Abstract
Objectives:
To determine whether preferences for future attempts at life-sustaining treatment change over time in a consistent and predictable manner.
Design:
Observational cohort study.
Setting:
Community
Participants:
189 community-dwelling persons age ≥ 60 years with advanced cancer, heart failure, or chronic obstructive pulmonary disease.
Measurements:
Participants were asked, if faced with an illness exacerbation that would be fatal if untreated, whether they would: a) undergo high-burden therapy for a chance to avoid death and b) risk an impaired health state to avoid death. Interviews occurred at least every 4 months for up to 2 years.
Results:
When asked their willingness to undergo high-burden therapy for a chance to avoid death, 35% had an inconsistent preference trajectory (e.g. becoming more and then less willing over time or vice versa). The proportion with inconsistent trajectories increased to 48% and 49% when asked their willingness to risk physical or cognitive disability in order to avoid death. Participants with variable health states over time were more likely to have inconsistent trajectories; however, inconsistent trajectories were also common among those with stable health states.
Conclusions:
A large proportion of older persons with advanced illness have inconsistent trajectories of willingness to undergo burdensome therapy or risk an impaired health state for a chance to avoid death. This is explained in part by variability in their health state over time. However, the frequency of inconsistent trajectories even among those with stable health states suggests that preferences are influenced by transient factors rather than representing stable core values.
Keywords: Decision making, preferences, life-sustaining treatment
INTRODUCTION
The stability of patients' preferences for future attempts at life-sustaining treatment has been examined in a large number of studies, which have generally found that these preferences may change over time.1-11 These studies examined preferences at only two points in time and, therefore, could not elucidate the pattern of change in order to determine whether preferences change in a consistent and/or predictable fashion. Knowing the pattern of change in preferences is critical to evaluating the process of advance care planning, the process by which patients express their preferences for a future time in which they are no longer able to participate in medical decision-making. The finding of changing preferences has led to concerns about the validity of this process, because it suggests that patients may not be able to forecast their future preferences accurately.12, 13 If preferences change in a consistent direction and in response to changes in the patient's health, then a pattern of past response might be used to help patients understand how they might value future states of health.14, 15 If, however, changes in preferences do not occur in a consistent direction, as has been demonstrated in a more recent study examining the effects of hospitalization on preferences,16 then the challenges to advance care planning would be even greater, as prior patterns of response could not help to predict future responses.
We undertook a longitudinal study of older persons with advanced chronic disease to evaluate changes in treatment preferences over multiple points in time. We sought to determine the consistency of preference trajectories and to examine factors associated with consistent versus inconsistent trajectories.
METHODS
Participants
Participants for this study were members of a cohort of community-dwelling older persons with advanced chronic illness. The Human Investigations Committee of each of the hospitals participating in the study approved the study protocol, and each participant provided written informed consent. We screened sequential charts of persons age 60 years or older with a primary diagnosis of cancer, heart failure (HF), or chronic obstructive pulmonary disease (COPD) for the primary eligibility requirement: advanced illness, as defined by Connecticut Hospice criteria17 or SUPPORT criteria.18 Charts were identified according to the patient's age and primary diagnosis in the subspecialty outpatient practices in the greater New Haven area and in three hospitals: a university teaching hospital, a community hospital, and a VA hospital. Of the 26 practices approached for participation, 3 (12%) did not permit screening of their charts. An additional eligibility criterion used to define advanced disease19 was the need for assistance with at least one instrumental activity of daily living (IADL),20 determined during a telephone screen. Screening and enrollment was stratified by diagnosis to enroll approximately equal numbers of patients with cancer, HF, and COPD.
Of 548 patients identified by chart review, 78 did not receive the telephone screen because the physician refused permission to call (n=30), the patient died prior to call (n=24), the patient refused screening (n=18), or the patient could not be reached (n=6)). Of the 470 who were screened, 362 required IADL assistance. Exclusion criteria included cognitive impairment, as assessed by the Short Portable Mental Status Questionnaire21 and EXIT22 (n=77), and part-time Connecticut residence (n=6). Of 279 eligible patients, 2 died prior to participation and 51 refused participation. Non-participants did not differ from participants according to age or gender. Among eligible patients with HF, 8% refused participation, compared to 19% among patients with cancer and 25% among patients with COPD (p=.02). Of the 226 initial participants, 8 withdrew after the initial interview (4%), 26 died before completing a follow-up interview (12%), and 3 became too cognitively impaired, utilizing the same assessment as for eligibility, or too symptomatic to participate in follow-up interviews (1%), resulting in a cohort of 189 participants for the current study. Of the surviving 124 participants at the end of the first year of the study, 98 (79%) consented to a second year of participation, with no further withdrawals.
