Abstract
Morphine is recommended as a first-line opioid analgesic in the pain management of cancer patients. Accumulating evidence shows that morphine has anti-apoptotic activity, but its impact on the therapeutic applications of antineoplastic drugs is not well known. The present study was undertaken to test the hypothesis that morphine might antagonize the pro-apoptotic activity of DOX (doxorubicin), a commonly used antitumour drug for the treatment of neuroblastoma, in cultured SH-SY5Y cells. In the present study we demonstrated that morphine suppressed DOX-induced inhibition of cell proliferation and programmed cell death in a concentration-dependent, and naloxone as well as pertussis toxin-irreversible, manner. Further studies showed that morphine inhibited ROS (reactive oxygen species) generation, and prevented DOX-mediated caspase-3 activation, cytochrome c release and changes of Bax and Bcl-2 protein expression. The antioxidant NAC (N-acetylcysteine) also showed the same effects as morphine on DOX-induced ROS generation, caspase-3 activation and cytochrome c release and changes in Bax (Bcl-2-associated X protein) and Bcl-2 protein expression. Additionally, morphine was found to suppress DOX-induced NF-κB (nuclear factor κB) transcriptional activation via a reduction of IκBα (inhibitor of nuclear factor κB) degradation. These present findings support the hypothesis that morphine can inhibit DOX-induced neuroblastoma cell apoptosis by the inhibition of ROS generation and mitochondrial cytochrome c release, as well as by blockade of NF-κB transcriptional activation, and suggests that morphine might have an impact on the antitumour efficiency of DOX.
Keywords: apoptosis, doxorubicin (DOX), morphine, nuclear factor κB, reactive oxygen species, SH-SY5Y cell
Abbreviations: Bax, Bcl-2-associated X protein; DAPI, 4′,6-diamidino-2-phenylindole; DCFH2-DA, 2,7-dichlorodihydrofluorescein diacetate; DOX, doxorubicin; HE, hydroethidine; IκBα, inhibitor of nuclear factor κB; NAC, N-acetylcysteine; NF-κB, nuclear factor κB; PI, propidium iodide; ROS, reactive oxygen species; SRB, sulforhodamine B
INTRODUCTION
Pain relief is a fundamental and formidable task in the treatment of cancer patients because most cancer patients have severe pain. The great majority of these patients require orally administered opioid analgesics for appropriate pain control [1]. Morphine has been shown to be a potent opioid analgesic with the characteristics of being the most widely available in a variety of oral formulations, has several routes of administration and it avoids the clinically relevant ceiling effect to analgesic. Therefore it is recommended as a first-line analgesic in the WHO (World Health Organization) Cancer Pain Relief Guidelines [2], and is commonly used for the treatment of pain in patients with cancers. In addition to the well-recognized analgesic effect, accumulating evidence demonstrates morphine to have anti-apoptotic activity. For example, morphine has been shown to delay normal cell death in the avian ciliary ganglion [3], to protect astrocytes from apoptosis triggered by apoptosis-promoting agents [4], and to increase the proliferation of tumour cells [5–7], as well as to promote breast tumour growth [8]. Moreover, morphine has also been reported to suppress lymphocyte apoptosis triggered by actinomycin, a chemotherapeutic agent used for the treatment of cancers [9]. Despite the widespread use of morphine to treat pain in patients with cancers, little is known about the impact of morphine on the therapeutic applications of antineoplastic drugs.
DOX (doxorubicin) is a broad-spectrum antitumour drug that is widely used for the treatment of various cancers [10,11]. ROS (reactive oxygen species) generation has been observed in a variety of tumour cell systems following DOX treatment [12–15]. ROS have been implicated in cell death regulation [16]. Not only can apoptosis be induced by exposing cells to exogenous oxidants [17], but also many chemical and physical agents, such as anticancer drugs, capable of inducing cell death are known to generate ROS. Therefore ROS derived from redox activation have been proposed to be responsible for DOX-induced apoptosis [18]. NF-κB (nuclear factor κB) is a ubiquitous nuclear transcription factor that plays a major regulatory role in the balance between cell survival and apoptosis via expression of its target genes [19]. Previously, a growing body of evidence has shown that NF-κB activation mediates DOX-induced apoptosis in a myriad of cell systems [11,20–22]. These results demonstrate that NF-κB activation and IκBα (inhibitor of nuclear factor κB) degradation are early events activated by DOX, and that NF-κB activation is essential for the pro-apoptotic role of DOX. It has been reported that ROS are involved in DOX-induced cytochrome c release and caspase-3 activation [12–15] and NF-κB translocation [20].
Because cancer patients often need to be treated with morphine and antineoplastic drugs such as DOX concurrently, it is important to know whether the anti-apoptotic activity of morphine would be harmful to the therapeutic efficiency of anticancer drugs against tumour cells. Based on the evidence that morphine inhibits NF-κB activation [23,24], and displays anti-apoptotic activity as mentioned above, we hypothesized that morphine would attenuate the ability of DOX to induce apoptosis. Therefore the present study was undertaken to test the hypothesis using the cultured neuroblastoma line SH-SY5Y cells as a model to detect the possible effects of morphine on DOX-induced apoptosis. In the present study we report that morphine inhibited the pro-apoptotic activity of DOX through partial suppression of DOX-induced ROS generation, mitochondrial cytochrome c release and NF-κB transcriptional activation. These findings suggest the need for further study into the effects of morphine on patients receiving chemotherapeutic agents such as DOX for the treatment of cancer.
