Abstract
Injuries caused by brown spiders (Loxosceles genus) are associated with dermonecrotic lesions with gravitational spreading and systemic manifestations. The venom has a complex composition containing many different toxins, of which metalloproteases have been described in many different species of this genus. These toxins may degrade extracellular matrix constituents acting as a spreading factor. By using a cDNA library from an Loxosceles intermedia venom gland, we cloned and expressed a 900 bp cDNA, which encoded a signal peptide and a propeptide, which corresponded to a 30 kDa metalloprotease, now named LALP (Loxosceles astacin-like protease). Recombinant LALP was refolded and used to produce a polyclonal antiserum, which showed cross-reactivity with a 29 kDa native venom protein. CD analysis provided evidence that the recombinant LALP toxin was folded correctly, was still in a native conformation and had not aggregated. LALP addition to endothelial cell cultures resulted in de-adhesion of the cells, and also in the degradation of fibronectin and fibrinogen (this could be inhibited by the presence of the bivalent chelator 1,10-phenanthroline) and of gelatin in vitro. Sequence comparison (nucleotide and deduced amino acid), phylogenetic analysis and analysis of the functional recombinant toxin revealed that LALP is related in both structure and function to the astacin family of metalloproteases. This suggests that an astacin-like toxin is present in a animal venom secretion and indicates that recombinant LALP will be a useful tool for future structural and functional studies on venom and the astacin family.
Keywords: astacin, Loxosceles astacin-like protease (LALP), metalloprotease, spider, toxin, venom
Abbreviations: IPTG, isopropyl β-D-thiogalactoside; LALP, Loxosceles astacin-like protease; LB, Luria–Bertani; Ni-NTA, Ni2+-nitrilotriacetate; SPAN, Strongylocentrotus purpuratus astacin-like protease
INTRODUCTION
Injuries caused by spiders of the genus Loxosceles (brown spiders) typically result in necrotic skin lesions and some systemic-level symptoms, including intravascular haemolysis, acute renal failure and disseminated intravascular coagulation [1,2].
Brown spiders have a worldwide distribution and have been found in Europe, America, Asia, Australia and Africa. Some explanation for this general flourishing may be offered by the biology of these spiders. They survive in adverse conditions, withstand temperatures ranging from 8 to 43 °C [1] and have a predilection towards both dead scavenged prey and live prey [3].
Loxoscelism or necrotic arachnidism are the names used to describe lesions and symptoms induced by bites from spiders of the Loxosceles genus. Loxoscelism is indicated by a dermonecrotic lesion with gravitational spreading [1,2]. The mechanism by which the venom causes dermonecrosis is currently under investigation. There is evidence for the role of an inflammatory response, involving neutrophils and other leucocytes, which triggers aseptic coagulative tissue necrosis [4,5]. This inflammatory response seems to be dependent on an endothelial cell agonist effect caused by the venom that triggers indirect and dysregulated leucocyte activation [6]. Several constituents of Loxosceles spider venom have been identifed previously, including hyaluronidases, serine proteases, metalloproteases and phospholipase D family members [1,2,7–12]. Of these constituents, phospholipase D, also called dermonecrotic toxin, can induce red blood cell haemolysis, platelet aggregation, renal disturbances, macroscopic dermonecrosis and diffuse inflammatory response at the site of injection under laboratory conditions [1,13,14]. Today, it is well-established that phospholipase D comprises a family of toxins present in several Loxosceles species and exists as different isoforms [1,10,11].
Metalloproteases in Loxosceles venom were first identified in Loxosceles intermedia. Feitosa et al. [7], working with eletrostimulated venom, described two molecules in the venom with fibronectinolytic, fibrinogenolytic (20–28 kDa molecule) and gelatinolytic (32–35 kDa molecule) activity. Metalloprotease activity in the venom was studied further by da Silveira et al. [15], who showed the same metalloprotease-dependent fibronectinolytic, fibrinogenolytic and gelatinolytic activities in extracts from L. intermedia and Loxosceles laeta gland venom. Additionally, Zanetti et al. [16] described this fibrinogenolytic and metalloprotease-dependent activity in venom from Loxosceles gaucho and L. laeta, and purified a 30 kDa molecule with fibrinogenolytic activity from L. intermedia venom. The action of L. intermedia venom on fibrinogen was demonstrated to have a complete cleaving effect on denatured fibrinogen, compared with a partial cleavage activity on intact fibrinogen (α and β chains), suggesting that susceptibility to the venom was due to the conformation of the substrate. There have also been metalloproteases identified in Loxosceles rufescens venom which possess caseinolytic, gelatinolytic and fibrinogenolytic activities [9].
In the present paper, we report the identification, cloning, expression, purification and functional characterization of a metalloprotease characterized as an astacin-like toxin isolated from the L. intermedia venom gland. These results strengthen the previous data that described metalloproteases in Loxosceles venoms and indicate for the first time the presence of an astacin-like enzyme as a venom toxin constituent.
EXPERIMENTAL
Materials and reagents
Polyclonal antibodies against L. intermedia crude venom toxins and the recombinant toxin, LALP (Loxosceles astacin-like protease), were produced in rabbits as described previously [17,18]. Crude venom from L. intermedia was extracted from wild-caught spiders following the method in Feitosa et al. [7]. Fibronectin was purified from fresh human plasma by affinity chromatography on gelatin-Sepharose (Sigma) [19]. Human fibrinogen and gelatin were also purchased from Sigma.
cDNA library construction
The venom gland cDNA library was constructed as described by Chaim et al. [14]. Briefly, venom gland mRNAs from 200 adult L. intermedia spiders were purified using the FastTrack 2.0 mRNA isolation kit (Invitrogen). The cDNAs were then synthesized using the SuperScript Plasmid System with Gateway Technology for cDNA Synthesis and Cloning (Invitrogen), cloned into NotI and SalI pre-cut pSPORT1 vector and transformed into Escherichia coli DH5α cells. Transformants were selected on LB (Luria–Bertani) agar plates containing 100 μg/ml ampicillin.
cDNA library screening
Randomly chosen colonies (approx. 100 clones) were inoculated into LB broth containing 100 μg/ml ampicillin and grown overnight at 37 °C (with aeration). From this, recombinant plasmids were purified using QIAprep Spin Miniprep Kit (Qiagen). The cloned cDNAs were sequenced on both strands using ABI PRISM® BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). Reactions were analysed using an ABI 377 automatic sequencer (Applied Biosystems). The T7 and SP6 promoter regions were used for priming the sequencing reactions. The cDNA sequences were analysed, and the putative protein products from these sequences were used to search the GenBank® protein databases on the NCBI (National Center for Biotechnology Information) website (http://www.ncbi.nlm.nih.gov/) [20].
