Abstract
Summary:
Individuals with spinal cord injury (SCI) often experience bone loss and muscle atrophy. Muscle atrophy can result in reduced metabolic rate and increase the risk of metabolic disorders. Sublesional osteoporosis predisposes individuals with SCI to an increased risk of low-trauma fracture. Fractures in people with SCI have been reported during transfers from bed to chair, and while being turned in bed. The bone loss and muscle atrophy that occur after SCI are substantial and may be influenced by factors such as completeness of injury or time postinjury. A number of interventions, including standing, electrically stimulated cycling or resistance training, and walking exercises have been explored with the aim of reducing bone loss and/or increasing bone mass and muscle mass in individuals with SCI. Exercise with electrical stimulation appears to increase muscle mass and/or prevent atrophy, but studies investigating its effect on bone are conflicting. Several methodological limitations in exercise studies with individuals with SCI to date limit our ability to confirm the utility of exercise for improving skeletal status. The impact of standing or walking exercises on muscle and bone has not been well established. Future research should carefully consider the study design, skeletal measurement sites, and the measurement techniques used in order to facilitate sound conclusions.
Keywords: Spinal cord injuries, Bone loss, Muscle atrophy, Fractures, Osteoporosis, Functional electrical stimulation, Bone mineral density, Immobilization, Exercise, Mechanical loading
INTRODUCTION
Decreases in muscle activity and mechanical loading result in bone loss and muscle atrophy, as has been observed following spaceflight, bed rest, and aging (1–3). Osteoporosis and muscle atrophy are frequently cited complications occurring after a spinal cord injury (SCI) (4–8). The purpose of this review is to summarize the literature regarding changes in muscle and bone that occur following an SCI, as well as to review the interventions that have been studied for preventing or reversing these changes.
SOFT TISSUE CHANGES AFTER SCI
Changes in Muscle
After SCI, there is a rapid and dramatic loss of muscle mass below the level of the lesion (9–11). In individuals who were only 6 weeks post-SCI, average muscle cross-sectional areas (CSAs) were 18% to 46% lower than in control subjects (12). Prospective study of these patients up to 24 weeks post-SCI revealed further declines in average gastrocnemius and soleus muscle CSAs of 24% and 12%, respectively (12). Similarly, from 6 weeks to 24 weeks postinjury the average decreases in quadriceps, hamstrings, and adductor muscle CSAs were 16%, 14%, and 16%, respectively. Another prospective study, which employed dual-energy x-ray absorptiometry (DXA) to measure fat-free mass, documented a 15% loss of lower limb lean mass in the first year after SCI (11). Advancing age and duration of injury have been associated with less percentage lean mass (13). Muscle atrophy may be limited to sublesional areas; in a monozygotic twin study, trunk and leg lean masses were significantly lower in the twins with SCI, whereas arm lean mass was not significantly different when the twin pairs were compared (14). In individuals with SCI, fat-free soft tissue contains approximately 15% less muscle tissue than in control subjects; therefore, using DXA-measured fat-free mass as a surrogate for muscle mass may actually underestimate the muscle atrophy that occurs (15).
Reductions in muscle can result in decreased metabolic rate and increased fat storage if energy intake is not adequately adjusted relative to energy expenditure (16). For example, individuals with complete SCI had reduced energy expenditure compared with controls, and lesion level was correlated with basal metabolic rate and total daily energy expenditure (9,17). Reduced peripheral sympathetic nervous system activity in individuals with SCI may also contribute to reductions in resting metabolic rate. The potential influence of reduced sympathetic nervous system activity on resting metabolic rate was revealed by the observation that after adjusting for fat-free mass, fat mass, and age, resting metabolic rate was still lower in individuals with SCI when compared with control subjects (9).
Changes in Fat Mass
Reports of SCI-related changes in fat mass are inconsistent; some reports indicate that fat mass increases after SCI, and other reports indicate that there is no change in fat mass after SCI. A prospective study in individuals with acute SCI demonstrated trends toward increasing fat mass in the lower limbs after SCI; however, large dispersion of individual changes prevented any general conclusions (11). Conversely, a study of monozygotic twins demonstrated that the twins with SCI had more total body fat and percentage fat per unit increment in body mass index than the non-SCI twins (14). Absolute leg fat was reported to be similar in SCI and non-SCI twins (18). However another study reported that percentage fat in the legs was higher in SCI twins when compared with non-SCI twins (14); the discrepancy may be related to differences in reporting, as muscle atrophy in the legs in SCI twins would result in an apparent increase in percentage fat in the legs, even if fat mass remained constant. Several other reports in the literature confirm that fat mass in individuals with chronic SCI is increased relative to controls (10,13,16,19). Two other studies suggesting that fat mass is not different in individuals with SCI incorporated small sample sizes (9,20).
Several factors may explain the unpredictable nature of fat mass changes following SCI. Several different measurement methodologies have been employed, including DXA, total body electrical conductivity, and dilution of 3H2O and Na2 35SO4. Changes in fat mass may be variable and dependent on the interaction of a variety of patient-specific variables. For example, advancing age has been associated with less lean mass and increased fat mass in individuals with SCI but is mildly associated with these variables in controls (13). The level and completeness of the injury of all subjects can differ across studies. Activity level may also play an important role; sedentary SCI subjects were found to have significantly higher percentage and absolute body fat mass than active SCI subjects (20).
