Abstract
The normal PrPC (cellular prion protein) contains sLeX [sialyl-LeX (Lewis X)] and LeX. sLeX is a ligand of selectins. To examine whether PrPC is a ligand of selectins, we generated three human PrPC–Ig fusion proteins: one with LeX, one with sLeX, and the other with neither LeX nor sLeX. Only LeX-PrPC–Ig binds E-, L- and P-selectins. Binding is Ca2+-dependent and occurs with nanomolar affinity. Removal of sialic acid on sLeX-PrPC–Ig enables the fusion protein to bind all selectins. These findings were confirmed with brain-derived PrPC. The selectins precipitated PrPC in human brain in a Ca2+-dependent manner. Treatment of brain homogenates with neuraminidase increased the amounts of PrPC precipitated. Therefore the presence of sialic acid prevents the binding of PrPC in human brain to selectins. Hence, human brain PrPC interacts with selectins in a manner that is distinct from interactions in peripheral tissues. Alternations in these interactions may have pathological consequences.
Keywords: human brain, Lewis X (LeX) isotope, neural cell-adhesion molecule (NCAM), sialic acid, prion protein, selectin
Abbreviations: CHO, Chinese-hamster ovary; CNS, central nervous system; GPI, glycosylphosphatidylinositol; HRP, horseradish peroxidase; LeX, Lewis X; mAb, monoclonal antibody; MoCD24, mouse CD24; NCAM, neural cell-adhesion molecule; PNGase F, peptide N-glycosidase F; PrPC, cellular prion protein; PrPSc, scrapie prion protein; PSGL-1, P-selectin glycoprotein ligand-1; sLeX, sialyl-LeX; SPR, surface plasmon resonance
INTRODUCTION
The normal PrPC (cellular prion protein) is a highly conserved, GPI (glycosylphosphatidylinositol)-anchored, cell surface protein present on many cell types [1,2]. Currently, the normal physiological functions of PrPC remain unclear. PrPC binds metal and functions as a metal transporter [3,4]. PrPC has pro-apoptotic, as well as anti-apoptotic, activities [5,6]. Many different ligands for PrPC have been identified using bacteria-produced recombinant PrPC, yeast two-hybrid system, biochemical cross-linking and transfected cell lines, which overexpress PrPC [7]. Consequently, the significance of these interactions remains unclear.
The human PrPC has two highly conserved, N-linked glycosylation sites. The N-linked glycans on PrP are of the complex type, which contain terminal galactose, sialic acid and fucose attached to the innermost GlcNAc cores. Approximately 70% of the terminal galactose on PrPC is modified with sialic acid. Another feature of PrPC is that its GPI anchor contains sialic acids [8]. Rodent brain PrPC also contains sulfate [9]. More detailed studies using MS with purified hamster and murine PrPC found that both of the N-linked glycosylation sites are glycosylated, resulting in the generation of more than 30 glycostructures, and the glycans contain a trisaccharide, LeX (Lewis X), and/or a tetrasaccharide, sLeX (sialyl-Lewis X) [10,11]. Currently, the biological significance of glycosylation in PrPC function and the pathogenesis of prion diseases are not completely understood. All prion diseases are believed to share the same pathogenic mechanism, which is based on the conversion of the PrPC into the infectious and pathogenic PrPSc (scrapie prion protein) [2].
The selectins (CD62) are a family of cell surface molecules that are important in cell adhesion and migration [12–14]. There are three selectins: L-selectin (CD62L), which is found on most leucocytes; P-selectin (CD62P), which is present on activated platelets and endothelial cells; and E-selectin (CD62E), which is expressed exclusively on activated endothelial cells. All three selectins interact with carbohydrates in a Ca2+-dependent manner, and sialic acid is an essential component of the carbohydrate recognition complex. Of all the selectin ligands, the most extensively studied is sLeX [15].
Most studies on selectins have been focused on the roles they play in migration of blood cells and interactions between blood cells and the vascular system in inflammation. Much less is known about the role selectins play in other systems, such as the CNS (central nervous system). Previously, Huang et al. [16] reported the presence of an L-selectin ligand on myelin in the mouse CNS [16]. Interestingly, binding of L-selectin to myelin is sialic acid-independent. The ligand was thought to be a surface protein with a lipid anchor [16]. The levels of L-selectin have been reported to be up-regulated during early stages of prion diseases in animals [17]. Because mouse and rat brain PrPC contain sLeX and LeX epitopes, PrPC in the CNS may interact with selectins. In the present paper, we investigate whether human brain-derived PrPC contains LeX and/or sLeX epitopes and whether human PrPC interacts with selectins.
MATERIALS AND METHODS
Cell lines
CHO (Chinese-hamster ovary)-K1, LEC11 and LEC12 cells lines were provided by Dr Pamela Stanley of Albert Einstein College of Medicine (New York, NY, U.S.A.). LEC11 and LEC12 cell lines are glycosylation mutants derived from the CHO-K1 cell line and are known to express two different GDP-fucose:N-acetylgucosaminide 3-α-L-fusosyltransferases, which are responsible for the differences in expression of LeX and sLeX epitopes on these mutant cell lines [48].
Antibodies and other reagents
Anti-PrP mAbs (monoclonal antibodies) 7A12, 8H4 and 8F9 were produced, affinity-purified and biotinylated as previously reported [49]. mAb 7A12, mAb 8H4 and mAb 8F9 recognize amino acid residues 135–145, 175–185 and 225–231 respectively. These mAbs are specific for PrP and have been characterized extensively. Antibodies against E-, L- and P-selectins, recombinant human E-, L-, P-selectin–IgG1 Ig fusion proteins and human NCAM (neural cell-adhesion molecule)–Ig fusion protein were purchased from R&D Systems. All the fusion proteins were produced in NSO cells. Mouse IgM antibody, goat anti-mouse IgG–HRP (horseradish peroxidase) antibody and FITC–goat anti-mouse IgM were purchased from Chemicon. Mouse anti-human CD15 (clone HI98, IgM), rat anti-mouse CD24 and goat anti-rat IgG–HRP were purchased from BioLegend. Mouse anti-human CD15s (clone CSLEX1, IgM) was purchased from BD PharMingen. Neuraminidase was purchased from ICN. Protein G–agarose beads were purchased from Roche. PNGase F (peptide N-glycosidase F) was purchased from New England Biolabs. All reagents for SPR (surface plasmon resonance) assays were supplied by the Biocore™ Core Facility of the Cleveland Clinic Foundation (Cleveland, OH, U.S.A.) through Biacore™. Protein assay kits were purchased from Bio-Rad.