Recombinant protein expression
cDNA encoding for the putative mature metalloprotease toxin LALP was amplified by PCR using Pfu DNA polymerase (Fermentas). The forward primer used was 5′-CGCGTCGACAATGCTGTCAAATATGACCAG-3′ and the reverse primer was 5′-CGGGATCCTCACACATTTACATACGGACGT-3′. The forward primer contained a SalI restriction site (underlined) at the 5′ end, and the reverse primer contained an engineered BamHI restriction site (underlined) and the native stop codon (in bold) of the cDNA. The PCR product was digested using SalI and BamHI and gel-purified using the PerfectPrep Gel Cleanup kit (Eppendorf) The purified DNA was subcloned into pET-14b (Novagen) and digested with XhoI and BamHI. The correct construct was confirmed by PCR and sequencing. The recombinant construct was subsequently expressed as a fusion protein, with a 6×His tag and a 13-amino-acid linker, including a thrombin site, at the N-terminus. The final construct was the first asparagine residue of mature LALP preceded by a 6×His tag and a thrombin site. The expression construct was transformed into E. coli BL21(DE3)pLysS competent cells and plated on to LB agar plates containing 100 μg/ml ampicillin and 34 μg/ml chloramphenicol. Single colonies of LALP were used to inoculate LB broth (100 μg/ml ampicilin and 34 μg/ml chloramphenicol) and grown overnight at 37 °C. These cultures were diluted 1:100 into 1 litre of fresh LB broth containing ampicillin and chloramphenicol, and incubated at 37 °C until the D550 reached 0.5. Recombinant expression was induced by the addition of IPTG (isopropyl β-D-thiogalactoside) to a final concentration of 0.05 mM, and cells were incubated for 4 h at 37 °C with vigorous shaking. Cells were harvested by centrifugation at 4000 g for 7 min at 4 °C, before being resuspended in 40 ml of a denaturing lysis buffer (20 mM sodium phosphate, pH 7.8, 500 mM NaCl and 6 M guanidinium chloride) and frozen at −20 °C overnight.
Protein purification and refolding
Cell suspensions were thawed, and the lysed material was clarified by centrifugation at 9000 g for 30 min at 4 °C. The supernatants were incubated with 1 ml of Ni-NTA (Ni2+-nitrilotriacetate)–agarose beads for 30 min at room temperature (25 °C), with gentle agitation. The suspensions were loaded on to a column, and the packed gel was exhaustively washed with the following buffer (20 mM sodium phosphate, pH 8.0, 500 mM NaCl, 8 M urea and 20 mM imidazole). The recombinant protein was then eluted in 10 ml of elution buffer (20 mM sodium phosphate, pH 8.0, 500 mM NaCl, 8 M urea and 250 mM imidazole), and 1 ml fractions were collected and resolved by SDS/PAGE (12.5% gels) under reducing conditions. Fractions were pooled and dialysed against 10 mM sodium phosphate buffer (10 mM Na2HPO4/NaH2PO4, pH 7.4), for 16 h. After dialysis, the refolded recombinant protein was recovered from the supernatant by centrifugation at 9000 g for 30 min at 4 °C and quantified [21].
CD spectroscopy
For measurement of CD spectra, recombinant LALP was dialysed at 4 °C for 16 h against 10 mM sodium phosphate buffer, pH 7.4, and spectra were recorded in a Jasco J-810 spectropolarimeter (Jasco Corporation) using a 1 mm gap cuvette. Each spectrum (0.5 nm interval) was the average of eight measurements, performed at a rate of 50 nm/min using a response time of 8 s and a bandwidth of 1 nm. The temperature was kept constant at 25 °C. The secondary structures of the toxin were estimated from the spectra using the Contin method and the Self-consistent method as provided in Dicroprot 2000 1.0.4 software [22] (see Table 1).
Table 1. Secondary-structure estimation of LALP by using CD spectrophotometry and deconvolution of spectra using Dichroprot 2000 1.0.4 software.
| Estimated secondary structure of LALP (%) | ||||
|---|---|---|---|---|
| Method | α-Helix | β-Sheet | β-Turn | Other |
| Self-consistent method | 19.3 | 27.2 | 22.2 | 31.2 |
| Contin method | 19.0 | 27.0 | − | 54.0 |
Animals
Adult rabbits (weighing approx. 3 kg) from the Central Animal House of the Federal University of Paraná were used for in vivo experiments (production of polyclonal antibodies) using both crude venom and recombinant toxin. All experimental protocols using animals were performed according to the Principles of Laboratory Animal Care [NIH (National Institutes of Health) Publication number 85–23, revised 1985], Brazilian Federal Laws and Ethical Committee Agreement number 126 of Federal University of Paraná.
Gel electrophoresis, immunoblotting and ELISA
The protein concentration of samples was determined using the Coomassie Blue method (Bio-Rad), and experiments were performed five times. Protein concentration measurement was also done using the protein guanidine-denaturation method (6 M guanadinium chloride and 20 mM phosphate buffer, pH 6.5) and measurement at 280 nm. Consistent results were obtained using both methods. For protein analysis, SDS/PAGE (12.5% gels) was performed under reducing conditions, and, for protein detection, gels were stained with Coomassie Blue. For immunoblotting, proteins were transferred on to nitrocellulose filters overnight and immunostained with hyperimmune antisera, which reacts with LALP, or against crude venom toxins (as described above). Molecular-mass markers were purchased from Sigma. Antibody-capture ELISAs were performed as follows. Crude venom or purified recombinant toxin (10 μg/ml) was bound to the plate bottoms (Nunc MaxiSorp) for 2 h at room temperature under humid conditions. The plates were washed with PBS, and the remaining sites for protein binding on the plates were saturated with blocking buffer [3% (w/v) BSA in PBS] for 2 h at room temperature. After washing the plates with PBS, the primary antisera (against crude venom toxins or LALP) were incubated for 2 h at room temperature. The plates were then incubated with secondary antibodies conjugated to HRP (horseradish peroxidase) for 1 h at room temperature, and the colorimetric reaction was developed using OPD (o-phenylenediamine dihydrochloride) [17].