OSTEOPOROSIS IN SCI
Diagnostic Methods
DXA is the clinical “gold standard” for diagnosing osteoporosis. From a research perspective, DXA, peripheral quantitative computed tomography (pQCT), magnetic resonance imaging (MRI), and quantitative ultrasound have been used to characterize skeletal changes after SCI. Quantitative ultrasound may assess indices of bone strength that are independent of bone mineral density (BMD). Peripheral quantitative computed tomography enables researchers to evaluate volumetric BMD (in both cortical and trabecular compartments), as well as bone area and indices of bone geometry at appendicular sites. SCI may have dissimilar effects on the different bone compartments and/or on bone geometry.
Clinically, measurements of BMD are often expressed as T-scores (standard deviation units). The World Health Organization (WHO) defines osteoporosis as having a DXA-measured BMD T-score at the spine, hip, or radius that is 2.5 SD or greater below the mean of a healthy young adult reference population (21). However, the WHO T-score osteoporosis screening criteria using BMD measurements are based on the likelihood of hip fracture in non–spinal cord injured postmenopausal women and may only apply to that population, skeletal site, and measurement technique. Criteria for assessing fracture risk in the SCI population have yet to be defined via prospective studies. Further, confounding variables, such as heterotopic ossification or neuropathic changes, may falsely elevate BMD as measured by DXA, both in the spine (22) and hip (23). Therefore careful interpretation is warranted in the diagnosis of osteoporosis using DXA-measured BMD in the SCI population.
Skeletal Changes
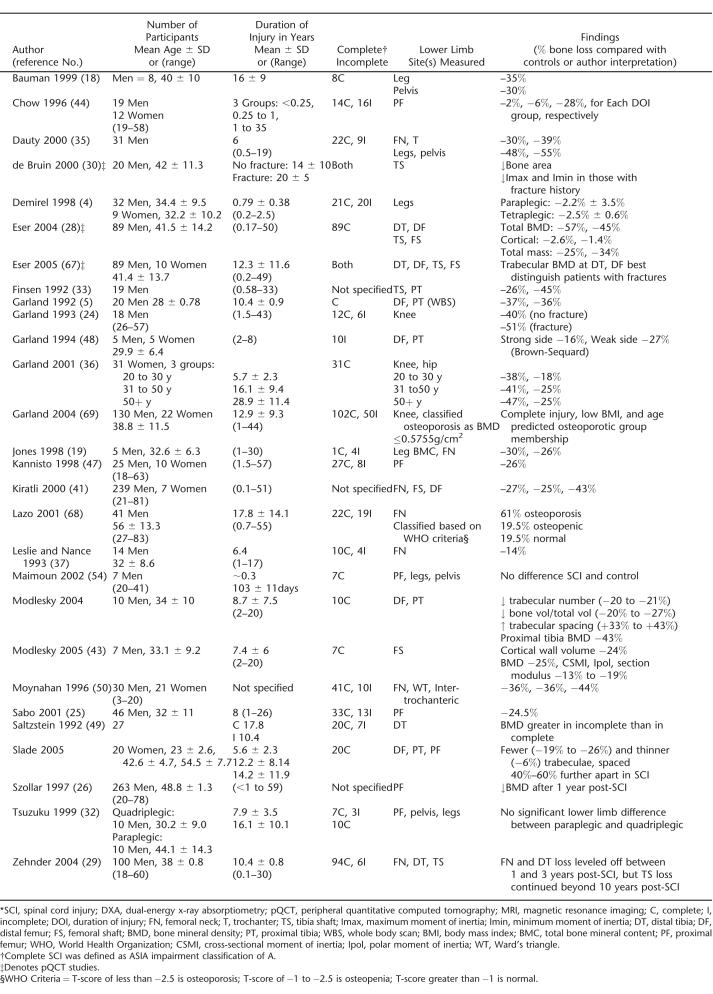
The magnitude of bone lost in the lower limbs following SCI is substantial and has been described in a number of cross-sectional studies using both DXA and pQCT (Table 1). Earlier research suggested that the rate of bone loss after SCI is rapid and linear in the acute stages, establishing a lower steady-state bone mass level 1 to 2 years after the event (5,24). Significant bone loss has been reported many years after SCI in other studies, indicating that bone loss may not plateau as previous studies had suggested (25–28). The time course of bone loss may depend on the bone compartment; at sites with a high proportion of trabecular bone, bone loss followed a log curve leveling off from 1 to 3 years postinjury, whereas at the tibial diaphysis, a cortical bone site, bone mass appeared to decrease progressively beyond 10 years postinjury (29).
Table 1.
Cross-Sectional Studies of Lower Limb Bone Loss After SCI Measured by DXA, Pqct, and MRI *
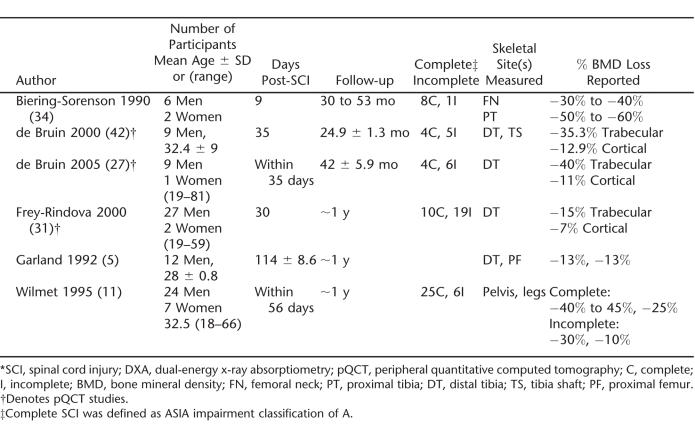
Significant loss of lower limb bone after SCI has been confirmed in a handful of prospective longitudinal studies (Table 2). Consistent with observations in cross-sectional studies, initial bone mass losses are greater in trabecular than in cortical compartments (11,30,31). In addition, there is considerable interindividual variability in the amount of bone loss that occurs after SCI. For example, tibial trabecular bone losses within 2 years of SCI ranged from 0.4% to 80%, and cortical bone changes ranged from a 1.7% increase to a 32.7% decrease (30).