Data collection
We interviewed patients in their homes, obtaining variables by patient self-report, at least every four months for up to two years. If the patient had a decline in health status, determined by a monthly telephone call, the next interview was scheduled immediately. We then conducted subsequent interviews every four months, unless the patient had another decline. We defined decline in health status as: 1) a new disability in a basic activity of daily living (ADL),23, 2) a prolonged hospitalization (≥ 7 days) or a hospitalization resulting in a discharge to nursing home or rehabilitation facility, or 3) introduction of hospice services.
Sociodemographic and advance planning variables were obtained at the baseline assessment. Sociodemographic variables included age, gender, race/ethnicity, education, sufficiency of monthly income,24 marital status, and living arrangement. Advance planning variables included whether the patient had a living will or a health care proxy. Health status variables were obtained at each interview and included self-rated health, measured on a 5-point scale ranging from “excellent” to “poor,”25 number of ADL disabilities, ranging from 1 to 7,23 and self-rated quality of life, measured on a 5-point scale ranging from “best possible” to “worst possible.”
The outcome variables were patients' treatment preferences assessed using the Willingness to Accept Life-Sustaining Treatment instrument (WALT).26 The WALT, building on a number of earlier instruments,27-29 was developed in order to ask patients about their treatment preferences by asking them to consider the trade-offs involved in the receipt of life-sustaining therapy. This approach was chosen because explicit consideration of the trade-offs involved in complex decisions has been shown to improve the quality of decision making.30 Moreover, several studies have shown that standard decision-analytic approaches to evaluate preferences are difficult for older persons to complete,31 may lead to illogical responses,32, 33 and may be particularly ill suited for the elicitation of preferences regarding life-sustaining treatment.34, 35
Participants were asked to consider an exacerbation of their illness, which, if left untreated, would result in their death. They were asked to consider whether they would want treatment in a series of 3 scenarios. In the first scenario, participants were asked to consider the trade-off of enduring high-burden treatment for a given chance to avoid death. They were asked whether they would want high-burden treatment if it would return them to current health. Participants who would choose to have treatment were then asked whether they wanted therapy as the likelihood of death versus a return to current health increased.
The second and third scenarios asked participants to consider the trade-off of risking a severely impaired health state in order to avoid death. Participants were asked whether they would want low-burden treatment resulting in either severe physical disability, described as being bedbound, or severe cognitive disability, described as not being aware of what is going on around you. Participants who would choose not to have therapy were then asked whether they would want therapy as the likelihood of return to current health versus disability increased. Clinically meaningful likelihoods: 1%, 10%, 50%, 90%, 99% chance of death or impaired health state were used, and participants were shown pie charts depicted the likelihood of a return to current health versus the adverse outcome. The participant's preference was the highest likelihood of death, physical, or cognitive impairment at which he/she would want to receive therapy. (See Appendix A for a complete description of the scenarios as presented to participants).
Data analysis
We defined four trajectories for WALT scores over time: a) unchanged: participant provided the same response at each interview, b) increased willingness to undergo life sustaining therapy: WALT score greater in subsequent interview than in prior interview at least once and either equal to or greater than prior interview at all other interviews, c) decreased willingness to undergo life-sustaining therapy: WALT score lower in subsequent interview than in prior interview at least once and either equal to or less than prior interview at all other interviews, d) variable: all other patterns of scores. We then divided these trajectories into categories of “consistent,” which included the unchanged, decreased willingness, and increased willingness trajectories, and “inconsistent” which included the variable trajectory.
We examined the relationship between consistent vs. inconsistent trajectories and three factors: a) the number of interviews the participant completed, b) the participant's initial response to the 3 scenarios presented in the WALT, and c) sociodemographic status, health status, and advance care planning. We used the Wilcoxon sign-rank test or chi-square test as appropriate in bivariate analysis, but we also present medians for ease of interpretation. For health status variables, we examined the relationship between trajectories in WALT scores and both the baseline value for the health state variables and the trajectory of the health state variables over time. Using the same strategy as the one utilized for WALT trajectories, we characterized trajectories of self-rated health, self-rated quality of life, and ADL status as unchanged, improved, declined, and variable. We limited these analyses to participants with 3 or more interviews (n=154), because only these participants could have a variable health state trajectory.
To examine the independent association among these variables and trajectories, we used logistic regression models for each of the WALT scenarios where the outcome variable was whether the trajectory was consistent or inconsistent. We included all independent variables associated with a consistent versus inconsistent trajectory in bivariate analysis with a significance level of P ≤ 0.2.
RESULTS
Patient population
Table 1 provides a description of the baseline characteristics of the study population. Participants completed a median of 4 interviews (range 2, 10; interquartile range 3, 7). Of the 189 participants, 154 (81%) completed 3 or more interviews, and 65% completed 4 or more.
Table 1.
