Abstract
Av3 is a short peptide toxin from the sea anemone Anemonia viridis shown to be active on crustaceans and inactive on mammals. It inhibits inactivation of Navs (voltage-gated Na+ channels) like the structurally dissimilar scorpion α-toxins and type I sea anemone toxins that bind to receptor site-3. To examine the potency and mode of interaction of Av3 with insect Navs, we established a system for its expression, mutagenized it throughout, and analysed it in toxicity, binding and electrophysiological assays. The recombinant Av3 was found to be highly toxic to blowfly larvae (ED50=2.65±0.46 pmol/100 mg), to compete well with the site-3 toxin LqhαIT (from the scorpion Leiurus quinquestriatus) on binding to cockroach neuronal membranes (Ki=21.4±7.1 nM), and to inhibit the inactivation of Drosophila melanogaster channel, DmNav1, but not that of mammalian Navs expressed in Xenopus oocytes. Moreover, like other site-3 toxins, the activity of Av3 was synergically enhanced by ligands of receptor site-4 (e.g. scorpion β-toxins). The bioactive surface of Av3 was found to consist mainly of aromatic residues and did not resemble any of the bioactive surfaces of other site-3 toxins. These analyses have portrayed a toxin that might interact with receptor site-3 in a different fashion compared with other ligands of this site. This assumption was corroborated by a D1701R mutation in DmNav1, which has been shown to abolish the activity of all other site-3 ligands, except Av3. All in all, the present study provides further evidence for the heterogeneity of receptor site-3, and raises Av3 as a unique model for design of selective anti-insect compounds.
Keywords: Av3 toxin, insecticidal toxin, receptor site-3, sea anemone, voltage-gated sodium channel (Nav)
Abbreviations: ApB, anthopleurin B; FTIR, Fourier-transform infrared; Nav, voltage-gated Na+ channel; DmNav, Drosophila melanogaster Nav; hNav, human Nav; rNav, rat Nav
INTRODUCTION
Navs (voltage-gated Na+ channels) are targeted by a large variety of toxins because of their critical role in generation and propagation of electrical signals in excitable tissues [1]. They are composed of a pore-forming α-subunit (∼260 kDa) associated with one or two β-subunits. The α-subunit consists of four domains (D1–D4), each made of six transmembrane segments (S1–S6) connected by intra- and extra-cellular loops [1]. A key feature of Navs is their ability to rapidly activate and inactivate upon cell membrane depolarization, leading to a transient increase in Na+ conductance [2]. The fast inactivation process is inhibited by a large variety of toxins from sea anemones, scorpions, spiders and marine snails, resulting in prolongation of the Na+ current [3]. Despite the vast differences in primary and tertiary structures, type I sea anemone toxins, scorpion α-toxins and spider δ-atracotoxins compete for binding to receptor site-3 on Navs [3–5]. Receptor site-3 has not yet been characterized, although substitutions at the short extracellular loop D4/S3-S4, particularly of a conserved negatively-charged residue (Glu-1613 in the rat brain channel rNav1.2, Asp-1428 in the rat skeletal muscle channel rNav1.4, and Asp-1612 in the rat cardiac muscle channel rNav1.5), have been shown to strongly affect the activity of site-3 toxins [6–8]. Furthermore, Asp-1612 of rNav1.5 has been proposed to interact with Lys-37 of the type I sea anemone toxin ApB (anthopleurin B) of Anthopleura xanthogrammica [7].
To date, three toxins that bind to receptor site-3 have been suggested to be insect-specific: the type I sea anemone toxin BgII of Bunodosoma granulifera [9], the scorpion α-toxin BjαIT of Hottentotta judaica [10] and the Tx4 (6-1) toxin from the spider Phoneutria nigriventer [11]. Although BgII was reported to selectively inhibit the inactivation of insect Navs [9], its high activity in the brain (0.78 nmol/kg of mouse), ability to compete well with scorpion α-toxins on binding to rat brain synaptosomes (Ki=9 nM) [12] and substantial activity in dorsal root ganglia of rats [13] raise concerns as to its selectivity. BjαIT has been reported to be toxic to insects and is highly active at the Drosophila melanogaster channel DmNav1 expressed in Xenopus laevis oocytes [10]. Yet, out of the nine mammalian Nav subtypes, it was only assayed on rNav1.2 and since it was injected intraperitoneally to mice in low concentrations, its lack of toxicity to mammals still needs to be confirmed [14]. Tx4 (6-1) was found to be active in house flies and inactive when injected directly into mouse brain or when assayed on rNav1.2 and rNav1.4 [11]. As this toxin exhibited weak activity at cockroach axons compared with scorpion α-toxins and, since it was not analysed by subcutaneous or intraperitoneal injections to mammals, its specificity for insects remains to be clarified.
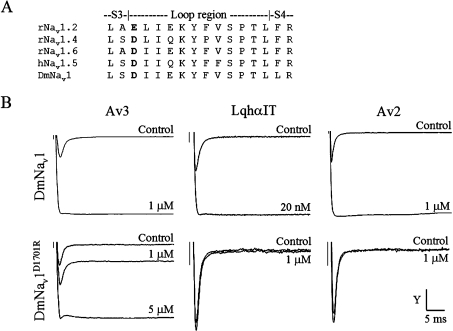
In addition to type I toxins, two more groups of toxins that inhibit inactivation of Na+ channels, type II and type III, have been described in sea anemones, yet their binding sites were not thoroughly studied [15,16]. The small (27 amino acids; Figure 1A) type III sea anemone toxin Av3 (previously named ATX III) from Anemonia viridis (previously named Anemonia sulcata) is structurally unique in that it is composed of only turn-based secondary-structure elements reticulated by three disulfide bonds, and lacks α-helices and β-strands (Figure 1A) [16,17]. This toxin was highly active on crustaceans and was inactive to mice [18,19], which raised the possibility of it also being highly active on other arthropods, such as insects. To investigate the insecticidal potential of Av3 and its binding site on the Nav, we established an expression system that enabled molecular dissection followed by thorough analyses of bioactivity. Toxicity, binding and electrophysiological assays of the recombinant toxin revealed that Av3 is a selective anti-insect site-3 toxin. Although it exhibits synergistic effects with site-4 ligands (such as scorpion β-toxins) as other site-3 toxins do [20,21], its binding to the insect receptor site was not affected by a D1701R substitution in D4/S3-S4 of the insect channel, which was shown to affect the binding and activity of all other site-3 toxins.