Generation of fusion proteins, transfection and purification of fusion proteins
Human full-length PrPC DNA (nt 97–780) was PCR-amplified with primers. The forward primer was 5′-TACCGCTTGGAACCGACGACCT-3′ and the reverse primer was 5′-AGAGACCATTATCCGGACTCTA-3′. The Ig fragment of human IgG1 was PCR-amplified with primers. The forward primer sequence was 5′-GCTCCCGCGCGGCAGTCAGAA-3′ and the reverse primer was 5′-TCATTTACCCGGAGACAGGGAG-3′. MoCD24 (mouse CD24) DNA was also PCR-amplified (nt 71–243) with forward primer (5′-TGTACCCGTCTCGCTACCACC-3′) and reverse primer (5′-CCTCGACGGTGGGGGAGACCAC-5′). All the PCR products were cloned into PGEM-T easy vector from Promega. To construct PrP–Ig and MoCD24–Ig, the PCR-amplified PrP and MoCD24 were cleaved with EcoRI and then inserted into the NotI site of mutated pBluescript II SK from Stratagene separately to create PrP-pBlu or MoCD24-pBlu. The Ig fragment was cleaved with SacII and SacI and then inserted into PrP-pBlu or MoCD24-pBlu to generate PrP-Ig-pBlu and MoCD24-Ig-pBlu. The constructs were cleaved further with HindIII and NotI and then inserted into pcDNA3.1/Hygro from Invitrogen to create PrP-Ig-pcDNA3.1 and MoCD24-Ig-pcDNA3.1.
Each construct was transfected individually into CHO, LEC11 and LEC12 cells with Lipofectamine™ 2000 from Invitrogen. Stable cell lines were selected and maintained with 50 μg/ml hygromycin (Invitrogen). Secreted PrPC–Ig and MoCD24–Ig proteins were purified with Protein G–agarose beads. The PrPC–Ig- and MoCD24–Ig-positive fractions were identified by goat anti-human IgG–HRP. The pooled proteins were quantified by ELISA and Western blots with anti-PrP, anti-mouse CD24 mAbs or anti-human IgG–HRP. The concentration of each fusion protein preparation was determined using a Bio-Rad protein assay kit.
Preparation of brain homogenates and treatment of brain homogenates or fusion proteins with enzymes
Brain tissues from non-CJD (Creutzfeldt–Jakob disease) controls were homogenized in 10 vol. (10%, v/w) of ice-cold lysis buffer (10 mM Tris, 150 mM NaCl, 1% Nonidet P40 and 0.5% sodium deoxycholate) in the presence of 1 mM PMSF [22]. After spinning at 4100 g for 10 min, the supernatants were stored in aliquots at −80°C [22]. Preparation of the anti-PrP mAb-coupled Protein G immunoaffinity column has been described in detail in [18]. An mAb 8H4-coupled column was used to enrich the total PrP population in total brain homogenate. Brain homogenates or affinity-purified fusion proteins were treated with neuraminidase to remove the sialic acid or with PNGase F to remove the N-linked glycan following the manufacturer's protocol (New England Biolabs). The treated brain homogenates were subjected for ELISA or precipitation.
ELISA
To detect LeX or sLeX epitopes on PrPC in human brain homogenate, the ELISA plate was coated with affinity-purified anti-PrP mAb 8H4 or 8F9 (2 μg/well) in duplicate and blocked with 4% (w/v) BSA in PBST (PBS with 0.05% Tween 20, pH 7.4), 40 μl of a 20% brain homogenate was added to each well, and incubated at room temperature (25°C) for 1 h with rocking. The bound PrP species were detected with anti-human CD15, CD15s (0.5 μg/μl, 1:300) or an irrelevant IgM for 1 h followed by a goat anti-mouse IgM–HRP antibody (1:500) and absorbance was read at 405 nm.
To detect LeX or sLeX epitopes on recombinant PrPC–Ig and MoCD24–Ig proteins expressed in CHO, LEC11 or LEC12 cells, purified fusion proteins from the respective cell lines were coated on the plate. The subsequent steps for detecting LeX and sLeX epitopes were the same as described above.
To detect interactions between selectins and PrPC–Ig or MoCD24–Ig fusion proteins, 2 μg/well human E-selectin–Ig, P-selectin–Ig, NCAM-1–Ig or 1.35 μg/well human L-selectin–Ig fusion proteins were coated on ELISA plates. PrPC–Ig and MoCD24–Ig were then loaded in the presence of 10 mM Ca2+. Bound PrPC–Ig was detected with biotinylated anti-PrP mAb 8H4 (0.5 μg/well) and detected further with streptavidin–HRP. The bound MoCD24–Ig was first detected by rat anti-mouse CD24 and then goat anti-rat IgG–HRP (BioLegend).
SPR
SPR experiments were performed at 25°C with a Biacore 3000 at The Cleveland Clinic Foundation Microbiology Core Facility. To detect binding between PrP–Ig and selectins, E-, L- and P-selectins were immobilized via amine groups at pH 4.5, or PrP–Ig was immobilized via amine groups at pH 4.0 on a CM5 chip. Binding between PrP–Ig and selectins in HBS-P solution (0.01 M Hepes, pH 7.4, 0.15 M NaCl and 0.005% Tween 20) with either Ca2+ or Mg2+ was measured at a flow rate of 20 μl/min. Regeneration was performed with 10 mM glycine (pH 3.0) at a flow rate of 20 μl/min. The data were simulated and the binding affinity of each interaction was obtained either through steady-state measurements if selectins were immobilized or through the Langmuir method if PrPC–Ig was immobilized.
Precipitation of brain-derived PrPC with selectin–Ig fusion proteins
Normal brain homogenate (40 μl) was incubated with 10 μg of E-selectin–Ig, L-selectin–Ig or P-selectin–Ig fusion proteins with 10 mM Ca2+ or EDTA at room temperature for 1 h. The beads were then washed five times in PBST at 1000 g for 2 min. Bound proteins were subjected to SDS/PAGE and Western blots. NCAM-1–Ig fusion protein was used as a control.
Western blotting
Proteins were separated by SDS/12% PAGE and then electrotransferred on to PVDF membranes. After blocking in blocking solution from Roche at 4°C overnight, detection was by biotinylated 7A12 or 8H4 mAb against PrP followed by streptavidin–HRP. Western blots were developed using SuperSignal® West Femto Maximum Sensitivity Substrate from Pierce.
Statistical methods
Two-tailed P values were based on two-sample Student's t test assuming unequal variance (Microsoft Excel 2003).
RESULTS
Human brain PrPC contains LeX and sLeX
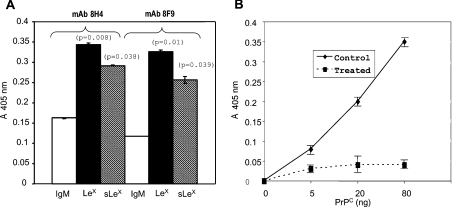
We first determined whether PrPC in human brain contains LeX and sLeX epitopes by ELISA. PrPC species in human brain homogenates were captured on ELISA plates with either anti-PrPC mAb 8H4 or mAb 8F9. These two mAbs react with two distinct epitopes on PrPC. An anti-CD15 (LeX) or an anti-CD15s (sLeX) mAb was used individually to react with captured PrPC. Both mAbs reacted significantly with the brain PrPC species. An irrelevant, control IgM antibody did not react with the captured PrPC species (Figure 1A). The ELISA also did not react with bacterial-produced, recombinant full-length human PrP (rHu-PrP23−231), which lacks the N-linked glycans, or with brain homogenates in which the PrPC species were depleted with anti-PrPC mAb (results not shown). We also confirmed the presence of the sLeX epitope on human PrPC by an alternative approach. Human PrPC species were first affinity-purified by anti-PrP mAb chromatography [18]. Purified PrPC species were treated either with PBS or with neuraminidase. After treatment, different amounts of treated PrPC were added to an ELISA plate, which had been precoated with an anti-CD15s mAb. A biotinylated anti-PrPC mAb, 8H4, was then used to detect bound PrPC. In the control PBS-treated homogenates, binding of anti-PrPC mAb 8H4 is detected in a PrPC concentration-dependent manner (Figure 1B). In contrast, when the purified human brain PrPC was first treated with neuraminidase to remove the sialic acid, binding of mAb 8H4 was greatly reduced (Figure 1B). No immunoreactivity was detected when rHu-PrP23−231 was added in place of brain-derived PrPC (results not shown). In summary, the ELISA is specific for both PrPC and N-linked glycans and human brain-derived PrPC contains both LeX and sLeX epitopes.
