Abstract
Rab GTPases are key regulators of intracellular membrane trafficking. We sought to elucidate the roles of Rab GTPases in Xenopus gastrulation, and found that a Xenopus homolog of Rab40 (XRab40) is required for normal gastrulation. XRab40 is localized at the Golgi apparatus and interacts with ElonginB/C and Cullin5 to form a ubiquitin ligase. XRab40/XCullin5 functions cooperatively and regulates the ubiquitination and localization of Rap2 GTPase. Furthermore, XRab40/XCullin5 regulates the membrane localization of Dishevelled (Dsh), a key signaling molecule in the Wnt pathway, through Rap2 and its effector Misshapen/Nck-interacting kinase (XMINK). XMINK interacts with Dsh, and is translocated to the plasma membrane by Wnt activation. We propose a novel signaling cascade consisting of XRab40/XCullin5, Rap2 and XMINK, which plays a crucial role in the regulation of the noncanonical Wnt pathway.
Keywords: gastrulation, Rab GTPase, ubiquitination, Wnt signaling, Xenopus
Introduction
Gastrulation is the first major morphogenetic process in Xenopus development that entails coordinated cell movements and, ultimately, the formation of three germ layers. During Xenopus gastrulation, mesodermal cells in an equatorial region involute to the inner region of the embryo and migrate on the blastocoel roof towards the animal poles. Axial mesoderm cells are first polarized and then intercalated in a mediolateral direction to cause dramatic extension of the dorsal marginal zone, which is defined as convergent extension (CE), to establish the anterior–posterior (A–P) axis of the body plan (Shih and Keller, 1992; Wallingford et al, 2002). It is known that the process of CE is regulated by the Wnt signaling pathway (Tada et al, 2002).
Wnts signal through the surface receptor complexes that include a seven transmembrane receptor, Frizzled (Fz). The cytoplasmic Dishevelled (Dsh) protein is located at a critical divergent point to branch into the canonical and the noncanonical Wnt pathways (Boutros and Mlodzik, 1999; Wharton, 2003). The canonical Wnt/β-catenin signaling pathway regulates the stabilization and the shuttle of β-catenin to the nucleus and subsequently regulates target gene transcription. An alternative pathway activates the noncanonical Wnt pathway and is known to control CE movements in Xenopus and zebrafish embryos (Kuhl, 2002; Tada et al, 2002). The activation of this pathway is known to involve the translocation of Dsh protein to the plasma membrane and the activation of Rho family GTPases and c-Jun N-terminal kinase (JNK) (Axelrod et al, 1998; Boutros et al, 1998; Li et al, 1999; Yamanaka et al, 2002; Habas et al, 2003).
Wnt signaling is known to be regulated by the ubiquitination system. β-catenin is a well-characterized substrate for ubiquitination. Recently, several ubiquitin ligases were identified to target Dsh for ubiquitination and proteasome degradation (Miyazaki et al, 2004; Simons et al, 2005; Angers et al, 2006). Also, adenomatous polyposis coli (APC) is downregulated by the ubiquitin–proteasome pathway in response to Wnt signaling (Choi et al, 2004). The ubiquitin ligase complexes have emerged as crucial regulators in the Wnt signal transduction pathway.
Ubiquitination is catalyzed by several enzymatic steps involving the E1-E2-E3 trio of enzymes to activate and deliver the ubiquitin molecules to the targeted substrate (reviewed by Hochstrasser, 2006). The Cullin-based ubiquitin ligases constitute one large class of E3 ubiquitin ligases. The Cullin proteins serve as scaffolds to recruit the substrate-binding proteins and the RING finger proteins with their N and C termini, respectively (reviewed by Petroski and Deshaies, 2005). Human cells express at least seven Cullins to form different ligase complexes. One of the Cullin ubiquitin ligases is the ElonginB/C-Cullin5-SOCS box complex. The ElonginB/C complex links the SOCS-box-containing proteins to Cullin5, which in turn, binds to the RING finger protein to form a multi-subunit complex with ubiquitin ligase activity. The SOCS-box-containing proteins are considered to confer substrate specificity. Rab GTPases belong to the Ras superfamily of small GTPases. They are known to lie at the very core of membrane trafficking to regulate intracellular vesicle transport and the trafficking of proteins between different organelles. There are at least 60 Rab proteins in humans, and many of them are expressed in specific cell types and localized in particular cell compartments. Several studies have been reported in which Rab proteins play a role in embryogenesis. It has been reported that endocytosis restricts the spreading and signaling range of Fgf8 in zebrafish (Scholpp and Brand, 2004). More recently, Rab5c have been reported to function in Wnt11-mediated endocytosis of E-cadherin (Ulrich et al, 2005). This endocytosis process is required for cell cohesion in mesendodermal cells during zebrafish gastrulation. Although the roles of some Rab proteins in development have been elucidated, there may be many Rab proteins whose functions during embryogenesis remain elusive.
Here, we report that a Xenopus homolog of Rab40, designated XRab40, plays an essential role during Xenopus gastrulation. Rat Rab40c has shown to be expressed in oligodendrocytes and localized in the recycling compartment (Rodriguez-Gabin et al, 2004); however, its function remains unknown. Intriguingly, we found that XRab40 forms a ubiquitin ligase complexed with Cullin5 at the Golgi apparatus. In addition, we identified Rap2 GTPase as a potential substrate for the XRab40/Cullin5 ubiquitin ligase. XRab40 plays an essential role in the noncanonical Wnt pathway by regulating Rap2 and its effector Misshapen (Msn)/Nck-interacting kinase (MINK). We also show that XRab40 regulates the membrane localization of Dsh that is necessary to mediate the Wnt signal transduction pathway. We propose a novel signaling cascade, including XRab40/Cullin5, Rap2 and MINK to modulate the Wnt signaling pathway.
Results
XRab40 plays an essential role during Xenopus gastrulation
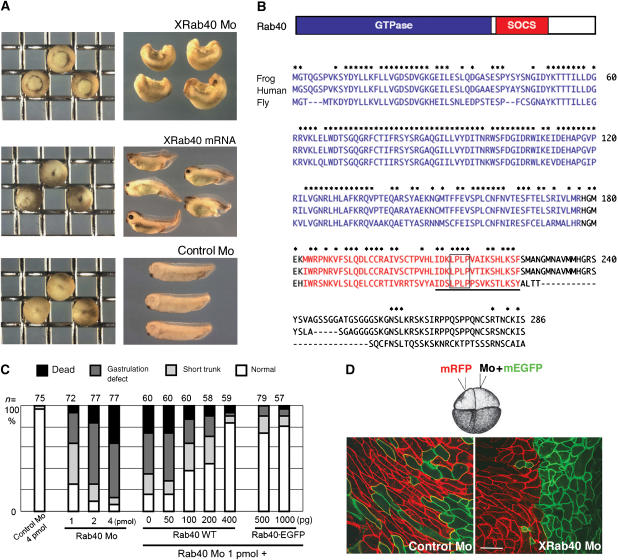
In an attempt to elucidate how Rab family proteins are involved in early Xenopus development, we screened Rab proteins expressed in Xenopus embryos. We searched complementary DNAs encoding Rab GTPases in databases, and found more than 30 Rab genes. Messenger RNAs were synthesized using these cDNAs as templates and injected into Xenopus embryos. Among them, we found that one cDNA encoding a Rab protein similar to mammalian Rab40 had strong inhibitory effects on gastrulation and the A–P axis elongation when it was overexpressed (Figure 1A and C). We thus focused on this Rab family protein, which we referred to as XRab40. XRab40 protein contains a GTPase domain and a SOCS (suppressors of cytokine signaling) box (reviewed by Kile et al, 2002) (Figure 1B). Proteins highly homologous to XRab40 were also found in other vertebrates and insects in the databases, suggesting the functional importance of this class of Rab GTPases.
Figure 1.