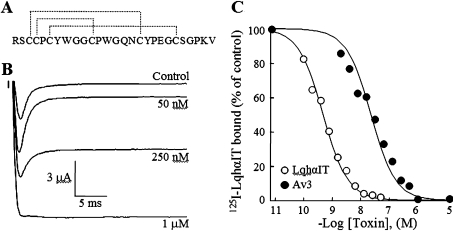
Figure 1. Characterization of the recombinant Av3.
(A) Amino acid sequence and disulfide bonds (broken lines) of Av3 [22]. (B) Effect of the recombinant Av3 on DmNav1 inactivation. Oocytes expressing DmNav1/TipE were clamped at −80 mV, and currents were elicited by step depolarization to −10 mV in the absence (control) and presence of the indicated recombinant Av3 concentrations. (C) Competition of the recombinant Av3 with 125I-LqhαIT on binding to cockroach neuronal membranes. The membranes (7 μg/ml) were incubated for 60 min at 22°C in the presence of 0.1 nM 125I-LqhαIT and increasing concentrations of toxin. Non-specific binding determined in the presence of 1 μM LqhαIT was subtracted.
MATERIALS AND METHODS
Bacterial strains, animals and purification of native Av3
Escherichia coli DH5α cells were used for plasmid expression, and the Rosettagami strain (DE3, pLys; Novagen) was employed for toxin expression in fusion with thioredoxin using the vector pET-32b (Novagen). Sarcophaga falculata blowfly larvae were bred in the laboratory. ICR white mice were purchased from Tel Aviv University. Native Av3 was purified using a Resource® 3 ml column (GE Healthcare) on an AKTA® Basic machine (GE Healthcare) from a venom fraction kindly provided by Professor L. Beress (Institute of Toxicology, University of Kiel, Kiel, Germany).
Plasmid and gene construction
A DNA sequence encoding an S tag, a thrombin-cleavage site, a His6 tag and an enterokinase cleavage site was cleaved out from pET-32b vector using MscI and NcoI and was replaced by a sequence encoding a His6 tag fused to a thrombin-cleavage site derived from pET-14b with an NcoI site downstream. A synthetic gene encoding Av3 was constructed from four overlapping oligonucleotide primers designed according to the published amino acid sequence [22] in a similar method described for the toxin Av2 [23]: 5′-TTCATATGCGATCGTGCTGCCCTTGCTATTGGGGTGGTTGCCCTTGGGGTCAGAAC-3′, 5′-TGCTATCCTGAAGGTTGCAGCGGTCCTAAGGTATAAGGATCC-3′, 5′-GGCAACCACCCCAATAGCAAGGGCAGCACGATCGCATATG-3′ and 5′-TTGGATCCTTATACCTTAGGACCGCTGCAACCTTCAGGATAGCAGTTCTGACCCCAAG-3′. The ligation mixture (1 μM) was used for PCR amplification of the Av3 gene using 5′-TTTTCCATGGCGCGCTCGTGCTGCCC-3′ and 5′-TT-GGATCCTTACTGCTTGCAGC-3′ (NcoI and BamHI restriction sites underlined respectively). After cleavage with NcoI and BamHI, the gene was ligated at the corresponding sites of the pET-32b derivative.
Expression and purification of recombinant Av3
E. coli Rosettagami cells were transformed with the final expression vector. Cells were grown on Luria broth at 37°C in the presence of 50 μg/ml ampicillin up to a D600 of 0.6 before IPTG (isopropyl β-D-thiogalactoside) was added to a final concentration of 0.5 mM and the temperature was lowered to 30°C. The cells were grown for additional 4 h, harvested by centrifugation at 5500 g for 6 min and frozen at −20°C. The cells were then suspended in binding buffer (PBS with 20 mM imidazole, pH 9.4) in the presence of a protease inhibitor cocktail (catalogue no. P2714, Sigma) and heated for 10 min at 80°C to denature cell proteins other than the thermally stable thioredoxin-tagged protein [24]. Then they were broken by sonication, centrifuged at 16000 g for 45 min, and the soluble fusion protein was purified on a HisTrap® column (GE Healthcare) connected to the AKTA® Basic machine. The column was washed by 15 column vol. of binding buffer, and the thioredoxin–Av3 fusion protein was eluted with a mixture of 60% binding buffer and 40% elution buffer (PBS with 500 mM imidazole, pH 9.4). The pH of the eluted fraction was adjusted to 8.0 by ethanoic (acetic) acid diluted in PBS, and thrombin (Sigma) was added to remove the thioredoxin tag, thus providing the final recombinant product bearing an N-terminal extension of Gly-Ser-Ser-Met-Ala. After an overnight incubation at 24°C and centrifugation at 16000 g for 30 min, the protein mixture was loaded on to a Resource® 3 ml column, and the recombinant Av3 was eluted with 27.5% acetonitrile (with 0.1% trifluoroacetic acid) in a reverse-phase HPLC linear gradient of 25–30% acetonitrile (in 1.5 ml/min over 5 column vol.). The recombinant Av3 constituted a major peak eluted with an average yield of 3 mg/l of E. coli culture.
Mutagenesis of Av3 and DmNav1
Mutations in Av3 were introduced via PCR using complementary oligonucleotide primers and the vector bearing the Av3 gene as the DNA template. All toxin mutants were produced in a similar fashion to the unmodified toxin. A ∼3 kb ApaI/NotI fragment of the gene encoding DmNav1 was cloned into pBluescript KS (Stratagene) and was used as a template for PCR-driven mutagenesis. The PCR product was digested with NcoI and HindIII and ligated into the corresponding restriction sites of the vector bearing the DmNav1 fragment. The sequence was verified, and the mutated gene fragment was subcloned into pAlter-DmNav1 (kindly provided by Dr J.W. Warmke, Merck, Whitehouse Station, NJ, U.S.A.) which was used for transcribing cRNA.
