Abstract
PolyP (inorganic polyphosphate) is a linear polymer of many tens or hundreds of orthophosphate residues found in a wide range of organisms, including bacteria, fungi, insects, plants and vertebrates. Despite its wide distribution in mammalian tissues and plasma, the biological functions of polyP on tumour metastasis and angiogenesis have not been previously examined. In the present study, we have shown that polyP effectively blocked in vivo pulmonary metastasis of B16BL6 cells by suppression of neovascularization, whereas it did not affect proliferation or adhesion to extracellular matrix proteins. PolyP not only inhibited bFGF (basic fibroblast growth factor)-induced proliferation and ERK (extracellular-signal-regulated kinase)/p38 MAPK (mitogen-activated protein kinase) activation of human endothelial cells, but also blocked the binding of bFGF to its cognate cell-surface receptor. Furthermore, polyP inhibited bFGF-induced in vitro and in vivo angiogenesis, suggesting that polyP possesses an anti-angiogenic activity. Since neovascularization is essential for tumour metastasis, our present findings clearly indicate that polyP has an in vivo anti-metastatic activity via its anti-angiogenic activity. Taken together with the fact that angiogenesis occurs under various normal and pathological conditions, our observations suggest that endogenous polyP may play a critical role during embryonic development, wound healing and inflammation, as well as in the progress of pathological diseases such as rheumatoid arthritis and cancer.
Keywords: angiogenesis, basic fibroblast growth factor (bFGF), endothelial cell, polyanion, inorganic polyphosphate (polyP), tumour metastasis
Abbreviations: bFGF, basic fibroblast growth factor; CAM, chorioallantoic membrane; ERK, extracellular-signal-regulated kinase; FBS, foetal bovine serum; Fc, fragment of IgG1; FGF R1, FGF receptor 1; FGF R1–Fc, a soluble chimaera protein consisting of the extracellular domain of the human FGF R1α and Fc region of human IgG1; HUVEC, human umbilical vein endothelial cell; MAPK, mitogen-activated protein kinase; MEM, minimal essential medium; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; PBST, PBS+0.05% Tween 20; polyP, inorganic polyphosphate
INTRODUCTION
Angiogenesis, the formation of new blood vessels from pre-existing vessels, occurs under various normal and pathological conditions. Although neovascularization in physiological processes is finely tuned by a balance of stimulatory and inhibitory factors, persistent and unregulated growth of new capillaries plays a prominent role in the development of pathological diseases including cancer and rheumatoid arthritis [1]. Among a large number of pro-angiogenic factors, bFGF (basic fibroblast growth factor), a heparin-binding polypeptide, is a potent endothelial mitogen. It stimulates angiogenesis in vivo and plays diverse functions during angiogenesis, wound healing and inflammation [2,3]. Tumour and stromal cells overexpress and secrete bFGF into the extracellular milieu. The binding of bFGF to a tumour or endothelial cell-surface receptor can stimulate cell growth, migration and other biological events through signal transduction cascades.
PolyP (inorganic polyphosphate) is a linear polymer of many tens or hundreds of orthophosphate residues linked by high-energy phosphoanhydride bonds [4,5]. PolyP has been found in every cell in nature. Although the biological functions of polyP have been mostly investigated in microorganisms, previous studies suggest that polyP has numerous biological functions in mammalian systems, such as the modulation of blood coagulation, mammalian cell proliferation via mTOR (mammalian target of rapamycin) activation, stimulation of osteoblast-like cell calcification, modulation of FGF activity via direct interaction and induction of apoptosis in plasma cells [6–10].
Polyanionic molecules including heparin, heparan sulfate and fucoidan have anti-angiogenic and anti-tumour activities that function by modulating the actions of growth factors released from tumour cells [11–14]. These observations suggest that polyP, a polyanionic molecule, should have an anti-angiogenic activity and can block tumour growth and metastasis. However, the biological functions of polyP with relation to tumour metastasis and neovascularization have not been studied. In the present study we show that polyP inhibits in vivo pulmonary metastasis by suppression of neovascularization without any apparent in vitro anti-proliferative or anti-adhesive activity on tumour cells. Furthermore, polyP has an anti-angiogenic activity: it inhibits bFGF-induced proliferation, ERK (extracellular-signal-regulated kinase) and p38 MAPK (mitogen-activated protein kinase) activation, capillary-like tube formation in human endothelial cells and in vivo angiogenesis via the blockage of bFGF binding to its cognate cell-surface receptor. These results suggest that polyP possesses novel anti-metastatic and anti-angiogenic activities.
