Abstract
The precise contribution of the cadherin–β-catenin synapse adhesion complex in the functional and structural changes associated with the pre- and postsynaptic terminals remains unclear. Here we report a requirement for endogenous β-catenin in regulating synaptic strength and dendritic spine morphology in cultured hippocampal pyramidal neurons. Ablating β-catenin after the initiation of synaptogenesis in the postsynaptic neuron reduces the amplitude of spontaneous excitatory synaptic responses without a concurrent change in their frequency and synapse density. The normal glutamatergic synaptic response is maintained by postsynaptic β-catenin in a cadherin-dependent manner and requires the C-terminal PDZ-binding motif of β-catenin but not the link to the actin cytoskeleton. In addition, ablating β-catenin in postsynaptic neurons accompanies a block of bidirectional quantal scaling of glutamatergic responses induced by chronic activity manipulation. In older cultures at a time when neurons have abundant dendritic spines, neurons ablated for β-catenin show thin, elongated spines and reduced proportion of mushroom spines without a change in spine density. Collectively, these findings suggest that the cadherin–β-catenin complex is an integral component of synaptic strength regulation and plays a basic role in coupling synapse function and spine morphology.
Keywords: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, spine morphology, synapse adhesion proteins, quantal scaling
Synaptic plasticity is a major means by which neuronal networks adapt to experience. Recent studies suggest that synapses also undergo activity-dependent structural remodeling that might subserve functional synaptic changes (1, 2). Stimulus protocols that induce durable forms of long-term potentiation produce a transient rise in the number of perforated synapses (3, 4) and axonal varicosities (5) and trigger the reorganization of the synaptic actin cytoskeleton to form new functional boutons (6). Dendritic spines also undergo stimulus-dependent active remodeling (7) by mechanisms that involve actin dynamics (8–10). Because the apposition of the presynaptic active zone and the postsynaptic density is always closely matched, remodeling of either the active zone or the dendritic spine must, at some point, accompany a parallel change in the opposite membrane specialization. How the two sides of the synaptic terminals undergo coordinated changes, however, remains to be established.
Several cell adhesion proteins have been identified at synapses where they are implicated in forming and/or maintaining synaptic junctions (11, 12). The best studied among such proteins are the classical cadherins, homophilic Ca2+-dependent adhesion molecules, which also play a role in axon outgrowth (13, 14), dendrite arborization (15), and target recognition (16). Whereas the extracellular domain of cadherins provides the direct intercellular link between apposing cells, strong adhesion by cadherins requires the dynamic intracellular connection to the actin cytoskeleton through β- and α-catenins (17, 18). After synaptogenesis, N-cadherin is found enriched at excitatory synapses in several brain regions (19). Within single synapses, the cadherin–β-catenin complex is present in areas surrounding or within the active zone and the postsynaptic density (20). These observations suggest that the cadherin–catenin complex could potentially regulate aspects of synapse function beyond contributing to synapse assembly in developing neurons. Accordingly, impairing the adhesive activity of cadherins by deletion of β-catenin or N-cadherin reduces the number of reserve pool synaptic vesicles at the presynaptic terminal, resulting in enhanced synaptic depression during repetitive stimulation (21, 22). Moreover, overexpressing an N-cadherin mutant lacking the extracellular domain or β-catenin mutants with an altered phosphorylation site alters spine morphology and synaptic efficacy (23–26). Analysis of αN-catenin knockout mice has also revealed a requirement for the cadherin link to the actin cytoskeleton in regulating spine dynamics (27).
Because cadherins mediate homophilic cell adhesion, it is assumed that any defects in the cadherin–catenin complex are exerted on both sides of the intercellular junction. Synapses, however, are asymmetric junctions. Hence, the intracellular actions of the cadherin–catenin complex could be regulated independently at the pre- and postsynaptic sides of the synaptic junction. Here we specifically examine the contribution of the endogenous, postsynaptic cadherin–catenin complex in regulating synaptic strength in cultured hippocampal pyramidal neurons. We use the Cre/loxP recombination system to inactivate the β-catenin gene after synaptogenesis. The key advantage of this approach is the impairment of cadherin-dependent adhesion that is not limited to N-cadherin but that targets all classic cadherins expressed at hippocampal synapses. Additionally, this method obviates the complications associated with overexpressing mutant forms of N-cadherin and β-catenin that must compete with endogenous proteins. We find that, upon postsynaptic β-catenin ablation, dendritic spines become thin and elongated, the amplitude of spontaneous glutamatergic synaptic currents [miniature excitatory postsynaptic currents (mEPSCs)] mediated by AMPA receptors (AMPARs) is reduced, and activity-dependent bidirectional scaling of the mEPSC amplitude is blocked. In contrast, the mEPSC frequency and the density of synaptic inputs and dendritic spines remain unaltered. Our results indicate that the postsynaptic cadherin–catenin complex plays a central role in regulating functional synaptic AMPARs.
Results
Loss of Endogenous β-Catenin Produces Subtle Changes in Synapse Morphology.
