Abstract
Arachidonic acid is a potential paracrine agent released by the uterine endometrial epithelium to induce PTGS2 [PG (prostaglandin)-endoperoxide synthase 2] in the stroma. In the present study, bovine endometrial stromal cells were used to determine whether PTGS2 is induced by arachidonic acid in stromal cells, and to investigate the potential role of PPARs (peroxisome-proliferator-activated receptors) in this effect. Arachidonic acid increased PTGS2 levels up to 7.5-fold within 6 h. The cells expressed PPARα and PPARδ (also known as PPARβ) (but not PPARγ). PTGS2 protein level was increased by PPAR agonists, including polyunsaturated fatty acids, synthetic PPAR ligands, PGA1 and NSAIDs (non-steroidal anti-inflammatory drugs) with a time course resembling that of arachidonic acid. Use of agonists and antagonists indicated PPARα (but not PPARδ or PPARγ) was responsible for PTGS2 induction. PTGS2 induction by arachidonic acid did not require PG synthesis. PTGS2 levels were increased by the PKC (protein kinase C) activators 4β-PMA and PGF2α, and the effects of arachidonic acid, NSAIDs, synthetic PPAR ligands and 4β-PMA were blocked by PKC inhibitors. This is consistent with PPAR phosphorylation by PKC. Induction of PTGS2 protein by 4β-PMA in the absence of a PPAR ligand was decreased by the NF-κB (nuclear factor κB) inhibitors MG132 and parthenolide, suggesting that PKC acted through NF-κB in addition to PPAR phosphorylation. Use of NF-κB inhibitors allowed the action of arachidonic acid as a PPAR agonist to be dissociated from an effect through PKC. The results are consistent with the hypothesis that arachidonic acid acts via PPARα to increase PTGS2 levels in bovine endometrial stromal cells.
Keywords: arachidonic acid, bovine uterus, endometrium, paracrine mechanism, peroxisome-proliferator-activated receptor (PPAR), prostaglandin-endoperoxidase synthase 2 (PTGS2), stromal cell
Abbreviations: ABAM, antibiotic/antimycotic; BADGE, bisphenol A diglycidyl ether; C/EBP, CCAAT/enhancer-binding protein; CRE, cAMP-response element; DGLA, dihomo-γ-linolenic acid; DMEM, Dulbecco's modified Eagle's medium; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; NSAID, non-steroidal anti-inflammatory drug; PG, prostaglandin; PKA, protein kinase A; PKC, protein kinase C; PPAR, peroxisome-proliferator-activated receptor; PPRE, PPAR-responsive element; PTGS, PG-endoperoxide synthase; PUFA, polyunsaturated fatty acid
INTRODUCTION
PTGS [PG (prostaglandin)-endoperoxidase synthases] 1 and 2 (previously known as cyclo-oxygenases or PGH synthases 1 and 2) are haem enzymes that catalyse the first two steps in the synthesis of prostanoids [1,2]. Their principal substrates are DGLA (dihomo-γ-linolenic acid; C20:3n−6), arachidonic acid (C20:4n−6) and eicosapentaenoic acid (C20:5n−3), essential fatty acids mostly esterified in membrane phospholipids. Both enzymes have cyclo-oxygenase and peroxidase activities, sequentially catalysing the cyclo-oxygenation of fatty acids to form PGG isomers, and the reduction of PGG to PGH. PTGS1 is expressed constitutively in the gastrointestinal tract, platelets, renal collecting tubules and seminal vesicles. PTGS2 is expressed constitutively in brain, testis, tracheal epithelium and kidney, but is inducible in the uterus, amnion, ovary, kidney and the central nervous system [1]. The two PTGS isoforms are the products of related genes; mature processed human PTGS1 consists of 576 amino acids and PTGS2 consists of 587 amino acids, and they share 60–65% amino acid sequence identity. Both enzymes are glycosylated to varying degrees.
PTGS1 maintains normal physiological functions through production of prostanoids and thromboxanes. PTGS2, being inducible in inflammation, fever and pain, produces the prostanoids responsible for these processes. Both of these enzymes have attracted attention as the sites of action of NSAIDs (non-steroidal anti-inflammatory drugs) [1]. PTGS2 is also implicated in the aetiology of cancers, notably of the colon and reproductive tract, and prostanoids play important roles in all aspects of uterine function in pregnant and non-pregnant mammals, including luteolysis, menstruation, implantation, the maternal recognition of pregnancy and decidualization, as well as in labour, parturition and postpartum uterine involution [3]. NSAIDs have been used to treat a range of disorders of these processes in women, including dysmenorrhoea, menorrhagia and preterm labour.
The role of PGs in luteolysis is well-established in ruminants. The uterine secretion of luteolytic episodes of PGF2α in response to activation of the oxytocin receptor is accompanied by an increase in the PTGS2 concentration in the endometrium [4,5]. This increase is most marked in the stroma [4]. At the time of luteolysis, endometrial oxytocin receptor expression is limited to the epithelium [6], and the question arises as to how up-regulation of PTGS2 occurs in the stroma. The experiments described in the present study were designed to investigate the control of PTGS2 expression in bovine endometrial stromal cells by a potential paracrine agent, arachidonic acid.
Paracrine relationships between the epithelium and the stroma have been suggested to control various uterine functions [4,7–9], including expression of PTGS2. For instance, in the rat, IL1α (interleukin 1α) acts in a paracrine manner to induce PTGS2 in stromal cells [9]. A second potential agent for this role is arachidonic acid. Arachidonic acid is a good candidate for a paracrine inducer of PTGS2 expression in the stroma at luteolysis, as it is produced in response to phospholipase C activation by oxytocin [10] and induces PTGS2 in other tissues, including mammary epithelial cells [11], bovine endometrial epithelial cells [12] and rat uterine stromal cells [13]. Arachidonic acid also plays paracrine and autocrine roles elsewhere in the reproductive system [14,15].
The PTGS2 gene upstream region contains numerous sequences controlling gene expression. Among these are sites activated by PPARs (peroxisome-proliferator-activated receptors), via PPREs (PPAR-responsive elements), and NF-κB (nuclear factor κB), as well as C/EBP (CCAAT/enhancer-binding protein), AP-2, CRE (cAMP-response element) and E-box sequences [11,16]. NF-κB sites are responsible for induction of PTGS2 expression by LPS (lipopolysaccharide) and pro-inflammatory cytokines [17]. PTGS2 is also induced following activation of PKC (protein kinase C) through NF-κB, C/EBP, CRE and E-box sites [18]. These enhancers are not all active in all tissues and, in some cases, their functions differ between cell types.
The control of PTGS2 expression by PPARs has been studied in detail. PPREs mediate increases in PTGS2 expression in a variety of cell lines [11,17,19]. PPARs are activated by their ligands, among which are arachidonic acid and other PUFAs (polyunsaturated fatty acids) [20–22], NSAIDs [23] and cyclopentenone PGs (such as PGA1 and PGJ2) [17]. There are at least three PPARs, PPARα, PPARδ (also known as PPARβ) and PPARγ, of which the PPARα and PPARδ isoforms are expressed in the bovine endometrium, although whether they are expressed in the stroma is not known [24]. Therefore activation of a PPAR is one mechanism by which arachidonic acid may induce PTGS2.
