Abstract
Activated mesangial cells are thought to play a pivotal role in the development of kidney fibrosis under chronic pathological conditions, including DN (diabetic nephropathy). Their prolonged survival may enhance the development of the disease since they express increased amounts of growth factors and extracellular matrix proteins. CTGF (connective tissue growth factor) is one of the growth factors produced by activated mesangial cells and is reported to play a key role in the pathogenesis of DN. Previous studies have shown that addition of exogenous CTGF to HMCs (human mesangial cells) rapidly activates ERK1/2 (extracellular-signal-regulated kinase 1/2) MAPK (mitogen-activated protein kinase) and JNK (c-Jun N-terminal kinase) MAPK, but not the p38 MAPK, despite the activation of the upstream kinases, MKK3/6 (MAPK kinase 3/6). The aim of the present study was to investigate whether the lack of phosphorylated p38 MAPK by CTGF has an anti-apoptotic effect on activated HMCs. We show that in HMC CTGF induces the rapid transcriptional activation and synthesis of MKP-1 (MAPK phosphatase-1), a dual specificity phosphatase that dephosphorylates p38 MAPK. This in turn prevents the anti-apoptotic protein, Bcl-2, from being phosphorylated and losing its function, leading to the survival of the cells. Knockout of MKP-1 protein in mesangial cells treated with CTGF, using siRNA (small interfering RNA) or antisense oligonucleotides, allows p38 MAPK activation and induces mesangial cell death.
Keywords: cell survival, connective tissue growth factor (CTGF), diabetic nephropathy (DN), mesangial cell, mitogen-activated protein kinase (MAPK) phosphatase-1 (MKP-1)
Abbreviations: CDK, cyclin-dependent kinase; CTGF, connective tissue growth factor; DN, diabetic nephropathy; ECM, extracellular matrix; ERK1/2, extracellular-signal-regulated kinase 1/2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HMC, human mesangial cell; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MKK3/6, MAPK kinase 3/6; MKP-1, MAPK phosphatase-1; PKC, protein kinase C; miRNA, microRNA; r-CTGF, recombinant CTGF; RNAi, RNA interference; RT, reverse transcriptase; siRNA, small interfering RNA; TGF-β, transforming growth factor-β; TrkA, tropomyosin receptor kinase A; XTT, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
INTRODUCTION
Glomerular mesangial cells are usually quiescent under normal physiological conditions. During acute kidney injury, these cells are thought to undergo a process termed ‘activation or dedifferentiation’, the result of which is that they become myofibroblast-like cells [1]. These have contractile properties, produce high levels of cytokines, chemokines and ECM (extracellular matrix) proteins, and are involved in matrix remodelling. These differentiated cells may then undergo spontaneous apoptosis, in keeping with data indicating that apoptosis mediates clearing of myofibroblasts from wounded skin [2] and of activated hepatic stellate cells following transient liver tissue injury [3]. However, prolonged survival of these cells under chronic injury conditions may lead to the mesangial hypercellularity and glomerular scarring that occurs in DN (diabetic nephropathy).
Numerous studies have shown that elevated levels of TGF-β (transforming growth factor-β) support the initiation and progression of the mesangial cell's differentiation programme. However, in the last 5–6 years it has become clear that CTGF (connective tissue growth factor), which is rapidly induced by TGF-β, as well as by other factors generated during chronic injury (e.g. reactive oxygen species and hypoxia), plays a key role in this programme [4–7].
Previously, we have shown that CTGF mediates several TGF-β-dependent changes in mesangial cells that are also reported to occur in DN. These are: (i) induction of the cellular hypertrophy due to induction of the CDKIs [CDK (cyclin-dependent kinase) inhibitors] p15, p21 and p27, leading to cell cycle arrest in the G1-phase [8], (ii) increased expression of ECM proteins [9], and (iii) increased expression of integrins on the cell surface to facilitate the deposition and assembly of ECM proteins [10].
In a previous study, we showed that addition of exogenous CTGF to mesangial cells activates several intracellular signalling pathways including MAPKs (mitogen-activated protein kinases) ERK1/2 (extracellular-signal-regulated kinase 1/2) MAPK and JNK (c-Jun N-terminal kinase) MAPK [11]. Interestingly, p38 MAPK was not activated. In the present study we show that p38 MAPK inactivation is critical for the survival of mesangial cells in the presence of CTGF. The underlying mechanism seems to involve the rapid induction of an immediate-early response gene encoding the dual specificity protein phosphatase 1 [MKP-1 (MAPK phosphatase-1)]. MKP-1 preferentially inactivates the p38 MAPK and thus prevents phosphorylation of the anti-apoptotic protein Bcl-2, thereby promoting the survival of HMCs (human mesangial cells).
MATERIALS AND METHODS
Cell cultures, antibodies and reagents
Primary normal adult HMCs (CC-2259, lot OF1548) were purchased from Cambrex Bio Science (http://www.cambrex.com), maintained in culture as described previously [9], and used at passage 9–10. r-CTGF (recombinant CTGF) was expressed as a fusion protein with a V5 tag as described previously [9]. Phospho-ERK1/2 pathway sampler, phospho-JNK pathway sampler and phosphor-p38 MAPK pathway sampler antibodies were from New England Biolabs (http://www.neb.uk.com). Bcl-2 monoclonal antibody was from Santa Cruz Biotechnology (http://www.autogenbioclear.com). Anti-MKP-1 antibody was from Sigma (http://www.sigma-aldrich.com). The MKP-1 ANTISENSE-RNAi-Combination-kit was from Biognostik (http://www.biognostik.com).
