Abstract
Adult human bone marrow stromal cells (hMSCs) grown in suspension culture gave rise to spheres of neural progenitor (NP) cells, capable of expressing both dopaminergic (DA) and GABAergic (GABA) traits. After transplantation into the Parkinsonian rat, human NPs and neurons were present at 2 weeks. Although no DA neurons appeared to survive transplantation, there were abundant GABA neurons present in the graft. By 4 weeks, however, all cells had died. Finding ways to prolong survival and promote the appropriate neurotransmitter phenotype is essential if hMSCs are to be clinically useful.
Keywords: Dopamine, GABA, Bone marrow, Progenitor cell, Parkinson's disease, Transdifferentiation, Transplantation
Parkinson's disease (PD) is a common neurodegenerative disorder that affects more than 2% of the population over the age of 65 (Dauer and Przedborski, 2003). PD is caused by the progressive neurodegeneration of dopamine (DA) neurons, leading to symptoms of tremor, rigidity, and bradykinesia (Dauer and Przedborski, 2003). Stem/progenitor cell transplantation is one of the most promising new therapeutic approaches for treating PD.
Finding a reliable source for the generation of human NPs for transplantation, however, has been difficult. Ethical questions have been raised regarding the use of both embryonic stem and fetal cells. Moreover, in neural transplantation, there is always a risk of immune rejection of allogeneic transplants. Thus, the best source of tissue for transplantation is patient-derived. For this reason, sources of autologous progenitor cells, such as bone marrow, have been investigated (Azizi et al., 1998; Brazelton et al., 2000; Mezey et al., 2000; Rieske et al., 2005; Woodbury et al., 2000). Although human bone marrow stromal cells (hMSCs) normally give rise to mesenchymal derivatives, such as osteoblasts, adipocytes, myoblasts, and chondrocytes (Caterson et al., 2002), recent studies indicated that these cells were capable of remarkable phenotypic plasticity. Thus, rodent and human MSCs can, under certain conditions, cross traditional germ layer boundaries to develop into neurons, cardiac muscle, and other types of cells (Azizi et al., 1998; Kohyama et al., 2002; Munoz-Elias et al., 2003; Sanchez-Ramos et al., 2000; Suon et al., 2004; Woodbury et al., 2000).
Studies have shown that rodent and human MSCs can be converted into process-bearing cells resembling neurons that express central nervous system (CNS) proteins when they are incubated in media containing a variety of different differentiation factors (Kohyama et al., 2002; Suon et al., 2004), raising the possibility that hMSCs have the plasticity to transdifferentiate into mature neurons, such as DA neurons. If so, they could represent an important source of cells for transplantation in PD.
In an attempt to potentially change hMSCs from a mesenchymal lineage to a neural lineage, in the present study, we have modified their growth in culture from an attached monolayer to a suspended sphere using a procedure similar to the one used for propagation of NPs. We sought to further refine growth conditions to promote the differentiation of DA neurotransmitter traits, like tyrosine hydroxylase (TH), in NP-hMSC spheres. In addition, we have examined their ability to express traits of other neurotransmitter systems such as GABA. Finally, we have studied their survival and capability to express CNS traits after transplantation into unilaterally 6-hydroxydopamine (6-OHDA) lesioned rats. hMSC spheres are clinically attractive because they can be expanded into large quantities in culture for transplantation into patients as autografts or allografts.
DA differentiation of bone marrow stromal spheres in culture
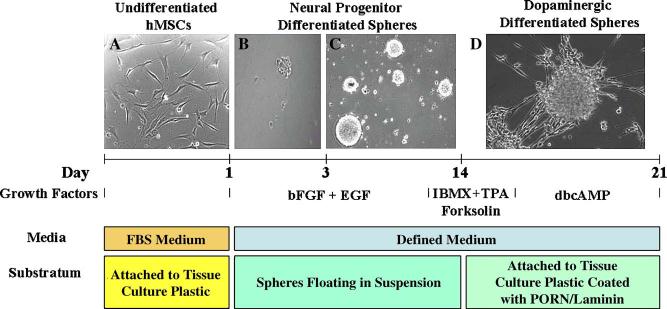
We began our studies by establishing growth conditions for growing hMSCs in spheres, similar to culture methods used to generate DA neurons from brain-derived hNPs. Undifferentiated hMSCs were initially grown in monolayer culture (Fig. 1A) as described previously (Caterson et al., 2002; Suon et al., 2004). To initiate sphere formation, hMSCs were mechanically dissociated and cultured in suspension in a defined serum-free growth medium containing epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). After 2 to 4 days in culture, cellular aggregates in the shape of spheres began to form (Fig. 1B). These grew larger and remained as floating spheres of variable size (Fig. 1C). We refer to cells in this stage as NP-hMSC spheres.