MATERIALS AND METHODS
Cell culture
HUVECs (human umbilical-vein endothelial cells) were isolated from human umbilical-cord veins and cultured as described previously [15]. B16BL6 melanoma cells were grown in MEM (minimal essential medium; Invitrogen) supplemented with 10% FBS (foetal bovine serum) and 100 units/ml penicillin/100 μg/ml streptomycin. Cells were grown at 37°C under a humidified air/CO2 (19:1) atmosphere.
Pulmonary metastasis
The pulmonary metastasis assay was performed as described previously [16]. Cultured B16BL6 melanoma cells were harvested and suspended in PBS with different concentrations of polyP (chain length 75 phosphate residues; Sigma). PolyP mixture (200 μl; 50000 cells) was injected intravenously via the tail vein into each male C57BL/6 mice (5–7 weeks old). After 14 days, lungs were isolated from mice and then photographed using a digital camera (Nikon). The number of metastasized colonies were counted. Two independent experiments were performed and each experiment conducted using seven mice per group.
Histological analysis
Animal study protocols were approved by the Institutional Animal Care and Use Committee at Pohang University of Science and Technology, Pohang, Republic of Korea.
Mice were killed and lungs were removed and fixed with 10% buffered formalin solution. They were embedded in paraffin and sectioned in 4-μm thicknesses. The paraffin sections were de-paraffinized with xylene and were stained with haematoxylin and eosin. Slides were examined and photographed using an Olympus BX51 microscope.
Cell survival assay
Cell survival was assayed by measuring the conversion of the yellow, water-soluble MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] into blue, water-insoluble formazan [17]. B16BL6 cells (1×104 cells/well) were plated on to 96-well plates. After overnight incubation, different concentrations of polyP in serum-free MEM was added to cells, and incubated for 24 h. MTT (final concentration 0.5 mg/ml) was added to the cells and incubated for a further 4 h. The absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 690 nm using a microplate reader (Bio-Rad). In this assay, the value obtained from the cells treated with serum-free MEM was considered 100% viable.
[3H]Thymidine incorporation assay
A [3H]thymidine incorporation assay was carried out as described previously [18]. Briefly, B16BL6 cells were seeded at a density of 1×104 cells on to a 96-well plate. After 12 h, cells were washed with serum-free MEM and incubated for 6 h in serum-free MEM. Different concentrations of polyP in MEM containing 5% FBS was added to the cells, and incubated for 24 h, followed by the addition of 0.5 μCi/well of [3H]thymidine (Amersham) for 6 h. For HUVECs, cells were seeded at a density of 5×103 cells/well on to a gelatin-coated 96-well plate. After 12 h, cells were washed with M199 and incubated for 6 h in M199 containing 1% FBS. bFGF (1 ng/ml; R&D Systems) with different concentrations of polyP in M199 containing 5% FBS was added to the cells, and incubated for 24 h, followed by the addition of 0.5 μCi/well of [3H]thymidine for 6 h. B16BL6 cells and HUVECs were washed, solubilized with 150 μl of 0.2 M NaOH/0.2% SDS at room temperature (25 °C) for 20 min, and then neutralized with 50 μl of 0.6 M HCl. Radioactivity was determined in a liquid scintillation counter.
Cell adhesion assay
A cell-adhesion assay was carried out as described previously [19]. Briefly, 96-well plates were coated with 10 μg/ml of vitronectin, collagen IV, fibrinogen, Matrigel or gelatin overnight at 4°C. Plates were washed three times with PBS followed by blocking non-specific sites with 3% BSA in PBS for 2 h. B16BL6 cells were radiolabelled with 50 μCi of [3H]thymidine/100 mm culture dish for 24 h in MEM containing 10% FBS. After labelling, cells were washed with PBS and suspended in serum-free MEM at a concentration of 5×105 cells/ml. Radiolabelled B16BL6 cells (5×104 cells/well) with different concentrations of polyP in serum-free MEM were seeded on to plates coated with matrix proteins. After 1 h incubation, the non-adherent cells were removed by washing three times with PBS. The adherent cells were solubilized with 150 μl of 1% SDS and counted for radioactivity. The percentage of cell adhesion was then calculated by dividing the radioactivity associated with adherent cells by the total radioactivity in 5×104 cells.