Cell culture conditions and LALP cytotoxicity
Rabbit subendothelial cells which were used in this study were maintained in liquid nitrogen with a low number of passages. After thawing, the cells were grown in monolayer cultures in RPMI 1640 medium containing penicillin (10000 IU/ml) and supplemented with 10% FCS (fetal calf serum). The cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Release of the cells from the dish was performed by treating cells with 0.25% pancreatin for 10 min. After cell counting, the cells were resuspended in 10 ml of medium and evaluated for cytotoxic effects in the presence or absence of LALP (5 μg/ml). Plates were photographed at 3 and 16 h after resuspension using an inverted microscope (Leica-DMIL), and changes in cell morphology were evaluated. Experiments were performed in triplicate.
Proteolytic effect of LALP on fibronectin, fibrinogen and gelatin
Human plasma fibronectin and human fibrinogen were incubated with LALP at a substrate/recombinant toxin ratio of 25:1 at 37 °C for 16 h. Negative-control substrates were incubated at 37 °C for 16 h in the absence of recombinant toxin. As an added control, we incubated protein substrates under similar conditions as described above, but with a recombinant toxin devoid of proteolytic activities and obtained under the same conditions as used for LALP. This was to discount the theory that LALP was contaminated with bacterial proteases during purification [14]. To ascertain whether LALP displayed any metalloprotease activity, substrates were incubated with recombinant LALP in the presence of 5 mM 1,10-phenanthroline (a bivalent chelator) under the conditions described above. Proteolysis of substrates was checked by heating the samples at 100 °C for 5 min and then analysing them by SDS/PAGE (7.5% gel for fibronectin, 10% gel for fibrinogen) under reducing conditions, alongside molecular-mass markers. Gels were subsequently stained using Coomassie Blue. Additionally, the proteolytic activity of LALP was assessed through zymography using SDS/PAGE (12% gel) copolymerized with gelatin (3 mg/ml). A sample of LALP (2.5 μg) was dissolved in Laemmli buffer in the absence of reducing agents and then electrophoresed at 4 °C. After electrophoresis, the gel was washed twice for 30 min in 2.5% (v/v) Triton X-100 to remove SDS and incubated overnight at 37 °C in 50 mM Tris/HCl, pH 7.3, 150 mM NaCl and 1 mM CaCl2 and then stained with Coomassie Blue. A clear zone of substrate lysis against a blue background stain indicated the presence of proteolytic activity.
Statistical analysis
Statistical analyses were performed using ANOVA post-hoc Tukey test for average comparisons using the GraphPad InStat program, version 3.00 for Windows 2000. Statistical significance was established at P<0.05.
RESULTS
Molecular cloning of an astacin-like metalloprotease from L. intermedia venom gland
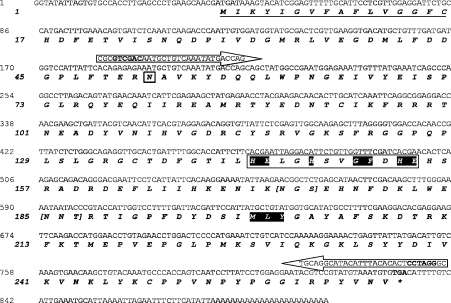
By screening clones of a cDNA library from L. intermedia venom gland, a cDNA encoding for an astacin-like metalloprotease toxin was isolated. The cDNA was identified using a protein-BLAST search, and the putative protein product from this cDNA was named LALP. Sequence analysis revealed a putative hydrophobic signal peptide consisting of 16 amino acids [23], a propeptide of 35 amino acids (deduced from homology with other astacin-like metalloproteases) [24] and a complete mature protein of 213 amino acids containing the astacin-family motifs HEXXHXXGFXHE (the enzymatic catalytic domain) and MXY (methionine-turn), separated by 44 amino acids [25]. The complete cDNA sequence of LALP was 900 bp, and the calculated molecular mass of the full non-active protein was 28.6 kDa, with a pI of 6.09. Two putative N-glycosylation sites were also identified (Figure 1).
Figure 1. Molecular cloning of a metalloprotease toxin LALP from a L. intermedia venom gland cDNA library.
Nucleotide and deduced amino acid sequences of cDNA of LALP from L. intermedia venom gland. The predicted signal peptide is underlined in the protein sequence. Annealing positions for primers used for subcloning into the expression vector are indicated by arrows. Asparagine (in a box) is the first amino acid of the mature protein, with 213 amino acids. The consensus sequences for astacin family members, HEXXHXXGFXHE, containing the zinc-binding motif is highlighted in another box, and the methionine-turn MXY (highlighted) is also shown. An asterisk indicates the stop codon (TGA) in bold. The square brackets mark two potential N-glycosylation sites.
Phylogenetic relationship to and multiple alignment analysis of the cDNA-deduced amino acid sequence of LALP with other astacin family members
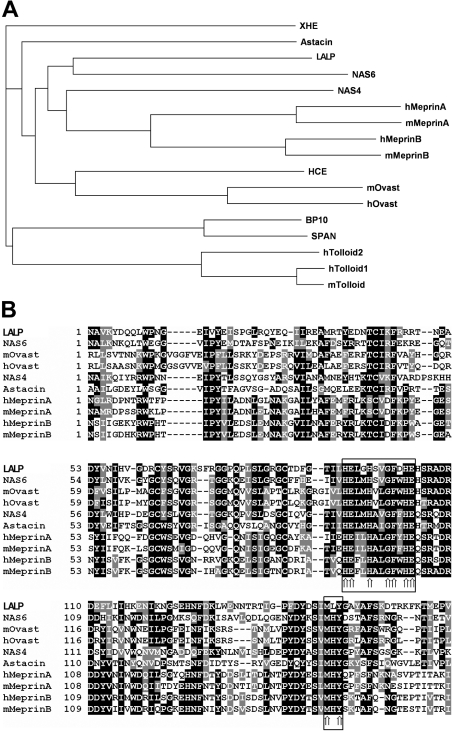
To explore further the structural and evolutionary relationships among the newly identified toxin and other members of the astacin family, we performed a phylogenetic tree analysis. Results (Figure 2A) established among LALP and other astacin-like cloned cDNAs that LALP had the greatest similarity to NAS-6 (GenBank® accession number NP_503121), a nematode astacin protease family member, with an overall identity of 35.0% with LALP. On the basis of phylogenetic analysis, LALP was found to be homologous with other astacin family members from a variety of species, including human and mouse meprins, nematode astacin NAS-4, mouse and human ovastacins, choriolytic hatching enzyme and astacin. Further analysis of the predicted amino acid sequences additionally confirmed the presence of conserved features between these different molecules (specifically for catalytic domains containing the zinc-binding motif) and strengthened the hypothesis that LALP is an astacin family member (Figure 2B).