RNA extraction and RT (reverse transcriptase)–PCR analysis
Total RNA was extracted from mesangial cells using the RNAzol B method (AMS Biotechnology, Abingdon, Oxfordshire, U.K.). Equal amounts of total RNA (3 μg) from each sample were reverse-transcribed into cDNA using Superscript II RNase H+ RT (Gibco BRL, Paisley, U.K.) and random primers. Equal amounts of the reverse transcription reaction were subjected to PCR amplification as described previously [9]. Amplification was started with 5 min denaturation at 94°C followed by 30 PCR cycles. Each cycle consisted of 60 s at 94°C, 60 s at 55°C and 60 s at 72°C. The final extension was for 10 min at 72°C. A control omitting the reverse transcription step was also performed. The sequences of the primers used to amplify MKP-1 were 5′-CCGGAGCTGTGCAGCAAA-3′ and 5′-CTCCACAGGGATGCTCTT-3′, yielding a PCR product of 284 bp.
Western blotting
Cells grown in culture flasks were lysed in reducing SDS/PAGE loading buffer [250 mM Tris/HCl, pH 6.8, 6% SDS, 30% glycerol, 0.1% Bromophenol Blue, 0.1 M dithiothreitol, 0.1 mg/ml PMSF and 1×proteinase inhibitor cocktail (Roche) added immediately before use] and immediately scraped off the plate. Cell lysates were sonicated for 10 s to shear DNA. Samples were boiled for 5 min and resolved on 4–12% gradient gels by SDS/PAGE. Proteins were transferred on to a PVDF membrane filter (Immobilin-P; Millipore) using a Bio-Rad transfer apparatus. Blots were incubated in blocking buffer containing 1× TBS (137 mM Tris/HCl, pH 6.8, 2.7 mM KCl and 25 mM NaCl) and 0.1% Tween 20 with 5% (w/v) non-fat dry milk for 1 h. Immunodetection was performed by incubating the blots in primary antibody at the appropriate dilution in antibody dilution buffer [1× TBS and 0.1% Tween 20 with 5% (w/v) BSA], overnight at 4°C. Blots were then washed three times with washing buffer (1× TBS and 0.1% Tween 20) and incubated with secondary horseradish peroxidase-conjugated antibodies for 1 h at room temperature (20–25°C). Bound antibodies were visualized using the enhanced chemiluminescence reagent Luminol (Autogen Bioclear, Wiltshire, U.K.). Prestained molecular mass standards (Amersham International, Amersham, U.K.) were used to monitor protein migration.
Immunofluorescence staining
Cells grown on coverslips were fixed with 3.7% (w/v) paraformaldehyde and permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature. Coverslips were then incubated overnight at 4°C with serum (5% in PBS) from the same species as that in which the secondary antibody was raised. After this they were incubated with primary antibodies (at optimum dilution in PBS containing 3% BSA) for 1 h at 37°C. Coverslips were then washed and incubated in the dark for 1 h with fluorescein-conjugated secondary antibody (Sigma–Aldrich, Poole, U.K.). After staining, the coverslips were mounted on glass slips with anti-fade mounting media (Vector Laboratories, Peterborough, U.K.) and examined using a fluorescence microscope.
Use of antisense oligonucleotides
Phosphothioate MKP-1 antisense oligonucleotide (2 μM, as recommended by the manufacturer; sequence ID: 2.05983, Biognostik), or a CG-matched randomized sequence oligonucleotide (2 μM, as a negative control; sequence ID: C5278), was added directly to cultures of equal numbers of cells, 24 h prior to any other treatment.
siRNA (small interfering RNA) transfection
HMCs were plated at 2.4×104 cells/well in 24-well plates, 24 h before transfection. Cells were then transfected with MKP-1 siRNA, or with a luciferase siRNA as a non-specific control, using 0.7 μl of Lipofectamine™ 2000 (Invitrogen) per well according to the manufacturer's instructions. Three different MKP-1-specific siRNAs were tested at different concentrations (10–50 nM) for their ability to reduce MKP-1 expression in HMCs stimulated with 20 ng/ml CTGF for 1 h (quiescent cells express only very low levels of MKP-1). The optimum concentration of siRNAs in the cell culture medium was determined to be 30 nM. At 6 h post-transfection, growth medium was replaced with serum-free medium. Cells were then incubated at 37°C for 24 h before being assessed for MKP-1 gene knock-down. All three siRNAs caused a reduction in MKP-1 gene expression but to different levels. Silencer 20639 was the most effective and was used in the reported experiments (sense 5′-CCACCACCGUGUUCAACUUtt-3′; antisense 5′-AAGUUGAACACGGUGGUGGtt-3′).
Cell viability assay
Mesangial cell death was determined using the cell proliferation kit II {XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide]} (Roche). The method is based on the cellular reduction of XTT by mitochondrial dehydrogenases to an orange formazan product that can be measured spectrophotometrically. Briefly, XTT labelling mixture (1 ml of XTT labelling reagent and 20 μl of electron coupling reagent) was prepared immediately before use. Aliquots of 150 μl of the XTT labelling mixture were added to cells grown in a 24-well plate in a final volume of 300 μl of culture medium per well. Cells were then incubated for different periods of time at 37°C after which absorbance at 450 nm was measured with a multiwell spectrophotometer.
Statistical analysis
Results were compared using Student's unpaired t test. A P value of 0.05 or less was regarded as denoting a significant difference.