Fig. 1.
Generation of DA neurons from adult hMSC spheres. Cells from the hMPC 32F line were propagated in a protocol similar to the cultivation of neural progenitor cells. HMPC 32F were mechanically dissociated and cultured in suspension in a serum-free growth medium containing epidermal growth factor and basic fibroblast growth factor. Spheres were further differentiated by treating them in serum-free medium containing a differentiation cocktail (200 nM TPA + 250 μM IBMX + 50 μM Forskolin) for 3 h and dibutyryl cAMP for 7 days.
After 14 days in suspension, NP-hMSC spheres were plated on polyornithine/laminin (PORN/Ln)-coated slides in serum-free medium containing a DA differentiation cocktail (200 nM TPA + 250 μM IBMX + 50 μM Forskolin) for 3 h and 1 mM dibutyryl cAMP for 7 days (Fig. 1D) as described in our previous studies (Du and Iacovitti, 1997a,b; Suon et al., 2004). The DA-differentiated hMSC (DA-hMSC) spheres attached within hours and cells at the outer margins of each sphere began to migrate away from the primary site of attachment and began to elicit neuritic-like processes (Fig. 1D).
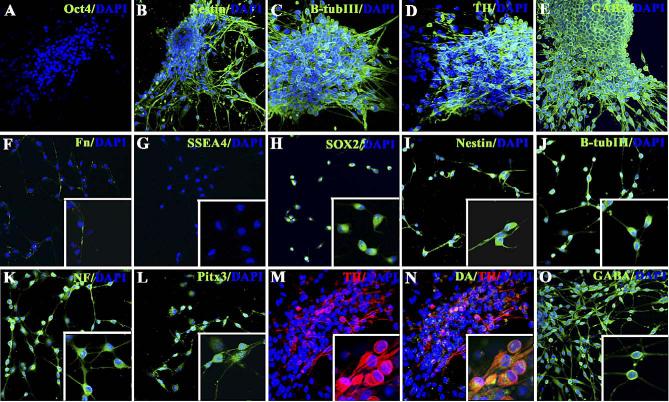
In order to determine the phenotype of hMSC cells in spheres or in adherent cells which migrated away from spheres, cultures were fixed and evaluated for the presence of cell-type-specific markers using immunocytochemistry (Fig. 2) and PCR analysis (Fig. 3). We expected that commitment to the neuronal lineage would result in the down-regulation of mesenchymal gene products and up-regulation of neural-associated proteins. As anticipated, we found that NP-hMSC spheres and DA-hMSC spheres expressed low levels of the mesenchymal marker fibronectin as compared to undifferentiated hMSCs (Figs. 2F and 3) and higher levels of the NP markers, nestin, Musashi 1, and Sox-2 (Figs. 2B, H, I and 3). Not surprisingly, pluripotent embryonic stem cell markers, such as Oct-4 and SSEA-4 (Figs. 2A and G), were not present. DA-hMSC spheres further expressed detectable levels of the neuronal markers (β-tubulin III and NF) (Figs. 2C, J, K and 3) as well as markers of a DA phenotype (Nurr-1, Pitx-3, TH, AADC and GIRK2) (Figs. 2D, L-N and 3). Indeed, when neurons in DA-hMSC spheres were double-labeled and counted, 15% ± 3% of all β-tubulin III positive neurons were found to be TH positive. Surprisingly, when sister cultures were stained for the presence of the neurotransmitter GABA, essentially all cells stained positively (Figs. 2E, O). Taken together, these data indicate that, when hMSCs are grown and differentiated in culture as NPs, cells differentiate into GABA neurons, a subset of which co-express a DA phenotypic traits.
