Abstract
OBJECTIVE
To review the evidence on vitamin D (VTD) insufficiency and deficiency from a Canadian perspective and to highlight some of the known and evolving implications of insufficiency or deficiency for health.
QUALITY OF EVIDENCE
PubMed was searched for articles on VTD insufficiency or deficiency and the role they play in various diseases and conditions. Level I and II evidence indicates that lack of VTD has a major role in short- and long-latency diseases.
MAIN MESSAGE
The long winters in Canada and lack of exposure to the sun contribute to lower levels of VTD among Canadians in late winter and spring. Currently recommended levels of fortification and supplementation are likely not high enough to restore adequate levels of VTD in the body. Repletion and maintenance therapy might be needed.
CONCLUSION
Many Canadians are at risk of VTD insufficiency or deficiency. Assessment of VTD status is important because optimal levels of VTD have been determined for various conditions. Low levels of VTD have negative implications for bone health and the health of other cell types.
RÉSUMÉ
OBJECTIF
Faire le point sur les données concernant l’insuffisance/ladéficience en vitamine D (VTD) d’un point de vue canadien et rappeler certaines des conséquences connues ou présentement à l’étude de ce problème pour la santé.
QUALITÉ DES PREUVES
On a consulté PubMed à la recherche d’articles sur l’insuffisance ou la déficience en VTD et sur leur rôle dans diverses maladies ou conditions. Des preuves de niveaux I et II indiquent qu’un manque de VTD joue un rôle important dans des maladies d’apparition rapide ou lente.
PRINCIPAL MESSAGE
Les longs hivers canadiens et le manque d’exposition au soleil contribuent à abaisser les niveaux de VTD chez les Canadiens en fin d’hiver et au printemps. Il est probable que les niveaux d’aliments enrichis ou de suppléments actuellement recommandés ne soient pas suffisants pour assurer des niveaux adéquats de VTD dans l’organisme. Il pourrait être nécessaire de restaurer et de maintenir les réserves.
CONCLUSION
Plusieurs Canadiens sont à risque d’insuffisance ou de déficience en VTD. L’évaluation du bilan de la VTD est important parce que des niveaux optimaux de VTD ont été établis pour diverses conditions. Les bas niveaux de VTD ont des effets négatifssur la santé de l’os et sur celle d’autres types de cellules.
This article reviews the guidelines for adequate intake of vitamin D (VTD), some of the basic physiology of VTD, and the relevance of the VTD receptor in some disease states. The definition of VTD status, and the prevalence, etiology, and treatment of inadequate status for various diseases will be discussed.
Quality of evidence
MEDLINE was searched using the words “vitamin D” and “vitamin D receptor” combined with “insufficiency,” “deficiency,” “osteoporosis,” “neuromuscular function,” “falls,” “cardiovascular disease,” “autoimmune disease,” “diabetes,” “cancer,” and “treatment.” Articles containing levels I, II, and III evidence were found. Original studies reviewed in this paper are listed in Table 1.1–53
Table 1.
Articles reviewed, number of subjects, design, outcomes, and comments
| STUDY | N | DESIGN | OUTCOME | COMMENTS |
|---|---|---|---|---|
| Krejs et al1 | 10 | Intestinal perfusion study before and after administration of VTD | Calcium and magnesium absorption increased 2%– 300% from baseline | None |
| Zittermann et al2 | 68: 34 controls, 34 patients with congestive heart failure | Case-control study | With lower VTD levels (P < .001), PTH levels (P < .001) and inflammatory markers (P < .001) were raised | Lower VTD levels were seen in patients with more severe congestive heart failure |
| Latham et al3 | 2496 | Systematic review | NS reduction in falls among patients receiving VTD | None |
| Chui et al4 | 126 | Univariate and multivariate regression analysis | Positive correlation of VTD levels with insulin sensitivity (P < .0001); negative effect on beta cell function (P < .0045) | Subjects with VTD deficiency are at higher risk of insulin resistance |
| Barger-Lux et al5 | 116 | Open-label treatment groups: 1000 IU VTD3, 10 000 IU VTD3, 50 000 IU VTD3 | Raised 25(OH)D levels by 29 nmol/L, 146 nmol/L, and 643 nmol/L, respectively | 8 weeks before steady state achieved |
| Chapuy et al6 | 1569 | Population prevalence study (cross-sectional study) of VTD and PTH levels | Parathyroid secretion initiated when serum 25(OH)D falls below 78 nmol/L | 14% of the population had wintertime levels <30 nmol/L |
| Moussavi et al7 | 318 | Population prevalence study (cross-sectional study) of VTD deficiency in Iran | 46.2% had levels <50 nmol/L (72.1% of women and 18.3% of men) | 95% of women had levels <80 nmol/L |
| Rucker et al8 | 188 | Population prevalence study (cross-sectional study) of VTD and PTH levels in western Canada | 97% of subjects had levels <80 nmol/L at some time during the year; levels were lower during fall, winter, and spring than during summer | 34% had levels <40 nmol/L sometime during the year; levels were taken 4 times yearly |
| Pasco et al9 | 3280 | Cross-sectional study of seasonal periodicity of serum VTD, PTH, and fractures in Australia | In winter, VTD levels were lower (P < .001) and falls were more likely to result in fractures (P < .001) | VTD levels of <28 nmol/L were found in 14% of subjects in winter |
| Lebrun et al10 | 160 | Cross-sectional study in Manitoba | 43% of children and 76% of mothers had levels <25 nmol/L | 70% of mothers drank no milk; 24% were intolerant of milk |
| Waiters et al11 | 121: 22 whites, 51 Inuit, 37 Native Canadians* | Cross-sectional study of mothers and newborns in Inuvik | Average 25(OH)D levels at time of delivery were 50.1 nmol/L in Natives and 59.8 nmol/L in non-Natives | Plasma levels of 25(OH)D in newborns averaged only 67% of levels in mothers |
| Vieth et al12 | 796 | Cross-sectional study in Toronto, Ont, of women aged 18–35 y | 21% of women reporting no consumption of VTD, 26% of women reporting <200 IU, and 20% reporting >200 IU of VTD were deficient (<40 nmol/L) during winter months | Recommended intake is too low to prevent VTD insufficiency and deficiency; deficiency could be determined only by laboratory tests, not by dietary history |
| Roth et al13 | 90 | Cross-sectional study in children presenting to a emergency department in Edmonton, Alta | 34% of patients had VTD levels <40 nmol/L, 6% had levels <25 nmol/L (deficiency) | Levels taken at end of winter |
| Thomas et al14 | 290 | Cross-sectional study in consecutive medical inpatients | 57% considered deficient in VTD (<37.5 nmol/L); 22% severely deficient (<20 nmol/L) | 37% of patients who consumed more than the recommended intake of VTD were deficient |
| Kauppinen- Makelin et al15 | 205: 106 inpatients, 99 outpatients | Cross-sectional study in consecutive medical inpatients and outpatients | 70% of female and 61% of male inpatients had levels <37.5 nmol/L, and 44% of female and 37% of male outpatients had levels <37.5 nmol/L | Inpatients were more deficient in VTD than outpatients |
| Hochwald et al16 | 296 | Cross-sectional study of consecutive medical inpatients in Israel | 26.27% of inpatients had levels <37.5 nmol/L | Even in a sunny country, >25% of patients were deficient in VTD |