Production of additional toxins
The toxins LqhαIT (of the scorpion Leiurus quinquestriatus hebraeus), Bj-xtrIT (of the scorpion H. judaica) and its mutant E15R, and Av2 (of the sea anemone A. viridis) were produced in recombinant forms as described in [23,25,26].
Mass spectrometry
The mass of the recombinant Av3 (after thrombin cleavage) was determined at the Maiman Proteome Research Institute, Tel-Aviv University, using a Voyager DE-STR MALDI–TOF (matrix-assisted laser-desorption ionization–time of flight) MS system (Applied Biosystems).
Toxicity assays
Four-day-old blowfly larvae (body mass 150±20 mg) were injected inter-segmentally. A positive result was scored when immobilization and contraction were observed after 10 min. Five concentrations of each toxin were injected into larvae (nine larvae in each group) in three independent experiments. ED50 (50% effective dose) values were calculated according to the sampling and estimation method of Reed and Muench [27]. Av3 in 0.9% NaCl was injected subcutaneously to female ICR mice (body mass 20±3 g), which were monitored for 24 h.
Competition binding experiments
Neuronal membranes were prepared from heads of adult Periplaneta americana cockroaches [28]. Membrane protein concentration was determined by a Bio-Rad protein assay, using BSA as standard. Radio-iodinated LqhαIT was prepared by lactoperoxidase (Sigma) using 10 μg of toxin and 0.5 mCi of carrier-free Na125I (GE Healthcare) following a published protocol [29]. The mono-iodotoxin was purified and its concentration was determined as described previously [23,28]. The composition of media used in the binding assays and termination of the reactions has been described previously [28]. Non-specific toxin binding was determined in the presence of excess (1 μM) unlabelled toxin. Competition binding assays were performed and data were analysed as described in [23].
CD spectroscopy
CD spectra were recorded at 25°C using a model 202 CD spectrometer (Aviv Instruments). Toxins (150 μM) were dissolved in 10 mM sodium phosphate buffer, pH 7.0, and their spectra were measured using a quartz cell of 0.1-mm light path. Each spectrum was measured three times, averaged, and a blank spectrum of the buffer run under similar conditions was subtracted.
FTIR (Fourier-transform infrared) spectroscopy
IR spectra were recorded using a Nexus 470 FTIR spectrometer (Thermo Fisher Scientific). The toxins were dissolved in 2H2O to a concentration of 4 mg/ml and desiccated on a disposable ST-IR card made of polytetrafluoroethylene (Thermo Fisher Scientific). The recordings were made in 4 cm−1 resolution and were averaged from 2000 scans. The absorbance was determined by the OMNIC 6.0a analysis program (Thermo Fisher Scientific).
Electrophysiological assays
The pNa200 vectors bearing the genes encoding for the neuronal channels rNav1.2a and rNav1.6 were a gift from Dr A. Goldin (Department of Anatomy and Neurobiology, University of California, Irvine, CA, U.S.A.). The pAlter vectors bearing the cDNAs encoding rNav1.4 and hNav1.5 (human Na+ channel) were a gift from Dr R. G. Kallen (Department of Biochemistry and Biophysics, University of Pennsylvania, Philadelphia, PA, U.S.A.). cRNAs encoding rNav1.2, rNav1.4, hNav1.5, rNav1.6 and DmNav1 Na+ channel α-subunits and the auxiliary subunits β1 and TipE were transcribed in vitro using T7 RNA-polymerase and the mMESSAGE mMACHINE® system (Ambion) and injected into X. laevis oocytes as described in [30]. The molar ratios for α/β subunits injected were 1:5 and 1:1 for the mammalian and insect channels respectively. Currents were measured 1–4 days after injection, and data were acquired as described previously [23]. Currents were elicited by depolarization to −10 mV from a holding potential of −80 mV in the presence of several toxin concentrations. At each toxin concentration, currents were allowed to reach a steady-state level before the final measurement.
Three-dimensional models
The three-dimensional models were drawn using DeepView/PDB viewer (version 3.7 by GlaxoSmithKline) and were rendered by PovRay™ (version 3.6 by Persistence of Vision Raytracer). The Av3 and LqhαIT NMR-based structures are available at the Protein Data Bank (http://www.pdb.org) under codes 1ANS and 1LQI respectively. The Av2 modelling has been described previously [23]. The size of the bioactive surface of Av3 was calculated in the DeepView/PDB viewer and compared with that of LqhαIT.
RESULTS
Characterization of recombinant Av3
Recombinant Av3 was characterized by several criteria. The primary structure bearing an N-terminal extension (Gly-Ser-Ser-Met-Ala) obtained after cleavage by thrombin of the fusion polypeptide produced in E. coli was confirmed by MS and amino acid analysis (results not shown). Toxicity assays on blowfly larvae revealed contraction paralysis at a potency comparable with that of the scorpion α-toxin LqhαIT and the type I sea anemone toxin Av2 [23,25]. Similarly to Av2 and unlike LqhαIT, the contraction caused by Av3 at ED50 values was irreversible. The toxicity of the recombinant Av3 to insects was akin to that of the native toxin (ED50=2.65±0.46 compared with 1.15±0.02 pmol/100 mg of larvae respectively). Unlike Av2 and LqhαIT, and in accordance with previous reports [19], the recombinant Av3 was inactive when injected subcutaneously at a high dose into mice (up to 5940 nmol/kg of mouse).