Figure 1. Human brain PrPC had LeX and sLeX epitopes.
(A) Human brain-derived PrPC species were first captured with either anti-PrPC mAb 8H4 (epitope between amino acid residues 175 and 185) or 8F9 (epitope between amino acid residues 225 and 231). Plates were then incubated with an irrelevant mouse IgM, anti-CD15 (LeX) or anti-CD15s (sLeX) mAbs. The bound antibody was detected further with HRP-conjugated anti-mouse IgM antibody (IgM–HRP). Both anti-CD15 and anti-CD15s but not IgM antibodies reacted significantly with the captured PrPC. Therefore human brain-derived PrPC contains both LeX and sLeX epitopes. Results are the means±S.D. for three experiments (n=12 for each treatment). P-values represent comparisons between binding of IgM control and binding of anti-CD15 or anti-CD15s antibodies. (B) Affinity-purified human brain PrPC were first treated with PBS or neuraminidase. After treatment, identical amounts of proteins were added into ELISA plates that had been precoated with anti-human CD15s antibody. Captured PrPs were then detected with a biotinylated 8H4 antibody. The bound mAb 8H4 antibody was reacted further with a streptavidin-conjugated HRP. mAb 8H4 did not react with the neuraminidase-treated PrPC, indicating that the anti-CD15s mAb failed to capture the neuraminidase-treated PrPC. Results are the means±S.D. for three experiments (n=12 for each treatment).
Chimaeric human PrPC–Ig fusion proteins produced in CHO, LEC11 and LEC12 cell lines
We generated an expression plasmid, which encodes a chimaeric fusion protein containing the full-length human PrPC (without the GPI anchor) and the Fc region of human IgG1. We then stably transfected this construct into three well-characterized cell lines, CHO, LEC11 and LEC12. The LEC11 and LEC12 cell lines are glycosylation mutants, which were derived from the parental CHO cell line. By RIA, it was found that the parental cell line, CHO, expresses neither LeX nor sLeX epitopes, the LEC11 cell line has more sLeX than LeX epitopes and the LEC12 cell line has LeX epitope [19].
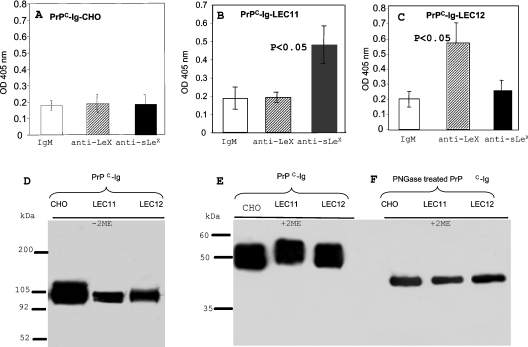
We then purified the PrPC–Ig-CHO, PrPC–Ig-LEC11 and PrPC–Ig-LEC12 fusion proteins from the culture supernatants of the respective cell lines by Protein G-affinity chromatography, and determined whether these PrPC–Ig fusion proteins express either LeX or sLeX epitopes by ELISA. Plates were first precoated with an anti-PrP mAb, 8H4. Identical amounts of purified PrPC–Ig-CHO, PrPC–Ig-LEC11 or PrPC–Ig-LEC12 fusion proteins were then added on to the plates. Antibodies specific for CD15 (LeX) or CD15S (sLeX) or an irrelevant IgM were then added to react with the bound PrPC–Ig fusion proteins. We did not detect any binding with PrPC–Ig-CHO; thus this fusion protein lacks both LeX and sLeX epitopes (Figure 2A). Interestingly, PrPC–Ig-LEC11 reacted with anti-CD15s (sLeX) but not with anti-CD15 (LeX) mAb (Figure 2B), while PrPC–Ig-LEC12 reacted only with anti-CD15 (LeX) but not anti-CD15s (sLeX) mAb (Figure 2C). Similar amounts of PrPC–Ig proteins were captured with anti-PrP mAb because comparable levels of binding were detected when an anti-human Ig antibody instead of anti-CD15 or anti-CD15s was used to detect bound PrPC–Ig (results not shown). Therefore PrPC–Ig-CHO lacks both LeX and sLeX, PrPC–Ig-LEC11 has sLeX but lacks detectable LeX, and PrPC–Ig-LEC12 contains LeX epitope but is deficient in sLeX epitope.
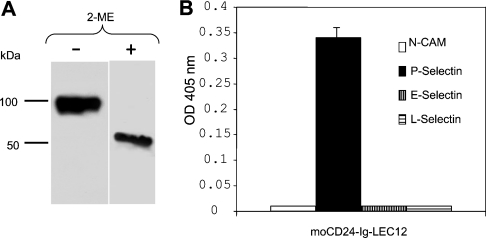
Figure 2. Expression of PrPC–Ig fusion proteins in CHO, LEC11 and LEC12 cell lines.
(A–C) Equal amounts of affinity-purified PrPC–Ig fusion proteins from CHO, LEC11 or LEC12 cells were added on to ELISA plates that had been coated with anti-PrPC mAb 8H4. Bound fusion proteins were then detected with either mouse IgM, anti-CD15 or anti-CD15s antibodies. The bound antibody was detected further with goat anti-mouse IgM–HRP. Neither LeX nor sLeX epitope was detected on PrPC–Ig CHO, only sLeX was detected on PrPC–Ig LEC11, and only LeX was detected on PrPC–Ig-LEC12. Results are the means±S.D. for four experiments (n=16 for each sample). (D) Affinity-purified PrPC–Ig fusion proteins were applied to SDS/PAGE under non-reducing conditions and then immunoblotted with mAb 8H4. Under non-reducing conditions, the apparent molecular mass of the fusion protein is approx. 100 kDa. (E, F) Affinity-purified PrPC–Ig fusion proteins were treated with PBS or PNGase F, then applied to SDS/PAGE under reducing conditions and immunoblotted with mAb 8H4. PNGase F treatment reduced the molecular mass of the PrPC–Ig chimaera from 52 kDa to approx. 42 kDa. OD, absorbance.
Under reducing conditions in SDS/PAGE, the three fusion proteins migrated as proteins with a molecular mass of approx. 52 kDa (Figure 2D). Under non-reducing conditions in SDS/PAGE, the three proteins had an apparent molecular mass of 100 kDa, corresponding to the fusion protein dimer, which doubles the expected molecular mass of human PrPC combined with the Fc region of human IgG1 (Figure 2E). When the fusion proteins were treated with PNGase to remove the N-linked glycans, they migrated as proteins with a molecular mass of approx. 42 kDa (Figure 2F). We did not detect any differences in the binding of multiple anti-PrP mAbs (n=6) between PrPC–Ig and brain-derived PrPC; therefore it is unlikely that addition of the Ig portion has drastically altered the overall conformation of the PrPC (results not shown).