RESULTS
CTGF induces p38 MAPK dephosphorylation
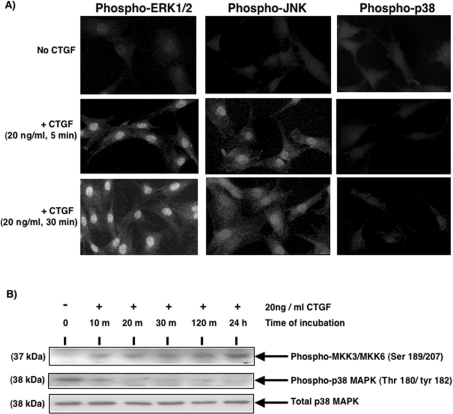
Stimulation of HMCs with 20 ng/ml r-CTGF rapidly activates the classical MAPK (ERK1/2) and JNK but not the p38 MAPK, as reported previously [11]. Figure 1(A) confirms this finding. However, Western-blot analysis indicates that the addition of CTGF to serum-starved HMC cultures rapidly activates MKK3/6 (MAPK kinase 3/6), the upstream kinases that are known to activate the p38 MAPK [12] (Figure 1B). Western-blot analysis also indicates that CTGF induces a rapid dephosphorylation of the basal active p38 MAPK. This was maintained up to 24 h after CTGF stimulation. Total p38 MAPK levels did not change over the time course of the experiment.
Figure 1. Activation of MAPK signalling pathways by CTGF in HMC.
(A) Cells were grown on coverslips and serum-starved for 48 h prior to incubation in the absence or presence of 20 ng/ml r-CTGF for 5 or 30 min. Cells were fixed, permeabilized, probed with anti-phospho-ERK1/2, JNK and p38 MAPK primary antibodies, and then with fluorescein-conjugated secondary antibody, as described in the Materials and methods section. Results are representative of three separate experiments. (B) Serum-starved HMCs were incubated in the presence of r-CTGF for the periods of time indicated, after which the cells were lysed. Equal amounts of lysate protein were subjected to SDS/PAGE and analysed by Western blotting using phospho-specific antibodies against the p38 MAPK and the phosphokinases upstream of it, MKK3/6, as well as antibody against the total p38 MAPK. Results are representative of three separate experiments
CTGF induces the rapid expression of MKP-1 in HMC
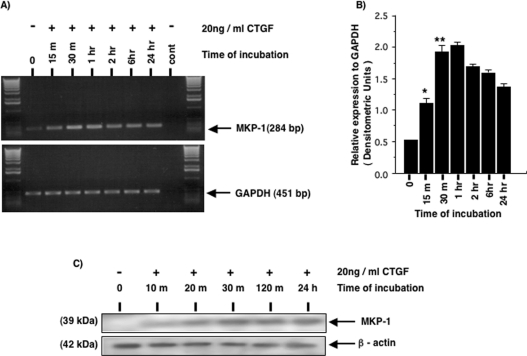
It is known that MAPKs, once activated, can be inactivated by dual specificity protein phosphatases. p38 and JNK MAPKs are preferentially inactivated by MKP-1 [13]. However, in vitro studies have shown that MKP-1 is most selective for p38 MAPK at physiological levels of expression where it directly binds to the p38 kinases [13,14]. At high levels of expression MKP-1 can also inactivate JNK and even ERK1/2 but at low levels [13,14]. MKP-1 is an immediate-early gene product that is potently induced in response to both growth factors and stress stimuli. Thus we investigated whether CTGF is able to induce the expression level of MKP-1 in HMCs. MKP-1 is constitutively expressed at a very low level in serum-starved HMCs (Figures 2A and 2B). Upon CTGF stimulation, MKP-1 mRNA levels were rapidly induced within 15 min and reach a maximum at approx. 60 min, followed by a gradual decline. Figure 2(C) shows that MKP-1 protein levels were also rapidly induced in CTGF-stimulated HMCs, mirroring the increase in the mRNA. However, MKP-1 protein was barely detectable in the untreated cells. CTGF induction of MKP-1 was sustained, with the protein detectable up to 24 h after CTGF addition.
Figure 2. CTGF induces the rapid expression of MKP-1 in HMC.
Serum-starved HMC were incubated in the presence or absence of 20 ng/ml r-CTGF for the periods of time indicated, after which the cells were used for RNA extraction and RT–PCR analysis (A, B) or lysed and used for SDS/PAGE and analysed by Western blotting (C). (A) A representative gel showing RT–PCR analysis of MKP-1 with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a housekeeping gene. (B) Densitometry of RT–PCR analysis of MKP-1 expression from three experiments. (C) Western-blot analysis using an anti-MKP-1 antibody. Membranes were stripped and reprobed with an anti-β-actin antibody to control for sample loading. Results are representative of three separate experiments. *P<0.0008, **P<0.0001 compared with unstimulated cells in (B).