| Lee et al17 | 53 | Analysis of dietary intake in Canadian long-term care | 70% of nursing-home patients consumed inadequate amounts of VTD through diet alone | Supplementation is necessary in these settings |
| Liu et al18 | 155 | Cross-sectional study in Toronto; prevalence and seasonal variation in long-term care | 9% of subjects had VTD levels <25 nmol/L in September; 18% had similar levels after the winter | <25 nmol/L is considered high risk for osteomalacia |
| Haney et al19 | 35 | Cross-sectional study in internal medicine residents | 74% had VTD levels <50 nmol/L in spring compared with 26% in fall | 69% of residents took in <400 IU/d of VTD |
| Holick et al20 | 1536 | Cross-sectional study of postmenopausal women in North America | Serum VTD was <50 nmol/L in 18%, <62.5 nmol/L in 36%, and <75 nmol/L in 52% of women | >50% of women taking osteoporosis therapy had inadequate VTD levels |
| Gaugris et al21 | 11 023 | Systematic review of VTD status in postmenopausal women with osteoporosis | 50%–70% of women with a fracture had VTD levels <37.5 nmol/L | High prevalence of low VTD levels in women with a history of fractures |
| Matsuoka et al22 | 40 | Randomized controlled trial | VTD levels lower in sunscreen users (40.2 nmol/L) than controls (91.3 nmol/L) (P <.001) | Lower 25(OH)D levels suggest lower VTD stores |
| Lo et al23 | 14: 7 healthy, 7 with fat malabsorption | Controlled trial. Intestinal absorption study before and after VTD radiolabeled | Absorption reduced from 60% in normal subjects to <18% (pancreatitis) in study subjects, 0% in those with bilary obstruction, and <50% in those with celiac disease | Various conditions involving malabsorption result in VTD insufficiency or deficiency |
| Jones et al24 | 209 | Double-blind, placebo-controlled study | 19% reduction in absorption of VTD in treated group | Unlikely to have substantial reduction with cutaneous production of VTD |
| Binet and Kooh25 | 17 | Case review in Toronto | Native people* and immigrants at risk of VTD deficiency | Rickets is still a public health issue |
| Bischoff-Ferrari et al26 | 19 114: 9294 in hip and other fracture trial, 9820 in non- vertebral fracture trials | Meta-analysis of randomized controlled trials of fracture prevention | RR 0.74 (95% CI 0.61–0.88); reduced hip fracture by 26%; RR 0.77 (95% CI 0.68–0.89); reduced nonvertebral fracture by 23% | 700–800 IU/d of VTD reduces risk of hip and nonvertebral fractures; 400 IU/d does not |
| Dawson-Hughes et al27 | 389 | Randomized, double-blind, placebo-controlled study | Prevalence of fractures in placebo group was 10% compared with 4% in treatment group (P = .02) | 500 mg of calcium and 700 IU of VTD reduced incidence of nonvertebral fractures |
| Chapuy et al28 | 583 | Multicentre, randomized, double- masked, placebo-controlled confirmatory study | Prevalence of fractures in placebo group was 11.1% compared with 6.9% in treatment group ( P = .07, NS) | 1200 mg of calcium and 800 IU of VTD reduced incidence of nonvertebral fractures |
| Porthouse et al29 | 3314 | Randomized controlled trial of primary prevention | No evidence that calcium and VTD reduced fractures in community-dwelling older women | Only 63% of subjects were taking the supplements at 12 mo (poor compliance); no baseline or follow-up VTD levels taken |
| Grant et al30 | 5292 | Randomized, placebo-controlled trial of secondary fracture prevention | No evidence for secondary prevention of fractures with use of VTD or combined VTD and calcium; baseline 25(OH)D level rose from 38 to 62.25 nmol/L in treatment group | Only 60% had compliance rates of >80% of tablets taken; only 60 patients had baseline and follow-up 25(OH)D levels taken |
| Dhesi et al31 | 139 | Randomized, double-blind, placebo-controlled study | With treatment, significant change in choice reaction time (P < .01), postural sway ( P < .02), and aggregate functional performance time (P < .05) | NS difference in falls; small trial |
| Bischoff-Ferrari et al32 | 1237, 5 trials reviewed | Meta-analysis of double-blind, randomized controlled trials | VTD reduced risk of falling by 22% | Number needed to treat was 15 to prevent 1 fall |
| Bischoff-Ferrari et al33 | 4100 | Cross-sectional, population-based survey | 2.5-m walk test (P = .001 for trend) and sit-to-stand test (P = .017 for trend); comparison of highest to lowest quartile 25(OH)D levels | In ambulatory patients, active or inactive concentrations of 40–94 nmol/L of 25(OH)D resulted in better lower- extremity musculoskeletal function |
| Sato et al34 | 96 | Randomized placebo-controlled trial | 1000 IU of VTD2 resulted in 59% reduction in falls (P = .049) in patients with long-standing stroke | VTD levels were deficient with 25(OH)D levels <25 nmol/L |
| Al Faraj and Al Mutairi35 | 341 | Cross-sectional interventional study | 299 (83% of total) with 25(OH)D levels <22.5 nmol/L and idiopathic back pain had a 100% improvement in symptoms when treated with 5000–10 000 IU of VTD until 25(OH)D levels were normal | In 299 patients, VTD levels were clearly deficient; very high doses were used for repletion therapy with no side effects |
| Al-Allaf et al36 | 87 | Case-control study | 25(HO)D levels <20 nmol/L were more common in fibromyalgia patients than in controls (P = .015) | Unclear whether low VTD levels are causative in fibromyalgia or result from the disease |
| Plotnikoff and Quigley37 | 150 | Cross-sectional population study | 93% of patients with persistent nonspecific musculoskeletal pain had 25(OH)D levels <30 nmol/L | Osteomalacia is a known cause of nonspecific musculoskeletal pain |
| Hyppönen et al38 | 10 821 | Study of children given 2000 IU of VTD supplements | Regular supplementation resulted in a 78% reduction in risk of developing type 1 diabetes later in life | A subset receiving supplementation with >2000 IU of VTD had an 86% RR39 |
| Pfiefer et al40 | 148 | Randomized placebo-controlled trial of blood-pressure therapy supplementing with VTD | 800 IU of VTD supplementation decreased systolic hypertension by 9.3% (P < .01) | Short-term study (8 weeks). No statistical benefit on diastolic blood pressure |
| Van den Berghe et al41 | 124 | Randomized controlled trial; comparison of 200 and 500 IU of VTD | C-reactive protein levels fell significantly in the group taking the higher dose (P <.05) | 25(HO)D levels were deficient and did not normalize with 200 IU of VTD |
| Forman et al42 | 216 313 | Summary of 3 large prospective cohort studies | Higher VTD intake was not associated with lower risk of incident hypertension | Patients followed up for 8 years |
| Garland et al43 | Unstated | Summary of 63 epidemiologic studies: 30 of colon cancer, 13 of breast cancer, 26 of prostate cancer, and 7 of ovarian cancer | 25(OH)D levels <75 nmol/L double the risk of those with levels >75 nmol/L; women in lowest quartile of VTD intake had 5 times the risk of developing breast cancer than those in highest quartile. In a study on prostate cancer (19 000 men), incidence was 70% higher among those with 25(OH)D levels <40 nmol/L than among those with levels >40 nmol/L | No studies showed an increase in cancer rates with VTD, but some showed no effect |