Fig. 2.
Characterization of cells in human MSC spheres. Immunocytochemical localization of Oct-4 (A), nestin (B;I), β-tubulin III (C; J), Fn (F), SSEA4 (G), Sox-2 (H), NF (K), Pitx-3 (L), TH (D, M, N), DA (N), and GABA (E, O) in DA-hMSC spheres (A–E) or in cells which migrated away from adherent DA-hMSC spheres (F–O). All stained cultures were mounted in Prolong Gold for nuclear staining with DAPI (Invitrogen). High power (40×) insets are included in panels F–O.
Fig. 3.
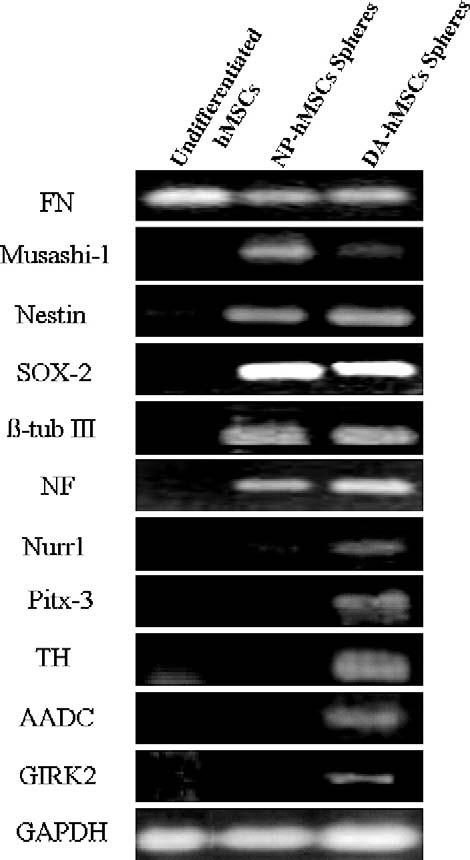
RT-PCR analysis indicated that NP-hMSC spheres expressed neuronal markers (nestin, Nurr-1, and β-tubulin III). In contrast, only DA-hMSC spheres expressed specific midbrain DA markers (Pitx-3, AADC, GIRK2, and TH).
Transplantation of bone marrow stromal spheres
As with DA-differentiated brain-derived neurons, our pilot studies indicated that lifting and transplanting attached DA-hMSC spheres would be difficult and cells were likely too mature to survive engraftment (data not shown). Thus, in our in vivo studies, we used an earlier stage of development, NP-hMSC spheres, as our source of cells for transplantation into the striatum of 6-OHDA-treated rats (n = 4). In Fig. 4A, the NPhMSC spheres were immunostained with human nuclear protein (hunu) to identify transplanted tissue. We found that NP-hMSC spheres survived short-term (1–2 weeks) and integrated well into the host striatum, with little migration away from the graft. Although some cells in the graft expressed the NP marker nestin (Fig. 4B) and the neuronal marker β-tubulin III (Fig. 4D), even with appropriate “priming” in culture, hMSC-derived neurons were incapable of further differentiation into TH-expressing cells in vivo (Figs. 4E-F). In contrast, however, many engrafted hunu+ hMSCs stained for GABA (Figs. 4G-I). However, none of these cells survived long term in the grafts, by 4 weeks, all cells had died (data not shown).
Fig. 4.