CTGF enhances the levels of Bcl-2 protein in HMCs
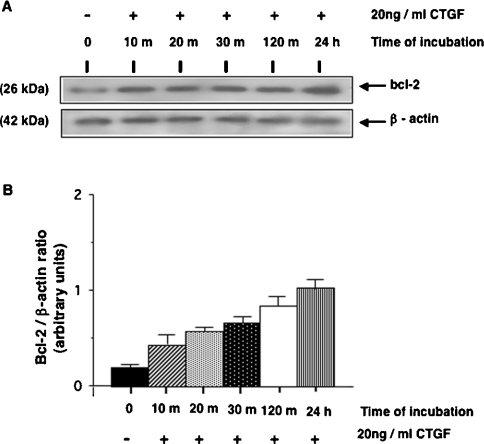
The correlation between MKP-1 induction and the inactivation of p38 MAPK suggests that MKP-1 may play a significant role in mediating the dephosphorylation of this kinase in CTGF-stimulated HMCs. p38 MAPK has been reported as one of the kinases responsible for the phosphorylation of the anti-apoptotic protein Bcl-2, which leads to loss of its anti-apoptotic potential [15] and an increase in its proteolytic degradation via a proteosomal pathway [16]. Thus we investigated the relative abundance of the Bcl-2 protein in HMCs stimulated with CTGF. Figure 3 shows that CTGF stimulation rapidly enhances the level of Bcl-2 protein in HMCs and that this increased level was maintained over the time course of the experiment. No clear shift in the mobility of Bcl-2 protein was observed. Attempts to detect Bcl-XL protein expression level in HMCs were not successful (results not shown).
Figure 3. CTGF enhances the levels of Bcl-2 protein in HMCs.
Serum-starved HMCs were incubated in the presence or absence of 20 ng/ml r-CTGF for the periods of time indicated, after which the cells were lysed. (A) Equal amounts of lysate protein were subjected to SDS/PAGE and analysed by Western blotting using anti-Bcl-2 antibody. Membranes were stripped and reprobed with an anti-β-actin antibody to control for sample loading. (B) Densitometric analysis of Bcl-2 expression from three separate experiments. Results shown are means±S.E.M.
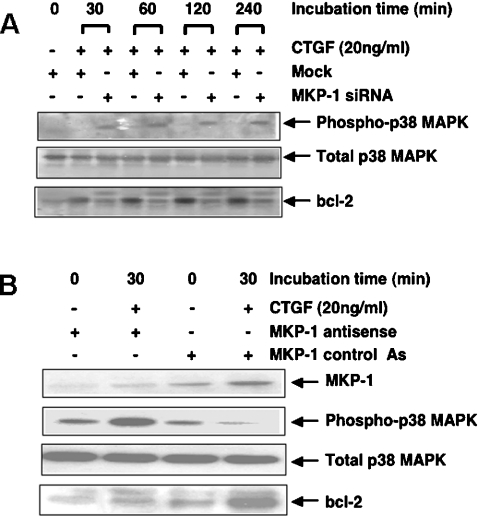
To confirm the prominent role of MKP-1 in the dephosphorylation/inactivation of p38 MAPK and thus the protection of Bcl-2, we decided to knock-down MKP-1 protein in cells stimulated with CTGF. For this we used an ANTISENSE-RNAi-Combination-kit, which includes siRNAs and antisense oligonucleotides specifically designed for the human MKP-1 gene. This allowed us to cross-validate our results, as both techniques work through independent mechanisms of action. At 24 h after MKP-1 siRNA transfection or MKP-1 antisense oligonucleotide transfection, cells were incubated with or without 20 ng/ml CTGF for 1 h. To control for non-specific effects of siRNA delivery, controls were included in which cells were either mock-transfected (transfection reagent only) or transfected with luciferase siRNA, while a CG-matched randomized sequence oligonucleotide was used as a control for the specificity of the antisense delivery.
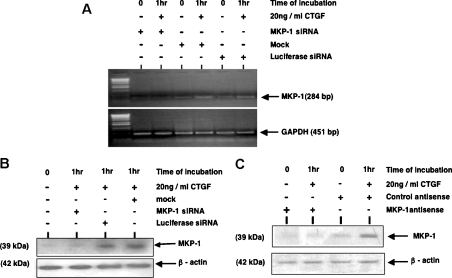
MKP-1 expression was induced in response to CTGF stimulation in cells that were either mock-transfected or transfected with a non-specific siRNA (Figures 4A and 4B). In contrast, transfection with a specific siRNA led to a significant reduction of MKP-1 expression in HMCs treated with CTGF for 1 h at both the mRNA and protein levels. MKP-1 RNA inhibition correlates well with the high reduction in the protein level, indicating that the response is a classical RNAi (RNA interference) effect and not an miRNA (microRNA) effect.
Figure 4. Efficiency of MKP-1 protein knock-down in HMCs using siRNA and antisense oligonucleotide approaches.
At 24 h after transfection with MKP-1 siRNA, or MKP-1 antisense oligonucleotides, cells were incubated with or without 20 ng/ml r-CTGF for 1 h. To control for non-specific effects of siRNA delivery, controls were included in which cells were either mock-transfected (transfection reagent only) or transfected with luciferase siRNA, whereas a CG-matched randomized sequence oligonucleotide was used as a control for the specificity of the antisense delivery. At the end of the incubation time, total RNA was extracted and used for semi-quantitative RT–PCR (A). In parallel experiments, cells were lysed and protein was subjected to SDS/PAGE and analysed by Western blotting using anti-MKP-1 antibody (B, C). Membranes were stripped and reprobed with an anti-β-actin antibody to control for sample loading. Results are representative of three separate experiments.
Figure 4(C) shows that MKP-1 antisense oligonucleotide, but not the control oligonucleotide, also induces a marked reduction in MKP-1 protein expression level in HMCs treated with CTGF for 1 h.
