Abstract
NOX4 is an enigmatic member of the NOX (NADPH oxidase) family of ROS (reactive oxygen species)-generating NADPH oxidases. NOX4 has a wide tissue distribution, but the physiological function and activation mechanisms are largely unknown, and its pharmacology is poorly understood. We have generated cell lines expressing NOX4 upon tetracycline induction. Tetracycline induced a rapid increase in NOX4 mRNA (1 h) followed closely (2 h) by a release of ROS. Upon tetracycline withdrawal, NOX4 mRNA levels and ROS release decreased rapidly (<24 h). In membrane preparations, NOX4 activity was selective for NADPH over NADH and did not require the addition of cytosol. The pharmacological profile of NOX4 was distinct from other NOX isoforms: DPI (diphenyleneiodonium chloride) and thioridazine inhibited the enzyme efficiently, whereas apocynin and gliotoxin did not (IC50>100 μM). The pattern of NOX4-dependent ROS generation was unique: (i) ROS release upon NOX4 induction was spontaneous without need for a stimulus, and (ii) the type of ROS released from NOX4-expressing cells was H2O2, whereas superoxide (O2−) was almost undetectable. Probes that allow detection of intracellular O2− generation yielded differential results: DHE (dihydroethidium) fluorescence and ACP (1-acetoxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine) ESR measurements did not detect any NOX4 signal, whereas a robust signal was observed with NBT. Thus NOX4 probably generates O2− within an intracellular compartment that is accessible to NBT (Nitro Blue Tetrazolium), but not to DHE or ACP. In conclusion, NOX4 has a distinct pharmacology and pattern of ROS generation. The close correlation between NOX4 mRNA and ROS generation might hint towards a function as an inducible NOX isoform.
Keywords: hydrogen peroxide, NADPH oxidase (NOX), NOX4, NOX4 inhibitor, reactive oxygen species (ROS), superoxide
Abbreviations: ACP, 1-acetoxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine; CMV, cytomegalovirus; DHE, dihydroethidium; DMEM, Dulbecco's modified Eagle's medium; DPI, diphenyleneiodonium chloride; HBSS, Hanks balanced salt solution; HEK-293, human embryonic kidney; HRP, horseradish peroxidase; NBT, Nitro Blue Tetrazolium; NOX, NADPH oxidase; PKC, protein kinase C; ROS, reactive oxygen species; tet, tetracycline; TGF, transforming growth factor
INTRODUCTION
NOX4 is a member of the NOX (NADPH oxidase) family of NADPH oxidases. These enzymes transport electrons from cytoplasmic NADPH across cell and organelle membranes to generate superoxide (O2−) and downstream ROS (reactive oxygen species). NOX enzymes have been shown to be involved in the regulation of a wide range of physiological functions, including cell death and survival, differentiation, proliferation, Ca2+ signalling, gene expression and migration (reviewed in [1,2]).
NOX4 was first described in 2000 [3,4]. The enzyme is highly expressed in kidney, particularly in the tubular system [3]. However, NOX4 is also expressed in many other cell types, including endothelial cells [5], osteoclasts [6], smooth muscle cells [7], fibroblasts [8], mesangial cells [9], adipocytes [10], pancreatic islets [11] and embryonic stem cells [12]. It has been suggested to be involved in stress signals in kidney [9,13] and smooth muscle cells [14], TGFβ (transforming growth factor β)-induced differentiation [8,15,16], insulin signalling [10], oxygen-sensing [17,18], cardiac differentiation [12] and transcriptional regulation [19,20].
NOX4 expression is increased by TGFβ stimulation in fibroblasts [8,15], as well as by angiotensin stimulation of mesangial cells in the kidney [9]. These stimulations were paralleled by an increase in ROS generation, suggesting that NOX4 activity is regulated, at least in part, at the transcriptional level.
Although NOX1, NOX2 and NOX3 activity depends on the presence of activator or organizer subunits (NOXAs and NOXOs) [2], NOX4 is active in cells that do not express such cytoplasmic subunits. NOX4 does require the membrane-associated subunit p22phox for function [21]; however, unlike NOX1–NOX3, it does not require the organizer subunit-interacting proline-rich domain of p22phox [22]. Data concerning its dependence on the small GTPase Rac and on the need for cell stimulation are contradictory. Although some indirect data raise the possibility of Rac involvement, reconstitution data in a variety of cell types suggest that NOX4 activity does not require Rac and is constitutive [21,23,24].
This constitutive activity of NOX4 upon heterologous expression is an interesting, but experimentally challenging, feature. It raises the possibility that NOX4 might be an inducible NOX isoform, regulated at the level of mRNA, rather than through post-translational protein modifications. However, this feature also presents a challenge for the study of NOX4 owing to the toxicity associated with sustained ROS generation, as is evident from cellular senescence upon heterologous NOX4 expression [3,4].
In the present paper, we report the generation of a cell line that expresses NOX4 upon tet (tetracycline) induction. We use this system to study basic features of NOX4 activity and its unusual pattern of ROS generation.
EXPERIMENTAL
Materials
DMEM (Dulbecco's modified Eagle's medium), HBSS (Hanks balanced salt solution), Gateway cloning system including pENTRY and DEST 30 vectors, p221 vector, Superscript II reverse transcriptase, fetal calf serum, blasticidin, neomycin (G418, geneticin), tet, cloning primers, calcein and Amplex Red were purchased from Invitrogen. RNeasy columns and DNase were purchased from Qiagen. Scopoletin, HRP (horseradish peroxidase), luminol, cytochrome c, DPI (diphenyleneiodonium chloride), thioridazine and apocynin were purchased from Sigma–Aldrich. Human kidney RNA was purchased from Clontech.
Cloning of human NOX4
Reverse transcription was carried out on human kidney RNA with Superscript II reverse transcriptase, according to the manufacturer's instructions. The full-length human NOX4 was amplified by PCR using the primers NOX4_F, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCACCATGGCTGTGTCCTGGAGG-3′, and NOX4_R, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCTCAGCTGAAAGACTCTTTATTGTATTC-3′.
The PCR product was cloned into a p221 vector and transferred into a pDEST 30 vector carrying a tet-on CMV (cytomegalovirus) promoter using the Gateway cloning system, according to the manufacturer's instructions.
