Abstract
Biliary atresia is an inflammatory fibrosclerosing lesion of the bile ducts that leads to biliary cirrhosis and is the most frequent indication for liver transplantation in children. The pathogenesis of biliary atresia is not known; one theory is that of a virus-induced, subsequent autoimmune-mediated injury of bile ducts. The aim of this study was to determine whether autoreactive T cells and autoantibodies specific to bile duct epithelia are present in the rotavirus (RRV)- induced murine model of biliary atresia and whether the T cells are sufficient to result in bile duct inflammation. In vitro analyses showed significant increases in IFN-γ–producing T cells from RRV-diseased mice in response to bile duct epithelial autoantigen. Adoptive transfer of the T cells from RRV-diseased mice into naïve syngeneic SCID recipients resulted in bile duct–specific inflammation. This induction of bile duct pathology occurred in the absence of detectable virus, indicating a definite response to bile duct autoantigens. Furthermore, periductal immunoglobulin deposits and serum antibodies reactive to bile duct epithelial protein were detected in RRV-diseased mice. In conclusion, both cellular and humoral components of autoimmunity exist in murine biliary atresia, and the progressive bile duct injury is due in part to a bile duct epithelia–specific T cell–mediated immune response. The role of cellular and humoral autoimmunity in human biliary atresia and possible interventional strategies therefore should be the focus of future research.
Biliary atresia (BA) is a disease of the intrahepatic and extrahepatic bile ducts that begins in infancy and is characterized by periductal inflammation with fibrosis and eventual bile duct obstruction.1-4 Because of the progressive nature of this disease, up to 80% of BA patients will eventually require liver transplantation for survival, and therefore it is the most common indication for liver transplantation in children.5 A recent study has shown that of the 20% or so who are alive with their native liver for at least 20 years after the portoenterostomy, progressive bile duct injury and subsequent biliary cirrhosis is present in more than 95% of these patients.6
The cause of biliary atresia is not known. Current theories regarding its pathogenesis include defects in bile duct development,7 viral infection of the biliary tree,8-10 and immune-mediated bile duct injury.11-13 We hypothesized that the pathogenesis of BA may involve both a primary perinatal hepatobiliary viral infection and secondary generation of an autoreactive T cell–mediated bile duct injury.11 It is proposed that after infection with a cholangiotropic virus that resulted in initial bile duct epithelial damage, autoimmune-mediated inflammation and injury to bile duct epithelia would persist despite clearance of the virus. The factors eliciting the cellular autoimmunity may include bystander activation or molecular mimicry.
In addition to T cell–mediated immunity, humoral autoimmunity also plays a role in most autoimmune diseases. Tissue-specific autoimmune diseases such as multiple sclerosis and type 1 juvenile diabetes involve predominantly T-cell–mediated effector mechanisms of tissue injury, and the associated autoantibodies are largely markers of the autoimmune process.14,15 To test the theory of viral-induced autoimmunity in BA and investigate both the cellular and humoral autoimmune responses, we used the Rhesus group A rotavirus (RRV)-induced murine model of BA.
In the RRV-induced murine model of BA, newborn mice infected with RRV develop progressive inflammation and obstruction of the extrahepatic bile duct by 2 weeks of age.16 The inflammatory injury of the bile ducts persists despite clearance of RRV from the liver and is associated with death by 3 to 4 weeks of life.16-18 The significance of the immune response in this model was elucidated by Shivakumar et al.,18 who reported that interferon gamma (IFN-γ) knockout mice were protected from the progressive inflammation and obstruction of the bile ducts with improved long-term survival.
The aim of this study was to determine whether auto-reactive T cells and autoantibodies specific to bile duct epithelia are present in the RRV-induced murine model of BA and whether the T cells are sufficient to cause bile duct inflammation and injury. The techniques used to address this hypothesis included in vitro studies to determine the antigen specificity of activated T cells, adoptive transfer of T cells from RRV-diseased mice into naïve SCID mice to establish transfer of biliary disease, and Western blot analysis to detect serum antibodies reactive to bile duct epithelia.
Materials and Methods
Isolation of Liver and Spleen T Cells
All animals received care in accordance with NIH “Guide for Care and Use of Laboratory Animals” (publication #86-25 1985) through the UCDHSC Center for Laboratory Animals. Timed-pregnant female BALB/cJ mice were purchased from virus-free colonies of Jackson Laboratory, Maine. Forty microliters of a Hank's Balanced Salt Solution solution (BSS—control group) or 1.0 × 106 pfu/mL Rhesus rotavirus strain MMU 18006 (RRV—experimental group) was injected intraperitoneally into newborn mice at 12 to 18 hours of life. Mice were sacrificed on day 14, and liver, extrahepatic bile duct, and spleen were removed. Pooled tissues from 15 to 20 mice were used for each experiment. Spleen and liver tissue was homogenized over a wire mesh and red blood cells lysed. Liver immune cells were enriched by Percoll gradient (40/60).
Analysis of IFN-γ Production by ELISPOT Assay
ELISPOT assays were performed on plates coated with murine IFN-γ capture monoclonal antibody (eBio-science, CA) and blocked with media [RPMI/10% fetal calf serum (FCS)]. Responder T cells were purified by autoMACS magnetic bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany) (purity of 85%-90%) and rested for 72 hours in culture with murine interleukin 15 (IL-15), 100 ng/mL (eBioscience, San Diego, CA). Mitomycin C–treated stimulator splenocytes from naïve BALB/cJ mice (5 × 105 splenocytes/well) were cultured with responder T cells (2 × 105 cells/well) and cultured in the presence of media alone, UV-irradiated RRV (5 × 104 pfu/well), or bile duct epithelial cell line homogenate (5-100 μg/mL). The immortalized bile duct epithelial cell line, provided by Y. Ueno and N. LaRusso, Mayo Clinic, MN, was previously generated by transduction with the thermosensitive mutant SV40 large T antigen. [This transduction includes a genetic construct containing the large T antigen, retroviral long terminal repeats, and a viral packaging signal, Ψ. Only the large T antigen gene gives rise to protein.]19 Other controls included antigen-presenting cells (APCs) only, T cells only, normal BALB/cJ kidney epithelial homogenate (100 μg/mL), SV40 large T antigen only (1 μg/well, CHIMERx, Madison, WI), and phytohemagglutinin (PHA) (6 μg/mL).