Table 2.
Prospective Studies of Bone Loss After SCI Measured by DXA or pQCT *
Bone lost after SCI is site-specific, with the largest decrements visible in the lower limbs. Upper extremity loss is often only noted in tetraplegia; significant differences have been noted in upper extremity bone status when comparisons were made between paraplegia and tetraplegia (4,31–33). A prospective study demonstrated trabecular and cortical bone losses of 19% and 3% to 4%, respectively, at the radius 12 months after SCI in patients with tetraplegia (31). Lumbar spine BMD has been documented to be increased, decreased, or unchanged after SCI (18,25,34–38). However, CT scans of the spine in individuals with SCI revealed that bone loss had occurred, but bone loss was not apparent in DXA scans, perhaps because of confounding factors such as heterotopic ossification or neuropathic changes (22).
After SCI, there are bone structural changes in addition to losses of bone mass. For example, an MRI study demonstrated that men and women with longstanding complete SCI had reduced bone volume and trabecular number, and increased trabecular spacing at the distal femur and proximal tibia compared with controls (39,40). Alterations in bone area and bone geometry after SCI have also been reported (41,42). Another MRI study revealed endosteal erosion at mid-femur resulting in reduced cortical thickness, polar and cross-sectional moments of inertia, and section modulus in the SCI group compared with controls (43). An interaction between reduced mechanical loading and estrogen loss has been demonstrated; trabecular spacing in postmenopausal women with SCI was 34% less than in premenopausal women with SCI (P < 0.059) (40).
Broadband ultrasound attenuation (BUA) and speed of sound (SOS), measured in the lower limbs with quantitative ultrasound, have been demonstrated to be lower in individuals with SCI than in reference populations, and decreased with time post-SCI (29,44,45). However, one study demonstrated that SOS measurements at mid-tibia were not different in individuals with SCI compared with a reference population (46). The time course of change in BUA and SOS varied at each site; changes in ultrasound variables at the calcaneus leveled off 6 to 12 months postinjury, whereas tibia SOS decreased linearly with time postinjury, perhaps reflecting the type of bone represented at each site (ie, trabecular vs cortical) (29).
Several factors may influence the loss of bone after SCI. The degree of bone loss has been demonstrated to be associated with the degree of posttraumatic immobilization and the time postinjury (35,47). Individuals with incomplete SCI tend to lose less bone than individuals with complete SCI (4,25,36,48). The degree of mobility may be important: a cross-sectional study demonstrated that BMDs in individuals with SCI were positively correlated with mobility assessed via a mobility index ranging from complete paralysis to unlimited ambulation (49). Although increased spasticity may help to preserve muscle mass in individuals with SCI, it may not preserve bone (11). However, a study of pediatric individuals with SCI demonstrated that BMD was higher at the femoral neck and Ward's triangle among individuals with spasticity (50). A significant correlation between cortical bone volume and muscle volume was demonstrated in individuals with SCI and controls, indicating that muscle activity may play a role in maintaining bone mass (43). However, the cortical bone volume:muscle ratio was higher in SCI than in controls, indicating that the muscle loss after SCI was greater than the loss of cortical bone.
Bone Biochemical Changes
Histomorphometric data indicate that in the first 16 weeks of immobilization, trabecular osteoclastic resorption surfaces increase, returning to normal at approximately 40 weeks. Osteoblastic apposition rate and the thickness of the iliac cortices decrease over 40 weeks of immobilization (51). After SCI, bone formation markers remain at normal or slightly higher than normal levels. Osteocalcin levels increase to a peak several months after SCI but often remain within normal ranges (52,53). Serum procollagen I carboxyterminal propeptide levels within normal ranges have been reported up to 3 months after SCI (53). Bone alkaline phosphatase measured approximately 3 months postinjury was not significantly different from controls (54). However, high levels of alkaline phosphatase have been reported during the first year postinjury in individuals with SCI, which may reflect high levels of overall bone turnover (55).
Markers of bone resorption include urinary free and total pyridinoline (Pyr) and deoxypyridinoline (DPD) cross-links, type 1 collagen C-telopeptide (CTX), and N-telopeptide (NTX). After SCI, notable increases in bone resorption markers have been reported to occur as early as 2 weeks, reaching peak values 2 to 4 months after injury onset (53,54,56,57). Values did not return to baseline levels at 6 months postinjury (56). A cross-sectional study reported elevated levels of DPD in 30% of paraplegic individuals injured for greater than 10 years (29). These studies suggest that bone resorption increases after SCI with only small changes in bone formation, and the elevated resorption may persist beyond the acute stages of injury.
Systemic factors known to regulate bone and calcium homeostasis may become altered after SCI. Hypercalci-uria is often reported after SCI and may be reduced with re-ambulation (58,59). Ionized calcium has been demonstrated to increase into the hypercalcemic range after SCI, remaining there for 6 months together with a parallel increase in urinary calcium excretion (56). Increases in ionized calcium may result in suppression of parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D levels in the first 4 months to the first year after SCI (54,56,57). Predisposing factors for hypercalcemia in acute SCI include age less than 21 years, higher injury level, complete injury, and prolonged immobilization (60). In individuals with long-standing SCI, ionized calcium levels were not different from non-SCI controls (61). Vitamin D deficiency has been reported in individuals with chronic SCI, which may cause secondary hyperparathyroidism and subsequently increase bone resorption in these individuals (62).
