Abstract
Background/Objective:
Identify factors related to long-term survival, and quantify their effect on mortality and life expectancy.
Setting:
Model spinal cord injury systems of care across the United States.
Study Design:
Survival analysis of persons with traumatic spinal cord injury who are ventilator dependent at discharge from inpatient rehabilitation and who survive at least 1 year after injury.
Methods:
Logistic regression analysis on a data set of 1,986 person-years occurring among 319 individuals injured from 1973 through 2003.
Results:
The key factors related to long-term survival were age, time since injury, neurologic level, and degree of completeness of injury. The life expectancies were modestly lower than previous estimates. Pneumonia and other respiratory conditions remain the leading cause of death but account for only 31% of deaths of known causes.
Conclusions:
Whereas previous research has suggested a dramatic improvement in survival over the last few decades in this population, this is only the case during the critical first few years after injury. There was no evidence for such a trend in the subsequent period.
Keywords: Spinal cord injuries, Model systems, Tetraplegia, Ventilator dependence, Mortality, Life expectancies, National Spinal Cord Injury Statistical Center
INTRODUCTION
Several studies have reported on the long-term survival of persons with ventilator-dependent spinal cord injury (SCI) (1–5). In the most comprehensive of these, DeVivo and Ivie (5) calculated substantially reduced life expectancies for these individuals compared with persons in the general population of comparable age, sex, and race. They identified age at time of injury, time since injury, and high-level neurologically complete injury as additional prognostic factors; found a substantial trend toward improving survival rates occurring from 1973 through 1992; and documented the leading causes of death to be pneumonia and heart disease.
Two more recent studies evaluated ventilator dependency as a negative prognostic factor for survival among all persons with SCI (6,7). The authors adjusted for age, sex, race, level of injury, completeness of injury, cause of injury, sponsor of care, hospital where rehabilitation took place, and the trend toward improving survival rates over time. They found that persons with ventilator-dependent SCI were 39.5 times as likely to die during the first year after injury as other persons with SCI, and they had 2.61 times higher mortality rates thereafter (6). When health status, community integration, and economic status were also controlled, the odds of dying in any given year after the first anniversary of injury increased to 3.53 times higher for ventilator-dependent persons with SCI than other persons with SCI (7). Neither study evaluated interactions between ventilator status and other prognostic factors, leaving open the possibility that the effect of factors such as age, sex, race, and neurologic level varies according to ventilator-dependent status.
The 3 most recent of the cited studies relied on data from persons treated in any of the federally funded model SCI systems of care located throughout the United States (5–7). Using a more up-to-date version of the same database with additional patients and longer follow-up, we sought to (a) quantify the effects of additional prognostic factors on survival among ventilator-dependent persons with SCI, (b) update previous estimates of life expectancy, and (c) determine the leading causes of death. The factors considered were age, sex, race, etiology of injury, current calendar year, level of injury, American Spinal Injury Association (ASIA) grade of injury, and time since injury.
MATERIALS AND METHODS
Study Population
Data for this study were collected through the model SCI systems program and submitted to the National Spinal Cord Injury Statistical Center (NSCISC). As many as 25 SCI centers located around the United States have contributed to this database. Detailed descriptions of the history, eligibility criteria, data collection protocol, data quality control procedures, and current status of the NSCISC database have been published previously (8,9). This is a large and comprehensive source of data on SCI. Briefly, to be included in the database, individuals must have (a) had a clinically discernible degree of neurologic (spinal cord) impairment after a traumatic event, (b) been treated at a model system within 1 year of injury, (c) resided in the model system geographic catchment area at the time of injury, and (d) given informed consent.
To be included in this study, individuals also had to be ventilator dependent on discharge from inpatient rehabilitation. Ventilator dependence was defined as use of any type of mechanical ventilation to sustain daily respiration for at least part of the day. Twelve persons with implanted phrenic nerve stimulators at discharge were included in the study, and a few others may have had phrenic nerve stimulators implanted later. Emergency mouth-to-mouth or machine resuscitation, routine administration of oxygen, emergency “bagging,” periodic intermittent positive pressure breathing (IPPB) administration, or operative/postoperative ventilator support used for less than 7 days were not considered to constitute ventilator dependence. This definition of ventilator dependence is consistent with that used in all previous studies involving the NSCISC database. It is not possible with this database to distinguish individuals who are ventilator dependent 24 h/d from those who require it less often.
There were 1,014 persons meeting the above inclusion criteria, 595 of whom died during the 1973–2003 study period. Because the primary focus of this study was on adults, eligibility was further restricted to the 810 persons older than 20 years of age at injury. Long-term survival among children will be the subject of a separate study. Eligibility was further restricted to the 319 persons who survived at least 1 year after injury. This focus on long-term mortality enabled us to avoid consideration of transient short-term effects on survival and their possible interactions and eliminated the effect of the large mortality rates seen in the first 12 months after injury. All persons were at least 21 years of age during the study period. The average time to system admission was 42 days, with 70% arriving within 30 days and 85% within 60 days.