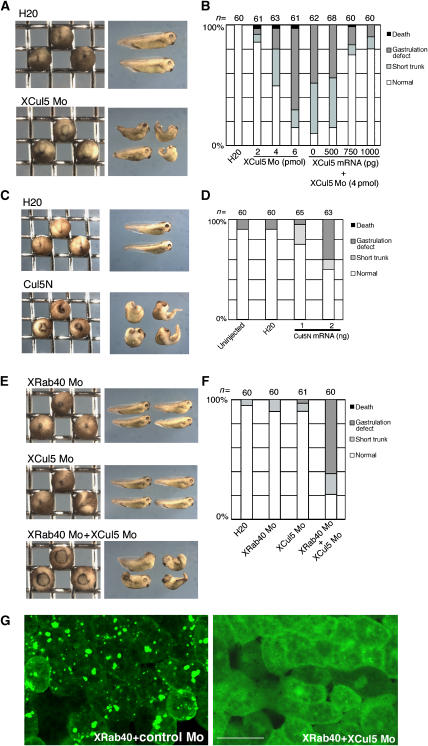
XRab40 is essential for Xenopus gastrulation. (A) XRab40 mRNA and XRab40 Mo caused gastrulation defects when expressed laterally in one of the two blastomeres in the two-cell stage embryos. (B) Schematic illustration of XRab40, which is comprised of a Rab GTPase domain and a SOCS box. Sequence alignments of Xenopus Rab40, human Rab40c and Drosophila Rab40 (GenBank accession no: AB262321, AAK61236, AAF48164, respectively). The GTPase domain (residues in blue) and the SOCS box (residues in red) are indicated. The Cul5 box is underlined. The box indicates the mutation site of the XRab40ΔCul5 box mutant. (C) Statistical data of gastrulation-defective phenotypes caused by XRab40 Mo and rescue experiments co-injected with XRab40 and EGFP-XRab40 mRNAs. (D) XRab40 Mo or control Mo (1 pmol each) was co-injected with membrane-tethered GFP into one of the two blastomeres in the four-cell stage embryos. Membrane-tethered RFP alone was injected into the other dorsal blastomere. Scale bar=50 μm.
RT–PCR and in situ hybridization analyses showed that XRab40 was maternally and ubiquitously expressed throughout various developmental stages (Supplementary Figure S1D and data not shown). To investigate the roles of XRab40 during Xenopus development, we designed a specific antisense morpholino oligonucleotide (Mo) to disrupt XRab40 function. XRab40 Mo-injected embryos showed a gastrulation-defective phenotype (Figure 1A and C). The specificity of the Mo was confirmed by using mRNAs with the Mo-sensitive and Mo-resistant sequences (Supplementary Figure S2A and B). The abnormal phenotypes can be effectively rescued by either XRab40 or EGFP-XRab40 mRNAs that are insensitive to the Mo, confirming the specific disruption of XRab40 function by the Mo. These phenotypic studies demonstrated that XRab40 is required for normal gastrulation.
XRab40 Mo inhibited gastrulation movements and elongation along the A–P axis, suggesting that XRab40 might be required for CE movements. To test this possibility, we observed CE movements of XRab40 morphant embryos (Figure 1D). At the onset of gastrulation, the dorsal marginal zone tissue was isolated and cultured on a fibronectin-coated dish (Kinoshita et al, 2003). In control-Mo-injected embryos, cells were polarized and intercalated. However, XRab40-Mo injected cells were not polarized or intercalated. XRab40 is required for cell polarization and migration during CE. The inhibition of CE may cause the gastrulation-defective phenotype.
In Mo-injected embryos, expressions of the mesodermal markers, chordin, gsc, Xnr3 and twisted were retained by using both in situ hybridization and RT–PCR analyses (Supplementary Figure S4A and B). However, Xbra expression was reduced in embryos with XRab40 knockdown, suggesting that XRab40 participates in mesoderm formation. It has been shown that Xbra plays an important role in the regulation of CE movements (Kwan and Kirschner, 2003). The reduction of Xbra expression by XRab40 Mo may account for the CE-defective phenotype. The activin/nodal and FGF signaling pathways induce Xbra expression in Xenopus embryos (Smith et al, 1991). As the expressions of gsc and chordin were not affected by XRab40 Mo (Supplementary Figure S4A and B) and phosphorylation of Smad2, the downstream signaling molecules of activin/nodal signaling was not influenced by XRab40 Mo in the immunoblotting result (Supplementary Figure S4C), XRab40 may not be required for the activin/nodal signaling pathway. We thus focused on the FGF signaling and found that XRab40 Mo had no effect on Xbra expression induced by embryonic FGF in animal caps (Supplementary Figure S4D) and did not cause any observable change in the localization of embryonic FGF or FGF receptor (Supplementary Figure S4E). XRab40 might be involved in an unknown mechanism required for Xbra induction.
XRab40 forms a ubiquitin ligase complex with ElonginB/C and Cullin5
To understand the molecular functions of XRab40, we tried to identify the interaction partners of XRab40 by mass spectrometry (MS) analysis and a yeast two-hybrid assay. For MS analysis, we identified ElonginB, ElonginC and Cullin5 as XRab40-associated proteins (data not shown). SOCS-box-containing proteins including Rar3, a mammalian Rab GTPase similar to XRab40, have been shown to bind to ElonginB/C and Cullin5 (Kamura et al, 2004). The result of the MS analysis strongly suggested that XRab40 also interacts with ElonginB/C and Cullin5 to form a ubiquitin ligase complex.
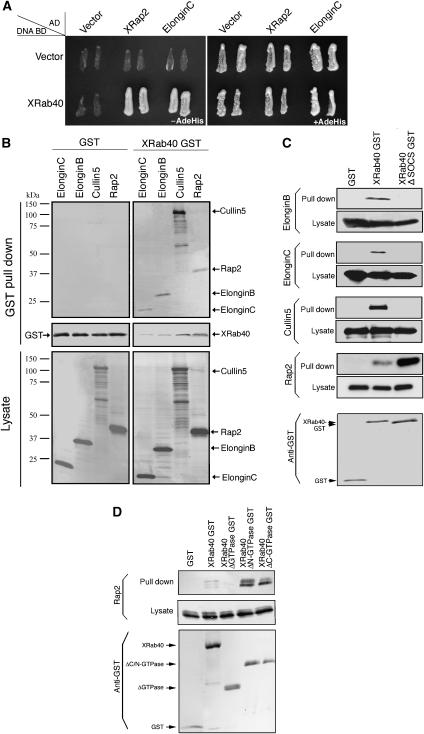
We also carried out a yeast two-hybrid assay. The screen identified ElonginC and Xenopus Rap2 (XRap2; Figure 2A). The identification of ElonginC is consistent with the result of the MS analysis. XRap2 has been reported to regulate the Wnt signaling pathway (Choi and Han, 2005). We further investigated the significance of XRab40 interaction with ElonginB, ElonginC, Cullin5 and Rap2.
Figure 2.
