Abstract
We have assessed the ability of the plant secretory pathway to handle the expression of complex heterologous proteins by investigating the fate of a hybrid immunoglobulin A/G in tobacco cells. Although plant cells can express large amounts of the antibody, a relevant proportion is normally lost to vacuolar sorting and degradation. Here we show that the synthesis of high amounts of IgA/G does not impose stress on the plant secretory pathway. Plant cells can assemble antibody chains with high efficiency and vacuolar transport occurs only after the assembled immunoglobulins have traveled through the Golgi complex. We prove that vacuolar delivery of IgA/G depends on the presence of a cryptic sorting signal in the tailpiece of the IgA/G heavy chain. We also show that unassembled light chains are efficiently secreted as monomers by the plant secretory pathway.
INTRODUCTION
Plants are an efficient and versatile system to express foreign proteins (Daniell et al., 2001) and can even produce complex, multimeric proteins in large amounts. One such example is the expression of a decameric, secretory IgA (Ma et al., 1995), a molecule composed of two IgA units (2 heavy and 2 light chains), a joining J chain, and a secretory component. It has been shown that transgenic tobacco cells are able to translocate all the IgA subunits in the endoplasmic reticulum (ER), where complex assembly occurs with high efficiency (Frigerio et al., 2000). The overall production of IgA is up to 8% of protein of a mature tobacco plant. This very high accumulation makes plants the system of choice for the expression of these molecules, which at present are produced only to minimal levels in transfected mammalian cells (Ma et al., 1995; Larrick et al., 2001). However, we have recently discovered that unlike in mammals, not all assembled antibody is secreted by plant cells. Rather, a significant proportion of the molecules (at least 80%, our unpublished observation) are retained intracellularly and eventually delivered to vacuoles, where they are ultimately fragmented and probably degraded. We have shown that vacuolar delivery is not the result of endocytosis of secreted polypeptides (Frigerio et al., 2000). All IgA assembly intermediates— from the decamer to the simplest, heterotetrameric unit— share this intracellular fate, and this transport and subsequent degradation in the vacuolar compartment can be inhibited by treatment with the fungal metabolite brefeldin A (BFA). In contrast, when the parent IgG molecule was expressed, it was efficiently and quantitatively secreted (Frigerio et al., 2000). Because the κ light chain is common to both IgG and IgA/G molecules, this led us to speculate that features of the IgA/G heavy chain might be responsible for its intracellular diversion. This could in turn be due either to a stress imposed on the ER, with subsequent mis-sorting or quality control delivery to the vacuole or to the presence of cryptic signals for vacuolar sorting.
In the present report we have tested these hypotheses. We have analyzed the fate of IgA molecules in transgenic tobacco plants or transiently transfected tobacco protoplasts. We demonstrate that assembled IgA molecules travel through the Golgi complex before reaching the vacuole. IgA/G transport to the vacuole is not due to stress imposed on the plant ER but is the result of a cryptic signal that resides in the C-terminal domain of the IgA/G heavy chains. In addition, we demonstrate that antibody light chains are expressed in excess of the heavy chains and that free light chains are secreted in their monomeric form.
MATERIALS AND METHODS
Transgenic Plants
Transgenic Nicotiana tabacum cv Xanthi plant lines expressing assembled IgG and IgA/G under the control of the cauliflower mosaic virus 35S-promoter have previously been described (Ma et al., 1994). For protoplast transfection experiments, wild-type N. tabacum cv Petit Havana SR1 was used. Plants were grown in axenic conditions under a 12-h light-dark regime.
Recombinant DNA
All DNA manipulations were performed using established procedures.
The full length IgA/G γ/α heavy chain was amplified from the binary vector pMON530 (Ma et al., 1994) using the PCR. The oligonucleotides 5′-ccatcgatggaatggacctgggttttt-3′ and 5′-ccctctagactagtagcataggccatc-3′, containing ClaI and XbaI restriction sites before the start codon and after the stop codon for cloning purposes, were used. The digested PCR products were ligated into a pUC-based vector downstream of the CaMV35S-promoter (Denecke et al., 1992). The resulting plasmid was designated pJLH38.
Glycosylation site mutations were produced using the “Quickchange” in vitro mutagenesis system (Stratagene, La Jolla, CA). Potential glycan sites mutations Ser 76 to Ala (Δglycan1 pJLH40), Thr 289 to Val (Δglycan2 pJLH41), Thr 526 to Ala (Δglycan3 pJLH42), and Ser 541 to Ala (Δglycan4 pJLH43) were introduced using the oligonucleotides 5′-actgtagacaattccgccacctcagcctaca-3′, 5′-tgtaggctgaggtggcggaattgtctacagt-3′, 5′-gagcag ctcaacagcgttttccgctcagtcag-3′, 5′-ctgactgagcggaaaacgctgttgagctgctc-3′, 5′-ttgcccatgaacttcgtccagaagaccatcga-3′, 5′-tcgatggtcttctggacgaagttcatgggcaa-3′, 5′-aaacccaccaatgtcgctgtgtctgtgatcatg-3′, or 5′-catgatcacagacacagcgacattggtgggttt-3′, respectively, using pJLH38 as a template. Multiple glycan mutant Δ3,4 was also produced using Quickchange in vitro mutagenesis system (Stratagene) with the oligonucleotides 5′-aaacccaccaatgtcgctgtgtctgtgatcatg-3′ and 5′-catgatcacagacacagcgacatt ggtgggttt-3′ and pJLH42 as template. The glycan mutant pJLH45, containing no glycosylation sites (Δ1,2,3,4) was produced by isolating the ClaI-NcoI fragment from pJLH40, the NcoI-EcoRI fragment pJLH41, and the EcoRI-XbaI fragment from pJLH44 and ligating them into the pUC vector previously cut with ClaI and XbaI.
Removal of the last 18 amino acids of the γ/α heavy chain (ΔC18 pJLH47) was achieved by PCR using the antisense oligonucleotide 5′-ccctctagactatttacccgacagacggtc-3′ producing a stop codon followed by an XbaI site at position 537 with the sense oligo 5′-gagcagctcaacagcgttttccgctcagtcag-3′. The resulting PCR product was cut with EcoRI and XbaI and ligated into the expression vector cut with ClaI and XbaI along with a ClaI-EcoRI fragment of pJLH38.
Phaseolin expression constructs T343F and Δ418 are described in Pedrazzini et al. (1997) and Frigerio et al. (1998a), respectively.