Data Collection
Demographic data were collected using a standardized protocol by trained individuals at each model system and sent to the NSCISC database. The neurologic examination was performed just before model system discharge in accordance with the most current version of the International Standards for Neurological Classification of SCI (10). Neurologic level of injury was defined as the most caudal segment of the spinal cord with normal sensory and motor function on both sides of the body. The ASIA Impairment Scale was used to categorize the extent of injury as either neurologically complete (Grade A), incomplete with sensory, but not motor, function preserved through the S4–S5 segment (Grade B), incomplete with motor function preserved and more than one half of key muscles below the neurologic level having a grade less than 3 of 5 (Grade C), or incomplete with motor function preserved and at least one half of key muscles below the neurologic level having a grade of at least 3 of 5 (Grade D). Before 1992, degree of completeness was determined using the Frankel scale; after 1992, this was determined using the ASIA impairment scale (11,12). Given the comparatively longer length of stay for ventilator-dependent persons, much of the neurologic recovery in this group will have occurred by discharge, so discharge neurologic level and ASIA Impairment Scale should be stable thereafter and will be a better predictor of long-term survival than neurologic status at initial hospital admission (12).
Date of death was reported to the NSCISC database by the model system data collectors whenever, in the course of their routine follow-up data collection activities, they determined an individual to be deceased. In addition, during March and April 2004, the staff of the NSCISC checked on the survival status of persons in the database whose identities were known (about one half the database) by searching the Social Security Death Index (SSDI) and several individual state death indices online at www.ancestry.com. Persons not reported as deceased by the model system and not found in the SSDI or state death indices were assumed to be alive on January 1, 2004. The SSDI alone has previously been found to be 92.4% sensitive and 99.5% specific for persons in the NSCISC database (13).
Causes of death are documented from any of 3 sources, including the death certificate, hospital discharge summary, or autopsy report. Primary (underlying) cause of death was determined using the same methods used by the National Center for Health Statistics for reporting United States vital statistics, except that any reference to either SCI or the original injury-producing event was excluded from consideration as a possible underlying cause of death. Therefore, any external causes of death will represent new unintentional injuries, suicides, or homicides that occur after the SCI. This approach is consistent with prior published reports using the NSCISC database (5,6,14,15).
Statistical Analysis
Using data on the final study population of 319 persons, 2 data sets were created. The first was a traditional survival data set (16) with 319 rows, 1 for each person. Columns correspond to the associated variables, including age, sex, level and completeness of injury, survival time, and an indicator of whether the person died during the study period. There were 121 deaths. Kaplan-Meier survival curves (16) were generated for several subsets of these data. These simple survival analyses were useful for preliminary analysis, but more powerful methods were used on the second database to address all the research questions of interest, as described below.
In the second data set, each person contributed 1 person-year of data for each year of the study that he or she was alive and met the eligibility criteria (17). The resulting data set had 1,986 person-years and (as in the other data set) 121 deaths. This data set was analyzed using logistic regression (18) to determine the factors related to the probability of dying in any given year and to quantify their effect on that probability. This approach, sometimes known as pooled repeated observations, is formally equivalent to a Cox model with time-varying covariates but is considerably more convenient to implement. The method can readily identify and account for a “time since injury” effect if one does indeed exist (we return to this point in Results). For further details of the method and discussion of its versatility, see Strauss et al (17) and the references cited there. On the basis of prior research and biologic plausibility, a number of plausible models were studied. We considered the main effects and 2-way interactions of age, sex, race, etiology, current calendar year, level of injury, ASIA grade of injury, and time since injury. For model selection, we used the deviance statistic (19) for nested models and the Akaike information criterion (19,20) otherwise.
The final model was used to compute the annual mortality probabilities, which were converted to mortality rates. The latter were used to construct life tables (21–23), which yielded the life expectancies by age and neurologic category.
The effect of a secular (time) trend or, equivalently, the effect of calendar year, was also studied. Three nonoverlapping data sets were considered separately: (a) the first 12 months after injury only, (b) the period from 12 to 36 months after injury, and (c) 3.0 years and later after injury. The final logistic regression model from above was used as the starting point for these studies.
To study the issue of a secular trend further, we repeated the Cox regression analyses carried out by DeVivo and Ivie (5), using data from 1973 through 1994 as they did, but using updated death information through January 2004 for some cases that were originally lost to follow-up. For further details on the data and methods, the reader is referred to the original study.
In the Discussion, we compare life expectancies, mortality rates, and excess death rates (EDRs) for persons who are ventilator dependent with the associated values for persons with similar SCIs who are not ventilator dependent. An EDR is simply the difference between 2 mortality rates and represents the extra risk of death caused by a factor. We note that, for a given SCI life expectancy at a specified age, the underlying mortality rates at all ages can be determined. It has been shown that the model of “proportional life expectancy” (24) can reasonably be used in SCI data.