The transactivation function of PPARα is affected by phosphorylation [25,26]. PPARα is activated through phosphorylation by PKA (protein kinase A) at serine residues principally in the DNA-binding domain [27] and by PKC at threonine and serine residues between the DNA-binding and ligand-binding domains [28]. Use of inhibitors and non-phosphorylatable mutant PPARs shows that phosphorylation at these sites is a prerequisite for PPAR transactivation function and that, if phosphorylation by PKC is blocked then PPAR ligands do not induce target gene transcription. PKC is activated by arachidonic acid and other PUFAs [29,30], and these compounds may therefore induce PTGS2 through increased PPAR phosphorylation in addition to their action as PPAR ligands.
We show in the present study that arachidonic acid induces PTGS2 in endometrial stromal cells, and we test further the hypothesis that PPARs are responsible for PTGS2 induction by arachidonic acid, determine which PPAR isoforms may be involved and investigate whether the effect of arachidonic acid as a PPAR ligand can be differentiated from its actions as an activator of PKC. Endometrial stromal cells of bovine origin have been used because of the role of oxytocin in luteolysis in this species [6] and as oxytocin receptor occupancy generates arachidonic acid [10]. The effects of the agents used were determined by measurement of protein levels, and no attempt was made to differentiate between effects on PTGS2 gene expression and PTGS2 transcript or protein turnover.
MATERIALS AND METHODS
Cell culture
Bovine endometrial stromal cells were isolated from a day 16 cycling Holstein–Friesian cow using pancreatin and dispase in calcium- and magnesium-free medium [31], and were maintained in DMEM (Dulbecco's modified Eagle's medium; Sigma) containing 10% (v/v) fetal bovine serum and 1% ABAM (antibiotic/antimycotic). These cells, which were phenotypically stable, were purified and maintained free of epithelial cell contamination by differential trypsinization, as confirmed by cytokeratin immunocytochemistry. The cells were grown in flasks to 60–80% confluence and passaged at intervals of 3–4 days. For testing the effects of PUFAs and other agents, cells were transferred to 24-well plates and the medium was changed to DMEM containing 10% (v/v) dextran-coated charcoal-stripped fetal bovine serum and 1% ABAM. In contrast with bovine endometrial epithelial cells, which produce PGF2α more rapidly than PGE2 in culture, stromal cells produce more PGE2 than PGF2α [32]. The ratio of PGE2 to PGF2α produced therefore confirmed the stromal origin of the cells.
The following reagents (from Sigma or Calbiochem) were added to culture medium to activate or inhibit PTGS2 expression, at the concentrations given in the Figure legends: the PUFAs arachidonic acid, DGLA, linoleic acid and conjugated linoleic acid; the synthetic PPAR agonists WY14643, ciprofibrate, methylclofenapate, bezafibrate, SB400455, ciglitazone and pioglitazone [33]; the PPARγ antagonists BADGE (bisphenol A diglycidyl ether) [34] and GW9662 [35]; the NSAIDs indomethacin and NS398; PGF2α and PGA1; the phorbol ester 4β-PMA and its inactive analogue 4α-phorbol-12,13-didecanoate; the PKC inhibitors RO318425 and calphostin C, and the NF-κB inhibitors MG132 [36], parthenolide [37] and sulfasalazine [38]. Conjugated linoleic acid (catalogue number O5507; Sigma) was a mixture of cis- and trans-9,11- and -10,12-octadecadienoic acids. PUFAs, indomethacin and PGs were dissolved in ethanol; all other compounds were added to culture medium in DMSO. Vehicle controls were used as appropriate. PUFAs were stored in ethanol under nitrogen at −20 °C in the dark.
In preparation for immunoblotting, the medium was removed from cells at the end of the incubation period, centrifuged for 10 min at 13000 g and the supernatants were stored at −20 °C for PG assay. Cells were washed once in 1 ml of ice-cold PBS containing 0.2 mM sodium orthovanadate and lysed for 20 min on ice in 0.1 ml of lysis buffer [63.5 mM Tris/HCl (pH 6.8), 10% (w/v) glycerol, 2% (w/v) SDS, 60 μg/ml leupeptin, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride and 2 mM sodium orthovanadate]. A portion (60 μl) of the lysed cell extract was diluted with 4 μl of 2-mercaptoethanol and 2 μl of 2% (w/v) Bromophenol Blue, heated in a boiling water bath for 5 min and stored at −20 °C. The remaining lysate was used for protein assay.
PCR
Total RNA extracted from stromal cells [39] was used in random hexamer- or specific primer-initiated reverse transcription (BIOLINE) and subsequently used for PCR. Pairs of PCR primers spanning different exons were designed to distinguish between the PPAR isoforms. For PPARα (GenBank® accession no. BT020756), a fragment of 105 bp was amplified using primers PPARα forward (bp 682–701) and PPARα reverse (bp 786–765) at an annealing temperature of 51 °C. PPARδ (GenBank® accession no. AF229357) gave a sequence of 159 bp using primers PPARδ forward (bp 351–371) and PPARδ reverse (bp 509–490) at an annealing temperature of 55 °C.
For PPARγ-2 (GenBank® accession no. Y12420), three sets of primers were used: PPARγ5 (forward; bp 972–992) with PPARγ2 (reverse; bp 1117–1136) or PPARγ6 (reverse; bp 1178–1199) and, located further 5′, PPARγ7 (forward; bp 383–393) and PPARγ8 (reverse; bp 606–626). The annealing temperature was 54 °C in all cases. All three primer sets were used directly in PCR from RNA reverse-transcribed using random hexamers or the specific reverse primers. A nested PCR approach was also used with cDNA generated using random hexamers or PPARγ6, followed by PCR with a combination of primers PPARγ7 and PPARγ6. This pair of primers generated a product of 840 bp, which was subsequently used as a template in nested PCR with primers PPARγ7 or PPARγ8 and PPARγ5 or PPARγ6.
All PCR reactions were run for 30 cycles. PPARα and PPARδ PCR products obtained from stromal cell RNA and PPARγ products obtained from spleen (the positive control tissue) were confirmed by sequencing.