| Munger et al44 | 187 563 | Summary of 2 prospective cohort studies | Supplementation with =400 IU of VTD resulted in a 41% decrease in incidence of multiple sclerosis | Dietary intake of VTD resulted in a lower reduction of 33% |
| Merlino et al45 | 29 368 | Prospective cohort study | Supplementation with =400 IU of VTD resulted in a 36% decrease in incidence of rheumatoid arthritis | Dietary intake resulted in a slightly lower reduction of 28% |
| Berwick et al46 | 528 | Population-based study of cutaneous melanoma | Intermittent sun exposure was associated with increased survival in melanoma patients | Antiproliferative effect of VTD |
| Kennedy et al47 | 966 | Cohort case-control study | Painful sunburn early in life increased melanoma, squamous cell carcinoma, and especially actinic keratosis | Lifelong moderate sun exposure decreased risk of melanoma |
| Linday et al48 | 94 | Case-control study | Supplement with ~700 IU of VTD significantly decreased upper respiratory tract infections over time (P < .042) | Decreased need for antibiotics in control group; compliance was only 70% |
| Wayse et al49 | 150 | Case-control study | Low VTD levels were associated with increased risk of severe acute lower respiratory infection: 25(OH)D <22.5 nmol/L (P < .001) | Despite abundant sunlight, 25(OH)D levels were deficient |
| Krall et al50 | 145 | Randomized controlled trial using calcium and VTD supplements | 13% of patients taking supplements lost teeth compared with 27% of patients not taking supplements | VTD was not independently related to risk of losing teeth |
| Vieth et al51 | 64 | Randomized comparison control study; 4000 IU of VTD compared with 600 IU (current recommended intake); based on 1-tail Mann-Whitney well-being score, (P = .034) | No side effects of high dose of VTD other than improved mood | 6-mo trials |
| Vieth et al52 | 61 | Randomized comparison control study; 1000 vs 4000 IU of VTD supplementation for 3 mo | Average 25(OH)D levels were 68.7 nmol/L and 96.4 nmol/L, respectively, after 3 mo | NS changes in serum calcium and urinary calcium excretion in patients taking high doses |
| Aloia et al53 | 208 | Randomized controlled trial in 50- to 70-year-old African- American women | Only 60% of women treated with 2000 IU of VTD daily achieved normal 25(OH)D levels after a year | 87% compliance for 1 y |
25(OH)D—25-hydroxyvitamin D, CI—confidence interval, IU—international units, NS—nonsignificant, PTH—parathyroid hormone, RR—risk reduction, VTD—vitamin D.
Native is used to refer to the indigenous and aboriginal inhabitants of Canada and their descendants.
Past and current (1997) guidelines for adequate intake of vitamin D (VTD) are shown in Table 2.54–57 Some have recommended that new guidelines for breastfed infants and people with osteoporosis are needed. New guidelines might be forthcoming after review of new data relating to our understanding of VTD and its role in chronic diseases with long latency periods.58
Table 2.
Canadian recommendations for adequate intake of vitamin D: 1975 to 2007.
Background
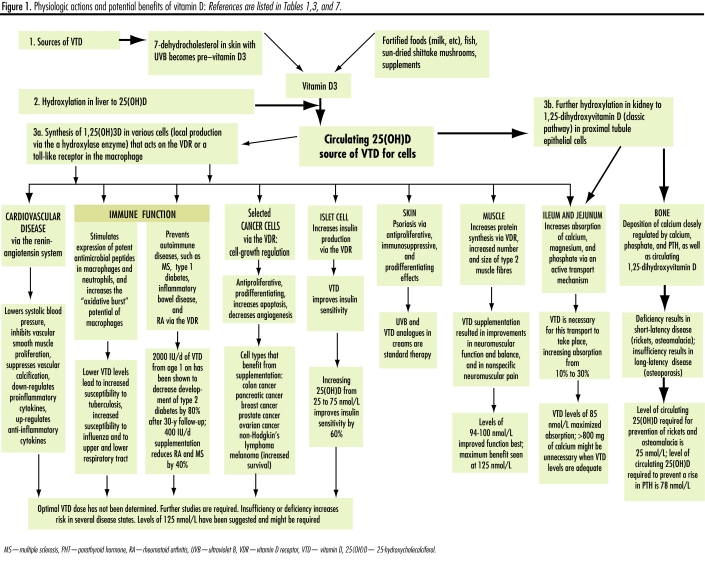
Vitamin D, a steroid hormone produced in the skin, has specific regulatory or functional effects on other parts of the body. Vitamin D is hydroxylated in the liver to 25-hydroxyvitamin D (25[OH]D) and further hydroxylated in the kidney to 1,25-dihydroxyvitamin D. Hydroxylation in the kidney is regulated closely by parathyroid hormone (PTH), hypocalcemia, and hypophosphatemia and is inhibited by 1,25-dihydroxyvitamin D.59 As well, 1,25-dihydroxyvitamin D (produced locally within cells) regulates gene transcription through nuclear high-affinity VTD receptors.60 These receptors are found in the classic target organs: gut, bone, kidney, and parathyroid61 and many other tissues as well, such as brain, breast, colon, heart, pancreas, prostate, skin, and immune system. Vitamin D regulates cell growth and maturation, inhibits renin production, stimulates insulin secretion, and modulates the function of activated T- and B-lymphocytes and macrophages62,63 (Table 3,1,2,4,33,59,62,64–82 Figure 1).
Table 3.
Studies of functions of vitamin D
| ORGAN OR SYSTEM | EFFECT OF SUFFICIENT VITAMIN D | EFFECT OF INSUFFICIENT OR DEFICIENT LEVELS OF VITAMIN D | OPTIMAL VITAMIN D INTAKE FOR HEALTH |
|---|---|---|---|
| Jejunum and ileum | Increases absorption of calcium and magnesium to 30%1 | Absorption of calcium and magnesium reduced to 10% | 85 nmol/L allows maximum absorption64,65; with adequate VTD levels, >800 mg of calcium might be unnecessary66 |
| Bone | Maintains calcium and phosphate homeostasis and is required for proper mineralization59 | Rickets or osteomalacia;62 short- latency disease | Rickets and osteomalacia are prevented when VTD levels are >25 nmol/L67 |
| Parathyroid | Regulates calcium and phosphate levels, controls conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D59 | Parathyroid hormone excretion increases as levels of VTD decrease resulting in secondary hyperparathyroidism, which in turn results in resorption of calcium from bone and exacerbates osteoporosis | Parathyroid hormone levels are dramatically suppressed when VTD levels are maintained at >50 nmol/L67; levels begin to rise when 25-hydroxyvitamin D levels fall <78 nmol/L |
| Cardiovascular system via VDR | Inhibition of vascular smooth-muscle proliferation; suppression of vascular calcification; down-regulation of pro- inflammatory cytokines; up-regulation of anti-inflammatory cytokines. VTD acts as a negative endocrine regulator of the renin-angiotensin system68 | Might contribute to congestive heart failure2,69; deficiency results in loss of calciotropic effect in long- latency disease | Currently unknown, but 2000–4000 IU of vitamin D3 are being suggested70 |
| Muscle via VDR | Modulates calcium transport, protein synthesis, and kinetics of muscle contraction71 | Muscle weakness, limb pain, and impaired physical function72; loss of calciotropic effect | Maximum neuromuscular performance achieved with VTD levels of 125 nmol/L33 |
| Skin via VDR | Production of calcitrol that regulates cellular function in keratocytes | Antiproliferative, immunosuppressive, and prodifferentiating effects | VTD analogues are used for psoriasis73 |
| Islet cells via VDR | Improvement in insulin sensitivity4 | Negative effect on beta cell function with reduced insulin secretion; loss of immune modulatory effect | Raising VTD levels from 25 to 75 nmol/L improves sensitivity by 60%; optimal level has not been determined |
| Certain cancer cell types mediated via VDR | Suppressed growth and increased apoptosis74,75; stabilized chromosomal structure and prevented DNA breakdown76 | Loss of antiproliferative effect | Optimal level undetermined |
| Immune system modulator | Stimulated expression of potent anti- microbial peptides, increased “oxidative burst” potential of macrophages77 | Increased susceptibility to influenza77 and tuberculosis78 | Optimal level undetermined; summer levels of 125 nmol/L likely required77 |
| Innate immune function | Increased production of cathelicidins effective against Escherichia coli, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Candida | Decreased wound barrier function | Optimal dose undetermined79–82 |
VTD—vitamin D, VDR—vitamin D receptor.