The portion of the region encoding the predicted mature portion of the IgG κ light chain was amplified and inserted between the blunted KpnI site and the BamHI site of pDE300d, a pUC19-based plasmid carrying a 35S promoter, the signal peptide of PR1b (Denecke et al., 1990), a polylinker, and a 3′nos polyadenylation site. This resulted in plasmid pSK3 and encodes a secreted form of the IgG κ light chain that is formed after cleavage of the PR1b signal peptide.
Protoplast Transfection
Protoplasts prepared from axenic leaves of tobacco (Nicotiana tabacum cv Petit Havana SRI), grown in sterile in vitro conditions under a 12-h light-dark regime, were subjected to polyethylene glycol– mediated transfections as described by Pedrazzini et al. (1997). Forty micrograms of each plasmid was used to transform 106 protoplasts in 1 ml. When only single antibody chains were expressed, the total amount of DNA was maintained constant among samples by adding 40 μg of empty expression vector pDHA (Tabe and Higgins, 1998). After transfection, cells were incubated at 25°C before metabolic labeling.
In Vivo Labeling of Protoplasts and Analysis of Expressed Polypeptides
Pulse-chase experiments were conducted by labeling protoplasts using ProMix (a mixture of 35S-Met and 35S-Cys; Amersham Biosciences, Piscataway, NJ) and chasing with an excess of cold amino acids for the times stated (Predrazzini et al. 1997). After harvesting at desired time points, protoplasts and incubation media were frozen and homogenized by adding two volumes of ice-cold homogenization buffer (150 mM Tris-HCl, 150 mM NaCl, 1.5 mM EDTA, and 1.5% (wt/vol) Triton X-100, pH 7.5) supplemented with Complete protease inhibitor cocktail (Roche Products Ltd., Welwyn Garden City, United Kingdom). Immunoprecipitation of expressed polypeptides was performed as described previously (Frigerio et al. 1998a) using rabbit polyclonal antisera raised against mouse IgG (whole molecule, Sigma Chemical, St. Louis, MO), γ heavy chain, κ light chain (Southern Biotechnology, Birmingham, AL), or bean phaseolin (Pedrazzini et al. 1997). Digestion of immunoprecipitated proteins with endoglycosidase H (Roche) was performed as described previously (Ceriotti et al., 1991).
For the analysis of the secreted free κ light chains by sedimentation velocity, after radioactive labeling and homogenization of cells, homogenates and incubation media were loaded on top of a continuous 5–25% (wt/vol) linear sucrose gradient made in 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, and 50 mM Tris-Cl, pH 7.5. Samples were centrifuged at 39,000 rpm in a SW40 Ti rotor (Beckman Instruments, Inc., Fullerton, CA) for 20 h at 20°C. κ light chain was then immunoselected from each gradient fraction. Immunoselected proteins were resolved by 15% (wt/vol) nonreducing or reducing SDS-PAGE and revealed by fluorography. The relative intensity of polypeptide bands was determined by densitometry using the Total Lab software package (Nonlinear Dynamics, Newcastle, UK).
RESULTS
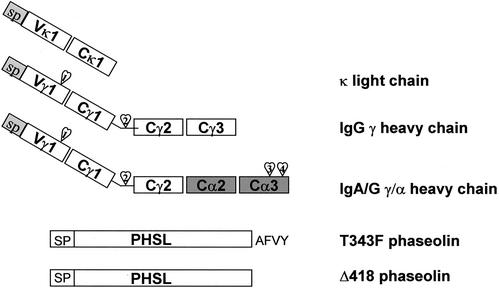
We have previously shown that the intracellular fate of decameric, secretory IgA/G is the same as its constituent tetrameric IgA/G subunit (Frigerio et al., 2000). We therefore decided to focus our efforts on the latter for simplicity of analysis. Tetrameric IgA/G consists of two κ light chains and two hybrid IgA/G chains. These polypeptides are schematically illustrated in Figure 1. The hybrid heavy chain (denominated γ/α) consists of the IgG γ variable domain and constant Cγ1 and Cγ2 domains from monoclonal IgG Guy's 13 (Ma et al., 1994), fused to the constant Cα2 and Cα3 domain from a secretory IgA (Ma et al., 1994). The Cα3 domain contains the C-terminal cysteine that is responsible for binding the J chain and contains regions necessary for contact with the secretory component (Mestecky and McGhee, 1987). The additional Cγ2 domain was originally added to provide an extra-affinity tag, to facilitate purification of the antibody from plant tissue (Ma et al., 1994). The presence or the absence of this extra domain does not affect the trafficking of the molecule (Ma et al., 1994, and our unpublished observations).
Figure 1.
Schematic representation of the constructs used in this study. All constructs were fused downstream of the CaMV35S promoter in the expression vector pJLH38 (see MATERIALS AND METHODS). SP, signal peptide. The hearts indicate the glycosylation sites in the heavy chains.
The Synthesis of High Amounts of IgA/G Does Not Impose Stress on the Secretory Pathway of Mesophyll Cells
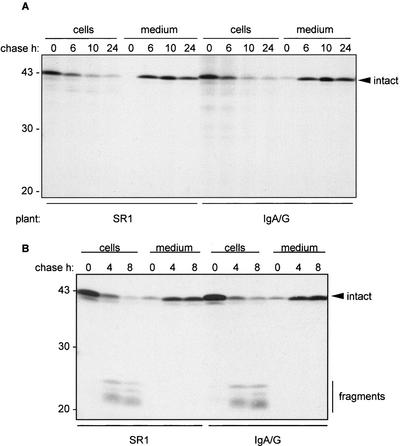
Is the observed vacuolar delivery of a proportion of SIgA/G the result of stress imposed to the secretory system by the high expression of this heterologous protein? Could vacuolar targeting be the effect of saturation of secretion? To test these possibilities, protoplasts from plants expressing SIgA/G were transiently transfected with plasmids encoding different constructs of phaseolin, a well-characterized vacuolar storage protein from bean. The mutated phaseolin Δ418 lacks the vacuolar sorting signal of phaseolin and is efficiently secreted after traffic through the Golgi complex when expressed in tobacco (Frigerio et al., 1998a). If expression of the antibody were saturating the ER capacity and imposing stress onto the system to the point of reducing secretion and causing missorting to the vacuole, we would expect secretion of phaseolin Δ418 to be inhibited as well. However, Figure 2A shows that in the antibody-expressing cells, the fate of phaseolin is not affected, and the protein is still secreted with the same efficiency observed in SR1 cells where IgA/G is not coexpressed. In the vacuole of tobacco cells, phaseolin undergoes proteolytic fragmentation to polypeptides in the 20–25-kDa range. The appearance of these fragments is therefore indicative of vacuolar delivery (Pedrazzini et al., 1997). Because no fragmentation of phaseolin Δ418 in SigA/G-expressing cells occurred during the chase (Figure 2A), we conclude that vacuolar delivery of IgA cannot be attributed to a general partial missorting of secretory proteins to vacuoles in these cells.