Immunoblotting
Cell lysates (10 μg of protein) were subjected to electrophoresis on 10% (w/v) acrylamide gels [5% (w/v) stacking gels] before the proteins were electroblotted on to an Optitran BA-S 83 membrane (Schleicher and Schuell) in 25 mM Tris/HCl (pH 8.3) containing 148 mM glycine and 20% (v/v) methanol. For detection of PTGS2, membranes were probed with a goat polyclonal PTGS2 antibody (IgG; C-20, SC1745; Santa Cruz Biotechnology), diluted 1:250 in PBS containing 1% (w/v) non-fat dried skimmed milk powder (Marvel) and 0.5% (w/v) Tween 20. The PTGS1 antibody was from Santa Cruz Biotechnology (IgG; C-20, SC1752). The secondary antibody in both cases was HRP (horseradish peroxidase)-conjugated donkey anti-(goat IgG) (Santa Cruz Biotechnology), diluted 1:11000 in PBS containing 3% (w/v) non-fat dried skimmed milk powder (Marvel) and 0.5% (w/v) Tween 20, and visualization was by ECL® (Amersham Biosciences) using Kodak BioMax Light film. Colour markers (molecular masses, 29–205 kDa; Sigma) were used to identify the molecular masses of the proteins. The expected molecular masses of bovine PTGS1 and PTGS2 were 70 and 72 kDa respectively [5]. A PTGS2-blocking peptide (SC1745P; Santa Cruz Biotechnology) was incubated with the primary antibody at room temperature overnight. Band intensities were quantified using Kodak 1D digital image analysis software.
The PPARγ antibody was a goat polyclonal antibody raised against a peptide from the N-terminal end of the mouse PPARγ2 (G-18, SC22020; Santa Cruz Biotechnology). A sheep kidney extract was used as positive control for PPARγ immunoblotting. Pre-stained SeeBlue plus 2 markers (molecular masses 50, 64, 98 and 148 kDa; Invitrogen) were used to identify molecular masses. A PPARγ-blocking peptide (SC 22020P; Santa Cruz Biotechnology) was incubated with the primary antibody at room temperature overnight.
PG assays
PGE2 and PGF2α were assayed in spent culture medium by RIA [40]. Intra-assay coefficients of variation were 3.5 and 4.5% for the PGE2 and PGF2α assays respectively, and all samples were measured in a single assay to eliminate inter-assay variation.
Protein assay
Protein concentrations in lysates prepared for electrophoresis were measured by the BCA (bicinchoninic acid) method (Perbio).
Experimental design and analysis
Experiments were performed in 24-well plates, with replicate treatments of between three and eight wells. In time course experiments, compounds were added at various times before cell lysis so that all cells were cultured for the same length of time after plating. To account for differences in band intensity between immunoblots, all blots included at least two control samples, and experimental treatments were related to the control bands on each gel. All immunoblot bands were included for analysis. All experiments were performed at least three times. Statistical analysis of treatment effects was by ANOVA using Genstat version 8 (VSN International). Where data from more than one experiment have been pooled, this is indicated in the Figure legends. Where significant effects were detected (P<0.05), individual differences were tested using Bonferroni's multiple comparison post-hoc test. Values are given as means±S.E.M. In the Figures, significant treatment effects (from controls or zero-time treatments) are indicated by different letters above the bars.
RESULTS
Prostanoid production, PTGS isoforms and the effects of arachidonic acid
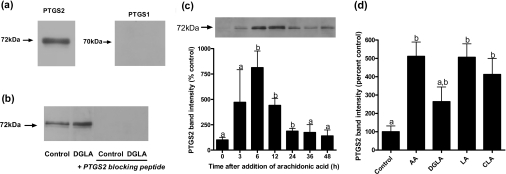
Endometrial stromal cells were tested for production of PGs and responses to PUFAs. Stromal cells released PGE2 and PGF2α into the culture medium, with the quantities of PGE2 exceeding those of PGF2α up to 100-fold (43.4±2.99 compared with 0.33±0.197 nmol/mg of protein respectively), measured after 48 h, and PTGS2 was detected at a molecular mass of 72 kDa by immunoblotting (Figure 1a). The antiserum was specific for PTGS2, as shown using the blocking peptide (Figure 1b), and PTGS1 was undetectable, as reported previously in bovine endometrium [5]. Arachidonic acid increased PTGS2 levels in stromal cells (Figure 1c), with the response to arachidonic acid peaking 6 h after addition to the medium and then declining, so that by 48 h the level of PTGS2 was close to that before treatment. The response to arachidonic acid was consistent but variable; in the seven experiments in which it was tested (Figures 1c, 1d, 2a–2d, 4b and 5b), the response ranged from 1.3–7.5-fold. This variation did not reflect passage number. In experiments to investigate whether PUFAs other than arachidonic acid increased PTGS2 levels, the effect was also observed with linoleic and conjugated linoleic acids [11,20–22,41], which induced PTGS2 within 6 h (Figure 1d), and there was a tendency for DGLA to have a similar effect. In agreement with previous observations [11,12,20], PUFAs were effective at micromolar concentrations; dose–response experiments in the range 1–100 μM showed that the effects of arachidonic acid, DGLA and conjugated linoleic acid were maximal at 50 μM, whereas that of linoleic acid peaked at 2 μM (results not shown).
Figure 1. PTGS levels and the effects of PUFAs.
(a) Immunoblotting demonstrated the presence of the 72 kDa molecular mass PTGS2, whereas PTGS1 was not detectable. (b) DGLA (50 μM) increased PTGS2 levels, and the PTGS2-blocking peptide confirmed the identity of the immunoblotted signal. (c) Arachidonic acid (AA; 50 μM) increased PTGS2 levels, the effect peaking at 6 h. (d) In a separate experiment, PTGS2 levels were increased at 6 h in cells treated with AA (50 μM), DGLA (50 μM), linoleic acid (LA; 2 μM) and conjugated linoleic acid (CLA; 50 μM). Representative immunoblots are shown in (a, b and c) as examples of results obtained at least in triplicate.
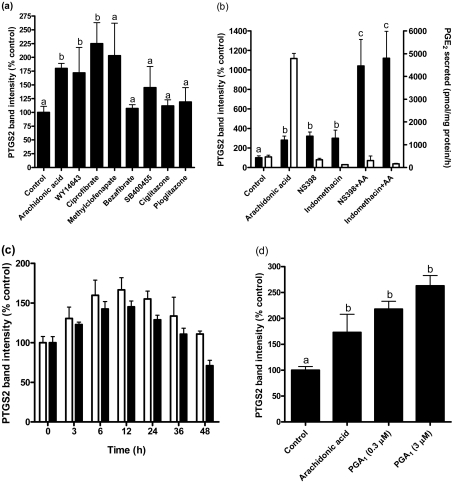
Figure 2. Effects of PPAR agonists and antagonists on PTGS2 levels.
(a) Bovine endometrial stromal cells were cultured with or without arachidonic acid (50 μM) and the synthetic PPARα agonists WY14643 (50 μM), ciprofibrate (50 μM) and methylclofenapate (50 μM), the PPARδ agonists bezafibrate (50 μM) and SB400455 (50 nM), and the PPARγ agonists ciglitazone (50 μM) and pioglitazone (1 μM). These concentrations have been shown to be effective in other cell types. PTGS2 was measured by immunoblotting after 6 h. The effects of WY14643 and ciprofibrate were statistically significant (P<0.05); the effect of methylclofenapate was not significant at 6 h, but reached significance (P<0.02) between 6 and 24 h (results not shown). Results were obtained from three experiments. (b) Effects of the PPARα/PPARγ agonist NSAIDs on PTGS2 levels. Closed bars, PTGS2 levels; open bars, PGE2 production. Stromal cells were cultured for 6 h with or without arachidonic acid (AA; 50 μM), NS398 (50 μM) or indomethacin (10 μM). PTGS2 level was increased by arachidonic acid (P<0.001), NS398 (P<0.001) and indomethacin (P<0.01). The increase in PGE2 production in response to arachidonic acid was blocked by NSAIDs. Results were obtained from four experiments. (c) Time course of the effects of arachidonic acid (50 μM; open bars) and indomethacin (10 μM; closed bars) on PTGS2 levels. The results were identical. (d) Effects of the PPARα/PPARδ/PPARγ agonist PGA1 on PTGS2 levels. Bovine endometrial stromal cells were cultured for 6 h with or without arachidonic acid (50 μM), PGA1 (0.3 μM) or PGA1 (3 μM). PTGS2 levels were increased in the presence of PGA1 (P<0.001).