MATERIALS AND METHODS
Reagents
Morphine hydrochloride was purchased from Qinghai Pharmaceutical General Factory. SRB (sulforhodamine B), DCFH2-DA (2,7-dichlorodihydrofluorescein diacetate), HE (hydroethidine), NAC (N-acetylcysteine), DAPI (4′,6-diamidino-2-phenylindole), DOX, AnnexinV-FITC/PI (propidium iodide) apoptosis detection kit and an anti-β-actin antibody were purchased from Sigma–Aldrich. Anti-IκBα, anti-NF-κB and anti-caspase-3 antibodies were supplied by Santa Cruz Biotechnology. Anti-Bcl-2, anti-Bax, anti-(cleaved caspase-3) and anti-(cytochrome c) antibodies were from Cell Signaling.
Cell culture
Human SH-SY5Y neuroblastoma cells were cultured in Dulbecco's modified Eagle's medium and F-12 medium (1:1, Gibco) with 10% (v/v) fetal bovine serum, and maintained at 37 °C with 95% humidified air and 5% CO2. All experiments were performed using logarithmically growing cells.
Cell viability assay
Cell viability was determined using the SRB assay [25]. Briefly, cells were plated at a density of 1×104 cells/well in 96-well plates and incubated overnight, and were then treated for 48 h with either vehicle or various concentrations of DOX (1–4 μM) in the absence or presence of increasing concentrations of morphine (50–400 μM). Morphine was added 1 h before DOX administration. At the end of treatment, cells were fixed with 10% (v/v) trichloroacetic acid, stained with 0.4% SRB solution, and the plate was read in a microplate reader at 520 nm (VERSAmax; Molecular Devices). Analysis was performed on triplicate wells, and the data presented is representative of three independent experiments.
Apoptosis assay
Apoptotic cells were quantified using an AnnexinV-FITC/PI kit and FACSCalibur flow cytometry as described previously [26]. Cells were plated at a density of 2×105 cells/well in six-well plates and incubated overnight, and were then treated with either vehicle or 4 μM DOX in the absence or presence of morphine (50–200 μM). Morphine was added 1 h before DOX administration. After 48 h of treatment, cells were washed with DPBS, detached, collected and resuspended in 500 μl of binding buffer [10 mM Hepes (pH 7.5), 2.5 mM CaCl2 and 140 mM NaCl], and incubated with 1 μg/ml Annexin V-FITC and 2 μg/ml PI for 10 min in the dark, then flow cytometric analysis was performed. A total of 10000 cells were acquired per sample, and data were analysed using CellQuest software (BD PharMingen). Cells in the early stages of apoptosis were Annexin V positive; whereas, cells that were Annexin V and PI positive were in the late stages of apoptosis.
H2O2 and O2•− assay
Cells were seeded in six-well plates at 2×105 cells/well and incubated overnight, and were then either treated with 4 μM DOX for a range of times from 1–48 h, or treated with 4 μM DOX in the presence of 200 μM morphine or 5 mM NAC for 24 h, or treated with vehicle (used as a control). Accumulation of intracellular O2•− and H2O2 was determined with the probes HE and DCFH2-DA respectively, as described previously [27,28]. At the end of treatment, cells were incubated with 5 μM HE or 10 μM DCFH2-DA for 20 min at 37 °C in a humidified atmosphere with 5% CO2. The fluorescence intensity (HE, FL-2 channel; DCFH2-DA, FL-1 channel) was measured by flow cytometry, and the data were analysed using CellQuest software.
Western blot analysis
Cells were seeded in 100-mm diameter dishes at 8×105 cells/dish and incubated overnight, and were then either treated with vehicle, 200 μM morphine, 5 mM NAC or 4 μM DOX alone, or treated with 200 μM morphine, 5 mM NAC in the presence of 4 μM DOX for 12 or 48 h. After treatment, cells were washed twice with cold PBS and solubilized in lysis buffer [50 mM Tris/HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 0.02% Na3N, 1% Nonidet P40, 1 mM PMSF and 2 μg/ml aprotinin). Protein concentrations were determined by the Lowry method [28a]. Protein samples (30 μg) were separated by SDS/PAGE (12% gels) and transferred on to a nitrocellulose membrane (Amersham Biosciences). The membrane was incubated with anti-caspase-3, anti-(cleaved caspase-3), anti-Bcl-2, anti-Bax, anti-IκBα or anti-β-actin as primary antibodies diluted in 5% non-fat milk in PBS with 0.1% Tween 20, followed by incubation with horseradish peroxidase-conjugated IgG (Calbiochem) as the secondary antibody. Visualization was carried out using an ECL® (enhanced chemiluminescence) kit (Amersham Biosciences).
Analysis of cytosolic cytochrome c
Cells were seeded in 100-mm diameter dishes at 8×105 cells/dish and incubated overnight, and were either treated with 4 μM DOX alone or treated with 4 μM DOX in combination with 200 μM morphine or 5 mM NAC for 48 h. Cytochrome c release from mitochondria into the cytosol was measured by Western blot analysis, as described previously [14]. Briefly, cells were harvested by centrifugation at 1000 g for 10 min at 4 °C. The cell pellets were washed twice with ice-cold PBS and resuspended with 5 vol of lysis buffer [20 mM Hepes-KOH (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1.0 mM sodium EDTA, 1.0 mM sodium EGTA, 250 mM sucrose and 500 μg/ml digitonin), supplemented with 0.1 mM PMSF and 10 mg/ml aprotinin. After incubation on ice for 5 min, the cells were homogenized and centrifuged at 1000 g for 10 min at 4 °C. The supernatants were centrifuged at 12000 g for 15 min at 4 °C. The supernatant was collected and added to an equal volume of 2×SDS sample buffer [100 mM Tris/HCl (pH 6.8), 4% SDS, 20% glycerol, 0.2% Bromophenol Blue and 200 mM dithiothreitol]. The cytosolic extract was separated by SDS/PAGE (12% gels), transferred on to a nitrocellulose membrane, and incubated with antibodies against cytochrome c.