Figure 2. Phylogenetic relationship and multiple alignment analysis of the cDNA-deduced amino acid sequences for the recombinant toxin LALP and other astacin-like metalloproteases.
Sequences were aligned using the ClustalW program (http://www.ebi.ac.uk/CLUSTAL). (A) Phylogenetic relationship of the cloned toxin to other astacin-like metalloproteases based on sequence data from GenBank®. The tree was constructed with the program ClustalW as described above. Proteins compared (and their GenBank® accession numbers) are as follows: NAS-6 (nematode astacin-6; NP_503121), hMeprinA (human meprin, α chain; NP_005579), mMeprinA (mouse meprin, α chain; NP_032611), hMeprinB (human meprin, β chain; NP_005916), mMeprinB (mouse meprin, β chain; NP_032612), NAS-4 (nematode astacin-4; AAB53827), mOvast (mouse ovastacin; CAD61264), hOvast (human ovastacin; CAD61265), HCE (high choriolytic hatching enzyme; C48826), Astacin (CAB43519), XHE (Xenopus hatching enzyme; BAA14003), BP10 (urchin blastula protease-10; CAA39673), SPAN (AAA30072), hTolloid1 (human tolloid-like 1; O43897), mTolloid (mouse tolloid-like; NP_033416) and hTolloid2 (human tolloid-like 2; Q9Y6L7). (B) Multiple alignment analysis of the amino acid domains of LALP and the astacin-family-related members as reported by phylogenetic analysis; identical amino acids are shaded in black, and conservative substitutions are in grey. Arrows in the boxes point to the astacin signature sequence and the methionine-turn.
Expression, purification and refolding of LALP
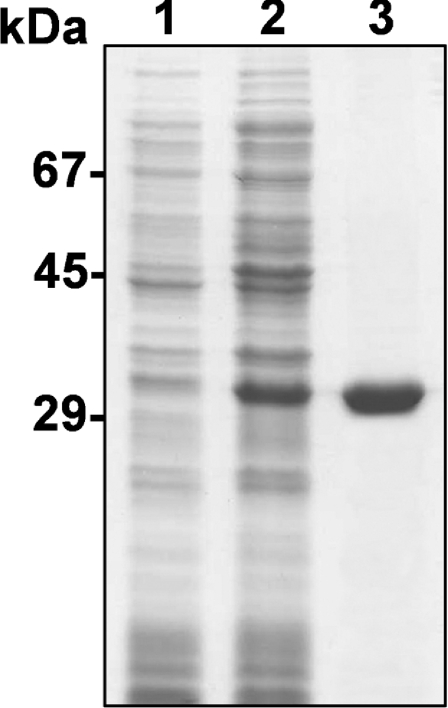
The recombinant metalloprotease, containing a N-terminal 6×His tag, was expressed in E. coli BL21(DE3)pLysS cells and was induced for 4 h with 0.05 mM IPTG. Recombinant toxin was purified by dissolving the insoluble fraction of cell lysates into 6 M guanidinium chloride and subsequently using Ni2+-chelating chromatography, resulting in a final concentration of LALP of 2.8 mg/l. As depicted in Figure 3, the SDS/PAGE mobility of the recombinant toxin after denaturation was 29 kDa. The difference between the predicted molecular mass and SDS/PAGE mobility for LALP is a consequence of the 6×His tag fusion peptide. The purified toxin refolded after dialysis against 10 mM sodium phosphate buffer, pH 7.4, as described in [21].
Figure 3. Expression and purification of recombinant LALP toxin analysed by SDS/PAGE (12% gel) under reducing conditions and Coomassie Blue staining.
Lane 1, E. coli BL21(DE3)pLysS cells collected by centrifugation and resuspended in Laemmli sample buffer 3.5 h prior to induction with 0.025 mM IPTG. Lane 2, the supernatant of cell lysates induced with 0.025 mM IPTG, obtained by freeze–thawing and sonication in extraction buffer before affinity chromatography using a Ni-NTA–agarose column. Lane 3, purified recombinant protein after elution through a Ni-NTA–agarose column. Molecular-mass standards are shown on the left.
CD spectroscopy
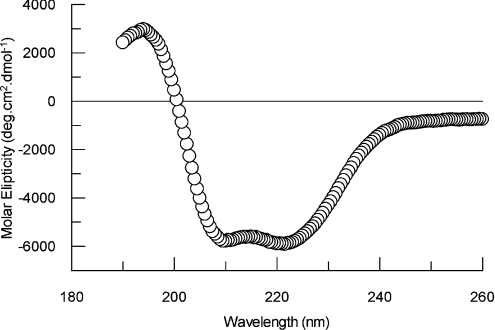
CD spectra of the purified recombinant LALP were obtained in order to analyse the correct refolding for the toxin and determine some structural details. As depicted in Figure 4, the spectrum corresponds to a protein with secondary structures of approx. 19% α-helix, 27% β-sheet and 54% other.
Figure 4. CD spectrum for purified recombinant LALP.
Spectra were obtained using toxin in 10 mM sodium phosphate buffer, pH 7.4, at 25 °C. Secondary structures were estimated using the Dichroprot 2000 1.0.4 software.
Immunological cross-reactivity of recombinant LALP and native venom toxins
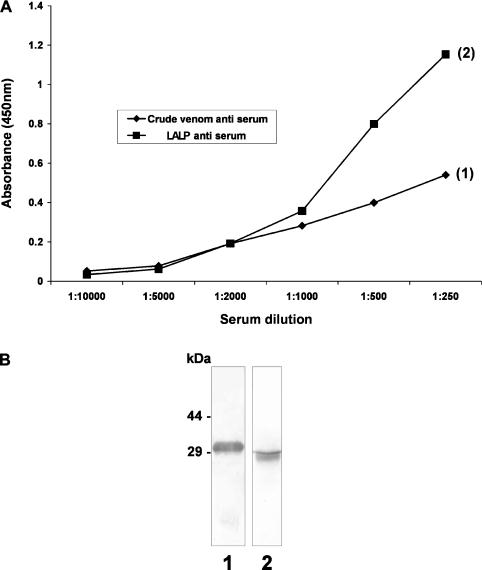
In order to evaluate further the relationship of the purified recombinant toxin with native venom toxins, we produced polyclonal antisera against whole-venom toxins or recombinant LALP and examined these for antigenic cross-reactivity between the recombinant toxin and whole venom. We performed an antibody-capture ELISA using antibodies that recognize non-denatured epitopes on the antigen and an immunoblot to identify antibodies that recognize denaturation-resistant epitopes on the antigen. As shown in Figure 5, antibodies against the whole-venom toxins cross-reacted with the recombinant toxin, and antibodies against LALP cross-reacted with a native venom toxin approx. 29 kDa in size, confirming that the whole venom contains a native protein which is similar to LALP, and this toxin is an important antigenic determinant.
