Abstract
The requirement of DAG (diacylglycerol) to recruit PKD (protein kinase D) to the TGN (trans-Golgi network) for the targeting of transport carriers to the cell surface, has led us to a search for new components involved in this regulatory pathway. Previous findings reveal that the heterotrimeric Gβγ (GTP-binding protein βγ subunits) act as PKD activators, leading to fission of transport vesicles at the TGN. We have recently shown that PKCη (protein kinase Cη) functions as an intermediate member in the vesicle generating pathway. DAG is capable of activating this kinase at the TGN, and at the same time is able to recruit PKD to this organelle in order to interact with PKCη, allowing phosphorylation of PKD's activation loop. The most qualified candidates for the production of DAG at the TGN are PI-PLCs (phosphatidylinositol-specific phospholipases C), since some members of this family can be directly activated by Gβγ, utilizing PtdIns(4,5)P2 as a substrate, to produce the second messengers DAG and InsP3. In the present study we show that βγ-dependent Golgi fragmentation, PKD1 activation and TGN to plasma membrane transport were affected by a specific PI-PLC inhibitor, U73122 [1-(6-{[17-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione]. In addition, a recently described PI-PLC activator, m-3M3FBS [2,4,6-trimethyl-N-(m-3-trifluoromethylphenyl)benzenesulfonamide], induced vesiculation of the Golgi apparatus as well as PKD1 phosphorylation at its activation loop. Finally, using siRNA (small interfering RNA) to block several PI-PLCs, we were able to identify PLCβ3 as the sole member of this family involved in the regulation of the formation of transport carriers at the TGN. In conclusion, we demonstrate that fission of transport carriers at the TGN is dependent on PI-PLCs, specifically PLCβ3, which is necessary to activate PKCη and PKD in that Golgi compartment, via DAG production.
Keywords: diacylglycerol (DAG), Golgi, GTP-binding protein βγ subunits (Gβγ), phospholipase C (PLC), protein kinase D (PKD), trafficking
Abbreviations: BFA, Brefeldin A; DAG, diacylglycerol; DGK, sn-1,2-diacylglycerol kinase; ER, endoplasmic reticulum; Gβγ, GTP-binding protein βγ subunits; GFP, green fluorescent protein; GPCR, G-protein-coupled receptor; GST, glutathione S-transferase; HA, haemagglutinin; HEK-293 cells, human embryonic kidney cells; HEK-293T cells, HEK-293 cells expressing the large T-antigen of SV40 (simian virus 40); IQ, ilimaquinone; m-3M3FBS, 2,4,6-trimethyl-N-(m-3-trifluoromethylphenyl)benzenesulfonamide; NDGA, nordihydroguaiaretic acid; NRK cell, normal rat kidney cell; PA, phosphatidic acid; PH domain, pleckstrin homology domain; PLC, phospholipase C; PI-PLC, phosphatidylinositol-specific PLC; PKC, protein kinase C; siRNA, small interfering RNA; TGN, trans-Golgi network; U73122, 1-(6-{[17-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione; VSV, vesicular stomatitis virus; VSV-G, VSV glycoprotein; wt, wild-type
INTRODUCTION
Since the discovery of IQ (ilimaquinone)'s role in Golgi fragmentation [1], a wide variety of new vesicle trafficking regulatory proteins have been found and their cellular localization and physical interactions described, which led to the understanding of how the pathway involving transport of cargo-filled vesicles was configured. These components include heterotrimeric GTP-binding proteins, specifically βγ subunits [2–4], protein kinases, like PKC (protein kinase C) [4] and PKD (protein kinase D) [3,5–7], and lipids, such as DAG (diacylglycerol) [8].
Even though the above mentioned are key regulatory components, some gaps are found that cannot be explained in a working model for vesicle trafficking regulation. One of these void spaces is the production of DAG, which is not only necessary to activate PKC, but also to recruit PKD to Golgi membranes in order to be activated and generate vesicle fission at the TGN (trans-Golgi network).
PI-PLCs [phosphatidylinositol PLCs (phospholipases C)] are a protein family composed of 13 members (β1–β4, γ1, γ2, δ1, δ3, δ4, ϵ, ζ, η1 and η2) [9], each one catalysing the production of the secondary messengers DAG and InsP3 from PtdIns(4,5)P2. Several of these proteins can be activated by the interaction of their PH domains (pleckstrin homology domains) with the βγ subunits of G-proteins [10–12]. This very interaction allows them to translocate to membranes, where they will find their main substrate to produce both secondary messengers, and later to activate downstream effectors, such as PKCs.
The fact that some PI-PLCs can be activated by Gβγ (GTP-binding protein βγ subunits), and activate PKCs via DAG production, makes these proteins excellent candidates to fill one important gap in the vesicle fission regulating pathway at the TGN.
In the present study, we describe for the first time that PI-PLC activity is necessary to regulate transport carrier fission, and that PLCβ3 is the specific member of this family involved in such a regulation, whose essential function is the DAG-dependent PKCη and PKD activation via Gβγ, necessary to sustain stable trafficking between TGN and the cell surface.
EXPERIMENTAL
cDNA constructs
pCDNA3.1 plasmids expressing FLAG–β1 and HA (haemagglutinin)–γ2, and with histidine tags were a gift from Silvio Gutkind [NIH (National Institutes of Health), Bethesda, MD, U.S.A.]. PKD plasmids were constructed by Dr Yusuke Maeda (Department of Immunoregulation, Research Institute for Microbial Diseases, University of Osaka, Osaka, Japan). FLAG–PKCη was a gift from Dr Motoi Ohba (Department of Microbiology, Showa University, Tokyo, Japan).
PLCβ1 expressing construct was kindly provided by Dr Sue Goo Rhee (Laboratory of Cell Signaling, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, U.S.A.), and PLCβ3 plasmid was a gift from Professor Pann Ghill Suh (Department of Life Science, Pohang University of Science and Technology, Pohang, South Korea).