For the T cell blocking experiments, CD4 blocking antibody GK 1.5 (1:200) (anti-L3T4a, IgG2b),20 CD8 blocking antibody CD8 (1:100) (clone 53-6.7, BD Pharmingen, San Diego, CA),21 and isotype control rat IgG2b were used. IFN-γ detection monoclonal antibodies were added and spots visualized by avidin-horseradish peroxidase and 3-amino-9-ethylcarbazole substrate reagent. The ELISPOT plates were analyzed with a CTL Immunospot Analyzer (Cellular Technology Ltd, Cleveland, OH), and results reported as mean ± SEM spot-forming units per well. ELISPOT assays were performed in triplicate and repeated twice. Differences between groups were analyzed statistically with Student t test for unpaired samples (InStat program, GraphPad Software, San Diego, CA) and considered significant for P values less than .05.
Adoptive Transfer Studies
Donor T Cell Preparation
Pooled liver or spleens from 15 to 20 two-week-old mice previously inoculated with BSS or RRV were used for each experiment. Mono-nuclear cells were isolated at the interface of the Percoll gradient, resuspended in FACS buffer, blocked with anti-CD16/32, and incubated with PE-CD45, APC-CD4, APC-CD8, and FITC-CD3 (BD Pharmingen). T cells were sorted on the MoFlo FACS (Dako Cytomation, Denmark) by gating on CD45+ leukocytes and selecting CD3+/CD4+/CD8+ T cells (final purity, 95%-99%). The average yield (mean ± SEM) of T cells from three separate experiments was as follows: BSS spleen T cells, 3.75 ± 0.87 × 106; BSS liver T cells, 6.6 ± 1.8 × 105; RRV spleen T cells, 2.7 ± 0.33 × 106; RRV liver T cells, 1.96 ± 0.16 × 106.
Adoptive Transfer
Six-week-old SCID C.B-17 mice (BALB/cJ) (Taconic Laboratories, Germantown, NY) were used as recipients of donor T cells. Four groups of recipient SCID mice received an intraperitoneal injection of donor T cells from either (1) BSS spleen (7 × 105 T cells/mouse); (2) RRV spleen (7 × 105 T cells/mouse); (3) BSS liver (4 × 105 T cells/mouse); or (4) RRV liver (5 × 105 T cells/mouse). Four recipient SCID mice were used per group (except BSS liver group—2 mice), and the adoptive transfer studies were repeated three times. Two weeks after adoptive transfer, the following was assessed:
Serum direct bilirubin level: Blood was collected from the renal artery and serum direct bilirubin determined with the Direct Bilirubin Test (Diagnostic Chemicals Ltd., Charlottetown, Price Edward Island, Canada). All results were based on at least three separate experiments of pooled mouse sera and reported as mean ± SEM.
Tissue histology: Tissue was formalin fixed, paraffin embedded, sectioned at 4 μm, and heat dried at 55°C for 20 minutes. Sections were stained with hematoxylineosin.
Immunohistochemistry studies: Endogenous peroxidases were blocked, proteinase K enzyme digestion performed, and serum free protein block (Open Biosystems, Huntsville, AL) applied. Rabbit anti-mouse CD3 monoclonal antibody (Lab Vision, Fremont, CA) or rat anti-mouse F4/80 monoclonal antibody (Serotec, Raleigh, NC) was applied followed by a second protein block. The secondary antibody was applied followed by labeled polymer-horseradish peroxidase conjugated, DAB and counterstained with hematoxylin.
Determination of Infectious Virus
Reverse Transcription Polymerase Chain Reaction Analysis of RRV mRNA Expression
RNA from donor liver immune cells (after Percoll isolation) and donor and recipient liver homogenates was purified and reverse transcription polymerase chain reaction (RT-PCR) was performed for actin or the RRV-derived gene VP-4 as previously described.17 All PCR work was repeated twice.
Infectious Virus Plaque Assay
Liver tissue from BSS and RRV inoculated donor mice and SCID recipients was weighed, homogenized, and plated on MA-104 cells in duplicate at increasing dilutions (10−1-10−5) as previously described.17 All plaque assays were performed in duplicate and repeated twice.
Indirect Immunofluorescence
Previously frozen liver sections (7 μm) from 2-week-old mice were fixed in 2% paraformaldehyde, incubated with 6% hydrogen peroxide, blocked with FcγR antibody (BD Biosciences, Rockville, MD), followed by incubation with goat-anti-mouse IgG-FITC (1:200) (Serotec). Controls included goat anti-human IgG-FITC (Biomeda, Foster City, CA) and goat serum IgG alone. Tissue was counterstained stained with Hoechst dye and positive fluorescent signal visualized under 200× magnification with the Zeiss Axioplan2 microscope (Thornwood, NY). Portal tracts were identified by characteristic vessel formation and bile duct epithelial nuclear staining and verified by serial sections with hematoxylin-eosin stain. The portal tracts were also easily identified because surrounding hepatocytes display a faint nonspecific background fluorescent staining and the portal tracts do not. Digital photographs were obtained with autocalibration of exposure time through the Zeiss KS 300 3.0 program. Exposure times were similar between BSS and RRV tissue samples. Five mouse livers from each group were analyzed, and this was repeated three times from separate experiments.