Western blotting
Total proteins were collected using M-PER (Pierce), separated by SDS/PAGE (10% gel), and transferred on to PVDF membrane. The blocked membrane was then incubated with the indicated antibodies, and the immunoreactive bands were visualized using a chemiluminescent substrate.
Binding of bFGF to its receptor
bFGF (100 ng/well) in 100 μl of PBS was immobilized to 96-well plates. The wells were washed and blocked with 3% BSA in PBST (PBS+0.05% Tween 20). Various concentrations of Fc (fragment of IgG1) or FGF R1–Fc [a soluble chimaera protein consisting of the extracellular domain of the human FGF R1α (FGF receptor 1) and Fc region of human IgG1; R&D Systems] in blocking buffer was added to the wells. After a 2 h incubation, the wells were washed three times with PBST, and then the number of bound Fc or FGF R1–Fc was determined by incubation with a peroxidase-conjugated anti-human IgG using a chemiluminescent substrate. To investigate the effect of polyP on the binding of FGF R1–Fc to the immobilized bFGF, FGF R1–Fc (100 ng/ml) with different concentrations of polyP in blocking buffer was added to the bFGF-coated wells, and incubated for 2 h. The number of bound receptors was determined by incubation with a peroxidase-conjugated anti-human IgG using a chemiluminescent substrate. For control experiments, we added peroxidase-conjugated anti-human IgG with different concentrations of polyP to the Fc-coated wells (50 ng/well), incubated for 2 h, and then determined the amount of bound peroxidase-conjugated anti-human IgG using a chemiluminescent substrate.
Capillary-like tube formation assay
A capillary-like tube formation assay was performed as described previously [20]. HUVECs were plated in 150 μl of 1:1 M199 medium and a Matrigel-coated 24-well plate (20000 cells/well) in 5% FBS with 5 ng/ml bFGF in the presence or absence of polyP. After a 4 h incubation, three randomly chosen fields (×10) from each sample were photographed. Using the Scion Image program, images were transferred to a computer and then total tube areas were measured in mm2.
CAM (chorioallantoic membrane) assay
To investigate whether polyP had in vivo anti-angiogenic activity, a CAM assay was carried out as described previously [20]. Briefly, bFGF with different concentrations of polyP in a type I collagen mixture was loaded on to one-quarter pieces of a 15-mm diameter Thermonox disc (Nunc), and samples were polymerized by warming. The disc was then loaded on to the 10-day-old embryo. After a 72 h incubation, the area around the loaded disk was photographed with a Nikon digital camera and the number of newly formed vessels was counted by two observers in a double-blind manner. Assays for each test sample were carried out using 10–12 eggs.
Statistical analysis
Values are presented as means from three replicate experiments, except for pulmonary metastasis experiments and the CAM assay. All values are expressed as means±S.D. P values were calculated from Student's t tests, based on comparisons with the appropriate control samples tested at the same time, *P<0.01 or **P<0.001 respectively.