To examine the specific role of endogenous postsynaptic β-catenin in maintaining synapse integrity and function, we prepared hippocampal cultures from mice homozygous for the β-catenin floxed allele (28). Transfecting freshly dissociated neurons with a plasmid encoding Cre-internal ribosome entry site (IRES)-GFP produced a gradual loss of β-catenin immunofluorescence signal. By 7 days in vitro (DIV) β-catenin was largely absent in neurites and substantially reduced in the cell body of GFP-positive neurons (Fig. 1A). A similar loss of β-catenin was observed when neurons were transfected at DIV8 and examined at DIV12 (data not shown). Because of low transfection efficiency, Cre was expressed in relatively few cells, which enabled us to assess the effects of postsynaptic β-catenin loss, since virtually all of the synaptic inputs onto a Cre-positive neuron came from nontransfected cells.
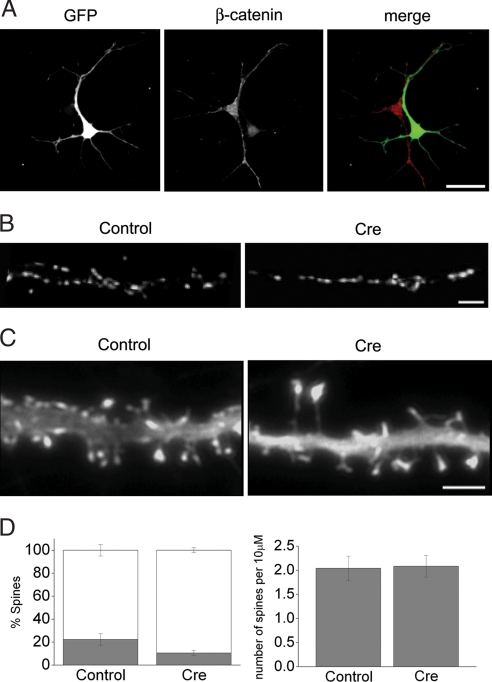
Fig. 1.
Synapse morphology in neurons ablated for postsynaptic β-catenin. (A) Dissociated cells from homozygous β-catenin-floxed mice were transfected with Cre-IRES-GFP and grown in culture. Cre-expressing neuron, identified by the GFP fluorescence (green), shows reduced β-catenin expression compared with a neighboring nontransfected neuron (red) at DIV7. (B) Representative synapsin I localization on dendrites of neurons transfected with control GFP or Cre-IRES-GFP. Postsynaptic β-catenin loss has no significant effect on synapse density (see Results). (C) Representative GFP-actin expression in dendrite segments of DIV18 β-catenin-floxed neurons. Coexpression of Cre produces elongated spines compared with a control cell. [Scale bars: 40 μm (A), 5 μm (B and C).] (D Left) Relative proportion of mushroom-shaped (gray bars) vs. filopodial (open bars) spines in control (n = 9) and Cre-positive (n = 13) neurons (P < 0.05). (Right) Comparison of mean spine density along 10-μm dendritic length between control (n = 9) and Cre (n = 13)-transfected neurons (P > 0.2).
We first asked whether synaptic inputs were maintained in dendrites after β-catenin ablation. To limit the possible effects arising from perturbing β-catenin function in synaptogenesis, transfection was carried out in DIV8–10 neurons in this and all of the subsequent experiments. Immunofluorescence labeling for synapsin I showed no significant difference in synapse density between Cre- and GFP-expressing control neurons at DIV14 (P > 0.2; control, 5.4 ± 0.3 puncta per 10 μm; Cre, 4.9 ± 0.3 puncta per 10 μm; n = 12 cells each). The ability to sustain synaptic inputs is thus not affected by the loss of postsynaptic β-catenin.
We next examined the effect of β-catenin loss on the spine morphology by comparing the pattern of GFP or GFP-actin fluorescence in neurons cotransfected with or without the Cre plasmid. Actin is highly enriched in postsynaptic terminals, and the shape of GFP-actin in the dendritic spines matches the spine morphology delimited by the plasma membrane (29). Whereas ablating postsynaptic β-catenin did not significantly change the overall synapse distribution, subtle changes in spine morphology were apparent at a later culture stage (DIV18) when dendritic spines were prominent. Compared with control cells that had abundant mushroom-shaped short spines, Cre-expressing neurons had thin and elongated spines (Fig. 1C), and the relative proportion of mushroom-shaped spines was reduced by half (Fig. 1D Left). Spine length measurements confirmed longer spines in Cre-expressing cells compared with controls (P < 0.05; control, 1.24 ± 0.04 μm; Cre, 1.47 ± 0.07 μm; n = 8 cells each). However, the spine density was not altered by the loss of β-catenin (Fig. 1D Right), consistent with the lack of change in synapsin I puncta density (see above). These observations are in agreement with previous studies in which overexpressing a dominant-negative N-cadherin or αN-catenin deletion produced similarly thin and elongated spines (24). Endogenous β-catenin thus participates in regulating spine morphology, likely by providing a dynamic link between cadherins and the spine actin cytoskeleton.
β-Catenin Modulates AMPA-Mediated Synaptic Currents.