Description of Baseline Characteristics of 189 Participants
| Diagnosis (%) | |
| Cancer | 30 |
| COPD | 39 |
| HF | 31 |
| Age (yrs ± SD) | 73 ± 7 |
| Education (yrs ± SD) | 12 ± 3 |
| White (%) | 92 |
| Women (%) | 45 |
| Married (%) | 56 |
| Lives alone (%) | 25 |
| Has a living will (%) | 55 |
| Self-rated health: Excellent/Very Good/Good (%) | 38 |
| Self-rated quality of life: Best possible/Good (%) | 65 |
| ≥ 2 hospitalizations in past year (%) | 46 |
| Intensive care unit admission in past year (%) | 33 |
| ≥ 1 ADL dependency | 33 |
ADL = Activities of Daily Living; COPD = Chronic obstructive pulmonary disease; HF = heart failure
Interview trajectories and trajectories according to number of interviews
When participants were asked their willingness to undergo high-burden therapy for a given chance to avoid death, 35% had an inconsistent trajectory of response (Table 2). The proportion of participants with inconsistent trajectories increased to 48% when asked their willingness to risk physical disability and to 49% when asked their willingness to risk cognitive disability in order to avoid death.
Table 2.
Frequency of Trajectories in Willingness to Undergo Life-Sustaining Therapy and Number of Interviews Completed by Participants with Each of These Trajectories:
| WILLINGNESS TO UNDERGO HIGH BURDEN THERAPY TO AVOID DEATH |
Number of interviews per subject | p-value | ||||
|---|---|---|---|---|---|---|
| Trajectory | n | % | Median | Minimum | Maximum | |
| Consistent | 122 | 65 | 3 | 2 | 10 | |
| Inconsistent | 67 | 35 | 7 | 3 | 10 | <.0001 |
| WILLINGNESS TO RISK PHYSICAL DISABILITY TO AVOID DEATH* |
Number of interviews per subject | p-value | ||||
| Trajectory | n | % | Median | Minimum | Maximum | |
| Consistent | 97 | 52 | 3 | 2 | 10 | |
| Inconsistent | 91 | 48 | 6 | 3 | 10 | <.0001 |
| WILLINGNESS TO RISK COGNITIVE DISABILITY TO AVOID DEATH* |
Number of interviews per subject | p-value | ||||
| Trajectory | n | % | Median | Minimum | Maximum | |
| Consistent | 95 | 51 | 3 | 2 | 9 | |
| Inconsistent | 93 | 49 | 6 | 3 | 10 | <.0001 |
n = 188 because of one missing trajectory.
In each scenario, participants with variable trajectories had a higher median number of interviews than those with other types of trajectories of response (Table 2). However, there was an overlap in the range of interviews completed by participants with the different types of trajectories.
Trajectories according to initial response
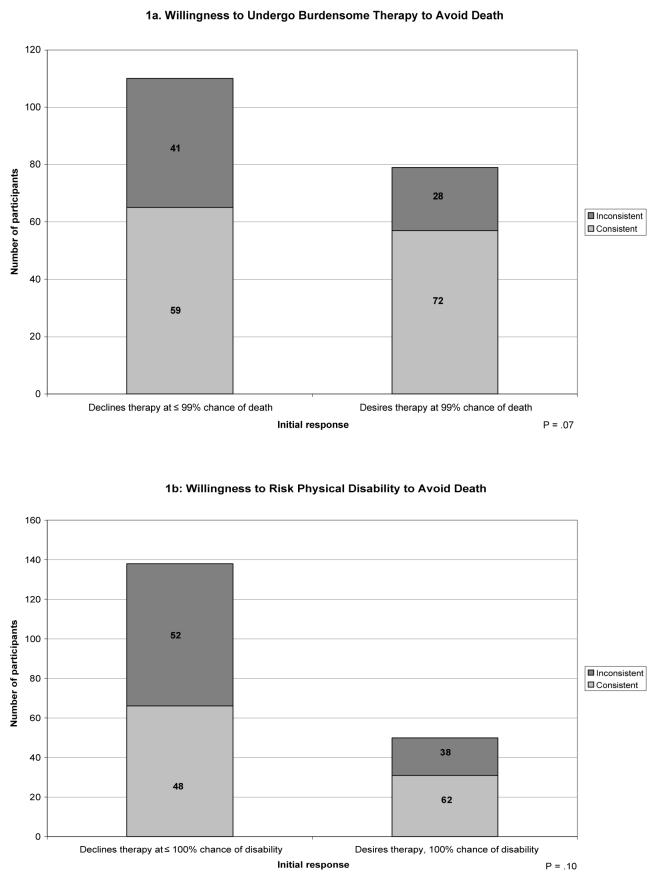
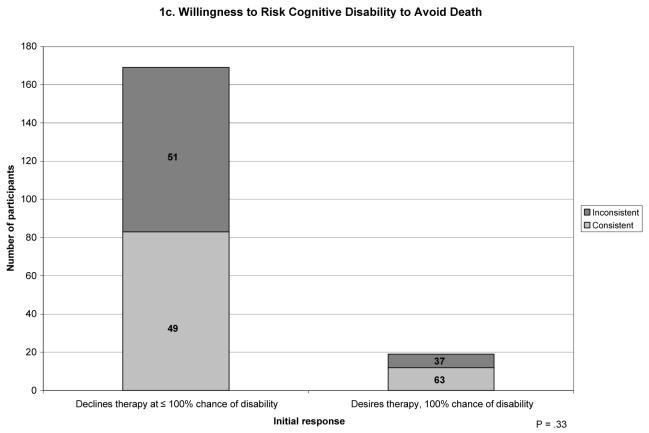
The frequencies of the preference trajectories differed according to participants' initial response (Figure 1). In each scenario, participants whose initial response was at the extreme of desiring therapy despite the highest likelihood of an adverse outcome were more likely to have a consistent trajectory of preferences compared to participants whose initial response was less extreme
Figure 1.