RESULTS AND DISCUSSION
PolyP blocks in vivo pulmonary metastasis of melanoma cells by the suppression of neovascularization
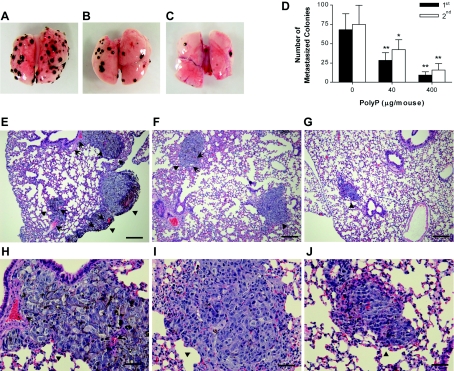
To investigate the effect of polyP on tumour metastasis, we used a previously established model of experimental lung metastasis by intravenously injecting B16BL6 melanoma cells with polyP. All mice without polyP treatment had evidence of lung metastasis, but the animals treated with polyP had a dramatic reduction in the number of metastasized lung colonies (Figures 1A–D). In the first experiment, mice treated with 0 μg of polyP/mouse, 40 μg of polyP/mouse and 400 μg of polyP/mouse had 68.0±20.7, 28.4±9.7 and 9.4±4.1 metastasized colonies per lung respectively (Figure 1D). Similar findings were also observed in the second experiment; mice treated with 0 μg of polyP/mouse, 40 μg of polyP/mouse and 400 μg of polyP/mouse had 74.8±24.8, 42.3±12.6 and 15.8±8.2 metastasized colonies per lung respectively. Overall, the reproducible inhibitory effect of polyP on lung metastasis in two independent studies suggests that polyP possesses in vivo anti-metastatic activity. Histological analyses also revealed that the number of metastasized colonies of mice treated with polyP was much lower than that of untreated (Figures 1E–J). These observations are consistent with the results shown in Figures 1(A)–1(D). Furthermore, polyP strongly inhibited tumour-induced neovascularization: polyP treatment caused a decreased microvessel density within and nearby metastasized colonies when compared with untreated controls. These results suggest that polyP has a potent in vivo anti-angiogenic activity, presumably resulting in the suppression of tumour growth and metastasis.
Figure 1. Effect of polyP on in vivo pulmonary metastasis of melanoma cells and tumour-induced neovascularization.
(A–D) Inhibition of in vivo pulmonary metastasis of melanoma cells by polyP. Cultured B16BL6 cells suspended in PBS with polyP were injected intravenously via the tail vein into mice. On day 14, the lungs were removed and photographed. Two independent experiments were performed and each experiment conducted using seven mice per group. (A) Untreated control; (B) treated with 40 μg of polyP/mouse; (C) treated with 400 μg of polyP/mouse; (D) the number of surface metastasized colonies from two independent experiments. (E–J) Suppression of neovascularization by polyP in lung metastasis. Lungs were formalin-fixed, embedded in paraffin, sectioned at 4 μm thickness, and stained with haematoxylin and eosin. Representative areas were photographed at ×100 (E–G) and ×400 (H–J). (E and H) Untreated control; (F and I) treated with 40 μg of polyP/mouse; and (G and J) treated with 400 μg of polyP/mouse. Arrowheads indicate melanoma cells and arrows represent microvessels within and nearby metastasized colonies. Scale bars, 200 μm (E–G) and 50 μm (H–J).
PolyP does not affect in vitro proliferation or adhesion of melanoma cells
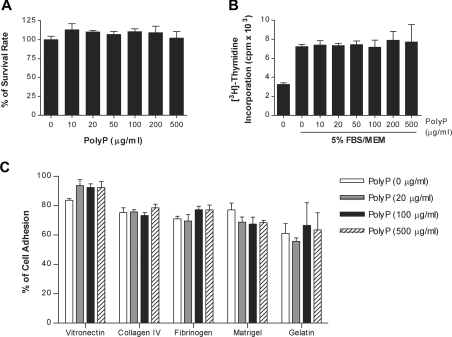
To determine the mechanisms by which polyP inhibits pulmonary metastasis, we investigated the effect of polyP on the survival and proliferation of melanoma cells using MTT and thymidine incorporation assays respectively. The presence of polyP at concentrations up to 500 μg/ml did not show any cytotoxic (Figure 2A) or anti-proliferative activity on B16BL6 cells (Figure 2B). We next investigated whether polyP blocked the adhesion of B16BL6 cells to the immobilized proteins that are components of the extracellular matrix. In the absence of polyP, 83.6%, 75.2%, 71.0%, 77.1% and 61.1% of the B16BL6 cells adhered to the wells coated with vitronectin, collagen IV, fibrinogen, Matrigel and gelatin respectively (Figure 2C). The presence of polyP at concentrations up to 500 μg/ml did not affect the adhesion of B16BL6 cells to these matrix proteins. Since the volume of mouse whole blood is around 4 ml, 40 μg of polyP/mouse and 400 μg of polyP/mouse used in the pulmonary metastasis experiments, are equivalent to about 10 μg/ml and 100 μg/ml respectively. Although we could not completely exclude the possibility that polyP influences melanoma cell survival, proliferation or adhesion to matrix proteins, our results indicate that polyP inhibits in vivo pulmonary metastasis not by its anti-proliferative or anti-adhesive activity on melanoma cells, but by its anti-angiogenic activity.