Figure 2.
The synthesis of high amounts of IgA/G is not imposing stress onto the secretory pathway of mesophyll cells. Protoplasts from wild-type (SR1) or transgenic tobacco plants expressing IgA/G were transiently transfected with plasmids encoding phaseolin Δ418 (A) or T343F (B). Cells were labeled for 1 h with 35S-methionine and -cysteine and chased for the indicated periods of time. Total cell homogenates or incubation media were immunoprecipitated with anti-phaseolin antiserum and polypeptides were visualized by SDS-PAGE and fluorography. The arrowhead indicates the position of intact phaseolin. The vertical bar in B indicates the position of vacuolar fragmentation products of phaseolin. Numbers at left indicate molecular mass markers in kilodaltons.
To further explore the possibility of a spurious effect due to stress on the secretory system, we wanted to test whether expression of IgA/G was affecting transport of a naturally vacuolar protein. We used T343F, a form of phaseolin that contains the normal vacuolar sorting signal of this protein and is efficiently transported to the vacuole via the Golgi complex also in tobacco (Pedrazzini et al., 1997). The appearance of fragmentation products during the chase indicated that in the IgA/G-expressing cells, phaseolin was still delivered to vacuoles, with an efficiency comparable to wild-type cells (Figure 2B). A proportion of T343F molecules was also secreted in the incubation medium. We have previously shown that this is due to saturation of the sorting machinery during transient expression (Frigerio et al., 1998a). In IgA/G expressing cells, there is no shift in the ratio vacuolar/secreted phaseolin with respect to nontransgenic SR1 cells (Figure 2B). Because sorting of phaseolin to the vacuole is a saturable process (Frigerio et al., 1998a), this result indicates that expression—and vacuolar delivery— of IgA/G is not competing with vacuolar sorting of phaseolin, suggesting that the two proteins are sorted to the vacuole by different mechanisms.
Intracellular Trafficking of IgA/G Is Faithfully Reproduced in Transiently Transfected Tobacco Protoplasts
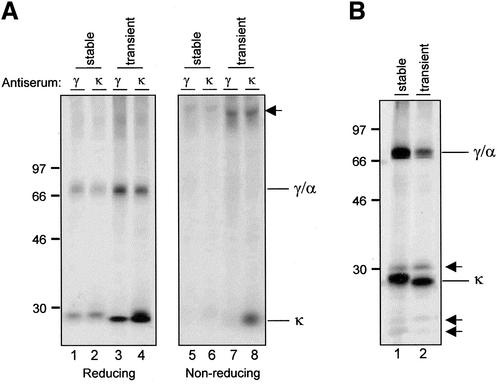
Transient expression in protoplasts has been successfully used to study the behavior and trafficking of proteins in the plant secretory pathway (Frigerio et al., 1998a, 1998b; Phillipson et al., 2001; Törmäkangas et al., 2001), although it has never been used for complex multimeric proteins. The transient expression system allows rapid testing of mutant constructs and would therefore be a valuable tool for the analysis of potential of trafficking mutants of IgA/G. To reliably use this technique in this study we first needed to prove that the behavior of the Ig chains was comparable in transiently and permanently transformed tobacco cells. To achieve these aims we cloned the Ig coding sequences (heavy and light chains, Figure 1), fused to the CaMV35S promoter, into a CaMV35S-driven vector for transient expression. Tobacco mesophyll protoplasts were transfected with plasmids encoding κ chain and IgA/G γ/α heavy chain. Transfected cells were metabolically labeled for 2 h with 35S-methionine and -cysteine and homogenized. Protoplasts from transgenic plants expressing IgA/G were also treated similarly, for comparison. Homogenates were immunoprecipitated with either anti-γ or anti-κ antisera. Anti-γ recognizes only the constant γ domains in the heavy chains, whereas anti-κ binds to the light chain only. Both of these antisera were capable of coselecting both heavy and light chains, indicating assembly of the IgA molecule after transient and permanent expression (Figure 3A, lanes 1– 4). Assembly is very efficient, as further confirmed by the fact that the molecules migrate with the mobility expected for fully assembled heterotetramers when resolved on nonreducing SDS-PAGE (Figure 3A, lanes 5– 8) with no free heavy chain detected in either transient of stably expressing protoplasts. An excess of free light chain can be observed in lane 8 and will be discussed further below. The discrepancy observed in the mobility of the κ chain between transient and stable expression is due to the fact that a different signal peptide (Denecke et al., 1992) was used to prepare the transient expression construct compared with the murine signal peptide used to generate transgenic plants (Ma et al., 1994 and see MATERIALS AND METHODS) Because the original sequence contains additional residues between the predicted signal peptide cleavage site and the first codon of mature κ chain (Ma et al., 1994), this probably results in a slightly different electrophoretic mobility.
Figure 3.
Permanently and transiently expressed IgA/G chains have the same fate in tobacco protoplasts. (A) Cells from IgA/G-expressing transgenic plants or SR1 protoplasts transfected with plasmids encoding κ and γ/α chains were labeled for 2 h with 35S-methionine and -cysteine. Total cell homogenates were immunoprecipitated with anti-γ or anti-κ antisera. Polypeptides were visualized by reducing or nonreducing SDS-PAGE and fluorography. The arrow indicates assembled IgA/G tetramers. (B) As in A, but cells were labeled for 16 h and cell homogenates immunoprecipitated with anti-IgG antiserum. The arrows indicate vacuolar fragmentation products. Numbers at left indicate molecular mass markers in kilodaltons.
As mentioned above, our observations in transgenic plants revealed that the IgA/G tetramer is partially delivered to vacuoles. This results in the appearance of degradation products after a long chase (Frigerio et al., 2000). To test whether this is also the case for transiently expressed IgA/G, we compared the phenotype of the molecules after a 16-h metabolic labeling (Figure 3B). Subsequent immunoprecipitation with anti-IgG, which recognizes the whole IgG molecule, reveals the presence of small polypeptides resulting from degradation (Figure 3B, arrows) when the IgA/G molecule is expressed transiently. The sizes of these fragments are comparable in both transiently and stably expressed antibody chains. We therefore conclude that fragments observed in transient expression are also the result of vacuolar delivery. Therefore, both the assembly and intracellular transport events of IgA/G are faithfully reproduced in transient expression using tobacco mesophyll protoplasts.