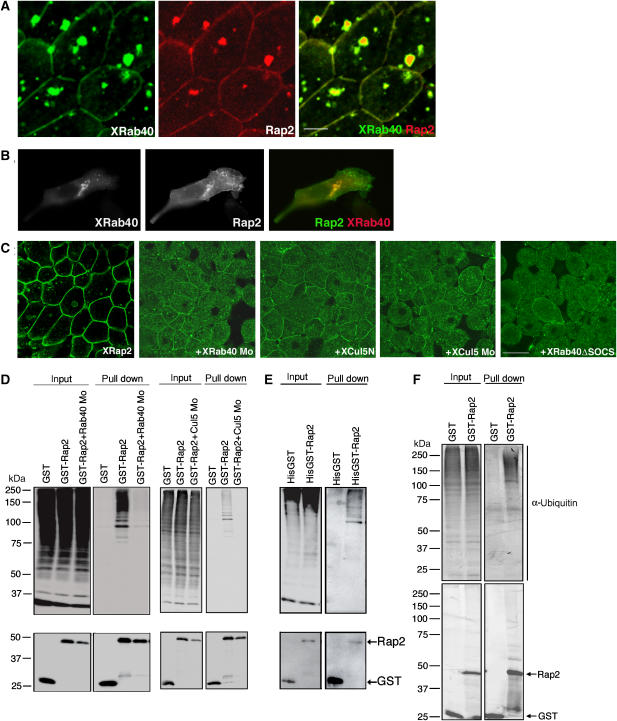
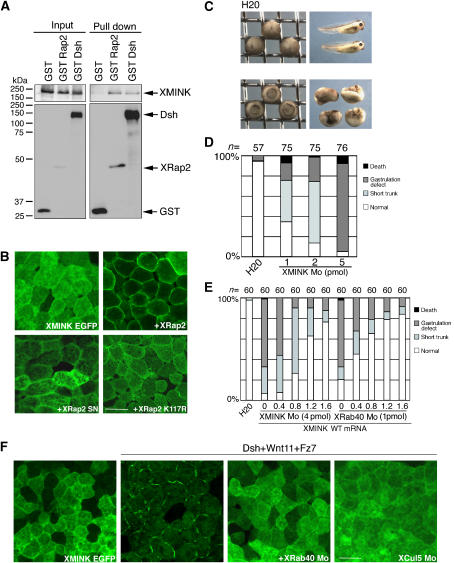
XRab40 binds to XElonginB/C, XCul5 and XRap2. (A) Yeast two-hybrid assay result of XRab40 with its interaction partners. Yeast cells were co-transformed with activation domain and DNA-binding domain of the indicated plasmids in the figure. The yeast two-hybrid analysis was performed by plating two independent colonies to grow on agar plates with (+) or without (−) adenine (Ade) and histine (His). The growth of yeast on agar plate (−AdeHis) indicated interaction. The agar plate (+AdeHis) is a control experiment. (B) Myc-XElonginB/C, XCul5 or XRap2 mRNAs was co-injected with GST-XRab40 or GST alone in the animal cap cells. (C) Myc-tagged mRNAs were co-injected with GST alone, GST-XRab40 or GST-XRab40 with the deletion of the SOCS box (XRab40 ΔSOCS-GST). (D) Myc-tagged XRap2 was co-injected with GST alone, GST-XRab40, GST-XRab40ΔGTPase, GST-XRab40ΔN- or ΔC GTPase in the animal cap cells.
First, we searched Xenopus homologs of ElonginB, ElonginC and Cullin5 in the databases and the cloned cDNAs having highest similarities to the mammalian genes. These were designated as XElonginB, XElonginC and XCul5 (Supplementary Figure S1A, B and C). RT–PCR and in situ hybridization showed that they were maternally and ubiquitously expressed during early Xenopus embryogenesis (Supplementary Figure S1D and data not shown).
The interactions were further validated by performing a GST pull-down assay in Xenopus embryos. XElonginB, XElonginC, XCul5 and XRap2 bound to XRab40 (Figure 2B). As a control, they did not have any nonspecific interaction with GST alone. Moreover, there was no comparable difference on the interaction levels between these proteins in both DMZ and VMZ cells (data not shown). We confirmed the interactions by using myc-tagged XRab40 and GST-tagged XElonginB, XElonginC, XCul5 and XRap2 (data not shown). We also demonstrated that XRab40 specifically interacted with XCul5, but not with other Cullins (Cul1, 2, 3 and 4B; data not shown). Deletion of the SOCS box of XRab40 abolished the interaction with XElonginB, XElonginC and XCul5, suggesting these proteins bound to the SOCS box of XRab40. XRap2, however, did not require the SOCS box for the interaction (Figure 2C). We examined whether the GTPase domain of XRab40 is required for the interaction of XRap2. We prepared three GTPase domain deletion mutants. The mutants were constructed with deletions of the whole (XRab40 ΔGTPase; 14–157 aa), the C-terminal half (XRab40 ΔN-GTPase; 14–90 aa) or the N-terminal half of the GTPase domains (XRab40 ΔC-GTPase; 91–157 aa). We tried a pull-down assay and found that XRap2 interacted with both XRab40 ΔN- and ΔC-GTPase, but not with XRab40 ΔGTPase (Figure 2D). It suggests that XRap2 binds to the GTPase domain of XRab40, but the interaction may not require the GTPase activity as XRab40 ΔN- and ΔC-GTPase, whose GTPase activities are expected to be disrupted, still bind to XRap2. Taken together, these results suggested that XRab40 forms a ubiquitin ligase complex interacting with XElonginB/C and XCul5, and that the GTPase domain of XRab40 may recognize its substrates, such as XRap2.
XRab40 is localized at the Golgi apparatus
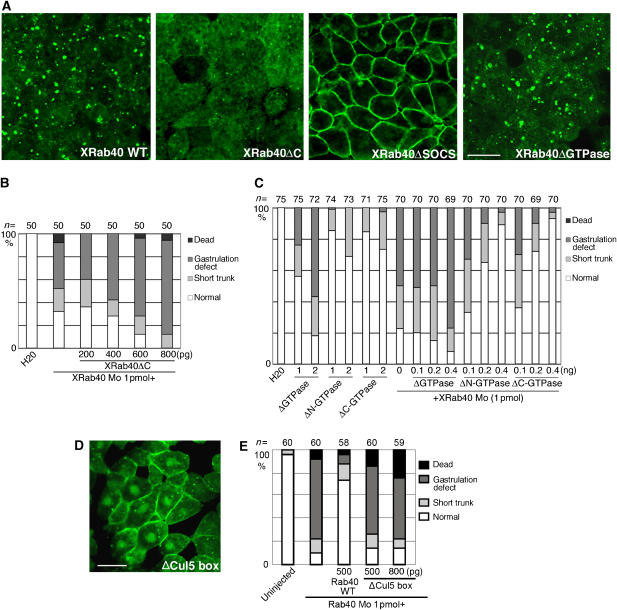
We then examined the intracellular localization of XRab40 by expressing the EGFP-tagged construct (EGFP-XRab40). EGFP-XRab40 was localized predominantly at the cytoplasmic vesicles and to a smaller extent, at the plasma membrane when expressed in the animal cap (ectodermal) cells of Xenopus embryos (Figure 3A). As we have confirmed that EGFP-XRab40 can efficiently rescue XRab40 Mo-injected embryos (Figure 1C) and the localization is not dependent on the level of expression, we expect that this localization must be physiologically significant. Endogenous XRab40 was also localized at the cytoplasmic vesicles and weakly at the plasma membrane in Xenopus animal cap cells (Supplementary Figure S3). We further conducted a domain analysis. Deletion of the C-terminal four amino acids (ΔC), supposedly required for lipid modification, altered the localization of XRab40 to the cytoplasm (Figure 3A). To directly analyze the importance of the membrane anchor for XRab40 function, we performed the rescue experiment of XRab40 Mo with XRab40ΔC (Figure 3B). We showed that XRab40ΔC could not rescue the defective phenotype of XRab40 Mo, suggesting that XRab40 membrane anchor is important for its function. XRab40 consists of a GTPase domain. The localization of XRab40 did not alter when the GTPase domain was deleted (Figure 3A). The GTPase activity of XRab40 might not be important for its localization. We further examined whether the GTPase domain deletion mutants cause CE-defective phenotype. Dorsally overexpression of XRab40 ΔGTPase caused gastrulation defects whereas both XRab40 ΔN- and ΔC-GTPase displayed a mild-short trunk phenotype (Figure 3C). Moreover, both XRab40 ΔN- and ΔC-GTPase could rescue the defective phenotype impaired by XRab40 Mo, whereas XRab40 ΔGTPase could not (Figure 3C). XRab40 was localized at the plasma membrane when the SOCS box was deleted (Figure 3A). It has been shown that Cul5 binds to the C-terminal half of the SOCS box, defined as the Cul5 box (Kamura et al, 2004). We replaced the highly conserved four amino-acid sequence ‘LPLP' with ‘AAAA' in the Cul5 box (Figure 1B). This mutant also failed to localize to the vesicles (Figure 3D). Importantly, this mutant lost the ability to rescue the XRab40-Mo-induced phenotype (Figure 3E). The SOCS box is essential for the control of XRab40 localization and function.
Figure 3.