Because postsynaptic β-catenin ablation did not compromise the ability of neurons to maintain presynaptic inputs, we next examined the effects of β-catenin loss on glutamatergic quantal responses by performing whole-cell patch-clamp recordings from control and Cre-expressing neurons. The mean mEPSC amplitude, which under our experimental conditions was largely carried by AMPAR, was reduced by ≈25% in Cre-transfected neurons compared with GFP-transfected control neurons (Fig. 2). The mean mEPSC frequency, however, was not significantly altered by the Cre expression (Fig. 2D). A lack of effect on mEPSC frequency, a parameter that is proportional to the density of functional boutons, is consistent with the observed lack of change in densities of spines and synapsin I puncta along dendrites of Cre-expressing cells. When the kinetics of mEPSCs were compared, both the rise and decay phases were slightly prolonged in Cre-transfected neurons compared with control neurons (rise time constant: control, 0.63 ± 0.02 ms; Cre, 0.73 ± 0.04 ms; P < 0.05; decay time constant: control, 3.2 ± 0.2 ms; Cre, 3.9 ± 0.2 ms; P < 0.05; n = 15 cells each; also see Fig. 2C). The slowed time course of mEPSCs could reflect changes in the synaptic structure or organization, including the composition of AMPAR subunits (30, 31).
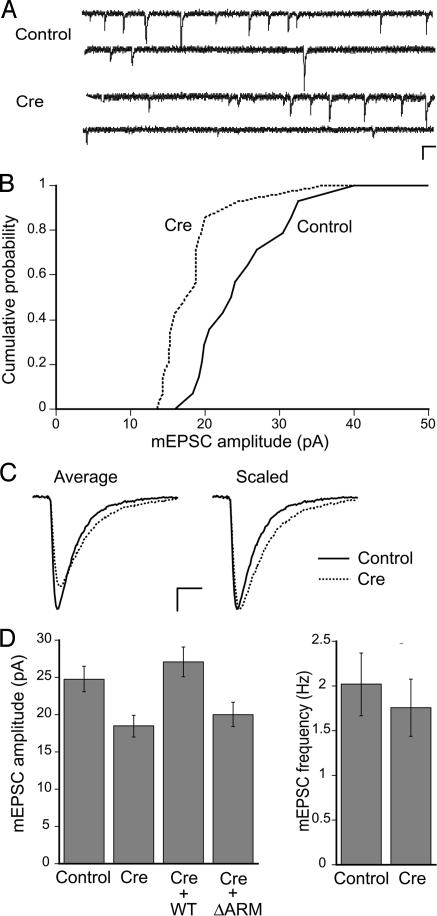
Fig. 2.
Effects of β-catenin loss on glutamatergic quantal responses. (A) Representative traces of mEPSCs from β-catenin-floxed neurons expressing GFP alone or Cre at DIV14. (Scale bars: 100 ms, 20 pA.) (B) Cumulative distribution plots of the mean mEPSC amplitude from GFP- (solid line) or Cre-expressing neurons (dashed line) (P < 0.05; n = 15 cells each). (C) Examples of average mEPSC trace from GFP- (solid line) or Cre-expressing neurons (dotted line). Traces on the right have been scaled to the peak. (Scale bars: 5 ms, 5 pA.) (D Left) Summary of mean mEPSC amplitude. Coexpression of WT β-catenin with Cre rescued the mean mEPSC amplitude to control levels (n = 8; P > 0.4 vs. control), but not by ΔARM (n = 9; P > 0.6 vs. Cre). (Right) Summary of mean mEPSC frequency. The mean mEPSC frequency is unaltered in Cre-expressing neurons compared with controls (P > 0.5; n = 15 cells each).
We sought to confirm that the reduced glutamatergic synaptic strength in Cre-expressing neurons was caused by the loss of endogenous β-catenin rather than by indirect effects of Cre expression. Coexpression of wild-type (WT) β-catenin with Cre fully restored the mean mEPSC amplitude to levels found in control neurons, whereas coexpression of a β-catenin mutant lacking the central armadillo repeat region, which mediates binding to cadherins and TCF/LEF transcription factors (ΔARM, see below), did not rescue the reduction in mEPSC size (Fig. 2D). The full rescue by WT β-catenin supports the role for this protein in modulating synaptic AMPARs. Furthermore, the requirement for the central armadillo domain suggests the importance of the linkage to cadherins and/or the contribution of β-catenin-dependent transcription in regulating the synaptic AMPA currents.
Overexpression of N-cadherin Mutant Reduces Quantal AMPA Responses.