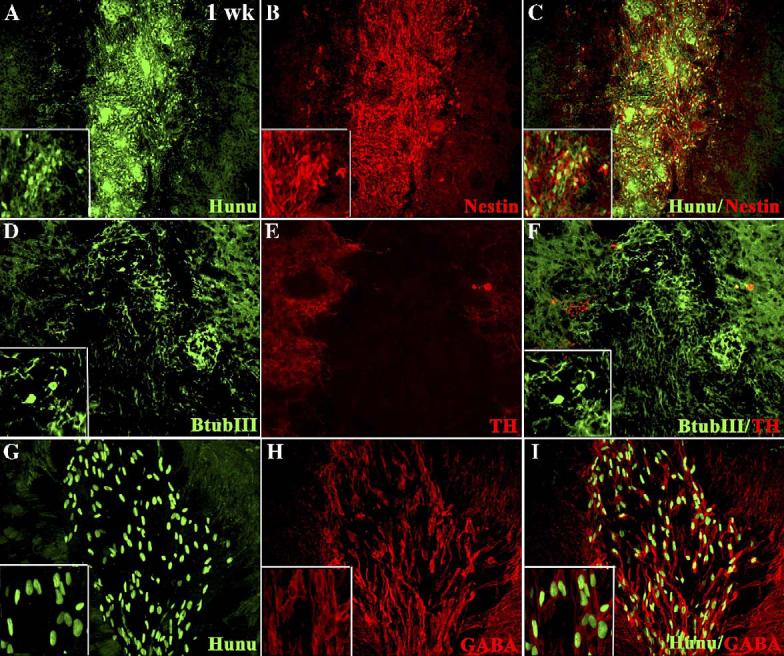
Characterization of NP-hMSC spheres 1 week after transplantation into the striatum of 6-OHDA-treated rats. Immunocytochemical localization of hunu (A), nestin (B), and images merged in panel C, β-III tub (D), TH (E), and images merged in panel F, hunu (G, GABA (H) and images merged (I)). High-power (40×) insets are included in panels A–D, F, G–I.
Recent evidence has suggested that progenitor cells derived from the bone marrow have the ability to generate either neurons or glia in vitro (Kohyama et al., 2002; Munoz-Elias et al., 2003; Sanchez-Ramos et al., 2000; Suon et al., 2004; Woodbury et al., 2000) and in vivo (Azizi et al., 1998; Brazelton et al., 2000; Deng et al., 2001; Eglitis and Mezey, 1997; Mezey et al., 2000). These promising results therefore led to the suggestion that hMSC might be a good candidate for stem cell therapy in PD. Indeed, hMSC can be easily obtained from bone marrow, expanded in culture and stored in universal donor cell banks for use in allogeneic or autologous transplantation. The latter possibility is exciting as immune suppression and prevention of the host immune response would not be necessary. In addition, hMSCs do not pose ethical concerns as fetal and embryonic stem cells.
However, for hMSCs to be useful in PD, they must express a fully differentiated DA phenotype. The results of the present study demonstrate that, when hMSCs are grown in culture as spheres, using a protocol similar to the one developed for the propagation of brain-derived NPs, they behave as NPs. By growing NP-hMSCs under conditions that increased their intracellular cAMP levels, they further expressed traits typical of neurons in vitro. Moreover, a subset of these neurons further express the transcription factors (Nurr-1, Pitx-3), enzymes (TH, AADC), and ion channels (GIRK2) specifically associated with a DA pheno-type. However, when examined further, we found that all cells expressing DA traits also stained for GABA. Although somewhat unexpected, in fact, the co-expression of DA traits in GABA neurons in vitro and in vivo has been shown previously by us (Iacovitti, 1991; Du and Iacovitti, 1995; Max et al., 1996; Stull and Iacovitti, 2001) and others (Gaspar et al., 1987; Kosaka et al., 1987; Jaeger and Joh, 1983; Satoh and Suzuki, 1990) in a variety of different neuronal systems.
Because attached DA-hMSC spheres were too mature for transplantation into the brain, we tested whether NP-hMSCs could complete the neuronal and/or neurotransmitter differentiation process in vivo. We found that NP-hMSC spheres survived short term (1–2 weeks) in the striatum of 6-OHDA-treated rats, integrated into the host parenchyma, and in some cases differentiated into β-tubulin III+ neurons. Although many of these neurons expressed a GABA phenotype, none simultaneously stained for TH. Possibly, the preferential differentiation of these neurons into GABA neurons owes to local differentiation factors present in the striatum, a structure which is intrinsically comprised of 90% GABA neurons (Mugnaini and Oertel, 1985). While no hMSCs survived long-term (4 week) in the graft, our results indicate that cells grown as NP-hMSC spheres can indeed develop into neurons in situ. Whether with longer time in the graft or treatment with additional factors prior to engraftment, hMSCs can develop into DA neurons, awaits further investigation.