The SOCS box domain localizes XRab40 to the Golgi apparatus. (A) Wild-type (XRab40 WT) or deletion constructs of EGFP-XRab40 was expressed in the animal regions. (B) Statistical data of phenotypic studies on XRab40 Mo and XRab40 ΔC mRNA. (C) Statistical data of phenotypic studies on overexpression of XRab40 ΔGTPase, XRab40 ΔN- or ΔC-GTPase mRNAs. (D) EGFP-XRab40 mRNA with deletion of the Cul5 box (ΔCul5 box) was injected in the animal cap cells. (E) Statistical data of phenotypic studies on XRab40 Mo with XRab40 ΔCul5. Scale bar=50 μm.
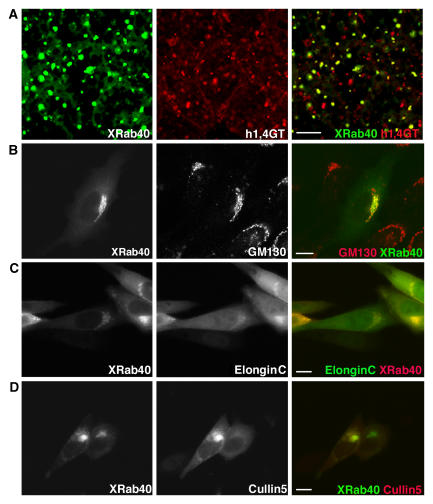
To further define where XRab40 is localized, we examined known subcellular localization markers in Xenopus embryonic cells and Chinese Hamster Ovary (CHO) cells. In Xenopus cells, XRab40 was found to colocalize with 1,4-Galactosyltransferase (h1,4GT), which is a marker of the Golgi apparatus (Yamaguchi and Fukuda, 1995; Figure 4A). We tested the colocalization of XRab40 with the N-terminally truncated EEA1, CD63, GP96 and hLBR, which are the markers for endosome (Gaullier et al, 1998; Patki et al, 1998), lysosomes (Metzelaar et al, 1991), endoplasmic reticulum (ER; Argon and Simen, 1999) and nuclear membrane (Haraguchi et al, 2001; Supplementary Figure S5), respectively. None of these organelle markers were colocalized with XRab40.
Figure 4.
XRab40 is localized at the plasma membrane and Golgi apparatus, and colocalized with ElonginB and Cullin5 in CHO cells. (A) EGFP-XRab40 was co-injected with RFP-h1,4GT. (B) XRab40 was expressed in CHO cells and then immunostained with GM130. XElonginC and XCullin5 were colocalized with XRab40 in CHO cells. Cells were transiently transfected with (C) RFP-XRab40 and EGFP-XElonginC and (D) EGFP-XRab40 and RFP-XCullin5. Scale bar=10 μm.
In CHO cells, we used a Golgi marker, GM130, to confirm the localization of XRab40 at the Golgi apparatus (Figure 4B). As we have shown that XElonginC and XCul5 physically interact with XRab40, we examined whether these proteins are colocalized in CHO cells. Although XElonginC and XCul5 were broadly distributed in the cytoplasm, they were apparently colocalized with XRab40 at the Golgi apparatus (Figure 4C and D), further implying that XRab40 forms a Golgi-localized ubiquitin ligase with these proteins.
Loss of XCul5 function phenocopied and synergized with the effect of XRab40 Mo
To analyze the role of XCul5 in Xenopus development, we used XCul5 Mo. The specificity of the Mo was confirmed (Supplementary Figure S2C and D). XCul5-depleted embryos showed defects in gastrulation and/or in the elongation of the A–P axis (Figure 5A). These phenotypes were rescued by XCul5 mRNA without the Mo-targeting sequence (Figure 5B), indicating that the phenotypes were caused by XCul5 depletion. We also injected mRNA encoding the C-terminally-truncated XCul5 mutant (Cul5N; Figure 5C and D). It is known that the N-terminal region of Cullin family proteins interacts with the proteins required for substrate recognition. The truncated C-terminal region is supposed to interact with the RING finger protein and an E2 ubiquitin conjugating enzyme, which are essential for the ubiquitination reaction (reviewed by Deshaies, 1999). Thus, Cul5N was expected to act as a dominant-negative mutant to inhibit the endogenous XCul5 function. As expected, Cul5N-injected embryos showed a phenotype very similar to XCul5 Mo (Figure 5C and D). The C-terminally truncated Cullin isoforms did not affect gastrulation (data not shown), suggesting the specific importance of XCul5 in this process. The striking similarities between the phenotypes elicited by XRab40 Mo, XCul5 Mo and XCul5N corroborate the idea that XRab40 and XCul5 function collectively during Xenopus gastrulation.
Figure 5.
XCul5 Mo phenocopies and synergizes with XRab40 knockdown. (A) XCul5 Mo (5 pmol) was laterally injected into one of the two blastomeres in the two-cell stage embryos. (B) Statistical data of gastrulation-defective phenotypes caused by XCul5 Mo and rescue experiments. (C) XCul5N mRNA (2 ng) was laterally injected into one of the two blastomeres in the two-cell stage embryos. (D) Statistical data of the gastrulation-defective phenotypes caused by XCul5N mRNA. (E) XRab40 Mo (0.5 pmol), XCul5 Mo (2 pmol) or mixed Mos was laterally injected into one of the two blastomeres in the two-cell stage embryos. (F) Statistical data of gastrulation-defective phenotypes. (G) EGFP-XRab40 was co-injected with either control Mo or XCul5 Mo (2 pmol each) in the animal cap cells. Scale bar=50 μm.
We then tested whether XCul5 Mo synergizes with the effect of XRab40 Mo. We injected XRab40 and XCul5 Mos at low doses that did not cause any defective phenotype when each was injected alone. Injection of the mixed Mos resulted in embryos showing a severe gastrulation-defective phenotype (Figure 5E and F). As additional evidence, the synergistic effect of XRab40 and XCul5 Mos strongly suggested that XRab40 and XCul5 cooperate during gastrulation.
Because the SOCS box is essential for the Golgi localization of XRab40 (Figure 3A), it is notable that the SOCS box-interacting protein may play an important role in the regulation of its localization. We then examined whether XCul5 is required for the localization of XRab40. XCul5 Mo inhibited the Golgi localization of XRab40 (Figure 5G and Supplementary Figure S3B). This result indicated that XCul5 determines the localization of XRab40 and this may be essential for XRab40 to function as a ubiquitin ligase with XCul5.
XRab40 regulates the ubiquitination and the localization of Rap2
We showed that XRab40 interacted with XRap2 in a SOCS box-independent manner. This result suggested that XRap2 could be a substrate for XRab40/XCul5 ubiquitin ligase. Thus, we further analyzed the relationship between XRab40 and XRap2. First, we observed the localization of XRap2 and XRab40 in both Xenopus animal cap explants (Figure 6A) and CHO cells (Figure 6B). It has been reported that XRap2 is localized at the plasma membrane and the cytoplasmic vesicles (Choi and Han, 2005). XRab40 was colocalized with XRap2 at these cytoplasmic vesicles and the plasma membrane. Next, we observed the change of localization of XRap2 by XRab40 and XCul5 knockdowns in the animal cap cells. XRab40 Mo, XCul5 Mo, XCul5N and XRab40 ΔSOCS were found to cause mislocalization of XRap2 to the cytoplasmic puncta (Figure 6C). The specific knockdowns of XRab40 and XCul5 by Mos causing mislocalization of XRap2 were confirmed by the retrieval of the normal localization of XRap2 when co-injected with the respective mRNAs (Supplementary Figure S6A). We further found that C-terminally truncated Cullins other than XCul5N did not affect the localization of XRap2 (Supplementary Figure S6B). Moreover, XRab40/XCul5 knockdowns do not cause any morphological influence on the Golgi apparatus as well as endosome and ER (Supplementary Figure S6C), suggesting the mislocalization of XRap2 by Mos may not be due to the disruption of the broad intracellular membrane vesicles. As Cullin5 is known as a ubiquitin ligase component, we investigated whether XRab40 and XCul5 regulate the ubiquitination of XRap2. The Western blot result showed that XRap2 was polyubiquitinated and XRab40 Mo, XCul5 Mo or Cul5N caused a reduction in XRap2 ubiquitination (Figure 6D and data not shown). This result suggests that XRap2 is a substrate for XRab40/XCul5 ubiquitin ligase.