Only Assembled IgA/G Is Found in Vacuoles
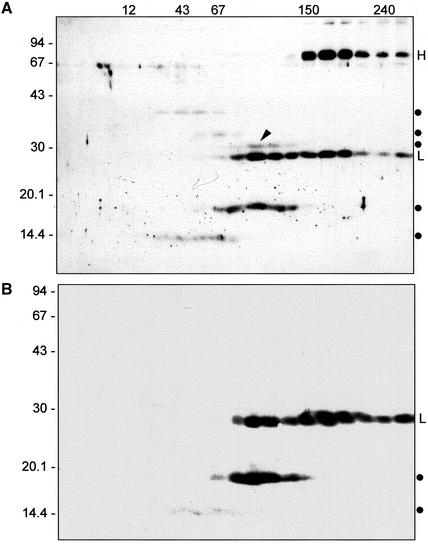
When a leaf extract is fractionated by velocity density centrifugation on sucrose gradient, the position of the IgA/G vacuolar fragments with respect to molecular weight markers indicates that neither of the fragments is monomeric (Figure 4A; the positions of fragments are indicated by dots at right; the bands that migrate at around 67 kDa on SDS-PAGE are not proteins: they are an artifact often visualized by ECL due to β-mercaptoethanol). Therefore all polypeptides delivered to vacuoles possess some degree of assembly, indicating that vacuolar delivery occurs after the antibody has assembled and is not a disposal route for orphan chains.
Figure 4.
Only assembled IgA/G molecules are delivered to vacuoles. Total leaf homogenates from transgenic plants expressing IgA/G were fractionated by sedimentation velocity centrifugation on a continuous 5–25% sucrose gradient. Fractions were resolved by SDS-PAGE and the gels subjected to immunoblotting with anti-IgG (A) or anti-κ chain (B) antisera. Immunoreactive polypeptides were visualized by ECL. Dots at right mark the position of the IgA/G fragments. H, heavy chain; L, light chain. Numbers at left and at the top indicate molecular mass marker in kilodaltons. The arrowhead indicates the glycosylated 30-kDa fragment.
Vacuolar Delivery of IgA/G Is Mediated by the Golgi Complex
In previous studies we have shown that vacuolar delivery of IgA/G is not the result of endocytosis of secreted proteins. This was demonstrated by the fact that increasing the volume of the incubation medium did not result in a decrease in the intensity of the intracellular fragmentation products (Frigerio et al., 2000). Likewise, incubating unlabeled protoplasts for 24 h with secretion medium isolated from extensively labeled protoplasts and therefore containing secreted, radioactive IgA/G did not result in the appearance of labeled intracellular Ig fragments (Santoro and Vitale, unpublished results). Both secretion and transport of IgA/G to the vacuole can be inhibited by the fungal metabolite BFA (Frigerio et al., 2000). BFA inhibits the formation of a number of vesicles that mediate traffic through the secretory pathway; we therefore used that evidence to conclude that IgA/G vacuolar delivery occurred through vesicle traffic. Recently, however, it has become clear that there exist alternative routes of traffic from the plant ER to vacuoles (Hara-Nishimura et al., 1998; Toyooka et al., 2000). We have shown that one of these routes, although prone to BFA inhibition, bypasses the Golgi complex (Frigerio et al., 2001). In the light of this new evidence it is clear that BFA inhibition alone cannot be used as a sole evidence for transport through the Golgi complex. Thus, in order to test whether IgA/G does indeed travel through the Golgi, we have studied the state of one of its N-linked glycans.
Analysis of the IgA/G degradation products (Figure 4) shows that one fragment has a molecular mass around 30 kDa (arrowhead in Figure 4A) and comigrates along the gradient with a proportion of intact light chains, strongly suggesting that the fragment is comprised of the variable and constant γ domains associated with the κ light chain to yield the Fab fragment originating from fragmentation of tetrameric IgA/G. Consistently, on protein blots, the 30-kDa fragment is not detected by anti-light (κ) chain antibodies (Figure 4B) or antibodies against the heavy (α) chains of IgA (our unpublished results). Because heavy chains are extensively glycosylated, we reasoned that the 30-kDa fragment (Figure 4A) could be used as a marker for traffic through the Golgi complex.
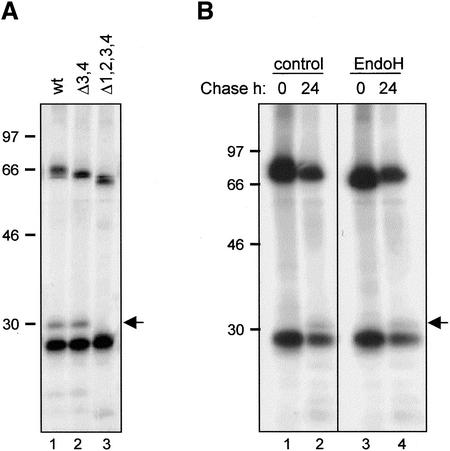
To determine whether the fragment was actually glycosylated, all glycosylation sites of the hybrid heavy chain were inactivated by site-specific mutagenesis (Δ1,2,3,4 in Figure 5A), and the mutated polypeptide was transiently coexpressed with κ chain in tobacco protoplasts. Protoplasts were labeled with 35S-labeled cysteine and -methionine for 16 h and immunoprecipitated with anti-IgG antibodies. The 30-kDa fragment was no longer detectable (Figure 5A, lane 3). The observed disappearance of the 30-kDa fragment upon mutation of the glycosylation sites is likely due to the fact that its mobility becomes very similar to that of the κ chain, resulting in comigration of the two polypeptides. The fact that the mobility shift is only detectable in the mutant γ/α chain where all four putative glycosylation sites have been removed (Figure 5A, lane 3) but not in a mutant chain carrying mutations in the two sites located in the α domain (Δ3,4, Figure 5A, lane 2) provides further evidence that the 30-kDa fragment originates from the γ domain of the hybrid γ/α heavy chain.
Figure 5.
Vacuolar delivery of IgA/G is mediated by the Golgi complex. (A) SR1 protoplasts were transfected with plasmids encoding κ chain and γ/α heavy chain (wt), γ/α lacking the two C-terminal glycosylation sites (Δ3,4) or γ/α lacking all four glycosylation sites (Δ1,2,3,4), respectively. Cells were pulse-labeled for 16 h, homogenized, and immunoprecipitated with anti-IgG antiserum. Proteins were visualized by SDS-PAGE and fluorography. (B) Cells were transfected with plasmids encoding κ and γ/α chains and treated as in A. Immunoprecipitates were subjected to treatment with endoglycosidase H (endo H) or buffer (control) before SDS-PAGE analysis. The arrow indicates the position of the 30-kDa vacuolar fragmentation product. Numbers at left indicate molecular mass markers in kilodaltons.