RESULTS
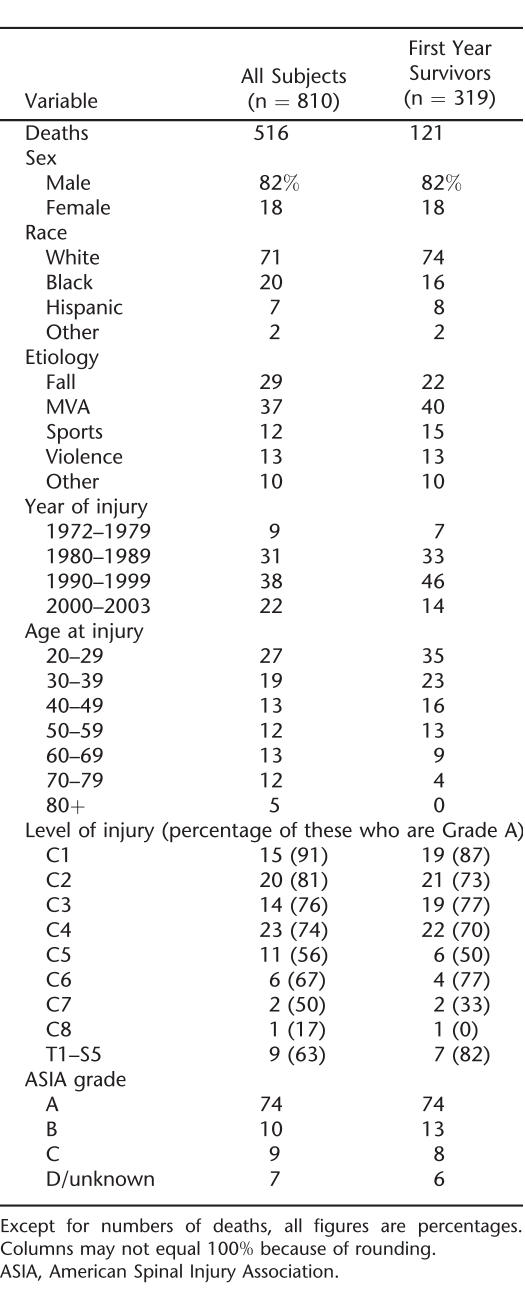
Before creating the 2 study data sets, the question of whether ventilator dependence has become more or less common in the entire NSCISC database over time was examined (details not shown). There was no significant trend, and overall, approximately 2% of persons in the database were ventilator dependent. Table 1 provides demographic and other descriptive statistics for the limited study population of ventilator-dependent patients.
Table 1.
Descriptive Statistics for Persons Injured at 20 Years of Age and Older Who Were Ventilator Dependent at Discharge From Rehabilitation
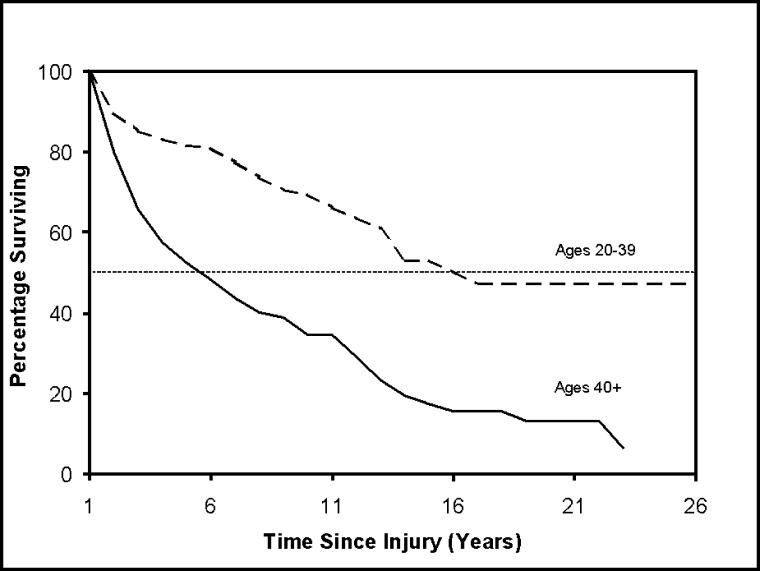
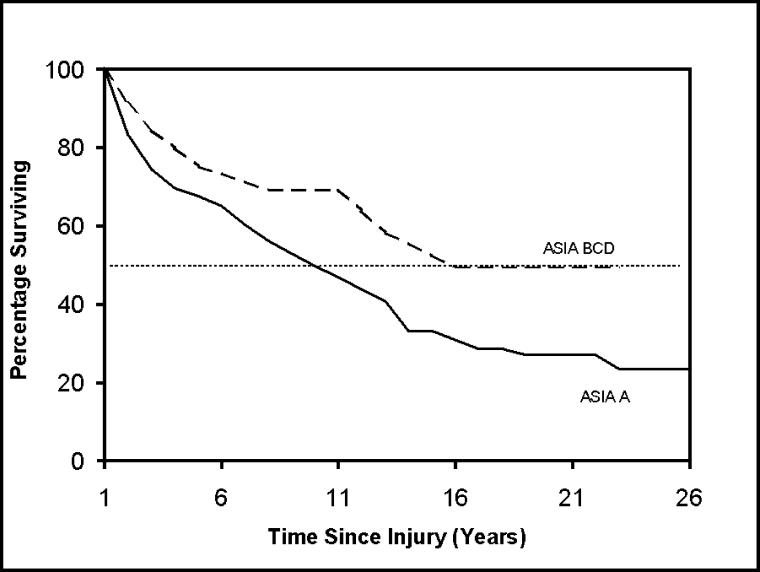
Figure 1 shows Kaplan-Meier survival curves for subjects 20 to 39 and 40+ years of age at the time of injury. As can be seen, older persons fared dramatically worse. Figure 2 shows curves by ASIA grade at the time of discharge from rehabilitation. Even in a population limited to ventilator-dependent persons, those with the most severe injury grade (ASIA A) had poorer survival. These curves can be compared statistically to determine whether they are significantly different (eg, using the log-rank test) (25). However, to consider multiple factors simultaneously, it was necessary to analyze the person-year data set using logistic regression.
Figure 1. Survival curves for ventilator-dependent persons with SCI who have already survived 1 year after injury by age at injury.
Figure 2. Survival curves for ventilator-dependent persons with SCI who have already survived 1 year after injury by ASIA grade.
The logistic regression modeling procedure identified 4 factors strongly related to survival: age, time since injury, and level and grade of injury. Other factors, including sex (odds ratio [OR] for men = 0.96, P = 0.57), race (OR for whites = 0.90, P = 0.59), and etiology of injury (OR for violence = 1.04, P = 0.89), were not practically or statistically significant.