Figure 2. Effects of polyP on in vitro proliferation and adhesion of melanoma cells.
(A) B16BL6 cells were treated with different concentrations of polyP in serum-free MEM for 24 h, and the number of viable cells was determined using the MTT assay. Cells treated with serum-free MEM were considered 100% viable. (B) B16BL6 cells were stimulated with 5% serum in the absence or presence of polyP for 30 h, and [3H]thymidine incorporation was measured during the last 6 h of incubation. (C) Radiolabelled B16BL6 cells with different concentrations of polyP in serum-free MEM were seeded on to 96-well plates coated with vitronectin, collagen IV, fibrinogen, Matrigel or gelatin. After 1 h incubation, the percentage of cell adhesion was determined as described in the Materials and methods section.
PolyP inhibits bFGF-induced proliferation and ERK/p38 MAPK activation of human endothelial cells
Since angiogenesis is essential for tumour growth and metastasis, and tumour cells produce high levels of bFGF that contribute to tumour neovascularization [2,3], we investigated the effects of polyP on bFGF-induced proliferation and cell signalling of endothelial cells. PolyP blocked bFGF-induced DNA synthesis in a dose-dependent manner and half-maximal inhibition occurred at lower than 5 μg/ml of polyP (Figure 3A). Additionally, the presence of polyP at concentrations up to 20 μg/ml did not show any cytotoxic activity on HUVECs (results not shown). PolyP also inhibited bFGF-induced ERK and p38 MAPK activation in a dose-dependent manner (Figure 3B). These results suggest that polyP possesses an anti-angiogenic activity.
Figure 3. Inhibition of bFGF-induced proliferation and ERK/p38 MAPK activation of endothelial cells by polyP.
(A) HUVECs were stimulated with bFGF in the absence or presence of polyP for 30 h. The amount of incorporated [3H]thymidine was measured during the last 6 h of incubation. (B) HUVECs were pre-incubated with polyP for 1 h and then stimulated with bFGF for 10 min. Activation of ERK and p38 MAPK was determined by Western blotting.
PolyP blocks the binding of bFGF to its receptor
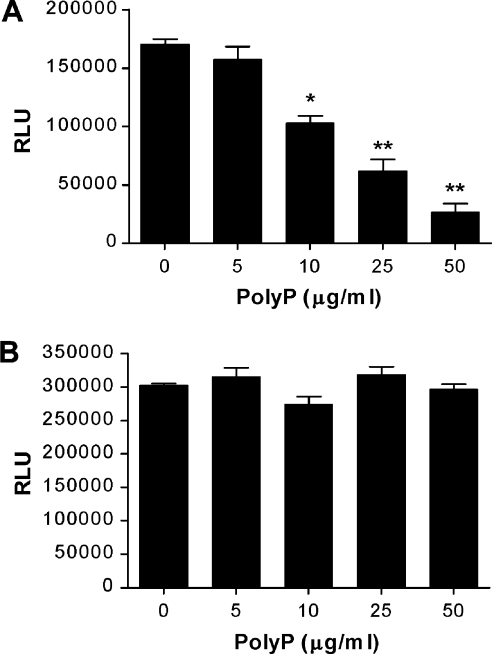
To determine the mechanism by which polyP blocks bFGF-induced proliferation and ERK/p38 MAPK activation of endothelial cells, we investigated the effect of polyP on the binding of bFGF to its cognate receptor. FGF R1–Fc, a soluble fusion protein containing the extracellular domain of FGF R1, specifically bound to the immobilized bFGF in a dose-dependent manner, whereas the control Fc did not bind to the immobilized bFGF (results not shown). PolyP blocked the interaction between bFGF and FGF R1–Fc in a dose-dependent manner (Figure 4A). However, the presence of polyP did not inhibit the interaction between Fc and peroxidase-conjugated anti-human IgG (Figure 4B). These results clearly indicate that polyP inhibits bFGF-induced proliferation, and ERK and p38 MAPK activation via the blockage of bFGF binding to its cognate cell-surface receptor.