Cell culture and transfections
HeLa ATCC cells and HEK-293T cells [HEK-293 cells (human embryonic kidney cells) expressing the large T-antigen of SV40 (simian virus 40)] were cultured in DMEM (Dulbecco's modified Eagle's medium; Cellgro), supplemented with 10% (v/v) FBS (fetal bovine serum; Gibco BRL). NRK cells (normal rat kidney cells) were grown in α-MEM (α modification of Eagle's medium; Cellgro). HeLa ATCC cells were transfected by established procedures by either calcium phosphate [13] or Lipofectamine™ 2000 (as recommended by Invitrogen). For both procedures, a final concentration of 4 or 0.5 μg respectively of plasmid or a combination of plasmids was transfected into 1.8×105 cells/well grown on coverslips previously coated with pronectin F (Biosource International) and covered with 500 μl of the corresponding culture media. Cells were fixed 24 h after transfection for immunofluorescence microscopy. In those cases where cell lysates for Western blot were necessary, the procedure for transfection was the same, scaled up for 100 mm cell culture plates.
The PI-PLC inhibitor U73122 [1-6-{[17-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione], its inactive analogue U73343 and the PI-PLC activator m-3M3FBS [2, 4, 6-trimethyl-N-(m-3-trifluoromethylphenyl)benzenesulfonamide] were obtained from Calbiochem.
Immunofluorescence microscopy and Western blot
The following antibodies were used: anti-FLAG and β-tubulin (Sigma–Aldrich), GST (glutathione S-transferase; Amersham Biosciences) and TGN38 (BD Biosciences). Anti-TGN46 antibody was a gift from Dr S. Ponnambalam (Centre for Structural Molecular Biology, University of Leeds, Leeds, U.K.). Anti-phospho-Ser744/Ser748 PKC/PKD was obtained from Cell Signaling Technology. Anti-phospho-Thr655 PKCη was from Biosource International. PLC antibodies were from Santa Cruz Biotechnology [PLCβ1 (G-12), PLCβ2 (H-255), PLCβ3 (C-20) and PLCγ1 (1249)], BD Transduction Laboratories (PLCδ1) and Upstate Cell Signaling Solutions (PLCϵ).
Transfected cells on coverslips were fixed for 10 min in 4% formaldehyde in PBS, blocked for 15 min with a solution containing 2.5% (v/v) horse serum, 0.02% sodium azide and 0.1% Tween 20 (blocking solution). Appropriate antibodies diluted in blocking buffer were added, and cells were incubated for 30 min. After washing twice with PBS/0.1% Tween 20, 1:500 dilutions in blocking buffer of the selected fluorescent goat antibody (Jackson ImmunoResearch Laboratories) were added for a 30 min incubation. The cells were washed twice again with PBS/Tween 20, and mounted on slips with gelvatol {140 mM NaCl, 10 mM KH2PO4/Na2HPO4, 25 g of polyvinyl alcohol, 50 ml of glycerol and 6.74 g of DABCO (1,4-diazadicyclo[2.2.2]octane), pH 8.6, for 200 ml}. The entire procedure was performed at room temperature (25 °C). Cells were visualized with a Nikon Microphot-FXA microscope. All the slips were observed with a ×60 objective, and the pictures were taken with a DP30 monochrome digital camera (Olympus). The images were analysed on a Windows PC with the software MagnaFIRE 2.1 (Optronics). The filters used were B2A (for Texas Red staining) and B2E [for Cy2 staining and GFP (green fluorescent protein)] (Nikon).
Western blots were prepared by following the manufacturer's protocol for Phospho-PKD/PKC (Ser744/Ser748) (Cell Signaling Technology). Secondary goat horseradish peroxidase-conjugated antibodies were obtained from Jackson ImmunoResearch Laboratories. The chemiluminescence reagent used to develop the blots was from PerkinElmer, and the imaging film, X-Omat Blue XB-1, was from Eastman Kodak.
All the densitometric scans were performed using the NIH Image 1.62 software.
Protein purification
HEK-293T cells were transfected with β1γ2 constructs with histidine tags by the calcium phosphate method into twenty 150 mm plates. Cells were washed 48 h after transfection with PBS and incubated for 30 min on ice with 1 ml of lysis solution (50 mM NaH2PO4, 500 mM NaCl, 1% CHAPS and 10 mM imidazole).
The cells were passed through a series of decreasing pore size gauge needles (18G11/2, 20G11/2 and 25G5/8) 20 times each, and lysed in a Dounce homogenizer on ice. Cell lysates were cleared by centrifugation at 6000 g for 30 min in a refrigerated centrifuge. The expressed proteins were purified using nickel–agarose columns and eluted from the column with imidazole following the manufacturer's protocol (Qiagen). Recombinant proteins were analysed by SDS/PAGE.
Purified proteins were dialysed overnight against buffer used in semi-permeabilization assays and stored at −80 °C in 50 μl aliquots.
Cell permeabilization
Cells were permeabilized as described previously [14]; 10 nM β1γ2 was used for each coverslip with semi-permeabilized cells.
Transport assays
HeLa cells were transfected with a plasmid codifying the tsO45 mutant VSV-G [VSV (vesicular stomatitis virus) glycoprotein] protein with a GFP tag. Five hours after transfection, cells were incubated overnight at 40 °C to allow accumulation of the G-protein in the ER (endoplasmic reticulum). Then, they were incubated for 2 h at 20 °C, allowing the G-protein to be transported from the ER to the Golgi. Cycloheximide (10 g/ml; Calbiochem) was added during the last hour of incubation to block the production of new protein. The cells were then incubated for 1 h at 32 °C, and prepared for immunofluorescence microscopy, to quantify localization of VSV-G protein at the cell surface.