Figure 5. Comparative immunological cross-reactivity of crude venom and recombinant toxins.
(A) Antibody-capture ELISAs were carried out using recombinant toxin LALP (1) or whole-venom toxins (2) at a concentration of 10 μg/ml, immobilized on a solid phase. Primary antisera against LALP or whole-venom toxins at the indicated concentrations (x-axis) were incubated for 2 h at room temperature, and the reaction developed as described in the Experimental section. (B) Purified recombinant toxin LALP (lane 1) and L. intermedia crude venom (lane 2), each at a concentration of 5.0 μg were resolved by SDS/PAGE (12.5% gel) under reducing conditions, transferred on to nitrocellulose membranes that were exposed to antisera against crude venom toxins (lane 1) or to LALP (lane 2). Molecular-mass markers are shown on the left (sizes in kDa). In both cases (ELISA and immunoblotting), cross-reactivity occurred among antibodies against crude venom toxins and recombinant toxin or antibodies against LALP with native venom toxins.
Effect of LALP on rabbit subendothelial cells
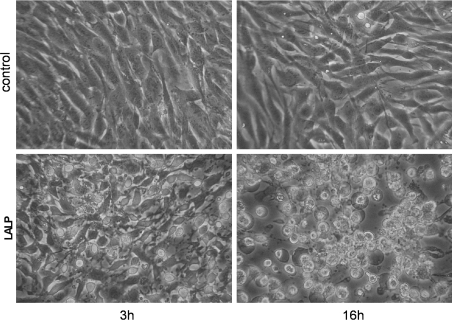
We reported previously the loss of adhesion of some cells, including rabbit aorta endothelial cells and MDCK (Madin–Darby canine kidney) cells, after exposure to Loxosceles whole venom [14,26,27]. This loss of adhesion caused by the presence of the venom seems to be dependent on proteolysis of extracellular matrix constituents, which mediate cell adhesion to substrata [7,8,15,26,28]. To test the relationship between whole-venom-induced cells' loss of adhesion and LALP, rabbit subendothelial cells were treated with purified LALP (5.0 μg/ml) for either 3 or 16 h under the same experimental conditions as for controls (absence of toxin). As shown in Figure 6, it is possible to observe loss of adhesion of cells following treatment with the recombinant toxin: treated cells are fully rounded up, lysed and detached from culture substrata, whereas the control cells are adherent and spaced out in a normal manner.
Figure 6. The effect of LALP on subendothelial cells.
Rabbit subendothelial cells were exposed to LALP (5.0 μg/ml) for 3 or 16 h. As a negative control, cells were grown under similar conditions and incubated with a recombinant toxin devoid of proteolytic activity, but obtained under similar conditions as for LALP. Morphological alterations of cells were observed under an inverted microscope. For LALP toxin-treated cells, cell spreading appears to be impaired, detachment from the substrate was observed, and cells were round and originated aggregates of debris in a time-dependent manner compared with absence of cytotoxic effects in the control sample (magnification ×200).
Protease effect of LALP on fibronectin, fibrinogen and gelatin
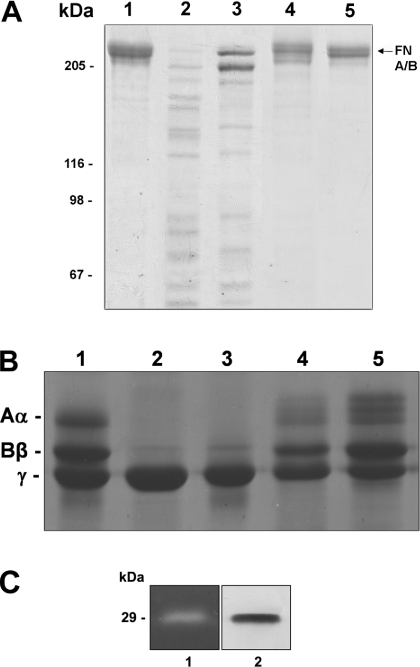
To evaluate further the role of LALP in the cytotoxic activity of the venom and to support the indication that proteolysis, loss of cell adhesion and cell lysis occur due to the presence of purified LALP, we treated purified fibronectin and fibrinogen (extracellular matrix molecules that participate in rabbit subendothelial cell adhesion and vessel wall integrity) with LALP. In addition, we tested the ability of 1,10-phenanthroline (a bivalent chelator) to block the enzymatic activity of LALP. As observed, LALP treatment resulted in the degradation of both fibronectin (Figure 7A) and fibrinogen (Figure 7B), whereas 1,10-phenanthroline abolished the proteolytic activity of LALP. A zymogram experiment containing co-polymerized gelatin further strengthened the hypothesis that LALP has proteolytic activity (Figure 7C).
Figure 7. Proteolytic effect of LALP on fibronectin, fibrinogen and gelatin.
Purified fibronectin (A) and fibrinogen (B) were incubated with LALP at 37 °C for 16 h, with a substrate/toxin ratio of 25:1. For the negative control, protein substrates were incubated under similar conditions with a recombinant toxin devoid of proteolytic activity, but obtained under the same conditions for LALP. Samples were subjected to SDS/PAGE (7.5% gel for fibronectin; 10% gel for fibrinogen) under reducing conditions, and gels were stained with Coomassie Blue. Lane 1, purified substrates without toxin treatment; lane 2, substrates incubated with whole venom; lane 3, substrates exposed to LALP; lane 4, substrates incubated with LALP in the presence of 1,10-phenanthroline (5 mM); lane 5, substrates incubated with a recombinant toxin without proteolytic activity. Positions of fibrinogen A(α), B(β) and γ chains and fibronectin A and B chains that co-migrate (FN A/B, arrow) are indicated. Molecular-mass markers are shown on the left. Additionally, the enzymatic function of the recombinant toxin was assessed through a zymogram copolymerized with gelatin (C). Lane 1 demonstrates the gelatinolytic activity of LALP, and lane 2 depicts an immunoblot using antibodies against LALP.