Binding of human PrPC–Ig fusion proteins to human selectin–Ig fusion proteins
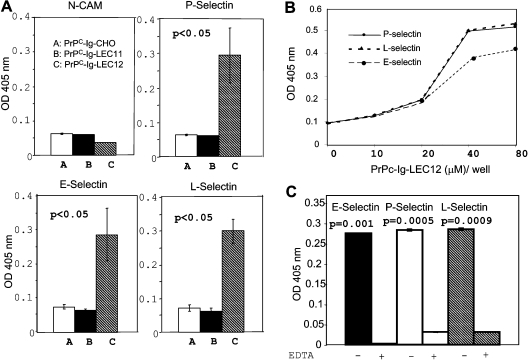
We next determined whether the three PrP–Ig fusion proteins (PrPC–Ig-CHO, PrPC–Ig-LEC11 and PrPC–Ig-LEC12) interact with human selectin–Ig fusion proteins: P-selectin–Ig, E-selectin–Ig and L-selectin–Ig. All selectin fusion proteins were produced in NSO cells. An NCAM–Ig fusion protein produced in NSO cells was included as a control. None of the PrPC–Ig fusion proteins reacted with NCAM–Ig (Figure 3A). On the other hand, PrPC–Ig-LEC12, which contains LeX, is able to bind all three selectins. Neither PrPC–Ig-CHO, which lacks both LeX and sLeX epitopes, nor PrPC–Ig-LEC11, which carries the sLeX epitope, was able to bind selectins. Binding to selectins is PrPC–Ig fusion protein concentration-dependent (Figure 3B), and requires Ca2+ (Figure 3C). Addition of EDTA, a Ca2+ chelator, inhibits binding.
Figure 3. PrPC–Ig from LEC12 cells binds E-, L- and P-selectins in a Ca2+-dependent manner.
(A) ELISA plates were precoated with identical amounts of E-selectin–Ig, L-selectin–Ig, P-selectin–Ig or NCAM–Ig fusion protein. Identical amounts of affinity-purified PrPC–Ig fusion proteins from CHO, LEC11 and LEC12 cells were then added to the plates. The bound PrPC–Ig fusion proteins were then detected with a biotinylated anti-PrPC mAb, 8H4. The bound antibody was detected further with streptavidin–HRP. Only PrPC–Ig fusion protein from LEC12 cells binds all three selectins. None of the PrPC–Ig fusion proteins bind NCAM. Results are the means±S.D. for four experiments (n=12). (B) Binding between selectins and PrPC–Ig-LEC12 is PrPC–Ig fusion protein concentration-dependent. (C) Binding between PrPC–Ig LEC12 and selectin–Ig fusion protein is eliminated in the presence of EDTA (10 mM). P-values between binding of PrPC–Ig LEC12 to selectin–Ig fusion proteins with or without EDTA were <0.005 for all three selectins. Results are the means±S.D. for three experiments (n=8 for each treatment). OD, absorbance.
Treatment with neuraminidase enables PrPC–Ig-LEC11 to bind selectins
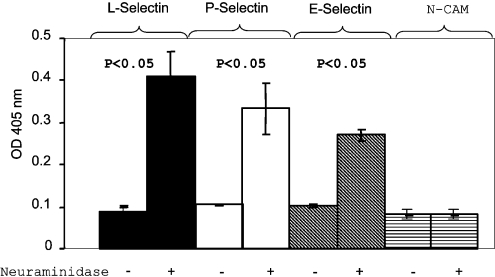
To determine whether the presence of sialic acid interferes with the binding of PrPC–Ig-LEC11 fusion protein to selectins, we treated this fusion protein with neuraminidase to remove the sialic acid residues. After neuraminidase treatment, PrPC–Ig-LEC11 is able to bind all three selectins (Figure 4). However, neuraminidase-treated PrPC–Ig-LEC11 remained unable to bind NCAM–Ig. These results provide strong evidence that the presence of sialic acid on PrPC–Ig-LEC11 prevents its binding to selectins.
Figure 4. Treatment with neuraminidase enables PrPC–Ig-LEC11 to bind all three selectins.
ELISA plates were precoated with identical amounts of E-selectin–Ig, L-selectin–Ig, P-selectin–Ig or NCAM–Ig fusion proteins. Equal amounts of affinity-purified, PBS-treated or neuraminidase-treated PrPC–Ig LEC11 were added on to the plates. The bound fusion proteins were then detected with a biotinylated anti-PrPC mAb, 8H4. Bound antibody was detected further with streptavidin–HRP. Results are the means±S.D. for four experiments (n=8 for each sample). Removal of sialic acid enables PrPC–Ig-LEC11 to bind all three selectin–Ig fusion proteins. Neuraminidase-treated PrPC–Ig-LEC11 remained unable to bind NCAM–Ig fusion protein. OD, absorbance.
Binding between PrPC–Ig-LEC12 and selectins is PrPC-specific
The Fc portion of the PrP–Ig fusion protein has one potential N-linked glycosylation site. Therefore the specificity of the interaction between PrPC–Ig-LEC12 and the selectins was validated further by using another fusion protein, mouse CD24–Ig fusion protein produced in LEC12 cells. We chose CD24 for the following reasons: (i) similar to PrPC, CD24 is a GPI-anchored protein; (ii) CD24 is present in the CNS as well as in peripheral tissues; and (iii) CD24 binds P-selectin [20]. Expressed CD24–Ig-LEC12 has detectable LeX epitope (results not shown). Under non-reducing conditions, CD24–Ig-LEC12 also has a molecular mass of approx. 98 kDa; in the presence of reducing agent, 2-mercaptoethanol, it has a molecular mass of approx. 49 kDa (Figure 5A). As has been reported, CD24–Ig-LEC12 binds P-selectin but not E- or L-selectins [21]. CD24 is highly conserved between mouse and human. Our results also suggest that mouse CD24 binds human P-selectin (Figure 5B). Therefore simply having an LeX epitope is insufficient for the fusion protein to bind all three selectins. From these experiments, we concluded that binding between PrPC–Ig-LEC12 and the three selectins requires both the LeX epitope on PrPC and the PrPC protein backbone.
Figure 5. Mouse CD24–Ig produced in LEC12 cells (MoCD24–Ig-LEC12) bound only P-selectin.
(A) Mouse CD24–Ig expressed in LEC12 cells exists as dimer under non-reducing conditions with an apparent molecular mass of 98 kDa. Its apparent molecular mass under reducing conditions is 49 kDa. (B) ELISA plates were precoated with identical amounts of E-selectin–Ig, L-selectin–Ig, P-selectin–Ig or NCAM–Ig fusion protein. Identical amounts of affinity-purified MoCD24–Ig-LEC12 fusion proteins were then added onto the plates. The bound MoCD24–Ig-LEC12 proteins were then detected with rat anti-mouse CD24 antibody and the binding was detected further with goat anti-rat IgG–HRP. MoCD24–Ig-LEC12 fusion protein reacts only with P-selectin. Results are the means±S.D. for three experiments (n=6 for each treatment).