We examined further the mode of Av3 action on the DmNav1 expressed in X. laevis oocytes. Av3 increased the peak Na+ current and inhibited the inactivation of the channel. A complete removal of inactivation was observed when 1 μM toxin was applied (Figure 1B). These effects were similar to those described previously for Av2 [23,31] and LqhαIT [14] on DmNav1 and for Av3 on lobster neurons [32], suggesting that Av3 is a site-3 toxin. In order to substantiate this finding, the recombinant Av3 was examined in competition binding assays against LqhαIT, a known marker of receptor site-3 on insect Navs [33]. Av3 competed well with LqhαIT on binding to a cockroach (P. americana) neuronal membrane preparation (Ki=21.4±7.1 nM), which established its classification as a site-3 toxin (Figure 1C). Av3 was analysed further on four mammalian Nav subtypes: rNav1.2, rNav1.4, hNav1.5 and rNav1.6. As shown in Figure 2, 10 μM toxin had no effect on rNav1.2, rNav1.4 or rNav1.6, and had a negligible effect on hNav1.5. A strong inhibition of Na+ current inactivation was obtained in the same oocyte expressing hNav1.5 upon further application of 1 μM Av2 (positive control; [23]). These results demonstrate that, in contrast with other site-3 toxins, Av3 clearly differentiates between insect and mammalian Navs.
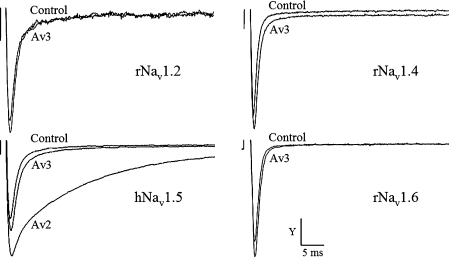
Figure 2. Analysis of the recombinant Av3 activity on mammalian Navs expressed in Xenopus oocytes.
Oocytes expressing rNav1.2a, rNav1.4, hNav1.5 and rNav1.6 were clamped at −80 mV, and currents were elicited by step depolarization to −10 mV. Av3 was applied at a concentration of 10 μM, and a minor effect was observed only in the cardiac channel. To examine the extent of inhibition of inactivation this channel undergoes in the presence of a cardio-active site-3 toxin [3,23], 1 μM Av2 was applied after 20 min when the maximal effect of Av3 was reached. The strong inhibitory effect on channel inactivation induced by Av2 emphasized the negligible effect of Av3 at the cardiac channel. The y-axis scale bar is 400 nA for rNav1.2, 500 nA for rNav1.4, 1.6 μA for hNav1.5 and 1.1 μA for rNav1.6.
Synergism of sea anemone toxins with site-4 toxins
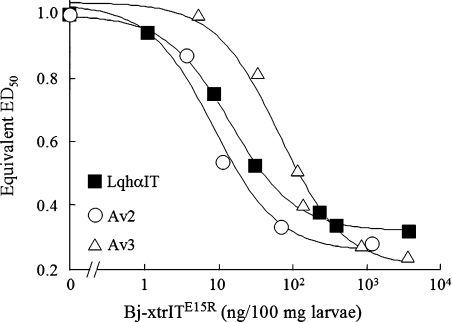
To examine further the similarities in activity between Av3 and other receptor site-3 toxins, we analysed whether Av3 induces synergistic effects with site-4 scorpion β-toxins, as was shown recently for the site-3 toxin LqhαIT [20]. A mixture of Av3 and the insect specific site-4 toxin Bj-xtrIT (1:1 molar ratio) reduced significantly the ED50 obtained for each of the toxins (Av3 ED50=2.65±0.46, Bj-xtrIT ED50=2.1±0.09, mixture ED50=0.2±0.07; all in pmol/100 mg of larvae). To discern a synergistic effect resulting from mere binding from that generated by the joint toxin activities [20], we injected into blowfly larvae Av3 mixed with Bj-xtrITE15R, a mutant that lost its toxicity, but retained high binding affinity for insect neuronal membranes [34]. Indeed, a synergistic effect that lowered the ED50 of Av3 3.7-fold was obtained (Figure 3). A similar synergistic effect was observed when Av2 was co-injected with Bj-xtrIT (results not shown) or its E15R non-toxic mutant (Figure 3). These results demonstrate that the synergism with site-4 ligands is a common trait of receptor site-3 toxins.
Figure 3. The toxicity of site-3 toxins is enhanced by Bj-xtrITE15R.
Dose–response curves for the toxicity in blowfly larvae of the site-3 toxins LqhαIT, Av2 and Av3 in the presence of increasing concentrations of the non-toxic site-4 ligand Bj-xtrITE15R. Each data point represents the ED50 equivalent of the site-3 toxin in the presence of the indicated dose of Bj-xtrITE15R.
Mutagenic dissection of Av3
Although the structure of Av3 differs from those of other site-3 toxins, the possibility that a subset of bioactive residues on their surfaces would be similar and explain their binding to a similar receptor site was examined. Sixteen residues of Av3, not including glycine, which participate in β- and γ-turns as well as chain reversals [17], and cysteine, which participate in disulfide bridging, were mutated to alanine. The mutants, produced according to a similar protocol described for the unmodified toxin, were assayed by injection into blowfly larvae, by competition binding studies using cockroach neuronal membranes and by CD spectral analysis.
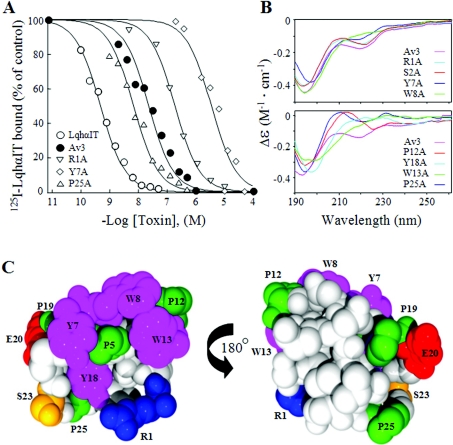
In general, the results of the binding assays were in good correlation with those obtained for the toxicity assays (Table 1). Substitutions R1A, P5A and P19A decreased the toxin affinity 5–10-fold, whereas the effects of Y7A, W8A, P12A, W13A and Y18A on toxin affinity were highly detrimental (over 80-fold in binding and above 9-fold in toxicity). Substitutions E20A, S23A and P25A surprisingly improved the toxin affinity (Table 1 and Figure 4A). To clarify whether these changes in activity were related to putative alterations in structure, the mutant toxins were analysed by CD spectroscopy. The CD pattern of P12A, W13A, Y18A and P19A altered in comparison with that of the unmodified recombinant Av3, while substitutions Y7A and W8A had only a minor effect (Figure 4B). Since Av3 lacks common secondary structure motifs such as α-helices and β-strands that contribute heavily to CD spectra of proteins [35], its CD pattern, which relies mainly on turn-based motifs, may be very sensitive to substitutions. Therefore we also analysed all mutants by FTIR spectroscopy and found a highly similar absorbance pattern with a major band at 1659 cm−1 (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/406/bj4060041add.htm) that may be attributed to β-turns [35].