DISCUSSION
Proteases of venomous animals have been identified as playing a major role in the harmful effects observed after bites, such as haemorrhage, necrosis, cytotoxicity and oedema [29,30]. The presence of proteolytic activity in the Loxosceles spider venom was demonstrated previously in electrostimulated venom of L. reclusa and L. intermedia [7,8,31,32]. The inhibitory effects of bivalent metal chelators, such as EDTA and 1,10-phenanthroline, indicated the presence of metalloproteases in the venom [7,15,16]. The idea of metalloproteases as components of brown spider venom was strengthened by the venom gland extract possessing proteolytic activity and a sensitivity to metal chelators. This eliminated criticism that electrostimulated venom could have been contamined with spider stomach egesta (containing hydrolytic enzymes) during extraction [15]. Moreover, the presence of metalloproteases in the venom of different Loxosceles species, such as L. intermedia, L. gaucho, L. deserta, L. laeta and L. rufescens provides evidence for metalloproteases being a biologically significant and conserved feature of the Loxosceles species venom [7,9,15,16,33].
The astacin family of metalloproteases, named after the prototypical digestive enzyme astacin from crayfish, Astacus astacus, consists of zinc peptidases [34,35]. The astacin family comprises structurally related digestive, extracellular or cell-surface-bound proteases that are involved in peptide processing and play a role in the activation of growth factors, degradation of polypeptides and the processing of extracellular molecules [34,36,37]. The zinc-binding domain with the consensus sequence HEXXHXXGXXHE and the methionine-turn MXY are both key elements in the astacin family [34,36,37]. Astacin-like enzymes have been detected in different species, such as human, mouse, rat, amphibians, fish, sea urchins, insects, molluscs and even bacteria [36,37], supporting their biological importance.
In the present paper, we have described the identification, cloning, heterologous expression, purification, immunological cross-reactivity and function of a metalloprotease toxin, LALP, from the venom gland of L. intermedia. On the basis of the predicted amino acid sequence, LALP is thought to be composed of a domain structure similar to that of astacin metalloproteases and other astacin-like molecules. The LALP primary structure includes a signal sequence, a propeptide region, an astacin-like protease catalytic domain and a characteristic methionine-turn domain, similar to those of other astacin-like metalloproteases [34,36,37].
Additionally, several pieces of evidence support the idea that LALP is an astacin-like metalloprotease. LALP possess an overall shared identity with astacin and other astacin-like family members. The molecular mass calculated from the deduced amino acid sequence (28.6 kDa) and from the SDS/PAGE mobility (29 kDa) of LALP is very similar to those described previously for metalloproteases in L. intermedia venom (28–33 kDa) [7,15] and other Loxosceles venom (30–35 kDa) [33]. The calculated pI for LALP (pI=6.09) is very similar to those described for other astacin-like proteases, such as HMP2 (pI=5.12), a cnidarian metalloprotease from Hydra vulgaris [38] and meprin A subunit (pI=5.2), which is a vertebrate astacin-like metalloprotease [39]. Other additional evidence which supports LALP as an astacin-like metalloprotease comes from its astacin signature HEXXHXXGFXHE (His92–Glu103), which is the zinc-binding domain catalytic site, and the methionine-turn MXY (Met148–Tyr150), an amino acid domain involved in the maintenance of the conformation of the enzyme [36,40]. Finally, biochemical studies demonstrated the proteolytic activity of LALP on proteins such as fibronectin, fibrinogen and gelatin, as well as loss of cellular adhesion from the culture substrate, which strongly suggests that this recombinant toxin is a closely related member of the astacin-like protease family.
Phylogenetic studies based on protein sequence analysis of several different astacin family members compared with LALP additionally suggests that LALP is as an astacin-like molecule. Recombinat LALP was shown to closely resemble astacin, the prototype member of the atascin-like protease family. The proteins with the highest amino acid sequence similarities to LALP were identifed to be NAS-4 and NAS-6, two nematode astacin protease family members from Caenorhabditis elegans (35.0% of amino acid identity), followed by SPAN (Strongylocentrotus purpuratus astacin-like protease) from S. purpuratus (32.0%). Additionally, LALP is 31.0% similar at the amino acid level with sea urchin blastula protease BP10. These data suggest that astacin in venom could have a physiological function in spiders, similar to that performed by other astacin family members.
Full-length LALP heterogeneously expressed in E. coli cells was purified using denaturing Ni2+-affinity chromatography and, using this approach, LALP was eluted in a pure form, as determined by SDS/PAGE resolution. The recombinant toxin was refolded followed dialysis against sodium phosphate buffer. From CD spectra, we can conclude that correct refolding of the recombinant toxin occurred, and, in addition, the data showed an estimated percentage for secondary structures of toxin. The results are typical of folded non-denatured and non-aggregated proteins.
Characterization of the antigenic cross-reactivity by ELISA and immunoblotting using antibodies against LALP suggested that the crude venom contained a similar protein of 29 kDa, which showed antigenic structural identity with recombinant LALP. Additionally, antibodies raised against whole-venom toxins produced a high cross-reactivity with LALP, suggesting that epitopes present in this toxin are strong antigenic determinants and corroborating the presence of this astacin-like toxin in the venom.
The mechanism by which Loxosceles venom causes gravitational spreading of dermonecrosis and dispersion of other venom toxins through the body is currently under investigation. The participation of proteases and hyaluronidases as spreading factors could be putatively inferred [1]. Hyaluronidases and proteases have been detected in the venom of many different animals (snakes, caterpillars, scorpions, bees and spiders [29,41,42,43]). These enzymes, by degrading extracellular matrix constituents, can act as spreading factors and facilitate the diffusion of other venom toxins.
Consistent with the structural characteristics of LALP, the functional analysis of the recombinant toxin was corroborated further by the loss of adhesion of endothelial cells on incubation with LALP, as well as its proteolytic activities on fibronectin, fibrinogen and gelatin and sensitivity to 1,10-phenanthroline, which blocked the proteolytic activity of the toxin.
Further work is required to evaluate the biological significance of native astacin-like venom toxin; and to answer the question of why a protease exists in the venom? We speculate that the presence of this toxin in the venom represents an adaptation of spiders to paralyse and kill insect prey as well as to serve as a defence against predators. Astacin, a digestive enzyme of the astacin family, shows a phylogenetic link to the LALP subgroup, which is in agreement with this hypothesis. The presence of astacin-like metallopeptidases in the digestive fluid of the spider Argiope aurantia [24] strengthens the idea of astacin-like molecule expression in venom by spiders and a digestive function for spider astacin-like metalloproteases. Additionally, digestive juice components (as is the case with proteases) may play a role in some of the cutaneous and systemic effects that occur following brown spider envenomation, as regurgitation can occur when spiders are crushed up against the skin as part of the bite process [44]. These proteases could act in synergy with other venom toxins involved in the deleterious effects after envenomation.