Figure 1.
Physiologic actions and potential benefits of vitamin D: References are listed in Tables 1,3, and 7.
Assessing VTD status
The major circulating metabolite of VTD is serum 25(OH)D, which has a half-life of between 10 and 19 days.5 It is the best indicator of VTD status and reflects levels from dietary intake and synthesis in the skin.83 Levels <25 nmol/L are generally considered deficient; levels <80 nmol/L are considered insufficient.84 There is some concern about the reliability and consistency of serum 25(OH)D laboratory results,85 although there has been some improvement in the quality of tests in the past few years. The 2 main assays commercially available are listed in Table 4.86,87 Liquid chromatography, which is the criterion standard, is not readily available.
Table 4.
Assessment of vitamin D levels
| ASSAY | MEASUREMENT | COMMENTS |
|---|---|---|
| Radioimmunoassay | Uses antibodies that recognize both 25(OH)D3 and 25(OH)D2 | Most commonly used assay; coefficient of variability in assay is 12%–18% in normal range of VTD (85–147.5 nmol/L) and 10%-25% in lower range of VTD (20–62.5 nmol/L)86 |
| Competitive binding protein assay | Reagent separates VTD from binding proteins | Often yields values about 30% higher (nonspecific) but might not detect 25(OH)D287 |
25(OH)D3—25-hydroxyvitamin D3, 25(OH)D2—25-hydroxyvitamin D2, VTD—vitamin D.
Prevalence of VTD insufficiency or deficiency
Globally, VTD insufficiency or deficiency has been noted in many countries, from high school students in Iran7 to healthy western Canadians.8 Substantial seasonal variability has been noted in both Canada and Australia above and below the 37th parallel, respectively, with up to 97% of Canadians having inadequate levels of VTD at some time during the winter or spring.8,9
Mothers and infants among native Canadian Cree in Manitoba have been found to be severely deficient in VTD, even in midsummer.10 In Inuvik, 48% of Inuit mothers were found to be deficient in VTD despite supplementation.11 Seasonal variations were found in a Canadian study of healthy women in Toronto, Ont, and supplementation with 400 IU of VTD did not prevent insufficiency in the winter.12 A study in Edmonton, Alta, showed that children and adolescents had low levels of VTD.13,88
Three studies in the United States, Finland, and Israel found that inpatients had insufficient or deficient levels of VTD.14–16 Only 30% of patients in 3 Canadian long-term care facilities got adequate amounts of VTD through diet alone.17 Long-term care residents in Toronto had VTD deficiency that increased from 9% in the fall to 18% in the spring.18 Not only are inpatients at risk, but internal medical residents who work long hours indoors are also.19 A global study of VTD in postmenopausal women with osteoporosis showed that levels were deficient in 28.4% of them. There was no significant difference in levels among community-dwelling people and nursing-home patients. Deficiency increases with age; about 50% of those aged 70 and 80% of those aged 90 are deficient.89 In a study of North American postmenopausal women, all taking an agent to treat or prevent osteoporosis and 59% taking =400 IU of VTD daily, 18% had levels below 50 nmol/L, and 52% had levels below 75 nmol/L. Despite supplementation, about 50% of women have suboptimal VTD levels.20 A systematic review of 30 articles written in the past 10 years on VTD inadequacy in menopausal women supports these findings.21
Etiology of VTD deficiency and insufficiency
The risk factors that contribute to low levels of VTD are numerous and are summarized in Table 5.10,22–24,90–110
Table 5.
Risk factors for low serum vitamin D levels
| RISK FACTORS | REASON |
|---|---|
| Inadequate exposure to the sun | |
| • Skin type | - Dark skin requires up to 5 times the length of exposure because of melanin content |
| • Season, latitude, angle of the sun | - People living at latitudes higher than the 37th parallel cannot get adequate amounts of UVB from the sun during winter months |
| • Use of sunscreen22,90 | - Continuous use of sunscreen with greater than factor 8 UVB protection22; controversial because of risk of skin cancer, but UVB decreases risk of internal cancer |
| • Time of day | - Ultraviolet B is at its maximum from 10:00 AM to 2:00 PM91; exposure to 1 minimal erythemal dose* in a swimsuit can provide the equivalent of 10 000 IU of VTD92 |
| • Covering the skin | For various religious or cultural reasons |
| Inadequate dietary intake | Limited intake of foods rich in VTD, such as oily fish and fish-liver oil, low intake of fortified foods or no use of supplements; strict vegans and non-milk drinkers are at higher risk93 |
| Obesity | Irreversible sequestration of VTD in the fat pool, especially if body mass index is >30 and person does little outdoor activity94 |
| Exclusive breastfeeding | Breast milk is low in VTD10,95; supplementing with 4000 IU of VTD has been shown to achieve adequate levels in both mother and child96 |
| Pregnancy | Adequate maternal VTD levels are required to ensure fetal bone health and general health of mother and child97–100 |
| Age | |
| • Decreased production of VTD through the skin | - A 70-year-old person’s skin can synthesize only 25% as much VTD as a young person’s101–102; conversion of 7-dehydrocholesterol in aging skin is considerably lessened103 |
| • Age-related lactose intolerance | - Reduced intake of fortified milk |
| • Immobility | - More time housebound or in hospital; many are institutionalized |
| • Aging kidneys | - Decreased renal conversion of VTD |
| Comorbid conditions | Malabsorption syndromes, such as Crohn disease, Whipple disease, cystic fibrosis, and sprue, as well as severe liver disease23 |
| Drug interactions | |
| • Drugs that impair VTD activation or increase its clearance | - Phenytoin, carbamazepine, rifampin, cimetidine, thiazides104–106; lithium raises parathyroid hormone levels and lowers levels of the active hormone 1,25-dihydroxyvitamin D107 |
| • Drugs that impair VTD absorption | - Mineral oil laxatives or fat substitutes, such as Olestra24; obesity management medications, such as orlistat108; or bile-acid sequestrants, such as cholestyramine and colestipol109 |
| Variations in metabolism of VTD | Some Indo-Asians have increased 24-hydroxylase activity that results in low serum levels of 25-hydroxyvitamin D110 |
IU—international units, UVB—ultraviolet B, VTD—vitamin D.