Figure 4. Inhibition of binding of bFGF to its receptor by polyP.
(A) FGF R1-Fc with different concentrations of polyP was added to bFGF-coated wells for 2 h. The amount of bound FGF R1-Fc was determined with a peroxidase-conjugated anti-human IgG using a chemiluminescent substrate. (B) A peroxidase-conjugated anti-human IgG with different concentrations of polyP was added to Fc-coated wells for 2 h. The amount of bound peroxidase-conjugated anti-human IgG was determined using a chemiluminescent substrate. RLU, relative luminescence unit.
PolyP inhibits bFGF-induced in vitro and in vivo angiogenesis
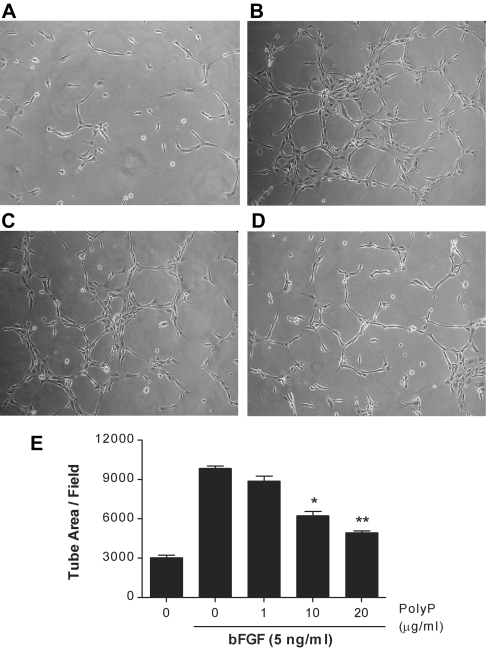
Since the central aspects of bFGF action on endothelial cells are its ability to stimulate in vitro angiogenesis by inducing the angiogenic morphogenesis of endothelial cells [2,3], we investigated the effects of polyP on bFGF-induced endothelial cell capillary-like tube formation. bFGF promoted the formation of capillary-like structures of HUVECs on Matrigel and polyP effectively blocked bFGF-induced angiogenesis (Figure 5). The ability of polyP to inhibit bFGF-induced in vivo angiogenesis was also examined using a chick CAM assay. When angiogenic bFGF (100 ng/egg) was loaded on to the chick CAM, it significantly induced neovascularization from pre-existing blood vessels over controls (Figure 6). PolyP effectively blocked bFGF-induced angiogenesis in a dose-dependent manner without any effect on the pre-existing blood vessels. The presence of 0.25, 1 and 4 μg of polyP per egg caused a 10.9%, 44.1% and 80.2% inhibition of bFGF-induced neovascularization respectively (Figure 6E). All of these results indicate that polyP has a potent in vitro and in vivo anti-angiogenic activity.
Figure 5. Inhibition of bFGF-induced in vitro angiogenesis by polyP.
After a 4 h incubation of HUVECs seeded on Matrigel, three randomly chosen fields from each sample were photographed and the tube length was measured. Representative photographs of (A) untreated controls, (B) treated with 5 ng/ml bFGF, (C) treated with bFGF+polyP (1 μg/ml) and (D) treated with bFGF+polyP (10 μg/ml). (E) Quantification of in vitro angiogenesis.
Figure 6. Inhibition of bFGF-induced in vivo angiogenesis by polyP.
bFGF (100 ng/egg) with different concentrations of polyP entrapped in type I collagen gels was loaded on to a CAM of day-10 chick embryos. After a 72 h incubation, discs and surrounding CAMs were photographed. Representative photographs of (A) untreated control, (B) treated with bFGF (100 ng/egg), (C) treated with bFGF+polyP (1 μg/egg) and (D) treated with bFGF+polyP (4 μg/egg). (E) Quantification of newly formed blood vessels. For each data point 10–12 eggs were used.