Table 1. Effects of mutations in Av3 on the binding affinity to cockroach neuronal membranes and on the toxicity to blowfly larvae.
The change in affinity of each mutant was obtained in competition with 125I-LqhαIT scorpion toxin on binding to cockroach neuronal membranes, and is represented by the Ki ratio (Kimut/Kiwt; Kiwt is 21.4±7.1 nM, n=3). Ki determination is described in the Materials and methods section and in Figure 4(A). Toxicity changes are represented as the ED50 ratio (ED50mut/ED50wt; ED50wt is 2.65±0.46 pmol/100 mg of larvae). ED50 determination is described in the Materials and methods section. rAv3 designates the recombinant toxin cleaved by thrombin.
| Toxin | Ki ratio | ED50 ratio |
|---|---|---|
| rAv3 | 1 | 1 |
| R1A | 5.9 | 5.4 |
| S2A | 0.85 | 2.5 |
| P5A | 8 | 11.6 |
| Y7A | >100 | 20 |
| W8A | 82 | 9.6 |
| P12A | >100 | 12.8 |
| W13A | >100 | 10.3 |
| Q15A | 1.3 | 3.4 |
| N16A | 3.7 | 1.3 |
| Y18A | >100 | 39 |
| P19A | 7.3 | 5.3 |
| E20A | 0.5 | 0.9 |
| S23A | 0.5 | 0.6 |
| P25A | 0.3 | 0.5 |
| K26A | 1.8 | 3.2 |
| V27A | 3.2 | 1.4 |
Figure 4. Effects of substitutions on the binding affinity and CD spectrum portray the bioactive surface of Av3.
(A) Competition curves of Av3 and its mutants with 125I-LqhαIT on binding to cockroach neuronal membranes. Membranes (7 μg/ml) were incubated at 22°C for 60 min with 0.1 nM 125I-LqhαIT and increasing concentrations of the various mutants. Non-specific binding, determined in the presence of 1 μM LqhαIT, was subtracted. The Ki values are Av3, 21.4±7.1 nM; R1A, 127±38 nM; Y7A, 6081±3703 nM; and P25A, 5.8±1.5 nM. (B) Far-UV CD spectra of recombinant Av3 and representative mutants. (C) Space-filled presentation of the Av3 structure based on PDB code 1ANS [17]. Residues whose substitution affected the binding affinity and activity of the toxin are coloured as follows: amino acids carrying aromatic side chains are magenta; those with aliphatic side chains are green; those with polar side chains are orange; positively charged residues are blue; and negatively charged residues are red.
The effect of a D1701R substitution in DmNav1 on the activity of site-3 toxins
The ability of Av3 to bind to receptor site-3 despite its different structure and bioactive surface from those of type I sea anemone toxins and scorpion α-toxins is intriguing and raises questions about its mode of interaction with the Nav. It was shown that the interactions of four scorpion α-toxins [6,8] as well as two type I sea anemone toxins [6,7] were affected by substituting for a conserved negatively charged residue in D4/S3-S4 (Asp-1428 in rNav1.4, Asp-1612 in rNav1.5 and Glu-1613 in rNav1.2; Figure 5A). Therefore we compared the effect of substituting for the equivalent residue in the insect channel DmNav1 (D1701R) on the activity of the three site-3 toxins Av3, Av2 and LqhαIT. Unexpectedly, while LqhαIT and Av2 lost their activity on DmNav1D1701R, the activity of Av3 decreased only 5-fold (Figure 5B).
Figure 5. Effects of Av3, LqhαιT and Av2 on DmNav1 and DmNav1D1701R.
(A) Sequence alignment of the S3-S4 extracellular loop of domain 4 in various Navs. rNav1.2 and rNav1.6, rat neuronal Na+ channel subtypes; rNav1.4, rat skeletal muscle Na+ channel; hNav1.5, human cardiac muscle channel; DmNav1, Drosophila melanogaster Na+ channel. The conserved negatively charged residue, whose substitution affects the activity of site-3 toxins is indicated in bold. (B) Xenopus oocytes expressing DmNav1 or DmNav1D1701R were clamped at −80 mV, and currents were elicited by step depolarization to −10 mV. The effects on inactivation were monitored on DmNav1 (upper panels) in the presence of 1 μM Av3 (y-axis scale bar=3 μA), 20 nM LqhαIT (y-axis scale bar=450 nA) or 1 μM Av2 (y-axis scale bar=3 μA). The effects on DmNav1D1701R (lower panels) were measured in the presence of 1 or 5 μM Av3 (y-axis scale bar=1.3 μA), 1 μM LqhαIT (y-axis scale bar=400 nA) or 1 μM Av2 (y-axis scale bar=400 nA).
DISCUSSION
The reported preference of Av3 for crustaceans [19], which are phylogenetically closely related to insects, and its ability to inhibit inactivation in lobster neurons [32], has raised the possibility that it is a site-3 toxin that would be highly active on insects. We were attracted by these pharmacological features, along with the small size (27 residues) and unique structure [17], to question further its selectivity and interaction with the Nav.