Additionally, the presence of a protease in venom can represent a biological mechanism to facilitate the diffusion of other important venom toxins through the bodies of victims by rendering tissue structures more permeable, and then acting in parallel with other biologically active toxins. Finally, a protease toxin in the venom could be important for the processing of other peptide-derived toxins that, after proteolysis, are active.
In summary, we have identified a novel brown spider venom toxin. Moreover, we have cloned, heterogeneously expressed and purified this toxin, which was characterized as an astacin-like protease. This toxin degrades extracellular matrix components and can act as a spreading factor. Together, these results reveal greater insight into loxoscelism and contribute to the further understanding of this metalloprotease family, which has now been identified to be a component of venom.
Acknowledgments
This work was supported by grants from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES [(Fundação) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior], FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), Fundação Araucária-PR, MEC-SESU (Ministério da Educação e Cultura do Brasil–Secretaria de Ensino Superior) and SETI-PR (Secretaria de Estado da Ciência, Tecnologia e Ensino Superior do Paraná), Brazil.
References
- 1.da Silva P. H., da Silveira R. B., Appel M. H., Mangili O. C., Gremski W., Veiga S. S. Brown spider and loxoscelism. Toxicon. 2004;44:693–709. doi: 10.1016/j.toxicon.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Swanson D. L., Vetter R. S. Loxoscelism. Clin. Dermatol. 2006;24:213–221. doi: 10.1016/j.clindermatol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Sandidge J. S. Arachnology: scavenging by brown recluse spiders. Nature. 2003;426:30. doi: 10.1038/426030a. [DOI] [PubMed] [Google Scholar]
- 4.Ospedal K. Z., Appel M. H., Neto J. F., Mangili O. C., Veiga S. S., Gremski W. Histopathological findings in rabbits after experimental acute exposure to the Loxosceles intermedia (brown spider) venom. Int. J. Exp. Pathol. 2002;83:287–294. doi: 10.1046/j.1365-2613.2002.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elston D. M., Eggers J. S., Schmidt W. E., Storrow A. B., Doe R. H., McGlasson D., Fischer J. R. Histological findings after brown recluse spider envenomation. Am. J. Dermatopathol. 2000;22:242–246. doi: 10.1097/00000372-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Patel K. D., Modur V., Zimmerman G. A., Prescott S. M., McIntyre T. M. The necrotic venom of brown recluse induces dysregulated endothelial cell-dependent neutrophil activation. J. Clin. Invest. 1994;94:631–642. doi: 10.1172/JCI117379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feitosa L., Gremski W., Veiga S. S., Elias M. C. Q. B., Graner E., Mangili O. C., Brentani R. R. Detection and characterization of metalloproteinases with gelatinolytic, fibronectinolytic and fibrinogenolytic activities in brown spider (Loxosceles intermedia) venom. Toxicon. 1998;36:1039–1051. doi: 10.1016/s0041-0101(97)00083-4. [DOI] [PubMed] [Google Scholar]
- 8.Veiga S. S., Feitosa L., Santos V. L. P., de Souza G. A., Ribeiro A. S., Mangili O. C., Porcionatto M. A., Nader H. B., Dietrich C. P., Brentani R. R., Gremski W. Effect of Loxosceles intermedia (brown spider) venom on basement membrane structures. Histochem. J. 2000;32:397–408. doi: 10.1023/a:1004031019827. [DOI] [PubMed] [Google Scholar]
- 9.Young A. R., Pincus S. J. Comparison of enzymatic activity from three species of necrotising arachnids in Australia: Loxosceles rufescens, Badumna insignis and Lampona cylindrata. Toxicon. 2001;39:391–400. doi: 10.1016/s0041-0101(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 10.da Silveira R. B., Pigozzo R. B., Chaim O. M., Appel M. H., Dreyfuss J. L., Toma L., Mangili O. C., Gremski W., Dietrich C. P., Nader H. B., Veiga S. S. Molecular cloning and functional characterization of two isoforms of dermonecrotic toxin from Loxosceles intermedia (brown spider) venom gland. Biochimie. 2006;88:1241–1253. doi: 10.1016/j.biochi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.da Silveira R. B., Pigozzo R. B., Chaim O. M., Appel M. H., Trevisan D. S., Dreyfuss J. L., Toma L., Dietrich C. P., Nader H. B., Veiga S. S., Gremski W. Two novel dermonecrotic toxins LiRecDT4 and LiRecDT5 from brown spider (Loxosceles intermedia) venom: from cloning to functional characterization. Biochimie. 2007;89:289–300. doi: 10.1016/j.biochi.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.da Silveira R. B., Chaim O. M., Mangili O. C., Gremski W., Dietrich C. P., Nader H. B., Veiga S. S. Hyaluronidases in Loxosceles intermedia (Brown spider) venom are endo-β-N-acetyl-D-hexosaminidases hydrolases. Toxicon. 2007;49:758–768. doi: 10.1016/j.toxicon.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Lee S., Lynch K. R. Brown recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA) Biochem. J. 2005;391:317–323. doi: 10.1042/BJ20050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaim O. M., Sade Y. B., da Silveira R. B., Toma L., Kalapothakis E., Chávez-Olórtegui C., Mangili O. C., Gremski W., Dietrich C. P., Nader H. B., Veiga S. S. Brown spider dermonecrotic toxin directly induces nephrotoxicity. Toxicol. Appl. Pharmacol. 2006;211:64–77. doi: 10.1016/j.taap.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.da Silveira R. B., Filho J. F. S., Mangili O. C., Veiga S. S., Gremski W., Nader H. B., Dietrich C. P. Identification of proteases in the extract of venom glands from brown spider. Toxicon. 2002;40:815–822. doi: 10.1016/s0041-0101(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 16.Zanetti V. C., da Silveira R. B., Dreyfuss J. L., Haoach J., Mangili O. C., Veiga S. S., Gremski W. Morphological and biochemical evidence of blood vessel damage and fibrinogenolysis triggered by brown spider venom. Blood Coagulation Fibrinolysis. 2002;13:135–148. doi: 10.1097/00001721-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E., Lane D. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. Antibodies: A Laboratory Manual. [Google Scholar]