Risk for Fractures
Low-energy fractures in individuals with SCI have been reported to occur during events that would not normally cause fracture, such as a transfer from bed to chair, or being turned in bed (63–65). Common fracture sites appear to be those around the knee, such as the distal femur or proximal tibia (64,66). The fracture rate in the SCI population has been reported to be from 1% to 21% of patients (6,63,64,66,67). Fracture prevalence has been reported to increase with time post-SCI, from 1% in the first 12 months to 4.6% in individuals >20 years postinjury (29).
Fractures are more likely to occur in individuals with lower than with upper motor neuron lesions, and they are more likely in individuals with complete injuries than incomplete injuries (66). Duration of injury and BMD have been suggested as predictors of fracture risk, in studies comparing individuals with SCI who do and do not have a history of fracture (29,68). Several studies (Table 1) have demonstrated that DXA-measured and pQCT-measured BMD or bone geometry can distinguish individuals with SCI who have had fractures from those who have not (42,67–69). However, the most appropriate method (DXA, pQCT), measurement site (proximal femur, distal femur, proximal tibia), variable (BMD, bone area, bone geometry), or threshold that should be used to define fracture risk in the SCI population has not been confirmed in a prospective study (70).
Complications related to fracture in the SCI population present an additional source of morbidity. Some complications reported in the literature include altered fracture healing, delayed union, malunion and non-union, pressure sores, infection, and osteomyelitis (6,64,65,71). In addition, diminished pain sensation may delay the seeking of medical advice; delays of 1 day to 4 weeks have been reported (72). Finally, complications and difficulties treating fracture in individuals with SCI may require prolonged immobilization and hospitalization. This necessity may cause further detriment to bone and moreover may result in lost wages, less social interaction, and reduced quality of life.
The risk of fracture for individuals with SCI who partake in activities such as functional electrical stimulation (FES), standing frames, and treadmill walking has not been studied extensively. A case report documented a femoral fracture that resulted from measurement of maximal isometric quadriceps torque using electrical stimulation (73). For individuals with SCI who participate in exercise or activities involving mechanical loading of the lower limbs, an assessment of risk, including history of fracture, BMD, and degree of loading, should be performed by a qualified physician before initiation of the proposed activities, and recommendations made accordingly.
FUNCTIONAL ELECTRICAL STIMULATION IN INDIVIDUALS WITH SCI
Effects on Muscle
Functional electrical stimulation can be used to produce isometric contractions (74–76), to facilitate gait (77,78), or to produce contractions against resistance during cycling or leg extensions in individuals with SCI (74,75,79–90). Despite variability in the intensity, duration, and frequency of the exercise interventions, the positive effects of FES exercise on muscle are fairly well established (74–76,84,89,91). For example, lower extremity muscle volume increased 10% after 6 months of FES cycle ergometry 2 to 3 times per week (89). More frequent bouts of exercise may have a greater effect on muscle; 7 FES cycle ergometry sessions per week in men with complete tetraplegia resulted in significant increases in lower limb muscle areas (+22%), along with significant increases in whole body percentage lean mass and reductions in percentage fat mass (84). FES muscle strengthening before FES cycle ergometry may also be advantageous for increasing muscle. An FES training program that began with quadriceps strengthening and progressed to concurrent arm ergometry and FES cycle ergometry produced significant increases in muscle cross-sectional areas (rectus femoris +31%, sartorius +22%, adductor magnus-hamstrings+39%, and vastus medialis-intermedius+31%) (91). In fact, the muscle-strengthening component may have the greatest impact: significant increases in quadriceps muscle protein synthetic rate were noted in 4 men with paraplegia after 10 weeks of quadriceps muscle strengthening, but the increase in muscle area after transition to a cycle ergometry program was not significantly different from the end of the first regimen (87).
FES-induced isometric contractions can also increase muscle cross-sectional area and maximal force, and improve fatigue resistance in individuals with complete SCI (76,92). However, isometric contractions may not be optimal for preventing or reversing muscle atrophy. FES cycle ergometry, but not isometric contractions with FES, prevented muscle atrophy when performed in the acute phase following SCI (74). In addition to its effects on muscle mass, FES cycle ergometry has been demonstrated to increase muscle fiber area and capillary number in individuals with motor complete SCI (82).
Effects on Bone
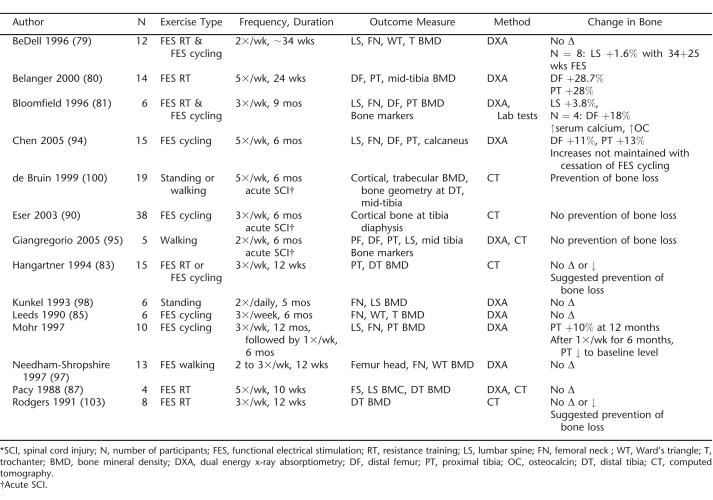
In contrast to its reported positive influence on muscle, the effects of FES exercise on bone in acute and chronic SCI are inconclusive (Table 3). Several studies have demonstrated no effect of FES strengthening or cycle ergometry on measures of bone health (79,81,85,87,90), whereas others have demonstrated increases in bone mass after FES-induced muscle strengthening (80) and FES cycle ergometry (93,94). These latter studies were longer in duration than many of the other studies, and 2 employed a higher exercise frequency (5 times per week). In addition, both of these studies measured BMD at one or both of the fracture-prone sites in individuals with SCI (80,93,94).