DGK (sn-1,2-diacylglycerol kinase) assay
Purified rat (Rattus norvegicus) Golgi membranes [15] were incubated for 30 min at 32 °C with or without m-3M3FBS or purified β1γ2, in PBS (final volume 50 μl). The samples were then brought to 0.8 ml with PBS, and homogenized with 3 ml of chloroform/methanol (2:1, v/v). After washing them with 0.2 vol. of 0.9% NaCl, they were centrifuged for 2 min at 5000 g, and the lower phase (lipids) was recovered. The samples were then dried with a nitrogen stream, and resuspended in kinase buffer (16 mM Tris/HCl, pH 7.4, 10 mM MgCl2, 100 μM ATP and 0.6 mM dithiothreitol), with 5 μCi of [γ-32P]ATP, and 10 units of recombinant Escherichia coli DGK (Calbiochem). The mixture was then incubated for another 30 min at 25 °C, washed twice with PBS, dried, and dissolved in 20 μl of chloroform/methanol (95:5). The final samples were applied to a TLC plate (silica gel H-60 on an aluminium sheet; Merck), and developed in a closed glass chamber with 200 ml of chloroform/methanol/acetic acid (65:15:5).
Finally, the plate was air-dried, and exposed against an X-Omat Blue XB-1 film (Eastman Kodak). Densitometric scans were performed using the NIH Image 1.62 software.
siRNA (small interfering RNA) transfections
HeLa cells were transfected with siRNAs (Dharmacon) against different PI-PLCs, using oligofectamine (as recommended by Invitrogen). The sequences of the transfected siRNAs were: PLCβ1, AACAAUUCUCCUUUCGUUUGA (GenBank® accession no. BC117231, nts 1275–1293); PLCβ2-a, AACCAUCAUCCUGUCGUUUGA (GenBank® accession no. NM_004573, nts 1506–1524); PLCβ2-b, AAAGAUGCAGCUCAACUCUGA (GenBank® accession no. NM_004573, nts 767–786); PLCβ2-c, AAAGAUCCUUGUGAAGCUCAA (GenBank® accession no. NM_004573, nts 749–768); PLCβ3, CCUGUGGCCUCAAAUUCAA (GenBank® accession no. BC032659, nts 448–466); PLCγ1, AAGAAUCGUGAGGAUCGUAUA (GenBank® accession no. NM_002660, nts 619–637); PLCδ1, AAACACGGAGUUUGCGUUUGA (GenBank® accession no. NM_006225, nts 2175–2193); PLCϵ, AAACACCAACCUGACAAUUGA (GenBank® accession no. NM_016341, nts 4398–4416).
Protein expression levels were measured by Western blot at 24, 48 and 72 h. At 72 h post-transfection, the levels of all the proteins were reduced more than 90% compared with non-transfected cells.
In those experiments where exogenous protein expression was desired (such as GST–PKD1, GFP–VSV-G tsO45, or rat PLCs), their transfection was performed with Lipofectamine™ 2000, 48 h after siRNA transfection, by incubating the cells for another 24 h before preparing cell extracts, fixing the cells for immunofluorescence, or shifting temperatures for transport assays.
RESULTS
βγ-dependent Golgi fragmentation and PKD1 activation are blocked by a specific PI-PLC inhibitor
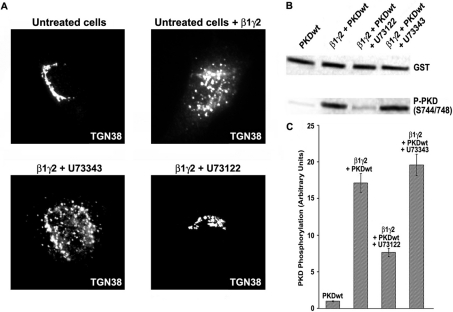
We have previously employed the semi-permeabilized cell assay to monitor the effect of total [2,3] and specific [4] Gβγ subunits in Golgi stability. In order to test our hypothesis that PI-PLCs are involved in Gβγ-dependent fission of transport carriers, we incubated NRK semi-permeabilized cells with the specific PI-PLC inhibitor U73122 for 10 min [16]. Then, purified β1γ2 complex was added to the cells, to incubate them again for 30 min at 32 °C, always in the presence of U73122. An inactive analogue of this inhibitor, U73343, was used as a negative control. The cells were then fixed, blocked, and incubated with TGN38 antibodies, in order to detect the TGN by immunofluorescence microscopy. As is shown in Figure 1(A) (lower left panel), cells treated with the inactive analogue U73343 displayed a completely fragmented Golgi, as was observed with cells incubated with β1γ2 alone (Figure 1A, upper right panel). However, cells incubated in the presence of the PI-PLC inhibitor U73122 showed an intact TGN (Figure 1A, lower right panel), the same as untreated cells (Figure 1A, upper left panel). The effect of the inhibitor was observed in all of the cells.
Figure 1. Inhibitory effect of U73122 on β1γ2-dependent Golgi fragmentation and PKD activation.
(A) Purified His–β1γ2 was added to semi-permeabilized NRK cells, previously incubated with U73122 (lower right panel) or U73343 (lower left panel). After 30 min, the cells were treated for immunofluorescence, and Golgi membranes were detected with anti-TGN48 antibodies. Untreated cells were incubated only with (upper right panel) or without (upper left panel) purified His–β1γ2. (B) HeLa cells were co-transfected with FLAG–β1–HA–γ2 and GST–PKD1, and incubated for 16 h in the presence of U73122 or U73343. Cell lysates were prepared and analysed by Western blot to monitor PKD1 activity (activation loop phosphorylation). (C) Densitometry of the experiment described in (B). All the experiments shown in (A) were repeated five times, and values in (C) are the means (±S.D., vertical bars) for four separate experiments.
To test if PLC inhibition was connected to our previously described pathway [4], we co-transfected HeLa cells with β1γ2 and GST–PKD1wt (wild-type), and incubated these cells in the presence of 5 μM U73122 or U73343 for 16 h after transfection. Then, whole cell extracts were prepared and analysed by SDS/PAGE followed by Western blot. Figure 1(B) (third lane from left) shows that in the presence of U73122, PKD1 activation loop phosphorylation was reduced to one-third (Figure 1C), in contrast with the activation level attained by expression of β1γ2 alone (Figure 1B, second lane from left) or combined with the inactive analogue U73343 (Figure 1B, fourth lane from left).
From the above results, we are able to postulate that PI-PLCs could operate as regulators of Gβγ/PKCη/PKD-dependent Golgi fragmentation.