After assessing the efficiency of both the siRNA and antisense approaches to knock-down MKP-1 protein in HMCs, similar experiments were performed in which the cells were lysed after different periods of time and equal amounts of total protein analysed for the expression level of phospho-p38 MAPK and Bcl-2. Figure 5(A) shows that in HMCs treated with CTGF, inhibition of MKP-1 expression by RNAi is accompanied by enhanced phosphorylation of p38 MAPK and a clear reduction in Bcl-2 level. This suggests that p38 MAPK signalling may be co-ordinating Bcl-2 gene expression, mRNA stability, translation efficiency or protein degradation. However, this was not investigated in the present study. Interestingly, a second band of a higher molecular mass (∼29 kDa) that cross-reacted with the Bcl-2 antibody can also be seen in the lysates from cells treated with siRNA, but not in those that were mock-treated. This may represent the p38 MAPK-dependent phosphorylated form of Bcl-2. Similar results were obtained using the MKP-1 antisense approach. The data in Figure 5(B) confirm that inhibition of MKP-1 expression in HMCs enhances CTGF-mediated p38 MAPK activation, which in turn plays a key role in reducing the level of the cell survival factor Bcl-2.
Figure 5. Targeted inhibition of MKP-1 expression enhances p38 MAPK activation and reduces Bcl-2 expression in HMCs treated with r-CTGF.
At 24 h after mock transfecting, or transfecting HMCs with MKP-1 siRNA (A) or treating them with MKP-1 antisense and control antisense oligonucleotides (B), cells were incubated with or without 20 ng/ml r-CTGF for different periods of time. Equal amounts of total protein were subjected to SDS/PAGE and analysed by Western blotting using anti-MKP-1, phospho-p38 MAPK, p38 MAPK and Bcl-2 antibodies. Results are representative of three separate experiments.
Modulation of MKP-1 protein levels is involved in CTGF-mediated survival activity in HMCs
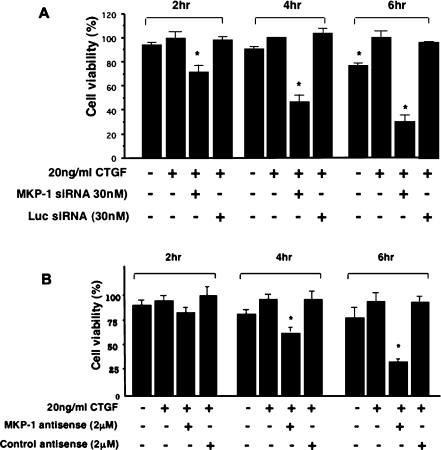
Next we investigated whether CTGF exerts a survival-promoting activity in HMCs. At 24 h after MKP-1 siRNA transfection or MKP-1 antisense oligonucleotide transfection, equal numbers of cells were incubated with or without 20 ng/ml CTGF for 2, 4 and 6 h. At the end of the incubation period, the proportions of surviving cells were quantified by XTT uptake and reduction, as described in the Materials and methods section. Figure 6(A) shows that cells treated with CTGF for 6 h were 100% viable, while cells in which MKP-1 phosphatase was knocked down by siRNA silencing show a decrease in viability by 25–70% within 2–6 h of incubation with the same concentration of CTGF. The control luciferase siRNA had no effect on HMC viability. Loss of viable cells due to serum deprivation, thereby decreasing the supply of survival factors of HMCs that were not treated with CTGF, was approx. 10–20% within 2–6 h.
Figure 6. Modulation of MKP-1 protein levels is involved in CTGF-mediated survival activity in HMCs.
At 24 h after (A) transfecting HMC with MKP-1 siRNA or luciferase (Luc) siRNA or (B) treating them with MKP-1 antisense or control antisense oligonucleotides, equal numbers of cells were incubated with or without 20 ng/ml r-CTGF for 2, 4 and 6 h. At the end of incubation period, the proportion of surviving cells was quantified by XTT uptake and reduction, as described in the Materials and methods section. Results are means±S.E.M. for three experiments, performed in triplicate. The number of cells treated with CTGF did not change during the course of the experiment and is expressed as 100% cell viability at each time point. *P<0.0001 compared with cells treated with CTGF only.
The role of MKP-1 in mediating CTGF survival-promoting activity was also confirmed using the MKP-1 antisense approach where cell viability decreased by ∼15–67% within 2–6 h of CTGF stimulation (Figure 6B).
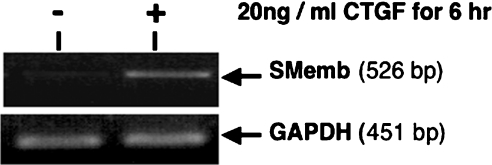
Differentiated HMCs adopting a myofibroblast-like phenotype have been reported to express de novo α-SMA (α-smooth muscle actin) and SMemb (the embryonic form of myosin heavy chain) genes [17,18]. To assess whether CTGF potentially protects differentiated/activated mesangial cells from apoptosis while promoting change to a myofibroblast phenotype, we investigated the expression of SMemb in HMCs treated with 20 ng/ml CTGF for 6 h. RT–PCR results (Figure 7) show that SMemb expression is clearly increased with this treatment.
Figure 7. CTGF promotes differentiation of HMCs.
Equal numbers of HMCs were incubated with or without 20 ng/ml r-CTGF for 6 h. At the end of the incubation period, total RNA was extracted, and equal amounts were reverse-transcribed and used for semi-quantitative PCR analysis for the myofibroblast marker, SMemb, as well as for the housekeeping gene GAPDH. Results are representative of three separate experiments.
DISCUSSION
It is now clear that CTGF is implicated in the pathogenesis of DN and it has been shown to mediate a wide range of disordered functions in HMCs [8–10,19]. However, the molecular mechanisms by which the growth factor functions are still poorly understood. In a previous study, we observed that, in HMC, CTGF rapidly activates the intra-signalling pathways ERK1/2 MAPK and JNK MAPK, but not the p38 MAPK, despite rapidly activating MKK3/6, the upstream activators of p38 MAPK. In the present paper, we confirmed this observation. As p38 MAPK has been reported to function as a pro-death signalling effector in some cell types [20], we addressed the hypothesis that CTGF-dependent p38 inactivation may protect mesangial cells from death.