Table 3.
Summary of Exercise Interventions Aimed at Improving Skeletal Status in SCI *
It may be that a minimum effective strain on bone is required to stimulate increases in BMD in the lower limbs after an SCI. Nine months of thrice weekly FES cycle ergometry failed to increase BMD at the femoral neck, distal femur, and proximal tibia in individuals with complete SCI. However, among those who could achieve a power output of greater than 18 W during cycle ergometry (n = 4), the average change in distal femur BMD was a statistically significant 17.8% increase (81).
STANDING OR WALKING AFTER SCI
Effects on Muscle and Bone
There are few published studies that report the effect of standing or walking interventions on muscle. A recent case series reported increases in lean mass and muscle area in individuals with acute SCI as a result of early weight bearing via body weight–supported treadmill training (95). Acute SCI was defined as SCI injury less than 1 year before baseline measurement. Increases in muscle fiber size and a shift of the fiber types toward a less fatigable fiber type profile after approximately 6 months of thrice-weekly body weight–supported treadmill training have been reported (96).
Studies of skeletal changes associated with weight-bearing activities after SCI are also limited. After 12 to 20 weeks of training with an ambulation device that combined FES and a modified walker, no significant increase in BMD was observed (97). Individuals with chronic SCI who participated in regular standing (with a standing frame) did not experience changes in BMD, but the average duration of the intervention was only 135 days (98). A cross-sectional study demonstrated that individuals with complete SCI who had performed standing during the acute phase postinjury, either with long leg braces, a standing frame, or a standing wheelchair, had better preserved BMD at the femoral shaft and/or proximal femur than those who had not (99). The data from this study are limited by a cross-sectional study design and participant self-selection to loading or nonloading groups.
Early weight-bearing after acute SCI by standing or treadmill walking (5 times weekly for 25 weeks) resulted in no loss or only moderate loss in trabecular bone compared with immobilized subjects, who lost 7% to 9% of trabecular bone at the tibia (100). However, the control group included only 4 individuals excluded from the intervention based on lower motor neuron involvement, immobilization for medical complications, or motivational issues, criteria that might make them more likely to experience bone loss than the weight-bearing group. Individuals participating in treadmill walking had motor incomplete (ASIA C or D) lesions (n = 4); individuals participating in standing had motor complete (ASIA A or B) lesions (n =5); and among individuals in the control group, 3 had motor complete lesions and 1 was classified as ASIA C (100). Trabecular bone density in individuals performing treadmill walking was not significantly different than a group that participated in passive standing (100). The results of this study suggest that early mobilization may reduce bone loss in the acute stages after SCI.
Preventing Bone Loss With Exercise In SCI: Things to Consider
Of the small number of studies demonstrating that mechanical loading might be beneficial for the skeleton after SCI, a common component was a longer study duration (ie, 6 months or greater) and/or an increased exercise frequency (ie, 5 times per week), which may not be practical in the clinical setting (80,86,100). The frequency, intensity, and/or duration of exercise in other published studies may not have been sufficient to have an effect on the skeleton. In accordance with recent research, shorter, more frequent exercise bouts may be the best strategy for increasing bone mineral (101). It is possible that once bone is lost in adults, it may be difficult to recover, particularly if substantial micro-architectural deterioration and/or permanent reductions in the mechanosensory abilities of bone cells have occurred. For example, only 24 hours of disuse was required for osteocytes to become hypoxic (102), suggesting that the ability of bone cells to detect loading may be impaired, even in acute SCI. As well, substantial micro-architectural deterioration has been reported after SCI (39). No exercise intervention has demonstrated restoration of bone micro-architecture after it has been lost. Currently, there are no interventions that have consistently demonstrated efficacy for preventing or reversing the dramatic bone loss occurring after SCI population that can easily be implemented in the clinical setting.
Since the distal femur and proximal tibia are the most common sites of fracture in SCI, an effort should be made to assess the impact of intervention at these sites, particularly because they have been demonstrated to respond to intervention (80,93). Finally, randomized, controlled designs are difficult in the SCI population because of the small number of subjects recruited and the high potential for drop out among subjects randomized to the control group. As well, unless a large number of participants are recruited, it is difficult to establish adequate matching between control and intervention groups because of interindividual variability in characteristics such as age, sex, level and completeness of lesion, and time postinjury. Limitations in study design restrict the conclusions that can be made regarding the effect of exercise on the skeleton and should be acknowledged.
SUMMARY
Individuals with SCI not only lose motor and/or sensory function, they experience dramatic muscle and bone changes. Functional electrical stimulation is a method of exercise that has been employed in the SCI population that has demonstrated some success in improving muscle, with less conclusive evidence that it has a positive effect on bone. Body weight–supported treadmill training has been explored in SCI with the intention of improving ambulation, but the potential benefits of this technique or of passive standing on the muscle and bone should be explored further. Future research should carefully consider the study design, the measurement sites, and the measurement techniques used in order to facilitate sound conclusions.
Footnotes
Lora Giangregorio is the recipient of a Health Research Partnership Fellowship Award from the Ontario March of Dimes and the Canadian Institutes for Health Research.