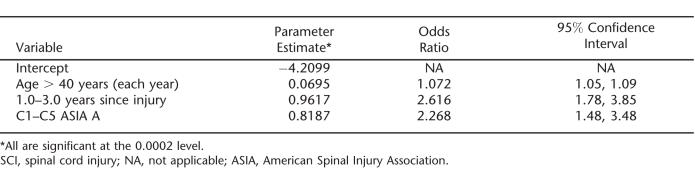
The final model is shown in Table 2. The most efficient coding of the age variable proved to be a roughly constant value from 20 to 40 years of age and a linear increase for those older than 40 years of age. The OR of 1.072 indicates that the risk of death increases by approximately 7% for every year of age over 40 years of age.
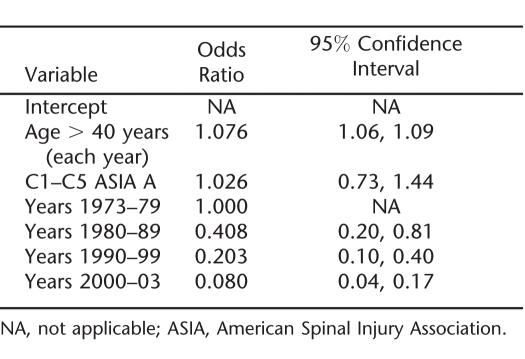
Table 2.
Final Logistic Regression Model of the Annual Probability of Dying for Ventilator-Dependent Persons With SCI Who Have Already Survived 1 Year After Injury
The C1–C5 ASIA A group had the highest mortality rates, with an OR of 2.268, indicating 2.268 times greater odds of dying than among ventilator-dependent persons who were not C1–C5 ASIA A. Other injury levels (C6 and below) and ASIA grades (B, C, D) were not substantially different from one another and were thus combined (they represent the reference group, with an odds ratio of 1.0). In particular, C1–C5 ASIA B injuries had a significantly better prognosis than C1–C5 ASIA A (OR = 0.45, P < 0.05), and C5 was similar to C1–C4. Our impression was that many persons with injuries at levels C6 and lower are eventually weaned from ventilator dependence after discharge, whereas comparatively fewer of the C1–C5 ASIA A persons are subsequently weaned. The time to weaning and factors that affect it are beyond the scope of this study, but we hope to report on that separately.
Time since injury proved to be an important factor for years 2 and 3 after injury (recall that Year 1 was excluded from the data in this analysis), the odds ratio of 2.6 indicating high mortality risk in the first years after injury. After 3 years after injury, there was no discernible relationship between time since injury and mortality, with other factors being equal.