U73122 affects transport of VSV-G from TGN to plasma membrane
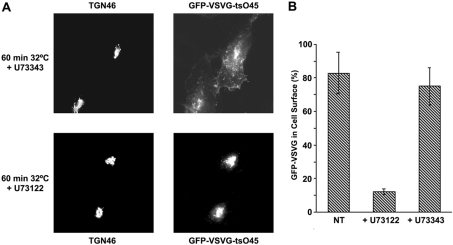
To address PI-PLCs involvement either in the regulation of the Golgi structure itself, or in changes in the trafficking equilibrium between the TGN and the plasma membrane, HeLa cells were transfected with the thermosensitive variant of G-protein from the VSV (VSV-G tsO45), attached to a GFP tag to track its intracellular trafficking by immunofluorescence. Cultures were treated with 5 μM U73122 or U73343 for 16 h after transfection, and subjected to temperature shifts. After accumulation of VSV-G at the TGN, cells were incubated at 32 °C for 60 min, and then assessed by immunofluorescence analysis.
It was observed that those cells whose transport was measured in the presence of the PI-PLC inhibitor U73122, showed a blockage in trafficking of GFP–VSV-G, since only approx. 10% of the transfected cells (Figure 2B) displayed this protein at the cell surface, while the remaining cells had VSV-G accumulated at the TGN (Figure 2A, lower panels). Cells incubated with the inactive analogue U73343 (Figure 2A, upper panels) and untreated cells (results not shown), exhibited VSV-G protein reaching the cell surface in approx. 70–90% of GFP–VSV-G-expressing cells (Figure 2B).
Figure 2. U73122 blocking effect on TGN to plasma membrane transport.
(A) HeLa cells were transfected with the thermosensitive mutant GFP–VSV-G tsO45. After accumulation of this protein in TGN at 20 °C, the cells were incubated at the permissive temperature for 60 min, and then analysed by immunofluorescence to monitor the localization of VSV-G. All the procedure was performed in the presence of U73122 or U73343, except for non-treated cells (NT). (B) Measurement of the experiment described in (A), depicted as a percentage of GFP–VSV-G-expressing cells where this protein has reached the cell surface. Values are the means (±S.D., vertical bars) for three separate experiments.
These results support our hypothesis that PI-PLCs are involved in βγ/PKCη/PKD-dependent vesicle fission at the TGN, therefore regulating transport between this Golgi compartment and the plasma membrane.
The PI-PLC activator m-3M3FBS induces Golgi apparatus fragmentation and PKD1 phosphorylation at its activation loop
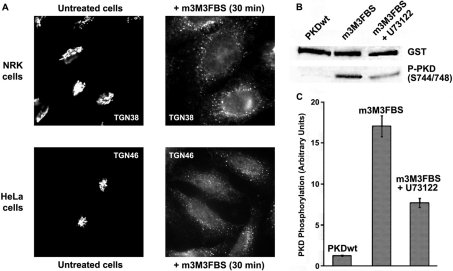
To reinforce further our theory that PI-PLCs are implicated in transport carrier formation at the TGN, we utilized a new compound, m-3M3FBS, which was described as the first specific PI-PLC activator [17]. We tested this compound in NRK and HeLa cells, incubating them with different concentrations of m-3M3FBS and at various time periods, and found that 50 μM of the drug induced a complete Golgi fragmentation in all its compartments (Figure 3A) after a 15–30 min incubation. NRK cells have shown more sensitivity to this compound, since they displayed complete Golgi fragmentation at lower concentrations (25 μM for 30 min) and at shorter incubation times (15 min at 50 μM) than HeLa cells.
Figure 3. PI-PLC activator m-3M3FBS induces Golgi fragmentation and PKD1 activation.
(A) NRK and HeLa cells were incubated respectively with 25 and 50 μM m-3M3FBS for 30 min. Then, the integrity of TGN structure was analysed by immunofluorescence. (B) Lysed extracts from GST–PKD1-transfected HeLa cells were analysed by Western blot to measure PKD1 activation. In some experiments, cells were incubated with U73122 before and during m-3M3FBS treatment (third lane from left). (C) Densitometry of the experiment described in (B). Values are means (±S.D., vertical bars) for three separate experiments.
We also monitored PKD1 phosphorylation in the presence of m-3M3FBS. HeLa cells transfected with GST–PKD1wt, were incubated with 50 μM m-3M3FBS for 30 min, and the cell extracts were then analysed by Western blot. Cells treated with this compound showed increased phosphorylation at the activation loop of PKD1 (Figure 3B, second lane from left), which correlates with our previously described observations that PI-PLCs are involved in the regulation of this kinase phosphorylation. When GST–PKD1wt-transfected cells were previously incubated for 10 min with the PI-PLC inhibitor U73122 (Figure 3B, third lane from left), the effect of m-3M3FBS on PKD1 phosphorylation was reduced to one third of the control (Figure 3C). Significantly, m-3M3FBS-induced Golgi fragmentation was also inhibited in cells previously treated with U73122 (results not shown).
We can also affirm that m-3M3FBS enhancement of Golgi fragmentation and PKD1 activation is directly related to its effect on PI-PLCs rather than to Ca2+ homoeostasis [18], since addition of a calcium chelator {BAPTA [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid]} showed no inhibitory effect on these processes (results not shown). These findings also correlate with the fact that m-3M3FBS activation of PLC, measured as inositol phosphate production [18], reached a peak just after 20 min of incubation with this compound, whereas the effect of Ca2+ was described to take place between 4 and 6 min after treatment [18], concurring with our observations that both PKD1 phosphorylation and Golgi fragmentation always required between 15 and 30 min of m-3M3FBS incubation to be detected.
