Abstract
Bacterial pathogens have developed sophisticated mechanisms of evading the immune system to survive in infected host cells. Central to the pathogenesis of Mycobacterium tuberculosis is the arrest of phagosome maturation, partly through interference with PtdIns signalling. The protein phosphatase MptpB is an essential secreted virulence factor in M. tuberculosis. A combination of bioinformatics analysis, enzyme kinetics and substrate-specificity characterization revealed that MptpB exhibits both dual-specificity protein phosphatase activity and, importantly, phosphoinositide phosphatase activity. Mutagenesis of conserved residues in the active site signature indicates a cysteine-based mechanism of dephosphorylation and identifies two new catalytic residues, Asp165, essential in catalysis, and Lys164, apparently involved in substrate specificity. Sequence similarities with mammalian lipid phosphatases and a preference for phosphoinositide substrates suggests a potential novel role of MptpB in PtdIns metabolism in the host and reveals new perspectives for the role of this phosphatase in mycobacteria pathogenicity.
Keywords: bacterial phosphatase, dual-specificity, lipid phosphatase, protein phosphatase, signalling
Abbreviations: DSP, dual-specificity protein phosphatase; EGFR, epidermal growth factor receptor; IFN-γ, interferon-γ; IPTG, isopropyl β-D-thiogalactoside; LB, Luria–Bertani; MAPK, mitogen-activated protein kinase; MTM, myotubularin; MTMR2, MTM-related protein 2; pNPP, p-nitrophenol phosphate; PTEN, phosphatase and tensin homologue deleted on chromosome 10; PTP, protein tyrosine phosphatase; TPTE, transmembrane phosphatase with tensin homology; TSP, triple-specificity protein phosphatase
INTRODUCTION
Mycobacterium tuberculosis is the causative agent of tuberculosis, a decimating disease affecting one third of the human population and causing around two million deaths every year (according to the World Health Organization, www.who.int). This intracellular pathogen survives and replicates primarily in host macrophages despite the antimicrobial functions of the macrophage. The primary defence against intracellular microbial pathogens is phagosomal maturation into phagolysosomes, leading to the acidification of lysosome contents and destruction of bacterial particles. Many pathogens have evolved sophisticated ways of evading this innate response to extend their survival in host cells. M. tuberculosis is a landmark example of such a strategy [1], since it can persist in phagocytes preventing the normal maturation to phagolysosomes and survive inside the host lungs in a latent asymptomatic state. In recent years, a number of studies have been directed at understanding the molecular mechanisms that allow M. tuberculosis survival in infected macrophages (reviewed in [2–4]). One proposed mechanism involves the host phosphoinositide, PtdIns3P, responsible for recruitment of the Rab5 effectors hVPS34 and EEA1 to endocytic organelles, an essential step in phagosome maturation [5]. Manipulation of phosphoinositide metabolism is emerging now as a common theme in bacterial pathogenesis [6].
Other mechanisms correlate infection of M. tuberculosis with disruption of several normal host signalling pathways involving MAPKs (mitogen-activated protein kinases), IFN-γ (interferon-γ), calcium signalling and apoptosis (reviewed in [4]). As phosphorylation and dephosphorylation of host proteins regulate these intracellular processes, it is not surprising that protein kinases and phosphatases play a role in pathogen infection and survival [3,4]. In fact, phosphatases are major virulence factors in other pathogenic bacteria such as Yersinia pseudotuberculosis, and Salmonella typhimurium [7,8]. Likewise, the M. tuberculosis secreted protein phosphatases MptpA and MptpB have been reported to be important for persistence of mycobacterial infection [9–10]. Disruption of the mptpB gene severely impairs the ability of the mutant strain to survive in both IFN-γ-activated macrophages and in guinea pigs [10]. The function of MptpB remains unclear, although a role in dephosphorylation of protein targets in the IFN-γ pathway has been suggested [10], the biological substrates of MptpB have not yet been elucidated. Therefore a true understanding of the enzymatic and substrate specificity of this phosphatase is essential to dissect the actual biological role of MptpB.