Western Blot Analysis
Protein sources for the Western blot analyses included UV-inactivated RRV (5 × 107 pfu/mL), bile duct epithelial cell line (2 mg/mL), control kidney homogenates (2 mg/mL), and SV40 large T antigen. Cells were lysed with Tris/Triton X-100/NaCl. Ten micrograms protein was loaded onto a NuPAGE 4-12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and run at 80 mVolts for 3 hours. A full-range Rainbow recombinant molecular weight marker was placed in an adjacent lane. The proteins were transferred onto a 0.2 μm nitrocellulose membrane and blocked with 0.05% powdered milk in Tris-buffered saline/0.05% Tween solution. Pooled sera from BSS or RRV mice were diluted in the milk blocking solution and incubated with the protein-containing membrane overnight at 4°C. Goat-anti-mouse IgG-peroxidase (KPL, Gaithersburg, MD) was added to the membranes for 1 hour at room temperature. The membranes were washed and equal volumes of luminescence reagent and oxidizing reagent (Santa Cruz Biotechnology, Santa Cruz, CA) were applied for 1 minute. A radiograph film was placed on top of the membrane and the film exposed for 1 minute. Western blot analysis was repeated three times on BSS and RRV sera obtained from separate experiments.
Results
Bile Duct Epithelial Antigen–Specific Autoreactive T Cells Reside Within 2-Week-Old RRV-Diseased Mice
To determine the presence of bile duct epithelia–specific autoreactive T cells, in vitro ELISPOT analyses were performed to identify IFN-γ–producing liver or spleen T cells in response to bile duct epithelial antigen. Spleen T cells were analyzed because naive lymphocytes are activated by antigen and differentiated into effector cells in the secondary lymphoid organs, that is, spleen, followed by re-circulation to the original site of injury. IFN-γ was chosen as the detection cytokine based on previous studies showing significant CD4+ T cell production of IFN-γ in the RRV-induced murine model of BA.17,18 Because T cells can die if immediately stimulated after isolation from animals because of their T cell activation status,22 purified liver or spleen T cells were rested for 72 hours in culture with IL-15. IL-15 prevents activation induced cell death of both CD4+ and CD8+ T cells in culture.22,23 The rested T cells (responders, 2 × 105 cells/ well) were then cultured for 48 hours with mitomycin-treated splenocytes from naïve adult BALB/cJ mice (stimulators, 5 × 105 cells/well) in media alone or with an antigen. Antigens tested included inactivated RRV (5 × 104 pfu/well), normal bile duct epithelia cell line homogenate (5-100 μg/mL), and control kidney epithelia homogenate (100 μg/mL), purified SV40 large T antigen (1 μg/well), and PHA (positive control, 6 μg/mL).
In vitro analyses of T cell antigen specificity revealed significant increases in IFN-γ–producing liver and spleen T cells from 2-week-old RRV-diseased mice in the presence of bile duct epithelial homogenate (bde) (Fig. 1). The increase in number of IFN-γ–producing T cells occurred in a dose–response fashion with increasing amounts of bile duct epithelial antigen (dose of bde/number of liver T cells: 25 μg/mL/49.6 ± 13.5, 100 μg/mL /116.7 ± 29.2; dose of bde/no. of spleen T cells: 5 μg/ mL/176.3 ± 40.8, 25 μg/mL/198.3 ± 18.7, 100 μg/mL/268 ± 71.0). Similar levels of IFN-γ–producing T cells were detected in cultures that contained inactivated RRV antigen, suggesting the presence of memory T cells to virus or molecular mimicry (245 ± 46.0 liver T cells; 158.3 ± 14.0 spleen T cells). There was low/undetectable IFN-γ within the following controls: media alone (no antigen), kidney epithelia homogenate, SV40 large T antigen, stimulators (APCs) alone, and responder T cells alone. Furthermore, cultured T cells from BSS-inoculated control mice produced low/undetectable IFN-γ in response to bile duct epithelia homogenate or RRV. In summary, bile duct epithelial antigen–specific autoreactive T cells reside within the liver and spleen of 2-week-old RRV-diseased mice.
Fig. 1.

Bile duct epithelial antigen–specific autoreactive T cells present in 2-week-old Rhesus rotavirus (RRV)-diseased mice. Purified T cells (rested with IL-15) from livers (A) or spleens (B) of 2-week-old mice previously inoculated with balanced salt solution (BSS) or RRV (responders, 2 × 105 cells/well) were cultured for 48 hours with mitomycin-treated naïve splenocytes (stimulators, 5 × 105 cells/well) in media alone or with antigen. Determination of activation was performed by measuring T cell production of interferon gamma (IFN-γ) using a standard ELISPOT technique. Both RRV-liver and RRV-spleen T cells produced significant amounts of IFN-γ in the presence of bile duct epithelia (bde) homogenate in a dose–response fashion, in comparison with no antigen control (*P < .05). Increased amounts of IFN-γ also were detected in cultures that contained inactivated RRV antigen, suggesting the presence of memory T cells to virus or molecular mimicry. Low IFN-γ was seen within the following controls: media alone (no antigen), kidney epithelia homogenate, SV40 antigen, antigen-presenting cells (APCs) alone, and T cells alone. Furthermore, BSS-liver and BSS-spleen T cells produced low/undetectable IFN-γ in response to multiple antigens. All responders (BSS and RRV groups) produced excess amounts of IFN-γ (TNTC) in the presence of phytohemagglutinin (PHA) (positive control; not shown).