The PI-PLC activator m-3M3FBS and purified β1γ2 complex stimulate similar levels of DAG production
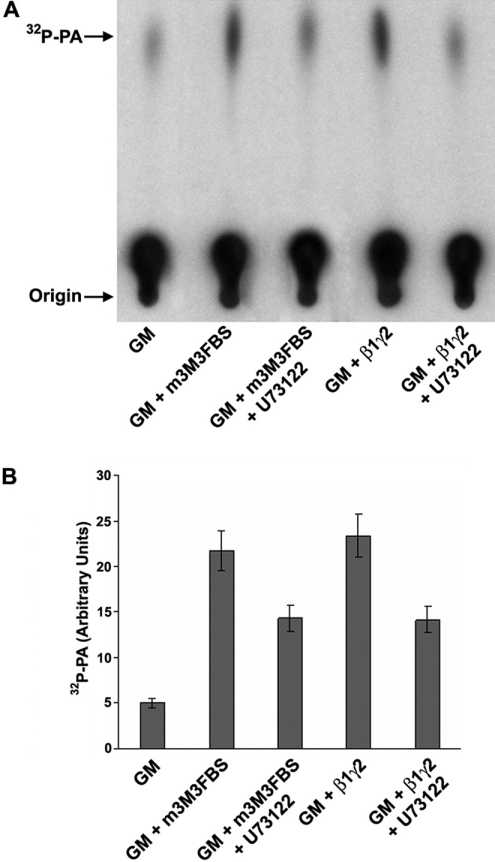
To verify the activity of m-3M3FBS as a PI-PLC activator, we tested DAG production in purified Golgi membranes previously treated with this compound. Rat liver purified Golgi membranes were incubated with 25 μM of m-3M3FBS for 30 min at 32 °C, with or without PI-PLC inhibitor U73122 addition. As a control we used purified β1γ2 alone or in combination with U73122. Then, membrane lipids were extracted with chloroform/methanol and incubated for 30 min at 32 °C in the presence of purified E. coli DGK and [γ-32P]ATP. DGK transforms all the DAG present in the extracted membranes in [32P]PA (phosphatidic acid), which can be separated from other lipids by TLC.
DAG-induced levels in Golgi membranes after incubation with m-3M3FBS were similar to those observed after addition of purified β1γ2 (Figures 4A and 4B, second and fourth lanes from left). Associated treatment with U73122 under both conditions caused a significant inhibitory effect on DAG production (Figures 4A and 4B, third and fifth lanes from left).
Figure 4. β1γ2 and m-3M3FBS induce DAG production at similar levels.
(A) Purified rat Golgi membranes (GM) were incubated with purified His–β1γ2 or m-3M3FBS in the presence or absence of U73122. Then, the membrane lipids were extracted and subjected to a DGK assay, as described in the Experimental section, and finally they were analysed by TLC for DAG production, which was measured as quantity of [32P]PA. (B) Densitometry of the experiment described in (A). Values are means (±S.D., vertical bars) for five separate experiments.
These experiments suggest that β1γ2 as well as m-3M3FBS activation of PI-PLCs render high levels of DAG, which, consecutively, induces PKCη/PKD phosphorylation, leading to a complete Golgi fragmentation due to destabilization of the vesicle trafficking balance between TGN and the cell surface [4].
PLCβ3 siRNA blocks transport from TGN to plasma membrane
Recently, several members of the PI-PLCs family were described as activation targets of heterotrimeric G-protein βγ subunits, among which we found PLCβ1 [19], PLCβ2 [20], PLCβ3 [20,21], PLCϵ [12], and PLCγ1 via PI3K (phosphoinositide 3-kinase) [22]. However, some PI-PLCs show no activation by βγ complexes, as is the case for PLCβ4 [23–25] and PLCδ1 [11,26].
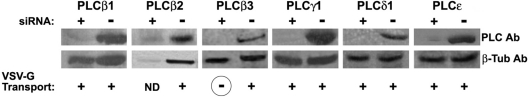
The data presented above support the involvement of PI-PLCs as key elements in the regulation of the formation of transport carriers. Thus the next question was posed as to which PLC family members were specifically involved in modulation of said formation. To address this matter, first, we had to establish which isoforms of PI-PLC were expressed in HeLa cells, by employing RT (reverse transcriptase)–PCR (results not shown) and Western blot (Figure 5). This cell line was found to express each tested protein family member, including PLCβ1–PLCβ4, PLCγ1 and PLCγ2, PLCδ1, and PLCϵ. Then, HeLa cells were transfected with various siRNAs directed against those PI-PLCs that could be activated by Gβγ, including PLCδ1 interference RNA as a negative control. As shown in Figure 5, siRNAs were able to block expression of all PI-PLCs probed, with the single exception of PLCβ2; in that case, we were unable to measure any effect due to occurrence of cell death shortly after transfection. Once the siRNA assays were set up in these knocked-out cells, GFP–VSV-G transport was measured, showing that only those cells transfected with the siRNA for PLCβ3 went through blocking of TGN to plasma membrane transport (Figures 5 and 6B).
Figure 5. Expression of different PI-PLCs' siRNAs, and their effect on VSV-G transport.
HeLa cells were transfected with siRNAs in order to block specific PI-PLCs. The resulting extracts prepared 72 h post-transfection were analysed by Western blot for each corresponding PLC (left upper lane for each protein), and then compared with non-transfected cellular extracts (right upper lane). As a control for protein expression, the extracts were monitored for β-tubulin expression (lower lanes). In a parallel experiment, siRNA-treated cells were transfected with GFP–VSV-G tsO45 48 h after siRNA transfection. After 24 h of VSV-G expression, cells were temperature-shifted as described in the Experimental section, and analysed by immunofluorescence to monitor the localization of VSV-G in the cell. Blocking of TGN to plasma membrane transport (circled ‘–’) was considered when less than 20% of GFP–VSV-G reached the cell surface, and normal transport (+) when more than 75% of the VSV-G-expressing cells had this protein localized at the plasma membrane. No data (ND) were collected from PLCβ2-siRNA-transfected cells, since they died 24–36 h post-siRNA transfection. Corresponding molecular masses in kDa are: PLCβ1: 134; PLCβ2: 130; PLCβ3: 132; PLCγ1: 142; PLCδ1: 83; and PLCϵ: 250. All siRNA Western blots and transport experiments were repeated four times.