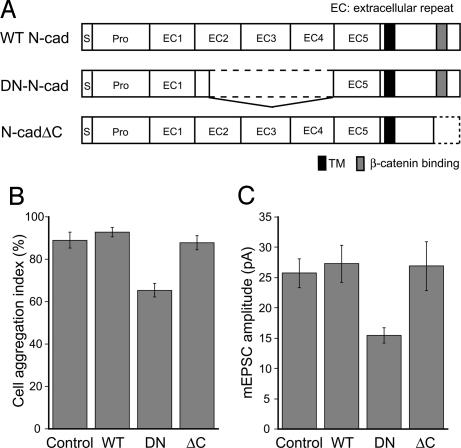
If the lack of rescue with the β-catenin ΔARM mutant reflects the involvement of postsynaptic cadherins in regulating synaptic AMPARs, then overexpressing a mutant form of N-cadherin [dominant-negative (DN)-N-cad] should mimic the effect of β-catenin loss on mEPSC size. The DN-N-cad carries a deletion within the extracellular domain that mediates the homophilic interaction between cadherins on the opposite sides of the membrane (Fig. 3A). Therefore, the DN-N-cad with the nonfunctional extracellular domain is expected to inhibit the adhesive activity of endogenous cadherins by competing for intracellular interactions with β-catenin and/or other cadherin-binding proteins. In contrast, overexpression of a mutant N-cadherin deleted for the C-terminal domain, including the β-catenin-binding region (N-cadΔC, Fig. 3A), should not compete with endogenous N-cadherin for β-catenin. We first confirmed the dominant-negative activity of DN-N-cad by transfecting HEK293 cells, which express endogenous N-cadherin, and using them in a cell dissociation assay that tests for cell adhesion. Exogenous expression of DN-N-cad but not WT N-cadherin or N-cadΔC significantly reduced Ca2+-dependent cell aggregation compared with control GFP-transfected cells (Fig. 3B). We then overexpressed WT N-cadherin, DN-N-cad, or N-cadΔC in cultured hippocampal neurons. Similarly to Cre transfections in β-catenin floxed cultures, low transfection efficiency ensured that few neurons overexpressed the cadherin construct in a given coverslip. Whole-cell recordings from transfected neurons showed that the mean mEPSC size was decreased by ≈30% in DN-N-cad-expressing cells relative to control GFP cells (Fig. 3C). In contrast, the mean quantal amplitude in neurons overexpressing the WT N-cadherin or N-cadΔC was not different from the control GFP neurons. That the overexpression of DN-N-cad can mimic the effect of β-catenin loss on synaptic AMPA currents suggests that regulation of quantal size by β-catenin requires its binding to cadherins.
Fig. 3.
DN N-cadherin mimics the effect of β-catenin loss. (A) Illustration of mutant N-cadherin constructs used. (B) Cell dissociation assay in HEK293 cells, comparing the efficacy of N-cadherin mutants for impairing Ca2+-dependent cell adhesion relative to control. WT, P > 0.2; DN-N-cad, P < 0.01; N-cadΔC, P > 0.7; n = 4 each. Cell aggregation index was defined as the percent ratio of [NTE − NTC]/NTE, where NTC and NTE were the cell particle number after the TC and TE treatments, respectively. (C) The mean mEPSC amplitudes from neurons expressing GFP alone (control, n = 15) compared with WT N-cadherin (WT, P > 0.3, n = 12), the DN N-cadherin (DN, P < 0.01, n = 14), or N-cadherinΔC (ΔC, P > 0.1, n = 10).
Domain Analysis of β-Catenin for Modulating Quantal AMPA Responses.
The structure of β-catenin consists of three major domains: (i) an N-terminal region that binds to α-catenin, which interacts with the actin cytoskeleton; (ii) a central armadillo repeat domain that binds to cadherins and TCF/LEF transcription factors; and (iii) a C-terminal domain that binds to PDZ proteins. To characterize the protein interaction domains of β-catenin in regulating synaptic AMPARs, we made a series of β-catenin deletion and point mutants that carry defects in each of the three domains (Fig. 4A). We first tested the binding of β-catenin mutants to N-cadherin by expressing the epitope-tagged WT or mutant β-catenin constructs in HEK293 cells. The endogenous N-cadherin efficiently bound to WT and mutant β-catenin with the exception of the ΔARM mutant lacking the cadherin-binding domain (Fig. 4B). We also tested the two N-terminal domain β-catenin mutants, ΔN and L132A, for their interaction with α-catenin. The ΔN has a deletion in the N-terminal α-catenin-binding domain, and the L132A carries a point mutation at the amino acid residue 132 that forms part of the α-catenin-binding interface (32). Accordingly, when WT or mutant β-catenin was coexpressed with αN-catenin in HEK293 cells, ΔN and L132A did not bind to αN-catenin, whereas WT, ΔARM, and C-terminal mutants did (Fig. 4B). Although both the N-terminal domain β-catenin mutants bound to endogenous N-cadherin as expected, given the loss of αN-catenin binding, these mutants would be deficient in linking cadherins to the actin cytoskeleton.
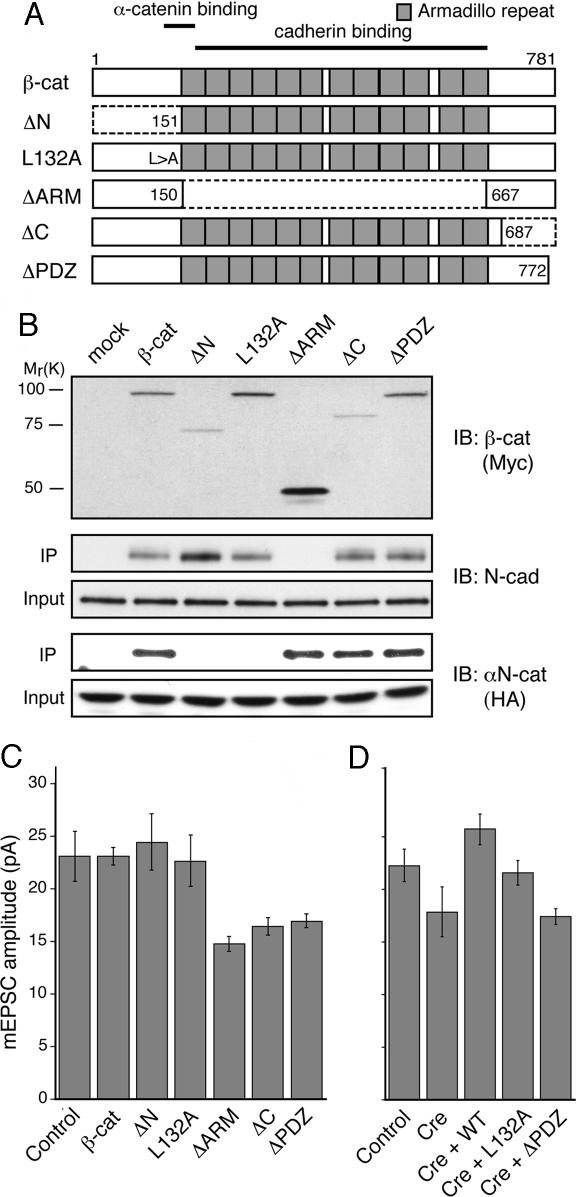
Fig. 4.