Bioinformatics analysis of MptpB reveals a large family of related proteins in bacterial and fungal species, including many pathogens. In-depth sequence analysis highlights common features to DSPs (dual-specificity protein phosphatases) and, surprisingly, a singular active site signature motif, reminiscent of eukaryotic lipid phosphatases. These observations prompted us to investigate further the biochemical and kinetic properties of this enzyme to define its substrate specificity. In the present study we provide experimental evidence that MptpB dephosphorylates phosphotyrosine and phosphoserine/threonine substrates, and importantly, phosphoinositides, thus exhibiting TSP (triple-specificity phosphatase) activity. The report of a new completely unexpected catalytic specificity for MptpB should be an aid in defining novel ways of addressing its mechanism of action in vivo, its potential role in host phosphoinositide metabolism, and to identify host targets for MptpB. In addition, this catalytic versatility provides new insights for future rational design of specific inhibitors of MptpB.
EXPERIMENTAL
Bioinformatics analysis
Multiple sequence alignments were performed using ClustalX [11], after initial identification of MptpB-related sequences using PSI-BLAST searches of GenBank® [12]. Analysis of the active site motif was performed using a ScanProsite search in SwissProt and TrEMBL (http://www.expasy.org/tools/scanprosite/).
Cloning and mutagenesis of MptpB
The open reading frame of Rv0153c, encoding MptpB, was amplified from M. tuberculosis H37Rv DNA. The oligonucleotide primers, forward (5′-CATATGGCTGTCCGTGAACTGCCGGGCGCG-3′) and reverse (5′-CTCGAGTCATCCGAGCAGCACCCCGCGCATC-3′) contained NdeI and XhoI restriction sites and the resulting MptpB-coding sequence was cloned into pET28a (Novagen) to generate an N-terminal His6-tagged expression construct. Site-directed mutagenesis of the following residues D82A, C160S, K164A, D165N and R166A were carried out using the QuikChange® Kit (Stratagene).
Overexpression and purification of recombinant proteins
Each construct (wild-type and mutants) was transformed into Escherichia coli strain BL21 (DE3) and grown in LB (Luria–Bertani) broth at 37 °C to mid-log phase. Expression was induced at 18 °C with 0.5 mM IPTG (isopropyl β-D-thiogalactoside) and the cells were harvested by centrifugation (4000 g for 15 min at 4 °C) 16 h later. The expressed His6–MptpB (wild-type and mutants) was purified by nickel-affinity chromatography. The supernatant from the bacterial lysate was loaded on to a 5 ml HiTrap column (Amersham Bioscience) in binding buffer (50 mM Hepes and 500 mM NaCl, pH 7) and the protein was recovered following on-column cleavage of the His6-tag with thrombin (Sigma) by washing with binding buffer. The recovered fractions were further purified on a MonoQ column using 20 mM Tris/HCl (pH 8) and eluted with 200 mM NaCl in the same buffer. The phosphatase from Trypanosoma brucei TbPTP1 has been recently characterized by our group as a tyrosine-specific phosphatase [13]. In the present study we used TbPTP1 as a control. TbPTP1 was amplified from T. brucei DNA and cloned into pET28a (Novagen). Recombinant TbPTP1 was expressed in E. coli strain BL21 DE3 CodonPlus™ RIPL, grown in LB broth at 37 °C and induced at 30 °C with 0.4 mM IPTG. Purification of His6-tagged TbPTP1 was performed by nickel-affinity chromatography using the same method as for MptpB.