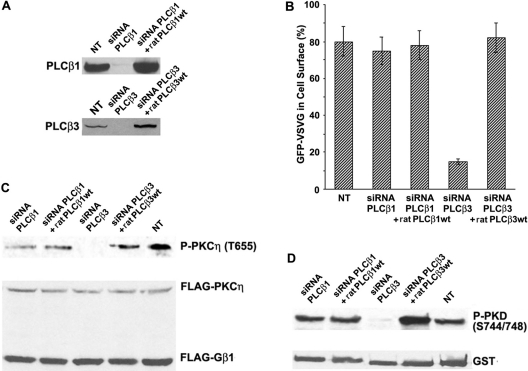
Figure 6. PLCβ3 siRNA blocks VSV-G transport and PKCη/PKD activation.
(A) Western blots showing the expression of PLCβ1 and PLCβ3 in normal cells (NT), siRNA-transfected cells, and siRNA-treated cells where the corresponding rat wild-type protein (ratPLCβ1 or 3 wt) was overexpressed for 24 h before cell lysate preparation. (B) Measurement of VSV-G transport in cells undergoing the same experimental conditions described in (A), shown as a percentage of GFP–VSV-G-expressing cells where this protein has reached the cell surface. Values are means (±S.D., vertical bars) for three separate experiments. (C) Measurement of PKCη activation (Phospho-PKCη Thr655) in extracts proceeding from the cells described in (A). (D) Same as (C) but for PKD1 activation (Phospho-PKD1 Ser744/Ser748). In order to determine activity of protein kinases in (C, D), cells were co-transfected with FLAG–β1–HA–γ2, FLAG–PKCη and GST–PKD1. All the experiments shown in (A, C, D) were repeated four times.
To analyse further the role of PLCβ3 in trafficking, we expressed this protein in siRNA-treated cells to examine the possibility of transport restoration. To determine the specificity of phospholipase activity, PLCβ1 was used as a control. In order to avoid siRNAs blockage on the exogenously added PLCs, we transfected plasmids expressing rat (R. norvegicus) isoforms, which share 92–97% protein similarity with human PLCs, although they show 10–15% nucleotide mismatch (2–3 bases of 19) at the region targeted to design the siRNAs, therefore avoiding interactions between the rat messenger and the human interference RNA sequences. Rat PLCs expression was confirmed by Western-blot analysis (Figure 6A). Figure 6(B) shows that VSV-G transport recovered to normal levels in PLCβ3 siRNA-treated cells when expressing rat wt protein. Addition of other PI-PLCs, such as PLCβ1, to the same PLCβ3-null cells did not restore transport levels to normal (results not shown), supporting a specific role of PLCβ3 in trafficking regulation.
We also monitored kinase activation under the conditions described above. Thus HeLa cells were co-transfected with wt FLAG–β1–HA–γ2 and FLAG–PKCη plus GST–PKD1, as previously described [4]. Blocking of PLCβ3 expression had an inhibitory effect on the phosphorylation/activation of PKCη (Figure 6C, third lane from left) and PKD1 (Figure 6D, third lane from left). Both kinases activation was restored after rat PLCβ3 overexpression in knocked-out cells (Figures 6C and 6D, fourth lane from left).
Finally, and based on the entirety of the experimental results described in the present study, we are able to conclude that PLCβ3 is a key regulator of transport carrier formation at the TGN.
DISCUSSION
Transport of uncoated vesicles from the TGN to the cell surface was believed to be unregulated, hence termed ‘constitutive transport’. During the last years, this concept has undergone progressive modifications, sustained by published results showing the involvement of various regulating proteins, especially those necessary for the fission of transport carriers at the TGN. One of these proteins, PKD, appeared as a key trafficking regulator, since it is capable of DAG-dependent translocation to the TGN, consecutively initiating a series of processes ending in vesicle fission [5,8,27].
Together with these findings, the involvement of Gβγ in trafficking regulation was also confirmed after reports of them as inducers of vesicle fission via PKD activation [3]. In addition, specific βγ subunits β1γ2 and β3γ2 were shown as responsible for stimulating novel PKCη-dependent PKD activation [4]. PKCη is a TGN-resident protein capable of both interacting with the PH domain of PKD and phosphorylating its activation loop. Production of DAG is necessary to activate PKCη and to recruit PKD to the TGN [8,28].
The aim of the present study was to elucidate the nature of the cellular source of Gβγ-induced DAG levels, required locally at the TGN for PKCη activation as well as PKD translocation. We proposed that the blocking of such component(s) should also be reflected as vesicle fission inhibition and lead to an accumulation of transport carriers at the TGN.
The perfect candidates that would fit this proposed pathway are the PI-PLCs. Some members of this family can be activated directly by Gβγ, producing DAG and InsP3 from PtdIns(4,5)P2. The DAG obtained can directly activate PKCη, and at the same time, recruit PKD to TGN membranes to subject it to phosphorylation by PKCη, finally producing the fission of transport carriers at the TGN.
Among the members of this increasingly growing PI-PLC family, only some of them could be expected to belong in this regulatory pathway, since only a group of them can be activated by heterotrimeric Gβγ. Our results show that PLCβ3 is responsible for DAG production via direct Gβγ activation. We cannot completely discard PLCβ2's role in trafficking regulation, since it has better affinity for βγ [29]. We have tried three siRNAs (PLCβ2-a, -b and -c) against different areas of PLCβ2, and in all cases the cells died between 24 and 36 h after transfection. This might suggest a possible involvement of PLCβ2 in the cell's vital processes. However, this result does not concur with a previous work showing that knockout mice for this protein are very similar to wt ones, especially in their survival rate [30]. Conversely, that study showed that different PLCβ2-null leucocyte populations had dissimilar behaviour with the same chemoattractant, demonstrating that the lack of PLCβ2 could have different effects depending on the cell type. Furthermore, there are no other reports describing the effect of PLCβ2 siRNA in cultured cells.
It is important to emphasize the effect of m-3M3FBS on Golgi structure. Only a small number of compounds capable of disassembling the Golgi apparatus have been described, such as nocodazole [31], BFA (Brefeldin A) [32], IQ [1] and NDGA (nordihydroguaiaretic acid) [33]. The mechanism of action leading to Golgi fragmentation is known for nocodazole and BFA, but not for IQ and NDGA. We demonstrate that in the case of m-3M3FBS, it seems clear that an overactivation of PI-PLCs by this compound leads to an excess of DAG production, which activates the protein kinases involved in vesicle fission, which ends in complete Golgi fragmentation. Even though we have not observed any effect of calcium at early incubation times in our experiments [18], we cannot discard its latest described role as a PKD activator, inducing DAG production via PLC [34].