Generation of inducible and stable cell lines
HEK-293 (human embryonic kidney) cells overexpressing a tet receptor (T-REx™; Invitrogen) were transfected with the NOX4-containing tet-on vector (NOX4-pDEST30) by the calcium phosphate method [25], and selected with 400 μg/ml neomycin (G418) starting 3 days after transfection. HEK-293 cells stably expressing NOX4 and NOX5 respectively were obtained by transfection of pcDNA3.1 with the full-length murine NOX4 or full-length human NOX5β cDNA inserted with neomycin as resistance gene, followed by clone selection after neomycin culture for 10–14 days [23].
Cell culture
All cells were cultured in DMEM containing 4.5 g/l glucose, supplemented with 10% foetal calf serum and 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C in air with 5% CO2. Selecting antibiotics, blasticidin (5 μg/ml) and neomycin (400 μg/ml), were maintained in transfected cell cultures throughout.
ROS measurements
Scopoletin, Amplex Red, cytochrome c reduction and luminol
Extracellular H2O2 was measured by either Scopoletin or Amplex Red. Scopoletin measurements were performed as described previously [26], with minor modifications. Briefly, detached cells (20000) were plated in 0.2 ml of HBSS containing 20 μM scopoletin and 0.5 unit/ml HRP, and where indicated, stimuli and/or inhibitors. The plate was kept at 37 °C, and fluorescence readings were taken every 1 min for 40–60 min, with excitation and emission wavelengths of 350 and 460 nm respectively, in a Victor3 Wallac or BMG Fluostar microplate reader.
Amplex Red measurements were carried out according to the manufacturer's instructions, with minor modifications. Briefly, detached cells (20000) were plated in 0.2 ml of HBSS containing 20 μM Amplex Red and 0.1 unit/ml HRP, and, where indicated, stimuli and/or inhibitors. The plate was kept at 37 °C, and fluorescence readings were taken every 1 min for 40–60 min, with excitation and emission wavelengths of 550 and 600 nm respectively, in a BMG Fluostar microplate reader.
Extracellular O2− was measured by either cytochrome c reduction or luminol, as described previously [23], with minor modifications. For cytochrome c, detached cells (20000) were plated in 0.2 ml of HBSS containing 150 μM cytochrome c, and, where indicated, stimuli and/or inhibitors. The plate was kept at 37 °C, and readings of the absorbance at 550 nm were taken every 1 min for 40–60 min in a Victor3 Wallac or BMG Fluostar microplate reader.
For luminol, detached cells (20000) were plated in 0.2 ml of HBSS containing 10 μM luminol, 0.5 unit/ml HRP, and, where indicated, stimuli and/or inhibitors. The plate was kept at 37 °C, and light emission was recorded continuously using a Victor3 Wallac or a BMG Fluostar microplate reader.
DHE (dihydroethidium)
Cells were plated in 96-well plates and, in the case of NOX4, induced with tet, 24 h before experiments. DHE (5 μM) was added to each well 5 min before addition of stimuli. Fluorescence (490 nm excitation and 600 nm emission) was recorded every 1 min for 60 min in a Fluostar microplate reader at 37 °C.
NBT (Nitro Blue Tetrazolium)
Cells were plated in 12-well plates and, in the case of T-REx™ NOX4, induced with tet 24 h before experiments. Cells were washed in HBSS and incubated with NBT (1.6 mg/ml) in HBSS at 37 °C for 45 min. After fixation in 100% methanol and a wash in methanol, the formazan precipitates were dissolved by addition of 560 μl 2M KOH and 480 μl DMSO. The amount of reduced NBT was quantified by determination of the absorbance at 630 nm. Photographs were taken before fixation, using a Nikon Coolpix CCD (charge-coupled device) camera (Nikon) mounted on an Axiovert S100 microscope (Zeiss) using a 100× objective.
Electron spin resonance
Cells were detached and resuspended in HBSS (supplemented with Ca2+ and Mg2+) to 1×107 cells/ml and incubated with 100 μM ACP (1-acetoxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine) for 45 min at 37 °C. For performing ESR measurements, aliquots of approx. 12 μl were transferred into 1.0 mm (internal diameter)/1.2 mm (external diameter) quartz capillary tubes from VitroCom (sample height of 50 mm) and sealed on both ends with Cha-Seal™ tube sealing compound (Medex International). ESR experiments were carried out at room temperature (25 °C) using an ESP300E spectrometer (Bruker BioSpin), which was equipped with a standard rectangular mode TE102 cavity. Instrument settings were: microwave power, 20 mW; microwave frequency, 9.775 GHz; magnetic field, 3480±60 G; modulation frequency, 100 kHz; modulation amplitude, 1.045 G; receiver gain, 2×103; time constant, 20.48 ms; conversion time, 40.96 ms; and total scan time, 41.9 s. Five-scan field-swept spectra were accumulated for each ESR trace.
Quantification of cell numbers
Cell numbers were determined by calcein fluorescence in order to calculate the amount of H2O2 produced/cells using 490 nm excitation and 520 nm emission filters in separate wells after 10 min of incubation with calcein (2.5 μM) at 37 °C and normalized to a standard curve of counted cell numbers.
ROS measurements in membrane preparations
Membrane were prepared as described previously [27]. Protein concentration was determined with Bradford reagent using BSA as a standard. ROS activity was determined with the Amplex Red method described above with 5 μg of protein/96-well containing 25 μM Amplex Red and 0.1 unit/ml HRP in HBSS (with Ca2+ and Mg2+).
Real-time PCR
Total RNA was isolated using RNeasy, according to the manufacturer's instructions. cDNA was synthesized from 0.5 μg of RNA, using a mix of oligo(dT) and random hexamers, with Superscript II reverse transcriptase, according to manufacturer's instructions. The cDNA equivalent to 5 ng of total RNA was robot-pipetted and PCR-amplified in a final volume of 10 μl in triplicates in an ABI PRISM 7700 detection system (PE-Applied Biosystems).
Primers for the reference genes TBP (TATA-box-binding protein), GusB (β-glucoronidase) and EEF1A1 (elongation factor-1α1) (see probe and primer sequences in Table 1), were designed by Primer Express 2.0 (Applied Biosystems). NOX4 was detected with primers and probe from Assay-on-Demand™ no. Hs00276431 from Applied Biosystems. The quantitative PCR experiments and analysis were performed with the help of the genomic platform, Genomic Research Laboratory, Geneva.