The PDZ-binding motif of β-catenin is important for regulating mEPSC size. (A) Schematic diagram of β-catenin deletion mutants. (B) Interaction of β-catenin deletion mutants with N-cadherin or αN-catenin in HEK293 cells. Western blots show coimmunoprecipitation (IP) of endogenous N-cadherin (Middle) and exogenous αN-catenin-HA (Bottom) with exogenously expressed Myc-tagged β-catenin mutants (Top). (C) Summary of mean mEPSC size in WT cultures overexpressing β-catenin constructs. Compared with control GFP neurons (n = 12), expression of WT (n = 10), ΔN (n = 10), or L132A (n = 10) had no effect (P > 0.7), whereas expression of ΔARM (n = 11), ΔC (n = 12), and ΔPDZ (n = 10) mutants reduced mEPSC amplitude (P < 0.05). (D) Summary of mean mEPSC amplitude in β-catenin-floxed neurons. The decreased mean mEPSC amplitude upon loss of β-catenin (P < 0.05; control, n = 9; Cre, n = 9; also see Fig. 2C) was rescued by coexpression of WT β-catenin (P > 0.1 vs. control; n = 7) or L132A (P > 0.7 vs. control; n = 9), but not by ΔPDZ (P < 0.01 vs. control n = 10) in parallel experiments.
Subsequently, we expressed the β-catenin mutants in cultured neurons. Double immunofluorescence labeling against epitope-tagged β-catenin and synapsin I confirmed synaptic localization of exogenously expressed β-catenins except for the ΔARM mutant, which showed diffuse staining (data not shown). Efficient targeting of β-catenin to synapses, therefore, likely requires cadherin binding. Whole-cell recordings of mEPSCs from transfected neurons showed a reduced mean mEPSC amplitude in cells overexpressing β-catenin ΔARM, ΔC, or ΔPDZ compared with control GFP-transfected cells (Fig. 4C). The central armadillo repeats and the C-terminal PDZ-binding motif of β-catenin are thus required for maintaining the basal level of synaptic AMPARs. Notably, despite the lack of apparent synaptic enrichment, the ΔARM mutant reduced the mEPSC size such that the level of overexpression achieved was apparently sufficient to uncouple β-catenin-binding protein(s) from the endogenous synaptic cadherin–β-catenin complex. In contrast to cells transfected with the ΔARM or C-terminal domain mutants, neurons expressing either the WT β-catenin or ΔN or L132A mutants that are impaired for αN-catenin binding, displayed mean mEPSC amplitudes that were not significantly different from the control GFP-transfected cells (Fig. 4C). These overexpression experiments suggest that modulation of synaptic AMPARs by β-catenin does not require its interaction to α-catenin. In further support, coexpression of WT β-catenin or the L132A mutant with Cre in β-catenin floxed neurons largely restored the mean mEPSC amplitude to levels found in control neurons, whereas coexpression of ΔPDZ did not rescue the reduction in mEPSC size (Fig. 4D). Taken together, the regulation of quantal AMPA responses by β-catenin depends on its interaction to cadherins and PDZ proteins, whereas the linkage to the actin cytoskeleton through α-catenin plays a lesser role.
Loss of Postsynaptic β-Catenin Impairs Quantal Synaptic Scaling.