The amount of sunlight to which a person can be exposed before the skin begins to turn slightly red. Minimal erythemal dose varies from person to person depending on skin type.
Classic effects of VTD insufficiency or deficiency on disease
Vitamin D deficiency causes rickets in children and osteomalacia in adults. Rickets cases are still being reported in Canada.25,111 Osteomalacia also still occurs, but its symptoms are much less specific and are easily missed.112 Vitamin D is used to treat osteoporosis, but studies using calcium and 400 IU of VTD showed little effect on fractures. Most but not all studies using calcium and 700 to 800 IU of VTD did show a reduction in fractures.26–28,113 No benefit was seen from 1000 mg of calcium and 800 IU of VTD in a primary prevention trial29 and a secondary prevention trial.30,114 Compliance was poor in both studies, and only 63% of patients were still taking treatment after 12 months in the former study, and only 1.1% of patients had baseline VTD levels taken in the latter study.
Supplementing with 400 IU of VTD for 8 weeks raised the measured 25(OH)D level by a mere 11 nmol/L in healthy men.5 To date, no studies have ensured that all subjects in treatment groups consistently had VTD levels >78 nmol/L. There is still great controversy over the benefit of VTD in fracture control.
Effects of insufficiency or deficiency on other disease states
Neuromuscular effects
Vitamin D acts on the VTD receptor in skeletal muscle cells by binding to the nuclear receptor and also to a cell membrane receptor, which results in numerous physiologic actions.71 Severe VTD deficiency is associated with muscle weakness, limb pain, and impaired physical function.3,31,115 A meta-analysis looking at ambulatory and institutionalized older patients found a reduction in falls of more than 20% with use of VTD. This effect was independent of calcium supplementation.32 In the most current multidose study of institutionalized older patients, supplementation with 800 IU of VTD resulted in a 72% reduction in falls.116 Another review found no such association.3 There is also evidence that idiopathic low back pain in patients with VTD deficiency markedly improves when VTD levels are restored.35 Low levels of VTD are also common in patients with fibromyalgia and chronic refractory nonspecific musculoskeletal pain.36,37,117
Type 1 and 2 diabetes
A prospective study (begun in 1966) using 2000 IU of VTD in children resulted in an 80% reduction in development of type 1 diabetes during the next 30 years.38 Studies using 400 IU of VTD early in life did not show a protective effect, and higher doses are being suggested.39,118 Increasing VTD levels from 25 to 75 nmol/L results in a 60% improvement in insulin sensitivity.4,119 Low VTD levels were also shown to have a negative effect on beta cell function.120 The improvement in insulin sensitivity was greater with VTD than improvement seen with either troglitzone (54%) or metformin (13%).4,121
Multiple sclerosis and rheumatoid arthritis
Living at higher than 37° latitude increases the risk of developing multiple sclerosis by >100%. Taking a multivitamin with 400 IU of VTD reduces the risk by 40%.44,122 Women taking a multivitamin with 400 IU of VTD reduced their risk of developing rheumatoid arthritis by 40%.45,122
Cardiovascular disease
Increased VTD levels suppress renin expression and renin levels and thus result in down-regulation of the renin-angiotensin system in animals.123 Several mechanisms have been suggested for VTD’s protective role in cardiovascular disease.68
Supplementation with calcium and VTD results in a substantial 9.3% decrease in systolic blood pressure and a 5.4% decrease in heart rate.40 Supplementing with VTD substantially reduces C-reactive protein levels in critically ill patients.41 Low VTD levels might contribute to congestive heart failure.2 In 3 large prospective cohort studies, however, higher intake of VTD was not associated with lower risk of hypertension.42 Clinical trials are needed to evaluate whether the morbidity and mortality associated with cardiovascular disease are reduced by optimal intake of oral VTD.
Cancer
Evidence from 63 observational studies indicates that inadequate VTD levels are a risk factor for certain types of cancer, such as breast, colon, ovarian, and prostate cancer.43,124,125 Vitamin D and VTD analogues can induce cell death in some cancer cell lines.74,75 Exposure to the sun might increase risk of skin cancer, but VTD has been shown to suppress growth and increase apop-tosis in melanoma cells.126 The risks and benefits of sun exposure are a topic of hot debate at this time.46,127,128
Psoriasis
Vitamin D analogues are used for psoriasis along with ultraviolet-B light. Treatment is successful because of the antiproliferative, immunosuppressive, and prodifferentiating effects of VTD.73,129
Sources of VTD
The best way to increase VTD levels is to expose the skin to the sun. This has never been known to cause toxicity because of self-regulatory factors in the skin. Other sources of VTD are listed in Table 6.47,91,130,131
Table 6.
Sources of vitamin D
| SOURCE | RISKS AND BENEFITS |
|---|---|
| Sun | Exposure has never been known to cause toxicity; however, risk of skin cancer increases with exposure47 |
| Oily fish or fish oils | High levels of vitamin A in fish oils (cod, halibut); sometimes high levels of mercury and other toxins (dioxins) are found in fish130,131 |
| Fortified foods, such as milk, soya milk, or rice milk (in some countries); cereal; orange juice | Lactose intolerance limits consumption of milk for some people; celiac disease limits consumption of cereal for some people |
| Shittake mushrooms (sun-dried)91 | Beneficial for those on a strict vegan diet |
| Supplements | Inexpensive (<5¢/d for 2000 international units of vitamin D3); vitamin D2 is ergocalciferol; vitamin D3 is cholecal-ciferol, which is 1.7 times as potent as ergocalciferol |
Treatment of VTD insufficiency and deficiency
The beneficial effects of VTD on various diseases are listed in Table 7.4,35,38,44,45,48–50,73,77,78,98,111,112,117,132–145 The question is, how can one vitamin influence so many disorders in a positive way? Just as abnormal levels of thyroid hormone can affect many cell systems, abnormal levels of VTD, a hormone, appear to affect many cell systems. Our understanding of the non-bone effects has greatly increased in the last 10 years.
Table 7.