Table 1. Primers and probes used in the present study.
| (a) | |
|---|---|
| Primer | Sequence |
| Hs-TBP_for | 5′-GCCCGAAACGCCGAATATA-3′ |
| Hs-TBP_rev | 5′-CGTGGCTCTCTTATCCTCATGA-3′ |
| Hs-GusB_for | 5′-CCACCAGGGACCATCCAAT-3′ |
| Hs-GusB_rev | 5′-AGTCAAAATATGTGTTCTGGACAAAGTAA-3′ |
| Hs-EEF1A1_for | 5′-AGCAAAAATGACCCACCAATG-3′ |
| Hs-EEF1A1_rev | 5′-GGCCTGGATGGTTCAGGATA-3′ |
| (b) | |
| Probe | Sequence |
| Hs-TBP_T | 5′-CCGCAGCAAACCGCTTGGGA-3′ |
| Hs-GusB_T | 5′-CCTGACTGACACCTCCAAGTATCCCAAGG-3′ |
| Hs-EEF1A1_T | 5′-CACCTGAGCAGTGAAGCCAGCTGCTT-3′ |
Statistics
Results are expressed as means±S.E.M. for three to eight experiments performed independently. Paired Student's t test or ANOVA were performed where indicated and P<0.05 was considered significant (*P<0.05; **P<0.01; ***P<0.001).
RESULTS
ROS generation in a tet-inducible NOX4 system
Because NOX4 is constitutively active upon heterologous expression, we generated a tet-inducible system that allowed controlled NOX4 expression. We used the T-REx™ system which is based on the expression of a tet repressor that binds to a modified CMV promoter and thereby maintains the latter in an inactive state. Tet binds to the tet repressor removing it from the promoter, thereby permitting transcription. As the tet-repressor-expressing HEK-293 cells (T-REx™ cells) were established with a blasticidin resistance, blasticidin was included continuously in the culture medium to ensure high level expression of the tet repressor, which is important to minimize residual expression (leaking).
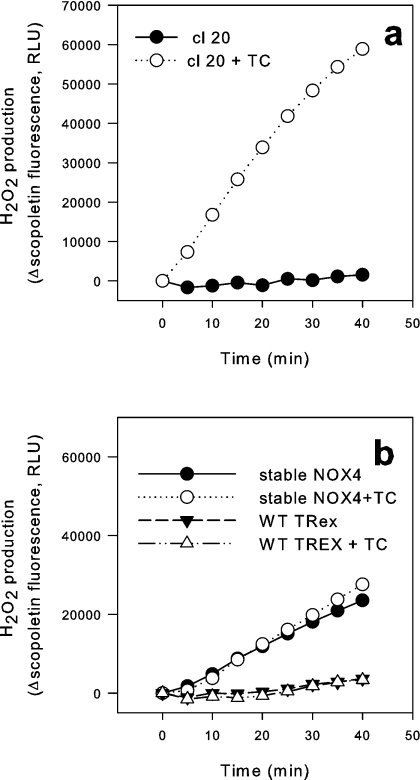
To generate inducible NOX4 cells, the T-REx™ cells were transfected with a vector construct carrying the full-length human NOX4 cDNA driven by the modified CMV promoter. After 14 days of neomycin selection, several clones were established and investigated for the release of ROS upon tet-induction using a scopoletin assay. Among six clones tested, the increase in the rate of ROS production upon tet induction ranged from no change to 161 times that of uninduced cells (results not shown). Figure 1(a) shows the clone selected for further experiments. In the absence of tet, there was no detectable release of ROS into the medium. Tet did not lead to ROS release in non-transfected cells (i.e. cells expressing the tet repressor only), nor did it enhance ROS release in cells stably expressing NOX4 (Figure 1b).
Figure 1. ROS release in tet-inducible NOX4 cells.
Tet repressor-expressing HEK-293 cells (T-REx™ cells) were transfected with an expression plasmid containing the human NOX4 sequence under the control of a modified CMV promoter as well as a neomycin selection cassette. After neomycin selection, clones (cl 20) were analysed for ROS release without or with tet (TC) (24 h) using a scopoletin fluorescence assay (a). (b) Scopoletin measurements in tet repressor-expressing HEK-293 cells (T-REx™) without NOX4 transfection as well as from stable cell lines expressing NOX4 continuously, with and without tet. Representative traces are shown; similar results were obtained on at least three independent occasions. RLU, relative light units; WT, wild-type.
Quantitative analysis of ROS release using the Amplex Red technique yielded the following results: non-transfected T-REx™ cells generated less than 0.2 fmol of H2O2/cell per h. Non-induced NOX4 T-REx™ cells produced 1.5±0.8 fmol of H2O2/cell per h, whereas tet-induced cells (1 μg/ml, 24 h) generated 83±24 fmol of H2O2/cell per h after a 24 h tet induction (n=6). To put these numbers into context, tet-induced NOX4 cells released roughly equal amounts of H2O2/cell as a reconstituted NOX2 system in HEK-293 cells (results not shown) or NOX5-expressing HEK-293 cells (see below), but approx 5–10-fold less than PMA-stimulated neutrophils (results not shown). Thus the capacity of NOX4 is probably in a similar range to that of other NOX enzymes.
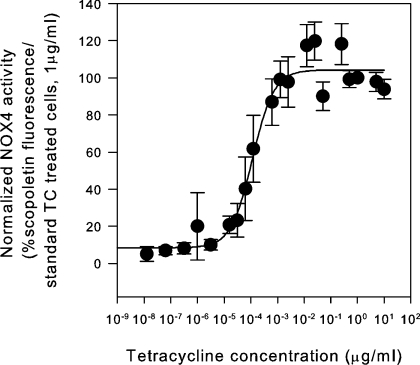
We next investigated the effect of tet concentration in the system. Cells were induced with tet concentrations ranging from 1.25 fg/ml to 10 μg/ml, and NOX4 activity was assessed by measuring ROS generation (Figure 2). In the absence of tet, background ROS generation was below 3% of that in cells induced with 1 μg/ml tet. The EC50 for tet was 91 pg/ml. We chose a concentration of 1 μg/ml for further experiments since this concentration was well within the plateau range without apparent toxic effects.
Figure 2. Induction of NOX4 mRNA and ROS release as a function of tet concentration in inducible NOX4 cells.
NOX4 T-REx™ cells (clone 20) were incubated for 24 h with tet (1.25 fg/ml to 10 μg/ml). The ROS release is presented as relative scopoletin fluorescence/h (100 corresponds to cells induced with 1 μg/ml tet, TC). The dose–response curve was fitted with a four-parameter Hill sigmoid curve for the determination of the EC50 (n=4).