Affinity of binding of PrPC–Ig-LEC12 to selectin–Ig
We next determined the affinity of binding of PrP–Ig-LEC12 to the selectins by SPR. Affinity was determined through steady-state measurements or through the Langmuir method. We found that PrPC–Ig-LEC12 binds to the three selectins with rather high affinity (Kd), ranging from approx. 66 nM for L-selectin, 220 nM for P-selectin and 340 nM for E-selectin (Table 1). The requirement for Ca2+ was also confirmed in these binding studies; replacement of Ca2+ with Mg2+ completely abolished the binding of PrPC–Ig-LEC12 to the selectins (results not shown).
Table 1. Binding affinity of PrPC–Ig-LEC12 for selectin–Ig.
After selectin–Ig (experiments 1–3) or PrP–Ig (experiment 4) was immobilized on a CM5 chip through the N-termini, PrP-Ig (experiments 1–3) or selectin-Ig (experiment 4) was flowed through to detect the interactions. Kd values were calculated with either steady-state kinetics (experiments 1–3) or Langmuir method (experiment 4). ND, not determined.
| Kd (nM) | |||||
|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Means±S.D. | |
| P-selectin | ND | 234 | 226 | 211 | 224±10.4 |
| E-selectin | 286 | 378 | 347 | ND | 337±46.8 |
| L-selectin | 80 | 60 | 67 | 57 | 66±10.23 |
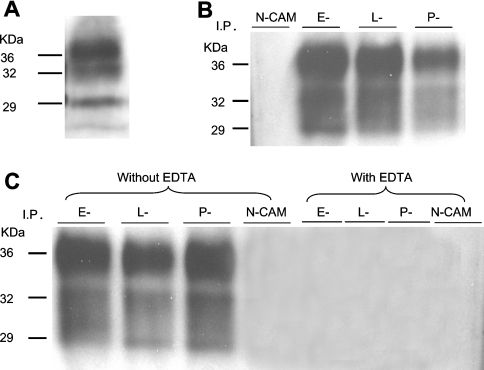
Interactions between brain-derived PrPC and selectins
When human brain homogenate was electrophoresed and immunoblotted with anti-PrP mAb 7A12, we observed the characteristic immunoreactive pattern with three bands (Figure 6A). The 36 kDa band is the full-length, fully glycosylated PrPC species, while the two smaller PrP species are mainly N-terminally truncated PrP species [22]. We next sought to verify that selectins indeed bind human brain-derived, native PrPC. Human brain homogenates were first incubated with one of the four fusion proteins: E-selectin–Ig, P-selectin–Ig, L-selectin–Ig or NCAM–Ig. Precipitated proteins were then separated by SDS/PAGE and immunoblotted with anti-PrP mAb 7A12. All three selectins bring down three PrPC species with molecular masses of 36, 32 and 29 kDa (Figure 6B). No immunoreactive band is detected in the sample incubated with NCAM–Ig. In another study, we also demonstrated that precipitation of native brain-derived PrPC by selectin–Ig is Ca2+-dependent (Figure 6C); in the presence of EDTA, no anti-PrP mAb immunoreactive band was detected in any of the samples. Collectively, these results provide conclusive evidence that human brain-derived PrPC interacts with all three selectins.
Figure 6. Human brain PrPC binds selectins and binding is Ca2+-dependent.
(A) Proteins in normal human brain homogenate were separated by SDS/PAGE and then immunoblotted with anti-PrPC mAb, 7A12. It showed the typical three bands at approx. 29, 32 and 36 kDa. (B) Proteins in normal human brain homogenates were precipitated with NCAM–Ig, E-selectin–Ig, L-selectin–Ig or P-selectin–Ig fusion protein. Precipitated proteins were then separated by SDS/PAGE and immunoblotted with anti-PrPC mAb, 7A12. All three selectins but not the NCAM–Ig fusion protein precipitated three proteins with apparent molecular masses of 29, 32 and 36 kDa, a pattern identical with the one seen in (A). (C) When precipitation with selectin–Ig was carried out in the presence of 10 mM EDTA, no PrP can be precipitated by selectin–Ig fusion proteins. Therefore binding of all three selectin–Ig fusion proteins to brain-derived PrPC is also calcium-dependent. I. P., immunoprecipitated.
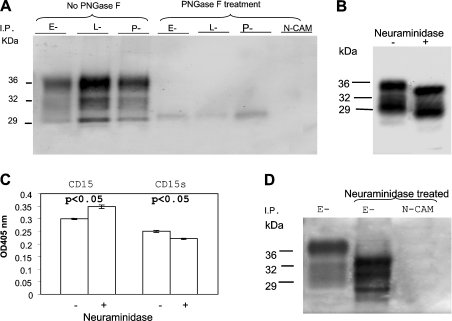
We next confirmed that the N-linked glycans on PrPC are important in the binding of PrPC to selectins. Brain homogenates were first treated with PNGase F to remove the N-linked glycans prior to precipitation with selectin–Ig fusion proteins. Removal of the N-linked glycans on PrPC drastically reduced the amounts of PrPC precipitated by the three selectin–Ig proteins. Therefore the interaction between PrPC and selectin is N-linked glycan-dependent (Figure 7A).
Figure 7. Selectin–Ig proteins do not react with brain-derived PrPC lacking N-linked glycans.
(A) Brain homogenate was treated with PBS or with PNGase F and then subjected to precipitation with either selectin–Ig or NCAM–Ig fusion proteins. The precipitated proteins were then separated by SDS/PAGE and immunoblotted with anti-PrPC mAb, 8H4. Treatment with PNGase F greatly reduced the amounts of PrPC precipitated by the three selectin–Ig fusion proteins. Therefore N-linked glycans on PrPC are critical for binding of selectin–Ig to PrPC. (B) Human brain homogenates were treated with PBS or neuraminidase. After treatment, proteins were separated by SDS/PAGE and then immunoblotted with anti-PrPC mAb, 8H4. Neuraminidase treatment reduced the apparent molecular mass of PrPC as compared with the non-treated PrPC proteins. (C) ELISA plates were precoated with anti-PrPC mAb, 8H4. Equal amounts of PBS-treated control homogenates or neuraminidase-treated brain homogenates were added onto the ELISA plate. An anti-CD15 mAb or anti-CD15s mAb were then added to react with the bound PrPC species. The bound anti-CD15 or anti-CD15s antibodies were detected with a goat anti-mouse IgM–HRP. Anti-CD15 mAb reacted more strongly with neuraminidase-treated brain homogenate. On the other hand, binding of anti-CD15s antibody was reduced in neuraminidase-treated brain homogenates. Results are the means±S.D. for two experiments (n=12 for each treatment). P-values represent comparisons between samples treated with or without neuraminidase. (D) PBS-treated or neuraminidase-treated brain homogenates were precipitated with E-selectin–Ig or NCAM–Ig. Precipitated proteins were then separated by SDS/PAGE and immunoblotted with anti-PrPC mAb, 8H4. E-selectin–Ig precipitated more PrPC protein from neuraminidase-treated brain homogenate than PBS-treated brain homogenate. The neuraminidase-treated, E-selectin–Ig-precipitated PrPC proteins also migrated slightly faster than the PBS-treated controls. I. P., immunoprecipitated.