As noted in Methods, we partitioned the data into (a) person-years for the first 12 months after injury, (b) those for the next 24 months, and (c) those for the period 3+ years after injury. For each, we used the logistic regression model of Table 2 and added terms for the calendar periods 1973–79, 1980–89, and so on.
Table 3 shows the results for the first postinjury year only. As can be seen, there is a strong secular trend for the odds on dying decreasing from approximately 1.0 to 0.4 to 0.2 to 0.08 over the 30-year period. This is a dramatic decline; other things being equal, the chance of dying in the first postinjury year was approximately 12 times higher in the 1970s than in the most recent period. After the first 3 years after injury, however, there does not seem to be a secular trend at all, except for an early reduction in the odds of mortality after 1979 (results not shown). We studied this using various models, with linear and indicator variables, and found no downward trend whatsoever since 1980. In fact, there was a small and statistically insignificant upward trend. Finally, the “middle” interval—12 to 36 months after injury—suggested an intermediate pattern, with some suggestion of improvement over the study period, but one that was not clearly or statistically significant (results not shown).
Table 3.
Logistic Regression Analysis for Secular Trend in the Odds of Dying During the First Year After Injury
In our reanalyses of the data of DeVivo and Ivie (5), we again found that the secular improvement in survival seems to be confined largely to the critical first year after injury. The overall result was a less pronounced, and not statistically significant, secular trend. Further analysis confirmed that there was no trend after the third postinjury year.
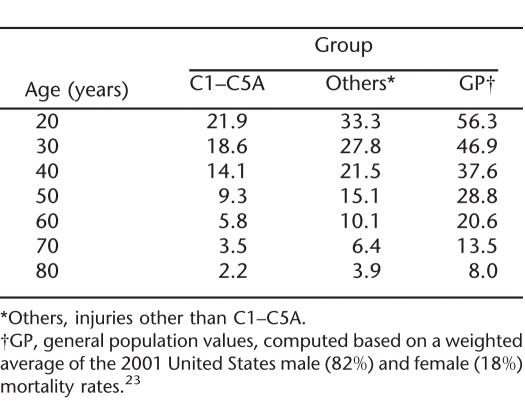
Life expectancies based on the model of Table 2 are shown in Table 4. These apply to persons with SCI who are ventilator dependent and are 3 or more years after injury. Table 4 provides life expectancies for long-term survivors. For persons who have not yet reached the 3-year mark, the life expectancies would be lower. As can be seen, life expectancy among the ventilator-dependent persons decreases both with age and severity of injury. For example, the life expectancy is 18.6 years for a 30 year old who has a C1–C5 ASIA A injury but only 2.2 years for an 80 year old.
Table 4.
Life Expectancies Based on the Model in Table 2, for Time Since Injury Greater Than 3.0 Years
Cause of death was known for 84 of 121 cases (69%). Known causes included 26 (31%) deaths caused by pneumonia and other respiratory diseases, 13 (15%) caused by heart disease, 10 (12%) caused by septicemia secondary to infections of either the urinary tract, pressure sores, or the gastrointestinal tract, 10 (12%) caused by symptoms and ill-defined conditions (typically with ICD-9-CM code 799.1, respiratory arrest), 8 (10%) caused by external events (5 unintentional injuries and 3 of unknown intent), 5 (6%) caused by diseases of the digestive system, 3 (4%) caused by pulmonary emboli, 3 (4%) caused by cerebrovascular disease, 2 (2%) caused by cancer, and 4 caused by other causes. There were insufficient sample sizes to assess any trends over time in causes of death.
DISCUSSION
Secular Trend
DeVivo and Ivie (5) previously studied the issue of a secular trend. They reported a strong trend, with survival of ventilator-dependent persons being much better for persons injured from 1986 through 1992 relative to 1973 through 1979. Using updated data through January 2004 and working with more sensitive methods described here, we found that the improvement seems to be confined largely to the critical first year after injury. This likely reflects great advances in acute care and rehabilitation during the critical first year after injury and undoubtedly led to a greater percentage of medically fragile patients surviving to Year 2 and onward.
That the trend in the reanalysis of DeVivo and Ivie (5) was limited to the early postinjury years is consistent with the findings of this study. It is reasonable to expect that improved acute trauma and rehabilitation medical care will have greatest impact in improving mortality during the period of greatest risk in the critical early period. However, improvements in longer-term rehabilitation for ventilator-dependent persons with SCI do not seem to have resulted in substantially improved long-term survival rates.
The issue of an overall secular trend in non–ventilator-dependent SCI survival has been recently studied (24). The findings were strikingly similar to this research: a strong trend toward improved survival during the first postinjury year and little if any such trend for the third and subsequent years.