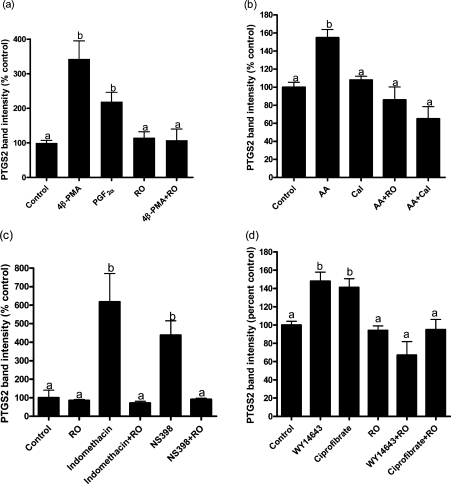
Figure 4. Effects of arachidonic acid, 4β-PMA, PGF2α, NSAIDs and synthetic PPARα agonists with and without PKC inhibitors.
Bovine endometrial stromal cells were cultured for 6 h with or without additions, and PTGS2 was measured by immunoblotting. (a) Effects of 4β-PMA (2μM), PGF2α (3 μM) and RO318425 (RO; 500 nM). In the absence of the PPAR ligand, PTGS2 level was increased by 4β-PMA (P<0.001) and PGF2α (P<0.05), and the effect of 4β-PMA was blocked by RO318425 (P<0.005). Results were obtained from four experiments. (b) PTGS2 level was increased by arachidonic acid (AA; P<0.001), and the effect of arachidonic acid was blocked by RO318425 (P<0.001) and calphostin C (Cal; 500 nM). Results were obtained from six experiments. Neither RO318425 (Figure 4a) nor calphostin C (Figure 4b) affected PTGS2 when added alone. (c) Indomethacin and NS398 increased PTGS2 levels (P<0.001 in both cases). The effects of NSAIDs were blocked by RO318425. Results were obtained from five experiments. (d) The effects of the synthetic PPARα agonists WY14643 (200 μM) and ciprofibrate (200 μM) were blocked by RO318425 (500 nM). Results were obtained from three experiments.
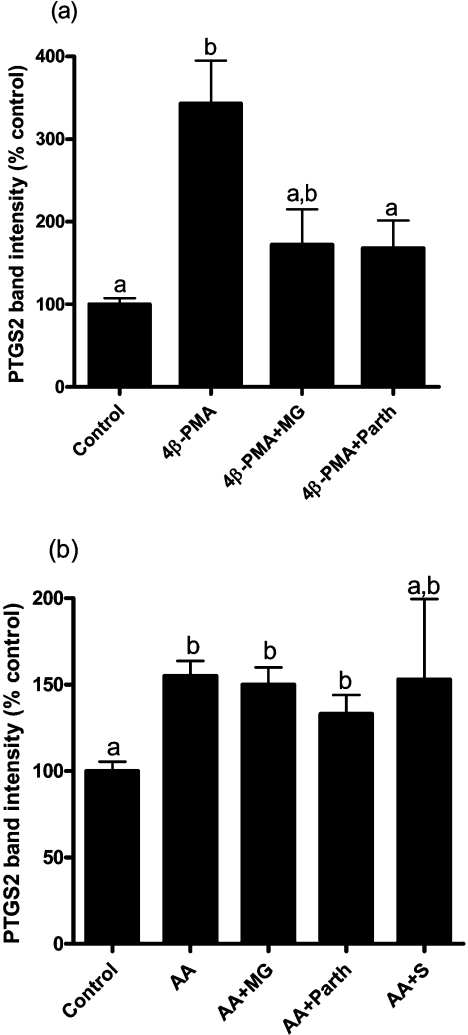
Figure 5. Effect of 4β-PMA on PTGS2 level, but not that of arachidonic acid, was decreased by NF-κB inhibitors.
(a) Bovine endometrial stromal cells were cultured in the absence of exogenous PPAR ligand for 6 h with 4β-PMA (2 μM) with or without the NF-κB inhibitors MG132 (MG; 30 μM) and parthenolide (Parth; 4 μM). Results were obtained from three experiments. (b) The effect of arachidonic acid (AA) on PTGS2 level (P<0.01) was not blocked by NF-κB inhibitors. Cells were cultured for 6 h with or without arachidonic acid (50 μM) and MG (30 μM), parthenolide (4 μM) or sulfasalazine (S; 1 μM). Results were obtained from six experiments.
Effects of PPAR agonists and antagonists
To test the hypothesis that PPARs are responsible for the induction of PTGS2 by PUFAs, a variety of established PPAR agonists [11,17,20,33] were used to determine whether they mimicked the effects of PUFAs (Figure 2). The synthetic PPARα and PPARγ agonists WY14643, ciprofibrate and methylclofenapate increased PTGS2 levels up to 2.2-fold at 6 h (Figure 2a). The effect of methylclofenapate was not significant at 6 h, but reached significance (P<0.02) between 6 and 24 h (results not shown). The PPARδ agonist bezafibrate (which is also a poor PPARα agonist) [33] and the selective PPARδ agonist SB400455 were ineffective, as were the PPARγ agonists ciglitazone (50 μM) and pioglitazone (1 μM). The PPARγ antagonists BADGE and GW9662 [34,35] did not block the effect of arachidonic acid (results not shown). Therefore it appeared that PPARα was responsible for the induction of PTGS2. Consistent with this, the NSAIDs indomethacin and NS398, which are mixed PPARα and PPARγ agonists [23,33], increased PTGS2 expression approx. 3-fold within 6 h (Figure 2b). The effects of arachidonic acid and the NSAIDs were additive rather than synergistic (i.e. there were no significant interactions; P>0.05; Figure 2b); at the concentrations tested, those of NS398 and indomethacin were additive (results not shown). The time courses of the effects of arachidonic acid and indomethacin were identical (Figure 2c) and, with the exception of methylclofenapate, the effects of the synthetic ligands used in Figure 2(a) were also higher at 6 than at 24 h (results not shown). The cyclopentanone prostanoid PGA1 [17] increased PTGS2 2.2- and 2.9-fold at 0.3 and 3 μM respectively, at 6 h (Figure 2d). As in rat endometrial stromal cells [13], the increase in PTGS2 in response to arachidonic acid was not due to increased prostanoid synthesis, as it occurred in the presence of NSAIDs (Figure 2b) when PGE2 synthesis was low (PGE2 production: 465 pmol per mg of protein/h in controls, 4791 pmol per mg of protein/h with arachidonic acid, and 162 pmol per mg of protein/h with arachidonic acid and indomethacin).