We also determined whether treatment of brain homogenates with neuraminidase increases the amount of PrPC available for selectin–Ig binding. Brain homogenate was first treated either with PBS or with neuraminidase. After treatment, each sample was then divided into multiple aliquots: one was immunoblotted with mAb 8H4 (Figure 7B), one was used to determine the binding of anti-CD15 (LeX) or anti-CD15s (sLeX) mAb in ELISA (Figure 7C), and remaining samples were precipitated with either E-selectin–Ig or NCAM–Ig, and then immunoblotted with anti-PrPC mAb 8H4 (Figure 7D). In samples that were immunoblotted with mAb 8H4, treatment with neuraminidase slightly increased the mobility of the PrP species, reflecting the removal of sialic acid residues (Figure 7B).
Accordingly, treatment of brain homogenate with neuraminidase increased the binding of anti-CD15 mAb but decreased the binding of anti-CD15s mAb (Figure 7C); these differences were rather small, but significant. As expected, the amount of PrPC precipitated with E-selectin–Ig was greater in the sample that had been previously treated with neuraminidase (Figure 7D). Based on densitometry measurements, the amounts of PrPC precipitated were approx. 20–40% (n=3) greater in neuraminidase-treated brain homogenates (results not shown). Again, neuraminidase-treated PrPC has a faster mobility in SDS/PAGE. No PrPC immunoreactivity was detected in the neuraminidase-treated sample first precipitated with NCAM–Ig. These results provide strong evidence that removal of sialic acid residues on human brain-derived PrPC increases the interactions between PrPC and selectins.
DISCUSSION
Human PrPC–Ig-LEC12 fusion protein with LeX structure binds E-, L- and P-selectin–Ig fusion proteins in a Ca2+-dependent manner. On the other hand, PrPC–Ig-LEC11 fusion protein with sLeX structure does not bind. However, removal of the sialic acid on PrPC–Ig-LEC11 enables it to bind all three selectins. Most importantly, all three selectin–Ig proteins but not control proteins also precipitated human brain-derived PrPC in a Ca2+ and N-linked glycan-dependent manner. Removal of sialic acid from brain-derived PrPC increases the amount of PrPC precipitated with selectins. Both the LeX structure and the protein portion of PrPC are important in binding to selectins. An irrelevant protein, mouse CD24–Ig-LEC12, which bears LeX, failed to bind either E- or L-selectins, but did bind P-selectin, a known ligand of CD24 [20]. We hypothesize that interactions between PrPC and selectins may be important in normal cellular physiology and/or pathophysiology under some conditions.
PrPC–Ig-LEC12 protein binds the three selectin–Ig proteins with Kd values in the nanomolar range: approx. 66 nM for L-selectin, 220 nM for P-selectin and 340 nM for E-selectin. These values are substantially higher than those reported earlier, with the exception of the binding of P-selectin to PSGL-1 (P-selectin glycoprotein ligand-1) on neutrophils [23]. The reported affinity between selectins and their respective ligands varies significantly, ranging from 70 nM for the binding of P-selectin to PSGL-1 to 7800 nM for the binding of the same selectin to sLeX [23,24]. Both selectin–Ig and PrPC–Ig proteins are dimeric, and thus bivalent. One may argue that their affinity may be overestimated. In fact, recombinant PrP does exist as a dimer [25]. Furthermore, PrPC is a GPI-anchored protein occupying microdomains on the cell membrane known as lipid rafts; each lipid raft is known to contain multiple GPI-anchored proteins [26]. Some selectins are also present on special domains, pseudopods, on the cell surface in multimeric forms [23]. Our conclusion that PrPC binds selectins with rather high affinity is also supported by our finding that all selectin–Ig proteins precipitated PrPC in human brain in a Ca2+-dependent manner.
The ability of PrPC with LeX epitopes to bind selectins is reminiscent of the binding of P-selectins to PSGL-1. On the surface of lymphocytic cells, a significant percentage of the PSGL-1 present is non-functional, not binding selectins due to the lack of proper glycans. Therefore, in PSGL-1, binding to selectins requires the presence of sLeX epitope; in contrast, binding of PrPC to selectins necessitates the absence of the sLeX epitope. Addition of sLeX on PrPC may be a mechanism by which the interaction between PrPC and selectins is regulated.
Whether interactions between selectins and PrPC are important in cellular physiology is not known. PrPC is broadly expressed on the surface of human peripheral blood cells, including T-cells, B-cells, monocytes, neutrophils, dendritic cells as well as platelets [27,28]. Furthermore, memory T-cells, activated T-cells and macrophages express more PrPC than naive, resting T-cells or unstimulated macrophages. PrPC on leucocytes participates in signal transduction [29]. Human PrPC is physically present in the ‘immune synapses’, which are critical for the interactions between T-lymphocytes and antigen-presenting cells [30]. On the other hand, L-selectins are present on most leucocytes and P-selectins are present on platelets. Therefore it is highly likely PrPC and selectins will encounter each other, either on the same cell surface or on different cell types. However, the consequences of these interactions are not clear at this time. Conversely, PrPC is present on cultured human umbilical-vein endothelial cells [31]. Human brain endothelial cells also express PrPC [32]. Importantly, expression of PrPC has been reported to be critical in the trans-endothelial migration of human monocytes [32]. Therefore selectins on blood cells can also interact with PrPC on endothelial cells, which may be important in cellular activation, adhesion or migration.
The nature of the N-linked glycans on PrPC is cell-type-dependent. In SDS/PAGE, PrPC species from human peripheral blood mononuclear cells migrate slower than brain-derived PrPC due to the difference of their N-linked glycans [33]. However, whether PrPC on human peripheral blood cells has LeX or sLeX epitopes is not known. Recently, we reported that the natures of N-linked glycans on PrPC change during normal aging in mouse [34]. Selective modification of the N-linked glycans on PrPC in different cell types or during normal aging will provide additional mechanisms for regulating the interactions between PrPC and selectins.
In contrast with human, most of the circulating lymphocytes in mice lack PrPC. PrPC is preferentially expressed on immature thymocytes, and in a subpopulation of bone marrow progenitor cells [35]. Since there is no obvious phenotypic alteration in leucocyte composition in peripheral or central lymphoid organs in mice lacking PrPC expression, interactions between PrPC on blood cells and selectins on vascular structures do not seem to be important in lymphocyte migration in mouse. However, a recent report found that PrPC−/− mice are deficient in recruitment of neutrophils after intraperitoneal injection of zymosan, suggesting that PrPC may be important in cell migration under certain conditions [36]. Another recent report found that bone marrow cells from PrPC−/− mice are less efficient in repopulating irradiated recipients, suggesting a possible defect in bone marrow stem cells in PrPC−/− mice [37]. Yet another report found that normal T-lymphocyte development is altered in mouse, which overexpresses PrPC on thymocytes [38]. Collectively, all these findings are also consistent with the interpretation that PrPC may be important in cell–cell interactions and cell migrations.