Time Since Injury
After controlling for the effects of age and level and extent of injury, we found that mortality rates in the fourth postinjury year and onward were unaffected by time since injury. This is similar to previous findings from the same database for ventilator-dependent persons. In their 1999 study, DeVivo et al (6) found diminishing gains in life expectancy for ventilator-dependent persons as time after injury increased through the fifth postinjury year, with no further gains thereafter. This conceivably reflects a survival of the fittest phenomenon where only those who are very healthy at the time of injury, are strongly motivated, and have good family support survive the first few years of very high mortality rates. However, those who do survive beyond the first few years tend to do relatively well thereafter and report reasonably good quality of life (26).
Other Prognostic Factors
Among the many prognostic factors that were evaluated in this study, age, level and completeness of injury, and time since injury were the key predictors of survival for persons who are ventilator dependent after SCI. Survival rates decrease with age, as indicated by the odds ratio of 1.072 for each year of age over 40 years of age. The comparable figure for those not ventilator dependent is 1.06 (6), although the difference is not statistically significant.
The absence of significant associations between sex or race and the annual likelihood of survival is also consistent with previous findings (5). Life expectancy is typically too short in this population for the usual effects of sex and race that are caused by differential levels of smoking, alcohol consumption, stress, exercise, etc, to have a significant impact. Preinjury differences in these factors between men and women and among the various racial and ethnic groups are often eliminated after such a severe injury occurs. Moreover, there is no evidence or reason to believe that any specific sex, racial, or ethnic group is at differentially high risk for pneumonia, septicemia, pulmonary embolism, or any of the other leading causes of death in this population or that the cause-specific case fatality rates once these complications occur would vary by sex or race. The one exception where differential risk by sex and race does exist is heart disease. However, most deaths categorized as caused by heart disease in this population are nonischemic cardiac deaths in relatively young people and may not be a true reflection of underlying heart disease (27).
Life Expectancy
Table 2 of the 1995 study by DeVivo and Ivie (5) notes a life expectancy of 23.6 years for a patient 30 years of age who is ventilator dependent and has survived at least 2 years after injury. A more recent study by DeVivo et al (6) reported a life expectancy of 28.5 years for a 25 year old who is ventilator dependent and at 5 years after injury. The value at age 30 years would be approximately 3 years lower (25.5 years). These figures were based on all persons who were ventilator dependent at discharge, but some patients, particularly those with lower injury levels, were subsequently weaned off the ventilator. This study concentrated on the individuals with the most severe injuries (C1–C5 ASIA A), in whom the proportion who are subsequently weaned will be lower. Our impression is that persons with C6 or lower injuries will often eventually be weaned. It is thus not surprising that this study gives a lower figure for life expectancy, 18.6 years, at age 30 years (Table 4). Conversely, the life expectancy of 27.8 years reported here for 30 year olds with other level/grade injuries (eg, C8 ASIA A or C3 ASIA B) is higher than the above figures (23.6 and 25.5 years). Undoubtedly, this is because the current figure is level/grade specific, whereas the previous figures are an average over all levels/grades.
Excess Mortality Associated With Ventilator Dependence
The excess mortality for persons with SCI who are ventilator dependent, compared with similarly disabled persons who are not ventilator dependent, is primarily caused by respiratory diseases. Mortality rates in these 2 groups can also be compared. For example, for a person 30 years of age with a C3 ASIA A injury, the life expectancy is 22.3 years (based on an adjustment to the figures in Table 14–3 of DeVivo and Stover) (27). As noted above, for ventilator dependence, it is 18.6 years. The EDR can be shown to be approximately 1% (see explanation in Methods). At age 50 years, the value is 2%. The EDR thus seems to increase with age, as is common in many conditions (22,28). These EDRs do not seem to have been explicitly published previously.
As an independent check of a related but less severe mortality risk factor than ventilator dependence, we undertook a separate study of persons who required a tracheostomy but who were not ventilator dependent. The data were from approximately 1000 persons with developmental disabilities (29–30), but not SCI, who required a tracheostomy compared with otherwise comparable persons who did not require a tracheostomy. The resulting EDR of roughly 1% did not seem to vary by age or severity of injury. That the EDR for tracheostomy was less than that for ventilator dependence makes clinical sense and serves to confirm both results.
We also compared these results with that of another group with catastrophic disability. In follow-up of a previous study of persons in the vegetative state (32) (ie, severe brain injury), we computed an EDR for ventilator dependence of approximately 2%.
Other Considerations
Our personal experience suggests that approximately 25% of persons with SCI and ventilator dependence have traumatic brain injury (TBI), and 25% require gastrostomy feeding. Both factors are known to be negative for survival (32–36). One might therefore ask whether some of the excess death rate for ventilator dependence (1% and 2% as derived above) is caused by these factors. Given that the EDR for tracheostomy dependence alone seems to be approximately 1%, however, any overestimation is likely small.