Benefits of vitamin D for various diseases, dosages, and comments
| DISEASE | DOSE OF VITAMIN D USED OR CHANGE IN LEVEL OF VITAMIN D | RISK REDUCTION OR IMPROVEMENT | COMMENTS |
|---|---|---|---|
| Rickets111 | Requires repletion therapy when diagnosed; usually prevented with VTD levels >25 nmol/L | Complete resolution of symptoms and signs (except in cases of vitamin D resistance132) | Adequate intake of calcium also needed |
| Osteomalacia112 | 800 IU required; patients might need up to 2200 IU for up to a year | Resolution of symptoms, including bone pain, especially in pelvis, lumbar spine, and ribs | |
| Psoriasis | Topical VTD creams | Plaque thickness and redness markedly improved by UVB and VTD analogues | First-line therapy worldwide73 |
| Multiple sclerosis44 | 400 IU/d | 40% risk reduction | |
| Rheumatoid arthritis45 | 400 IU/d | 40% risk reduction | |
| Type 1 diabetes38 | 2000 IU/d | 80% risk reduction | |
| Type 2 diabetes4 | VTD level raised from 25 to 75 nmol/L | 63% improvement in insulin sensitivity | |
| Gestational diabetes and hypertension during pregnancy98 | Individualized dosing to restore levels to >80 nmol/L | Marked improvement in insulin sensitivity and insulin production | |
| Birth weight133 | For each IU/d of VTD intake, birth weight increased | Birth weight increased by 11 g/IU of VTD | |
| Osteogenesis imperfecta | 6–8 IU/kg daily | Correction of deficiency status | Recommendation of the Kennedy Krieger Osteogenesis Imperfecta Clinic |
| Polycystic ovary disease134 | 50 000 IU of VTD weekly or biweekly | Normalized menstrual cycles in >50% of patients | Very small study |
| Premenstrual syndrome135 | 700 IU/d | 40% reduction in risk of having symptoms | Increased dietary calcium is known to decrease symptoms135 |
| Colon cancer136–138 | To achieve levels of 65-100 nmol/L | 40%–80% risk reduction with supplement; rectal cancer reduced by 48%; exposure to sunlight reduced risk by 38%137 | Increased dietary calcium is known to decrease risk, but benefit for >700 mg/d is minimal139 |
| Cancer of the prostate140 | Serum level of 25(OH)D =40– <60 nmol/L | 50% risk reduction125 | 1 study suggests >80 nmol/L might increase risk141 |
| Cancer of the pancreas142 | 300–450 IU/d compared with 150 IU/d | 43% risk reduction 22% risk reduction | Higher doses gave no further protection142 |
| Cancer of the breast | >50 nmol/L compared with 50 nmol/L | 50%–70% risk reduction143 | Sun exposure reduces mortality144 |
| Cancer of the ovary144,145 | Exposure to sunlight | 16% risk reduction; risk is 5 times higher among those living farther north in the United States | Despite these studies, more information is needed |
| Upper respiratory tract infections48 | 600–700 IU given as cod-liver oil | 50% risk reduction | Also given selenium and omega-3 fatty acids |
| Lower respiratory tract infections49 | Children with levels <25 nmol/L | 11 times more likely to be infected | |
| Seasonal influenza77 | Levels as high as 125 nmol/L have been suggested | Immune function improved in various immune cells | Clinical trials needed |
| Mycobacterium tuberculosis78 | To restore levels to normal physiologic levels, >100 nmol/L are suggested | Increased production of macrophages’ antimicrobial peptide cathelicidin kills Mycobacterium tuberculosis | Clinical trials needed |
| Idiopathic back pain35 | Restoring levels from <25–>80 nmol/L | 100% of deficient patients had pain resolve using 5000 IU/d of VTD | 340 patients (85%) had deficient levels of 25(OH)D |
| Nonspecific chronic musculoskeletal pain117 | Restoring levels from 21 nmol/L to normal levels | 67% of patients had complete resolution of symptoms | Diagnosis prior to VTD deficiency was somatization |
| Reduced tooth loss in the elderly | 400–600 IU of VTD and 1000 mg of calcium | 50% improvement in tooth retention over 2 y | Effect of VTD not assessed independently50 |
25(OH)D—25-hydroxyergocalciferol, IU—international units, UVB—ultraviolet B, VTD—vitamin D.
To maintain a healthy blood level of 25(OH)D (80 to 100 nmol/L), most healthy patients require at least 1000 IU of VTD each day if they do not get exposure to the sun.63,146 Topping up to adequate levels quickly is the goal. Recommended repletion therapy consists of 50 000 IU of vitamin D2 weekly for 8 weeks or 2000 IU of vitamin D3 daily for 8 weeks.147 Doses of 4000 IU of vitamin D3 have been used safely for several months, and there is evidence that doses up to 2000 IU/d can be considered safely (Table 851–53,148–153).52
Table 8. Source and dose of vitamin D, side effects, and potential toxicity:
Reported side effects of vitamin D include nausea, vomiting, headache, metallic taste, vascular or nephrocalcinosis, and pancreatitis. Reported contraindications to vitamin D include hypercalcemia in sarcoidosis; metastatic bone disease148; other granulomatous diseases, such as tuberculosis and Crohn disease (active phase) that have disordered vitamin D metabolism in activated macrophages149; and Williams syndrome150 (infantile hypercalcemia).
| SOURCE AND DOSE OF VITAMIN D* | SIDE EFFECT OR TOXICITY | COMMENTS |
|---|---|---|
| Maximum sun exposure | No known vitamin D toxicity, but too much exposure to UVB (burns) results in increased risk of skin cancer | 10 000 IU (oral equivalent easily achieved with full-body exposure and results in levels of 148–163 nmol/L); in lifeguards exposed to the sun, kidney stones are more common151 |
| About 10 to 15 min of sun exposure of hands and arms midday when sun is overhead needed to achieve daily requirement (about 400 IU) | No known side effects; too much exposure to UVB (burns) results in increased risk of skin cancer | Dark skin requires 4 times as much sun exposure to get the same dose |
| Use of 2000 IU in African Americans (after 1 y) | No known side effects | Failed to achieve a level of 80 nmol/L in 40% of patients53 |
| Use of 4000 IU for 6 mo | Improved mood the only side effect noted | Average level of 25-hydroxyvitamin D was 110 nmol/L,51 a level seen with adequate sun exposure; no increase in serum calcium noted |
| 4000 IU for 3 mo | No notable side effects52 | |
| Use of vitamin D2 (synthetic analogue) | Several metabolites with unknown side effects | Toxicity reported using higher levels152,153 |
UVB—ultraviolet B.
Vitamin D3 unless specified.
Conclusion
Low levels of VTD are considered a major public health problem in Canada, especially during the winter. Those with risk factors should be screened for low 25(OH)D levels and repletion therapy instituted if needed. Researchers have estimated that the oral dose of vitamin D3 to attain and maintain 25(OH)D levels >80 nmol/L is 2200 IU/d if baseline levels are 20 to 40 nmol/L, 1800 IU/d if levels are 40 to 60 nmol/L, and 1160 IU/d if levels are between 60 and 80 nmol/L.64
We need to ensure that patients have healthy blood levels of 25(OH)D to prevent levels of parathyroid hormone from rising and to maximize absorption of calcium, magnesium, and phosphate. Positive effects on bone are marginal at best unless patients consume at least 800 IU/d of VTD. The emerging and exciting role of the VTD receptor and the actions of VTD in maintaining health in other cell types have become more apparent during the last decade.
EDITOR’S KEY POINTS
Inadequate levels of vitamin D (VTD) have classically been associated with bone disorders, such as rickets, osteomalacia, and osteoporosis.
New research has demonstrated that VTD receptors are present throughout the body and that VTD has much broader effects than previously believed.
Current recommendations for VTD supplementation might be inadequate to ensure appropriate blood levels of VTD.
POINTS DE REPèRE DU RÉDACTEUR
Les niveaux inadéquats de vitamine D (VTD) ont généralement été associés à des anomalies osseuses comme le rachitisme, l’ostéomalacie et l’ostéoporose.
Les études récentes ont montré qu’il y a des récepteurs de VTD un peu partout dans l’organisme et que la VTD a des effets beaucoup plus étendus qu’on ne le croyait auparavant.
Les recommandations actuelles sur les suppléments de VTD pourraient donc ne pas assurer des niveaux sanguins adéquats de VTD.
Footnotes
This article has been peer reviewed.