Thus, the 30-kDa fragmentation product, derives from assembled IgA/G, and is N-glycosylated. As fragmentation occurs in the vacuolar compartment (Frigerio et al., 2000), we expect that the glycan present on the 30-kDa fragment product be of the complex (i.e., Golgi-modified) type, if the molecule has traveled through the Golgi complex en route to the vacuole. If this is the case, this glycan should be resistant to in vitro endoglycosidase H (endo H) digestion, because this enzyme removes high-mannose, N-linked glycans that are attached to glycoproteins in the ER but not glycans modified by Golgi enzymes. Indeed, treatment with endo H after transient expression of heavy and light chains, metabolic labeling, and subsequent immunoprecipitation does not affect the mobility of the 30-kDa fragment (Figure 5B, compare lanes 2 and 4), indicating that this glycan has acquired endo H resistance. It is, however, clear that the enzyme is active, because the mobility of the heavy chain is increased by endo H treatment at the end of the pulse (Figure 5B, compare lanes 1 and 3). This is expected because, shortly after synthesis, while still in the ER, the heavy chain glycans are in the high-mannose, endo H–sensitive form. The same results were obtained when the experiment was performed on protoplasts isolated from transgenic plants expressing IgA/G (our unpublished results).
On the basis of the results shown, we therefore conclude that transport of IgA/G to the vacuole occurs through the Golgi complex.
The C-terminal Domain of α Heavy Chain Determines Vacuolar Delivery
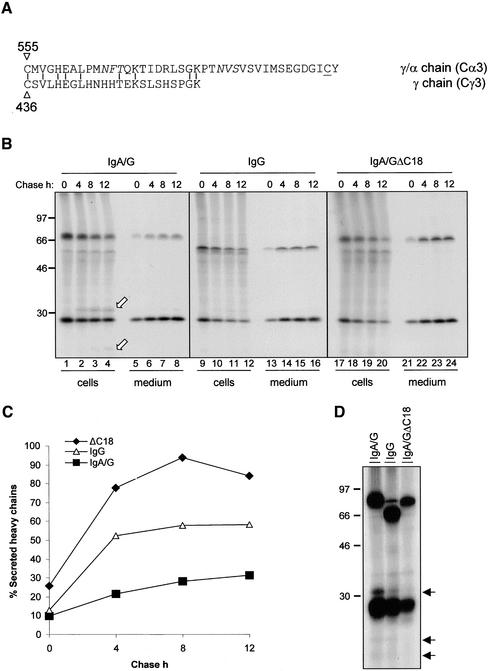
The work presented so far shows that the secretory pathway in plants expressing IgA/G is not overloaded and that the IgA/G tetrameric molecule is transported to the vacuole via the Golgi complex where it is subjected to proteolysis. The most likely explanation for IgA/G delivery to the vacuole is therefore the existence of positive, albeit cryptic, sorting information within the molecule. Clearly, this signal must be absent from the parent IgG as this is secreted efficiently (Frigerio et al., 2000). Therefore, it must be contained within the additional constant α domains of the γ/α heavy chain (Figure 1). We speculated that the signal must be exposed and therefore compared the C-terminal regions of the two heavy chains. Sequence alignment of the IgA/G Cα3 constant domain with the constant Cγ3 domain of its parent Guy's 13 IgG heavy chain (Ma et al., 1994) reveals that the Cα3 domain is 18 residues longer than Cγ3 (Figure 6A). This extension constitutes the tailpiece containing the cysteine residue required for J chain binding (Mattu et al., 1998). Given the heterogeneous nature of C-terminal vacuolar sorting signals (ctVSS; Vitale and Raikhel, 1999), the tailpiece might be recognized as a ctVSS within plant cells. To test this hypothesis, we deleted the 18-residue, C-terminal tail to produce a truncated heavy γ/α chain, denominated ΔC18. We cotransfected protoplasts with plasmids encoding the κ light chain and γ/α, γ, or ΔC18 heavy chains, respectively, and subjected them to pulse-chase analysis. When coexpressed with light chain, ΔC18 assembled correctly into tetramers (our unpublished results) and was secreted very efficiently (Figure 6, B and C). Given that free, unassembled light chains are secreted (see below), but unassembled heavy chains are completely retained in the endoplasmic reticulum (Nuttall et al., 2002), the amount of heavy chains recovered from the medium represents the actual levels of tetramer secretion. Note that although the extracellular amount of IgA/G heavy chains increases very slowly, to ∼30% of the initial labeled protein after a 12-h chase, the amount of secreted IgA/G containing ΔC18 heavy chains increases much more rapidly and reaches ∼90% of the synthesized chains during the chase (Figure 6C, and compare lanes 5– 8 with lanes 21–24 in Figure 6B). This rate and efficiency of secretion is comparable or even higher than that of IgG (Figure 6, B and C). The increased secretion of ΔC18 with respect to wild-type IgA/G was paralleled by the almost complete disappearance of vacuolar degradation fragments. This is visible in Figure 6B (compare lanes 2– 4, arrows, with lanes 18–20) and was further confirmed by overexposing gels of immunoprecipitates after a 16-h labeling (Figure 6D). This shows that deletion of the C-terminal 18 amino acids of the IgA/G heavy chain virtually abolishes vacuolar targeting and results in increased secretion of the assembled tetramers. Therefore, we conclude that the C-terminal tail of the hybrid γ/α chain is responsible for vacuolar delivery of IgA/G.
Figure 6.
Deletion of the Cα3 tailpiece results in secretion of IgA/G. (A) Comparison of the C-terminal tailpieces of the constant domains of IgA/G and IgG heavy chains. Amino acid sequence alignment was generated using the MegAlign program (DNASTAR Inc., Madison, WI). Vertical bars identify identical amino acid residues The N-glycosylation sites in γ/α are italicized. The J chain binding cysteine residue is underlined. (B) SR1 protoplasts were transfected with plasmids encoding κ chain and γ/α, γ, or γ/α ΔC18 heavy chains, respectively. Cells were pulse labeled for 1 h and chased for the indicated periods of time. Cell homogenates and incubation media were immunoprecipitated with anti-IgG antiserum. Proteins were visualized by SDS-PAGE and fluorography. Numbers at left indicate molecular mass markers in kilodaltons. White arrows indicate vacuolar fragmentation products. (C) The fluorograms shown in B were subjected to densitometry to quantify the amount of secreted heavy chains. Secreted heavy chains are expressed as percentage of total intracellular heavy chains immunoselected at 0-h chase. (D) Cells transfected as in B were labeled for 16 h, homogenized and immunoprecipitated with anti-IgG antiserum. Proteins were visualized by SDS-PAGE and fluorography. Numbers at left indicate molecular mass markers in kilodaltons. Arrows indicate vacuolar fragmentation products.