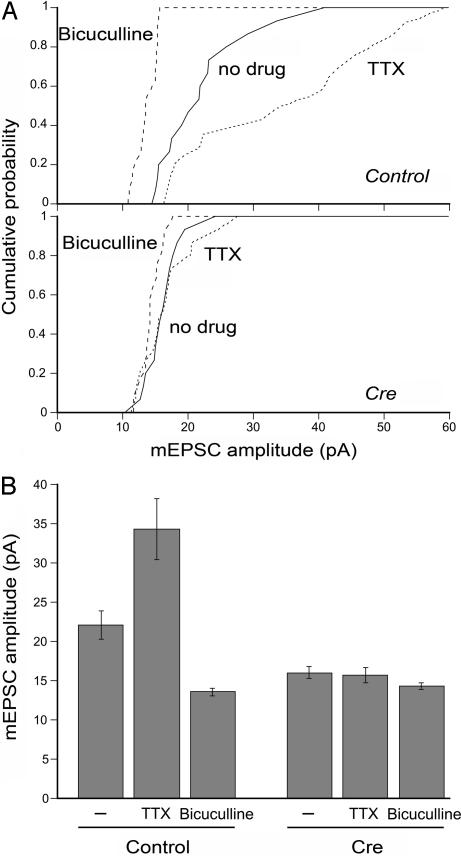
Given its role in regulating the basal synaptic strength, β-catenin could participate in activity-dependent modulation of quantal AMPA responses. Quantal synaptic scaling is a form of homeostatic plasticity that modifies synaptic strength bidirectionally to compensate for overall changes in neural network activity (33). To test whether the loss of β-catenin might also impair the activity-dependent scaling of mEPSC amplitude, neuronal cultures were treated for 2 days with tetrodotoxin (TTX) or bicuculline. As reported previously, in control neurons, TTX treatment increased the mean mEPSC amplitude by ≈50% relative to non-drug-treated cells, whereas bicuculline decreased the mean mESPC amplitude by ≈40% compared with non-drug-treated neurons (Fig. 5). In β-catenin-null neurons, however, neither a 2-day TTX nor bicuculline treatment produced a significant change in the mean mEPSC amplitude compared with non-drug-treated cells (Fig. 5). Suppressing overall network activity by blocking AMPARs for 2 days with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), similarly to TTX treatment, increased the mEPSC amplitude in control neurons but not in β-catenin-null neurons (data not shown). These results, therefore, suggest that in addition to regulating the basal mEPSC size, β-catenin may also be required for the bidirectional homeostatic modification of synaptic AMPARs. Notably, in parallel experiments, the mean mEPSC size in Cre-transfected neurons after no drug or TTX and bicuculline treatments was comparable with the mEPSC size in bicuculline-treated β-catenin-positive control neurons. Thus, the mechanism by which mEPSC amplitude is reduced by ablating β-catenin could share the mechanisms of bicuculline-induced, activity-dependent down-regulation of quantal amplitude.
Fig. 5.
Activity-dependent synaptic scaling is impaired upon β-catenin loss. (A) Cumulative distribution plots of the mean mEPSC amplitudes from untransfected control (Upper) or Cre-transfected neurons (Lower) after culturing with no drug (solid line), TTX (dotted line), or bicuculline (dashed line) for 2 days. (B) Summary of the mean mEPSC amplitudes after chronic activity manipulations. In control neurons, relative to no drug treatment (n = 16), mEPSC size was scaled up in TTX (P < 0.01; n = 15) or down in bicuculline (P < 0.01; n = 12). In β-catenin-null neurons, relative to no drug treatment (n = 16), neither chronic TTX (P > 0.6; n = 16) nor bicuculline (P > 0.1; n = 13) caused a significant change in the mean mEPSC size.
Discussion
At mature excitatory synapses, where unlike inhibitory synapses, N-cadherin expression persists after synaptogenesis, the cadherin–catenin complex has been previously implicated in dendritic spine morphogenesis (24, 25, 27). In this work, we demonstrated a role for the cadherin–catenin complex in regulating excitatory synaptic strength. Ablation of postsynaptic β-catenin reduced synaptic AMPA responses, and the functional change was distinct from the expected role for the cadherin–catenin complex in regulating the dendritic spine shape. The reduction in quantal responses was observed well before the time when morphologically mature spines were abundant. Moreover, surprisingly, the modulation of synaptic AMPA currents did not require the link from the cadherin–catenin complex to the actin cytoskeleton, which is closely coupled to dendritic spine morphology as exemplified by the consequences of αN-catenin deletion (24). By regulating synaptic AMPARs in addition to spine morphogenesis, the cadherin–catenin complex serves as a molecular device that couples the structure and function of excitatory synapses. Upon losing β-catenin, the reduction of functional synaptic AMPARs could bias synapses to form thin and elongated rather than mushroom-shaped spines. A recent study that examined a role specifically for N-cadherin in synaptic transmission using knockout neurons did not find a change in mEPSC amplitude (22). It is therefore possible that other classical cadherins, whose activity is compromised by β-catenin deletion, may also play a role in regulating quantal size. Alternatively, in this previous study, N-cadherin-null neurons were derived from embryonic stem cells such that additional compensatory mechanisms may have been recruited during differentiation and development.
In developing neurons, a major synaptic function for the cadherin–catenin complex is in synapse assembly. Our work, in contrast, focused on the regulation of functional glutamatergic transmission in a more mature neuronal network at 12–16 DIV (34). The reduction in mEPSC size occurred when β-catenin was ablated after the initial wave of synaptogenesis, and coexpression of WT β-catenin fully restored the mEPSC size to control levels. In addition, postsynaptic ablation of β-catenin did not change the density of presynaptic markers and spines. Therefore, synaptic junction per se was maintained despite the loss of strong cadherin-mediated adhesion, as expected from the redundant functions of multiple synapse adhesion proteins that promote synapse formation and maintenance. Although our findings suggest a direct role for β-catenin in regulating synaptic strength, given that synapses turn over continually, albeit at a low rate, we cannot exclude a contribution of developmental effects from synapses assembled in the absence of β-catenin.