Trajectories according to initial willingness to undergo life-sustaining therapy. Each bar represents the total number of participants according to their initial willingness to undergo life sustaining therapy. The subsections of each bar indicate the number of participants with consistent versus inconsistent trajectories. The numbers within each subsection indicate the proportion of participants who had that trajectory among all participants with a given initial willingness to undergo therapy.
Trajectories according to participant characteristics
Neither participants' sociodemographic characteristics nor their baseline health status, in terms of their ADL status, self-rated health, or self-rated quality of life, was associated with their preference trajectories. Completion of a living will or health care proxy was also not associated with their preference trajectories. Participants' primary diagnosis was associated with the type of trajectory (Table 3). In general, there were lower proportions of inconsistent trajectories among participants with cancer than among participants with COPD or HF. Participants' health state trajectories were also associated with their preference trajectories (Table 3). In general, participants with a variable health state trajectory were most likely to have an inconsistent trajectory of preferences, and participants with an unchanged health state were least likely.
TABLE 3.
Prevalence of Inconsistent Trajectories according to Patient's Diagnosis and Trajectories in Health Status:
| WILLINGNESS TO UNDERGO HIGH BURDEN THERAPY TO AVOID DEATH |
WILLINGNESS TO RISK PHYSICAL DISABILITY TO AVOID DEATH |
WILLINGNESS TO RISK COGNITIVE DISABILITY TO AVOID DEATH |
||||
|---|---|---|---|---|---|---|
| Inconsistent trajectory | p-value | Inconsistent trajectory | p-value | Inconsistent trajectory | p-value | |
| n (%) |
n (%) |
N (%) |
||||
| Diagnosis | ||||||
| Cancer (n=39) | 11 (28) | .08 | 18 (46) | .16 | 16 (41) | .02 |
| COPD (n=64) | 32 (50) | 41 (64) | 44 (69) | |||
| HF (n=51) | 24 (47) | 32 (63) | 33 (65) | |||
| ADL status | ||||||
| No change (n=54) | 22 (41) | .13 | 31 (57) | .02 | 27 (50) | .07 |
| Improved (n=6) | 4 (67) | 5 (83) | 5 (83) | |||
| Declined (n=41) | 13 (32) | 17 (42) | 23 (56) | |||
| Variable (n=53) | 28 (53) | 38 (72) | 38 (72) | |||
| Self-rated health | ||||||
| No change (n=15) | 3 (20) | .008 | 5 (33) | .007 | 8 (53) | .28 |
| Improved (n=29) | 9 (31) | 16 (55) | 18 (62) | |||
| Declined (n=30) | 10 (33) | 13 (43) | 14 (47) | |||
| Variable (n=80) | 45 (56) | 57 (71) | 53 (66) | |||
| Quality of life* | ||||||
| No change (n=25) | 9 (36) | .24 | 13 (52) | .49 | 12 (48) | .12 |
| Improved (n=19) | 5 (26) | 9 (47) | 8 (42) | |||
| Declined (n=18) | 9 (44) | 11 (61) | 11 (61) | |||
| Variable (n=91) | 45 (49) | 58 (64) | 61 (67) | |||
ADL = Activities of Daily Living; COPD = Chronic obstructive pulmonary disease; HF = Heart failure
n= 153 because of one missing observation.
In multivariable analysis, after adjusting for the number of interviews the participant had completed, an initial preference at the extreme of desiring therapy despite a high likelihood of adverse outcome was significantly associated with a decreased likelihood of an inconsistent preference trajectory for two of the scenarios (Table 4). There were trends toward an increased likelihood of inconsistent preference trajectories among participants with inconsistent trajectories of self-rated health and quality of life for the scenarios of willingness to risk disability to avoid death. The associations between preference trajectories and ADL trajectories demonstrated in bivariate analysis were not confirmed in multivariable analysis.
Table 4.