The toxicity, binding and electrophysiological assays have indicated that the recombinant Av3 is highly active on insect Navs (Figure 1) and may suggest that the Av3 receptor site of insect and crab Navs are similar. The lack of activity of Av3 on mammalian channels (Figure 2) fits well with the previously reported inactivity observed when the native toxin was injected intraperitoneally into mice [19]. Although very weak activity of Av3 on hNav1.5 was observed in high concentrations (Figure 2), the insensitivity of mice to high doses of the toxin (see the Results section) suggests that Av3 does not act on the cardiac muscle at physiologically relevant doses. It is noteworthy that the native Av3 was active at high concentrations (8.9 nmol/kg of mouse) when injected directly into the mammalian brain [36], and at very high concentrations was able to compete with anti-mammalian site-3 toxins on binding to rat brain synaptomsomes (Ki>100 μM) [19]. Still, our assays on two Navs that are common in the brain (rNav1.2 and rNav1.6) have shown that Av3 was practically inactive (Figure 2). Finally, the strong synergism exhibited by Av3 with the specific site-4 anti-insect toxin Bj-xtrIT has promise for developing new strategies in insect pest control. All of the above reasons establish Av3 as an attractive model for design of selective anti-insect insecticides targeted to receptor site-3.
The residues important for Av3 function are clustered on one hemisphere of the molecule surface (Figure 4C) and include the highly flexible Arg-1, a patch of hydrophobic residues, Pro-5, Tyr-7, Trp-8, Pro-12 and Trp-13, as well as Tyr-18 located in a cavity of the toxin molecule and only partially exposed to the solvent (Figure 4C) [17]. While the R1A substitution had no effect on the CD spectrum compared with the unmodified Av3, Y7A and W8A caused some minor alterations (Figure 4B) that may be explained by the location of these residues at the very heart of a distorted type-I β-turn, which contributes to the CD spectrum [37]. The other substitutions that affected the toxin binding affinity had a greater impact on the CD spectrum (Figure 4B), which could arise from structural perturbations. Since analysis of putative perturbations in structure by FTIR did not indicate significant alterations (Supplementary Figure 1), we suggest that the changes in Av3 activity as a result of amino acid substitutions may be related to the functional role of these residues in the interaction with the channel.
Substitutions E20A, S23A and P25A surprisingly improved the toxin affinity, suggesting that these residues interfere in some way with the binding process to the insect Nav receptor site. The identification of such residues demonstrates that improvement in the insecticidal potency of naturally occurring substances is feasible.
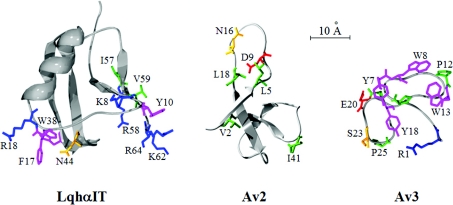
The bioactive surface of Av3 is much more condensed than those of the type I sea anemone toxins ApB [3] and Av2 [23]. While the bioactive surface of Av2 lacks aromatic residues, the bioactive surface of Av3 heavily depends on them (Figure 6). Moreover, some of the residues most critical for Av2 activity appear on the flexible Arg-14 loop [3,23], whereas such flexibility was not observed in Av3 [17]. The bioactive surface of Av3 [245 Å2 (1 Å=0.1 nm)] is considerably smaller than those of scorpion α-toxins (e.g. 430 Å2 for LqhαIT), whose bioactive surfaces are divided into two major amino acid clusters (Figure 6) [14,38]. These prominent differences imply that the three toxin types interact differently with receptor site-3 on DmNav1, providing further support for the suggestion that this site is in fact a macrosite [39], and highlight the advantage of Av3 as a probe for studying the interaction of a site-3 toxin with the insect Na+ channel.
Figure 6. Comparison of the bioactive surfaces of toxins that bind receptor site-3.
The residues of the bioactive surfaces toward insects of the scorpion α-toxin LqhαIT (based on [25,40]), the type I sea anemone toxin Av2 (based on [23]) and the type III sea anemone toxin Av3 (the present study) are presented as ‘sticks’ on a Cα ribbon structure. The PDB codes for the LqhαIT and Av3 structures are 1LQI [41] and 1ANS [17] respectively. The residues are coloured as described in Figure 4(C).
The ability of Av3 to displace LqhαIT in competition binding assays on insect neuronal membranes classifies it as a site-3 toxin. Thus Av3 joins a growing family of peptide toxins from sea anemones, scorpions and spiders that appeared via convergent evolution to recognize receptor site-3 and inhibit the inactivation process of the Nav [3]. The classification of Av3 as site-3 toxin is corroborated further by the synergistic effects that it exhibits with site-4 ligands, as was demonstrated recently with other toxins targeting this site [20], yet it differs markedly from all other site-3 toxins in that its activity was hardly affected by the D1701R mutation in DmNav1 (Figure 5B). To date, the only channel substitution that affected the activity of all site-3 toxins was assigned to this position on D4/S3-S4 (Figure 5A) [6–8]. Even conservative substitutions of this residue in rNav1.4 (D1428E) strongly affected the action of two scorpion α-toxins [8], and charge-inversion of this residue (E1613R in rNav1.2 and D1612R in rNav1.5) almost abolished the binding of both type I sea anemone toxins and scorpion α-toxins [6,7]. In the present study, we show that charge-inversion of the equivalent residue in the insect Nav indeed diminished the activity of LqhαIT and Av2, but had only little effect on Av3 activity (Figure 5B). This difference implies that despite its ability to compete in binding, Av3 interacts with receptor site-3 differently from the other toxins that recognize this site.
In summary, Av3 is a site-3 toxin and is synergistic with site-4 ligands. Yet, its bioactive surface differs markedly from those of other site-3 toxins, and its activity on DmNav1 is hardly affected by the D1701R mutation at D4/S3-S4. Therefore, despite the commonality among site-3 toxins, their ability to interact in various ways with the Nav illuminates a heterogeneous receptor. In addition, the smaller bioactive surface of Av3 and its selectivity to insect Navs compared with other site-3 toxins offers a convenient model for studying how to design selective anti-insect compounds.
Online data
Acknowledgments
We thank Professor E. Gazit and Dr I. Cherny, Tel Aviv University, for their help with the FTIR measurements, and Professor L. Beress, University of Kiel, for his gift of native toxins. This research was supported by grants from the United States-Israel Binational Agricultural Research and Development IS-3928-06 (M. G. and D. G.) and IS-4066-07 (D. G. and M. G.); by the Israeli Science Foundation grants 909/04 (M. G.) and 1008/05 (D. G.); and by the European Community Integrated Project LSH-2005-1.2.5-2 proposal No. 037592 - CONCO (M. G. and D. G.).