Phosphatase activity assays
The Malachite Green assay (Sigma) was used to determine the amount of free phosphate during the dephosphorylation assays with a range of substrates: phosphotyrosine peptides from EGFR (epidermal growth factor receptor; DADEpYLIPQQG) and insulin receptor (TRDIpYETDYYRK), phosphoserine peptide (RRApSVA), phosphothreonine peptide (KRpTIRR; Alta Bioscience, University of Birmingham), phosphoserine, phosphothreonine and phosphotyrosine (Sigma), IMP (inosine monophosphate), AMP, pNPP (p-nitrophenol phosphate; Sigma), diC8-PtdIns3P, diC8-PtdIns(3,4)P2, diC8-PtdIns(3,5)P2, diC8-PtdIns4P, diC8-PtdIns(4,5)P2, diC8PtdIns5P and diC8-PtdIns(3,4,5)P3 (all from Echelon Bioscience). Each reaction was prepared in triplicate in a 96-well microplate, containing 50 μl of the reaction mixture with 5 μg of enzyme (MptpB wild-type, mutants and TbPTP1), 0.05–2 mM substrate (3–500 μM range for phosphoinositides) and buffer (50 mM Tris/HCl, 50 mM Bis-Tris and 100 mM sodium acetate, pH 6). The reaction mixtures were incubated for 15 min at 37 °C prior to the addition of 50 μl of Malachite Green reagent (Sigma) and further incubated for 10 min at room temperature (20 °C). The absorbance was subsequently read at 620 nm and the mean values calculated. Control reactions containing no enzyme were included to measure the background level of phosphate. A phosphate standard curve was produced using known amounts of phosphate (25–3000 pmol of Sigma phosphate standard solution). Experimental points were interpolated in the standard curve to calculate the amount of phosphate released.
Kinetic analysis
The kinetic parameters Vmax and Km for each substrate were determined using non-linear regression fit of the initial velocity versus substrate concentration curve using Hyper32 (developed by Dr John Easterby, Department of Biochemistry, University of Liverpool, Liverpool, U.K.). The kcat was determined by dividing the Vmax by the molar enzyme concentration and the means±S.E.M. were calculated.
RESULTS AND DISCUSSION
MptpB-related bacterial and fungal phosphatases
A PSI-BLAST search with the full-length MptpB sequence identified orthologues in other mycobacterium species (Mycobacterium bovis, Mycobacterium flavescens, Mycobacterium vanbaalenii and Mycobacterium sp.MCS) and returned more than 150 related sequences in bacteria and fungi, most of which are currently annotated as hypothetical proteins. The multiple sequence alignment highlights the existence of a characteristic active site signature motif CX5R, present in the PTP (protein tyrosine phosphatase) superfamily, which forms the P-loop (phosphate-binding loop) (Figure 1A). The presence of the CX5R motif suggests a classification of these sequences as predicted phosphatases. However, the analysis failed to identify motifs conserved only in the tyrosine-specific phosphatases, such as the WPD motif that contains the catalytic aspartate residue or the Q-loop involved in catalysis [14]. Instead, the active site region of MptpB and related proteins resembles the extended signature motif described for a subfamily of PTPs, the DSPs that can efficiently dephosphorylate phosphotyrosine and phosphoserine/threonine substrates [15]. The extended signature comprises the P-loop motif and flanking hydrophobic residues (Figure 1A). Consistent with this observation, the overall sequence similarity of MptpB with tyrosine-specific PTPs, such as human PTP1B is only 6%, whereas similarity with known DSPs is 24% with VHR (vaccinia virus VH1-related dual-specific protein phosphatase), 25.8% with MKP3 (MAPK phosphatase 3) and 33.5% with IphP (dual-specific phosphatase from Nostoc commune). Therefore although MptpB is designated as a tyrosine phosphatase in the genome databases, sequence analysis suggests otherwise.
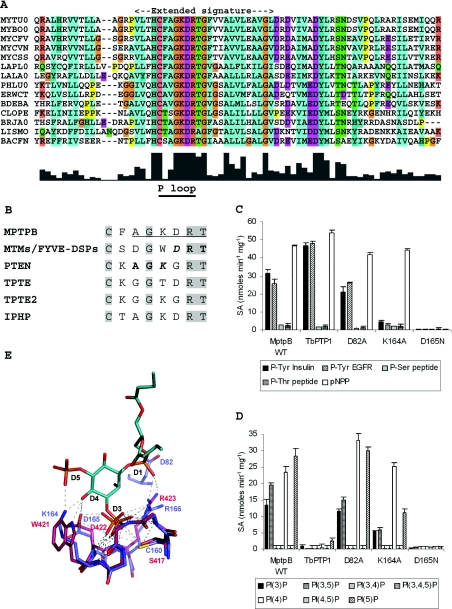
Figure 1. MptpB exhibits DSP and phosphoinositide phosphatase activity and displays sequence and structural similarities with eukaryotic lipid phosphatases.