Factors Associated with Inconsistent Preference Trajectories in Multivariable Analysis:
| WILLINGNESS TO UNDERGO HIGH BURDEN THERAPY TO AVOID DEATH |
WILLINGNESS TO RISK PHYSICAL DISABILITY TO AVOID DEATH |
WILLINGNESS TO RISK COGNITIVE DISABILITY TO AVOID DEATH |
|
|---|---|---|---|
| Adjusted Odds Ratio (95% Confidence Interval) |
|||
| Number of interviews | 1.9 (1.5, 2.4) | 1.7 (1.3, 2.2) | 1.9 (1.5, 2.4) |
| Diagnosis | |||
| Cancer | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| COPD | 2.0 (0.8, 5.4) | 1.7 (0.7, 4.2) | 2.5 (1.0, 6.1) |
| HF | 1.5 (0.5, 4.1) | 1.3 (0.5, 3.3) | 1.7 (0.7, 4.4) |
| Inconsistent ADL trajectory | 0.6 (0.2, 1.5) | 1.1 (0.4, 2.5) | 0.8 (0.3, 1.9) |
| Inconsistent SRH trajectory | 1.5 (0.7, 3.4) | 2.3 (1.0, 5.2) | -- |
| Inconsistent QOL trajectory | -- | -- | 1.8 (0.8, 3.8) |
| Initial preference at the extreme (wants therapy despite 99% chance of death/ 100% chance of disability) |
0.5 (0.2, 1.0) | 0.3 (0.1, 0.8) | 0.6 (0.2, 2.0) |
COPD = chronic obstructive pulmonary disease; HF = heart failure; ADL = activities of daily living; SRH = self-rated health; QOL = quality of life.
DISCUSSION
Among a cohort of older persons with advanced chronic illness interviewed at multiple time points, trajectories of preferences for potentially life-sustaining treatment, assessed in terms of participants' willingness to endure high-burden treatment for a given chance to avoid death or risk disability in order to avoid death, were frequently inconsistent. Many participants became more and then less willing (or vice-versa) over time to undergo future high-burden therapy or to risk severe disability. The likelihood of a consistent versus an inconsistent trajectory differed according to the trade-off under consideration, and according to the participant's primary diagnosis, nature of initial preference, and health state trajectory.
The inconsistency in preferences over time poses an extreme challenge to advance care planning. Advance directives, documents in which patients could detail their treatment preferences for times of incapacity, were proposed in order to allow patients to express what were assumed to be deeply held and therefore presumably stable values regarding medical treatment.36 Early reviews of the stability of end-of-life treatment preferences concluded either that preferences were indeed stable37 or that the rate of change was similar to other major life decisions, such as estate wills, and that stability could be improved by ensuring that decisions were well informed and carefully considered.38 More recent studies have demonstrated that preferences are only moderately stable.7, 9, 10 However, because changes in preferences have been associated with changes in the patient's health state,7, 9 the interpretation of this change is that it occurs in a consistent and predictable direction. The notion is that, because patients adapt to diminishing health,12 as their own health declines and states that they imagined as intolerable are actually experienced, older persons may become increasingly willing to undergo life-sustaining treatment.39
In contrast to the notion that preferences change in a consistent manner, the findings of this study demonstrate that many persons had an inconsistent pattern of preferences over time. The study supports several explanations for these findings. The first is that it is true that, as previously shown, changes in health status do affect preferences in a predictable way. However, in contrast to the assumption that the health state of persons with advanced chronic illness changes in a predictable way; ie: their health state steadily declines over time, in this study we found that health state may be variable over time. This explanation is supported by the finding that participants with inconsistent health state trajectories were more likely to have inconsistent preference trajectories than were participants with consistent health state trajectories. The frequency of inconsistent health state trajectories confirms prior work demonstrating variable functional status trajectories both among older persons in general40 and in the last year of life among persons dying with organ failure.41
However, the high frequency of inconsistent preference trajectories even among participants with consistent health state trajectories suggests that there are additional influences on preferences. These inconsistent trajectories supports prior work demonstrating that preferences are influenced by transient immediate circumstances and affective states that may change according to these circumstances.42, 43 One recent study, which examined preferences for life-sustaining treatment among a cohort of older persons before, shortly after, and then several months after being in the hospital demonstrated that hospitalization transiently changed preferences for a number of specific interventions.16 This notion of the potential importance of transient influences on preferences is further supported by the observation that the will of patients terminally ill with cancer to live fluctuates greatly over short time periods and is associated both with affective states of anxiety and depression and with the physical symptom of shortness of breath.44
The frequencies of inconsistent preference trajectories in the current study were greater than in a prior analysis performed in this cohort, which utilized a measure of treatment preferences based solely on a consideration of the health state that would result from treatment.45 This comparison suggests that the increased complexity of the choices participants were asked to consider in the present study also contributed to inconsistent trajectories. Although it is not surprising that a more complex choice would lead to less consistency in response, it is both critically important and highly challenging to determine why. It is possible that the complexity of the assessment may, by overwhelming the cognitive capacity and understanding of respondents, fail to reflect participants' core values and beliefs. The items used in this study demonstrated only moderate test-retest reliability, performed over a one-week period, with intraclass correlation coefficients of .49 -.77. However, if preferences are inherently unstable, this could also account for changes in response seen after one week, suggesting that test-retest reliability may not be a measure of the quality of assessment tools, and that alternative methods need to be used to determine how well these tools are understood.