We have identified a possible role for the cadherin–catenin complex in homeostatic scaling of synaptic strength, whose underlying molecular mechanisms are not well understood. The cadherin–catenin complex is present in the perijunctional region that circumscribes the postsynaptic density and the active zone (20). Given that homeostatic plasticity is accompanied by a change in the length of the active zone (35), the cadherin–catenin complex, by delimiting the junctional area, is positioned to facilitate the matching of the postsynaptic changes to the presynaptic changes. This facilitation could be accomplished, via the transsynaptic homophilic interaction of cadherins, by the intracellular linkage of the cadherin–catenin complex to the postsynaptic scaffold through the PDZ interactions of β-catenin, where the postsynaptic density is modulated coordinately with the presynaptic active zone assembly (36). A potential role for transsynaptic cadherin in regulating the junctional geometry is supported by the observed slowing of mEPSC time course that accompanies postsynaptic loss of β-catenin. In addition, a pronounced increase in the number of perforated synapses has been reported in the brains of mice ablated for β-catenin in vivo (21).
It has become increasingly clear that PDZ proteins serve as critical regulators of the trafficking of AMPARs (37). Our findings identify β-catenin as a further extension that links PDZ protein-dependent regulation of synaptic AMPARs to a synapse adhesion complex. A role for the PDZ-binding motif of β-catenin in the postsynaptic organization mirrors the presynaptic function for β-catenin in controlling the synaptic vesicle cluster (21), where, similarly to the postsynaptic side, the linkage of β-catenin to the presynaptic PDZ proteins, rather than its link to the actin cytoskeleton, is important. Among PDZ proteins that bind to the PDZ domain-binding motif of β-catenin are Velis, which in turn binds to CASK (38), and S-SCAM, which is also known as MAGI-2 (39). Our attempts to determine the contribution of Velis and S-SCAM in β-catenin-dependent regulation of synaptic AMPARs were inconclusive, and Par-3, whose binding to β-catenin has been recently reported (40), or additional as yet to be identified β-catenin-interacting PDZ proteins could provide a link to the AMPAR modification. Other non-PDZ interactions of β-catenin could also play a role. For instance, binding of cadherins and β-catenin to the LAR–liprin-α–GRIP complex through the N-terminal domain of β-catenin has been shown to facilitate the postsynaptic recruitment of AMPARs and the cadherin–β-catenin complex (41), whereas N-cadherin has also been reported to associate directly with AMPARs (42). Notably, Wnt signaling through β-catenin has been demonstrated to modulate AMPARs in Caenorhabditis elegans synapses (43). Although our experiments do not exclude the potential contribution of Wnt signaling, it is unlikely for the following reasons. In the present work, the reduction of mEPSC size occurred upon overexpressing either the ΔARM mutant, which was shown not to interfere with Wnt signaling of the endogenous β-catenin (44), or the ΔPDZ mutant, which was deleted for the region nonessential for Wnt signaling (45). Moreover, reduced mEPSC size was also observed when a DN N-cadherin was overexpressed.
It is of interest to consider the effects of manipulating postsynaptic β-catenin levels on mEPSC amplitude. Whereas a loss of β-catenin resulted in a decrease in mEPSC amplitude, overexpression of WT β-catenin or WT N-cadherin did not increase mEPSC amplitude (cf. Figs. 3C and 4C). In addition, a previous study reported a lack of change in mEPSC amplitude under conditions that recruited exogenous β-catenin to dendritic spines (23). Synaptic strength modulation thus appears not to be simply proportional to the level of postsynaptic β-catenin. Rather, these findings suggest that whereas β-catenin serves to maintain a functional pool of synaptic AMPARs, on its own, it may be insufficient to expand the functional capacity of the postsynaptic receptor pool. Components of the postsynaptic scaffold that interact with β-catenin and that become limiting upon overexpressing β-catenin are reasonable candidates that regulate the upper limit of postsynaptic strength.
In summary, our work demonstrates that the cadherin–β-catenin complex is not only important for synapse assembly and dendritic spine formation, but plays a central role in controlling the quantal AMPA responses. By regulating the abundance of functional synaptic AMPARs by its PDZ interaction domain and by controlling the shape of dendritic spines, the cadherin–β-catenin complex serves as bifunctional regulator that couples postsynaptic strength and spine morphology.
Materials and Methods
DNA Constructs.
Cre-GFP was a generous gift from Kenji Okuse (Imperial College, London, U.K.). N-cadherin and Myc-β-catenin were from Yasuyuki Fujita (MRC Laboratory for Molecular Cell Biology). αN-catenin was from Masatoshi Takeichi (Kyoto University, Japan) [for details of the plasmid modifications and mutant constructs, see supporting information (SI) Materials and Methods].
Hippocampal Cultures and Transfection.
Primary cultures of hippocampal neurons were prepared from P0–P2 rats or the β-catenin-floxed mice (28) as described previously (6). Unless otherwise noted, neurons were transfected at DIV8–10. In some experiments, cultures were treated with 1 μM TTX or 20 μM bicuculline for 48 h. For details, see SI Materials and Methods.
Immunocytochemistry and Image Analysis.
Immunocytochemical analysis was performed on DIV14–18 cultures (for details, see SI Materials and Methods). Fluorescence images were analyzed with the ImageJ software (National Institutes of Health, Bethesda, MD). Spine shape analysis was as described (46).