Although previous studies have shown survival of rodent MSC, no prior studies have demonstrated survival of differentiated human MSC in a transplant in the brain. Even so, survival of hMSCs in the graft was short-lived. One possibility is that major histocompatibility complex (MHC) Class I and II antigens on the surface of hMSCs may contribute to their death and transplants into nude rats or SCID mice may improve graft survival. However, the fact that human brain-derived NPs survive many months in vivo (Yang et al., 2004) suggests that immuno-rejection of xeno-transplanted tissue may not be the sole explanation for their rapid demise. Possibly, MSCs produce a variety of factors that alter the host environment, creating a more hostile milieu in which to survive. Our ongoing studies are addressing possible ways to improve and prolong the survival of differentiated hMSC spheres in vivo, an essential step towards their utility as replacement neurons in neurodegenerative disease.
Tissue culture
Tissue recovery of hMSCs and the establishment of the hMPC 32F line (Osyczka et al., 2002) was performed as described in Caterson et al. (2002) and as modified in Suon et al. (2004). hMPCs were grown in culture in media containing DMEM, 10% FBS, and penicillin/streptomycin at 37 °C: 5% CO2. hMPCs were mechanically dissociated and cultured as spheres in suspension containing DMEM/F12, 0.5% B27, basic FGF (50 ng/ml), EGF (50 ng/ml), and 1% penicillin/streptomycin for 14 days. Spheres were dissociated into single cells with a large bore fire bent pipette and plated on polyornithine/laminin (PL)-coated slides and treated for 3 h with a differentiation cocktail containing 200 nM of the protein kinase C activator 4β-12-O-tetradecanoylphorbol 13-acetate (TPA) + 250 μM of the phosphodiesterase inhibitor IBMX + 50 βM of the adenylate cyclase activator Forskolin in serum-free media followed by 7 days with dibutyryl cAMP (dbcAMP, 1 mM). After which, cells were fixed in 4% paraformaldehyde and evaluated by immunocytochemistry.
RT-PCR
Cultured hMSCs were harvested for RNA according to the Cells to cDNA™ II Kit Manual (Ambion) and reverse transcriptase (RT)-PCR (Promega). Briefly, total RNA was extracted from hMSC spheres and incubated with 100 μlof lysis buffer at 75 °C for 10 min. DNAse was added into each sample at a final concentration of 0.06 U/ml to digest genomic DNA at 37 °C for 60 min. The RT reaction was prepared by initially incubating 5 μl cell lysate, 4 μl dNTP mixture, 2 μl Oligo (dT), and 5 μl nuclease-free water. This mixture was incubated at 70 °C for 3 min. At the end of the incubation, the following reagents were added to the mixture: 2 μl 10× RT buffer, 1 μlMMLV RT, and 1 μl RNAse inhibitor. At this point, RT was conducted at 42 °C for 1 h. cDNA template (1 μl) was used in a 50 μl reaction volume with the AccessQuick RT-PCR System (Promega). The number of cycles varied from 25 to 40 cycles depending on the particular RNA abundance. The cycling parameters were: 94 °C, 1 min; 55 °C or 60 °C, 1 min; 72 °C, 1 min. The PCR cycle was preceded by an initial denaturation of 5 min at 94 °C and followed by a final extension of 10 min at 72 °C. Primers were as follows: GAPDH: 5′-TGACATCAAGAAGGTG GTGAAGC-3′ and 5′-CCCTGTTGCTGTAGCCGTATTC-3′; GIRK2: 5′-GGAACTGGAGATTGTGGTCAT-3′ and 5′-CATCACCATTCCTCTCTGTCA-3′;FN: 5′-TGTTATGGAGGAAGCCGAGGTTTT-3′ and 5′-CCCGATGCAGGTACAGTCCCAGAT-3′; NF: 5′-GAGCGCAAAGACTACCTGAAGA-3′ and 5′-CAGCGATTTCTATATCCAGAGCC-3′. The primers for AADC, Nurr-1, Pitx-3, and TH were taken from Zeng et al. (2004); for Nestin, Sox-2, and β-tubulin III from Ginis et al. (2004); for Musashi-1 from Sugiyama-Nakagiri et al. (2006).