Can the process of advance care planning overcome these challenges? It has been eloquently argued that, for some patients, the ability to indicate their preferred care in advance of decisional incapacity, ensures that they will end their lives according to the deeply held values with which they lived them.46 For patients with unchanging preferences, advance care planning may be a reliable process that reveals their deeply held and stable core values. Patients whose preferences are changing because of adaptation to declining health may be able to incorporate knowledge of how their preferences are changing into their valuation of future states of health and disability.14, 15 Whether patients whose preferences are influenced by variable trajectories of health and/or transient affective states can do the same is unknown and needs to be determined. These considerations suggest that the process of advance care planning would be greatly strengthened by asking patients to re-evaluate their preferences over time, with an explicit consideration of the reasoning underlying these preferences. This would serve to identify that subgroup of patients whose preferences reflect stable core values. For patients with more variable preferences, it would help them to recognize the factors influencing their preferences, which holds the potential to improve the advance care planning process.
The study was limited by the small numbers in the analyses examining health state trajectories and by the lack of racial and ethnic diversity in the study population, which decreases its generalizability.
Even with its potential limitations, our measure assessed preferences in a more systematic way than is typically done in clinical practice. That such an approach is characterized by a high degree of variability in response over time highlights the caution that must be applied to the interpretation of the treatment preferences that are elicited in clinical contexts. This variability also highlights the need to understand not only what patients' preferences may be but also how they have formulated these preferences and to recognize that inconsistency in the trajectory of treatment preferences is common.
ACKNOWLEDGMENTS
The authors thank Carm Joncas, RN and Barbara Mendes, RN for their extraordinary interviewing skills.
Financial Disclosures: This work was supported by grant PCC-98-070-1 from VA HSR&D, R01 AG19769 from the National Institute on Aging, P30 AG21342 from the Claude D. Pepper Older Americans Independence Center at Yale, and a Paul Beeson Physician Faculty Scholars Award. Dr. Fraenkel is supported by K23 AR048826 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fried is supported by K02 AG20113 from the National Institute on Aging.
Sponsor's Role: The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
Appendix
Scenario 1.
Think about if you were suddenly to get sick with an illness that would require you to be in the hospital for at least a month. It would either be that your [CHF, COPD, cancer] worsened, or you got sick with a different illness. In the hospital, you would need to have many minor tests, such as x-rays and blood draws, and you would require more tests, such as CT scans. You would need major therapies such as being in the intensive care unit, receiving surgery, or having a breathing machine. Without the treatment, you would not survive. If this treatment would get you back to your current state of health, would you want to have it?
If NO: Question complete.
If YES: Now, what if the doctor told you that there was a 50/50 chance that it would work and get you back to your current state of health. If it did not work, you would not survive. Without the treatment, then you would not survive for certain. Would you want the treatment?
If NO: Now what if the doctor told you there were a 90% (99%) chance that it would work and get you back to your current state of health and a 10% (1%) chance that it would not. Without the treatment, then you would not survive for certain. Would you want the treatment?
If YES: Now, what if the doctor told you there was a 10% (1%) chance that it would work and get you back to your current state of health and a 90% (99%) chance that it would not work. Without the treatment, then you would not survive for certain. Would you want the treatment?
Scenario 2.
Think again about if you were suddenly to get sick with an illness that would require you to be in the hospital for a few days to a week. It would either be that your [CHF, COPD, cancer] worsened, or you got sick with a different illness. In the hospital you would need to have minor tests, such as x-rays and blood draws, and therapies such as intravenous antibiotics and oxygen. However, this time, imagine that at the end of the treatment, you would be in a state where you would be bedbound. You would not be able to get up out of bed to the bathroom by yourself, and you would need help with all of your daily activities. Without the treatment, you would not survive. Would you want the treatment?
If YES: Question complete.
If NO: Now, what if the doctor told you that there was a 50/50 chance that it would get you back to your current state or would leave you bedbound. Without the treatment, then you would not survive for certain. Would you want the treatment?
If NO: Now what if the doctor told you there were a 90% (99%) chance that it would work and get you back to your current state of health and a 10% (1%) chance that it would leave you bedbound. Without the treatment, then you would not survive for certain. Would you want the treatment?
If YES: Now, what if the doctor told you there was a 10% (1%) chance that it would work and get you back to your current state of health and a 90% (99%) chance that it would leave you bedbound. Without the treatment, then you would not survive for certain. Would you want the treatment?