Time course of induction of mRNA and functional activity
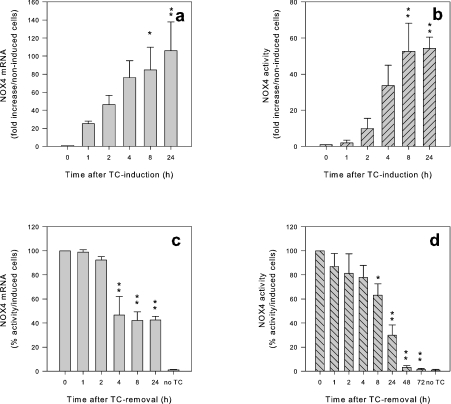
We investigated the time course of NOX4 induction by tet. Both, NOX4 mRNA and ROS production showed a time-dependent increase (Figures 3a and 3b). However, mRNA elevations were detected earlier than the increased ROS generation (1 and 2 h after tet addition respectively). The fact that ROS generation was detectable after 2 h suggests a relatively rapid transcriptional and translational processing of NOX4. Longer times of tet induction resulted in a slight reduction of activity, possibly reflecting cell toxicity (results not shown).
Figure 3. Close temporal correlation between induction of NOX4 mRNA and activity after tet-induction and omission.
T-REx™ NOX4 cells were induced for NOX4 by addition of 1 μg/ml tet (TC) for the times indicated (a and b). In (c and d), tet was withdrawn after 24 h, and the run-down was assessed at the indicated time points. (a) NOX4 mRNA was determined by quantitative PCR, normalized to three house-keeping genes and is shown as fold increase over non-induced NOX4 T-REx™ cells. (b) NOX4 activity was measured as H2O2 production/h using Amplex Red and is shown as fold increase in H2O2 produced over non-induced cells (n=4). (c) NOX4 mRNA was determined by quantitative PCR, normalized to three house-keeping genes and is shown as percentage of NOX4 mRNA after 24 h of tet-induction. (d) NOX4 activity was measured as H2O2 production/h using Amplex Red and is shown as percentage of H2O2 produced by tet-induced cells after 24 h of tet-induction (n=3–8). *P<0.05; **P<0.01.
In order to investigate the reversibility of the NOX4 induction, we followed NOX4 mRNA levels and ROS generation after tet withdrawal. NOX4 mRNA levels decreased by more than 50% after 4 h, whereas a 50% decrease in ROS required more than 8 h (Figures 3c and 3d).
NOX4 activity in membrane fractions: independence of cytosol and selectivity for NADPH over NADH
We next investigated ROS generation in membrane fractions without added cytosol using Amplex Red. As shown in Figure 4, a strong signal was only observed with NADPH as electron donor. The signal with NADH was only slightly above background values, demonstrating that NOX4 preferentially uses NADPH. Thus NOX4 is similar to other members of the NOX family, a true NADPH oxidase. No enhancement of activity was obtained by addition of cytosol (results not shown), confirming that NOX4 activity is independent of cytosolic subunits for function. Thus we conclude that NOX4 is, similarly to other members of the NOX family, selective for NADPH over NADH.
Figure 4. NOX4 is an NADPH-dependent oxidase.
T-REx™ NOX4 cells were induced with 1 μg/ml tet (TC) for 24 h, followed by lysis and membrane preparation. Membranes (5 μg/well; no addition of cytosol) were added to an Amplex Red mixture in a final volume of 200 μl. NADPH and NADH were added, and mixtures were incubated for 20 min at 37 °C before ROS production was measured by Amplex Red fluorescence. Results (means±S.E.M.) are shown as end values (n=3–4). **P<0.01.
Pharmacological profile of NOX4 activity
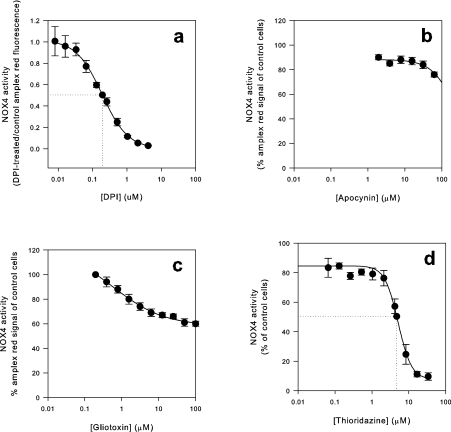
In order to characterize potential inhibitors, four known NOX2 inhibitors were tested for an effect on NOX4 activity (Figure 5). DPI, the most commonly used agent to block NOX activity, and thioridazine, an anti-psychotic drug that inhibits NOX2 activity in neutrophils [28], inhibited NOX4 with high to intermediate affinity, in a concentration range similar to that observed for NOX2. DPI blocked NOX4 activity at concentrations in the submicromolar range (IC50 of 0.2 μM), while thioridazine blocked at low micromolar concentrations (4 μM).
Figure 5. Effect of inhibitors on NOX4 activity.
T-REx™ NOX4 cells were induced with 1 μg/ml tet for 24 h before measurements. DPI (a), apocynin (b), gliotoxin (c) or thioridazine (d) were added at different concentrations, and NOX4 activity was measured by Amplex Red fluorescence. Fluorescence values from 50000 cells were recorded after 20 min and normalized to those obtained from NOX4-induced cells without inhibitor. A four-parameter Hill sigmoid curve was used for the determination of IC50 values. Results are means±S.E.M. (n=3).
In contrast, two other NOX2 inhibitors, which inhibit NOX2 in the low micromolar range inhibited NOX4 only with a very low affinity. Apocynin, which inhibits neutrophil ROS generation with an IC50 of approx. 10 μM [29], had an IC50 of >200 μM for NOX4. Gliotoxin, an Aspergillus fumigatus toxin inhibits NOX2 with an IC50 of 5–10 μM [30,31]. The effect of gliotoxin on NOX4 showed some low efficacy inhibition, but the maximal inhibition achieved was approx. 40% with 100 μM gliotoxin, therefore IC50 values could not be calculated. Thus NOX4 has a distinct profile of inhibition as compared with NOX2 (Table 2).
Table 2. A comparison of the effect of various inhibitors on NOX4 as well as on NOX2 and mitochondrial ROS generation.