REFERENCES
- Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135(suppl 7):1824S–1828S. doi: 10.1093/jn/135.7.1824S. [DOI] [PubMed] [Google Scholar]
- Giangregorio L, Blimkie CJ. Skeletal adaptations to alterations in weight-bearing activity: a comparison of models of disuse osteoporosis. Sports Med. 2002;32(suppl 7):459–476. doi: 10.2165/00007256-200232070-00005. [DOI] [PubMed] [Google Scholar]
- Vandenborne K, Elliott MA, Walter GA et al. Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve. 1998;21(suppl 8):1006–1012. doi: 10.1002/(sici)1097-4598(199808)21:8<1006::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998;36(suppl 12):822–825. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- Garland DE, Stewart CA, Adkins RH et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- Nottage WM. A review of long-bone fractures in patients with spinal cord injuries. Clin Orthop. 1981;155:65–70. [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86(suppl 1):350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002;32(suppl 2):65–74. doi: 10.2519/jospt.2002.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68(suppl 6):1223–1227. doi: 10.1093/ajcn/68.6.1223. [DOI] [PubMed] [Google Scholar]
- Nuhlicek DN, Spurr GB, Barboriak JJ, Rooney CB, el Ghatit AZ, Bongard RD. Body composition of patients with spinal cord injury. Eur J Clin Nutr. 1988;42(suppl 9):765–773. [PubMed] [Google Scholar]
- Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33(suppl 11):674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(suppl 4):373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003. pp. 2398–2407. [DOI] [PubMed]
- Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol. 2000;88(suppl 4):1310–1315. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol. 2004. [DOI] [PubMed]
- Sedlock DA, Laventure SJ. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia. 1990;28(suppl 7):448–454. doi: 10.1038/sc.1990.60. [DOI] [PubMed] [Google Scholar]
- Mollinger LA, Spurr GB, el Ghatit AZ et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66(suppl 7):420–426. [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int. 1999;10(suppl 2):123–127. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- Jones LM, Goulding A, Gerrard DF. DEXA: a practical and accurate tool to demonstrate total and regional bone loss, lean tissue loss and fat mass gain in paraplegia. Spinal Cord. 1998;36(suppl 9):637–640. doi: 10.1038/sj.sc.3100664. [DOI] [PubMed] [Google Scholar]
- Olle MM, Pivarnik JM, Klish WJ, Morrow JR., Jr Body composition of sedentary and physically active spinal cord injured individuals estimated from total body electrical conductivity. Arch Phys Med Rehabil. 1993;74(suppl 7):706–710. doi: 10.1016/0003-9993(93)90030-e. [DOI] [PubMed] [Google Scholar]
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(suppl 6):368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- Liu CC, Theodorou DJ, Theodorou SJ et al. Quantitative computed tomography in the evaluation of spinal osteoporosis following spinal cord injury. Osteoporos Int. 2000;11(suppl 10):889–896. doi: 10.1007/s001980070049. [DOI] [PubMed] [Google Scholar]
- Jaovisidha S, Sartoris DJ, Martin EM, Foldes K, Szollar SM, Deftos LJ. Influence of heterotopic ossification of the hip on bone densitometry: a study in spinal cord injured patients. Spinal Cord. 1998;36(suppl 9):647–653. doi: 10.1038/sj.sc.3100701. [DOI] [PubMed] [Google Scholar]
- Garland DE, Maric Z, Adkins RH, Stewart CA. Bone mineral density about the knee in spinal cord injured patients with pathologic fractures. Contemporary Orthop. 1993;26(suppl 4):375–379. [Google Scholar]
- Sabo D, Blaich S, Wenz W, Hohmann M, Loew M, Gerner HJ. Osteoporosis in patients with paralysis after spinal cord injury: a cross-sectional study in 46 male patients with dual-energy X-ray absorptiometry. Arch Orthop Trauma Surg. 2001;121(suppl 1–2):75–78. doi: 10.1007/s004020000162. [DOI] [PubMed] [Google Scholar]
- Szollar SM, Martin EM, Parthemore JG, Sartoris DJ, Deftos LJ. Densitometric patterns of spinal cord injury associated bone loss. Spinal Cord. 1997;35(suppl 6):374–382. doi: 10.1038/sj.sc.3100394. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43(suppl 2):96–101. doi: 10.1038/sj.sc.3101685. [DOI] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34(suppl 5):869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Zehnder Y, Luthi M, Michel D et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(suppl 3):180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Dietz V, Dambacher MA, Stussi E. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil. 2000;14(suppl 2):145–152. doi: 10.1191/026921500670532165. [DOI] [PubMed] [Google Scholar]
- Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38(suppl 1):26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- Tsuzuku S, Ikegami Y, Yabe K. Bone mineral density differences between paraplegic and quadriplegic patients: a cross-sectional study. Spinal Cord. 1999;37(suppl 5):358–361. doi: 10.1038/sj.sc.3100835. [DOI] [PubMed] [Google Scholar]
- Finsen V, Indredavik B, Fougner KJ. Bone mineral and hormone status in paraplegics. Paraplegia. 1992;30(suppl 5):343–347. doi: 10.1038/sc.1992.80. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Dauty M, Perrouin VB, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord–injured patients. Bone. 2000;27(suppl 2):305–309. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6(suppl 3):37–46. [Google Scholar]
- Leslie WD, Nance PW. Dissociated hip and spine demineralization: a specific finding in spinal cord injury. Arch Phys Med Rehabil. 1993;74:960–964. [PubMed] [Google Scholar]