That ventilator dependence is an independent predictor of diminished survival is not surprising. The association of mechanical ventilation with risk of pneumonia is well known, and this study confirmed pneumonia and other respiratory diseases as the most common cause of death for these patients. Aspiration should also be considered as a possible contributor to the risk for pneumonia in this patient population, given the increased risk of aspiration in patients with mechanical ventilation and tracheostomy (37). While the NSCISC database does not include information on the incidence of dysphagia and the method of alimentation, it should be assumed that individuals with high-level tetraplegia are at risk. The clinician's response to these survival data should be the institution of appropriate preventive measures aimed at improving the overall health of the patient with particular attention to the respiratory system. The safety of oral feeding should be assessed, and an alternative route of alimentation (feeding tube) should be provided when aspiration is documented. Pneumococcal and influenza vaccinations should be administered. Comprehensive periodic evaluations should include focused study of the respiratory system with physical examination, chest radiographs, pulmonary function assessments, and laboratory testing. While there is a lack of evidence-based guidance on the specifics of respiratory management of this patient population, a recent Clinical Practice Guideline on Respiratory Management After Spinal Cord Injury provides consensus expert opinion recommendations for the field that will hopefully lead to improved short- and long-term outcomes (38).
Weaknesses of this study are related to limitations of the NSCISC database. First, the database only captures data from approximately 13% of the persons who have sustained an SCI in the United States (8). Nonetheless, it could be argued that, because there is a relative overrepresentation of higher-level and more complicated cases in the Model System centers that contribute patient data to this database (9), it is likely to be more representative of this ventilator-dependent subpopulation than of the population of persons with SCI as a whole. Additionally, the database does not capture other data that may be of prognostic importance, such as amount of ventilator use, dysphagia, aspiration, smoking history, severity of concomitant injuries such as traumatic brain injury, and chest trauma. The database also has incomplete follow-up on ventilator status of some persons who may have been successfully weaned from the ventilator after discharge from rehabilitation, and the study was restricted to those who were dependent at discharge. To the extent that any persons included in this study were subsequently weaned, the life expectancy of confirmed long-term ventilator-dependent persons may be overestimated by the results reported here. Furthermore, the relatively small sample sizes at the various severity levels, and consequent random variation, led us to use a rather broad grouping of injuries (eg, C1–C5). Additional data and further research would allow for more precise modeling and estimates. Finally, causes of death were missing for 31% of the deaths where identifying information was unknown to the NSCISC staff, thereby precluding use of the National Death Index to ascertain the missing data. With so many missing causes of death, calculation of meaningful cause-specific standardized mortality ratios was not possible.
CONCLUSION
An appreciation of survival and life expectancy is central to the overall understanding of the impact of SCI, not only for the individual, but also for families, the community, and society as a whole. As they begin their experience with catastrophic disability in the trauma hospital intensive care setting and progress later in acute rehabilitation, patients and their family members often have mistaken and excessively pessimistic assumptions regarding the effect of SCI on life expectancy. This may be especially true in the case of ventilator-dependent high tetraplegia, the most critically injured category of this patient population. It is therefore important for clinicians to provide accurate evidence-based education on the topic to enable a more informed understanding of what lies ahead. These issues are also of interest to third-party payers, where information on survival and life expectancy is essential for planning resource allocation over the lifetime of the injured patient. While prevailing wisdom suggests that ventilator-dependent tetraplegia is associated with the most severe impact on life expectancy in SCI, changes in our health care system and ongoing refinements in our care of this patient population warrant a periodic reassessment of this important information.
Footnotes
This study was supported in part by National Institute on Disability and Rehabilitation Research grant #H133A011201, Office of Special Education and Rehabilitative Services, United States Department of Education, Washington, DC.