NF-κB translocation assay
NF-κB translocation was visualized using laser scanning confocal microscopy [29]. Briefly, SH-SY5Y cells grown on glass coverslips were pretreated with 200 μM morphine or 5 mM NAC for 1 h, and then treated with 4 μM DOX for 16 h. Thereafter, cells were fixed at room temperature (25 °C) with 4% (w/v) paraformaldehyde/PBS for 20 min, then permeabilized at room temperature by 0.2% (v/v) Triton X-100/PBS for 10 min and blocked with 1% BSA/PBS for 1 h. The cells were then incubated at 37 °C with a mouse anti-NF-κB p65 monoclonal antibody for 1 h. After washing slides with 0.1% Tween 20-PBS, the cells were incubated with an Alexa Fluor® 488 conjugated anti-mouse secondary antibody (Molecular Probes) for 1 h at 37 °C. The cells were then co-stained with DAPI in the dark. Scanning images were recorded with a laser confocal microscope (Leica Microsystems).
Statistical analysis
Data are presented as the means±S.E.M. Statistical differences were determined by ANOVA followed by post-hoc analysis for multiple comparisons or the Student's t test.
RESULTS
Morphine inhibits DOX-induced cytotoxicity and apoptosis
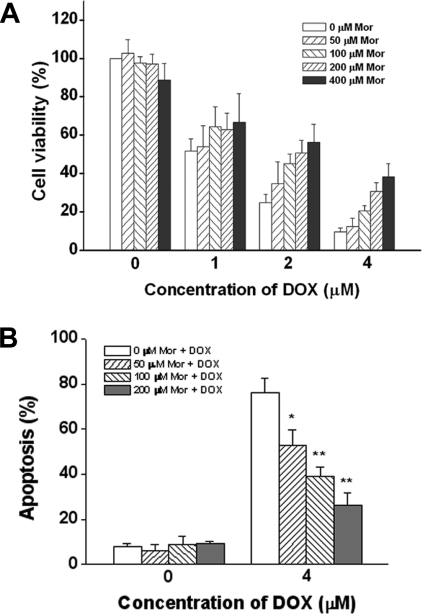
To determine the possible effect of morphine on the pro-apoptotic activity of DOX, SH-SY5Y cells were treated with DOX in the absence or presence of morphine. Cell viability was detected using the SRB assay. As shown in Figure 1(A), DOX alone substantially affected cell survival, whereas in the presence of morphine, cell viability was significantly enhanced in a concentration-dependent manner, indicating that morphine was able to inhibit cell death induced by DOX. Moreover, inhibition of the pro-apoptotic activity of DOX by morphine was further examined by an Annexin V/PI double staining assay. As shown in Figure 1(B), morphine was able to inhibit DOX-induced apoptosis in a concentration-dependent manner, with 50, 100 and 200 μM morphine inhibiting apoptosis by 26%, 39% and 53% respectively, which confirms the results obtained using the SRB assay.
Figure 1. Morphine protectes SH-SY5Y cells from DOX-induced cell death.
(A) Cells were treated with various concentrations of DOX for 48 h in the absence or presence of an increasing concentration of morphine (Mor), and cell viability was determined using the SRB assay as described in the Materials and methods section. (B) Cells were treated with 4 μM DOX in the presence of increasing concentrations of morphine (Mor) for 48 h, and the apoptotic cells were detected by flow cytometry as described in the Materials and methods section. Values are expressed as a percentage of the untreated control cell samples and represented as means±S.E.M. for at least three independent experiments performed in triplicate. *P<0.05 and **P<0.01 compared with DOX respectively.
Morphine inhibits DOX-induced ROS generation
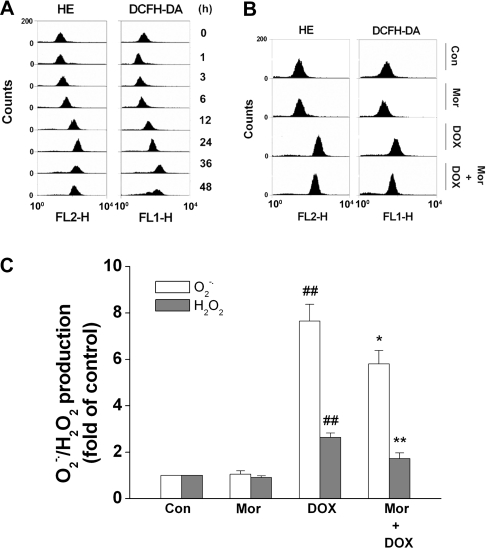
ROS generation has been demonstrated to be responsible for DOX-induced apoptosis [13,30,31]. To determine whether ROS is the mediator for DOX-induced apoptosis in SH-SY5Y cells, the cells were treated with 4 μM DOX for various times, as indicated in Figure 2. Intracellular O2•− and H2O2 levels were assessed by staining cells with HE and DCFH2-DA respectively, as described previously [28]. As shown in Figure 2(A), treatment of cells with DOX stimulated a significant increase in O2•− and H2O2 levels. An increase in ROS was detected as early as 3 h after DOX incubation, and the maximal enhancement was detected within 24 h and 36 h for O2•− and H2O2 respectively. Next, the effect of morphine on DOX-induced ROS generation was examined. When 200 μM morphine was present in conjunction with 4 μM DOX for 24 h, the generation of O2•− and H2O2 was significantly decreased by 24% and 35% respectively, compared with DOX alone (Figures 2B and 2C), indicating that morphine attenuated DOX-induced ROS generation.