PPAR expression
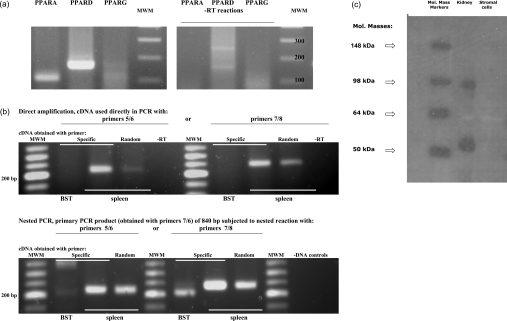
PCR revealed that PPARα and PPARδ transcripts were expressed in bovine endometrial stromal cells (Figure 3a). There was no evidence for expression of PPARγ. None of the pairs of PPARγ primers (PPARγ5/PPARγ6, PPARγ5/PPARγ2 and PPARγ7/PPARγ8) generated products in direct PCR from RNA of stromal cells, in contrast with a positive control tissue (bovine spleen; Figure 3b). Primers PPARγ7/PPARγ6 generated a longer PCR product of 840 bp if used with spleen cDNA, which was confirmed to be derived from PPARγ by sequencing. In contrast, there was no product with stromal cell cDNA regardless of whether specific primers or random hexamers were used as primers to generate cDNA. In nested PCR, where a larger product of 840 bp was used to generate a smaller internal product of 222–260 bp, again no amplification of PPARγ was seen in stromal cell samples. The absence of PPARγ in stromal cells was confirmed by immunoblotting (Figure 3c). Two bands were present in the positive-control lane, which were consistent with PPARγ (molecular mass, 52 kDa) and a dimer (molecular mass, 92 kDa). Both bands disappeared upon adding the PPARγ-blocking peptide (results not shown).
Figure 3. Identification of PPARs.
Total RNA extracted from stromal cells or bovine spleen (positive control) was analysed by PCR. (a) Initial analysis for PPAR isoforms in stromal cells. PPARα (PPARA) and PPARδ (PPARD) were expressed, but not PPARγ (PPARG). The expected products of 105 bp and 159 bp for PPARα and PPARδ, obtained using the PPARα and PPARδ primers described in the Materials and methods section, were verified by sequencing. No product was obtained for PPARγ using primers PPARγ5 and PPARγ2. Right-hand panel represents control reactions without reverse transcriptase. (b) Further analysis for PPARγ. Total RNA from stromal cells (BST) and spleen was reverse-transcribed using the specific primer PPARγ6 (Specific) or random hexamers (Random), and subsequently used in direct amplification (upper panel) or in nested PCR (lower panel) with primers PPARγ5/PPARγ6 (5/6) or PPARγ7/PPARγ8 (7/8). In direct amplification, spleen gave products of the expected sizes, which were verified by sequencing, but no product was obtained with stromal cells. To confirm the lack of amplification in stromal cells, a nested approach was subsequently used. The first round of nested PCR (lower panel) using primers PPARγ7/PPARγ6 (7/6) generated a product of 840 bp, which was then used as a template with primers PPARγ5/PPARγ6 (5/6) or PPARγ7/PPARγ8 (7/8). RNA from spleen generated a positive reaction, but stromal cells failed to generate a product of the appropriate size. MWM, molecular-mass markers; -RT, controls without reverse transcriptase; -DNA, controls without cDNA with primer pairs PPARγ5/PPARγ6 and PPARγ7/PPARγ8. (c) Immunoblotting for PPARγ. Lane 1, molecular-mass markers (in kDa); lane 2, control extract of ovine kidney tissue; lane 3, untreated stromal cells.
Role of protein kinases in the induction of PTGS2
The additive effects of arachidonic acid and NSAIDs (Figure 2b) suggested that they may act via different mechanisms. This is consistent with the fact that PPAR activation requires phosphorylation [25–28], as arachidonic acid is an activator of PKC [29,30], one of the protein kinases reported to phosphorylate PPARα [28]. An effect of arachidonic acid via PKC was supported by the results of treating cells with compounds which activate or inhibit PKC (Figure 4). The phorbol ester 4β-PMA increased the levels of PTGS2 protein up to 7.6-fold (mean of four experiments, 3.4-fold). The inactive isomer 4α-phorbol-12,13-didecanoate (2 μM) was ineffective (results not shown). PGF2α [which activates PKC via the FP receptor (PGF2 receptor)] [42] increased PTGS2 protein 2.2-fold. The effect of 4β-PMA was blocked by RO318425 (Figure 4a). The effect of arachidonic acid was blocked by RO318425 and calphostin C (500 nM; Figure 4b). RO318425 also prevented the induction of PTGS2 by indomethacin or NS398 (Figure 4c) and by WY14643 or ciprofibrate (Figure 4d).
PKC induces PTGS2 independently of PPARs
On the basis of the experiments described above, it was not possible to dissociate a potential effect of arachidonic acid as a PPAR ligand from an effect on PPAR phosphorylation via basal or activated PKC. However, in addition to phosphorylating PPARs, in the absence of an exogenous PPAR ligand PKC appeared to act through a second pathway to induce PTGS2. This was revealed by blocking the PTGS2 induction pathway downstream of PKC. When cells were treated with inhibitors of the NF-κB pathway [36–38], the effect of 4β-PMA was decreased approx. 70% by both MG132 and parthenolide (Figure 5a). In contrast, none of the NF-κB inhibitors used (MG132, parthenolide or sulfasalazine) affected PTGS2 induction by arachidonic acid (Figure 5b). In these experiments, none of the NF-κB inhibitors decreased the basal level of PTGS2 when added alone (results not shown).
DISCUSSION
Arachidonic acid induced PTGS2 in endometrial stromal cells, as in epithelial cells [12]. This effect provides an explanation for the increase in PTGS2 in endometrial stroma at luteolysis [4], and is consistent with the proposed paracrine action of arachidonic acid within the endometrium.
Five separate pieces of evidence from the present study support the hypothesis that PPARs are involved in the effect of arachidonic acid on PTGS2 levels: (i) a wide variety of PPAR agonists mimicked the action of arachidonic acid on PTGS2, including other PUFAs, PGA1, NSAIDs and synthetic pharmaceutical agents; (ii) these compounds acted with time courses similar to that of arachidonic acid, leading to increased PTGS2 levels 6 h after treatment; (iii) agents expected to act via PPAR phosphorylation (4β-PMA and PGF2α) also increased PTGS2 levels; (iv) consistent with PPAR function requiring phosphorylation, the effects of arachidonic acid and NSAIDs were both inhibited by RO318425; and (v) the effect of arachidonic acid was not blocked by NF-κB inhibitors, whereas, in the absence of a PPAR ligand, that of 4β-PMA was prevented, suggesting that arachidonic acid acted independently of PKC as well as via PKC. Linoleic acid increased PTGS2 levels at a lower concentration than other PUFAs, possibly reflecting its greater efficacy in causing PPARα heterodimerization [20]. Although a PPRE has not yet been identified upstream of the coding sequence in the bovine PTGS2 gene, these findings are consistent with the hypothesis that PUFAs induce PTGS2 expression in stromal cells via PPARs, as in other cell lines. The interactions postulated to occur in the experiments described in the present study are summarized in Scheme 1.