Unassembled Light Chains Are Efficiently Secreted as Monomers
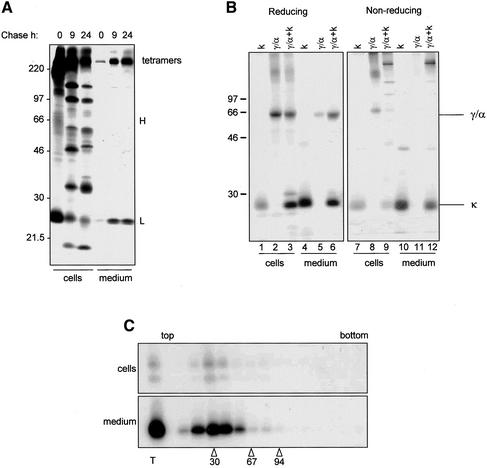
When protoplasts from transgenic plants expressing IgA/G are subjected to pulse-chase labeling and IgA/G are analyzed by SDS-PAGE under nonreducing conditions, it can be observed that at the end of the 1-h pulse only a very minor proportion of heavy chains is recovered as unassembled polypeptides, indicating very rapid and efficient assembly (Figure 7A). Conversely, a relevant proportion of light chains is still monomeric, indicating that in these plants light chains are produced in excess with respect to heavy chains. Analysis of the protoplast incubation medium shows that, besides the heterotetramers, unassembled light chains are also secreted (Figure 7A). To test this, we transiently transformed tobacco protoplasts with plasmid encoding the κ chain, the γ/α heavy chain, or both chains together and subjected them to metabolic labeling for 16 h. Protoplasts were then homogenized and immunoprecipitated with anti-IgG. When both chains are coexpressed, immunoprecipitation with anti-IgG antiserum reveals the presence of a larger amount of κ light chains compared with heavy chain in both the cells and the incubation medium (Figure 7B, lanes 3 and 6). When the samples are resolved on nonreducing SDS-PAGE (Figure 7B, lanes 7–12), it appears evident that the excess κ light chain observed is free κ light chain not associated with heavy chain. In mammalian cells there is evidence that free unassembled light chain can be secreted as covalent or noncovalent dimer (Leitzgen et al., 1997). From the experiment in nonreducing conditions (Figure 7B, lanes 10 and 12), it is clear that there are no disulfide-linked dimers of κ. When the κ chain is expressed alone, it is mainly recovered from the incubation medium (Figure 7B, lanes 1 and 4). Conversely, the γ/α chain remains mainly intracellular when κ chain is not coexpressed (Figure 7B, lanes 2 and 5; Nuttall et al., 2002). To determine if noncovalent dimerization occurs before secretion of κ chain in plants, we transfected protoplasts with κ chain and metabolically labeled them for 16 h. We then loaded cell homogenates and incubation medium onto linear 5–25% sucrose gradients and subjected them to sedimentation velocity centrifugation, along with molecular mass markers. Gradient fractions were then immunoprecipitated with anti-IgG antiserum (Figure 7C). Clearly, the vast majority of secreted light chain is retrieved in fractions corresponding to a mass around 30 kDa, a size compatible with the monomeric form. We conclude that κ is efficiently secreted as a monomer in tobacco mesophyll protoplasts. This is in agreement with the finding that vacuolar delivery of assembled IgA/G depends on the presence of the C-terminal portion of α heavy chains.
Figure 7.
Unassembled light chain is secreted as a monomer. (A) Protoplasts from transgenic plants expressing IgA/G were pulse-labeled for 1 h and chased for the indicated periods of time. Cell homogenates and incubation media were immunoprecipitated with anti-IgG antiserum and proteins analyzed by nonreducing SDS-PAGE and fluorography. (B) SR1 protoplasts were transfected with plasmids encoding κ chain, γ/α heavy chain, or both chains together and labeled for 16 h. Cell homogenates and incubation media were immunoprecipitated with anti-IgG and proteins resolved by reducing or nonreducing SDS-PAGE and fluorography. (C) SR1 protoplasts were transfected with plasmid encoding κ chain and labeled for 16 h. The cell homogenate and incubation medium were subjected to sedimentation velocity centrifugation on a linear 5–25% sucrose gradient. Gradient fractions were immunoprecipitated with anti-IgG antiserum and polypeptides resolved by SDS-PAGE and fluorography. T, total (unfractionated) sample. Numbers at the bottom indicate molecular mass markers in kilodaltons.
DISCUSSION
In this work we have studied the fate of a hybrid secretory immunoglobulin IgA/G and gained further insight on how plants respond to the expression of a complex heterologous protein. The efficiency of IgA/G assembly in the plant ER is virtually 100% (Frigerio et al., 2000, and here). In this respect, the plant ER is a very versatile and efficient folding compartment for foreign proteins, as shown by other studies (Frigerio et al., 1998b; Crofts et al., 1999; Frigerio et al., 2001). Production of biologically active SIgA/G in transgenic tobacco leaves reaches ∼8% of total soluble protein (Ma et al., 1995). However, we have previously shown that part of the protein is directed to the vacuole instead of being secreted into the apoplast (Frigerio et al., 2000). Vacuolar SIgA/G is not an irrelevant proportion, it is detectable at the electron microscope level and accumulates as proteolytic fragments (Frigerio et al., 2000). We do not know whether fragmentation is then followed by full degradation, but the fragments are unlikely to have the same activity as the intact molecules. Understanding the mechanism that leads to vacuolar delivery of these immunoglobulins would therefore increase our knowledge on how plants handle foreign secretory proteins and more in general on plant vacuolar delivery mechanisms, but would also allow us to plan strategies to increase the production of SIgA/G.
The Capacity of the Plant Secretory Pathway Is Very High
By transiently expressing vacuolar or secreted forms of phaseolin we have shown that the secretory pathway of IgA/G plants still has spare translocational and traffic activity. Therefore, delivery of IgA/G to the vacuole is not due to overloading of the endomembrane system. This is consistent with the current view that in plants, secretion is the default pathway for proteins introduced into the secretory pathway and lacking sorting signals (Denecke et al., 1990). We have also shown here that at least one of the heavy chain fragments present in the vacuole has N-linked oligosaccharide chains that, similarly to secreted intact IgA/G, have undergone modifications in the Golgi complex. This implies that the Golgi complex mediates vacuolar delivery of the heterologous protein and that most probably the sorting occurs within or at the exit of this compartment.