Figure 6.
XRab40 regulates the localization and ubiquitination of XRap2. (A) EGFP-XRab40 was coexpressed with RFP-XRap2 in the animal regions. Scale bar=20 μm. (B) CHO cells were transiently transfected with RFP-XRab40 and EGFP-XRap2. (C) EGFP-XRap2 mRNA was coexpressed with either XRab40 Mo (1 pmol), XCul5N mRNA, XCul5 Mo or XRab40 ΔSOCS in the animal cap cells. Scale bar=50 μm. (D) GST-XRap2 mRNA was co-injected with myc-ubiquitin mRNA and either with XRab40 Mo or XCul5 Mo in the animal regions. Three independent experiments were carried out with the same conclusion. (E) 6 × histidine and GST-tagged XRap2 mRNA was coexpressed with myc-ubiquitin mRNA in the animal regions. (F) GST alone or GST-XRap2 was expressed in the animal regions. The immunoblotting results of antibodies against ubiquitin (upper panel) and GST (bottom panel) are shown.
We next sought to determine whether XRap2 is directly ubiquitinated by precipitation of XRap2 under the denaturation condition and XRap2 ubiquitination was still detected (Figure 6E), strongly suggesting XRap2 is likely to be directly ubiquitinated. We also demonstrated that Rap2 is ubiquitinated by endogenous ubiquitin, which is detected by immunoblotting with two anti-ubiquitin antibodies (Figure 6F and data not shown). As Rap2 stability is not affected by XRab40/XCul5 knockdowns (Figure 6D), XRab40/XCul5 is unlikely to regulate the degradation of XRap2. In addition, both XRab40 ΔN- and ΔC-GTPase rescued the reduction of XRap2 ubiquitination by XRab40 Mo, but XRab40 ΔGTPase did not (Supplementary Figure S7A). XRab40 ΔGTPase overexpression caused mislocalization of XRap2, whereas XRab40 ΔN- and XRab40 ΔC-GTPase did not (data not shown). These results suggest that the GTPase activity of XRab40 might not be required for its function. We speculate that ubiquitination of XRap2 is required for its localization. We then examined each of the potential ubiquitination sites in XRap2. The XRap2KR mutants were constructed by the substitution of the lysine to arginine residues. We demonstrated that one of the mutants, XRap2 K117R, showed increased cytoplasmic punctate appearance whereas XRap2 is mainly localized at the plasma membrane (Supplementary Figure S7B). Moreover, this mutant caused a reduction in ubiquitination when compared with wild-type XRap2 (Supplementary Figure S7C). These results further strengthen the idea that the ubiquitination of Rap2 is required for its localization. Knockdowns of XRab40/XCul5 inhibit the ubiquitination of XRap2, which consequently lead to the mislocalization of XRap2.
XRab40 regulates a Rap2 effector, STE20-like kinase XMINK
We then questioned how XRab40 and XRap2 might be involved in the regulation of Xenopus gastrulation. Several proteins have been identified as Rap2 effectors. One of the Rap2 effectors is STE20-like kinase, Traf2- and Nck-interacting kinase (TNIK). TNIK is activated by directly interacting with Rap2 (Taira et al, 2004). Interestingly, TNIK is similar to Drosophila Msn, which has been shown to be involved in the planar cell polarity (PCP) pathway functioning downstream of the Fz receptor (Paricio et al, 1999). Recently, zebrafish Msn has been shown to play an essential role in epiboly during gastrulation through regulating actin cytoskeleton (Köppen et al, 2006). However, the functions of this class of protein kinases in Xenopus embryogenesis have not yet been elucidated. Database searches revealed that Xenopus expresses a gene encoding a protein kinase highly homologous to mammalian TNIK-related kinase. The amino acid sequence of this protein is 79% identical to MINK and 66% identical to TNIK (Supplementary Figure S1E and F). We therefore referred to this kinase as XMINK. RT–PCR analysis showed that it was expressed maternally and throughout various Xenopus developmental stages (Supplementary Figure S1G).
First, we examined whether XMINK binds to XRap2 and found that indeed they have an interaction (Figure 7A). We then observed the subcellular localization of XMINK. XMINK was evenly distributed in the cytoplasm when expressed alone (Figure 7B). When coexpressed with XRap2, XMINK is mainly localized in the plasma membrane, suggesting that XRap2 may control the localization of XMINK. We also assessed whether the GTPase activity is important for Rap2 to function. We coexpressed XMINK with XRap2 SN, the constitutively inactive form of XRap2. In contrast to wild type, XRap2 SN failed to translocate XMINK to the plasma membrane (Figure 7B), indicating that the active GTP-bound form of Rap2 is required to exert its function. In addition, Rap2 K117R failed to translocate XMINK to the plasma membrane (Figure 7B). It suggests that proper localization of XRap2 is a prerequisite to its function. To clarify the function of XMINK in Xenopus embryogenesis, we next injected XMINK Mo into Xenopus embryos. The specificity of the Mo was examined (Supplementary Figure S2E). The XMINK Mo-injected embryos showed a gastrulation-defective phenotype, which was rescued by XMINK mRNA with no Mo-targeting sequence (Figure 7C–E). This result indicates that XMINK is essential for normal gastrulation. To assess whether XRab40 and XMINK shared the same signaling pathway, we performed a rescue experiment of XRab40 Mo using XMINK mRNA (Figure 7E). The gastrulation defect found in XRab40-depleted embryos was rescued by XMINK mRNA. To make the epistatic relationship between XMINK, XRab40 and XRap2 clearer, we tested whether XRab40 and/or XRap2 can rescue XMINK-depleted embryos. We demonstrated that XRab40 and XRap2 could not rescue the defective phenotype of XMINK Mo (data not shown). These phenotypic observations imply that XRab40 and XMINK are in a common signaling cascade where XMINK acts downstream of XRab40.
Figure 7.
XRab40/XCul5 regulates XMINK function. (A) Myc-XMINK mRNA was co-injected with GST alone, GST-XRap2 or GST-Dsh in the animal cap cells. (B) EGFP-XMINK was injected alone or together with XRap2, XRap2 SN or XRap K117R mRNAs in the animal regions. (C) XMINK Mo caused gastrulation defects when dorsally injected into two blastomeres in the four-cell stage embryos. (D) Statistical data of gastrulation-defective phenotypes caused by XMINK Mo. (E) Statistical data of the rescue experiment of XMINK (4 pmol) and XRab40 (1 pmol) Mos by XMINK mRNA. (F) EGFP-XMINK was injected in the animal regions alone, co-injected with the mRNAs or together with Mos (2 pmol each). Scale bar=50 μm.
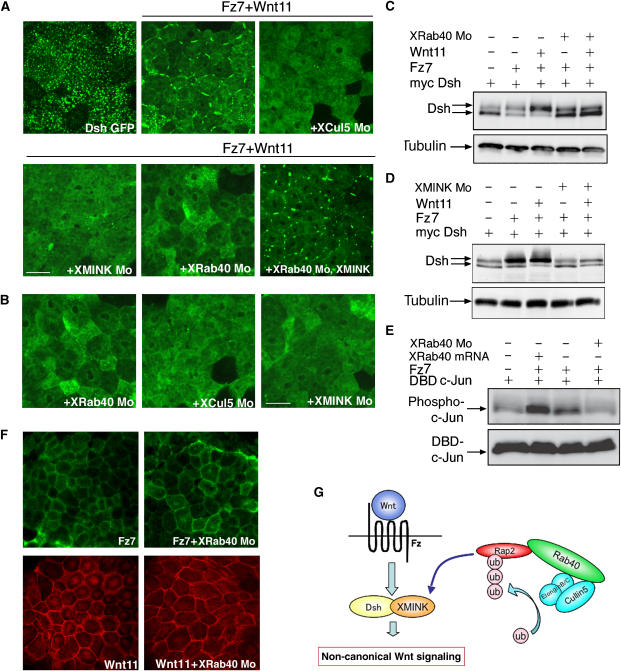
As Drosophila Msn functions in the PCP pathway downstream of Fz, XMINK might also play a role in noncanonical Wnt signaling. To test this possibility, we first examined whether XMINK binds to Dsh. As shown in Figure 7A, Dsh specifically associates with XMINK. Furthermore, we found that XMINK also translocated from the cytoplasm to the plasma membrane when coexpressed together with Wnt11, Fz7 and Dsh, and this translocation was inhibited by XRab40 and XCul5 Mos (Figure 7F). These results suggest a potential crosstalk between the XRab40-XMINK signaling cascade and the Wnt pathway.