MKP-1 phosphatase is a member of the family of inducible dual specificity phosphatases, dsPTPs [21], and is a potential deactivator for p38 MAPK [13]. Although serum-starved HMCs express only very low levels of MKP-1, stimulation with CTGF resulted in a rapid and sustained induction of MKP-1 expression. This rapid induction is consistent with data reported in other experimental systems [22–24]. MKP-1 has also been reported to be involved in the anti-apoptotic effect of retinoids in mesangial cells incubated with H2O2 [25] as well as in the restriction of TNF (tumour necrosis factor)-induced apoptosis in rat mesangial cells [26].
Interestingly, it has been reported that activation of the TrkA (tropomyosin receptor kinase A) receptor may be crucial for MKP-1 gene expression, independently of the cellular origin or type on which the receptor is active [27]. HMCs express the TrkA receptor and CTGF has been shown to activate it [11]. Activation of the ERK1/2 MAPK pathway and PKC (protein kinase C) were reported to be involved in the induction of MKP-1 gene expression [25,27].
p38 MAPK is one of several kinases including CDC2 (cell division cycle 2 kinase), CDK6, PKCα and JNK, which can phosphorylate the anti-apoptotic protein Bcl-2 [28–31] at serine and threonine residues located in the loop region between the α1- and α2-helices of the molecule [32,33]. This phosphorylation impairs the anti-apoptotic function of the protein by reducing its ability for heterodimerization with Bax (Bcl-2–associated X protein), the pro-apoptotic protein [15]. Homodimers of Bax then form pores in the mitochondrial membrane that release cytochrome c and other pro-apoptotic factors into the cytoplasm. Accordingly, Bcl-2 phosphorylation by p38 MAPK can lead to cytochrome c release from the mitochondria, caspase activation and apoptotic cell death [34]. On the other hand, continuous deactivation of p38 MAPK maintains the functional and structural properties of Bcl-2 protein and was found to be important for the survival-promoting activity of NGF (nerve growth factor) in the lymphoblastoid CESS B-cell line [34]. Phosphorylation of Bcl-2 can also render the protein susceptible to proteolytic degradation [16]. In the present study, we show that CTGF-mediated rapid and continuous deactivation of p38 MAPK is accompanied by enhanced levels of Bcl-2. The direct role of MKP-1 in mediating this p38 MAPK inactivation was assessed by MKP-1 protein knock-down experiments. Due to the recent growing concerns about siRNAs causing off-target effects by acting in an miRNA-like manner or by inducing an interferon response [32,35–37], and since such effects are not caused by the antisense oligonucleotides, we used the antisense–RNAi-combination techniques to cross-validate our results. Here we show that selective blockade of MKP-1 expression in HMCs by both techniques resulted in the same observed phenotype in response to CTGF. This phenotype is characterized by sustained activation of p38 MAPK and a decreased expression level of Bcl-2, accompanied by a reduction in cell viability.
In the present study we were not able to detect any mitogenic activity for CTGF in HMCs (results not shown), consistent with our previous finding that the growth factor is a hypertrophic factor that promotes mesangial cell cycle arrest in the G1-phase [8]. Although CTGF has also been reported to have a non-mitogenic anti-apoptotic function in CEFs (chicken embryo fibroblasts) [38], it does not promote the survival of NIH 3T3 cells [38]. Moreover, it induces apoptosis in human breast cancer cells [39]. This may indicate that the survival-promoting function of CTGF may be cell-type-specific, or that cells are CTGF-responsive only at a certain stage of their differentiation.
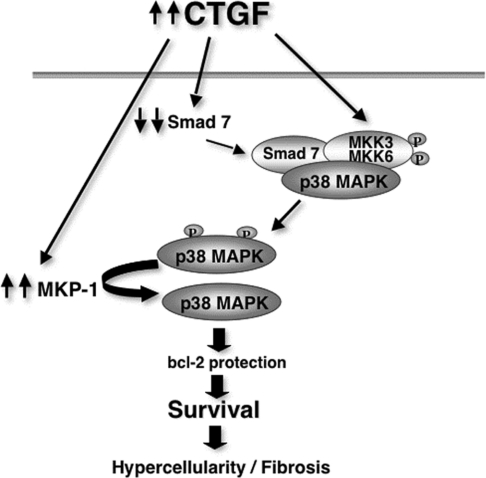
Mesangial cells are usually quiescent but, in response to injury, the cells undergo a process of differentiation into myofibroblast-like cells as an important step in the tissue repair programme. These cells undergo spontaneous apoptosis during transient injury. In contrast, their prolonged survival during chronic injury may lead to hypercellularity and amplification of fibrosis. In the present study, we presented in vitro evidence that addition of r-CTGF at a concentration of 20 ng/ml (500 pM) promotes the survival of activated HMCs. Since CTGF expression level is highly up-regulated in the glomeruli of DN patients [19], this may account for the mesangial glomerular hypercellularity seen in DN. In conclusion, we propose that CTGF orchestrates several cellular mechanisms in order to activate, and then protect the activated HMC from cell death. Figure 8 represents a model to illustrate this. Elevated expression of CTGF under diabetic conditions induces the re-expression of several embryonic genes including the SMemb myosin heavy chain isoform, and the fibronectin EDA+ splice variant [10], leading to dedifferentiation of mesangial cells into myofibroblasts. At the same time, CTGF activates MKK3/6, the upstream kinases that activate p38 MAPK.