Immunocytochemistry
Fixed slides were processed using polyclonal antibodies to Fibronectin (Fn; Polysciences; 1:5,000), Octamer-Binding Transcription Factor 4 (Oct-4; Chemicon; 1:50), nestin (Chemicon; 1:200), SRY-Related HMG-Box Gene 2 (Sox-2; Chemicon; 1:200), β-tubulin III (β-tub III; Sigma; 1:200), Neurofilament (NF; Sigma; 1:250), Paired-like Home-domain Transcription Factor 3 (Pitx-3; Chemicon; 1:100), and TH (Pel-freez; 1:100), human nuclear protein (Hunu; Chemicon; 1:100), SSEA-4 (Chemicon; 1:100), GABA (Chemicon: 1:200), and DA (Abcam; 1:100). Secondary antibodies (donkey anti-rb-FITC; donkey anti-mouse-FITC) were purchased from Jackson Immunoresearch. All antibodies were used at a 1:100 dilution and staining analyzed on a Nikon-Scanalytics Image System and a Zeiss LSM 510 META Confocal Image System. To quantify the number of DA neurons in cultures, stained cells (TH, β-tubulin III) were counted in 3–4 representative fields, at a magnification of 20×, in 3 wells in triplicate experiments. Data were expressed as a percentage of TH/β-tubulin III cells ± SD.
6-OHDA lesions
All procedures involving live animals were carried out in accordance with IACUC guidelines. As described previously (Jin and Iacovitti, 1995), twelve Fischer 344 rats (Taconic) were used for these studies, 4 animals per time point. Briefly, rats were anesthetized with sodium pentobarbital (30 mg/kg, intraperitoneal), placed in a stereo-taxic apparatus (Kopf Instruments) and a 26-gauge Hamilton syringe containing 6-hydroxydopamine (6-OHDA) (Sigma; 20 μg/ml in 4 μl PBS containing 0.2 mg/ml ascorbate) was lowered into the right median forebrain bundle (AP: −4.4 mm, ML: −1.2 mm, DV: −7.8 mm from bregma). The 6-OHDA solution was gradually injected at a rate of 1 μl/min. All lesions were verified 3 and 6 weeks later by assessment of rotational behavior in an automated rotometer system (Columbus Instruments) following amphetamine challenge (5 mg/kg, i.p.). Only rats with consistent and stable lesions (>10 ipsilateral turns/min on multiple tests) were used for transplantation studies.
Transplantation procedures
Animals with verified lesions were implanted with 10 μl of a suspension of neural progenitor differentiated hMSC spheres. Cells were deposited at two depths along the needle track (AP: +1.2 mm, ML: −2.7 mm, DV: −5.4 mm and −4.9 mm) in the hope of generating a continuous dorsal to ventral strand of transplanted cells in the striatum on the side ipsilateral to the 6-OHDA lesion as described previously (Yang et al., 2004). All transplant recipients received cyclosporin A (10 mg/kg i.p.) daily, beginning 3 days prior to transplantation.
Acknowledgments
We thank Dr. Keith Danielson (Department of Orthopaedic Surgery, Thomas Jefferson University) for generously providing the stromal cell line hMPC 32F for these studies. This work was supported by NIH NS32519; NIH NS 43309.