Defective proteins introduced into the secretory pathway are often dislocated from the ER into the cytosol using the Sec61 translocation channel in reverse and are finally degraded by the proteasome (Chevet et al., 2001). It has however been shown both in plants and yeast that structurally defective or foreign nonvacuolar proteins can also be directed to vacuoles, in processes that could be alternative quality control mechanism with respect to dislocation to the cytosol (Coleman et al., 1996; Hong et al., 1996; Bagga et al., 1997). In plants the mechanism could involve direct autophagy of portions of the ER by the vacuole (Coleman et al., 1996; Bagga et al., 1997). We have also shown that overaccumulation of a protein in the plant ER due to addition of the ER localization signal KDEL can lead to vacuolar delivery without Golgi complex involvement (Frigerio et al., 2001). A role of such mechanisms in IgA/G vacuolar delivery is however ruled out by our observation that IgA/G fragments have Golgi-modified oligosaccharide chains. Quality control vacuolar delivery in yeast can instead be mediated by the Golgi complex and has shown to be dependent on the vacuolar sorting receptor Vps10p. This receptor also mediates sorting of a number of yeast natural vacuolar enzymes through clathrin-coated vesicle delivery from the Golgi complex to prevacuolar endosomes (Hong et al., 1996). The receptor would therefore be involved both in normal vacuolar sorting and in quality control, possibly through distinct recognition activities (Hong et al., 1996). We therefore cannot rule out that SIgA/G is also partially directed to the vacuole by a form of Golgi-mediated quality control, although, as explained below, we favor the hypothesis of the presence of a cryptic vacuolar sorting signal.
A Signal for Vacuolar Delivery in the IgA Heavy Chains
We have deleted the last 18 amino acids from the hybrid heavy chain. When this truncated chain was coexpressed with light chain we observed efficient secretion of the assembled immunoglobulin and could not detect fragmentation. The deleted segment is part of the Cα3 constant domain of IgA and is an extension with respect to the IgG heavy chain. Therefore, the segment contains a signal that determines vacuolar delivery of Ig tetramers. We do not know if this signal is also sufficient. We intend to test this by fusing it to reporter secretory proteins although probably many random C-terminal sequences can lead to vacuolar delivery in plant cells (Dombrowski et al., 1993; Neuhaus et al., 1994). Therefore, even if the Cα3 tailpiece should prove sufficient to deliver reporter proteins to the plant vacuole, this would not mean that the signal is the result of a selective pressure for the delivery of IgA to a similar hydrolytic compartment in animal cells.
The deleted fragment contains the cysteine residue that forms a disulfide bridge with the J chain (Mattu et al., 1998). This residue is therefore necessary for IgA dimerization and must be maintained in an engineered heavy chain. The free cysteine, however, is not responsible for vacuolar delivery because dimers of IgA/G, where the cysteine is engaged in J chain interaction, have the same intracellular fate as the individual units (Frigerio et al., 2000). Therefore, it could be possible to mutagenize the C termini of the heavy chain to inhibit vacuolar sorting without affecting dimerization. A number of natural propeptide vacuolar sorting signals have been identified, both for storage and vegetative vacuoles (see Vitale and Raikhel, 1999 for a review). A comparison with the C-terminal segment of IgA shows that the IgA pentapeptide VSVSV is a repetition of dipeptides (VS or SV) that are present three times in the C-terminal propeptides of tobacco β-glucanase (one SV and two VS) and once in potato PT20 (VS; Koide et al., 1999; Vitale and Raikhel, 1999). It may therefore be possible that just by chance the IgA sequence is recognized by one of the plant vacuolar sorting mechanisms. The fact that vacuolar delivery of IgA/G is only partial could be explained considering that IgAs have clearly not evolved as vacuolar proteins: the fortuitous signal would therefore be inefficient. It has been shown that mutagenesis of natural plant sorting signals often results in decreased but not abolished vacuolar sorting, whereas full deletion causes efficient secretion (Dombrowski et al., 1993; Neuhaus et al., 1994).
These observations are not in favor of vacuolar delivery of IgA/G because of quality control. It seems more likely that the protein is misrecognized as a natural vacuolar protein. This would be a more favorable situation for site-directed mutagenesis aimed to obtain complete secretion.
The κ Light Chains Do Not Have the Same Behavior in Mammalian and Plant Cells
We have shown that tobacco protoplasts efficiently secrete unassembled κ chains. Light chains are also secreted when expressed in mammalian cells in the absence of heavy chains, and their secretion requires dimerization (Leitzgen et al., 1997). These homodimers do not form when heavy chains are also present, but the variable and first constant domain are similar enough in the two types of chains to allow this assembly to occur in the absence of competing heavy chains. Tobacco protoplasts instead secrete monomeric light chains. This was demonstrated both by transient expression in the absence of heavy chains and in the transgenic IgA/G plants we have analyzed, where light chains are synthesized in excess with respect to their partners. Secretion of monomeric or homodimeric heavy chains seems negligible. In mammalian cells, before assembly with the light chains, heavy chains are quantitatively associated with BiP and retained in the ER. Indeed, we have recently shown that this is also the case in plant cells (Nuttall et al., 2002). However, the behavior of light chains is clearly different in plant and mammalian cells. It has been reported that an unusual mAb, comprised exclusively of light chains, is recovered both as monomers and dimers from hybridoma cell cultures (Masat et al., 1994). It has been suggested, however, that the detection of monomers would be the result of partial dissociation of dimers during purification (Leitzgen et al., 1997). We do not think that this explains our results, because we never observed any dimer formation in our experiments. It has been hypothesized that monomers of light chains are retained in the ER until dimerization because they would interact with the ER chaperone machinery (Leitzgen et al., 1997). Some features of the mammalian and plant ER folding machineries may therefore be different, contrary to previous indications from a number of studies (Vitale and Denecke, 1999).
Biotechnological Perspectives
The ultimate goal of secretory antibody expression in plants is to produce large amounts of protein in a way that allows for easy purification. Protein secretion by the roots is one promising approach (Borisjuk et al., 1999). Maximizing SIgA/G secretion is therefore an attractive goal. We have identified the signal that prevents a vast proportion of the immunoglobulin molecules from being secreted. Deletion of this signal leads to hybrid IgA/G secretion at levels comparable to IgG secretion, indicating that no further intracellular processes exist to prevent exocytosis. This is the first essential step toward the production of a fully secreted complex immunoglobulin. We are aware that complete deletion of the C-terminal tail, which also contains the C-terminal cysteine involved in J chain binding, is too drastic as it also prevents assembly of the decameric molecule. We are currently aiming to develop a set of heavy chain mutants that are devoid of vacuolar sorting information but are still able to allow decamer assembly.