Figure 2. Morphine antagonizes DOX-mediated enhancement of intercellular O2•− and H2O2 levels in SH-SY5Y cells.
(A) Cells were treated with 4 μM DOX for the indicated time periods, and the intracellular O2•− and H2O2 levels were detected by flow cytometry using 5 μM HE and 10 μM DCFH2-DA as fluorescent probes as described in the Materials and methods section. The Figure is representative of four independent experiments yielding similar results. (B and C) Cells were treated with either 4 μM DOX alone or with 4 μM DOX in combination with 200 μM morphine (Mor) for 24 h, and then the intracellular O2•− and H2O2 levels were detected. (B) Representative image of five independent experiments yielding similar results. (C) Quantification of O2•− and H2O2 generation. Values are means±S.E.M. for at least three independent experiments performed in triplicate (##P<0.01 compared with control; *P<0.05 and **P<0.01 compared with DOX alone).
Morphine inhibits DOX-induced caspase-3 activation, cytochrome c release and changes in the protein levels of Bax and Bcl-2
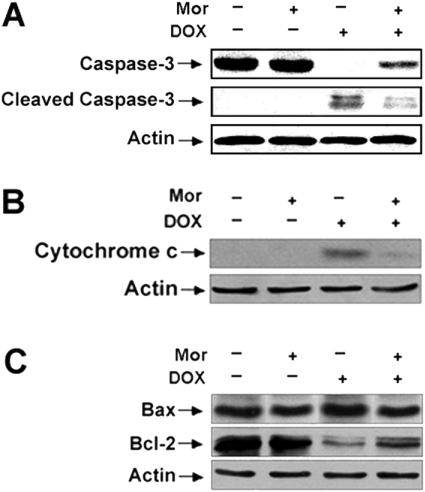
Mitochondria are a major source of the generation of ROS and also serve as the target of ROS during the apoptotic process [32]. To examine the molecular mechanism of morphine against DOX-mediated apoptosis, the effects of morphine on DOX-induced caspase-3 activation, cytochrome c release and the changes in the protein levels of Bax and Bcl-2 were determined using Western blot analysis. First, to confirm activation of caspase-3, the cleavage of procaspase-3 to its subunits was assessed. As shown in Figure 3(A), treatment of the cells with 4 μM DOX for 48 h induced the cleavage of procaspase-3 (32 kDa) to its 17- and 19-kDa subunits in these cells. However, cleavage of procaspase-3 to its subunits could be dramatically inhibited in cells treated with DOX in the presence of morphine, indicating that DOX-activated caspase-3 and that this activation could be prevented by morphine.
Figure 3. Morphine inhibites DOX-induced caspase-3 activation, cytochrome c release and changes in Bax and Bcl-2 protein levels in SH-SY5Y cells.
Cells were treated with either 4 μM DOX alone or with 4 μM DOX in combination with 200 μM morphine (Mor) for 48 h. Extracts from cells were subjected to SDS/PAGE (12% gels) and immunoblotted with (A) anti-procaspase-3 or anti-(cleaved caspase-3); (B) anti-cytochrome c and (C) anti-Bax or anti-Bcl-2 antibodies. Cytochrome c was isolated from the cytosol and measured by Western blotting as described in the Materials and methods section. Anti-actin antibodies were used as a control for equal loading.
The release of cytochrome c from mitochondria to the cytosol is essential for caspase-3 activation [33]. Next, the effect of DOX treatment on cytochrome c release was examined. The cytosolic fractions from cells were isolated, and the presence of cytochrome c was detected using an anti-cytochrome c antibody. Treatment of cells with DOX for 48 h led to large amounts of cytochrome c release into the cytosol compared with control cells. However, in the presence of morphine DOX was unable to induce significant cytochrome c release (Figure 3B).
Bcl-2 family proteins play an important role in regulating cytochrome c release and caspase-3 activation. Bcl-2, an anti-apoptotic protein, prevents the release of cytochrome c from mitochondria, whereas Bax, a pro-apoptotic protein, promotes the release of cytochrome c from mitochondria. Therefore the effects of treatment with DOX on the protein levels of Bcl-2 and Bax were further assessed. As shown in Figure 3(C), treatment of cells with DOX induced a marked decrease in the protein level of Bcl-2 and a robust increase in the protein level of Bax. When morphine was co-administered with DOX, it was able to inhibit the DOX-induced reduction of Bcl-2 protein and enhancement of Bax protein. Morphine itself had no significant impact on caspase-3 activation, cytochrome c release and the changes in the levels of Bcl-2 and Bax proteins.
Morphine inhibits DOX-induced IκBα degradation and NF-κB translocation
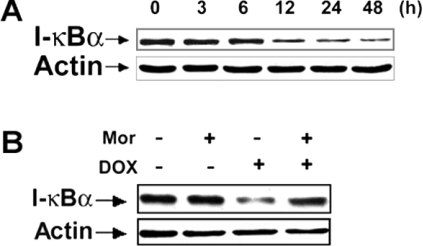
Previous studies have demonstrated that NF-κB plays a pro-apoptotic role in DOX-induced apoptosis in tumour and endothelial cells [11,20,21]. To study whether the inhibitory effects of morphine on DOX-induced apoptosis could be related to the inhibition of NF-κB activation, the effects of morphine on DOX-induced IκBα degradation and NF-κB translocation were examined. IκBα degradation plays a key role in mediating activation of the transcription factor NF-κB [34]. First, IκBα degradation was determined following treatment with DOX for various times. As shown in Figure 4(A), treatment of SH-SY5Y cells with 4 μM DOX yielded a significant decrease in IκBα levels after 6 h, with a maximal reduction at 48 h. Next, the effect of morphine on IκBα degradation induced by DOX was examined. As shown in Figure 4(B), treatment of the cells with 4 μM DOX in the presence of 200 μM morphine over 12 h markedly decreased DOX-induced IκBα degradation.