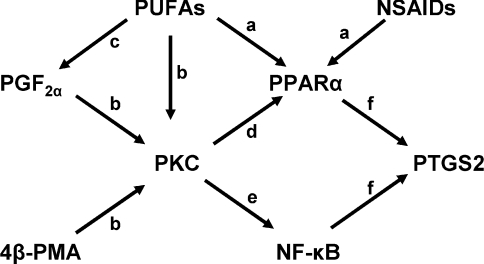
Scheme 1. Proposed mechanisms controlling PTGS2 levels in bovine endometrial stromal cells.
(a) PUFAs and NSAIDs are PPARα ligands. (b) PUFAs, PGF2α and 4β-PMA activate PKC. (c) PUFAs are converted into prostanoids, including PGF2α. (d) PKC phosphorylates PPARα, a step required for subsequent activation of PPARα by ligand occupancy; if PKC is inhibited PPAR ligands do not induce PTGS2. (e) PKC also activates NF-κB and, if NF-κB is inhibited in the absence of PPAR ligand, activation of PKC does not induce PTGS2. (f) PPARα and NF-κB increase PTGS2 levels. PPARA, PPARα.
PPARα and PPARδ were expressed in stromal cells, but there was no evidence for expression of PPARγ by either PCR or immunoblotting. This is consistent with the previous demonstration of PPARα and PPARδ, and the absence of PPARγ, in whole bovine endometrium and in an endometrial epithelial cell line (BEND cells) by Northern blotting [24]. The PPARγ probe used by MacLaren et al. [24] was derived by PCR from adipose tissue cDNA using primers corresponding closely to the PPARγ5/PPARγ2 or PPARγ5/PPARγ6 primer pairs used in the present study. The overlap between the two products was approx. 150 out of 240 bp. The absence of PPARγ in the endometrium is consistent with this isoform being principally found in adipose tissue [33].
Synthetic PPARδ agonists were ineffective in increasing PTGS2 levels and, therefore, since PPARγ was absent, PPARα was most probably responsible for the effect on PTGS2 levels. This was confirmed by showing that PTGS2 levels increased in response to the PPARα agonists WY14643, methylclofenapate and ciprofibrate, but not in response to appropriate concentrations of the PPARγ agonists ciglitazone and pioglitazone. The lack of involvement of PPARδ was compatible with the effect of indomethacin, which activates PPARα and PPARγ, but not PPARδ [33]. The conclusion that PPARα modulates PTGS2 levels in stromal cells is consistent with the observation that WY14643 induces PTGS2 in a bovine endometrial epithelial cell line [24].
The effects of the agonists and antagonists used in the present study were generally (but not always) consistent with their reported specificities. For instance, the PPARγ agonist pioglitazone, which is also a weak PPARα agonist [43], increased PTGS2 levels at 50 μM but not at 1 μM. The PPARγ antagonists BADGE and GW9662 also acted as partial PPARα agonists [34,44,45], but did not block the stimulatory effect of arachidonic acid. MK886, which is a PPARα antagonist in some systems [46], augmented, rather than prevented, the effects of arachidonic acid and indomethacin, and stimulated PTGS2 levels when added alone at 1–50 μM (results not shown). Indomethacin and MK886 are both substituted chlorophenyl indole derivatives and it is therefore not surprising that they have similar effects in some cells. Furthermore, MK886 is a potent lipoxygenase inhibitor and may have mimicked other lipoxygenase inhibitors, such as nordihydroguaiaretic acid, which also increased PTGS2 in stromal cells (E. L. R. Sheldrick, unpublished work), as in rat fibroblasts and murine keratinocytes [47,48]. Clearly, as noted previously [49], lipoxygenase inhibitors have cell- and tissue-specific effects on PPARs. Unfortunately, more specific PPARα antagonists which could be used to test the effect of inhibiting PPARα are not currently available.
PPAR transactivation function depends upon phosphorylation. The principal protein kinases responsible are MAPK (mitogen- activated protein kinase), PKA and PKC [25–28], and PUFAs are activators of PKC [29,30]. The consensus amino acid sequences subject to phosphorylation in PPARs have been studied mostly in humans and mice, but potential phosphorylation sites for each of these serine/threonine kinases are conserved in the corresponding bovine proteins. There are two PKC consensus sites in bovine PPARα (GenBank® accession no. BT020756) at Thr131 (FFRRTIRLK) and Ser348 (EFLKSLRKP), which are identical in the human and bovine proteins. Therefore bovine PPARα would be expected to undergo phosphorylation by PKC in the same way as the human protein [25,28]. Consistent with this, the effects of arachidonic acid, NSAIDs and PPAR activators were blocked by the PKC inhibitors RO318425 and (in the case of arachidonic acid) calphostin. This is consistent with the requirement for a basal level of phosphorylation for a PPAR response to ligand occupancy. We did not attempt to determine which of the many isoforms of PKC mediated the effects of 4β-PMA and PGF2α.
The results described above were insufficient to differentiate between an action of arachidonic acid as a PPAR ligand [20,21] and an effect through activation of PKC [29,30], and arachidonic acid and other PUFAs may have acted via both mechanisms. Actions of PUFAs exerted through two mechanisms (i.e. as PPAR ligands and through activation of PKC) might be expected to cause additive responses, and these were observed when indomethacin or NS398 was added with arachidonic acid (Figure 2b). In certain cell types, 4β-PMA-activated PKC induces PTGS2 expression via NF-κB [17], in addition to any effects resulting from phosphorylation of PPARs. We therefore tested whether inhibitors of NF-κB (MG132 and parthenolide) prevented the effect of 4β-PMA on PTGS2 levels in stromal cells. The results showed that both inhibitors decreased the response to 4β-PMA (Figure 5). In contrast, the effect of arachidonic acid was unaffected by the NF-κB blockers MG132, parthenolide or sulfasalazine (which was not tested with 4β-PMA). Inhibitors of NF-κB could therefore be used to dissociate the action of arachidonic acid from that of 4β-PMA. Since 4β-PMA is thought to be specific in activating PKC, this shows that arachidonic acid increases PTGS2 levels by more than one mechanism. Although NSAIDs of the fenamate group (but not indomethacin) block PTGS2 expression induced by TNFα (tumour necrosis factor α) or LPS in HT-29 human colon adenocarcinoma cells through inhibition of NF-κB [17], it is unlikely that the NSAIDs used in the present study (indomethacin and NS398) affected NF-κB.