References
- 1.Catterall W. A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 2.Ulbricht W. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 2005;85:1271–1301. doi: 10.1152/physrev.00024.2004. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal K. M., Seibert A. L. Voltage-gated sodium channel toxins: poison, probes, and future promise. Cell. Biochem. Biophys. 2003;38:215–238. doi: 10.1385/CBB:38:2:215. [DOI] [PubMed] [Google Scholar]
- 4.Catterall W. A., Beress L. Sea anemone toxin and scorpion toxin share a common receptor site associated with the action potential sodium ionophore. J. Biol. Chem. 1978;253:7393–7396. [PubMed] [Google Scholar]
- 5.Little M. J., Zappia C., Gilles N., Connor M., Tyler M. I., Martin-Eauclaire M. F., Gordon D., Nicholson G. M. δ-Atracotoxins from Australian funnel-web spiders compete with scorpion α-toxin binding but differentially modulate alkaloid toxin activation of voltage-gated sodium channels. J. Biol. Chem. 1998;273:27076–27083. doi: 10.1074/jbc.273.42.27076. [DOI] [PubMed] [Google Scholar]
- 6.Rogers J. C., Qu Y., Tanada T. N., Scheuer T., Catterall W. A. Molecular determinants of high affinity binding of α-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel α subunit. J. Biol. Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- 7.Benzinger G. R., Kyle J. W., Blumenthal K. M., Hanck D. A. A specific interaction between the cardiac sodium channel and site-3 toxin anthopleurin B. J. Biol. Chem. 1998;273:80–84. doi: 10.1074/jbc.273.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Leipold E., Lu S., Gordon D., Hansel A., Heinemann S. H. Combinatorial interaction of scorpion toxins Lqh-2, Lqh-3, and LqhαIT with sodium channel receptor sites-3. Mol. Pharmacol. 2004;65:685–691. doi: 10.1124/mol.65.3.685. [DOI] [PubMed] [Google Scholar]
- 9.Bosmans F., Aneiros A., Tytgat J. The sea anemone Bunodosoma granulifera contains surprisingly efficacious and potent insect-selective toxins. FEBS Lett. 2002;532:131–134. doi: 10.1016/s0014-5793(02)03653-0. [DOI] [PubMed] [Google Scholar]
- 10.Arnon T., Potikha T., Sher D., Elazar M., Mao W., Tal T., Bosmans F., Tytgat J., Ben-Arie N., Zlotkin E. BjαIT: a novel scorpion α-toxin selective for insects: unique pharmacological tool. Insect Biochem. Mol. Biol. 2005;35:187–195. doi: 10.1016/j.ibmb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.de Lima M. E., Stankiewicz M., Hamon A., de Figueiredo S. G., Cordeiro M. N., Diniz C. R., Martin-Eauclaire M.-F., Pelhate M. The toxin Tx4(6-1) from the spider Phoneutria nigriventer slows down Na+ current inactivation in insect CNS via binding to receptor site 3. J. Insect Physiol. 2002;48:53–61. doi: 10.1016/s0022-1910(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 12.Loret E. P., Menendez R., Mansuelle P., Sampieri F., Rochat H. Positively charged amino acid residues located similarly in sea anemone and scorpion toxins. J. Biol. Chem. 1994;269:16785–16788. [PubMed] [Google Scholar]
- 13.Salceda E., Garateix A., Soto E. The sea anemone toxins BgII and BgIII prolong the inactivation time course of the tetrodotoxin-sensitive sodium current in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2002;303:1067–1074. doi: 10.1124/jpet.102.038570. [DOI] [PubMed] [Google Scholar]
- 14.Gordon D., Karbat I., Ilan N., Cohen L., Kahn R., Gilles N., Dong K., Stühmer W., Tytgat J., Gurevitz M. The differential preference of scorpion α-toxins for insect or mammalian sodium channels: implications for improved insect control. Toxicon. 2007;49:452–472. doi: 10.1016/j.toxicon.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Norton R. S. Structure and structure–function relationships of sea anemone proteins that interact with the sodium channel. Toxicon. 1991;29:1051–1084. doi: 10.1016/0041-0101(91)90205-6. [DOI] [PubMed] [Google Scholar]
- 16.Honma T., Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Mar. Biotechnol. 2006;8:1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoleras N., Norton R. S. Three-dimensional structure in solution of neurotoxin III from the sea anemone Anemonia sulcata. Biochemistry. 1994;33:11051–11061. doi: 10.1021/bi00203a001. [DOI] [PubMed] [Google Scholar]
- 18.Beress L., Beress R., Wunderer G. Isolation and characterization of three polypeptides with neurotoxic activity from Anemonia sulcata. FEBS Lett. 1975;50:311–314. doi: 10.1016/0014-5793(75)80517-5. [DOI] [PubMed] [Google Scholar]
- 19.Schweitz H., Vincent J. P., Barhanin J., Frelin C., Linden G., Hugues M., Lazdunski M. Purification and pharmacological properties of eight sea anemone toxins from Anemonia sulcata, Anthopleura xanthogrammica, Stoichactis giganteus, and Actinodendron plumosum. Biochemistry. 1981;20:5245–5252. doi: 10.1021/bi00521a023. [DOI] [PubMed] [Google Scholar]
- 20.Cohen L., Lipstein N., Gordon D. Allosteric interaction between scorpion toxin receptor sites on voltage-gated Na channels imply a novel role for weakly active components in arthropod venom. FASEB J. 2006;20:1933–1935. doi: 10.1096/fj.05-5545fje. [DOI] [PubMed] [Google Scholar]