Figure 8. Model showing the cellular mechanisms orchestrated by CTGF to protect activated HMCs from apoptosis.
Smad7, which is known to have an inhibitory effect on TGF-β signalling, also has an important role as a pro-apoptotic molecule and its overexpression in rat mesangial cells induces apoptosis [40]. Smad7 acts as a scaffolding protein, interacting with TAK1 (TGF-β-activated kinase 1), MKK3 and p38 MAPK to facilitate p38 activation and apoptosis [41]. CTGF promotes continuous inactivation of p38 MAPK by down-regulating the expression of Smad7 [42] and up-regulating the expression of MKP-1, thus preventing the phosphorylation of Bcl-2, preserving its anti-apoptotic function and promoting cell survival.
Acknowledgments
We thank the U.K. Medical Research Council for the financial support.
References
- 1.Johnson R., Floege J., Yoshimura A., Iida H., Couser W., Alpers C. The activated mesangial cell: a glomerular ‘myofibroblast’? J. Am. Soc. Nephrol. 1992;2:S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 2.Desmouliere A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Enzan H. Proliferation of Ito cells (fat-storing cells) in acute carbon tetrachloride liver injury. A light and electron microscopic autoradiographic study. Acta Pathol. Japan. 1985;35:1301–1308. doi: 10.1111/j.1440-1827.1985.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 4.Park S.-K., Kim J.-a., Seomun Y., Choi J., Kim D.-H., Han I.-O., Lee E. H., Chung S.-K., Joo C.-K. Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem. Biophys. Res. Commun. 2001;284:966–971. doi: 10.1006/bbrc.2001.5058. [DOI] [PubMed] [Google Scholar]
- 5.Higgins D. F., Biju M. P., Akai Y., Wutz A., Johnson R. S., Haase V. H. Hypoxic induction of CTGF is directly mediated by Hif-1. Am. J. Physiol. Renal. Physiol. 2004;287:F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 6.Mason R. M., Wahab N. A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 7.Abdel Wahab N., Mason R. M. Connective tissue growth factor and renal diseases: some answers, more questions. Curr. Opin. Nephrol. Hypertens. 2004;13:53–58. doi: 10.1097/00041552-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Wahab N., Weston B. S., Roberts T., Mason R. M. Connective tissue growth factor and regulation of the mesangial cell cycle: role in cellular hypertrophy. J. Am. Soc. Nephrol. 2002;13:2437–2445. doi: 10.1097/01.asn.0000031828.58276.02. [DOI] [PubMed] [Google Scholar]
- 9.Wahab N. A., Yevdokimova N., Weston B. S., Roberts T., Li X., Brinkman H., Mason R. M. Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem. J. 2001;359:77–87. doi: 10.1042/0264-6021:3590077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weston B. S., Wahab N. A., Mason R. M. CTGF mediates TGF-β-induced fibronectin matrix deposition by upregulating active α5β1 integrin in human mesangial cells. J. Am. Soc. Nephrol. 2003;14:601–610. doi: 10.1097/01.asn.0000051600.53134.b9. [DOI] [PubMed] [Google Scholar]
- 11.Wahab N. A., Weston B. S., Mason R. M. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 2005;16:340–351. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- 12.Garrington T. P., Johnson G. L. Organization and regulation of mitogen-activated protein kinase signalling pathways. Curr. Opin. Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 13.Franklin C. C., Kraft A. S. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 14.Hutter D., Chen P., Barnes J., Liu Y. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: critical role of the p38 C-terminal domain in its negative regulation. Biochem. J. 2000;352:155–163. [PMC free article] [PubMed] [Google Scholar]
- 15.Haldar S., Chintapalli J., Croce C. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 16.Chadebech P., Brichese L., Baldin V., Vidal S., Valette A. Phosphorylation and proteasome-dependent degradation of Bcl-2 in mitotic-arrested cells after microtubule damage. Biochem. Biophys. Res. Commun. 1999;262:823–827. doi: 10.1006/bbrc.1999.1291. [DOI] [PubMed] [Google Scholar]
- 17.Makino H., Kashihara N., Sugiyama H., Kanao K., Sekikawa T., Okamoto K., Maeshima Y., Ota Z., Nagai R. Phenotypic modulation of the mesangium reflected by contractile proteins in diabetes. Diabetes. 1996;45:488–495. doi: 10.2337/diab.45.4.488. [DOI] [PubMed] [Google Scholar]
- 18.Kishino M., Kimura A., Yamaguchi K., Ohtani H., Yamada Y., Takahashi T., Mune M., Mimura K., Maeda T., Matsumura S., et al. Increased expression of a brain/embryo-type myosin heavy chain isoform (MIIB2) in mesangial proliferative glomerulonephritis. Kidney Int. 1996;49:1350–1359. doi: 10.1038/ki.1996.191. [DOI] [PubMed] [Google Scholar]
- 19.Wahab N. A., Schaefer L., Weston B. S., Yiannikouris O., Wright A., Babelova A., Schaefer R., Mason R. M. Glomerular expression of thrombospondin-1, transforming growth factor β and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia. 2005;48:2650–2660. doi: 10.1007/s00125-005-0006-5. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser R. A., Bueno O. F., Lips D. J., Doevendans P. A., Jones F., Kimball T. F., Molkentin J. D. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J. Biol. Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 21.Keyse S. An emerging family of dual specificity MAP kinase phosphatases. Biochim. Biophys. Acta. 1995;1265:152–160. doi: 10.1016/0167-4889(94)00211-v. [DOI] [PubMed] [Google Scholar]