(A) Alignment of a subset of MptpB-related sequences. Alignments prepared with ClustalX. M. tuberculosis (MYTU0, MptpB), M. bovis (MYBO0), M. flavescens (MYCFV), M. vanbaalenii (MYCVN), M. species (MYCSS), Photorhabdus luminescens (PHLU0), Erwinia carotovora (ERWCT), Bdellovibrio bacteriovorus (BDEBA), Clostridium perfringensgi (CLOPE), Lactobacillus plantarum (LAPL0), Lactococcus lactis (LALA0), Bradyrhizobium japonicum (BRJA0), Listeria monocytogenes (LISMO), Bacteroides fragilis (BACFN). (B) Alignment of P-loop motifs in lipid phosphatases. Indicated are the conserved ‘AGK’ motif found in PTEN and the ‘DRT’ from MTMs (bold), where K and D (shown in italics) are catalytic residues. MptpB has a unique combination of both signatures, with a conserved ‘AGKDRT’ motif (underlined). The DSP IphP from cyanobacteria [27] also contains the conserved ‘AGKDRT’ motif. (C) Specific activity (SA) for MptpB wild-type and mutants towards phosphorylated substrates. Each assay was performed in triplicate. Values are means±S.E.M. The activity of the tyrosine-specific phosphatase from T. brucei (TbPTP1) is shown as a control. (D) Enzyme activity of MptpB wild-type and mutants towards phosphoinositide substrates. (E) Stick representation of MptpB (purple) [25] superimposed into the active site of MTMR2 (pink) with bound PtdIns(3,5)P2 [22]. Catalytic residues and Asp82 are labelled and interactions are displayed as dashed lines. Generated with PyMol (DeLano Scientific LLC). PI, PtdIns; P-Ser, phosphoserine; P-Thr, phosphothreonine; P-Tyr, phosphotyrosine; WT, wild-type.
Furthermore, all bacterial and fungal MptpB-related sequences contain two strictly conserved residues in the P-loop: Lys164 and Asp165 (MptpB numbering). Such a feature is unusual in both classical tyrosine-specific PTPs and DSPs. A ScanProsite search using the regular expression pattern HCX2GKDR[T/A] found in the active site signature revealed eukaryotic orthologues of the lipid phosphatases PTEN (phosphatase and tensin homologue deleted on chromosome 10), TPTE (transmembrane phosphatase with tensin homology), TPTE2 [16], MTMs (myotubularins), and FYVE-DSP1/2 [17–18]. The alignment of the P-loop motif from these lipid phosphatases underlines the similarities with MptpB in this region (Figure 1B). The conserved lysine residue is also found in PTEN and PTEN-related phosphatases and it is important in substrate binding [19,20]. The conserved aspartate residue, found in MTMs and FYVE-DSP1/2 acts as the general acid in catalysis [21,22]. The combination of PTEN and MTM features in the active site suggests that MptpB may also share similar enzymatic properties. MTMs, FYVE-DSP1/2 and PTEN display both DSP and phosphoinositide phosphatase activity [17–19,23]. To test this we carried out a full investigation of the kinetic properties and substrate specificity of MptpB.
MptpB exhibits DSP activity
Recombinant MptpB was used for kinetic analyses towards a panel of phosphosubstrates (see the Experimental section). The catalytic activity of MptpB towards pNPP and the EGFR peptide was optimal at pH 6, both in the absence and presence of dithiothreitol (results not shown). MptpB dephosphorylates phosphotyrosine and phosphoserine/threonine substrates, with higher catalytic efficiency towards phosphopeptides as indicated by higher kcat/Km values (Table 1). A higher catalytic rate, kcat, is observed for phosphotyrosine peptide substrates, whereas lower Km values are found for phosphoserine/threonine peptides, indicating enhanced binding affinity (Table 1). Dephosphorylation of phosphotyrosine peptides is approx. 3.2 times more efficient than dephosphorylation of phosphoserine/threonine peptides. This is in good agreement with the ratio observed for other DSPs [24], and in contrast with the rates of dephosphorylation for tyrosine-specific PTPs commonly up to 105 times higher for phosphotyrosine substrates than for phosphoserine/threonine substrates [24]. Overall, the catalytic profile obtained from our kinetic analysis shows that MptpB has DSP activity with a preference for phosphotyrosine substrates.
