Scholar]
- 8.Grenyer R, Orme CDL, Jackson SF, Thomas GH, Davies RG, Davies TJ, Jones KE, Olson VA, Ridgely RS, Rasmussen PC, et al. Nature. 2006;444:93–96. doi: 10.1038/nature05237. [DOI] [PubMed] [Google Scholar]
- 9.Ceballos G, Ehrlich PR. Proc Natl Acad Sci USA. 2006;103:19374–19379. doi: 10.1073/pnas.0609334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamoreux JF, Morrison JC, Ricketts TH, Olson DM, Dinerstein E, McKnight MW, Shugart HH. Nature. 2006;440:212–214. doi: 10.1038/nature04291. [DOI] [PubMed] [Google Scholar]
- 11.Orme CDL, Davies RG, Burgess M, Eigenbrod F, Pickup N, Olson VA, Webster AJ, Ding TS, Rasmussen PC, Ridgely RS, et al. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. [DOI] [PubMed] [Google Scholar]
- 12.Ceballos G, Ehrlich PR, Soberon J, Salazar I, Fay JP. Science. 2005;309:603–607. doi: 10.1126/science.1114015. [DOI] [PubMed] [Google Scholar]
- 13.Balmford A, Moore JL, Brooks T, Burgess N, Hansen LA, Williams P, Rahbek C. Science. 2001;291:2616–2619. doi: 10.1126/science.291.5513.2616. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues ASL, Andelman SJ, Bakarr MI, Boitani L, Brooks TM, Cowling RM, Fishpool LDC, da Fonseca GAB, Gaston KJ, Hoffmann M, et al. Nature. 2004;428:640–643. doi: 10.1038/nature02422. [DOI] [PubMed] [Google Scholar]
- 15.Hurlbert AH, White EP. Ecol Lett. 2005;8:319–327. [Google Scholar]
- 16.McPherson JM, Jetz W. Glob Ecol Biogeogr. 2007 in press. [Google Scholar]
- 17.Graham CH, Hijmans RJ. Glob Ecol Biogeogr. 2006;15:578–587. [Google Scholar]
- 18.Lawes MJ, Piper SE. South Afric J Sci. 1998;94:207–210. [Google Scholar]
- 19.Rahbek C, Graves GR. Proc Natl Acad Sci USA. 2001;98:4534–4539. doi: 10.1073/pnas.071034898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurlbert AH, Haskell JP. Am Nat. 2003;161:83–97. doi: 10.1086/345459. [DOI] [PubMed] [Google Scholar]
- 21.Rahbek C. Ecol Lett. 2005;8:224–239. [Google Scholar]
- 22.Ferrier S, Powell GVN, Richardson KS, Manion G, Overton JM, Allnutt TF, Cameron SE, Mantle K, Burgess ND, Faith DP, et al. BioScience. 2004;54:1101–1109. [Google Scholar]
- 23.Jetz W, Wilcove DS, Dobson AP. PLoS Biol. 2007;5:1211–1219. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurlbert AH, White EP. Glob Ecol Biogeogr. 2007 in press. [Google Scholar]
- 25.McPherson JM, Jetz W, Rogers DJ. Ecol Model. 2006;192:499–522. [Google Scholar]
- 26.Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL. Science. 2006;313:58–61. doi: 10.1126/science.1127609. [DOI] [PubMed] [Google Scholar]
- 27.Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 28.Larsen FW, Rahbek C. Biodivers Conserv. 2003;12:599–614. [Google Scholar]
- 29.Shriner SA, Wilson KR, Flather CH. Ecol Appl. 2006;16:1660–1673. doi: 10.1890/1051-0761(2006)016[1660:rnborh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Chape S, Blyth S, Fish L, Fox P, Spalding M. 2003 United Nations List of Protected Areas; International Union for Conservation of Nature and Natural Resources; Gland, Switzerland. 2003. p. ix.p. 44. and United National Monitoring Programme World Conservation Monitoring Centre, Cambridge, UK. [Google Scholar]
- 31.Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford, UK: Oxford Univ Press; 2003. [Google Scholar]
- 32.Brown JH. Macroecology. Chicago: Univ of Chicago Press; 1995. [Google Scholar]
- 33.Ding TS, Yuan HW, Geng S, Koh CN, Lee PF. J Biogeogr. 2006;33:683–693. [Google Scholar]
- 34.Lees DC, Kremen C, Andriamampianina L. Biol J Linn Soc. 1999;67:529–584. [Google Scholar]
- 35.Lyons SK, Willig MR. Ecology. 2002;83:47–58. [Google Scholar]
- 36.Hawkins BA, Diniz-Filho JAF, Jaramillo CA, Soeller SA. J Biogeogr. 2006;33:770–780. [Google Scholar]
- 37.Shvarts EA, Pushkaryov EV, Krever VG, Ostrovsky MA. J Biogeogr. 1995;22:907–914. [Google Scholar]
- 38.del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol 1. Barcelona: Lynx Edicions; 1992. [Google Scholar]
- 39.del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol 3. Barcelona: Lynx Edicions; 1996. [Google Scholar]
- 40.del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol 4. Barcelona: Lynx Edicions; 1997. [Google Scholar]
- 41.del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol 5. Barcelona: Lynx Edicions; 1999. [Google Scholar]
- 42.del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol 6. Barcelona: Lynx Edicions; 2001. [Google Scholar]
- 43.del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. Vol 7. Barcelona: Lynx Edicions; 2002. [Google Scholar]
- 44.Elliott A, Sargatal J, del Hoyo J. Handbook of the Birds of the World. Vol 2. Barcelona: Lynx Edicions; 1994. [Google Scholar]
- 45.Fry CH, Keith S, Urban EK. The Birds of Africa. Vol 6. London: Academic; 2000. [Google Scholar]
- 46.Keith S, Urban EK, Fry CH. The Birds of Africa. Vol 4. London: Academic; 1992. [Google Scholar]
- 47.Urban EK, Fry CH, Keith S. The Birds of Africa. Vol 5. London: Academic; 1997. [Google Scholar]
- 48.Simpson K, Day N. Birds of Australia. Princeton, NJ: Princeton Univ Press; 2004. [Google Scholar]
- 49.Barrett G, Silcocks A, Barry S, Cunningham R, Poulter R. The New Atlas of Australian Birds. Melbourne: Birds Australia; 2003. [Google Scholar]
- 50.Harrison JA, Allan DG, Underhill LG, Herremans M, Tree AJ, Parker V, Brown CJ, editors. The Atlas of Southern African Birds. Johannesburg: Birdlife South Africa; 1997. [Google Scholar]
- 51.Nogués-Bravo D, Araújo MB. Glob Ecol Biogeogr. 2006;15:452–460. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.