CD4+ T Cell Blockade Abrogates IFN-γ Production From T Cells of RRV-Diseased Mice Stimulated With Bile Duct Epithelial Antigen
We next determined whether the CD4+ T cell subset was responsible for the increased IFN-γ production within CD3+ T cells of diseased mice stimulated with self bile duct epithelial homogenate (as shown previously). Significant increases in IFN-γ–producing CD4+ T cells in RRV-diseased mice in the first 2 weeks after RRV infection have been shown.17 CD4+ T cells recognize extracellular proteins (such as bile duct homogenate) that have been endocytosed into vesicles, processed, and presented in the context of MHC class II. Conversely, CD8+ T cells recognize small peptide sequences from intracellular cytosolic proteins (such as viruses) in the context of MHC class I.24 Furthermore, most T cell–mediated autoimmune diseases are driven by the CD4+ T cell subset. Based on this knowledge, we hypothesized that the CD4+ T cell was the necessary subset activated in the presence of bile duct epithelia homogenate. Purified CD3+ T cells from the spleen of 2-week-old RRV-diseased mice were placed in culture with mitomycin C–treated naïve splenocytes, bile duct homogenate alone or with anti-CD4, anti-CD8a, or IgG2b (isotype control). In the presence of CD4 blocking antibody, significant abrogation of T cells producing IFN-γ was seen (13.3 ± 2.0 T cells) compared with untreated cells (149.3 ± 14.7), isotype control (141.7 ± 26.5), or cells treated with anti-CD8 (134.7 ± 17.8) (Fig. 2). This study confirms that the bile duct epithelial antigen-specific autoreactive cells are CD4+ T cells.
Fig. 2.

CD4+ T cell blockade abrogates interferon gamma (IFN-γ) production from T cells of Rhesus rotavirus (RRV)-diseased mice stimulated with bile duct epithelial antigen. Purified CD3+ T cells (rested with IL-15) from spleens of 2-week-old mice previously inoculated with RRV (responders, 4 × 105 cells/well) were cultured for 48 hours with mitomycin-treated naïve splenocytes (stimulators, 5 × 105 cells/well) with bile duct epithelial antigen [100 μg/mL bile duct epithelial homogenate (bde)] alone or in combination with anti-CD4, anti-CD8, or IgG2b (isotype control). Determination of activation was performed by measuring T cell production of IFN-γ using the ELISPOT technique. Marked suppression of T cells producing IFN-γ was observed in the presence of CD4 blocking antibody. This suppression was not found in cultures with CD8 blocking antibody or isotype control.
Adoptively Transferred T Cells From RRV-Diseased Mice Lead to Periductal Infiltrates and Mild Direct Hyperbilirubinemia in SCID Recipients
We next sought to determine whether adoptive transfer of these autoreactive T cells from 2-week-old RRV-diseased mouse liver and spleen would lead to ductal inflammation in the recipient T cell–deficient SCID mouse. Four groups of SCID recipients received highly purified donor T cells from either the liver or spleen of 2-week-old BSS-inoculated mice or RRV-diseased mice. Two weeks after adoptive transfer, the recipients were killed and the extra-hepatic bile ducts and livers analyzed for cellular infiltrates. No periductal inflammation was observed in the SCID recipients of donor T cells from the liver or spleen of BSS-inoculated mice (Fig. 3A-B). Conversely, all SCID recipients of donor T cells from RRV-diseased livers or spleens had intense periductal infiltrates of the extrahepatic bile ducts and large intrahepatic ducts (Fig. 3C-D). To determine whether the inflammation was uniquely localized to the bile duct epithelium, we analyzed other epithelial tissues within the SCID recipient for immune cell infiltrates. No evidence was seen of inflammation within or surrounding the ductal epithelia of pancreatic ducts or renal tubular epithelia in SCID recipients of donor T cells from RRV-diseased mice (Fig. 3E-F). Therefore, adoptive transfer of T cells from RRV-diseased mice into SCID recipients resulted in an inflammatory infiltrate targeted specifically to bile duct epithelia.
Fig. 3.

SCID recipients of adoptively transferred liver T cells from Rhesus rotavirus (RRV)-diseased mice have prominent periductal infiltrates. Adult SCID C.B-17 mice received 4 to 5 × 105 liver T cells or 8 × 105 spleen T cells from 2-week-old balanced salt solution (BSS)-inoculated or RRV-diseased mice. Shown here are representative hematoxylineosin–stained sections from the extrahepatic ducts and porta hepatis regions of SCID mice, 2 weeks after adoptive transfer. Black arrows point to bile ducts. The SCID mice that received BSS liver T cells did not have periductal infiltrates of the extrahepatic bile ducts (A) or large intrahepatic ducts (B). In contrast, SCID recipients of RRV liver T cells (C-D) or spleen T cells (not shown) had prominent immune cell infiltrates surrounding both the extrahepatic ducts and large intrahepatic ducts. No evidence of inflammation was seen within or surrounding the ductal epithelia of pancreatic ducts or renal tubular epithelia in SCID recipients of donor T cells from RRV-diseased mice (Fig. 2E-F).
To determine whether the bile duct inflammation was associated with a clinical indicator of hepatobiliary dys-function, direct bilirubin was measured in all SCID recipients. A twofold increase was seen in the serum level of direct bilirubin in the SCID recipients of donor RRV-diseased liver T cells (0.96 ± 0.47 mg/dL) compared with BSS liver T cells (0.48 ± 0.29 mg/dL) and a significant 10-fold increase (P < .05) in direct bilirubin in the SCID recipients of donor RRV-diseased spleen T cells (1.27 ± 0.39 mg/dL) compared with BSS spleen T cells (0.13 ± 0.13 mg/dL). This mild direct hyperbilirubinemia parallels the periductal inflammation observed in the SCID recipients.