Competing interests
None declared
References
- 1.Krejs GJ, Nicar MJ, Zerwekh JE, Norman DA, Kane MG, Pak CY. Effect of 1,25-dihydroxyvitamin D3 on calcium and magnesium absorption in the healthy human jejunum and ileum. Am J Med. 1983;75(6):973–6. doi: 10.1016/0002-9343(83)90877-x. [DOI] [PubMed] [Google Scholar]
- 2.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(1):105–12. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 3.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51(9):1219–26. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 4.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 5.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–30. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 6.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 7.Moussavi M, Heidarpour R, Aminorraya A, Pournaghshband Z, Amini M. Prevalence of vitamin D deficiency in Isfahani high school students in 2004. Horm Res. 2005;64(3):144–8. doi: 10.1159/000088588. [DOI] [PubMed] [Google Scholar]
- 8.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ. 2002;166(12):1517–24. [PMC free article] [PubMed] [Google Scholar]
- 9.Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR, et al. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res. 2004;19(5):752–8. doi: 10.1359/JBMR.040125. Epub 2004 Jan 19. [DOI] [PubMed] [Google Scholar]
- 10.Lebrun JB, Moffatt ME, Mundy RJ, Sangster RK, Postl BD, Dooley JP, et al. Vitamin D deficiency in a Manitoba community. Can J Public Health. 1993;84(6):394–6. [PubMed] [Google Scholar]
- 11.Waiters B, Godel JC, Basu TK. Perinatal vitamin D and calcium status of northern Canadian mothers and their newborn infants. J Am Coll Nutr. 1999;18(2):122–6. doi: 10.1080/07315724.1999.10718839. [DOI] [PubMed] [Google Scholar]
- 12.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55(12):1091–7. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 13.Roth DE, Martz P, Yeo R, Prosser C, Bell M, Jones AB. Are national vitamin D guidelines sufficient to maintain adequate blood levels in children? Can J Public Health. 2005;96(6):443–9. doi: 10.1007/BF03405185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 15.Kauppinen-Makelin R, Tahtela R, Loyttyniemi E, Karkkainen J, Valimaki MJ. A high prevalence of hypovitaminosis D in Finnish medical in- and outpatients. J Intern Med. 2001;249(6):559–63. doi: 10.1046/j.1365-2796.2001.00847.x. [DOI] [PubMed] [Google Scholar]
- 16.Hochwald O, Harman-Boehm I, Castel H. Hypovitaminosis D among inpatients in a sunny country. Isr Med Assoc J. 2004;6(2):82–7. [PubMed] [Google Scholar]
- 17.Lee LT, Drake WM, Kendler DL. Intake of calcium and vitamin D in 3 Canadian long-term care facilities. J Am Diet Assoc. 2002;102(2):244–7. doi: 10.1016/s0002-8223(02)90057-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu BA, Gordon M, Labranche JM, Murray TM, Vieth R, Shear NH. Seasonal prevalence of vitamin D deficiency in institutionalized older adults. J Am Geriatr Soc. 1997;45(5):598–603. doi: 10.1111/j.1532-5415.1997.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 19.Haney EM, Stadler D, Bliziotes MM. Vitamin D insufficiency in internal medicine residents. Calcif Tissue Int. 2005;76(1):11–6. doi: 10.1007/s00223-004-0025-0. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 21.Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS. Vitamin D inadequacy among post-menopausal women: a systematic review. QJM. 2005;98(9):667–76. doi: 10.1093/qjmed/hci096. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch Dermatol. 1988;124(12):1802–4. [PubMed] [Google Scholar]
- 23.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644–9. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 24.Jones DY, Miller KW, Koonsvitsky BP, Ebert ML, Lin PY, Jones MB, et al. Serum 25-hydroxyvitamin D concentrations of free-living subjects consuming Olestra. Am J Clin Nutr. 1991;53(5):1281–7. doi: 10.1093/ajcn/53.5.1281. [DOI] [PubMed] [Google Scholar]
- 25.Binet A, Kooh SW. Persistence of Vitamin D-deficiency rickets in Toronto in the 1990s. Can J Public Health. 1996;87(4):227–30. [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 27.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–6. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 28.Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–64. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 29.Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–8. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 31.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33(6):589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20(3):187–92. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 35.Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine. 2003;28(2):177–9. doi: 10.1097/00007632-200301150-00015. [DOI] [PubMed] [Google Scholar]
- 36.Al-Allaf AW, Mole PA, Paterson CR, Pullar T. Bone health in patients with fibro-myalgia. Rheumatology (Oxford) 2003;42(10):1202–6. doi: 10.1093/rheumatology/keg356. [DOI] [PubMed] [Google Scholar]
- 37.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 38.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 39.Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr. 2005;135(2):323–5. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 40.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 41.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88(10):4623–32. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- 42.Forman JP, Bischoff-Ferrari HA, Willett WC, Stampfer MJ, Curhan GC. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension. 2005;46(4):676–82. doi: 10.1161/01.HYP.0000182662.82666.37. [DOI] [PubMed] [Google Scholar]
- 43.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 45.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 46.Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberle C, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97(3):195–9. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120(6):1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 48.Linday LA, Shindledecker RD, Tapia-Mendoza J, Dolitsky JN. Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites. Ann Otol Rhinol Laryngol. 2004;113(11):891–901. doi: 10.1177/000348940411301108. [DOI] [PubMed] [Google Scholar]
- 49.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58(4):563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 50.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111(6):452–6. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 51.Vieth R, Kimball S, Hu A, Walfish PG. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–94. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 53.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165(14):1618–23. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Committee for the revision of Dietary Standards for Canada. Dietary standard for Canada. Ottawa, Ont: Canadian Publishing Centre, Supply and Services Canada; 1976. [Google Scholar]
- 55.Committee for the revision of Dietary Standards for Canada. Dietary standard for Canada. Recommended intakes for Canadians. Ottawa, Ont: Canadian Publishing Centre, Supply and Services Canada; 1983. [Google Scholar]
- 56.Scientific Review Committee. Nutrition recommendations. Ottawa, Ont: Canadian Government Publishing Centre, Supply and Services Canada; 1990. [Google Scholar]
- 57.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- 58.Whiting SJ, Calvo MS. Dietary recommendations for vitamin D: a critical need for functional end points to establish an estimated average requirement. J Nutr. 2005;135(2):304–9. doi: 10.1093/jn/135.2.304. [DOI] [PubMed] [Google Scholar]
- 59.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 60.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 61.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihy-droxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206(4423):1188–90. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 62.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 63.Holick MF. The influence of vitamin D on bone health across the life cycle. J Nutr. 2005;135(11):2726S–7S. doi: 10.1093/jn/135.11.2726S. [DOI] [PubMed] [Google Scholar]
- 64.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97(1–2):13–9. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 66.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294(18):2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 67.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86(3):1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 68.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94(4):483–92. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 69.Vieth R, Kimball S. Vitamin D in congestive heart failure. Am J Clin Nutr. 2006;83(4):731–2. doi: 10.1093/ajcn/83.4.731. [DOI] [PubMed] [Google Scholar]