Related to the fact that NRK cells are more sensitive to m-3M3FBS compared with HeLa cells, it is important to remark that IQ, a metabolite, first isolated from the Taiwanese marine sponge Hippospongia metachromia, induces Golgi fragmentation in NRK cells [1,2,35], but needs to be used at higher concentrations in HeLa cells to produce a perturbation of the Golgi apparatus (appearance of tubes emanating from the Golgi), although the phenotype is not as dramatic as in NRK cells (G. Guizzunti, personal communication). Since both compounds are involved in the regulation of βγ/PKD-dependent vesicle fission ([2]; the present study), we can speculate that different cell lines react with them with diverse degrees of sensitivity, probably due to distinct levels of regulation or expression of the regulatory components involved.
The next significant question to answer is where this entire pathway assembles in the subcellular scenario. Previous results have shown the presence of endogenous PLCβ3 in Golgi membranes, whereas the presence of PLCβ2 in that organelle is not completely clear [36]. If we assume that a still unknown signal is originated on the cell surface via GPCRs (G-protein-coupled receptors), we can speculate that the signal responsible for transducing that signal from the plasma membrane to Golgi could be G-protein βγ subunits. Gβγ have been described to translocate from the cell surface to the Golgi complex by GPCR activation [37–39]. Even if we still ignore both the signal and the putative GPCR involved in the activation of this regulatory pathway, we now have enough information to design a model (Figure 7) that will allow us to seek new components, including PKD's downstream effectors.
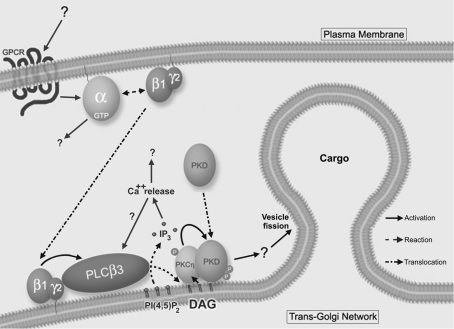
Figure 7. Model for TGN to plasma membrane transport regulation.
We propose that a still undiscovered signal is generated at the cell surface via GPCR. This causes the activation of a G-protein, allowing βγ subunits to translocate to the TGN, where they are able to activate PLCβ3 by binding to its PH domain. The activation of this phospholipase leads to the formation of DAG, which has two roles at the TGN: to directly activate PKCη, and to allow PKD1 to translocate to this Golgi compartment by direct binding to its C1a domain. Then, PKCη binds to the PH domain of PKD, which enables its phosphorylation by the former at its activation loop. Finally, PKD1 activation induces cargo-filled vesicle fission at the TGN by phosphorylation of still unknown downstream effectors.
Our future goal is then to understand how and when this regulatory pathway is triggered. These circumstances should relate to cellular requirements according to protein trafficking and localization necessities during growing and development. In addition, we will aim to find novel PKD effectors, particularly those substrates that upon PKD phosphorylation might participate in the regulation of fission events at the TGN.
With our description of PLCβ3 as a new key protein in transport carrier formation, we are another step closer to unveiling how these processes are regulated in the cell.
Acknowledgments
I am grateful to Dr Vivek Malhotra (University of California at San Diego) for his complete support and understanding, and especially to Dr Omar Adrián Coso (Institute of Physiology, Molecular Biology and Neurosciences, Buenos Aires, Argentina) for his thorough revision and always useful constructive criticism of this paper. Part of this work was performed at the laboratory of Dr Vivek Malhotra (Center for Molecular Genetics, University of California at San Diego) and funded by grants GM53747 and GM46224 from the NIH, and the remaining part was developed at the Laboratory of Neurobiology, INIMEC-CONICET, Córdoba, Argentina, and supported by an INIMEC institutional grant. A. M. D. A. is currently an Assistant Researcher from the CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Argentina. I dedicate this work to Dr Marcela Beatriz Ortiz de Díaz.
References
- 1.Takizawa P. A., Yucel J. K., Veit B., Faulkner D. J., Deerinck T., Soto G., Ellisman M., Malhotra V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell. 1993;73:1079–1090. doi: 10.1016/0092-8674(93)90638-7. [DOI] [PubMed] [Google Scholar]
- 2.Jamora C., Takizawa P. A., Zaarour R. F., Denesvre C., Faulkner D. J., Malhotra V. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 3.Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J. R., Faulkner D. J., Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 4.Díaz Añel A. M., Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 2005;169:83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liljedahl M., Maeda Y., Colanzi A., Ayala I., Van Lint J., Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 6.Yeaman C., Ayala M. I., Wright J. R., Bard F., Bossard C., Ang A., Maeda Y., Seufferlein T., Mellman I., Nelson W. J., Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghanekar Y., Lowe M. Protein kinase D: activation for Golgi carrier formation. Trends Cell Biol. 2005;15:511–514. doi: 10.1016/j.tcb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Baron C. L., Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 9.Nakahara M., Shimozawa M., Nakamura Y., Irino Y., Morita M., Kudo Y., Fukami K. A novel phospholipase C, PLC(eta)2, is a neuron-specific isozyme. J. Biol. Chem. 2005;280:29128–29134. doi: 10.1074/jbc.M503817200. [DOI] [PubMed] [Google Scholar]
- 10.Razzini G., Brancaccio A., Lemmon M. A., Guarnieri S., Falasca M. The role of the pleckstrin homology domain in membrane targeting and activation of phospholipase Cbeta(1) J. Biol. Chem. 2000;275:14873–14881. doi: 10.1074/jbc.275.20.14873. [DOI] [PubMed] [Google Scholar]