- 22.Duff J. L., Monia B. P. Mitogen-activated protein (MAP) kinase is regulated by the MAP kinase phosphatase (MKP-1) in vascular smooth muscle cells. J. Biol. Chem. 1995;270:7161–7166. doi: 10.1074/jbc.270.13.7161. [DOI] [PubMed] [Google Scholar]
- 23.Xu Q., Konta T., Kitamura M. Retinoic acid regulation of mesangial cell apoptosis. Exp. Nephrol. 2002;10:171–175. doi: 10.1159/000058343. [DOI] [PubMed] [Google Scholar]
- 24.Brondello J.-M., Brunet A., Pouyssegur J., McKenzie F. R. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J. Biol. Chem. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y., Kang B., Williamson J. Resistance to TNF-α cytotoxicity can be achieved through different signalling pathways in rat mesangial cells. Am. J. Physiol. 1999;276:C435–C441. doi: 10.1152/ajpcell.1999.276.2.C435. [DOI] [PubMed] [Google Scholar]
- 26.Peinado-Ramon P., Wallen A., Hallbrook F. MAP kinase phosphatase-1 mRNA is expressed in embryonic sympathetic neurons and is upregulated after NGF stimulation. Brain Res. Mol. Brain Res. 1998;56:256–267. doi: 10.1016/s0169-328x(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 27.Ryser S., Tortola S., van Haasteren G., Muda M., Li S., Schlegel W. MAP kinase phosphatase-1 gene transcription in rat neuroendocrine cells is modulated by a calcium-sensitive block to elongation in the first exon. J. Biol. Chem. 2001;276:33319–33327. doi: 10.1074/jbc.M102326200. [DOI] [PubMed] [Google Scholar]
- 28.Pathan N., Aime-Sempe C., Kitada S., Basu A., Haldar S., Reed J. C. Microtubule-targeting drugs induce bcl-2 phosphorylation and association with Pin1. Neoplasia. 2001;3:550–559. doi: 10.1038/sj.neo.7900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan M., Goodwin M., Vu T., Brantley-Finley C., Gaarde W. A., Chambers T. C. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK Cascade. J. Biol. Chem. 2000;275:29980–29985. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 30.Ojala P. M., Yamamoto K., Castanos-Velez E., Biberfeld P., Korsmeyer S. J., Makela T. P. The apoptotic v-cyclin–CDK6 complex phosphorylates and inactivates Bcl-2. Nat. Cell Biol. 2000;2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 31.Ruvolo P. P., Deng X., Carr B. K., May W. S. A functional role for mitochondrial protein kinase C α in Bcl2 phosphorylation and suppression of apoptosis. J. Biol. Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 32.Scacheri P. C., Rozenblatt-Rosen O., Caplen N. J., Wolfsberg T. G., Umayam L., Lee J. C., Hughes C. M., Shanmugam K. S., Bhattacharjee A., Meyerson M., Collins F. S. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1892–1897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang B., Minn A., Muchmore S., Fesik S., Thompson C. Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torcia M., De Chiara G., Nencioni L., Ammendola S., Labardi D., Lucibello M., Rosini P., Marlier L. N. J. L., Bonini P., Sbarba P. D., et al. Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release. J. Biol. Chem. 2001;276:39027–39036. doi: 10.1074/jbc.M102970200. [DOI] [PubMed] [Google Scholar]
- 35.Doench J. G., Petersen C. P., Sharp P. A. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snove J. O., Holen T. Many commonly used siRNAs risk off-target activity. Biochem. Biophys. Res. Commun. 2004;319:256–263. doi: 10.1016/j.bbrc.2004.04.175. [DOI] [PubMed] [Google Scholar]
- 37.Bridge A. J., Pebernard S., Ducraux A., Nicoulaz A.-L., Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- 38.Gygi D., Zumstein P., Grossenbacher D., Altwegg L., Luscher T. F., Gehring H. Human connective tissue growth factor expressed in Escherichia coli is a non-mitogenic inhibitor of apoptosis. Biochem. Biophys. Res. Commun. 2003;311:685–690. doi: 10.1016/j.bbrc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 39.Hishikawa K., Oemar B. S., Tanner F. C., Nakaki T., Luscher T. F., Fujii T. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J. Biol. Chem. 1999;274:37461–37466. doi: 10.1074/jbc.274.52.37461. [DOI] [PubMed] [Google Scholar]
- 40.Okado T., Terada Y., Tanaka H., Inoshita S., Nakao A., Sasaki S. Smad7 mediates transforming growth factor-β-induced apoptosis in mesangial cells. 2002;62:1178, 1186. doi: 10.1111/j.1523-1755.2002.kid583.x. [DOI] [PubMed] [Google Scholar]
- 41.Edlund S., Bu S., Schuster N., Aspenstrom P., Heuchel R., Heldin N.-E., ten Dijke P., Heldin C.-H., Landstrom M. Transforming growth factor-β 1 (TGF-β)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-β-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol. Biol. Cell. 2003;14:529–544. doi: 10.1091/mbc.02-03-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahab N. A., Weston B. S., Mason R. M. Modulation of the TGFβ/Smad signaling pathway in mesangial cells by CTGF/CCN2. Exp. Cell Res. 2005;307:305–314. doi: 10.1016/j.yexcr.2005.03.022. [DOI] [PubMed] [Google Scholar]