XRab40 is involved in the noncanonical Wnt pathway
We then investigated the effects of XRab40 in the Wnt pathway. Upon activation of the Wnt pathway, Dsh is phosphorylated and translocated to the plasma membrane in Xenopus embryonic cells (Axelrod et al, 1998; Rothbacher et al, 2000). JNK is also known to be activated (Yamanaka et al, 2002). We expressed GFP-Dsh in the animal cap cells. It is known that Dsh shows punctate distribution in the cytoplasm (Schwarz-Romond et al, 2005; Figure 8A). When the Wnt pathway was activated, Dsh was localized at the plasma membrane. However, XRab40, XCul5 and XMINK Mos were found to inhibit the translocation of Dsh. The translocation of Dsh was retrieved when co-injected with the respective mRNAs (Supplementary Figure S8A). XMINK mRNA rescued the inhibition of translocation of Dsh to the plasma membrane by XRab40 Mo (Figure 8A). It is not surprising as the XRab40 Mo-defective phenotype was ameliorated by co-injection with XMINK mRNA. This indicates XMINK functions downstream of XRab40 to regulate the Wnt pathway.
Figure 8.
XRab40 is involved in the noncanonical Wnt pathway. (A, B) The localization of GFP-Dsh was shown. mRNAs and Mos were injected in the animal regions (XRab40 Mo, 2 pmol). (C–E) mRNAs were injected in the animal caps (XRab40 Mo, 2 pmol) and the lysates were subjected to imunnoblotting. (F) EGFP-Fz7 was expressed with or without XRab40 Mo (top panel). Myc-Wnt11 was expressed with or without XRab40 Mo and subjected to immunostaining (bottom panel). (G) Schematic illustration of how XRab40/XCul5-XRap2-XMINK-Dsh act in the noncanonical Wnt pathway. Scale bar=50 μm.
Interestingly, XRab40, XCul5 and XMINK Mos also inhibited the punctate localization of Dsh even in the absence of Wnt signaling (Figure 8B). The punctate localization of Dsh inhibited by XCul5 and XMINK Mos were rescued by co-injection with the respective mRNAs (Supplementary Figure S8B). Inhibition of XRap2 has also been reported to cause similar cytoplasmic distribution of Dsh (Choi and Han, 2005).
In addition, we showed that XRab40 and XMINK Mos inhibited the phosphorylation of Dsh (Figure 8C and D), and that XRab40 Mo inhibited the activation of JNK by Wnt signaling (Figure 8E). Inhibition effects of XRab40 and XMINK Mos on Dsh phosphorylation were rescued by co-injection with XRab40 and XMINK mRNAs, respectively (data not shown). These results suggest that punctate localization is a prerequisite for Dsh to transduce Wnt signaling and that XRab40, XCul5, XRap2 and XMINK are required for the punctate localization of Dsh. The localizations of Wnt11 and Fz7 did not change by XRab40 Mo (Figure 8F), suggesting that the defect in the Wnt pathway might not be due to the failure to transport the ligand or the receptor.
Whereas XRap2 was first placed in the Wnt/β-catenin pathway (Choi and Han, 2005), it may also participate in noncanonical Wnt signaling. To examine the effects of XRap2 downregulation during Xenopus gastrulation, we dorsally expressed XRap2 SN and XRap2 K117R in embryos. Both XRap2 SN- and XRap2 K117R-injected embryos disrupted gastrulation (Supplementary Figure S9A and B). Notably, the defective phenotype was effectively rescued by the expression of wild-type XRap2. XMINK could also rescue the CE-defective phenotype of the XRap2 mutants, suggesting XMINK acts downstream of XRap2 (Supplementary Figure S9A and B). The XRab40 and XCul5 morphant embryos were also rescued by XRap2 (data not shown), implying XRap2 is downstream of XRab40/XCul5. We further speculated whether XRap2 SN and XRap2 K117R affect CE movements. Both XRap2 SN- and XRap2 K117R-injected cells were not polarized or intercalated (Supplementary Figure S9C). Moreover, the expressions of chordin, gsc, Xnr3 and Xbra in XRap2 SN- and XRap2 K117R-injected embryos did not change (Supplementary Figure S9D). Taken together, these results suggest that XRap2 is involved in CE movement without affecting mesodermal induction. We then performed a TOP-FLASH assay to investigate the canonical pathway. Both XRab40 knockdown and overexpression had no effect on the transcriptional activation of TOP-FLASH (Supplementary Figure S9E), indicating that XRab40 was not involved in canonical Wnt signaling. This is consistent with the result that XRab40 Mo did not affect the expression of Xnr3 and siamois, which are regulated by the canonical Wnt pathway (Brannon and Kimelman, 1996; Carnac et al, 1996; McKendry et al, 1997; Supplementary Figure S4B).
Discussion
Here, we analyzed the biological functions of XRab40 and found that it plays an essential role in noncanonical Wnt signaling. A key finding in this study is that XRab40 interacts with XCul5 forming a ubiquitin ligase at the Golgi apparatus. We also showed that XRab40/XCul5 regulates the ubiquitination and intracellular localization of XRap2. Moreover, we illustrated that XMINK, an effector of XRap2, is implicated in the Wnt/Fz signaling pathway by controlling the localization of Dsh.
XRab40 forms a ubiquitin ligase complex with XCul5 and XElonginB/C
The Rab40 family members commonly present a number of structural features distinctive from other Rab GTPases. These features include a C-terminal extension containing the SOCS-box domain. We found that XRab40 binds to XElonginB/C and XCul5 through the SOCS box. This domain was initially identified in SOCS proteins that negatively regulate the cytokine signaling pathways. A large number of SOCS box proteins, containing various other motifs, including GTPase, WD40 repeat, ankyrin repeat and SPRY domains, have also been identified (Masuhara et al, 1997; Hilton et al, 1998). Some of the SOCS box-containing proteins, including human Rar3/Rab40c, have been shown to interact with ElonginB/C and Cul5 (Kamura et al, 2004), which is consistent with our observation. In addition to the physical interaction between XRab40 and XCul5, we also showed that these proteins are closely related in terms of their functions. Our results also reinforce the notion that XCul5 and XRab40 form a ubiquitin ligase complex and cooperate with each other to exert their cellular functions.
The phenotypic observations of XCul5 Mo indicated that it is also essential for Xenopus gastrulation. Several recent studies suggest the requirements of Cullin-based E3 ligases in Xenopus development. For instance, Cullin1 is required for the correct allocation of cell fate in neural crest cells (Voigt and Papalopulu, 2006). The Cullin3-KLHL12 complex targets Dsh protein for degradation (Angers et al, 2006). In our study, we demonstrate that XCul5 has functions distinct from Cullin1 and 3 in Xenopus development.
XRap2 as a substrate of XRab40/XCul5 ubiquitin ligase
We identified XRap2 as a strong candidate for the substrate of XRab40/XCul5 ubiquitin ligase. We showed that it was polyubiquitinated in a manner dependent on both XRab40 and XCul5. XRab40 and XCul5 regulate the localization of XRap2. Either loss of XRab40 or XCul5 function led to a drastic change in the localization of XRap2. In addition, the Rap2 K117R mutant, which is less ubiquitinated, also changed its localization. Our data suggest that the ubiquitination of Rap2 is required to regulate its localization. There is accumulating evidence showing that polyubiquitination regulates localization and trafficking of membrane proteins (reviewed by d'Azzo et al, 2005). For example, EGF receptor, E-cadherin and M-cadherin are ubiquitinated on the plasma membrane and internalized to the endocytic compartment (Levkowitz et al, 1998; Fujita et al, 2002; Palacios et al, 2005; Charrasse et al, 2006). The ubiquitination regulated by XRab40/XCul5 ubiquitin ligase on the Golgi apparatus may be one of the important mechanisms to determine the localization of membrane proteins.