In the present study, we have shown that polyP has anti-metastatic and anti-angiogenic activities. PolyP blocked in vivo pulmonary metastasis of B16BL6 melanoma cells by reducing microvessel density without any apparent in vitro anti-proliferative or anti-adhesive activity on tumour cells. PolyP also inhibited bFGF-induced proliferation, ERK/p38 MAPK activation, capillary-like tube formation in endothelial cells and in vivo angiogenesis via the blockage of bFGF binding to its cell-surface receptor. Although we could not exclude the other mechanisms, the anti-angiogenic activity of polyP may result in its anti-metastatic activity. However, Shiba et al. [6] recently demonstrated that polyP (chain length 65 phosphate residues) directly interacts with bFGF and facilitates bFGF binding to its cell-surface receptor, resulting in the augmentation of the mitogenic activity of bFGF on fibroblast cells. The discrepancies in results might be explained by different conditions used in each experiment. Shiba et al. [6] used a somewhat higher concentration of polyP (∼105 μg/ml) in the absence of serum, whereas we investigated the effects of polyP (chain length 75 phosphate residues) at less than 20 μg/ml in the presence of serum. Another possible explanation is that the effects of polyP on bFGF are cell-type-specific. However, the precise mechanisms by which polyP antagonize bFGF actions on endothelial cell remains to be elucidated.
PolyP is present in mammalian tissues, serum and plasma, and previous studies have suggested that it may have diverse roles in mammalian systems (e.g. modulation of blood coagulation, stimulation of osteoblast-like cell calcification, and modulation of mammalian cell proliferation and apoptosis [6–10]). The present study provides the first evidence that polyP has another function, namely an anti-angiogenic activity that antagonizes the action of bFGF on endothelial cells.
Neovascularization is crucial for sustained tumour growth, because it allows oxygenation and nutrient perfusion to the tumour as well as removal of waste. Angiogenesis is also responsible for the increased tumour cell entry into circulation and metastasis [1]. The acquisition of an angiogenic phenotype is considered decisive for tumour progression, and anti-angiogenic molecules are able to block tumour growth and metastasis [21], suggesting that development of inhibitors which block the formation of new blood vessels can be an attractive therapeutic approach for the treatment of both primary and metastatic cancer. Tumour cells produce high levels of bFGF that contribute to the development of solid tumours by promoting tumour neovascularization. Binding of bFGF to their endothelial cell-surface receptors can stimulate endothelial cell growth, migration, tube formation and other biological events through signal transduction cascades, resulting in the stimulation of angiogenesis [2,22]. Therefore molecules that block the bFGF action may be useful for the treatment of cancer [23,24]. The concentrations of polyP in plasma and serum have been reported to be 12–14 μM and 39–49 μM in serum (in terms of phosphate residues) [25]. Taken together with the fact that angiogenesis occurs under various normal and pathological conditions and that polyanionic molecules such as heparan sulfate can modulate the actions of various kinds of growth factors, including bFGF [11–14], our present observations suggest that endogenous polyP may play diverse roles during embryonic development, wound healing and inflammation, as well as in the progression of pathological diseases such as rheumatoid arthritis and cancer. Furthermore, polyP may be a candidate for developing an anti-angiogenic agent for the treatment of angiogenesis-related diseases.
Acknowledgments
This work was supported by grants from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (02-PJ1-PG3-20801-0016), the POSTECH Research Fund, and KOSEF (the Korea Science and Engineering Foundation) funded by the Korea government (MOST) (R15-2004-033-05001-0). We are grateful to Mr. Hee-Yeoul Park (Postech Biotech Center, Pohang University of Science and Technology, Pohang, Republic of Korea) for histological analysis.