REFERENCES
- Whiteneck G. Long-term outlook for persons with high quadriplegia. In: Whiteneck G, Adler C, Carter RE, et al., editors. The Management of High Quadriplegia. New York: Demos Publications; 1989. pp. 353–361. [Google Scholar]
- Carter RE. Experiences with high tetraplegia. Paraplegia. 1979;17:140–146. doi: 10.1038/sc.1979.28. [DOI] [PubMed] [Google Scholar]
- Carter RE. Experience with ventilator dependent patients. Paraplegia. 1993;31:150–153. doi: 10.1038/sc.1993.28. [DOI] [PubMed] [Google Scholar]
- Wicks AB, Menter RR. Long-term outlook in quadriplegic patients with initial ventilator dependency. Chest. 1986;90:406–410. doi: 10.1378/chest.90.3.406. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Ivie SC. Life expectancy of ventilator-dependent persons with spinal cord injuries. Chest. 1995;108:226–232. doi: 10.1378/chest.108.1.226. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Krause JS, DeVivo MJ, Jackson AB. Health status, community integration, and economic risk factors for mortality after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1764–1773. doi: 10.1016/j.apmr.2004.06.062. [DOI] [PubMed] [Google Scholar]
- Stover SL, DeVivo MJ, Go BK. History, implementation, and current status of the national spinal cord injury database. Arch Phys Med Rehabil. 1999;80:1365–1371. doi: 10.1016/s0003-9993(99)90246-0. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med. 2002;25:335–338. doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- American Spinal Injury Association . International Standards for Neurological Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association; 2002. [Google Scholar]
- Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis L, Ungar G. The value of postural reduction in the initial management of closed injuries to the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Ditunno JF, Donovan WH, Maynard F. Neurologic recovery after traumatic spinal cord injury: data from the model spinal cord injury systems. Arch Phys Med Rehabil. 1999;80:1391–1396. doi: 10.1016/s0003-9993(99)90249-6. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL, Black KJ. Prognostic factors for 12-year survival after spinal cord injury. Arch Phys Med Rehabil. 1992;73:156–162. [PubMed] [Google Scholar]
- DeVivo MJ, Kartus PL, Stover SL, et al. Cause of death for patients with spinal cord injuries. Arch Intern Med. 1989;149:1761–1766. [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74:248–254. [PubMed] [Google Scholar]
- Collett D. Modelling Survival Data in Medical Research. London: Chapman and Hall; 1994. [Google Scholar]
- Strauss DJ, Shavelle RM, DeVivo MJ, Day S. An analytic method for longitudinal mortality studies. J Insurance Med. 2000;32:217–225. [PubMed] [Google Scholar]
- Hosmer D, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London: Chapman and Hall; 1989. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- Schoen R. Modelling Multigroup Populations. New York: Plenum Press; 1988. [Google Scholar]
- Anderson TW. Life Expectancy in Court: A Textbook for Doctors and Lawyers. Vancouver, Canada: Teviot Press; 2002. [Google Scholar]
- Arias E. United States Life Tables, 2001. National Vital Statistics Reports. no 14. vol 52. Bethesda, MD: National Center for Health Statistics; 2004. [PubMed] [Google Scholar]
- Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J. Asymptomatically efficient rank invariant procedures. J R Stat Soc (A) 1972;135:185–207. [Google Scholar]
- Fuhrer MJ, Carter RE, Donovan WH, Rossi CD, Wilkerson MA. Postdischarge outcomes for ventilator-dependent quadriplegics. Arch Phys Med Rehabil. 1987;68:353–356. [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL. Long-term survival and causes of death. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal Cord Injury. Gaithersburg, MD: Aspen Publishers; 1995. pp. 289–316. [Google Scholar]
- Strauss DJ, Vachon PJ, Shavelle RM. Estimation of future mortality rates and life expectancy in chronic medical conditions. J Insur Med. 2005;37:20–34. [PubMed] [Google Scholar]
- Strauss DJ, Shavelle RM, Anderson TW. Life expectancy of children with cerebral palsy. Pediatr Neurol. 1998;18:143–149. doi: 10.1016/s0887-8994(97)00172-0. [DOI] [PubMed] [Google Scholar]
- Strauss DJ, Shavelle RM. Life expectancy of adults with cerebral palsy. Dev Med Child Neurol. 1998;40:369–375. [PubMed] [Google Scholar]
- Strauss DJ, Ashwal S, Shavelle RM, Eyman RK. Prognosis for survival and improvement of children with developmental disabilities. Pediatrics. 1997;131:712–717. [PubMed] [Google Scholar]
- Strauss DJ, Shavelle RM, Ashwal S. Life expectancy and median survival time in the permanent vegetative state. Pediatr Neurol. 1999;21:626–631. doi: 10.1016/s0887-8994(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Shavelle RM, Strauss DJ, Day SM, Ojdana KA. Life expectancy. In: Zasler ND, Katz D, Zafonte R, editors. Brain Injury Medicine: Principles and Practice. New York: Demos Medical Publishing; 2006. [Google Scholar]
- Brown AW, Leibson CL, Malec JF, Perkins PK, Diehl NN, Larson DR. Long-term survival after traumatic brain injury: a population-based analysis. NeuroRehabilitation. 2004;19:37–43. [PubMed] [Google Scholar]
- Harrison-Felix C, Whiteneck G, DeVivo M, Hammond FM, Jha A. Mortality following rehabilitation in the traumatic brain injury model systems of care. NeuroRehabilitation. 2004;19:45–54. [PubMed] [Google Scholar]
- Baguley I, Slewa-Younan S, Lazarus R, Green A. Long-term mortality trends in patients with traumatic brain injury. Brain Injury. 2000;14:505–512. doi: 10.1080/026990500120420. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Johnston MV, Brown J, O'Connor KC, Jarosz P. Predictors of dysphagia after spinal cord injury. Arch Phys Med Rehabil. 1999;80:1101–1105. doi: 10.1016/s0003-9993(99)90068-0. [DOI] [PubMed] [Google Scholar]
- Consortium for Spinal Cord Medicine Respiratory management following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2005;28:259–293. doi: 10.1080/10790268.2005.11753821. [DOI] [PubMed] [Google Scholar]