Acknowledgments
This work was supported by a grant from the BBSRC (88/C13441) and a grant from the British Council/Ministero dell'Universita' e della Ricerca Scientifica e Tecnologica (ROM/889/99/10). J.N. is indebted to a BBRSC Quota studentship.
References
- Bagga, S., Adams, H.P., Rodriguez, F.D., Kemp, J.D., and Sengupta-Gopalan, C. (1997). Coexpression of the maize γ-zein and β-zein genes results in stable accumulation of δ-zein in endoplasmic reticulum-derived protein bodies fromed by β-zein. Plant Cell 9, 1683-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisjuk, N.V., Borisjuk, L.G., Logendra, S., Petersen, F., Gleba, Y., and Raskin, I. (1999). Production of recombinant proteins in plant root exudates. Nat. Biotechnol. 17, 466-469. [DOI] [PubMed] [Google Scholar]
- Ceriotti, A., Pedrazzini, E., Fabbrini, M.S., Zoppè, M., Bollini, R., Vitale, A. (1991). Expression of wild-type and mutated vacuolar storage protein phaseolin in Xenopus oocytes reveals relationships between assembly and intracellular transport. Eur. J. Biochem. 202, 959-968. [DOI] [PubMed] [Google Scholar]
- Chevet, E., Cameron, P.H., Pelletier, M.F., Thomas, D.Y., and Bergeron, J.J. (2001). The endoplasmic reticulum: integration of protein folding, quality control, signaling and degradation. Curr. Opin. Struct. Biol. 11, 120-124. [DOI] [PubMed] [Google Scholar]
- Coleman, C., Herman, E.M., Takasaki, K., and Larkins, B.A. (1996). The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell 8, 2335-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11, 2233-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell, H., Streatfield, S., and Wycoff, K. (2001). Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 6, 219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Botterman, J., and Deblaere, R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2, 51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11, 2345-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski, J.E., Schroeder, M.R., Bednarek, S.Y., and Raikhel, N.V. (1993). Determination of the functional elements within the vacuolar targeting signal of barley lectin. Plant Cell 5, 587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., de Virgilio, M., Prada, A., Faoro, F., and Vitale, A. (1998a). Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10, 1031-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Vitale, A., Lord, J.M., Ceriotti, A., and Roberts, L.M. (1998b). Free ricin A chain, proricin and native toxin have different cellular fates when expressed in tobacco protoplasts. J. Biol. Chem. 273, 14194-14199. [DOI] [PubMed] [Google Scholar]
- Frigerio, L., Vine, N.D., Pedrazzini, E., Hein, M.B., Wang, F., Ma, J.K.-C., and Vitale, A. (2000). Assembly, secretion and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol. 123, 1483-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Pastres, A., Prada, A., and Vitale, A. (2001). Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13, 1109-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998). Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10, 825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, E., Davidson, A.R., and Kaiser, C.A. (1996). A pathway for targeting soluble misfolded proteins to the yeast vacuole. J. Cell Biol. 135, 623-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide, Y., Matsuoka, K., Ohto, M., and Nakamura, K. (1999). The N-terminal propeptide and the C-terminus of the precursor to 20-kilo-dalton potato tuber protein can function as different types of vacuolar sorting signals. Plant Cell Physiol. 40, 1152-1159. [DOI] [PubMed] [Google Scholar]
- Larrick, J.W., Yu, L., Naftzger, C., Jaiswal, S., and Wycoff, K. (2001). Production of secretory IgA antibodies in plants. Biomol. Eng. 18, 87-94. [DOI] [PubMed] [Google Scholar]
- Leitzgen, K., Knittler, M.R., and Haas, I.G. (1997). Assembly of immunoglobulin light chains as a prerequisite for secretion. J. Biol. Chem. 272, 3117-3123. [DOI] [PubMed] [Google Scholar]
- Ma, J.K.-C., Lehner, T., Stabila, P., Fux, C.I., and Hiatt, A. (1994). Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur. J. Immunol. 24, 131-138. [DOI] [PubMed] [Google Scholar]
- Ma, J.K.-C., Hiatt, A., Hein, M., Vine, N.D., Wang, F., Stabila, P., von Dolleweerd, C., Mostov, K., and Lehner, T. (1995). Generation and assembly of secretory antibodies in plants. Science 268, 716-719. [DOI] [PubMed] [Google Scholar]
- Masat, L., Wabl, M., and Johnson, J.P. (1994). A simpler sort of antibody. Proc. Natl. Acad. Sci. USA 91, 893-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattu, T.S. et al. (1998). The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J. Biol. Chem. 273, 2260-2272. [DOI] [PubMed] [Google Scholar]
- Mestecky, J., and McGhee, J. (1987). Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 40, 153-245. [DOI] [PubMed] [Google Scholar]
- Neuhaus, J.-M., Pietrzak, M., and Boller, T. (1994). Mutation analysis of the C-terminal vacuolar targeting peptide of tobacco chitinase: low specificity of the sorting system, and gradual transition between intracellular retention and secretion into the extracellular space. Plant J. 5, 45-54. [DOI] [PubMed] [Google Scholar]
- Nuttall, J., Vine, N., Hadlington, J., Drake, P., Frigerio, L., and Ma, J.K.-M. (2002). ER-resident chaperone interactions with recombinant antibodies in transgenic plants. Eur J. Biochem. 269, 6042-6051. [DOI] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bielli, A., de Virgilio, M., Frigerio, L., Pesca, M., Faoro, F., Bollini, R., Ceriotti, A., Vitale, A. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9, 1869-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson, B.A., Pimpl, P., daSilva, L.L., Crofts, A.J., Taylor, J.P., Movafeghi, A., Robinson, D.G., and Denecke, J. (2001). Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13, 2005-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe, L., and Higgins, T.J.V. (1998). Engineering plant protein composition for improved nutrition. Trends Plant Sci. 3, 282-286. [Google Scholar]
- Törmäkangas, K., Hadlington, J.L., Pimpl, P., Hillmer, S., Brandizzi, F., Teeri, T., and Denecke, J. (2001). A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 13, 2021-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka, K., Okamoto, T., and Minamikawa, T. (2000). Mass transport of proform of a KDEL-tailed cysteine protease (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J. Cell Biol. 148, 453-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Denecke, J. (1999). The endoplasmic reticulum— gateway of the secretory pathway. Plant Cell 11, 615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Raikhel, N.V. (1999). What do proteins need to reach different vacuoles? Trends Plant Sci. 4, 149-155. [DOI] [PubMed] [Google Scholar]