Figure 4. Morphine inhibites DOX-mediated degradation of IκBα in SH-SY5Y cells.
(A) Cells were treated with 4 μM DOX for increasing time periods as indicated. Total cell lysates were resolved by SDS/PAGE (12% gels) and then immunoblotted to detect IκBα. (B) SH-SY5Y cells were treated with 4 μM DOX for 12 h in the absence or presence of 200 μM morphine (Mor), and then harvested for Western blot analysis. The image is a representative immunoblot for IκBα from three independent experiments yielding similar results. Anti-actin antibodies were used as a control for equal loading.
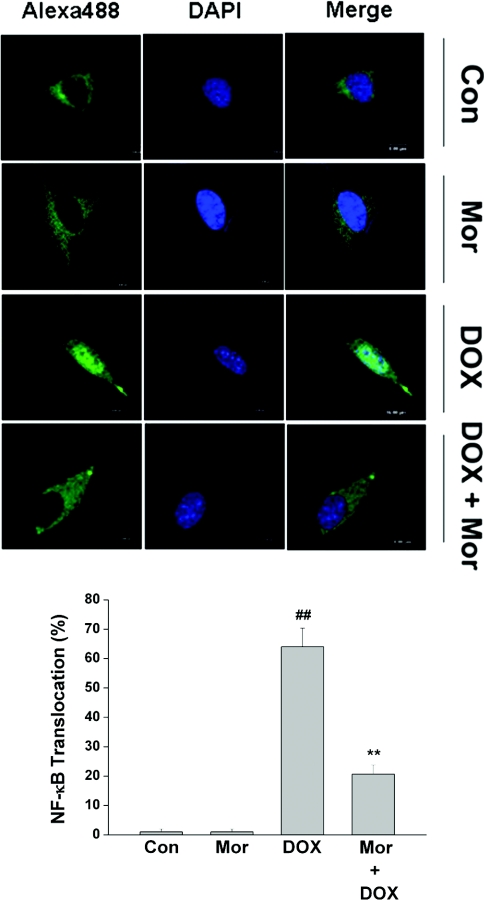
To examine the effect of morphine on DOX-induced translocation of NF-κB to the nucleus, the immunofluorescence of p65 protein was observed by confocal microscopy, which provides a visual detection of the location of NF-κB in SH-SY5Y cells. In the cells not treated by DOX, NF-κB resides predominantly in the cytoplasm (Figure 5, panel labelled ‘Con’). After a 16 h treatment with 4 μM DOX, the translocation of NF-κB from the cytoplasm to nuclei was apparent, which was monitored by tracking a strong white fluorescence in the nuclei. However, the translocation of NF-κB into nuclei was inhibited in the presence of morphine (Figure 5).
Figure 5. Morphine inhibites DOX-induced NF-κB p65 translocation in SH-SY5Y cells.
SH-SY5Y cells grown on glass coverslips were pretreated with 200 μM morphine (Mor) for 1 h, and then treated with 4 μM DOX for 16 h. After treatment, cells were immunostained and visualized as described in the Materials and methods section. The images are representative of three independent experiments yielding similar results. Green, NF-κB p65; dark blue, nucleus; white, NF-κB p65 in the nucleus. Values are means±S.E.M. for three independent experiments (##P<0.01 compared with control; **P<0.01 compared with DOX).
NAC inhibits DOX-mediated ROS generation, caspase-3 activation and cytochrome c release, but does not affect IκBα degradation and NF-κB translocation induced by DOX
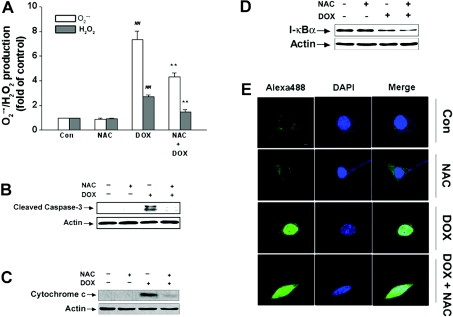
Antioxidants such as NAC and glutathione have been reported to inhibit anticancer drugs such as anthracyclines (daunorubicin) or DOX-induced tumour cell apoptosis [35,36]. To confirm the protective mechanism of morphine on DOX-induced apoptosis, the effects of NAC on DOX-mediated ROS generation, caspase-3 activation and cytochrome c release were determined. Similar to the effect of morphine administration, addition of exogenous NAC also greatly decreased DOX-stimulated intracellular generation of O2•− and H2O2 (Figure 6A), and dramatically blocked DOX-induced caspase-3 activation and cytochrome c release from mitochondria (Figures 6B and 6C). These results support the notion that the protective effects of morphine might be related to its inhibitory effects on ROS generation.
Figure 6. Effects of NAC on DOX-triggered cell death signalling.