Leucocyte infiltration is rarely seen in prion diseases, but activation of microglial cells has been speculated to be important in prion diseases. PrPC is expressed on microglial cells, neurons and astrocytes. Activated astrocytes express increased levels of E- and P-selectins [39]. In prion disease, neurons and astrocytes have been shown to recruit microglia [40]. In PrPSC-infected mouse brain, there were early up-regulation of L-selectin expression and down-regulation of P-selectin expression [17,41]. In vitro, direct cell-to-cell contact is required for PrPSc spreading from one infected cell to another [42]. Migration and/or adhesion of microglia cells involving PrP and selectins may play a role in the spread of PrPSc.
Both cell-surface PrPC and selectins have signal transduction capability [43,44]. Binding of PrPC in the CNS by anti-PrPC antibody activates apoptosis and neurodegeneration in mice [45]. Both soluble selectins and PrPC are present in the CNS [46,47]. Potentially, binding of soluble selectins to PrPC may have pathological consequences similar to those of cross-linking with anti-PrPC antibody. Conversely, binding of soluble PrPC to selectins may further activate selectin-bearing cells, such as glial cells, astrocytes or endothelial cells.
By expressing PrPC–Ig in cell lines with different capacity to add unique N-linked glycostructures, we have been able to identify novel binding partners of PrPC, the selectins. Three important issues remain to be addressed: (i) identifying the precise nature of the N-linked glycans that are important in these interactions, which will require much more detailed biochemical analysis; (ii) investigating whether interactions between PrPC and selectins are important in the normal physiology of the CNS; and (iii) determining whether these interactions are important in the pathogenesis of prion diseases. Experiments using sLeX-deficient mice may provide new insights into these questions.
Acknowledgments
This work was supported in part by NIH (National Institutes of Health) grant NS-045981-02 and an award/contract from the U.S. Department of Army, DAMD17-03-1-0286. We thank Dr Satya P. Yadav of The Biocore™ Core Facility of The Cleveland Clinic Foundation, for performing the binding studies, and Dr Eric D. Roush of BioCore™ Co. for help in evaluating the binding results. We also thank Dr Ajit Varki of UCSD (University of California San Diego) for providing E- and L-selectin–Ig fusion proteins from HEK-293 cells (human embryonic kidney cells) and suggestions, and Dr Michael Lamm for a careful reading of this paper. This work is supported in part by NIH grant NS-045981-01 and an award/contract from the U.S. Department of Army, DAMD17-03-1-0286 (to M. S. S.).
References
- 1.Weissmann C., Enari M., Klohn P. C., Rossi D., Flechsig E. Molecular biology of prions. Acta Neurobiol. Exp. 2002;62:153–166. doi: 10.55782/ane-2002-1434. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner S. B. Prions. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D. R., Qin K., Herms J. W., Madlung A., Manson J., Strome R., Fraser P. E., Kruck T., von Bohlen A., Schulz-Schaeffer W., et al. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 4.Pauly P. C., Harris D. A. Copper stimulates endocytosis of the prion protein. J. Biol. Chem. 1998;273:33107–33110. doi: 10.1074/jbc.273.50.33107. [DOI] [PubMed] [Google Scholar]
- 5.Paitel E., Fahraeus R., Checler F. Cellular prion protein sensitizes neurons to apoptotic stimuli through Mdm2-regulated and p53-dependent caspase 3-like activation. J. Biol. Chem. 2003;278:10061–10066. doi: 10.1074/jbc.M211580200. [DOI] [PubMed] [Google Scholar]
- 6.Bounhar Y., Zhang Y., Goodyer C. G., LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem. 2001;276:39145–39149. doi: 10.1074/jbc.C100443200. [DOI] [PubMed] [Google Scholar]
- 7.Lasmezas C. I. Putative functions of PrPC. Br. Med. Bull. 2003;66:61–70. doi: 10.1093/bmb/66.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Stahl N., Baldwin M. A., Hecker R., Pan K. M., Burlingame A. L., Prusiner S. B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry. 1992;31:5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- 9.Chen S., Mange A., Dong L., Lehmann S., Schachner M. Prion protein as trans-interacting partner for neurons is involved in neurite outgrowth and neuronal survival. Mol. Cell. Neurosci. 2003;22:227–233. doi: 10.1016/s1044-7431(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 10.Rudd P. M., Endo T., Colominas C., Groth D., Wheeler S. F., Harvey D. J., Wormald M. R., Serban H., Prusiner S. B., Kobata A., Dwek R. A. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13044–13049. doi: 10.1073/pnas.96.23.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stimson E., Hope J., Chong A., Burlingame A. L. Site-specific characterization of the N-linked glycans of murine prion protein by high-performance liquid chromatography/electrospray mass spectrometry and exoglycosidase digestions. Biochemistry. 1999;38:4885–4895. doi: 10.1021/bi982330q. [DOI] [PubMed] [Google Scholar]
- 12.Varki A. Selectin ligands. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe J. B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosen S. D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 15.Tamatani T., Suematsu M., Tezuka K., Hanzawa N., Tsuji T., Ishimura Y., Kannagi R., Toyoshima S., Homma M. Recognition of consensus CHO structure in ligands for selectins by novel antibody against sialyl Lewis X. Am. J. Physiol. 1995;269:H1282–H1287. doi: 10.1152/ajpheart.1995.269.4.H1282. [DOI] [PubMed] [Google Scholar]
- 16.Huang K., Kikuta A., Rosen S. D. Myelin localization of a central nervous system ligand for L-selectin. J. Neuroimmunol. 1994;53:133–141. doi: 10.1016/0165-5728(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z. Y., Baker C. A., Manuelidis L. New molecular markers of early and progressive CJD brain infection. J. Cell. Biochem. 2004;93:644–652. doi: 10.1002/jcb.20220. [DOI] [PubMed] [Google Scholar]
- 18.Pan T., Wong B. S., Liu T., Li R., Petersen R. B., Sy M. S. Cell-surface prion protein interacts with glycosaminoglycans. Biochem. J. 2002;368:81–90. doi: 10.1042/BJ20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard D. R., Fukuda M., Fukuda M. N., Stanley P. The GDP-fucose:N-acetylglucosaminide 3-α-L-fucosyltransferases of LEC11 and LEC12 Chinese hamster ovary mutants exhibit novel specificities for glycolipid substrates. J. Biol. Chem. 1987;262:16830–16837. [PubMed] [Google Scholar]
- 20.Sammar M., Aigner S., Hubbe M., Schirrmacher V., Schachner M., Vestweber D., Altevogt P. Heat-stable antigen (CD24) as ligand for mouse P-selectin. Int. Immunol. 1994;6:1027–1036. doi: 10.1093/intimm/6.7.1027. [DOI] [PubMed] [Google Scholar]
- 21.Sammar M., Aigner S., Altevogt P. Heat-stable antigen (mouse CD24) in the brain: dual but distinct interaction with P-selectin and L1. Biochim. Biophys. Acta. 1997;1337:287–294. doi: 10.1016/s0167-4838(96)00177-x. [DOI] [PubMed] [Google Scholar]