Characteristics of NOX4-dependent ROS generation
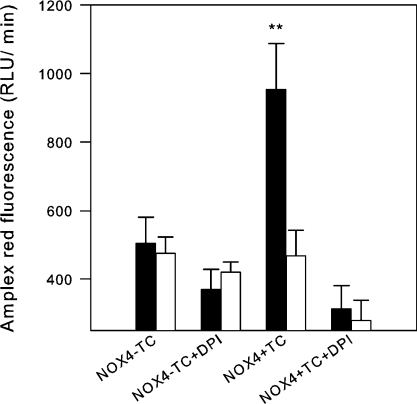
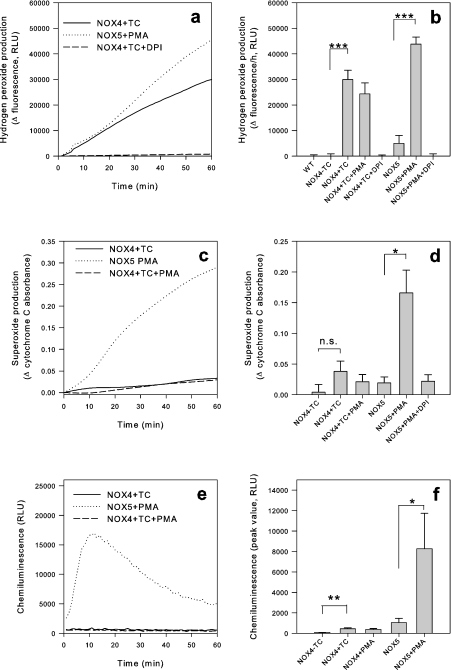
When different techniques to measure ROS release from cells were applied, we found an unusual pattern in NOX4-expressing cells. NOX4 activity induced a strong signal with probes that detect extracellular H2O2, such as scopoletin (Figures 1 and 2) and Amplex Red (Figures 3, 6a and 6b), but not with probes that detect extracellular O2−, such as cytochrome c reduction (Figures 6c and 6d) and HRP-dependent luminol-enhanced chemiluminescence (Figures 6e and 6f). This contrasts with the behaviour of other NOX enzymes, such as NOX5, which yields a strong signal with probes detecting O2− (Figures 6c–6f). Note that in these studies, PMA-stimulated NOX5 activation was used; the PMA effects on NOX5-expressing cells have characteristics comparable with the previously described ionomycin effects (results not shown, and [32]). Note also that NOX4 activity is independent of or even slightly inhibited by PMA (Figure 6b).
Figure 6. H2O2 is the major oxidative metabolite measured from NOX4-induced cells.
Cells (20000/well) were added to detection mixtures for Amplex Red (H2O2 detection), cytochrome c (O2− detection) and luminol (O2− detection) respectively. Signals were recorded for 60 min. DPI was used at 5 μM final concentration where indicated. (a, c and e) Representative traces from each set of experiments. (b, d and f) Means±S.E.M. from accumulated signals after 1 h (Amplex Red and cytochrome c) and peak signal (luminol) (n=3–8). n.s., not significant; RLY, relative light units; TC, tetracycline. *P<0.05; **P<0.01; ***P<0.001.
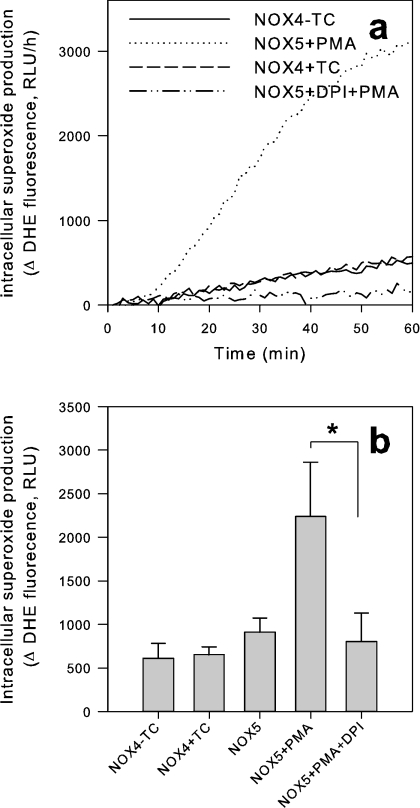
Our results suggest that NOX4 either generates H2O2 directly or that it generates intracellular O2− which dismutates intracellularly to the membrane-permeant H2O2. To distinguish between these possibilities, we used techniques to detect intracellular O2−. The DHE method has been used widely to detect intracellular O2− generation. Indeed, the non-fluorescent membrane-permeant DHE enters cells freely. Upon interaction with O2−, the membrane-impermeant ethidium cation is liberated, which becomes fluorescent upon intercalating DNA. As shown in Figure 7, no NOX4 signal was detected with the DHE method, whereas there was a clear NOX5 signal. These results are interesting, but they do not completely exclude intracellular O2− generation by NOX4. Indeed, a recent study demonstrated that the DHE method does not allow detection of O2− generated by NOX2 in the phagosome [33].
Figure 7. DHE does not detect a signal from NOX4-expressing cells.
DHE (5 μM) was added to adherent cells and fluorescence was measured for 60 min (Figure 9a). (a) Trace from representative experiment; (b) Δ values after 60 min (means±S.E.M., n=3–5). *P<0.05. RLU, relative light units; TC, tetracycline.