Table 1. Kinetic parameters of MptpB towards different phosphorylated substrates (all values are means±S.E.M.).
P-Ser, phosphoserine; P-Thr, phosphotheonine; P-Tyr, phosphotyrosine.
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (M−1·s−1)×105 |
|---|---|---|---|
| P-Tyr | 0.291±0.01 | 60.00±1.26 | 2.06±0.05 |
| P-Ser | 0.44±0.08 | 18.32±0.93 | 0.41±0.04 |
| P-Thr | 0.253±0.01 | 12.50±0.47 | 0.50±0.04 |
| P-Tyr insulin | 0.217±0.04 | 159.57±9.22 | 7.36±0.74 |
| P-Tyr EGFR | 0.327±0.03 | 158.31±4.27 | 4.84±0.24 |
| P-Ser peptide | 0.078±0.01 | 18.08±0.11 | 2.32±0.30 |
| P-Thr peptide | 0.076±0.01 | 9.12±0.11 | 1.20±0.34 |
| 5′ AMP | 1.749±0.07 | 60.97±1.55 | 0.35±0.01 |
| 5′ IMP | 1.196±0.05 | 88.40±2.38 | 0.74±0.02 |
| pNPP | 0.170±0.01 | 134.70±4.88 | 7.91±0.10 |
MptpB exhibits lipid phosphatase activity
The similarity between the active site signature from MptpB with those of MTMs and PTEN suggested that MptpB might also display lipid phosphatase activity. To test this, the activity of the enzyme was assayed against different phosphoinositides (Table 2). MptpB readily dephosphorylated PtdIns3P, PtdIns(3,5)P2, PtdIns4P and PtdIns5P, with higher efficiency for PtdIns3P and PtdIns(3,5)P2, but showed comparable Km values for the four phosphoinositide substrates, indicating similar binding affinity. Phosphoinositide substrates exhibited lower Km values than the phospho-amino acid or phosphopeptide substrates tested (Tables 1 and 2), thus indicating that MptpB has higher affinity for lipid substrates. The kcat/Km values for phosphoinositide dephosphorylation are also higher than for peptidic substrates. Interestingly, MptpB appears to have a broad specificity as it dephosphorylates single phosphate substitutions at the D3, D4 and D5 positions. However, the enzyme exhibited no activity against PtdIns(3,4,5)P3, PtdIns(3,4)P2 or PtdIns(4,5)P2, showing an apparent selectivity against phosphates at the D4 position of the inositol head when in combination with another phosphate group at D3 or D5. This broad phosphoinositide activity is in contrast with mammalian lipid phosphatases that exhibit a much more restricted specificity [20,23].
Table 2. Kinetic parameters for MptpB wild-type and K164A dephosphorylation of phosphoinositides (all values are means±S.E.M.).
n.a., no detectable activity.