References
- 1.Carmeliet P., Jain R. K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Powers C. J., McLeskey S. W., Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 3.Burger P. E., Coetzee S., McKeehan W. L., Kan M., Cook P., Fan Y., Suda T., Hebbel R. P., Novitzky N., Muller W. A., Wilson E. L. Fibroblast growth factor receptor-1 is expressed by endothelial progenitor cells. Blood. 2002;100:3527–3535. doi: 10.1182/blood.V100.10.3527. [DOI] [PubMed] [Google Scholar]
- 4.Brown M. R., Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornberg A., Rao N. N., Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Shiba T., Nishimura D., Kawazoe Y., Onodera Y., Tsutsumi K., Nakamura R., Ohshiro M. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J. Biol. Chem. 2003;278:26788–26792. doi: 10.1074/jbc.M303468200. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Fraley C. D., Faridi J., Kornberg A., Roth R. A. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11249–11254. doi: 10.1073/pnas.1534805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawazoe Y., Shiba T., Nakamura R., Mizuno A., Tsutsumi K., Uematsu T., Yamaoka M., Shindoh M., Kohgo T. Induction of calcification in MC3T3-E1 cells by inorganic polyphosphate. J. Dent. Res. 2004;83:613–618. doi: 10.1177/154405910408300806. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Ruiz L., Gonzalez-Garcia I., Castro C., Brieva J. A., Ruiz F. A. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica. 2006;91:1180–1186. [PubMed] [Google Scholar]
- 10.Smith S. A., Mutch N. J., Baskar D., Rohloff P., Docampo R., Morrissey J. H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zugmaier G., Lippman M. E., Wellstein A. Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J. Natl. Cancer Inst. 1992;84:1716–1724. doi: 10.1093/jnci/84.22.1716. [DOI] [PubMed] [Google Scholar]
- 12.Danesi R., Del Bianchi S., Soldani P., Campagni A., La Rocca R. V., Myers C. E., Paparelli A., Del Tacca M. Suramin inhibits bFGF-induced endothelial cell proliferation and angiogenesis in the chick chorioallantoic membrane. Br. J. Cancer. 1993;68:932–938. doi: 10.1038/bjc.1993.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyanagi S., Tanigawa N., Nakagawa H., Soeda S., Shimeno H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003;65:173–179. doi: 10.1016/s0006-2952(02)01478-8. [DOI] [PubMed] [Google Scholar]
- 14.Presta M., Leali D., Stabile H., Ronca R., Camozzi M., Coco L., Moroni E., Liekens S., Rusnati M. Heparin derivatives as angiogenesis inhibitors. Curr. Pharm. Des. 2003;9:553–566. doi: 10.2174/1381612033391379. [DOI] [PubMed] [Google Scholar]
- 15.Gho Y. S., Kleinman H. K., Sosne G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 1999;59:5128–5132. [PubMed] [Google Scholar]
- 16.Hart I. R., Fidler I. J. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980;40:2281–2287. [PubMed] [Google Scholar]
- 17.Vistica D. T., Skehan P., Scudiero D., Monks A., Pittman A., Boyd M. R. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51:2515–2520. [PubMed] [Google Scholar]
- 18.Kim Y. M., Hwang S., Kim Y. M., Pyun B. J., Kim T. Y., Lee S. T., Gho Y. S., Kwon Y. G. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 19.Genersch E., Schuppan D., Lichtner R. B. Signaling by epidermal growth factor differentially affects integrin-mediated adhesion of tumor cells to extracellular matrix proteins. J. Mol. Med. 1996;74:609–616. doi: 10.1007/s001090050064. [DOI] [PubMed] [Google Scholar]
- 20.Kim C. W., Lee H. M., Lee T. H., Kang C., Kleinman H. K., Gho Y. S. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 21.Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 22.Friesel R. E., Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 1995;9:919–925. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- 23.Kovalenko D., Yang X., Nadeau R. J., Harkins L. K., Friesel R. Sef inhibits fibroblast growth factor signaling by inhibiting FGFR1 tyrosine phosphorylation and subsequent ERK activation. J. Biol. Chem. 2003;278:14087–14091. doi: 10.1074/jbc.C200606200. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa T., Takayama K., Takakura N., Kitano S., Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002;9:633–640. doi: 10.1038/sj.cgt.7700478. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz B., Leuck J., Kohl D., Muller W. E., Schroder H. C. Anti-HIV-1 activity of inorganic polyphosphates. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 1997;14:110–118. doi: 10.1097/00042560-199702010-00003. [DOI] [PubMed] [Google Scholar]