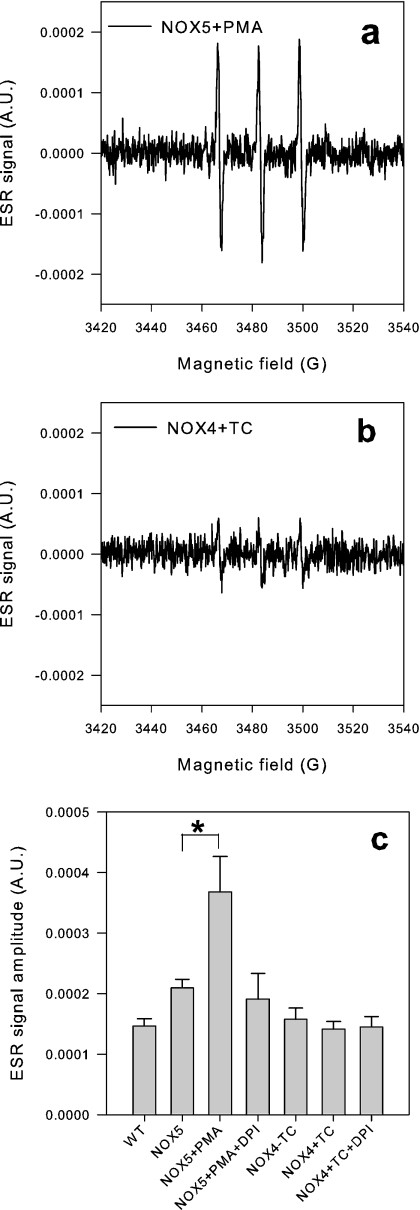
We next investigated NOX4-dependent ROS generation using ESR. With appropriate spin-trap agents, ESR can specifically detect O2−. ACP is an ESR spin-trap agent that has been shown to enter cells in its ester form which is then hydrolysed by cytosolic esterases. This hydrolysed form is able to interact with intracellular O2− [34,35]. Thus ACP is thought to be an appropriate spin-trap agent to measure intracellular O2− generation. NOX4 activity did not lead to a detectable ESR signal, whereas significant signals could be detected in PMA-stimulated NOX5 cells (Figure 8). However, the design of ACP (i.e. hydrolysis by cytosolic esterases) makes it suited to the detection of cytoplasmic O2− generation, and not for measuring O2− generation within intracellular organelles.
Figure 8. ESR detects O2− in NOX5 cells, but not in NOX4-induced cells.
Cells were detached and resuspended in HBSS to 106/ml, incubated with the spin probe ACP (100 μM) for 45 min at 30 °C before cell suspension was drawn up in capillaries and placed in the ESR spectrometer. (a and b) Representative ESR traces from NOX5 cells stimulated with PMA- and tet (TC)-induced NOX4 cells respectively. (c) Mean±S.D. of peak ESR signal for each condition (n=3). *P<0.05. A.U., arbitrary units.
Thus the DHE and the ESR results reasonably exclude NOX4-dependent O2− generation in the cytosol, and probably also in the nucleus and inner mitochondrial space, but they do not exclude the possibility that NOX4-dependent O2− generation takes place within some other intracellular organelle.
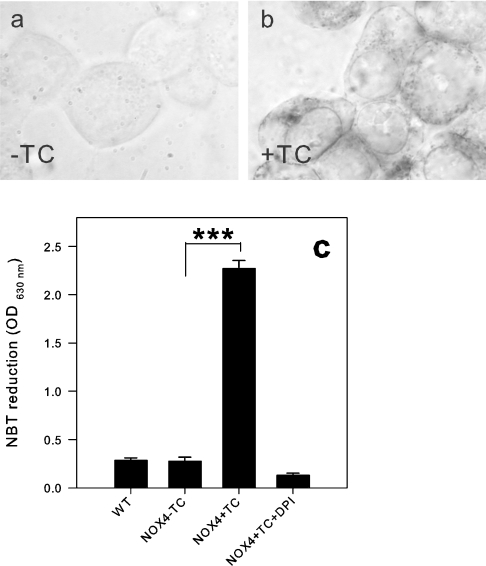
One of the oldest and most established methods to detect intracellular O2− generation is the reduction of NBT to formazan, a dark blue precipitate. As NBT needs to be reduced, only O2− (which may act as electron donor or acceptor), but not H2O2 (which is exclusively an oxidizing agent), is capable of reacting with NBT. Furthermore, NBT preferentially detects intracellular O2− [36]. As assessed by a quantitative NBT test (Figure 9), tet-induced NOX4 cells elicited a significant NBT reduction, as compared with non-induced cells. This signal was blocked completely by DPI (5 μM). The NOX4-dependent NBT signal is a good argument in favour of O2− generation. We cannot, however, formally exclude a diaphorase activity (i.e. NBT would receive electrons directly from NOX4 without a O2− intermediate). We also analysed the subcellular distribution of formazan precipitates by light microscopy. No formazan precipitates were observed in uninduced cells (Figure 9a). In tet-induced cells (Figure 9b), the precipitates showed a punctate staining within the cytoplasm. In many cells, this staining was in the perinuclear region, but occasionally also in the cell periphery. The nucleus was systematically spared, but the nuclear envelope (which is part of the endoplasmic reticulum) showed a strong staining.
Figure 9. Intracellular ROS detection with NBT is positive for NOX4-expressing cells.
NBT (1.6 mg/ml) was added to cells and plates were incubated at 37 °C for 45 min. Micrographs show NBT tests from (a) non-induced NOX4 cells and (b) tet (TC)-induced NOX4 cells. (c) Quantification of dissolved formazan by absorbance quantification at 630 nm from non-induced and tet (TC)-induced NOX4 cells (n=3–5). ***P<0.001. OD, absorbance; WT, wild-type.
DISCUSSION
In the present study, an inducible cell line was used to analyse basic biochemical properties of the NOX family NADPH oxidase NOX4. Given the fact that NOX4 generates substantial amounts of ROS without a need for cell stimulation [3,4,21], a stable expression system has inherent dangers: (i) damage to DNA which could lead to cellular senescence [3] or, in cells with deficient p53 function (e.g. HEK-293 cells), to an accumulation of DNA mutations; (ii) damage to mitochondria which may lead to increased mitochondrial ROS generation; and (iii) permanent up-regulation of antioxidant-responsive genes, which would make it difficult to distinguish the acute NOX4 effects from a long-term antioxidant response. Thus an inducible NOX4 system, as described in the present paper, is preferable to cells stably expressing NOX4.
An additional advantage of the inducible system is that it offers the possibility of studying the temporal relationship between mRNA levels and enzyme function. One of the striking results of our study is that NOX4 mRNA levels are closely followed by NOX4 activity. Unfortunately, there are presently no antibodies that reliably recognize human NOX4. Thus it is currently not possible to calculate protein half-life. However, our results based on NOX4 function suggest that the NOX4 protein is rapidly (i.e. within a time frame of hours) synthesized and degraded. Note that this is in stark contrast with available data on NOX2, where the protein and its function persist for several days after transcriptional activity is shut down [37]. Obviously, a heterologous expression system cannot determine whether NOX4 is regulated on a transcriptional level [8,15] in endogenously NOX4-expressing cells. However, our results clearly demonstrate that NOX4 has the potential to function as an inducible NOX, or ‘iNOX’.