| Wild-type | K164A | |||||
|---|---|---|---|---|---|---|
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (M−1·s−1)×105 | Km (mM) | kcat (s−1) | kcat/Km (M−1·s−1)×105 |
| PtdIns3P | 0.018±0.002 | 38.5±0.93 | 21.48±1.27 | 0.0055±0.003 | 14.6±0.40 | 2.64±1.13 |
| PtdIns(3,5)P2 | 0.028±0.001 | 75.4±1.00 | 27.23±0.65 | 0.022±0.003 | 12.7±0.62 | 5.79±0.56 |
| PtdIns(3,4)P2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| PtdIns(3,4,5)P3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| PtdIns4P | 0.074±0.004 | 100.3±2.74 | 13.50±0.48 | 0.103±0.013 | 82.6±6.07 | 8.0±0.56 |
| PtdIns(4,5)P2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| PtdIns5P | 0.064±0.004 | 80.5±2.13 | 12.66±0.48 | 0.086±0.016 | 35.9±2.81 | 4.16±0.70 |
| pNPP | 0.170±0.010 | 134.7±4.88 | 7.91±0.10 | 0.087±0.009 | 163.9±4.30 | 18.8±0.87 |
Mutagenesis of the active site signature residues
Next, we wanted to explore the potential implication of conserved P-loop residues (Cys160, Lys164, Asp165 and Arg166) in catalysis or substrate binding. As expected, the C160S and R166A mutated enzymes exhibit less than 1% of the wild-type activity for any of the substrates tested, including pNPP and phosphoinositides. This confirms that these residues are essential for enzymatic activity, presumably in a cysteine-based mechanism of catalysis similar to PTPs and DSPs [15], where the cysteine residue is the nucleophile and the arginine residue contributes to substrate binding.
Enzymatic assays with the D165N mutant showed that it displays less than 0.1% of wild-type activity for pNPP or peptide substrates (Figure 1C). Furthermore, the D165N mutant showed <3% activity towards phosphoinositides (Figure 1D). On the basis of this new experimental evidence we propose Asp165 as a putative catalytic residue for MptpB. Supporting this, the analogous conserved aspartate residue in MTMs and FYVE-DSP1/2 (Figure 1B) was found to be essential for catalysis in MTMR2 [21]. Interestingly, mutation of Asp82 to an alanine residue showed similar activity to the wild-type enzyme with all substrates tested (Figures 1C and 1D). This residue was proposed previously as the general acid in catalysis [25], although experimental evidence has not been reported to date. We have established that Asp82 is not the general acid in catalysis.
The presence of Lys164 in the P-loop is intriguing. Typically, only one basic residue, the conserved arginine residue, is necessary in the active site to direct binding of the negatively charged phosphosubstrates. Additional basic residues (e.g. lysine residues) are uncommon, and only found in the lipid phosphatases PTEN and TPTE (Figure 1B), which bind substrates with more than one negatively charged phosphate group. To investigate the functional role of Lys164, we mutated it to an alanine residue and tested its activity towards substrates (Table 2, Figures 1C and 1D). The activity of K164A is severely impaired towards phosphotyrosine peptides, with less than 10% of the wild-type activity (Table 2, Figure 1C), but remains unaltered towards pNPP and phosphoserine/threonine peptides. Dephosphorylation of lipids is also reduced in a substrate-dependent manner. The largest reduction is observed for PtdIns(3,5)P2, with a 70% decrease in activity with respect to wild-type, in contrast, there is little effect on dephosphorylation of PtdIns4P (Table 2, Figure 1D). Kinetic analysis with K164A showed that the kcat/Km values decreased by more than one order of magnitude for all phosphoinositides with respect to the wild-type enzyme, but Km values remained similar. On the other hand, a significant reduction in the catalytic rate was observed for PtdIns3P and PtdIns(3,5)P2 (Table 2). These results indicate a potential role for Lys164 in catalysis, albeit in a substrate-dependent manner. The analogous lysine residue in the lipid phosphatase PTEN, Lys128, is important in lipid binding [20]. Docking of PtdIns(3,5)P2 to the active site of MptpB suggests that the wide active site could easily accommodate PtdIns(3,5)P2 (Figure 1E), and that binding might be favoured through interactions of the D5 phosphate with a basic residue such as Lys164. In contrast, PtdIns(3,4,5)P3 would not bind in the active site because the D4 phosphate would clash with the catalytic Asp165 and Lys164 side chains. New insights regarding the role of this residue should be revealed when the three-dimensional structure of MptpB in complex with a lipid substrate is solved.
The enzymatic activity towards PtdIns3P, PtdIns4P and PtdIns5P indicates that all three monophosphate substrates bind in the active site, although different binding modes or alternative binding orientations of the phosphoinositol head must occur. The lack of activity for PtdIns(3,4)P2, PtdIns(4,5)P2 and PtdIns(3,4,5)P3 could be rationalized by the fact that contiguous biphosphate substitutions (3,4; 4,5 or 3,4,5) would probably result in stearic clashes with Asp165, whereas non-contiguous substitutions (3,5), may be favoured by interactions with Lys164 (Figure 1E).