Cells were treated with 4 μM DOX in the absence or presence of 5 mM NAC for different time periods (24 h for ROS, 48 h for cytochrome c, 12 h for IκBα and 16 h for NF-κB p65 respectively) and then the amount of intracellular ROS, caspase-3, cytochrome c, IκBα and NF-κB p65 were determined as described in the Materials and methods section. (A) NAC inhibited DOX-mediate ROS generation. Values are means±S.E.M. for three independent experiments (##P<0.01 compared with control; **P<0.01 compared with DOX alone). (B) NAC inhibited DOX-mediated caspase-3 cleavage. (C) NAC inhibited DOX-mediated enhancement of cytosolic cytochrome c levels. (D) NAC failed to inhibit DOX-mediated IκBα degradation. (E) NAC was unable to inhibit DOX-mediated NF-κB p65 translocation. The images are representative of three independent experiments yielding similar results. Green, NF-κB p65; dark blue, nucleus; white, NF-κB p65 in the nucleus.
Because ROS have been viewed previously as general messengers for signal-induced NF-κB activation [20,37], we further studied whether DOX-induced NF-κB activation and translocation was associated with ROS generation. Surprisingly, concurrent treatment of cells with NAC and DOX for 12 h had no effect on the DOX-induced decrease in IκBα protein expression levels (Figure 6D). Moreover, NAC also did not block the DOX-induced translocation of NF-κB from the cytoplasm to nuclei after 16 h incubation (Figure 6E). These data indicate that ROS were probably not involved in DOX-mediated NF-κB activation and suggest that inhibition of the IκBα degradation and NF-κB translocation by morphine might not be associated with its inhibitory effect on ROS generation.
DISCUSSION
In the present study, we demonstrated that morphine was able to inhibit DOX-mediated cytotoxicity and apoptosis in a dose-dependent manner. The underlying mechanisms are associated with the reduction of DOX-induced apoptosis by the inhibition of ROS generation and mitochondrial cytochrome c release, and blockade of NF-κB transcriptional activation. This occurred at concentrations that could be achieved as a result of orally administered morphine for pain control by cancer patients [1,2].
ROS generation is viewed as one of the main mechanisms of anthracycline cytotoxicity [18]. It has been demonstrated that daunorubicin- and DOX-induced apoptosis can be blocked by antioxidants, such as curcumin, NAC and glutathione [15,35,36]. To evaluate possible mechanisms responsible for morphine inhibition of apoptosis, we first studied ROS generation and found a significant increase in O2•− and H2O2 levels in cells treated for various times with DOX. Morphine effectively suppressed ROS accumulation in the DOX-treated SH-SY5Y cells. We further demonstrated that morphine suppressed DOX-induced caspase-3 activation, cytochrome c release and changes in Bax and Bcl-2 protein expression. The inhibition of ROS generation by morphine was correlated with its inhibitory effects on DOX-induced alterations of caspase-3 activation, cytochrome c release and changes in Bax and Bcl-2 protein expression levels, indicating that ROS might be a mediator for DOX-induced apoptosis, and decreases in ROS production might lead to inhibition of DOX-induced apoptosis in SH-SY5Y cells. The role of ROS as a mediator for DOX-induced apoptosis was further confirmed by treatment of cells with NAC, an exogenous thiol antioxidant. Pretreatment of cells with NAC also significantly attenuated DOX-induced ROS production, caspase-3 activation and cytochrome c release. Many reports have demonstrated that cytochrome c-dependent caspase-3 activation is an important mechanism responsible for ROS-induced apoptosis in vivo and in vitro [38]. The findings of the present study support the hypothesis that morphine suppresses DOX-induced apoptosis by, at least in part, blocking DOX-induced ROS formation, thereby leading to a decrease in cytochrome c release and attenuation of caspase-3 activation.
Besides causing apoptosis by ROS generation, DOX can induce apoptosis via several other mechanisms including the formation of ceramide [36], up-regulation of p53 function [39] and activation of NF-κB [11,20–22]. NF-κB is a ubiquitous nuclear transcription factor that plays a major regulatory role in apoptosis and inflammation. It resides in an inactive state in the cytoplasm as a heterotrimer consisting of p50, p65 and the inhibitory subunit of NF-κB (IκBα) [40]. Upon exposure of cells to cytotoxic agents including ligands of the cell surface death receptors such as tumour necrosis factor and Fas, and to genotoxic agents as well as chemotherapeutic drugs, IκBα subsequently undergoes phosphorylation, ubiquitination and degradation, causing the release and translocation of the NF-κB complex into the nucleus [41]. Activated NF-κB then binds to specific DNA sequences in promoters and thus regulates the expression of a number of genes which mediate the inflammatory response, apoptosis and carcinogenesis. Although a body of studies has documented NF-κB as an anti-apoptotic molecule [42,43], it has been also demonstrated to play a key role in mediating DOX-induced apoptosis in various cancer cells [11,20–22]. Since ROS serve as an upstream mediator for activation of NF-κB [20,37], and morphine inhibits NF-κB [23,24], we further studied whether inhibition of NF-κB activation and translocation is also implicated in the mechanism of morphine antagonism of DOX-induced apoptosis. Indeed, the present data demonstrated that DOX induced IκBα degradation and NF-κB transcriptional activation prior to cell death, as has been reported previously [11]. Morphine significantly inhibited IκBα degradation and NF-κB translocation into nuclei induced by DOX in SH-SY5Y cells. Distinct from the observations in other cell systems that ROS generation leads to the activation of NF-κB [20,44], we found that NAC, an antioxidant, has no significant effect on DOX-mediated IκBα degradation and NF-κB activation and translocation, suggesting that ROS formation might not be responsible for DOX-induced NF-κB activation in SH-SY5Y cells. The tumour suppressor p53 has been reported to cause NF-κB activation in cells that express wild-type p53 [45]. SH-SY5Y cells contain wild-type p53, and DOX has also been shown to increase p53 levels in SH-SY5Y cells [46]. Activation of p53, which in turn promotes apoptosis by activation of NF-κB, has been found in other tumour cells treated with DOX [20]. Efforts are underway in our laboratory to determine whether p53- and ceramide-dependent mechanisms are implicated in DOX-induced NF-κB activation in SH-SY5Y cells.