- 11.Rebecchi M. J., Pentyala S. N. Structure, function and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 12.Wing M. R., Houston D., Kelley G. G., Der C. J., Siderovski D. P., Harden T. K. Activation of phospholipase C-epsilon by heterotrimeric G protein betagamma-subunits. J. Biol. Chem. 2001;276:48257–48261. doi: 10.1074/jbc.C100574200. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J., Fritsch E. F., Maniatis T. 2nd edn. Plainview, NY: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 14.Acharya U., Mallabiabarrena A., Acharya J. K., Malhotra V. Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell. 1998;92:183–192. doi: 10.1016/s0092-8674(00)80913-7. [DOI] [PubMed] [Google Scholar]
- 15.Slusarewicz P., Hui N., Warren G. Purification of rat liver Golgi stacks. In: Celis J. E., editor. Cell Biology: A Laboratory Handbook, vol. 1. Orlando, FL: Academic Press; 1994. pp. 509–516. [Google Scholar]
- 16.Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 17.Bae Y. S., Lee T. G., Park J. C., Hur J. H., Kim Y., Heo K., Kwak J. Y., Suh P. G., Ryu S. H. Identification of a compound that directly stimulates phospholipase C activity. Mol. Pharmacol. 2003;63:1043–1050. doi: 10.1124/mol.63.5.1043. [DOI] [PubMed] [Google Scholar]
- 18.Krjukova J., Holmqvist T., Danis A. S., Akerman K. E., Kukkonen J. P. Phospholipase C activator m-3M3FBS affects Ca2+ homeostasis independently of phospholipase C activation. Br. J. Pharmacol. 2004;143:3–7. doi: 10.1038/sj.bjp.0705911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer J. L., Graber S. G., Waldo G. L., Harden T. K., Garrison J. C. Selective activation of phospholipase C by recombinant G-protein alpha- and beta gammasubunits. J. Biol. Chem. 1994;269:2814–2819. [PubMed] [Google Scholar]
- 20.Fogg V. C., Azpiazu I., Linder M. E., Smrcka A., Scarlata S., Gautam N. Role of the gamma subunit prenyl moiety in G protein beta gamma complex interaction with phospholipase Cbeta. J. Biol. Chem. 2001;276:41797–41802. doi: 10.1074/jbc.M107661200. [DOI] [PubMed] [Google Scholar]
- 21.Murthy K. S., Coy D. H., Makhlouf G. M. Somatostatin receptor-mediated signaling in smooth muscle. Activation of phospholipase C-beta3 by Gbetagamma and inhibition of adenylyl cyclase by Galphai1 and Galphao. J. Biol. Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- 22.Ferry X., Eichwald V., Daeffler L., Landry Y. Activation of betagamma subunits of G(i2) and G(i3) proteins by basic secretagogues induces exocytosis through phospholipase Cbeta and arachidonate release through phospholipase Cgamma in mast cells. J. Immunol. 2001;167:4805–4813. doi: 10.4049/jimmunol.167.9.4805. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H., Wu D., Simon M. I. Activation of phospholipase C beta 4 by heterotrimeric GTP-binding proteins. J. Biol. Chem. 1994;269:7593–7596. [PubMed] [Google Scholar]
- 24.Lee C. W., Lee K. H., Lee S B., Park D., Rhee S. G. Regulation of phospholipase C-beta 4 by ribonucleotides and the alpha subunit of Gq. J. Biol. Chem. 1994;269:25335–25338. [PubMed] [Google Scholar]
- 25.Kim M. J., Min D. S., Ryu S. H., Suh P. G. A cytosolic, galphaq- and betagamma-insensitive splice variant of phospholipase C-beta4. J. Biol. Chem. 1998;273:3618–3624. doi: 10.1074/jbc.273.6.3618. [DOI] [PubMed] [Google Scholar]
- 26.Rhee S. G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda Y., Beznoussenko G. V., Van Lint J., Mironov A. A., Malhotra V. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J. 2001;20:5982–5990. doi: 10.1093/emboj/20.21.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ron D., Kazanietz M. G. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. [PubMed] [Google Scholar]
- 29.Runnels L. W., Scarlata S. F. Determination of the affinities between heterotrimeric G protein subunits and their phospholipase C-beta effectors. Biochemistry. 1999;38:1488–1496. doi: 10.1021/bi9821519. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H., Kuang Y., Wu Y., Xie W., Simon M. I., Wu D. Roles of phospholipase C beta2 in chemoattractant-elicited responses. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7971–7975. doi: 10.1073/pnas.94.15.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogalski A. A., Bergmann J. E., Singer S. J. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J. Cell Biol. 1984;99:1101–1109. doi: 10.1083/jcb.99.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 33.Yamaguchi T., Yamamoto A., Furuno A., Hatsuzawa K., Tani K., Himeno M., Tagaya M. Possible involvement of heterotrimeric G proteins in the organization of the Golgi apparatus. J. Biol. Chem. 1997;272:25260–26266. doi: 10.1074/jbc.272.40.25260. [DOI] [PubMed] [Google Scholar]
- 34.Kunkel M. T., Toker A., Tsien R. Y., Newton A. C. Calcium-dependent regulation of protein kinase D revealed by a genetically-encoded kinase activity reporter. J. Biol. Chem. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya U., McCaffery J. M., Jacobs R., Malhotra V. Reconstitution of vesiculated Golgi membranes into stacks of cisternae: requirement of NSF in stack formation. J. Cell Biol. 1995;129:577–589. doi: 10.1083/jcb.129.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blayney L., Gapper P., Rix C. Identification of phospholipase C beta isoforms and their location in cultured vascular smooth muscle cells of pig, human and rat. Cardiovasc. Res. 1998;40:564–572. doi: 10.1016/s0008-6363(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 37.Akgoz M., Kalyanaraman V., Gautam N. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J. Biol. Chem. 2004;279:51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 38.Azpiazu I., Akgoz M., Kalyanaraman V., Gautam N. G protein betagamma11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the alpha subunit type. Cell. Signalling. 2005;18:1190–1200. doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akgoz M., Kalyanaraman V., Gautam N. G protein betagamma complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction. Cell. Signalling. 2006;18:1758–1768. doi: 10.1016/j.cellsig.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]