Our study revealed that XRab40 and XRap2 regulate XMINK localization. Several studies have shown that Rap2 selectively binds to MINK-related kinases, TNIK and mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4). Rap2 promotes the autophosphorylation and translocation of TNIK (Taira et al, 2004) and enhances the activation of JNK with MAP4K4 (Machida et al, 2004). Our result is consistent with these studies, suggesting the activation mechanism of MINK and related kinases by Rap2 may be conserved.
XRab40 is required for the Wnt signaling pathway
We demonstrate that XRab40, XCul5 and XMINK Mos prevent the translocation of Dsh to the plasma membrane induced by Wnt and Fz7, indicating that these are required for the noncanonical Wnt pathway. In addition, XMINK rescued the inhibition of Dsh translocation to the plasma membrane by XRab40 Mo, implying that XRab40/XCul5, XRap2 and XMINK constitute a signaling cascade to regulate Wnt signaling. Besides Wnt and Fz7 expressions, the membrane translocation of XMINK requires Dsh, suggesting Dsh and XMINK function in a mutually dependent manner. We found that Wnt signaling does not affect the interactions among XRab40, XCul5, XRap2 and XMINK. Activation of Wnt signaling did not change the localization of XRab40 and XRap2 (data not shown). These results suggest that Wnt signaling may not affect this signaling cascade, but crosstalk with each other by regulating XMINK and Dsh.
Although XRap2 has been reported to regulate the canonical Wnt pathway (Choi and Han, 2005), we found that XRap2 may play a role in the noncanonical Wnt pathway. We found that XRab40 did not affect the canonical Wnt pathway in the TOP-FLASH assay (Supplementary Figure S9E). Rap2 may be required for both canonical and noncanonical signaling, whereas XRab40 is required for XRap2 function in the noncanonical pathway, but is dispensable for its function in the canonical Wnt pathway. It has been shown that Dsh is translocated to the plasma membrane in the noncanonical pathway, but not in the canonical pathway (Axelrod et al, 1998). In order for Rap2 to regulate the noncanonical pathway, Rab40 may be required to localize Rap2 properly.
When Wnt signaling is not activated, Dsh is localized at the cytoplasmic puncta. Interestingly, loss of function of the XRab40–XMINK pathway disrupts the punctate localization of Dsh. It has been shown that endogenous Dsh is also localized to the cytoplasmic puncta in Xenopus cells (Cheyette et al, 2002). Dsh localization is known to be functionally significant and correlated with its activity to transduce the Wnt signaling cascade (Miller et al, 1999; Capelluto et al, 2002). Previous work has demonstrated that proper localization of Dsh is essential for the noncanonical Wnt pathway to control CE (Park et al, 2005). Taken together, the punctate localization of Dsh may be a prerequisite for Dsh to transduce Wnt signaling, and XRab40, XCul5, XRap2 and XMINK may be required for maintaining Dsh to localize to the puncta.
Why is the punctate localization of Dsh important? One possibility is that Wnt signaling might control membrane trafficking to regulate Dsh localization and the XRab40–XMINK pathway could participate in the regulation of this machinery. In fact, XRab40 and XRap2 are localized to the vesicles. MINK-related kinase TNIK has also been shown to localize at vesicle structures with Rap2 (Taira et al, 2004). In Drosophila, Msn, highly similar to vertebrate MINK and TNIK, acts downstream of Fz and Dsh, and specifically activates the JNK in the Wnt/PCP pathway (Paricio et al, 1999). Cul5 has been reported to be required for wing formation genetically interacting with the Wnt/Wingless signaling pathway in Drosophila (Ayyub et al, 2005). However, it has not yet been reported that Cul5 is involved in the PCP pathway. It would be interesting to know whether the regulatory mechanism of the PCP pathway by Rab40-Cul5 and MINK/Msn is conserved in different organisms.
MINK and TNIK have been shown to regulate actin cytoskeleton (Fu et al, 1999, Taira et al, 2004). Msn is required for epithelial morphogenesis through the regulation of the actin/myosin 2-based cell constriction at the epidermal marginal cells, and is conserved in both Drosophila and zebrafish (Köppen et al, 2006). Our previous work demonstrated that the noncanonical Wnt pathway regulates actin cytoskeleton and protrusive activity in Xenopus embryonic cells (Iioka et al, 2004). These findings suggest that XMINK may be one of the important components that link Wnt signaling to actin regulation. It has been shown that the noncanonical Wnt pathway is essential for CE movements (Kuhl, 2002; Tada et al, 2002). Our data suggest that XRab40/XCul5 is implicated in the noncanonical Wnt signaling pathway and is required for the CE process. However, we also observed that XRab40 knockdown reduced Xbra expression. Xbra is important for mesoderm induction and cell migration (Smith et al, 1991; Kwan and Kirschner, 2003), suggesting that XRab40 knockdown may cause the gastrulation defect due to the reduction of Xbra expression as well as the inhibition of the Wnt pathway. We demonstrated that Xbra failed to rescue the CE-defect phenotype caused by XRab40 Mo (data not shown). The reduction of Xbra might account for one of the factors that caused the CE-defect, but expression of Xbra alone is not sufficient to rescue XRab40-induced-defective phenotype. It is still unknown how XRab40 affects Xbra expression. XRab40/XCul5 may have multiple substrates, some of which may affect Xbra expression and more studies will be needed to show its regulatory mechanism.
In conclusion, our data demonstrate that XRab40 and XCul5 form a ubiquitin ligase complex at the Golgi apparatus and act as a prerequisite for the noncanonical Wnt pathway through the regulation of XRap2 and XMINK. The XRab40–XMINK pathway is required for controlling the membrane localization of Dsh that is important to transduce the Wnt signaling (Figure 8G). Our findings reveal that the Golgi-localized XRab40/XCul5 ubiquitin ligase complex may serve as a novel mechanism to regulate the Wnt pathway that is essential for gastrulation.
Materials and methods
Plasmids, RNA synthesis and Mos
Plasmids for the expression in Xenopus and CHO cells were constructed using pCS2+ or pCS2+-based epitope-tagging vectors. Capped mRNAs were synthesized using mMESSAGE mMACHINE kit (Ambion). Detailed plasmid construction, antisense Mos and in situ hybridization are described in Supplementary Data.
Pull-down assay and Western blotting
Cell lysates were prepared in PBS containing 0.1% Triton-X100 and a 1/200 volume of protease inhibitor cocktail (No. P8340, Sigma). The lysates were cleared by centrifugation. The supernatants were then incubated with Glutathione Sepharose beads (No. 17-0756-01, Amersham Biosciences) or Ni beads (No. 8901–1, Clontech) at 4°C for 2 h. The purified complex was washed five times with cold lysis buffer or with 4 M urea when purified by Ni beads. Proteins were separated by electrophoresis. Specific proteins were detected by primary antibodies against myc (1:1000; No. SC-40, Santa Cruz Biotechnology), GST (1:500; No. SC-138, Santa Cruz Biotechnology), His (1:500; Qiagen), GFP (1:100; No. 04363–24, Nacalai tesque Inc.), ubiquitin (1:500; MFK-001 and MFK-003, Nippon Bio-test Laboratories Inc.) or phosho-Smad2 (1:1000; No. 06–829, Upstate Biotechnology) and then secondary antibody of goat anti-mouse IgG or anti-rabbit IgG labeled with horseradish peroxidase (1:4000) and detected by ECL Detection Reagent, following the manufacturer's procedures (Amersham Biosciences Ltd.)