Periductal Infiltrates Within SCID Recipients of RRV-Diseased T Cells Consist of Both Adoptively Transferred Donor CD3+ T Cells and Recipient Macrophages
Confirmation of the presence of adoptively transferred T cells within the periductal infiltrates of SCID recipients was determined by immunohisto-chemistry staining of CD3 cell surface expression. Minimal CD3+ T cells were seen surrounding the bile ducts in SCID recipients of BSS-inoculated liver T cells (2.4 ± 0.68 CD3+ cells/ductal profile; Fig. 4A) compared with heavy infiltrates in recipients of RRV-diseased liver T cells (39 ± 6.45 CD3+ cells/ductal profile; Fig. 4B). The adoptively transferred T cells were found to be in close proximity to bile duct epithelia (Fig. 4C), occasionally invading between the epithelia (Fig. 4D). Interestingly, lymphocyte permeation into bile duct epithelium has been previously described in human biliary atresia.25
Fig. 4.
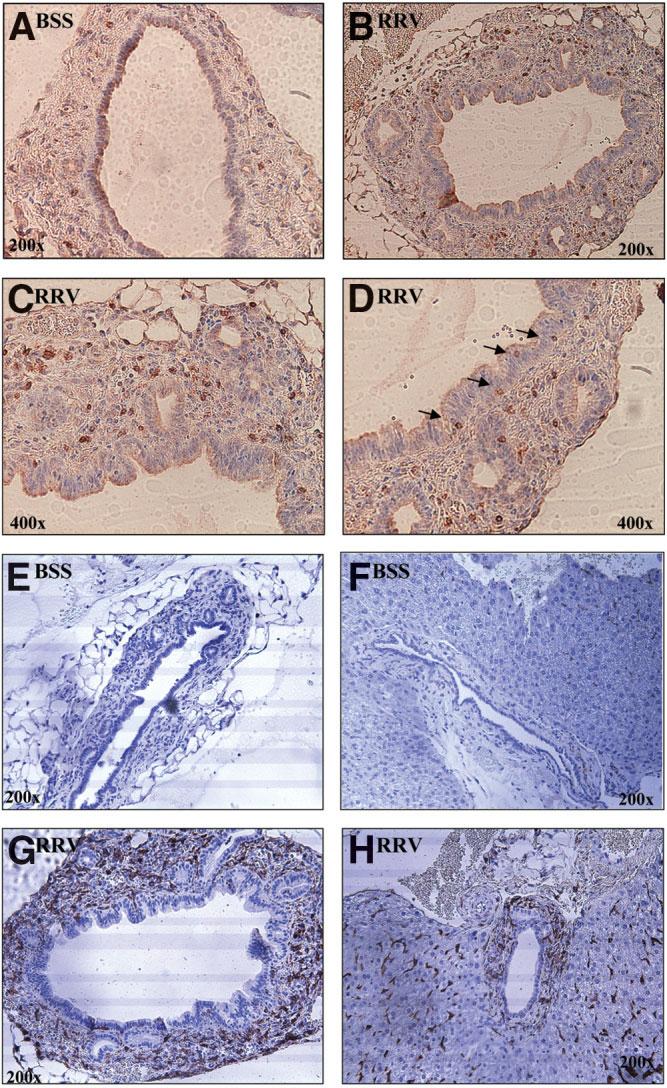
Periductal infiltrates within SCID recipients of Rhesus rotavirus (RRV) liver T cells contained the adoptively transferred donor CD3+ T cells and recipient macrophages. Representative sections from SCID recipients of balanced salt solution (BSS) control or RRV liver T cells were stained for antibody to CD3 or F4/80 (shown in brown) to identify adoptively transferred T cells and recipient macrophages, respectively. Few CD3+ T cells were seen surrounding the extrahepatic bile ducts in SCID recipients of BSS liver T cells (A). In contrast, the extrahepatic bile duct epithelia from SCID recipients of RRV liver T cells were surrounded by an abundance of CD3+ T cells (B-C; 400× magnified view). T cells also were found invading in between the bile duct epithelia (D, 400× ). SCID recipients of BSS liver T cells did not have F4/80+ cells (macrophages) surrounding either the extrahepatic bile ducts (E) or large intrahepatic ducts (F) In contrast, a marked influx of recipient macrophages was observed surrounding the extrahepatic ducts (G) and large intrahepatic ducts (H) in SCID recipients of RRV liver T cells. Note the F4/80+ staining of sinusoidal macrophages in the parenchyma of both groups.
Within the SCID recipients of RRV-diseased T cells, the degree of periductal inflammation observed by hematoxylin-eosin stain was subjectively greater than what could be accounted for by the T cells detected by immunohistochemistry. Therefore, we hypothesized that recipient macrophages may be activated and attracted by T cell cytokines or chemokines and infiltrate into the site of injury. A marked influx of activated recipient macrophages (F4/80 positive cells) was observed surrounding the extrahepatic bile ducts and large intrahepatic ducts in SCID recipients of T cells from RRV-diseased (Fig. 4G-H) but not BSS control mice (Fig. 4E-F).
Lack of Evidence of RRV Infection in SCID Mice With Adoptive Transfer of T Cells From RRV-Diseased 2-Week-Old Mice
To ensure no contamination of infectious RRV within the adoptive transfer studies, donor T cells and recipient SCID livers were analyzed for the presence of RRV. RNA expression of the RRV structural protein VP-4 was absent in both the pooled donor liver T cells from 2-week-old RRV-diseased mice and in the liver homogenates of recipient SCID mice (Fig. 5A). Verification of lack of transfer of RRV was performed with infectious plaque assays that revealed undetectable levels (<10 pfu/mL) of infectious virus from the 2-week-old RRV-liver donor T cells and recipient SCID liver homogenates. One-week-old RRV liver homogenates (positive control) had 0.5 × 106 ± 0.08 × 106 pfu/mL (Fig. 5B). These findings not only validate previous reports of viral clearance by 2 weeks of age16-18 but also confirm that no transmission of infectious RRV occurred with the adoptive transfer studies.
Fig. 5.