The concentrations at which prostanoids were added to culture medium (0.3–3 μM) were comparable with the levels achieved through endogenous prostanoid production by the cells themselves (up to 0.3 μM; Figure 1). As a result, it is possible that, if they acted via cell-surface receptors, endogenous PGs may have affected PTGS2 levels in these experiments. They are unlikely to have acted as PPAR ligands, as PGF2α and PGE2 are poor in this respect [11].
The increase in PTGS2 level in the mammary gland in response to a high dietary n−6 PUFA intake [50] raises the possibility that the effect of arachidonic acid on PTGS2 expression in uterine cells may occur in vivo. If, as proposed in the present study, uterine PTGS2 levels are controlled by PPARs, then NSAIDs administered to treat reproductive disorders may result in detrimental increases in PTGS2 in the tissues in which they are intended to reduce prostanoid synthesis.
Acknowledgments
We thank Dr David Bell (University of Nottingham) for kindly providing certain PPAR agonists. We thank Martin Bishton, Matthew Elmes, Mandeep Kandola and Yasmin Mussaddeq for assistance in the laboratory, and Pat Fisher for deriving the cells. This work was funded by the BBSRC (Biotechnology and Biological Sciences Research Council) and the Wellcome Trust.
References
- 1.Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Smith W. L., Garavito R. M., DeWitt D. L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 3.Sales K. J., Jabbour H. N. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction. 2003;126:559–567. doi: 10.1530/rep.0.1260559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boos A. Immunohistochemical assessment of prostaglandin H-synthase in bovine endometrial biopsy samples collected throughout the oestrous cycle. Anim. Reprod. Sci. 1998;51:261–273. doi: 10.1016/s0378-4320(98)00075-x. [DOI] [PubMed] [Google Scholar]
- 5.Arosh J. A., Parent J., Chapdelaine P., Sirois J., Fortier M. A. Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the estrous cycle. Biol. Reprod. 2002;67:161–169. doi: 10.1095/biolreprod67.1.161. [DOI] [PubMed] [Google Scholar]
- 6.Robinson R. S., Mann G. E., Lamming G. E., Wathes D. C. The effect of pregnancy on the expression of uterine oxytocin, oestrogen and progesterone receptors during early pregnancy in the cow. J. Endocrinol. 1999;160:21–33. doi: 10.1677/joe.0.1600021. [DOI] [PubMed] [Google Scholar]
- 7.Wathes D. C., Mann G. E., Payne J. H., Riley P. R., Stevenson K. R., Lamming G. E. Regulation of oxytocin, oestradiol and progesterone receptor concentrations in different uterine regions by oestradiol, progesterone and oxytocin in ovariectomized ewes. J. Endocrinol. 1996;151:375–393. doi: 10.1677/joe.0.1510375. [DOI] [PubMed] [Google Scholar]
- 8.Salamonsen L. A. Local regulators and the establishment of pregnancy: a review. Reprod. Fertil. Dev. 1992;4:125–134. doi: 10.1071/rd9920125. [DOI] [PubMed] [Google Scholar]
- 9.Bany B. M., Kennedy T. G. Role of interleukin 1 in the regulation of cyclooxygenase gene expression in rat endometrial stromal cells. J. Reprod. Fertil. 1999;115:125–131. doi: 10.1530/jrf.0.1150125. [DOI] [PubMed] [Google Scholar]
- 10.Flint A. P. F., Leat W. M. F., Sheldrick E. L., Stewart H. J. Stimulation of phosphoinositide hydrolysis by oxytocin and the mechanism by which oxytocin controls prostaglandin synthesis in the ovine endometrium. Biochem. J. 1986;237:797–805. doi: 10.1042/bj2370797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meade E. A., McIntyre T. M., Zimmerman G. A., Prescott S. M. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J. Biol. Chem. 1999;274:8328–8334. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 12.Parent J., Villeneuve C., Fortier M. A. Evaluation of the contribution of cyclooxygenase 1 and cyclooxygenase 2 to the production of PGE2 and PGF2α in epithelial cells from bovine endometrium. Reproduction. 2003;126:539–547. doi: 10.1530/rep.0.1260539. [DOI] [PubMed] [Google Scholar]
- 13.Prigent-Tessier A., Pageaux J.-F., Fayard J.-M., Lagarde M., Laugier C., Cohen H. Arachidonic acid up-regulates and prostaglandin E2 down-regulates the expression of pancreatic-type phospholipase A2 and prostaglandin-endoperoxide synthase 2 in uterine stromal cells. Eur. J. Biochem. 1996;241:872–878. doi: 10.1111/j.1432-1033.1996.00872.x. [DOI] [PubMed] [Google Scholar]
- 14.Cooke B. A., Dirami L., Chaudry L., Choi M. S. K., Abayasekara D. R. E., Phipp L. Release of arachidonic acid and the effects of corticosteroids on steroidogenesis in rat testis Leydig cells. J. Steroid Biochem. Mol. Biol. 1991;40:465–471. doi: 10.1016/0960-0760(91)90216-r. [DOI] [PubMed] [Google Scholar]
- 15.Ronco A. M., Moraga P. F., Llanos M. N. Arachidonic acid release from rat Leydig cells: the involvement of G protein, phospholipase A2 and regulation of cAMP production. J. Endocrinol. 2002;172:95–104. doi: 10.1677/joe.0.1720095. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Antaya M., Boerboom D., Lussier J. G., Silversides D. W., Sirois J. The delayed activation of the prostaglandin G/H synthase-2 promoter in bovine granulosa cells is associated with down-regulation of truncated upstream stimulatory factor-2. J. Biol. Chem. 1999;274:35037–35045. doi: 10.1074/jbc.274.49.35037. [DOI] [PubMed] [Google Scholar]
- 17.Paik J. H., Ju J. H., Lee J. Y., Boudreau M. D., Hwang D. H. Two opposing effects of non-steroidal anti-inflammatory drugs on the expression of the inducible cyclooxygenase. J. Biol. Chem. 2000;275:28173–28179. doi: 10.1074/jbc.M002329200. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y.-L., Wiltbank M. C. Transcriptional regulation of cyclooxygenase-2 gene in ovine large luteal cells. Biol. Reprod. 2001;65:1565–1572. doi: 10.1095/biolreprod65.5.1565. [DOI] [PubMed] [Google Scholar]
- 19.Ledwith B. J., Pauley C. J., Wagner L. K., Rokos C. L., Alberts D. W., Manam S. Induction of cyclooxygenase-2 expression by peroxisome proliferators and non-tetradecanoylphorbol 12,13-myristate-type tumor promoters in immortalized mouse liver cells. J. Biol. Chem. 1997;272:3707–3714. doi: 10.1074/jbc.272.6.3707. [DOI] [PubMed] [Google Scholar]
- 20.Forman B. M., Chen J., Evans R. M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely G. B., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., Lehmann J. M. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toomey S., Roche H., Fitzgerald D., Belton O. Regression of pre-established atherosclerosis in the apoE−/− mouse by conjugated linoleic acid. Biochem. Soc. Trans. 2003;31:1075–1079. doi: 10.1042/bst0311075. [DOI] [PubMed] [Google Scholar]