Yeast two-hybrid screening and mass spectrometric analysis
For yeast two-hybrid screening, XRab40 lacking the C-terminal four amino acids was cloned into pGBKT7 vector (Clontech Laboratories) to construct the bait plasmid. Screening was performed according to the manufacturer's instruction using the Xenopus oocyte MATCHMAKER cDNA and Mouse 11 day embryo MATCHMAKER cDNA libraries (Clonetech Laboratories). Flag-tagged XRab40 was expressed in HEK293 cells and immunoprecipitated by anti-Flag antibody. The precipitated complex was analyzed using liquid chromatography followed by tandem mass spectrometry (LC-MS/MS) (Natsume et al, 2002).
Supplementary Material
Supplementary Data
Supplementary References
Acknowledgments
We thank Professor Randall Moon (University of Washington School of Medicine) for plasmid constructs for the TOP-FLASH assay and also Dr Haraguchi (Kansai Advanced Research Center, Japan) for the human lamin B receptor plasmid construct. We thank Professor Naoto Ueno (National Institute for Basic Biology, Japan) and members in his laboratory for advice, suggestions and help throughout the course of this work. This work was supported by Grant-in-Aid for Scientific Research (Kakenhi) to NK and MO and grants from the Naito foundation to MO and from the New Energy and Industrial Technology Development Organization (NEDO) to SI and TN.
References
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, Maccoss MJ, Moon RT (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt–beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol 8: 348–357 [DOI] [PubMed] [Google Scholar]
- Argon Y, Simen BB (1999) GRP94, an ER chaperone with protein and peptide binding properties. Semin Cell Dev Biol 10: 495–505 [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N (1998) Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev 12: 2610–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyub C, Sen A, Gonsalves F, Badrinath K, Bhandari P, Shashidhara LS, Krishna S, Rodrigues V (2005) Cullin-5 plays multiple roles in cell fate specification and synapse formation during Drosophila development. Dev Dyn 232: 865–875 [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M (1999) Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev 83: 27–37 [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109–118 [DOI] [PubMed] [Google Scholar]
- Brannon M, Kimelman D (1996) Activation of Siamois by the Wnt pathway. Dev Biol 180: 344–347 [DOI] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M (2002) The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419: 726–729 [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P (1996) The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development 122: 3055–3065 [DOI] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouviere C (2006) RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol Biol Cell 17: 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT (2002) Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell 2: 449–461 [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Costantini F, Jho EH, Joo CK (2004) Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by Axin. J Biol Chem 279: 49188–49198 [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK (2005) Rap2 is required for Wnt/beta-catenin signaling pathway in Xenopus early development. EMBO J 24: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Azzo A, Bongiovanni A, Nastasi T (2005) E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 6: 429–441 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ (1999) SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol 15: 435–467 [DOI] [PubMed] [Google Scholar]
- Fu CA, Shen M, Huang BC, Lasaga J, Payan DG, Luo Y (1999) TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J Biol Chem 274: 30729–30737 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4: 222–231 [DOI] [PubMed] [Google Scholar]
- Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R (1998) FYVE fingers bind PtdIns(3)P. Nature 394: 432–433 [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17: 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T, Koujin T, Segura-Totten M, Lee KK, Matsuoka Y, Yoneda Y, Wilson KL, Hiraoka Y (2001) BAF is required for emerin assembly into the reforming nuclear envelope. J Cell Sci 114: 4575–4585 [DOI] [PubMed] [Google Scholar]
- Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA (1998) Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA 95: 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser M (2006) Lingering mysteries of ubiquitin-chain assembly. Cell 124: 27–34 [DOI] [PubMed] [Google Scholar]
- Iioka H, Ueno N, Kinoshita N (2004) Essential role of MARCKS in cortical actin dynamics during gastrulation movements. J Cell Biol 164: 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, Conaway JW, Nakayama KI (2004) VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev 18: 3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ (2002) The SOCS box: a tale of destruction and degradation. Trends Biochem Sci 27: 235–241 [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N (2003) PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev 17: 1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppen M, Fernandez BG, Carvalho L, Jacinto A, Heisenberg CP (2006) Coordinated cell-shape changes control epithelial movement in zebrafish and Drosophila. Development 133: 2671–2681 [DOI] [PubMed] [Google Scholar]
- Kuhl M (2002) Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin Cell Dev Biol 13: 243–249 [DOI] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW (2003) Xbra functions as a switch between cell migration and convergent extension in the Xenopus gastrula. Development 130: 1961–1972 [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev 12: 3663–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D (1999) Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem 274: 129–134 [DOI] [PubMed] [Google Scholar]
- Machida N, Umikawa M, Takei K, Sakima N, Myagmar BE, Taira K, Uezato H, Ogawa Y, Kariya K (2004) Mitogen-activated protein kinase kinase kinase kinase 4 as a putative effector of Rap2 to activate the c-Jun N-terminal kinase. J Biol Chem 279: 15711–15714 [DOI] [PubMed] [Google Scholar]
- Masuhara M, Sakamoto H, Matsumoto A, Suzuki R, Yasukawa H, Mitsui K, Wakioka T, Tanimura S, Sasaki A, Misawa H, Yokouchi M, Ohtsubo M, Yoshimura A (1997) Cloning and characterization of novel CIS family genes. Biochem Biophys Res Commun 239: 439–446 [DOI] [PubMed] [Google Scholar]
- Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC (1991) CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem 266: 3239–3245 [PubMed] [Google Scholar]
- McKendry R, Hsu SC, Harland RM, Grosschedl R (1997) LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev Biol 192: 420–431 [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT (1999) Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol 146: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Fujita T, Ozaki T, Kato C, Kurose Y, Sakamoto M, Kato S, Goto T, Itoyama Y, Aoki M, Nakagawara A (2004) NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J Biol Chem 279: 11327–11335 [DOI] [PubMed] [Google Scholar]
- Natsume T, Yamauchi Y, Nakayama H, Shinkawa T, Yanagida M, Takahashi N, Isobe T (2002) A direct nanoflow liquid chromatography-tandem mass spectrometry system for interaction proteomics. Anal Chem 74: 4725–4733 [DOI] [PubMed] [Google Scholar]
- Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C (2005) Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 25: 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M (1999) The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J 18: 4669–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Gray RS, Sato A, Habas R, Wallingford JB (2005) Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr Biol 15: 1039–1044 [DOI] [PubMed] [Google Scholar]
- Patki V, Lawe DC, Corvera S, Virbasius JV, Chawla A (1998) A functional PtdIns(3)P-binding motif. Nature 394: 433–434 [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gabin AG, Almazan G, Larocca JN (2004) Vesicle transport in oligodendrocytes: probable role of Rab40c protein. J Neurosci Res 76: 758–770 [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE (2000) Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J 19: 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpp S, Brand M (2004) Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr Biol 14: 1834–1841 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M (2005) The Wnt signaling effector dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci 118: 5269–5277 [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R (1992) Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development 116: 901–914 [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG (1991) Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67: 79–87 [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP (2002) Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol 13: 251–260 [DOI] [PubMed] [Google Scholar]
- Taira K, Umikawa M, Takei K, Myagmar BE, Shinzato M, Machida N, Uezato H, Nonaka S, Kariya K (2004) The Traf2- and Nck-interacting kinase as a putative effector of Rap2 to regulate actin cytoskeleton. J Biol Chem 279: 49488–49496 [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP (2005) Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 9: 555–564 [DOI] [PubMed] [Google Scholar]
- Voigt J, Papalopulu N (2006) A dominant-negative form of the E3 ubiquitin ligase Cullin-1 disrupts the correct allocation of cell fate in the neural crest lineage. Development 133: 559–568 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM (2002) Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell 2: 695–706 [DOI] [PubMed] [Google Scholar]
- Wharton KA Jr (2003) Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol 253: 1–17 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Fukuda MN (1995) Golgi retention mechanism of beta-1,4-galactosyltransferase. Membrane-spanning domain-dependent homodimerization and association with alpha- and beta-tubulins. J Biol Chem 270: 12170–12176 [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E (2002) JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3: 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Supplementary References