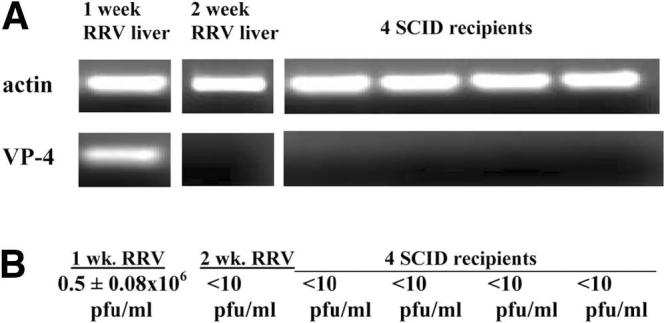
Absence of mRNA expression of Rhesus rotavirus (RRV) structural protein VP-4 and actively replicating virus in donor RRV-liver T cell preparations or recipient SCID mice livers. (A) Reverse transcription polymerase chain reaction (RT-PCR): RNA from 1-week-old liver homogenates of RRV-diseased mice (positive control), pooled donor liver immune cells (Percoll isolation) from 2-week-old RRV-diseased mice, and liver homogenates from four SCID recipients of RRV-diseased liver T cells were extracted and used in RT-PCR reactions for actin (control) and VP-4 (structural protein of RRV). PCR evidence of RRV RNA was found in the 1-week-old RRV-diseased liver homogenates but absent in the donor T cell preparations from 2-week-old RRV-diseased mice as well as SCID recipients, 2 weeks after adoptive transfer of RRV liver T cells. (B) Infectious plaque assay: One-week-old RRV liver homogenates (positive control) had 0.5 × 106 ± 0.08 × 106 pfu/mL infectious virus whereas undetectable levels (<10 pfu/mL) of infectious virus were observed in the 2-week-old RRV-liver donor T cells and recipient SCID liver homogenates.
Peri-ductal IgG Immune Deposits and Serum Antibodies Reactive to Bile Duct Epithelial Proteins Are Evident in 2-Week-Old RRV-Diseased Mice
The in vitro and adoptive transfer studies described provided complementary evidence for cellular autoimmunity in murine BA. We next determined whether humoral autoimmunity was also present in this disease. Autoantibodies exist in many autoimmune diseases and may function primarily as markers of the autoimmune response. Indirect immunofluorescence was employed to detect IgG immune deposits in liver tissue. RRV-diseased mice had detectable IgG immune deposits within the portal tracts that was not observed in the BSS control mice (Fig. 6A) or isotype controls (data not shown). The periductal immune deposits in RRV-diseased mice livers were observed at 2 weeks of age, a time point when infectious virus is no longer detectable in the liver. Therefore, the immunoglobulin likely was reactive with bile duct epithelia and not residual viral particles.
Fig. 6.
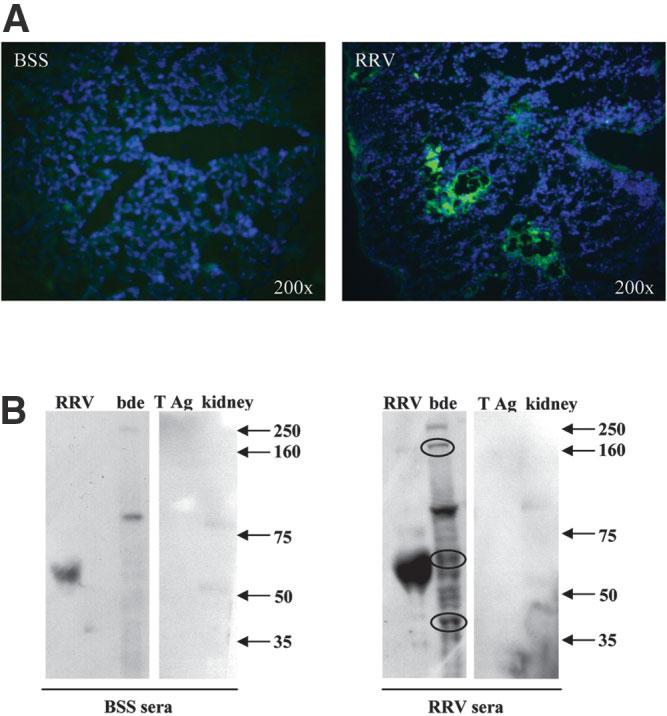
Peri-ductal IgG immune deposits and serum antibodies reactive to bile duct epithelial proteins are evident in 2-week-old Rhesus rotavirus (RRV)-diseased mice. Liver tissue and serum from 2-week-old mice previously inoculated with balanced salt solution (BSS) or RRV were obtained for immunohistochemistry and Western blot analysis, respectively. (A) Indirect immunofluorescence: Liver sections were analyzed for the presence of IgG immune deposits on bile duct epithelia by standard immunofluorescent techniques. Tissue was incubated with goat anti-mouse IgG-fluorescein isothiocyanate (FITC) (green) and counterstained stained with Hoechst dye (blue) to detect cellular nuclei. RRV-diseased mouse livers had detectable IgG immune deposits surrounding bile duct epithelia within portal tracts. No detectable IgG deposits were seen in livers from BSS control mice. (B) Western blot analysis: Irradiated RRV and bile duct epithelial cell line homogenate (and controls SV40 large T antigen and kidney epithelia homogenate) were separated by gel electrophoresis, blotted onto nitrocellulose paper, and incubated with either BSS or RRV sera. Antibodies reactive to protein were visualized by chemiluminescence (dark bands on radiograph). Both the BSS and RRV sera contained nonspecific antibodies reactive to (1) a viral protein at approximately 60 kd molecular weight; (2) bile duct epithelial proteins of approximately 80 kd and 250 kd; and (3) kidney epithelial proteins of 50 kd and 80 kd. Neither sera from BSS or RRV reacted with large T antigen. Importantly, the RRV sera, and not the BSS sera, contained antibodies reactive to multiple bile duct epithelial proteins, including strong reactivity to proteins of approximately 40, 60, and 160 kd (circled bands). These bands represent novel bile duct epithelial autoantigens bound by serum antibodies from 2-week-old RRV-diseased mice.