Electrophysiology.
Whole-cell patch-clamp recordings were performed from 12 to 16 DIV neurons as described (47). Kinetic parameters of mEPSC were determined by simultaneously fitting the individual mEPSC trace with the sum of two exponentials, with each exponential corresponding to the rise and decay phases.
HEK Cell Dissociation Assay and Immunoprecipitation.
Confluent HEK cells transiently expressing the exogenous N-cadherin constructs were treated with 0.01% trypsin with 2 mM Ca2+ (TC) or 2 mM EGTA (TE) in Hepes-buffered saline at 37°C for 30 min and dissociated by trituration. Ca2+-dependent cell adhesive activity was quantified by calculating the index: 1 − [NTC/NTE.], where NTC and NTE are the cell particle number after the TC and TE treatment, respectively. Immunoprecipitations were carried out by incubating HEK cell lysates with anti-Myc-agarose for 1 h (for details, see SI Materials and Methods).
Statistics.
All data are shown as the mean ± SEM. Statistical significance was determined by the two-tailed Student t test or one-way ANOVA.
Supplementary Material
Acknowledgments
We thank Drs. Kenji Okuse, Yasuyuki Fujita, and Masatoshi Takeichi for the plasmids, and Yasuyuki Fujita, Tiago Branco, and Kevin Staras for comments on the manuscript. This work was supported by a Human Frontier Science Program Long-Term Fellowship (to T.O.), the Medical Research Council (to Y.G.), and National Institute of Mental Health Grant MH66676 (to Y.G.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- DIV
days in vitro
- DN
dominant-negative
- IRES
internal ribosome entry site
- mEPSC
miniature excitatory postsynaptic current
- TTX
tetrodotoxin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702334104/DC1.
References
- 1.Yuste R, Bonhoeffer T. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 2.Dillon C, Goda Y. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 3.Neuhoff H, Roeper J, Schweizer M. Eur J Neurosci. 1999;11:4241–4250. doi: 10.1046/j.1460-9568.1999.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 5.De Paola V, Arber S, Caroni P. Nat Neurosci. 2003;6:491–500. doi: 10.1038/nn1046. [DOI] [PubMed] [Google Scholar]
- 6.Colicos MA, Collins BE, Sailor MJ, Goda Y. Cell. 2001;107:605–616. doi: 10.1016/s0092-8674(01)00579-7. [DOI] [PubMed] [Google Scholar]
- 7.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Homma KJ, Poo MM. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Scheiffele P. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 12.Yamagata M, Sanes JR, Weiner JA. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 13.Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- 14.Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Malenka RC. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 16.Fannon AM, Colman DR. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner BM. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 18.Gates J, Peifer M. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Benson DL, Tanaka H. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungling K, Eulenburg V, Moore R, Kemler R, Lessmann V, Gottmann K. J Neurosci. 2006;26:6968–6978. doi: 10.1523/JNEUROSCI.1013-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murase S, Mosser E, Schuman EM. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 24.Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 25.Okamura K, Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, Zeng LH, Colman DR, Miki N. J Cell Biol. 2004;167:961–972. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozdagi O, Valcin M, Poskanzer K, Tanaka H, Benson DL. Mol Cell Neurosci. 2004;27:509–521. doi: 10.1016/j.mcn.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe K, Chisaka O, Van Roy F, Takeichi M. Nat Neurosci. 2004;7:357–363. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- 28.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Development (Cambridge, UK) 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 29.Roelandse M, Welman A, Wagner U, Hagmann J, Matus A. Neuroscience. 2003;121:39–49. doi: 10.1016/s0306-4522(03)00405-6. [DOI] [PubMed] [Google Scholar]
- 30.Cathala L, Holderith NB, Nusser Z, DiGregorio DA, Cull-Candy SG. Nat Neurosci. 2005;8:1310–1318. doi: 10.1038/nn1534. [DOI] [PubMed] [Google Scholar]
- 31.Thiagarajan TC, Lindskog M, Tsien RW. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Aberle H, Schwartz H, Hoschuetzky H, Kemler R. J Biol Chem. 1996;271:1520–1526. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- 33.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 34.Renger JJ, Egles C, Liu G. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 35.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR. Neuron. 2000;25:93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- 37.Bredt DS, Nicoll RA. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 38.Perego C, Vanoni C, Massari S, Longhi R, Pietrini G. EMBO J. 2000;19:3978–3989. doi: 10.1093/emboj/19.15.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura W, Yao I, Iida J, Tanaka N, Hata Y. J Neurosci. 2002;22:757–765. doi: 10.1523/JNEUROSCI.22-03-00757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, Lin CM, Chou CS, Chia W, Modolell J, et al. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. Nat Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- 42.Nuriya M, Huganir RL. J Neurochem. 2006;97:652–661. doi: 10.1111/j.1471-4159.2006.03740.x. [DOI] [PubMed] [Google Scholar]
- 43.Dreier L, Burbea M, Kaplan JM. Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 44.Funayama N, Fagotto F, McCrea P, Gumbiner BM. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orsulic S, Peifer M. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Simoni A, Griesinger CB, Edwards FA. J Physiol (London) 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales M, Colicos MA, Goda Y. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.