- Szollar SM, Martin EME, Parthemore JG, Sartoris DJ, Deftos LJ. Demineralization in tetraplegic and paraplegic man over time. Spinal Cord. 1997;35:223–228. doi: 10.1038/sj.sc.3100401. [DOI] [PubMed] [Google Scholar]
- Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19(suppl 1):48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos Int. 2004. [DOI] [PubMed]
- Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev. 2000;37(suppl 2):225–233. [PubMed] [Google Scholar]
- de Bruin ED, Herzog R, Rozendal RH, Michel D, Stussi E. Estimation of geometric properties of cortical bone in spinal cord injury. Arch Phys Med Rehabil. 2000;81:150–156. [PubMed] [Google Scholar]
- Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005;36(suppl 2):331–339. doi: 10.1016/j.bone.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Chow YW, Inman C, Pollintine P, et al. Ultrasound bone densitometry and dual energy X-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord. 1996;34(suppl 12):736–741. doi: 10.1038/sc.1996.134. [DOI] [PubMed] [Google Scholar]
- Warden SJ, Bennell KL, Matthews B, et al. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporos Int. 2002;13(suppl 7):586–592. doi: 10.1007/s001980200077. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Webber CE. Speed of sound in bone at the tibia: is it related to lower limb bone mineral density in spinal cord–injured individuals? Spinal Cord. 2004;42(suppl 3):141–145. doi: 10.1038/sj.sc.3101570. [DOI] [PubMed] [Google Scholar]
- Kannisto M, Alaranta H, Merikanto J, Kroger H, Karkkainen J. Bone mineral status after pediatric spinal cord injury. Spinal Cord. 1998;36(suppl 9):641–646. doi: 10.1038/sj.sc.3100665. [DOI] [PubMed] [Google Scholar]
- Garland DE, Foulkes GD, Adkins RH, Stewart CA, Yakura JS. Regional osteoporosis following incomplete spinal cord injury. Contemporary Orthop. 1994;28(suppl 2):134–139. [Google Scholar]
- Saltzstein RJ, Hardin S, Hastings J. Osteoporosis in spinal cord injury: using an index of mobility and its relationship to bone density. J Am Paraplegia Soc. 1992;15(suppl 4):232–234. doi: 10.1080/01952307.1992.11761524. [DOI] [PubMed] [Google Scholar]
- Moynahan M, Betz RR, Triolo RJ, Maurer AH. Characterization of the bone mineral density of children with spinal cord injury. J Spinal Cord Med. 1996;19(suppl 4):249–254. doi: 10.1080/10790268.1996.11719441. [DOI] [PubMed] [Google Scholar]
- Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res. 1974;17(suppl 1):57–73. doi: 10.1007/BF02547214. [DOI] [PubMed] [Google Scholar]
- Pietschmann P, Pils P, Woloszczuk W, Lessan D, Stipicic J. Increased serum osteocalcin levels in patients with paraplegia. Paraplegia. 1992;30:204–209. doi: 10.1038/sc.1992.56. [DOI] [PubMed] [Google Scholar]
- Uebelhart D, Hartmann D, Vuagnat H, Castanier M, Hachen H, Chantraine A. Early modifications of biochemical markers of bone metabolism in spinal cord injury patients: a preliminary study. Scand J Rehabil Med. 1994;26:197–202. [PubMed] [Google Scholar]
- Maimoun L, Couret I, Micallef JP, et al. Use of bone biochemical markers with dual-energy x-ray absorptiom-etry for early determination of bone loss in persons with spinal cord injury. Metabolism. 2002;51(suppl 8):958–963. doi: 10.1053/meta.2002.34013. [DOI] [PubMed] [Google Scholar]
- Bergmann P, Heilporn A, Schoutens A, Paternot J, Tricot A. Longitudinal study of calcium and bone metabolism in paraplegic patients. Paraplegia. 1977;15(suppl 2):147–159. doi: 10.1038/sc.1977.20. [DOI] [PubMed] [Google Scholar]
- Roberts D, Lee W, Cuneo RC, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol. 1998;83:415–422. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia. 1995;33(suppl 11):669–673. doi: 10.1038/sc.1995.140. [DOI] [PubMed] [Google Scholar]
- Kaplan PE, Roden W, Gilbert E, Richards L, Goldschmidt JW. Reduction of hypercalciuria in tetraplegia after weight-bearing and strengthening exercises. Paraplegia. 1981;19(suppl 5):289–293. doi: 10.1038/sc.1981.55. [DOI] [PubMed] [Google Scholar]
- Kaplan PE, Gandhavadi B, Richards L, Goldschmidt J. Calcium balance in paraplegic patients: influence of injury duration and ambulation. Arch Phys Med Rehabil. 1978;59(suppl 10):447–450. [PubMed] [Google Scholar]
- Maynard FM. Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil. 1986;67(suppl 1):41–44. [PubMed] [Google Scholar]
- Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Vitamin D, parathormone, and calcitonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil. 1994;75(suppl 7):766–769. [PubMed] [Google Scholar]
- Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44(suppl 12):1612–1616. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36(suppl 11):790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62(suppl 9):418–423. [PubMed] [Google Scholar]
- Freehafer AA, Hazel CM, Becker CL. Lower extremity fractures in patients with spinal cord injury. Paraplegia. 1981;19(suppl 6):367–372. doi: 10.1038/sc.1981.69. [DOI] [PubMed] [Google Scholar]
- Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. Am J Surg. 1962;103:732–739. doi: 10.1016/0002-9610(62)90256-8. [DOI] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86(suppl 3):498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39(suppl 4):208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27(suppl 3):202–206. doi: 10.1080/10790268.2004.11753748. [DOI] [PubMed] [Google Scholar]
- Bauman WA. Risk factors for osteoporosis in persons with spinal cord injury: what we should know and what we should be doing. J Spinal Cord Med. 2004;27(suppl 3):212–213. doi: 10.1080/10790268.2004.11753750. [DOI] [PubMed] [Google Scholar]