To confirm the specificity of the immunoglobulin deposits, we analyzed the reactivity of antibodies from the sera of BSS control and RRV-diseased mice by Western blot techniques. Irradiated RRV, bile duct epithelial cell line homogenate, SV40 large T antigen, or nonspecific kidney epithelia homogenate control proteins were separated by gel electrophoresis, blotted onto nitrocellulose paper, and incubated with either BSS or RRV sera (pooled from 10 mice each). Antibodies reactive to a protein were visualized by chemiluminescence (dark bands on radiograph). Both the BSS and RRV sera contained antibodies reactive to a viral protein at approximately 60 kd molecular weight (Fig. 6B). In the RRV sera, this is most likely anti-RRV antibody, whereas in the BSS sera, this may reflect passively transferred maternal antibodies that contain anti-viral antibodies cross-reactive to RRV. The BSS and RRV sera also both contained nonspecific antibodies reactive to two bile duct epithelial proteins (80 kd and 250 kd) and two kidney epithelial proteins (faint bands at 80 kd and 50 kd). No antibody reactivity to the large T antigen was seen. The RRV sera, and not the BSS sera, contained antibodies reactive to multiple unique bile duct epithelial proteins, including strong reactivity to proteins of approximately 40, 60, and 160 kd molecular weight (Fig. 6B, circled). These bands represent novel bile duct epithelial autoantigens that serum antibodies from 2-week-old RRV-diseased mice are binding to, lending support for further evidence of autoimmunity in murine BA.
Discussion
The role of autoimmunity in the pathogenesis of biliary atresia has been proposed in the past; however, to our knowledge this has never been resolved. This study shows a clear autoimmune component, demonstrating the generation of bile duct–specific cellular and humoral autore-activity in the RRV-induced murine model of BA. In vitro studies showed the presence of bile duct epithelial antigen–specific T cells from livers and spleens of RRV-diseased mice. The source of the bile duct epithelial antigens was from a crude cell line homogenate, which contains hundreds of proteins. The peptide sequence of the target autoantigen within this homogenate was not identified in these initial investigations and will form the basis of future studies aimed at determining the target epitope(s).
Adoptive transfer of T cells from RRV-induced BA mice into SCID recipients resulted in periductal inflammation composed of donor T cells and recipient macrophages. The periductular immune cell types found in the SCID recipients are similar to that found in both the murine model17,18 and the human disease.1-4 Administration of 5 to 7 × 105 hepatic or splenic T cells from diseased neonatal mice into adult SCID mice led to consistent and reproducible bile duct inflammation and elevated serum bilirubin values but without complete bile duct obstruction. In contrast, in the original neonatal murine model, complete bile duct obstruction is seen by 2 weeks of age. The less severe bile duct injury seen in the adoptive transfer model is likely attributable to a number of factors: First, the recipient SCID mice were adults with larger extrahepatic bile duct lumens compared with neonates that may require substantially more inflammation to completely obstruct the lumen. Transferring enough T cells into a neonatal mouse peritoneum was not technically possible (data not shown); thus, adult mice were used as the recipients in our experiments. Second, as noted above, a relatively small number of T cells were available for transfer and may not have been sufficient to elicit complete luminal obstruction. In most adoptive transfer studies, greater numbers of T cells have been used for transfer, from 5 × 106 up to 1.5 × 107 T cells per recipient.26-28 Finally, full penetrance of disease probably requires the initial RRV infection of bile duct epithelia with activation of the host innate immune system in addition to the autoreactive T cell–mediated bile duct injury that was demonstrated in the adoptive transfer experiments.
Evidence for humoral autoimmunity was obtained by establishing the presence of periductal IgG deposits and serum autoantibodies reactive to bile duct epithelial proteins in RRV-diseased mice. Both IgG and IgM deposits have been previously described along the basement membrane of bile duct epithelia within extrahepatic bile duct remnants in human BA.29 In future investigations, the novel bile duct epithelial proteins identified by Western blot analysis will be purified and analyzed to identify candidate autoantigenic peptides.
In humans, the progressive nature of BA supports the role of a chronic immune-mediated attack directed at bile ducts. Little direct evidence has been found of autoimmunity in human BA. Studies of a possible link between HLA type and BA have yielded conflicting results.30-32 Because of the rarity of BA (approximately 1:8,000-18,000 live births)33 and the paucity of liver and bile duct tissue available for research, the use of murine models provides valuable insight into the potential mechanisms of bile duct injury in this devastating disease. In this study, we show that the progressive bile duct injury is attributable in part to bile duct epithelia–specific T cell–mediated autoimmunity and that serum autoantibodies reactive to bile duct epithelial proteins are present in murine BA. The role of cellular and humoral autoimmunity in human BA and possible interventional strategies therefore should be the focus of future research.
Acknowledgment
The authors thank Patsy Ruegg, IHC Tech, Denver, CO, for her work on the histology and immunohistochemistry.
Supported in part by grants NIH-NIDDK KO8 DK60710 (2002-2006) and RO3 DK069818 (2004-2006) and the March of Dimes Foundation, March of Dimes Basil O'Connor Young Investigator Award (2005-2007).
Abbreviations
- BA
biliary atresia
- RRV
Rhesus rotavirus
- IFN-γ
interferon gamma
- BSS
balanced salt solution
- IL-15
interleukin 15
- APC
antigen-presenting cells
- RT-PCR
reverse transcription polymerase chain reaction
- bde
bile duct epithelial homogenate
Footnotes
Presented as an oral presentation in a Plenary Session at the American Association for the Study of Liver Diseases Annual Meeting, November, 2005 and received the American Liver Foundation Pediatric Research Award.