- 22.Pan T., Li R., Wong B. S., Liu T., Gambetti P., Sy M. S. Heterogeneity of normal prion protein in two-dimensional immunoblot: presence of various glycosylated and truncated forms. J. Neurochem. 2002;81:1092–1101. doi: 10.1046/j.1471-4159.2002.00909.x. [DOI] [PubMed] [Google Scholar]
- 23.Ushiyama S., Laue T. M., Moore K. L., Erickson H. P., McEver R. P. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J. Biol. Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
- 24.Poppe L., Brown G. S., Philo J. S., Nikrad P. V., Shah B. H. Conformation of sLeX tetrasaccharide, free in solution and bound to E-, P-, and L-selectin. J. Am. Chem. Soc. 1997;119:1727–1736. [Google Scholar]
- 25.Knaus K. J., Morillas M., Swietnicki W., Malone M., Surewicz W. K., Yee V. C. Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nat. Struct. Biol. 2001;8:770–774. doi: 10.1038/nsb0901-770. [DOI] [PubMed] [Google Scholar]
- 26.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 27.MacGregor I., Hope J., Barnard G., Kirby L., Drummond O., Pepper D., Hornsey V., Barclay R., Bessos H., Turner M., Prowse C. Application of a time-resolved fluoroimmunoassay for the analysis of normal prion protein in human blood and its components. Vox Sang. 1999;77:88–96. doi: 10.1159/000031082. [DOI] [PubMed] [Google Scholar]
- 28.Perini F., Frangione B., Prelli F. Prion protein released by platelets. Lancet. 1996;347:1635–1636. doi: 10.1016/s0140-6736(96)91128-9. [DOI] [PubMed] [Google Scholar]
- 29.Stuermer C. A., Langhorst M. F., Wiechers M. F., Legler D. F., Von Hanwehr S. H., Guse A. H., Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 30.Paar C., Wurm S., Pfarr W., Sonnleitner A., Wechselberger C. Prion protein resides in membrane microclusters of the immunological synapse during lymphocyte activation. Eur. J. Cell Biol. 2007;86:253–264. doi: 10.1016/j.ejcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Starke R., Drummond O., MacGregor I., Biggerstaff J., Gale R., Camilleri R., Mackie I., Machin S., Harrison P. The expression of prion protein by endothelial cells: a source of the plasma form of prion protein? Br. J. Haematol. 2002;119:863–873. doi: 10.1046/j.1365-2141.2002.03847.x. [DOI] [PubMed] [Google Scholar]
- 32.Viegas P., Chaverot N., Enslen H., Perriere N., Couraud P. O., Cazaubon S. Junctional expression of the prion protein PrPC by brain endothelial cells: a role in trans-endothelial migration of human monocytes. J. Cell Sci. 2006;119:4634–4643. doi: 10.1242/jcs.03222. [DOI] [PubMed] [Google Scholar]
- 33.Li R., Liu D., Zanusso G., Liu T., Fayen J. D., Huang J. H., Petersen R. B., Gambetti P., Sy M. S. The expression and potential function of cellular prion protein in human lymphocytes. Cell. Immunol. 2001;207:49–58. doi: 10.1006/cimm.2000.1751. [DOI] [PubMed] [Google Scholar]
- 34.Goh A. X., Li C., Sy M. S., Wong B. S. Altered prion protein glycosylation in the aging mouse brain. J. Neurochem. 2007;100:841–854. doi: 10.1111/j.1471-4159.2006.04268.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu T., Li R., Wong B. S., Liu D., Pan T., Petersen R. B., Gambetti P., Sy M. S. Normal cellular prion protein is preferentially expressed on subpopulations of murine hemopoietic cells. J. Immunol. 2001;166:3733–3742. doi: 10.4049/jimmunol.166.6.3733. [DOI] [PubMed] [Google Scholar]
- 36.de Almeida C. J., Chiarini L. B., da Silva J. P., Silva P. M. E., Martins M. A., Linden R. The cellular prion protein modulates phagocytosis and inflammatory response. J. Leukocyte Biol. 2005;77:238–246. doi: 10.1189/jlb.1103531. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C. C., Steele A. D., Lindquist S., Lodish H. F. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2184–2189. doi: 10.1073/pnas.0510577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jouvin-Marche E., Attuil-Audenis V., Aude-Garcia C., Rachidi W., Zabel M., Podevin-Dimster V., Siret C., Huber C., Martinic M., Riondel J., et al. Overexpression of cellular prion protein induces an antioxidant environment altering T cell development in the thymus. J. Immunol. 2006;176:3490–3497. doi: 10.4049/jimmunol.176.6.3490. [DOI] [PubMed] [Google Scholar]
- 39.Hurwitz A. A., Lyman W. D., Guida M. P., Calderon T. M., Berman J. W. Tumor necrosis factor α induces adhesion molecule expression on human fetal astrocytes. J. Exp. Med. 1992;176:1631–1636. doi: 10.1084/jem.176.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marella M., Chabry J. Neurons and astrocytes respond to prion infection by inducing microglia recruitment. J. Neurosci. 2004;24:620–627. doi: 10.1523/JNEUROSCI.4303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker C. A., Manuelidis L. Unique inflammatory RNA profiles of microglia in Creutzfeldt–Jakob disease. Proc. Natl. Acad. Sci. U.S.A. 2003;100:675–679. doi: 10.1073/pnas.0237313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanu N., Imokawa Y., Drechsel D. N., Williamson R. A., Birkett C. R., Bostock C. J., Brockes J. P. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 2002;12:523–530. doi: 10.1016/s0960-9822(02)00722-4. [DOI] [PubMed] [Google Scholar]
- 43.Crockett-Torabi E. Selectins and mechanisms of signal transduction. J. Leukocyte Biol. 1998;63:1–14. [PubMed] [Google Scholar]
- 44.Mouillet-Richard S., Ermonval M., Chebassier C., Laplanche J. L., Lehmann S., Launay J. M., Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 45.Solforosi L., Criado J. R., McGavern D. B., Wirz S., Sanchez-Alavez M., Sugama S., DeGiorgio L. A., Volpe B. T., Wiseman E., Abalos G., et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science. 2004;303:1514–1516. doi: 10.1126/science.1094273. [DOI] [PubMed] [Google Scholar]
- 46.Wong B. S., Green A. J., Li R., Xie Z., Pan T., Liu T., Chen S. G., Gambetti P., Sy M. S. Absence of protease-resistant prion protein in the cerebrospinal fluid of Creutzfeldt–Jakob disease. J. Pathol. 2001;194:9–14. doi: 10.1002/path.872. [DOI] [PubMed] [Google Scholar]
- 47.Whalen M. J., Carlos T. M., Kochanek P. M., Wisniewski S. R., Bell M. J., Carcillo J. A., Clark R. S., DeKosky S. T., Adelson P. D. Soluble adhesion molecules in CSF are increased in children with severe head injury. J. Neurotrauma. 1998;15:777–787. doi: 10.1089/neu.1998.15.777. [DOI] [PubMed] [Google Scholar]
- 48.Campbell C., Stanley P. The Chinese hamster ovary glycosylation mutants LEC11 and LEC12 express two novel GDP-fucose:N-acetylglucosaminide 3-α-L-fucosyltransferase enzymes. J. Biol. Chem. 1984;259:11208–11214. [PubMed] [Google Scholar]
- 49.Zanusso G., Liu D., Ferrari S., Hegyi I., Yin X., Aguzzi A., Hornemann S., Liemann S., Glockshuber R., Manson J. C., et al. Prion protein expression in different species: analysis with a panel of new mAbs. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8812–8816. doi: 10.1073/pnas.95.15.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]