In addition, this inhibitory effect of morphine was not antagonized by the opioid antagonist naloxone across a range of concentrations from 1 to 100 μM, and by the G-protein inhibitor pertussis toxin at the concentrations of 10 and 100 ng/ml (results not shown). It is generally believed that at concentrations of naloxone and pertussis toxin as employed in the present study, a classical morphine response mediated by opioid receptors would be suitably blocked. The results suggest that the typical opioid receptor-coupled signalling cascade is not involved.
Taken together, the present study provides the first evidence that morphine significantly suppresses DOX-induced apoptosis through inhibition of ROS accumulation and mitochondrial cytochrome c release, and blockade of NF-κB transcriptional activation in SH-SY5Y cells.
Acknowledgments
This work was supported by the National Basic Research Program grant from the Ministry of Science and Technology of China G2003CB515401, the National Science Fund for Distinguished Young Scholar from the National Natural Science Foundation of China 30425002 and funds provided by the Chinese Academy of Sciences.
References
- 1.Ryan M., Moynihan T. J., Loprinzi C. L. As-needed morphine: yes, but at what dose and at what interval? J. Clin. Oncol. 2005;23:3849–3852. doi: 10.1200/JCO.2005.02.360. [DOI] [PubMed] [Google Scholar]
- 2.Davis M. P., Walsh D., Lagman R., LeGrand S. B. Controversies in pharmacotherapy of pain management. Lancet Oncol. 2005;6:696–704. doi: 10.1016/S1470-2045(05)70317-X. [DOI] [PubMed] [Google Scholar]
- 3.Meriney S. D., Gray D. B., Pilar G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. doi: 10.1126/science.2990029. [DOI] [PubMed] [Google Scholar]
- 4.Kim M. S., Cheong Y. P., So H. S., Lee K. M., Kim T. Y., Oh J., Chung Y. T., Son Y., Kim B. R., Park R. Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase) Biochem. Pharmacol. 2001;61:779–786. doi: 10.1016/s0006-2952(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa M., Tanno K., Kamo A., Takayanagi Y., Sasaki K. Enhancement of tumor growth by morphine and its possible mechanism in mice. Biol. Pharm. Bull. 1993;16:762–766. doi: 10.1248/bpb.16.762. [DOI] [PubMed] [Google Scholar]
- 6.Moon T. D. The effect of opiates upon prostatic carcinoma cell growth. Biochem. Biophys. Res. Commun. 1988;153:722–727. doi: 10.1016/s0006-291x(88)81154-9. [DOI] [PubMed] [Google Scholar]
- 7.Simon R. H., Arbo T. E. Morphine increases metastatic tumor growth. Brain Res. Bull. 1986;16:363–367. doi: 10.1016/0361-9230(86)90057-2. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K., Kshirsagar S., Chang L., Schwartz R., Law P. Y., Yee D., Hebbel R. P. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 9.Suzuki S., Chuang L. F., Doi R. H., Chuang R. Y. Morphine suppresses lymphocyte apoptosis by blocking p53-mediated death signaling. Biochem. Biophys. Res. Commun. 2003;308:802–808. doi: 10.1016/s0006-291x(03)01472-4. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz D. A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 11.Bian X., McAllister-Lucas L. M., Shao F., Schumacher K. R., Feng Z., Porter A. G., Castle V. P., Opipari A. W., Jr NF-κB activation mediates doxorubicin-induced cell death in N-type neuroblastoma cells. J. Biol. Chem. 2001;276:48921–48929. doi: 10.1074/jbc.M108674200. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani H., Tada-Oikawa S., Hiraku Y., Kojima M., Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–1453. doi: 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Tsang W. P., Chau S. P., Kong S. K., Fung K. P., Kwok T. T. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003;73:2047–2058. doi: 10.1016/s0024-3205(03)00566-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang G. W., Klein J. B., Kang Y. J. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J. Pharmacol. Exp. Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- 15.Somasundaram S., Edmund N. A., Moore D. T., Small G. W., Shi Y. Y., Orlowski R. Z. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 16.Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol. Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 17.Goldkorn T., Balaban N., Shannon M., Chea V., Matsukuma K., Gilchrist D., Wang H., Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J. Cell Sci. 1998;111:3209–3220. doi: 10.1242/jcs.111.21.3209. [DOI] [PubMed] [Google Scholar]
- 18.Muller I., Niethammer D., Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity. Int. J. Mol. Med. 1998;1:491–494. doi: 10.3892/ijmm.1.2.491. [DOI] [PubMed] [Google Scholar]
- 19.Luo J-L., Kamata M., Karin M. IKK/NF-κB signaling: balancing life and death-a new approach to cancer therapy. J. Clin. Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S., Kotamraju S., Konorev E., Kalivendi S., Joseph J., Kalyanaraman B. Activation of nuclear factor-κB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem. J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashikawa K., Shishodia S., Fokt I., Priebe W., Aggarwal B. B. Evidence that activation of nuclear factor-κB is essential for the cytotoxic effects of doxorubicin and its analogues. Biochem. Pharmacol. 2004;67:353–364. doi: 10.1016/j.bcp.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Müller I., Pfister S. M., Grohs U., Zweigner J., Handgretinger R., Niethammer D., Bruchelt G. Receptor activator of nuclear factor κB ligand plays a nonredundant role in doxorubicin-induced apoptosis. Cancer Res. 2003;63:1772–1775. [PubMed] [Google Scholar]