- Garland DE. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop. pp. 86–101. [PubMed]
- Ingram RR, Suman RK, Freeman PA. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia. 1989;27:133–139. doi: 10.1038/sc.1989.20. [DOI] [PubMed] [Google Scholar]
- Hartkopp A, Murphy R, Mohr T, Kjoer M, Biering-Sorensen F. Bone fracture during electrical stimulation of the quadriceps in a spinal cord injured subject. Arch Phys Med Rehabil. 1998;79:1133–1136. doi: 10.1016/s0003-9993(98)90184-8. [DOI] [PubMed] [Google Scholar]
- Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36(suppl 7):463–469. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80(suppl 4):394–396. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- Kagaya H, Shimada Y, Sato K, Sato M. Changes in muscle force following therapeutic electrical stimulation in patients with complete paraplegia. Paraplegia. 1996;34(suppl 1):24–29. doi: 10.1038/sc.1996.4. [DOI] [PubMed] [Google Scholar]
- Gallien P, Brissot R, Eyssette M, et al. Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia. 1995;33(suppl 11):660–664. doi: 10.1038/sc.1995.138. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Sato K, Abe E, et al. Clinical experience of functional electrical stimulation in complete paraplegia. Spinal Cord. 1996;34(suppl 10):615–619. doi: 10.1038/sc.1996.110. [DOI] [PubMed] [Google Scholar]
- BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation–induced lower extremity cycling on bone density of spinal cord–injured patients. Am J Phys Med Rehabil. 1996;75(suppl 1):29–34. doi: 10.1097/00002060-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81(suppl 8):1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19(suppl 1):61–68. doi: 10.1016/8756-3282(96)00109-3. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37(suppl 4):264–268. doi: 10.1038/sj.sc.3100785. [DOI] [PubMed] [Google Scholar]
- Hangartner TN, Rodgers MM, Glaser RM, Barre PS. Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev. 1994;31(suppl 1):50–61. [PubMed] [Google Scholar]
- Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273(suppl 3, pt 2):R1072–R1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- Leeds EM, Klose KJ, Ganz W, Serafini A, Green BA. Bone mineral density after bicycle ergometry training. Arch Phys Med Rehabil. 1990;71(suppl 3):207–209. [PubMed] [Google Scholar]
- Mohr T, Andersen JL, Biering-Sorensen F, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(suppl 1):1–16. doi: 10.1038/sj.sc.3100343. [DOI] [PubMed] [Google Scholar]
- Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci (Lond) 1988;75(suppl 5):481–487. doi: 10.1042/cs0750481. [DOI] [PubMed] [Google Scholar]
- Ragnarsson KT. Physiologic effects of functional electrical stimulation-induced exercises in spinal cord–injured individuals. Clin Orthop. 1988. pp. 53–63. [PubMed]
- Skold C, Lonn L, Harms-Ringdahl K, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord–injured individuals. J Rehabil Med. 2002;34(suppl 1):25–32. doi: 10.1080/165019702317242677. [DOI] [PubMed] [Google Scholar]
- Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E. Effect of electrical stimulation–induced cycling on bone mineral density in spinal cord–injured patients. Eur J Clin Invest. 2003;33(suppl 5):412–419. doi: 10.1046/j.1365-2362.2003.01156.x. [DOI] [PubMed] [Google Scholar]
- Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80(suppl 12):1531–1536. doi: 10.1016/s0003-9993(99)90326-x. [DOI] [PubMed] [Google Scholar]
- Gerrits HL, Hopman MT, Sargeant AJ, Jones DA, De Haan A. Effects of training on contractile properties of paralyzed quadriceps muscle. Muscle Nerve. 2002;25(suppl 4):559–567. doi: 10.1002/mus.10071. [DOI] [PubMed] [Google Scholar]
- Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61(suppl 1):22–25. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJ. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil. 2005;27(suppl 22):1337–1341. doi: 10.1080/09638280500164032. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Hicks AL, Webber CE, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43(suppl 11):649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- Stewart BG, Tarnopolsky MA, Hicks AL, et al. Treadmill training–induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2004;30(suppl 1):61–68. doi: 10.1002/mus.20048. [DOI] [PubMed] [Google Scholar]
- Needham-Shropshire BM, Broton JG, Klose J, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 Ambulation System: Part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil. 1997;78:799–803. doi: 10.1016/s0003-9993(97)90190-8. [DOI] [PubMed] [Google Scholar]
- Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil. 1993;74(suppl 1):73–78. [PubMed] [Google Scholar]
- Goemaere S, Van Laere M, De Neve P, Kaufman JM. Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporos Int. 1994;4(suppl 3):138–143. doi: 10.1007/BF01623058. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil. 1999;80(suppl 2):214–220. doi: 10.1016/s0003-9993(99)90124-7. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exerc. 2002;34(suppl 2):196–202. doi: 10.1097/00005768-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Dodd JS, Raleigh JA, Gross TS. Osteocyte hypoxia: a novel mechanotransduction pathway. Am J Physiol. 1999;277(suppl 3, pt 1):C598–C602. doi: 10.1152/ajpcell.1999.277.3.C598. [DOI] [PubMed] [Google Scholar]
- Rodgers MM, Glaser RM, Figoni SF, Hooker SP, Ezenwa BN, Collins SR, Mathews T, Suryaprasad AG, Gupta SC. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation—induced knee extension exercise training. J Rehabil Res Dev. 1991;28(4):19–26. doi: 10.1682/jrrd.1991.10.0019. [DOI] [PubMed] [Google Scholar]