Conclusions
In the present study we describe the new findings on the TSP activity of MptpB, a virulence factor from M. tuberculosis. Our kinetic characterization provides experimental evidence that MptpB exhibits DSP activity and unexpected phosphoinositide phosphatase activity for a broad range of lipids. This is the first report of phosphoinositide phosphatase activity for MptpB. Previously, a partial characterization of MptpB detected tyrosine phosphatase activity [26], although lipid substrates were not tested in that study. The broad specificity towards phosphoinositides observed for MptpB could be important for in vivo targeting of a wide range of host phosphoinositides or as part of a more sophisticated pleiotropic effect coupling phosphoinositide turnover with protein-mediated cell-signalling events. The different enzymatic activities observed in vitro may not reflect true relative activities in vivo, where other factors may modulate the physiological function of MptpB. Hence, the biologically relevant substrates of MptpB in vivo will still need to be investigated and both protein and lipid substrates considered. The molecular factors responsible for controlling phophoinositide levels in mycobacterium-infected macrophages have not yet been fully defined, and from the evidence presented in the present study MptpB may play an important role. Future investigations will be directed at studying the potential targeting of MptpB to membrane phosphoinositides and to define the molecular basis for phosphoinositide binding. The newly identified enzymatic activity of MptpB opens new horizons to test its potential role in host phosphoinositide metabolism and phagosome maturation arrest, and provides exciting new directions for pharmacological intervention.
Acknowledgments
We thank Dr. J. Bella (Faculty of Life Sciences, University of Manchester, Manchester, U.K.), Professor V. Rodwell (Department of Biochemistry, Purdue University, West Lafayette, IN, U.S.A), Professor H. Charbonneau (Department of Biochemistry, Purdue University, West Lafayette, IN, U.S.A) and Dr J. Friesen (Illinois State University, Normal, IL, U.S.A.) for helpful suggestions on the manuscript, and the MRC (Medical Research Council) and STFC (Science and Technology Facilities Council) for a CASE award to N. B.
References
- 1.Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusner D. J. Mechanisms of mycobacterial persistence in tuberculosis. Clin. Immunol. 2005;114:239–247. doi: 10.1016/j.clim.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Hestvik A. L., Hmama Z., Av-Gay Y. Mycobacterial manipulation of the host cell. FEMS Microbiol. Rev. 2005;29:1041–1050. doi: 10.1016/j.femsre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Koul A., Herget T., Klebl B., Ullrich A. Interplay between mycobacteria and host signalling pathways. Nat. Rev. Microbiol. 2004;2:189–202. doi: 10.1038/nrmicro840. [DOI] [PubMed] [Google Scholar]
- 5.Fratti R. A., Backer J. M., Gruenberg J., Corvera S., Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilbi H. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell. Microbiol. 2006;8:1697–1706. doi: 10.1111/j.1462-5822.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 7.Guan K., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–539. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 8.Kaniga K., Uralil J., Bliska J. B., Galan J. E. A secreted tyrosine phosphatase with modular effector domains in bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 1996;21:633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 9.Castandet J., Prost J. F., Peyron P., Astarie-Dequeker C., Anes E., Cozzone A. J., Griffiths G., Maridonneau-Parini I. Tyrosine phosphatase MptpA of Mycobacterium tuberculosis inhibits phagocytosis and increases actin polymerization in macrophages. Res. Microbiol. 2005;156:1005–1013. doi: 10.1016/j.resmic.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Singh R., Rao V., Shakila H., Gupta R., Khera A., Dhar N., Singh A., Koul A., Singh Y., Naseema M., et al. Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol. Microbiol. 2003;50:751–762. doi: 10.1046/j.1365-2958.2003.03712.x. [DOI] [PubMed] [Google Scholar]