Potential conflict of interest: Nothing to report.
References
- 1.Mack CL, Tucker RM, Sokol RJ, Karrer FM, Kotzin BL, Whitington PF, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport M, Gonde C, Redkar R, Koukoulis G, Tredger M, Mieli-Vergani G, et al. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36:1017–1025. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 3.Tracy TF, Dillon P, Fox ES, Minnick K, Vogler C. The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg. 1996;31:121–125. doi: 10.1016/s0022-3468(96)90333-4. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H, Puri P, O'Briain DS, Surana R, Miyano T. Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg. 1997;32:590–593. doi: 10.1016/s0022-3468(97)90714-4. [DOI] [PubMed] [Google Scholar]
- 5.Karrer FM, Price MR, Bensard DD, Sokol RJ, Narkewicz MR, Smith DJ, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996;131:493–496. doi: 10.1001/archsurg.1996.01430170039006. [DOI] [PubMed] [Google Scholar]
- 6.Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology. 2005;41:366–371. doi: 10.1002/hep.20547. [DOI] [PubMed] [Google Scholar]
- 7.Tan CEL, Driver M, Howard ER, Moscoso GJ. Extrahepatic biliary atresia: a first trimester event? Clues from light microscopy and immunohistochemistry. J Pediatr Surg. 1994;29:808–814. doi: 10.1016/0022-3468(94)90377-8. [DOI] [PubMed] [Google Scholar]
- 8.Morecki R, Glaser JH, Cho S, Balistreri WF, Horwitz MS. Biliary atresia and reovirus type 3 infection. N Engl J Med. 1982;307:481–484. doi: 10.1056/NEJM198208193070806. [DOI] [PubMed] [Google Scholar]
- 9.Tyler KL, Sokol RJ, Oberhaus SM, Lee M, Karrer FM, Narkewicz MR, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 10.Riepenhoff-Talty M, Gouvea V, Evans MJ, Svensson L, Hoffenberg E, Sokol RJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174:8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 11.Sokol RJ, Mack CL. Etiopathogenesis of biliary atresia. Semin Liver Dis. 2001;21:517–524. doi: 10.1055/s-2001-19032. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz MS, Sarvetnick N. Viruses, host responses and autoimmunity. Immunol Rev. 1999;169:241–253. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber RA, Kleinman RE. Genetics, immunology and biliary atresia: an opening or a diversion? J Pediatr Gastroenterol Nutr. 1993;16:111–113. doi: 10.1097/00005176-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–96. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 15.Abbas AK, Lichtman AH. Cellular and Molecular Immunology. Saunders; Philadelphia, PA: 2003. pp. 189–215. [Google Scholar]
- 16.Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhno I, Rossi T, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200–209. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mano Y, Ishii M, Kisara N, Kobayashi Y, Ueno Y, Kobayashi K, et al. Duct formation by immortalized mouse cholangiocytes: an in vitro model for cholangiopathies. Lab Invest. 1998;78:1467–1468. [PubMed] [Google Scholar]
- 20.Wilde DB, Marrack P, Kappler J, Dialynas DP, Fitch FW. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK 1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983;131:2178–2183. [PubMed] [Google Scholar]
- 21.Hathcock KS. T cell depletion by cytotoxic elimination. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley and Sons; New York: 1991. pp. 3.4.1–3.4.3. [DOI] [PubMed] [Google Scholar]
- 22.Vella A, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. PNAS. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niedbala W, Wei X, Liew FY. IL-15 induces type 1 and type 2 CD4 and CD8 T cells proliferation but is unable to drive cytokine production in the absence of TCR activation or IL-12/IL-4 stimulation in vitro. Eur J Immunol. 2002;32:341–347. doi: 10.1002/1521-4141(200202)32:2<341::AID-IMMU341>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Abbas AK, Lichtman AH. Cellular and Molecular Immunology. Saunders; Philadelphia, PA: 2003. pp. 90–92. [Google Scholar]
- 25.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg. 1995;30:515–518. doi: 10.1016/0022-3468(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 26.Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M. Analysis of the roles of CD4 and CD8 T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 27.Zekzer D, Wong S, Ayalon O, Millet I, Altieri M, Shintani S, et al. GAD-reactive CD4 Th1 cells induce diabetes in NOD/SCID mice. J Clin Invest. 1998;101:68–73. doi: 10.1172/JCI119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wicker LS, Miller BJ, Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic mice. Diabetes. 1986;35:855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- 29.Hadchouel M, Hugon RN, Odievre M. Immunoglobulin deposits in the biliary remnants of extrahepatic biliary atresia: a study by immunoperoxidase staining in 128 infants. Histopathology. 1981;5:217–221. doi: 10.1111/j.1365-2559.1981.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 30.Silveira TR, Salzano FM, Donaldson PT, Mieli-Vergani G, Howard ER, Mowat AP. Association between HLA and extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1993;16:114–117. doi: 10.1097/00005176-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 31.A-Kader HH, El-Ayyouti M, Hawas S, Abdalla A, Al-Tonbary Y, Bassiouny M, et al. HLA in Egyptian children with biliary atresia. J Pediatr. 2002;141:432–433. doi: 10.1067/mpd.2002.127506. [DOI] [PubMed] [Google Scholar]
- 32.Donaldson PT, Clare M, Constantini PK, Hadzic N, Mieli-Vergani G, Howard E, et al. HLA and cytokine gene polymorphisms in biliary atresia. Liver. 2002;22:213–219. doi: 10.1046/j.0106-9543.2002.01647.x. [DOI] [PubMed] [Google Scholar]
- 33.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]