To our knowledge, this is the first study of NOX4 activity in a broken cell system. It demonstrates unequivocally that NOX4 is functional in the absence of cytosol, reinforcing the concept that NOX4 activity is independent of cytosolic subunits. Importantly, this system also allowed the study of the NOX4 electron source. It is now clear that NOX2, NOX5 and NOX1 use NADPH specifically, and not NADH, as electron donor (reviewed in [2]). However, it has been suggested that ROS-generating enzymes, in the vascular system, might prefer NADH over NADPH [38], and for that reason several authors still use the term ‘NAD(P)H oxidase’. The present study reveals that NOX4 is indeed an NADPH oxidase with a preference for NADPH over NADH. Thus we suggest that the term NAD(P)H oxidase should no longer be used when referring to NOX enzymes.
The inhibitor analysis shown in the present study is, to our understanding, the first pharmacological profiling of NOX4. Not unexpectedly, DPI, a large-spectrum inhibitor of electron transporters including various NOX enzymes, mitochondrial oxidase and xanthine oxidase (reviewed in [2]) was found to inhibit NOX4. The following arguments suggest that the DPI effect is not due to mitochondrial inhibition: (i) inhibition of ROS generation occurred in the submicromolar range, whereas inhibition of mitochondrial oxidase occurs with an IC50 of approx. 10 μM DPI [39,40]; (ii) no ROS generation was detected unless NOX4 expression was induced by tet. Thioridazine, another efficient NOX4 inhibitor, lacks specificity, as it also inhibits mitochondrial oxidase and xanthine oxidase in an almost identical concentration range [41,42]. In contrast, neither apocynin nor gliotoxin are efficient NOX4 inhibitors. In the case of apocynin, this is expected, as apocynin is thought (i) to inhibit NOX2 subunit assembly, and (ii) to require myeloperoxidase-dependent metabolism for full activity [43]. The mode of action of gliotoxin is poorly understood, but clearly it is not a useful NOX4 inhibitor. In conclusion, NOX4 displays a unique pharmacological profile, distinct from that of NOX2 (Table 2).
The atypical pattern of NOX4-dependent ROS generation identified in the present study is most compatible with NOX4-dependent O2− generation within a tight intracellular compartment, devoid of DNA. The alternative explanation, namely direct H2O2 generation by NOX4 appears less likely since: (i) NOX enzymes are mono-electron transporters that transport single electrons across a membrane (reviewed in [2]); and (ii) H2O2 does not reduce NBT and thus could not account for the observed NBT signal. This conclusion is also in line with a recent study analysing ROS generation in smooth muscle cell membranes [44]. Localization studies of NOX4 are notoriously difficult. Antibody studies suggest a localization close to focal adhesions, within the nucleus or in the endoplasmic reticulum [20,21,24]. However, the specificity of currently available anti-NOX4 antibodies is not undisputed. The use of tagged NOX4 is also not without pitfalls, as it is hard to exclude the possibility that the tag alters cellular localization. In addition, C-terminal tags impair NOX4 activity (B. Banfi and K.-H. Krause, published work and [21]). With this limitation in mind, N-terminally eGFP (enhanced green fluorescent protein)-tagged NOX4 yielded interesting results: fluorescence displayed a punctate pattern consistent with intracellular membranes, possibly the endoplasmic reticulum [21,45]. Our data also support the hypothesis that NOX4 is localized in intracellular membranes. The subcellular localization of NBT precipitation is thought to reflect at least to some extent the site of O2− generation as the reduced NBT is water-insoluble and precipitates rapidly [36]. Thus the punctate localization of the formazan precipitations, in particular around the nuclear envelope (Figure 8) is consistent with previous studies which report NOX4 localization in the ER. An ER localization is consistent with the DHE findings. Although DHE can freely cross cell membranes, the ethidium cation generated after reduction by O2− is impermeant. Thus failure of DHE to detect a signal suggests that O2− is generated within subcellular compartments devoid of nucleic acids, such as the endoplasmic reticulum.
Fundamentally, there are at least two possible theories to explain the physiological need for an O2−-producing enzyme within intracellular membranes. NOX4 might regulate the redox environment within an intracellular organelle; this would be particularly pertinent for the endoplasmic reticulum which is supposed to maintain an oxidizing environment in order to ensure proper post-translational processing of proteins. NOX4 might, however, also regulate protein oxidation in the region surrounding the organelle through diffusion of H2O2. In this case, NOX4 might generate signals involving regulation of activity of kinases, phosphatases and transcription factors.
Acknowledgments
We thank the collaborators from the genomics platform of the NCCR (National Centers of Competence in Research), Geneva for assistance with the design of the quantitative PCR experiments, Bianca Mottironi for helpful discussions, and Marie-Claude Jaquot for technical assistance. We thank Katarzyna Pierzchała for kind assistance in performing ESR measurements. Vincent Jaquet and Sten Theander are acknowledged for their careful reading of the manuscript. This work was supported by grants from the Swiss National Science Foundations: MHV (Marie Heim-Vögtlin) grant (to L. S.) and project grant 3100A0-103725 (to K.-H. K.).