- 11.Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szoor B., Wilson J., McElhinney H., Tabernero L., Matthews K. R. Protein tyrosine phosphatase TbPTP1: a molecular switch controlling life cycle differentiation in trypanosomes. J. Cell Biol. 2006;175:293–303. doi: 10.1083/jcb.200605090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen J. N., Mortensen O. H., Peters G. H., Drake P. G., Iversen L. F., Olsen O. H., Jansen P. G., Andersen H. S., Tonks N. K., Moller N. P. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson M. D., Denu J. M. Molecular reactions of protein phosphatases-insights from structure and chemistry. Chem. Rev. 2001;101:2313–2340. doi: 10.1021/cr000247e. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Rossier C., Morris M. A., Scott H. S., Gos A., Bairoch A., Antonarakis S. E. A testis-specific gene, TPTE, encodes a putative transmembrane tyrosine phosphatase and maps to the pericentromeric region of human chromosomes 21 and 13, and to chromosomes 15, 22, and Y. Hum. Genet. 1999;105:399–409. doi: 10.1007/s004390051122. [DOI] [PubMed] [Google Scholar]
- 17.Zhao R., Qi Y., Zhao Z. J. FYVE-DSP1, a dual-specificity protein phosphatase containing an FYVE domain. Biochem. Biophys. Res. Commun. 2000;270:222–229. doi: 10.1006/bbrc.2000.2417. [DOI] [PubMed] [Google Scholar]
- 18.Zhao R., Qi Y., Chen J., Zhao J. Z. FYVE-DSP2, a FYVE domain-containing dual specificity protein phosphatase that dephosphorylates phosphotidylinositol 3-phosphate. Exp. Cell Res. 2001;265:329–338. doi: 10.1006/excr.2001.5185. [DOI] [PubMed] [Google Scholar]
- 19.Maehama T., Dixon J. E. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee J. O., Yang H., Georgescu M. M., Di Cristofano A., Maehama T., Shi Y., Dixon J. E., Pandolfi P., Pavletich N. P. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 21.Begley M. J., Taylor G. S., Kim S. A., Veine D. M., Dixon J. E., Stuckey J. A. Crystal structure of a phosphoinositide phosphatase, MTMR2: insights into myotubular myopathy and Charcoat-Marie-Tooth syndrome. Mol. Cell. 2003;12:1391–1402. doi: 10.1016/s1097-2765(03)00486-6. [DOI] [PubMed] [Google Scholar]
- 22.Begley M. J., Taylor G. S., Brock M. A., Ghosh P., Woods V. L., Dixon J. E. Molecular basis for substrate recognition by MTMR2, a myotubularin family phosphoinositide phosphatase. Proc. Natl. Acad. Sci. U.S.A. 2006;103:927–932. doi: 10.1073/pnas.0510006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor G. S., Maehama T., Dixon J. E. Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8910–8915. doi: 10.1073/pnas.160255697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savle P. S., Shelton T. E., Meadows C. C. A., Potts M., Gandour R. D., Kennelly P. J. N-(Cyclohexanecarboxyl)-O-phospho-L-serine, a minimal substrate for the dual-specificity protein phosphatase IphP. Arch. Biochem. Biophys. 2000;376:439–448. doi: 10.1006/abbi.2000.1750. [DOI] [PubMed] [Google Scholar]
- 25.Grundner C., Ng H. L., Alber T. Mycobacterium tuberculosis protein tyrosine phosphatase PtpB structure reveals a diverged fold and a buried active site. Structure. 2005;13:1625–1634. doi: 10.1016/j.str.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Koul A., Choidas A., Treder M., Tyagi A. K., Drlica K., Singh Y., Ullrich A. Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. J. Bacteriol. 2000;182:5425–5432. doi: 10.1128/jb.182.19.5425-5432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howell L. D., Griffiths C., Slade L. W., Potts M., Kennelly P. J. Substrate specificity of IphP, a cyanobacterial dual-specificity protein phosphatase with MAP kinase phosphatase activity. Biochemistry. 1996;35:7566–7572. doi: 10.1021/bi9600409. [DOI] [PubMed] [Google Scholar]