- 21.Gurevitz M., Karbat I., Cohen L., Ilan N., Kahn R., Turkov M., Stankiewicz M., Stühmer W., Dong K., Gordon D. The insecticidal potential of scorpion β-toxins. Toxicon. 2007;49:473–489. doi: 10.1016/j.toxicon.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Martinez G., Kopeyan C. Toxin III from Anemonia sulcata: primary structure. FEBS Lett. 1977;84:247–252. doi: 10.1016/0014-5793(77)80699-6. [DOI] [PubMed] [Google Scholar]
- 23.Moran Y., Cohen L., Kahn R., Karbat I., Gordon D., Gurevitz M. Expression and mutagenesis of the sea anemone toxin Av2 reveals key amino acid residues important for activity on voltage-gated sodium channels. Biochemistry. 2006;45:8864–8873. doi: 10.1021/bi060386b. [DOI] [PubMed] [Google Scholar]
- 24.Guo Q. R., Wei D. Z., Tong W. Y. Partial purification of human parathyroid hormone 1–84 as a thioredoxin fusion form in recombinant Escherichia coli by thermoosmotic shock. Protein Expression Purif. 2006;49:32–38. doi: 10.1016/j.pep.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Zilberberg N., Gordon D., Pelhate M., Adams M. E., Norris T. M., Zlotkin E., Gurevitz M. Functional expression and genetic alteration of an α scorpion neurotoxin. Biochemistry. 1996;35:10215–10222. doi: 10.1021/bi9528309. [DOI] [PubMed] [Google Scholar]
- 26.Froy O., Zilberberg N., Gordon D., Turkov M., Gilles N., Stankiewicz M., Plehate M., Loret E., Oren D. A., Shaanan B., Gurevitz M. The putative bioactive surface of insect-selective scorpion excitatory neurotoxins. J. Biol. Chem. 1999;274:5769–5776. doi: 10.1074/jbc.274.9.5769. [DOI] [PubMed] [Google Scholar]
- 27.Reed L., Muench H. A simple method of estimating fifty-percent endpoint. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 28.Gilles N., Krimm I., Bouet F., Froy O., Gurevitz M., Lancelin J. M., Gordon D. Structural implications on the interaction of scorpion α-like toxins with the sodium channel receptor site inferred from toxin iodination and pH-dependent binding. J. Neurochem. 2000;75:1735–1745. doi: 10.1046/j.1471-4159.2000.0751735.x. [DOI] [PubMed] [Google Scholar]
- 29.Rochat C., Tessier M., Miranda F., Lissitzky S. Radioiodination of scorpion and snake neurotoxins. Anal. Biochem. 1977;82:532–548. doi: 10.1016/0003-2697(77)90192-0. [DOI] [PubMed] [Google Scholar]
- 30.Shichor I., Zlotkin E., Ilan N., Chikashvili D., Stuhmer W., Gordon D., Lotan I. Domain 2 of Drosophila para voltage-gated sodium channel confers insect properties to a rat brain channel J. Neurosci. 2002;22:4364–4371. doi: 10.1523/JNEUROSCI.22-11-04364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warmke J. W., Reenan A. G. R., Wang P., Qian S., Arena J. P., Wang J., Wunderler D., Liu K., Kaczorowski G. J., van der Ploeg L. H. T., et al. Functional expression of Drosophila para sodium channels: modulation by membrane protein TipE and toxin pharmacology. J. Gen. Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartung K., Rathmayer W. Anemonia sulcata toxins modify activation and inactivation of Na+ currents in a crayfish neurone. Pflugers Arch. 1985;404:119–125. doi: 10.1007/BF00585406. [DOI] [PubMed] [Google Scholar]
- 33.Gordon D., Zlotkin E. Binding of α scorpion toxin to insect sodium channels is not dependent on membrane potential. FEBS Lett. 1993;315:125–128. doi: 10.1016/0014-5793(93)81147-r. [DOI] [PubMed] [Google Scholar]
- 34.Karbat I., Cohen L., Gilles N., Gordon D., Gurevitz M. Conversion of a scorpion toxin agonist into an antagonist highlights an acidic residue involved in voltage sensor trapping during activation of neuronal Na+ channels. FASEB J. 2004;18:683–689. doi: 10.1096/fj.03-0733com. [DOI] [PubMed] [Google Scholar]
- 35.Vass E., Hollosci M., Besson F., Buchet R. Vibrational spectroscopic detection of β- and γ-turns in synthetic and natural peptides and proteins. Chem. Rev. 2003;103:1917–1954. doi: 10.1021/cr000100n. [DOI] [PubMed] [Google Scholar]
- 36.Schweitz H. Lethal potency in mice of toxins from scorpion, sea anemone, snake and bee venoms following intraperitoneal and intracisternal injection. Toxicon. 1984;22:308–311. doi: 10.1016/0041-0101(84)90032-1. [DOI] [PubMed] [Google Scholar]
- 37.Greenfield N. J. Methods to estimate the conformation of proteins and polypeptides from circular dichroism data. Anal. Biochem. 1996;235:1–10. doi: 10.1006/abio.1996.0084. [DOI] [PubMed] [Google Scholar]
- 38.Bosmans F., Tytgat J. Voltage-gated sodium channel modulation by scorpion α-toxins. Toxicon. 2007;49:142–158. doi: 10.1016/j.toxicon.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon D., Martin-Eauclaire M. F., Cestele S., Kopeyan C., Carlier E., Ben Khalifa R., Pelhate M., Rochat H. Scorpion toxins affecting sodium current inactivation bind to distinct homologous receptor sites on rat brain and insect sodium channels. J. Biol. Chem. 1996;271:8034–8045. doi: 10.1074/jbc.271.14.8034. [DOI] [PubMed] [Google Scholar]
- 40.Karbat I., Frolow F., Froy O., Gilles N., Cohen L., Turkov M., Gordon D., Gurevitz M. Molecular basis of the high insecticidal potency of scorpion α-toxins. J. Biol. Chem. 2004;279:31679–31686. doi: 10.1074/jbc.M402048200. [DOI] [PubMed] [Google Scholar]
- 41.Tugarinov V., Kustanovich I., Zilberberg N., Gurevitz M., Anglister J. Solution structures of a highly insecticidal recombinant scorpion α-toxin and a mutant with increased activity. Biochemistry. 1997;36:2414–2424. doi: 10.1021/bi961497l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.