Scenario 3.
Think again about if you were suddenly to get sick with an illness that would require you to be in the hospital for a few days to a week. It would either be that your [CHF, COPD, cancer] worsened, or you got sick with a different illness. In the hospital you would need to have minor tests, such as x-rays and blood draws, and therapies such as intravenous antibiotics and oxygen. Now imagine that the treatment would leave you in a state where your mind would not be working, such that you would not be aware of what was going on around you or be able to recognize your loved ones. Without the treatment, you would not survive. Would you want the treatment?
If YES: Question complete.
If NO: Now, what if the doctor told you that there was a 50/50 chance that it would get you back to your current state or would leave you unaware. Without the treatment, then you would not survive for certain. Would you want the treatment?
If NO: Now what if the doctor told you there were a 90% (99%) chance that it would work and get you back to your current state of health and a 10% (1%) chance that it would leave you unaware. Without the treatment, then you would not survive for certain. Would you want the treatment?
If YES: Now, what if the doctor told you there was a 10% (1%) chance that it would work and get you back to your current state of health and a 90% (99%) chance that it would leave you unaware. Without the treatment, then you would not survive for certain. Would you want the treatment?
REFERENCES
- 1.Everhart MA, Pearlman RA. Stability of patient preferences regarding life-sustaining treatments. Chest. 1990;97:159–164. doi: 10.1378/chest.97.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein MD, Stocking CB, Antel JP, et al. Amyotrophic lateral sclerosis and life-sustaining therapy: patients' desires for information, participation in decision making, and life-sustaining therapy. Mayo Clin Proc. 1991;66:906–913. doi: 10.1016/s0025-6196(12)61577-8. [DOI] [PubMed] [Google Scholar]
- 3.Danis M, Garrett J, Harris R, et al. Stability of choices about life-sustaining treatments. Ann Intern Med. 1994;120:567–573. doi: 10.7326/0003-4819-120-7-199404010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Emanuel LL, Emanuel EJ, Stoeckle JD, et al. Advance directives. Stability of patients' treatment choices. Arch Intern Med. 1994;154:209–217. doi: 10.1001/archinte.154.2.209. [DOI] [PubMed] [Google Scholar]
- 5.Carmel S, Mutran EJ. Stability of elderly persons' expressed preferences regarding the use of life-sustaining treatments. Soc Sci Med. 1999;49:303–311. doi: 10.1016/s0277-9536(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld KE, Wenger NS, Phillips RS, et al. Factors associated with change in resuscitation preference of seriously ill patients. The SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Arch Intern Med. 1996;156:1558–1564. [PubMed] [Google Scholar]
- 7.Ditto PH, Smucker WD, Danks JH, et al. Stability of older adults' preferences for life-sustaining medical treatment. Health Psychol. 2003;22:605–615. doi: 10.1037/0278-6133.22.6.605. [DOI] [PubMed] [Google Scholar]
- 8.Kohut N, Sam M, O'Rourke K, et al. Stability of treatment preferences: although most preferences do not change, most people change some of their preferences. J Clin Ethics. 1997;8:124–135. [PubMed] [Google Scholar]
- 9.Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors Study. J Am Geriatr Soc. 2004;52:577–582. doi: 10.1111/j.1532-5415.2004.52165.x. [DOI] [PubMed] [Google Scholar]
- 10.Lockhart LK, Ditto PH, Danks JH, et al. The stability of older adults' judgments of fates better and worse than death. Death Stud. 2001;25:299–317. doi: 10.1080/07481180126279. [DOI] [PubMed] [Google Scholar]
- 11.Weissman JS, Haas JS, Fowler FJ, Jr., et al. The stability of preferences for life-sustaining care among persons with AIDS in the Boston Health Study. Med Decis Making. 1999;19:16–26. doi: 10.1177/0272989X9901900103. [DOI] [PubMed] [Google Scholar]
- 12.Ubel PA, Loewenstein G, Schwarz N, et al. Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24:S57–62. doi: 10.1037/0278-6133.24.4.S57. [DOI] [PubMed] [Google Scholar]
- 13.Fagerlin A, Schneider CE. Enough. The failure of the living will. Hastings Cent Rep. 2004;34:30–42. [PubMed] [Google Scholar]
- 14.Ubel PA, Loewenstein G, Jepson C. Disability and sunshine: can hedonic predictions be improved by drawing attention to focusing illusions or emotional adaptation? J Exp Psychol Appl. 2005;11:111–123. doi: 10.1037/1076-898X.11.2.111. [DOI] [PubMed] [Google Scholar]