- 18.Luciano M. N., Silva P. H., Chaim O. M., Santos V. P., Franco C. R. C., Soares M. F. S., Zanata S. M., Mangili O. C., Gremski W., Veiga S. S. Experimental evidence for a direct cytotoxicity of Loxosceles intermedia (brown spider) venom on renal tissue. J. Histochem. Cytochem. 2004;52:455–467. doi: 10.1177/002215540405200404. [DOI] [PubMed] [Google Scholar]
- 19.Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int. J. Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 20.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lippman D. J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gmachl M., Kreil G. Bee venom hyaluronidase is homologous to a membrane protein of mammalian sperm. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3569–3573. doi: 10.1073/pnas.90.8.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deleage G., Geourjon C. An interactive graphic program for calculating the secondary structure content of proteins from dichroism spectrum. Comput. Appl. Biosci. 1993;2:197–199. doi: 10.1093/bioinformatics/9.2.197. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen H., Engelbrecht J., Brunak S., von Heijne G. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 1997;8:581–599. doi: 10.1142/s0129065797000537. [DOI] [PubMed] [Google Scholar]
- 24.Foradori M. J., Tillinghast E. K., Smith J. S., Townley M. A., Mooney R. E. Astacin family metallopeptidases and serine peptidase inhibitors in spider digestive fluid. Comp. Biochem. Physiol. 2006;143:257–268. doi: 10.1016/j.cbpb.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bode W., Gomis-Ruth F. X., Stöcker W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and shoud be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331:134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- 26.Veiga S. S., Zanetti V. C., Braz A., Mangili O. C., Gremski W. Extracellular matrix molecules as targets for brown spider venom toxins. Braz. J. Med. Biol. Res. 2001;34:843–850. doi: 10.1590/s0100-879x2001000700002. [DOI] [PubMed] [Google Scholar]
- 27.Paludo K. C., Gremski L. H., Veiga S. S., Chaim O. M., Gremski W., Buchi D. F., Nader H. B., Dietrich C. P., Franco C. R. C. The effect of brown spider venom on endothelial cell morphology and adhesive structures. Toxicon. 2006;47:844–853. doi: 10.1016/j.toxicon.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Veiga S. S., Zanetti V. C., Franco C. R. C., Trindade E. S., Porcionatto M. A., Mangili O. C., Gremski W., Dietrich C. P., Nader H. B. In vivo and in vitro cytotoxicity of brown spider venom for blood vessel endothelial cells. Thromb. Res. 2001;102:229–237. doi: 10.1016/s0049-3848(01)00254-7. [DOI] [PubMed] [Google Scholar]
- 29.Fox J. W., Bjarnason J. B. Atrolysins: metalloproteinases from Crotalus atrox venom. Methods Enzymol. 1995;248:368–387. doi: 10.1016/0076-6879(95)48024-2. [DOI] [PubMed] [Google Scholar]
- 30.Wu W. B., Chang S. C., Liau M. Y., Huang T. F. Purification, molecular cloning and mechanism of action of graminelysin I, a snake-venom-derived metalloproteinase that induces apoptosis of human endothelial cells. Biochem. J. 2001;357:719–728. doi: 10.1042/0264-6021:3570719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskafi F. M., Norment B. R. Physiological action of Loxosceles reclusa venom on insect larvae. Toxicon. 1976;14:7–12. doi: 10.1016/0041-0101(76)90114-8. [DOI] [PubMed] [Google Scholar]
- 32.Jong Y. S., Norment B. R., Heitz J. R. Separation and characterization of venom components in Loxosceles reclusa. II. Protease enzyme activity. Toxicon. 1979;16:529–537. doi: 10.1016/0041-0101(79)90227-7. [DOI] [PubMed] [Google Scholar]
- 33.Barbaro K. C., Knysak I., Martins R., Hogan C., Winkel K. Enzymatic charactarization, antigenic cross-reactivity and neutralization of dermonecrotic activity of five Loxosceles spider venoms of medical importance in the Americas. Toxicon. 2005;45:489–499. doi: 10.1016/j.toxicon.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Dumermuth E., Sterchi E. E., Jiang W., Wolz R., Bond J. S., Flannery A. V., Beynon R. J. The astacin family of metalloendopeptidases. J. Biol. Chem. 1991;266:21381–21385. [PubMed] [Google Scholar]
- 35.Stöcker W., Grams F., Baumann U., Reinemer P., Gomis-Ruth F. X., Mckay D. B., Bode W. The metzincins: topological and sequential relations between the astacins, admalysis, serralysis and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bond J. S., Beynon R. J. The astacin family of metalloendopeptidases. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohrlen F., Bond J. S., Stöcker W. Other astacin homologues. In: Barrett A. J., Rawlings N. D., Woessner J. F., editors. Handbook of Proteolytic Enzymes. 2nd edn. London: Elsevier; 2004. [Google Scholar]
- 38.Yan L., Kaiyin F., Zhang J., Dexter S., Sarras P. Identification and characterization of Hydra metalloproteinase 2 (HMP2): a meprin-like astacin metalloprotease that functions in foot morphogenesis. Development. 2000;127:129–141. doi: 10.1242/dev.127.1.129. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W., Gorbea C. M., Flannery A. V., Beynon R. J., Grant G. A., Bond J. S. The subunit of meprin A. Molecular cloning and sequencing, diferential expression in inbred mouse strains, and evidence for divergent evolution of the α and β subunits. J. Biol. Chem. 1992;267:9185–9193. [PubMed] [Google Scholar]
- 40.Sarras M. P., Jr BMP-1 and the astacin family of metalloproteases: a potencial link between the extracellular matrix, growth factors and pattern formation. BioEssays. 1996;18:439–442. doi: 10.1002/bies.950180604. [DOI] [PubMed] [Google Scholar]
- 41.Kreil G. Hyaluronidases – a group of neglected enzymes. Protein Sci. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouveia A. I. C. B., da Silveira R. B., Nader H. B., Dietrich C. P., Gremski W., Veiga S. S. Identification and partial characterization of hyaluronidases in Lonomia obliqua venom. Toxicon. 2005;45:403–410. doi: 10.1016/j.toxicon.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn-Nentwig L., Schaller J., Nentwig W. Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae) Toxicon. 2004;43:543–553. doi: 10.1016/j.toxicon.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Futrell J. M. Loxoscelism. Am. J. Med. Sci. 1992;304:261–267. doi: 10.1097/00000441-199210000-00008. [DOI] [PubMed] [Google Scholar]