REFERENCES
- Azizi SA, Stokes D, Augellia BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—Similarities to astrocyte grafts. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Danielson K, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol. Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem. Biophys. Res. Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- Du X, Iacovitti L. Synergy between growth factors and neurotransmitters required for catecholamine differentiation in brain neurons. J. Neurosci. 1995;15:5420–5427. doi: 10.1523/JNEUROSCI.15-07-05420.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Iacovitti L. Multiple signaling pathways direct the initiation of tyrosine hydroxylase gene expression in cultured brain neurons. Mol. Brain Res. 1997a;50:1–8. doi: 10.1016/s0169-328x(97)00149-6. [DOI] [PubMed] [Google Scholar]
- Du X, Iacovitti L. Protein kinase C activators work in synergy with specific growth factors to initiate tyrosine hydroxylase gene expression in striatal neurons in culture. J. Neurochem. 1997b;68:564–569. doi: 10.1046/j.1471-4159.1997.68020564.x. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvert A, Krieger-Poulet M, Bori-Vol-tatorni C. Tyrosine hydroxylase immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci. Lett. 1987;80:257–262. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura I, Thies S, Brandenberge R, Gerechi-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev. Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Iacovitti L. Effects of a novel differentiation factor on the development of catecholamine traits in noncatecholamine neurons from various regions of the rat brain: studies in tissue culture. J. Neurosci. 1991;11:2403–2409. doi: 10.1523/JNEUROSCI.11-08-02403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger CB, Joh TH. Transient expression of tyrosine hydroxylase in some neurons of the developing inferior colliculus of the rat. Dev. Brain Res. 1983;11:128–132. doi: 10.1016/0165-3806(83)90208-0. [DOI] [PubMed] [Google Scholar]
- Jin BK, Iacovitti L. Dopamine differentiation factors produce partial motor recovery in 6-hydroxydopamine lesioned rats. Neurobiol. Dis. 1995;2:1–12. doi: 10.1006/nbdi.1995.0001. [DOI] [PubMed] [Google Scholar]
- Kohyama J, Abe H, Shimazaki T, Koizuma A, Nakashima K, Gojo S, Taga T, Okano H, Hata J, Umezawa A. Brain from bone: efficient “meta-differentiation” of marrow stromal-derived mature osteoblasts to neurons with noggin or a demethylating agent. Differentiation. 2002;68:235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu J-Y, Ottersen OP, Storm-Mathisen J, Hama K. Catecholamine neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactives in various brain regions of the rat. Exp. Brain Res. 1987;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Max SR, Bossio A, Iacovitti L. Co-expression of tyrosine hydroxylase and glutamic acid decarboxylase in dopamine differentiation factor-treated striatal neurons in culture. Dev. Brain Res. 1996;91:140–142. doi: 10.1016/0165-3806(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABA-ergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Pt I. Vol. 4. Elsevier; Amsterdam: 1985. GABA and Neuropeptides in the CNS. [Google Scholar]
- Munoz-Elias G, Woodbury D, Black I. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21:437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- Osyczka AM, Noth U, O'Connor J, Caterson EJ, Yoon K, Danielson KG, Tuan RS. Multilineage differentiation of adult human bone marrow progenitor cells transduced with human papilloma virus type 16 E6/E7 genes. Calcif. Tissue Int. 2002;71:447–458. doi: 10.1007/s00223-001-1090-2. [DOI] [PubMed] [Google Scholar]
- Rieske P, Krynska B, Azizi SA. Human fibroblast-derived cell lines have characteristics of embryonic stem cells and cells of neuro-ectodermal origin. Differentiation. 2005;73:474–483. doi: 10.1111/j.1432-0436.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Satoh J, Suzuki K. Tyrosine hydroxylase-immunoreactive neurons in the mouse cerebral cortex during the postnatal period. Dev. Brain Res. 1990;53:1–5. doi: 10.1016/0165-3806(90)90119-j. [DOI] [PubMed] [Google Scholar]
- Stull ND, Iacovitti L. Sonic hedgehog and FGF8: inadequate signals for the differentiation of dopamine phenotype in mouse and human neurons culture. Exp. Neurol. 2001;169:36–43. doi: 10.1006/exnr.2001.7640. [DOI] [PubMed] [Google Scholar]
- Sugiyama-Nakagiri Y, Akiyama M, Shibata S, Okano H, Shimizu H. Expression of RNA-binding protein Musashi in hair follicle development and hair cycle progression. Am. J. Pathol. 2006;168:80–92. doi: 10.2353/ajpath.2006.050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suon S, Jin H, Donaldson AE, Caterson EJ, Tuan RS, Deschennes G, Marshall C, Iacovitti L. Transient differentiation of adult human bone marrow cells into neuron-like cells in culture: development of morphological and biochemical traits is mediated by different molecular mechanisms. Stem Cells Dev. 2004;13:625–635. doi: 10.1089/scd.2004.13.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop EJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yang M, Donaldson AE, Marshall CE, Shen J, Iacovitti L. Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant. 2004;13:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Cai J, Chen J, Luo Y, You Z-B, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen C-J, Rao MS, Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]