References
- 1.Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Geiszt M., Kopp J. B., Varnai P., Leto T. L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 5.Ago T., Kitazono T., Ooboshi H., Iyama T., Han Y. H., Takada J., Wakisaka M., Ibayashi S., Utsumi H., Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 6.Yang S., Madyastha P., Bingel S., Ries W., Key L. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 7.Ellmark S. H., Dusting G. J., Fui M. N., Guzzo-Pernell N., Drummond G. R. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Cucoranu I., Clempus R., Dikalova A., Phelan P. J., Ariyan S., Dikalov S., Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 9.Gorin Y., Ricono J. M., Kim N. H., Bhandari B., Choudhury G. G., Abboud H. E. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am. J. Physiol. Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 10.Mahadev K., Motoshima H., Wu X., Ruddy J. M., Arnold R. S., Cheng G., Lambeth J. D., Goldstein B. J. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchizono Y., Takeya R., Iwase M., Sasaki N., Oku M., Imoto H., Iida M., Sumimoto H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006;80:133–139. doi: 10.1016/j.lfs.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Stouffs M., Serrander L., Banfi B., Bettiol E., Charnay Y., Steger K., Krause K. H., Jaconi M. E. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorin Y., Block K., Hernandez J., Bhandari B., Wagner B., Barnes J. L., Abboud H. E. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J. Biol. Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 14.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J. C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturrock A., Cahill B., Norman K., Huecksteadt T. P., Hill K., Sanders K., Karwande S. V., Stringham J. C., Bull D. A., Gleich M., et al. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 16.Hu T., Ramachandrarao S. P., Siva S., Valancius C., Zhu Y., Mahadev K., Toh I., Goldstein B. J., Woolkalis M., Sharma K. Reactive oxygen species production via NADPH oxidase mediates TGF-β-induced cytoskeletal alterations in endothelial cells. Am. J. Physiol. Renal Physiol. 2005;289:F816–F825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maranchie J. K., Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-α transcriptional activity in von Hippel–Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–9193. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y. M., Kim B. J., Chun Y. S., So I., Choi H., Kim M. S., Park J. W. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell. Signalling. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Brar S. S., Kennedy T. P., Sturrock A. B., Huecksteadt T. P., Quinn M. T., Whorton A. R., Hoidal J. R. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am. J. Physiol. Cell Physiol. 2002;282:C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda J., Nakagawa K., Yamasaki T., Nakamura K., Takeya R., Kuribayashi F., Imajoh-Ohmi S., Igarashi K., Shibata Y., Sueishi K., Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 21.Martyn K. D., Frederick L. M., von Loehneysen K., Dinauer M. C., Knaus U. G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Kawahara T., Ritsick D., Cheng G., Lambeth J. D. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 23.Banfi B., Molnar G., Maturana A., Steger K., Hegedus B., Demaurex N., Krause K. H. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 24.Ambasta R. K., Kumar P., Griendling K. K., Schmidt H. H., Busse R., Brandes R. P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 25.Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 26.De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J. Immunol. Methods. 1985;78:323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- 27.Banfi B., Tirone F., Durussel I., Knisz J., Moskwa P., Molnar G. Z., Krause K. H., Cox J. A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 28.Traykov T., Hadjimitova V., Goliysky P., Ribarov S. Effect of phenothiazines on activated macrophage-induced luminol-dependent chemiluminescence. Gen. Physiol. Biophys. 1997;16:3–14. [PubMed] [Google Scholar]
- 29.van den Worm E., Beukelman C. J., van den Berg A. J., Kroes B. H., Labadie R. P., Van Dijk H. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur. J. Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsunawaki S., Yoshida L. S., Nishida S., Kobayashi T., Shimoyama T. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 2004;72:3373–3382. doi: 10.1128/IAI.72.6.3373-3382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida S., Yoshida L. S., Shimoyama T., Nunoi H., Kobayashi T., Tsunawaki S. Fungal metabolite gliotoxin targets flavocytochrome b558 in the activation of the human neutrophil NADPH oxidase. Infect. Immun. 2005;73:235–244. doi: 10.1128/IAI.73.1.235-244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jagnandan D., Church J. E., Banfi B., Stuehr D. J., Marrero M. B., Fulton D. J. Novel mechanism of activation of NADPH oxidase 5 (NOX5): calcium sensitization via phosphorylation. J. Biol. Chem. 2006;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldi M., Moroni P., Paape M. J., Bannerman D. D. Evaluation of assays for the measurement of bovine neutrophil reactive oxygen species. Vet. Immunol. Immunopathol. 2007;115:107–125. doi: 10.1016/j.vetimm.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Takeshita K., Fujii K., Anzai K., Ozawa T. In vivo monitoring of hydroxyl radical generation caused by X-ray irradiation of rats using the spin trapping/EPR technique. Free Radical Biol. Med. 2004;36:1134–1143. doi: 10.1016/j.freeradbiomed.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama H., Itoh O., Aoyama M., Obara H., Ohya H., Kamada H. In vivo EPR imaging by using an acyl-protected hydroxylamine to analyze intracerebral oxidative stress in rats after epileptic seizures. Magn. Reson. Imaging. 2000;18:875–879. doi: 10.1016/s0730-725x(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 36.Berridge M. V., Herst P. M., Tan A. S. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 37.Borregaard N. Development of neutrophil granule diversity. Ann. N.Y. Acad. Sci. 1997;832:62–68. doi: 10.1111/j.1749-6632.1997.tb46237.x. [DOI] [PubMed] [Google Scholar]
- 38.Griendling K. K., Minieri C. A., Ollerenshaw J. D., Alexander R. W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 39.Hool L. C., Di Maria C. A., Viola H. M., Arthur P. G. Role of NAD(P)H oxidase in the regulation of cardiac L-type Ca2+ channel function during acute hypoxia. Cardiovasc. Res. 2005;67:624–635. doi: 10.1016/j.cardiores.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Trush M. A. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues T., Santos A. C., Pigoso A. A., Mingatto F. E., Uyemura S. A., Curti C. Thioridazine interacts with the membrane of mitochondria acquiring antioxidant activity toward apoptosis: potentially implicated mechanisms. Br. J. Pharmacol. 2002;136:136–142. doi: 10.1038/sj.bjp.0704672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadjimitova V., Traykov T., Mileva M., Ribarov S. Effect of some psychotropic drugs on luminol-dependent chemiluminescence induced by O2−, ·OH, HOCl. Z. Naturforsch. 2002;57:1066–1071. doi: 10.1515/znc-2002-11-1220. [DOI] [PubMed] [Google Scholar]
- 43.Stolk J., Hiltermann T. J., Dijkman J. H., Verhoeven A. J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 44.Clempus R. E., Sorescu D., Dikalova A. E., Pounkova L., Jo P., Sorescu G. P., Lassegue B., Griendling K. K. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2006;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Buul J. D., Ferdandez-Borja M., Anthony E. C., Hordijk P. L. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signaling. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 46.Hancock J. T., Jones O. T. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem. J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hampton M. B., Winterbourn C. C. Modification of neutrophil oxidant production with diphenyleneiodonium and its effect on bacterial killing. Free Radical Biol. Med. 1995;18:633–639. doi: 10.1016/0891-5849(94)00181-i. [DOI] [PubMed] [Google Scholar]
- 48.Ali M. H., Pearlstein D. P., Mathieu C. E., Schumacker P. T. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L486–L496. doi: 10.1152/ajplung.00389.2003. [DOI] [PubMed] [Google Scholar]
- 49.Kweon Y. O., Paik Y. H., Schnabl B., Qian T., Lemasters J. J., Brenner D. A. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J. Hepatol. 2003;39:38–46. doi: 10.1016/s0168-8278(03)00178-8. [DOI] [PubMed] [Google Scholar]