- 23.Roy S., Cain K. J., Chapin R. B., Charboneau R. G., Barke R. A. Morphine modulates NFκB activation in macrophages. Biochem. Biophys. Res. Commun. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- 24.Welters I. D., Menzebach A., Goumon Y., Cadet P., Menges T., Hughes T. K., Hempelmann G., Stefano G. B. Morphine inhibits NF-κB nuclear binding in human neutrophils and monocytes by a nitric oxide-dependent mechanism. Anesthesiology. 2000;92:1677–1684. doi: 10.1097/00000542-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Karl E., Warner K., Zeitlin B., Kaneko T., Wurtzel L., Jin T., Chang J., Wang S., Wang C. Y., Strieter R. M., et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-κB and CXC chemokines. Cancer Res. 2005;65:5063–5069. doi: 10.1158/0008-5472.CAN-05-0140. [DOI] [PubMed] [Google Scholar]
- 26.Yamochi T., Yamochi T., Aytac U., Sato T., Sato K., Ohnuma K., McKee K. S., Morimoto C., Dang N. H. Regulation of p38 phosphorylation and topoisomerase IIα expression in the B-cell lymphoma line Jiyoye by CD26/dipeptidyl peptidase IV is associated with enhanced in vitro and in vivo sensitivity to doxorubicin. Cancer Res. 2005;65:1973–1983. doi: 10.1158/0008-5472.CAN-04-2611. [DOI] [PubMed] [Google Scholar]
- 27.Wen J., You K. R., Lee S. Y., Song C. H., Kim D. G. Oxidative stress-mediated apoptosis. J. Biol. Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z., Leonard S. S., Huang C. S., Vallyathan V., Castranova V., Shi X. L. Role of reactive oxygen species and MAPKs in vanadate-induced G2/M phase arrest. Free Radical Biol. Med. 2003;34:1333–1342. doi: 10.1016/s0891-5849(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 28a.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Diao L. R., Zhang B. H., Fan J. K., Gao X., Sun S. G., Yang K., Xin D., Jin N. H., Geng Y. Q., Wang C. Herpes virus proteins ICP0 and BICP0 can activate NF-κB by catalyzing IκBα ubiquitination. Cell Signalling. 2005;17:217–229. doi: 10.1016/j.cellsig.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Kalivendi S. V., Konorev E. A., Gunningham S., Vanamala S. K., Kaji E. H., Joseph J., Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem. J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spallarossa P., Garibaldi S., Altieri P., Fabbi P., Manca V., Nasti S., Rossettin P., Ghigliotti G., Ballestrero A., Patrone F., et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J. Mol. Cell Cardiol. 2004;37:837–846. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q., Chai Y. C., Mazumder S., Jiang C., Macklis R. M., Chisolm G. M., Almasan A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ. 2003;10:323–334. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skulachev V. P. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett. 1998;423:275–280. doi: 10.1016/s0014-5793(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 34.Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol. Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansat-de Mas V., Bezombes C., Quillet-Mary A., Bettaieb A., de Thonel d'Orgeix A., Laurent G., Jaffrezou J. P. Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol. Pharmacol. 1999;56:867–874. doi: 10.1124/mol.56.5.867. [DOI] [PubMed] [Google Scholar]
- 36.Gouaze V., Mirault M-E., Carpentier S., Salvayer R., Levade T., Andrieu-Abadie N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol. Pharmacol. 2001;60:488–496. [PubMed] [Google Scholar]
- 37.Schulze-Osthoff K., Los M., Baeuerle P. A. Redox signalling by transcription factors NF-κB and AP-1 in lymphocytes. Biochem. Pharmacol. 1995;50:735–741. doi: 10.1016/0006-2952(95)02011-z. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang S., Lynch M. C., Kochevar I. E. Caspase-8 mediated caspase-3 activation and cytochrome c release during singlet oxygen-induced apoptosis of HL-60 cells. Exp. Cell Res. 1999;250:203–212. doi: 10.1006/excr.1999.4501. [DOI] [PubMed] [Google Scholar]
- 39.Wang S., Eugene A., Konorev E. A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H2O2- and p53-dependent pathways. J. Biol. Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- 40.Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 41.Chen F., Castranova V., Shi X., Demers L. M. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the intiation of diseases. Clin. Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 42.Romano M. F., Avellino R., Petrella A., Bisogni R., Romano S., Venuta S. Rapamycin inhibits doxorubicin-induced NF-κB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur. J. Cancer. 2004;40:2829–2836. doi: 10.1016/j.ejca.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Joyce D., Bouzahzah B., Fu M., Albanese C., D'Amico M., Steer J., Klein J. U., Lee R. J., Segall J. E., Westwick J. K., et al. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-κB-dependent pathway. J. Biol. Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt K. N., Amstad P., Cerutti P., Baeuerle P. A. The role of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-κB. Chem. Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 45.Ryan K. M., Ernst M. K., Rice N. R., Vousden K. H. Role of NF-κB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 46.Di Bartolomeo S., Di Sano F., Piacentini M., Spinedi A. Apoptosis induced by doxorubicin in neurotumor cells is divorced from drug effects on ceramide accumulation and may involve cell cycle-dependent caspase activation. J. Neurochem. 2000;75:532–539. doi: 10.1046/j.1471-4159.2000.0750532.x. [DOI] [PubMed] [Google Scholar]