- 23.Na H.-K., Surh Y.-J. Peroxisome proliferator-activated receptor γ (PPARγ) ligands as bifunctional regulators of cell proliferation. Biochem. Pharmacol. 2003;66:1381–1391. doi: 10.1016/s0006-2952(03)00488-x. [DOI] [PubMed] [Google Scholar]
- 24.MacLaren L. A., Guzeloglu A., Michel F., Thatcher W. W. Peroxisome proliferator-activated receptor (PPAR) expression in cultured bovine endometrial cells and response to omega-3 fatty acid, growth hormone and agonist stimulation in relation to series 2 prostaglandin production. Domest. Anim. Endocrinol. 2006;30:155–169. doi: 10.1016/j.domaniend.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Juge-Aubry C. E., Hammar E., Siegrist-Kaiser C., Pernin A., Takeshita A., Chin W. W., Burger A. G., Meier C. A. Regulation of the transcriptional activity of the peroxisome proliferator-activated receptor α by phosphorylation of a ligand-independent trans-activating domain. J. Biol. Chem. 1999;274:10505–10510. doi: 10.1074/jbc.274.15.10505. [DOI] [PubMed] [Google Scholar]
- 26.Barger P. M., Browning A. C., Garner A. N., Kelly D. P. p38 Mitogen-activated protein kinase activates peroxisome proliferator-activated receptor α. J. Biol. Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 27.Lazennec G., Canaple L., Saugy D., Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol. Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanquart C., Mansouri R., Paumelle R., Fruchart J.-C., Staels B., Glineur C. The protein kinase C signaling pathway regulates a molecular switch between transactivation and transrepression activity of the peroxisome proliferator-activated receptor α. Mol. Endocrinol. 2004;18:1906–1918. doi: 10.1210/me.2003-0327. [DOI] [PubMed] [Google Scholar]
- 29.Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan W. A., Blobe G. C., Hannun Y. A. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell. Signalling. 1995;7:171–184. doi: 10.1016/0898-6568(94)00089-t. [DOI] [PubMed] [Google Scholar]
- 31.Murakami S., Shibaya M., Takeuchi K., Skarzynski J., Okuda K. A passage and storage system for isolated bovine endometrial epithelial and stromal cells. J. Reprod. Dev. 2003;49:531–538. doi: 10.1262/jrd.49.531. [DOI] [PubMed] [Google Scholar]
- 32.Asselin E., Lacroix D., Fortier M. A. IFN-τ increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro. Mol. Cell. Endocrinol. 1997;132:117–126. doi: 10.1016/s0303-7207(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 33.Corton J. C., Anderson S. P., Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu. Rev. Pharmacol. Toxicol. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi T., Waki H., Kamon J., Murakami K., Motojima K., Komeda K., Miki H., Kubota N., Terauchi Y., Tsuchida A., et al. Inhibition of RXR and PPARγ ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 2001;108:1001–1013. doi: 10.1172/JCI12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J. T., Welch J. S., Ricote M., Binder C. J., Willson T. M., Kelly C., Witztum J. L., Funk C. D., Conrad D., Glass C. K. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 36.Wu W.-T., Chi K.-H., Ho F.-M., Tsao W.-C., Lin W.-W. Proteosome inhibitors up-regulate haem oxygenase-1 gene expression: requirement of p38 MAPK (mitogen-activated protein kinase) activation but not of NF-κB (nuclear factor κB) inhibition. Biochem. J. 2004;379:587–593. doi: 10.1042/BJ20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bork P. M., Schmitz M. L., Kuhnt M., Escher C., Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- 38.Wahl C., Liptay S., Adler G., Schmid R. M. Sulfasalazine: a potent and specific inhibitor of nuclear factor κB. J. Clin. Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chomczynski P., Sacchi N. Single-step method RNA isolation by acid guanidinum thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 40.Leung S. T., Cheng Z., Sheldrick E. L., Derecka K., Flint A. P. F., Wathes D. C. The effects of lipolysaccharide and interleukins-1α, -2 and -6 on oxytocin receptor expression and prostaglandin production in bovine endometrium. J. Endocrinol. 2001;168:497–508. doi: 10.1677/joe.0.1680497. [DOI] [PubMed] [Google Scholar]
- 41.Moya-Camarena S. Y., Vanden Heuvel J. P., Belury M. A. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARα. J. Lipid Res. 1999;40:1426–1433. [PubMed] [Google Scholar]
- 42.Abayasekara D. R. E., Jones P. M., Persaud S. J., Michael A. E., Flint A. P. F. Prostaglandin F2α activates protein kinase C in human ovarian cells. Mol. Cell. Endocrinol. 1993;91:51–57. doi: 10.1016/0303-7207(93)90254-h. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto J., Kimura H., Moriyama S., Odaka H., Momose Y., Sugiyama Y., Sawada H. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem. Biophys. Res. Comm. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 44.Leesnitzer L. M., Parks D. J., Bledsoe R. K., Cobb J. E., Collins J. L., Consler T. G., Davis R. G., Hull-Ryde E. A., Lenhard J. M., Patel L., et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 45.Seimandi M., Lemaire G., Pillon A., Perrin A., Carlavan I., Voegel J. J., Vignon F., Nicolas J.-C., Balaguer P. Differential responses of PPARα, PPARδ, and PPARγ reporter cell lines to selective PPAR synthetic ligands. Anal. Biochem. 2005;344:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Kehrer J. P., Biswal S. S., La E., Thuillier P., Datta K., Fischer S. M., Vanden Heuvel J. P. Inhibition of peroxisome-proliferator-activated receptor (PPAR)α by MK886. Biochem. J. 2001;356:899–906. doi: 10.1042/0264-6021:3560899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuwata H., Yamamoto S., Miyazaki Y., Shimbara S., Nakatani Y., Suzuki H., Ueda N., Yamamoto S., Murakami M., Kudo I. Studies on a mechanism by which cytosolic phospholipase A2 regulates the expression and function of type IIA secretory phospholipase A2. J. Immunol. 2000;165:4024–4031. doi: 10.4049/jimmunol.165.7.4024. [DOI] [PubMed] [Google Scholar]
- 48.Maldve R. E., Kim Y., Muga S. J., Fischer S. M. Prostaglandin E2 regulation of cyclooxygenase expression in keratinocytes is mediated via cyclic nucleotide-linked prostaglandin receptors. J. Lipid Res. 2000;41:873–881. [PubMed] [Google Scholar]
- 49.Thuillier P., Brash A. R., Kehrer J. P., Stimmel J. B., Leesnitzer L. M., Yang P., Newman R. A., Fischer S. M. Inhibition of peroxisome-proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem. J. 2002;366:901–910. doi: 10.1042/BJ20020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badawi A. F., El-Sohemy A., Stephen L. L., Ghoshal A. K., Archer M. C. The effect of dietary n-3 and n-6 polyunsaturated fatty acids on the expression of cyclooxygenase 1 and 2 and levels of p21ras in rat mammary glands. Carcinogenesis. 1998;19:905–910. doi: 10.1093/carcin/19.5.905. [DOI] [PubMed] [Google Scholar]