- 15.Damschroder LJ, Zikmund-Fisher BJ, Ubel PA. The impact of considering adaptation in health state valuation. Soc Sci Med. 2005;61:267–277. doi: 10.1016/j.socscimed.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 16.Ditto PH, Jacobson JA, Smucker WD, et al. Context changes choices: a prospective study of the effects of hospitalization on life-sustaining treatment preferences. Med Decis Making. 2006;26:313–322. doi: 10.1177/0272989X06290494. 2006. [DOI] [PubMed] [Google Scholar]
- 17.The Connecticut Hospice Inc. Summary Guidelines for Initiation of Advanced Care. John Thompson Institute; Branford, CT: 1996. [Google Scholar]
- 18.Murphy DJ, Knaus WA, Lynn J. Study population in SUPPORT: patients (as defined by disease categories and mortality projections), surrogates, and physicians. J Clin Epidemiol. 1990;43:11S–28S. doi: 10.1016/0895-4356(90)90213-9. [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 24.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22:337–356. [PubMed] [Google Scholar]
- 25.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 26.Fried TR, Bradley EH, Towle VR. Assessment of patient preferences: Integrating treatments and outcomes. J Gerontol B Psychol Sci Soc Sci. 2002;57:S348–354. doi: 10.1093/geronb/57.6.s348. 2002. [DOI] [PubMed] [Google Scholar]
- 27.Danis M, Mutran E, Garrett JM, et al. A prospective study of the impact of patient preferences on life-sustaining treatment and hospital cost. Crit Care Med. 1996;24:1811–1817. doi: 10.1097/00003246-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Pearlman RA, Cain KC, Patrick DL, et al. Insights pertaining to patient assessments of states worse than death. J Clin Ethics. 1993;4:33–41. [PubMed] [Google Scholar]
- 29.Dales RE, O'Connor A, Hebert P, Sullivan K, et al. Intubation and mechanical ventilation for COPD: development of an instrument to elicit patient preferences. Chest. 1999;116:792–800. doi: 10.1378/chest.116.3.792. [DOI] [PubMed] [Google Scholar]
- 30.Janis IL, Mann L. A psychological analysis of conflict, choice, and commitment. The Free Press; New York: 1985. Decision-making. [Google Scholar]
- 31.Tsevat J, Dawson NV, Wu AW, et al. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279:371–375. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 32.Souchek J, Stacks JR, Brody B, et al. A trial for comparing methods for eliciting treatment preferences from men with advanced prostate cancer: results from the initial visit. Med Care. 2000;38:1040–1050. doi: 10.1097/00005650-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Bravata DM, Nelson LM, Garber AM, et al. Invariance and inconsistency in utility ratings. Med Decis Making. 2005;25:158–167. doi: 10.1177/0272989X05275399. 2005. [DOI] [PubMed] [Google Scholar]
- 34.Mehrez A, Gafni A. Quality-adjusted life years, utility theory, and healthy-years equivalents. Med Decis Making. 1989;9:142–149. doi: 10.1177/0272989X8900900209. [DOI] [PubMed] [Google Scholar]
- 35.Llewellyn-Thomas HA. Investigating patients' preferences for different treatment options. Can J Nurs Res. 1997;29:45–64. [PubMed] [Google Scholar]
- 36.Emanuel LL, Emanuel EJ. The Medical Directive. A new comprehensive advance care document. JAMA. 1989;261:3288–3293. doi: 10.1001/jama.261.22.3288. [DOI] [PubMed] [Google Scholar]
- 37.Miles SH, Koepp R, Weber EP. Advance end-of-life treatment planning. A research review. Arch Intern Med. 1996;156:1062–1068. [PubMed] [Google Scholar]
- 38.Emanuel L. Advance directives: what have we learned so far? J Clin Ethics. 1993;4:8–16. [PubMed] [Google Scholar]
- 39.Emanuel LL. Advance directives and advancing age. J Am Geriatr Soc. 2004;52:641–642. doi: 10.1111/j.1532-5415.2004.52177.x. [DOI] [PubMed] [Google Scholar]
- 40.Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci. 2003;58:70–75. doi: 10.1093/gerona/58.1.m70. [DOI] [PubMed] [Google Scholar]
- 41.Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 42.Loewenstein G, Schkade D. Wouldn't it be nice? Predicting future feelings. In: Kahneman D, Diener E, Schwarz N, editors. Well-Being: The Foundations of Hedonic Psychology. Russell Sage Foundation; New York: 1999. pp. 85–108. [Google Scholar]
- 43.Loewenstein G. Projection bias in medical decision making. Med Decis Making. 2005;25:96–105. doi: 10.1177/0272989X04273799. [DOI] [PubMed] [Google Scholar]
- 44.Chochinov HM, Tataryn D, Clinch JJ, et al. Will to live in the terminally ill. Lancet. 1999;354:816. doi: 10.1016/S0140-6736(99)80011-7. [DOI] [PubMed] [Google Scholar]
- 45.Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890–895. doi: 10.1001/archinte.166.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine C. She died the same way she lived: planning well in advance. New York Times. 2005 Dec 6;:F5. [PubMed] [Google Scholar]