- 70.Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev. 2006;11(1):25–33. doi: 10.1007/s10741-006-9190-8. [DOI] [PubMed] [Google Scholar]
- 71.Pedrosa MA, Castro ML. Role of vitamin D in the neuro-muscular function. Arq Bras Endocrinol Metabol. 2005;49(4):495–502. doi: 10.1590/s0004-27302005000400005. [DOI] [PubMed] [Google Scholar]
- 72.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424–30. doi: 10.1001/archinte.166.4.424. [DOI] [PubMed] [Google Scholar]
- 73.Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(Suppl 4):11–5. doi: 10.1111/j.1600-0625.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 74.Sergeev IN. Calcium signaling in cancer and vitamin D. J Steroid Biochem Mol Biol. 2005;97(1–2):145–51. doi: 10.1016/j.jsbmb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Elias J, Marian B, Edling C, Lachmann B, Noe CR, Rolf SH. Induction of apop-tosis by vitamin D metabolites and analogs in a glioma cell line. Recent Results Cancer Res. 2003;164:319–32. doi: 10.1007/978-3-642-55580-0_22. [DOI] [PubMed] [Google Scholar]
- 76.Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;475(1–2):69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 77.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D–mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 79.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 80.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komatsuzawa H, Ouhara K, Yamada S, Fujiwara T, Sayama K, Hashimoto K, et al. Innate defences against methicillin-resistant Staphylococcus aureus (MRSA) infection. J Pathol. 2006;208(2):249–60. doi: 10.1002/path.1898. [DOI] [PubMed] [Google Scholar]
- 82.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125(1):108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 83.Hollis BW. Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it. Calcif Tissue Int. 1996;58(1):4–5. doi: 10.1007/BF02509538. [DOI] [PubMed] [Google Scholar]
- 84.Hanley DA, Davison KS. Vitamin D insufficiency in North America. J Nutr. 2005;135(2):332–7. doi: 10.1093/jn/135.2.332. [DOI] [PubMed] [Google Scholar]
- 85.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–7. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 86.Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90(2):635–40. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 87.Hollis BW. Comparison of commercially available (125)I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem. 2000;46(10):1657–61. [PubMed] [Google Scholar]
- 88.Harkness LS, Bonny AE. Calcium and vitamin D status in the adolescent: key roles for bone, body weight, glucose tolerance, and estrogen biosynthesis. J Pediatr Adolesc Gynecol. 2005;18(5):305–11. doi: 10.1016/j.jpag.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Reginster JY. The high prevalence of inadequate serum vitamin D levels and implications for bone health. Curr Med Res Opin. 2005;21(4):579–86. doi: 10.1185/030079905X41435. [DOI] [PubMed] [Google Scholar]
- 90.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 91.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–48S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 92.Lehmann B. The vitamin D3 pathway in human skin and its role for regulation of biological processes. Photochem Photobiol. 2005;81(6):1246–51. doi: 10.1562/2005-02-02-IR-430. [DOI] [PubMed] [Google Scholar]
- 93.Lamberg-Allardt C, Karkkainen M, Seppanen R, Bistrom H. Low serum 25-hydroxyvitamin D concentrations and secondary hyperparathyroidism in middle-aged white strict vegetarians. Am J Clin Nutr. 1993;58(5):684–9. doi: 10.1093/ajcn/58.5.684. [DOI] [PubMed] [Google Scholar]
- 94.Holick MF. Vitamin D deficiency in obesity and health consequences. Curr Opin Endocrinol Diabetes Obes. 2006;13(5):412–8. [Google Scholar]
- 95.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142(2):169–73. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- 96.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 Suppl):1752S–8S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 97.Pawley N, Bishop NJ. Prenatal and infant predictors of bone health: the influence of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1748S–51S. doi: 10.1093/ajcn/80.6.1748S. [DOI] [PubMed] [Google Scholar]
- 98.Rutz HP. Hypovitaminosis D, insulin resistance and hypertension in pregnancy. Eur J Clin Nutr. 2005;59(6):805–6. doi: 10.1038/sj.ejcn.1602128. [DOI] [PubMed] [Google Scholar]
- 99.Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’? Br J Nutr. 1998;79(4):315–27. doi: 10.1079/bjn19980055. [DOI] [PubMed] [Google Scholar]
- 100.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 101.Cerimele D, Celleno L, Serri F. Physiological changes in ageing skin. Br J Dermatol. 1990;122(Suppl 35):13–20. doi: 10.1111/j.1365-2133.1990.tb16120.x. [DOI] [PubMed] [Google Scholar]
- 102.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135(2):310–6. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 103.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–8. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramsay RE, Slater JD. Effects of antiepileptic drugs on hormones. Epilepsia. 1991;32(Suppl 6):S60–7. doi: 10.1111/j.1528-1157.1991.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 105.Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Effects of thiazide- and loop-diuretics, alone or in combination, on calcitropic hormones and biochemical bone markers: a randomized controlled study. J Intern Med. 2001;250(2):144–53. doi: 10.1046/j.1365-2796.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 106.Odes HS, Fraser GM, Krugliak P, Lamprecht SA, Shany S. Effect of cimetidine on hepatic vitamin D metabolism in humans. Digestion. 1990;46(2):61–4. doi: 10.1159/000200333. [DOI] [PubMed] [Google Scholar]
- 107.Rosenblatt S, Chanley JD, Segal RL. The effect of lithium on vitamin D metabolism. Biol Psychiatry. 1989;26(2):206–8. doi: 10.1016/0006-3223(89)90025-5. [DOI] [PubMed] [Google Scholar]
- 108.Czerwienska B, Kokot F, Franek E, Irzyniec T, Wiecek A. Effect of orlistat therapy on carbohydrate, lipid, vitamin and hormone plasma levels in obese subjects. Pol Arch Med Wewn. 2004;112(6):1415–23. [PubMed] [Google Scholar]
- 109.Knodel LC, Talbert RL. Adverse effects of hypolipidaemic drugs. Med Toxicol. 1987;2(1):10–32. doi: 10.1007/BF03259858. [DOI] [PubMed] [Google Scholar]
- 110.Awumey EM, Mitra DA, Hollis BW, Kumar R, Bell NH. Vitamin D metabolism is altered in Asian Indians in the southern United States: a clinical research center study. J Clin Endocrinol Metab. 1998;83(1):169–73. doi: 10.1210/jcem.83.1.4514. [DOI] [PubMed] [Google Scholar]
- 111.Wharton B, Bishop N. Rickets. Lancet. 2003;362(9393):1389–400. doi: 10.1016/S0140-6736(03)14636-3. [DOI] [PubMed] [Google Scholar]
- 112.Primary vitamin D deficiency in adults. Drug Ther Bull. 2006;44(4):25–9. doi: 10.1136/dtb.2006.44425. [DOI] [PubMed] [Google Scholar]
- 113.Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119(4 Suppl 1):S3–S11. doi: 10.1016/j.amjmed.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 114.Francis RM. Calcium, vitamin D and involutional osteoporosis. Curr Opin Clin Nutr Metab Care. 2006;9(1):13–7. doi: 10.1097/01.mco.0000196140.95916.3a. [DOI] [PubMed] [Google Scholar]
- 115.Montero-Odasso M, Duque G. Vitamin D in the aging musculoskeletal system: an authentic strength preserving hormone. Mol Aspects Med. 2005;26(3):203–19. doi: 10.1016/j.mam.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 116.Broe KE, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55(2):234–9. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 117.De Torrente de la Jara G, Pecoud A, Favrat B. Female asylum seekers with musculoskeletal pain: the importance of diagnosis and treatment of hypovita-minosis D. BMC Fam Pract. 2006;7:4. doi: 10.1186/1471-2296-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–6. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 119.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209(4458):823–5. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 120.Luong K, Nguyen LT, Nguyen DN. The role of vitamin D in protecting type 1 diabetes mellitus. Diabetes Metab Res Rev. 2005;21(4):338–46. doi: 10.1002/dmrr.557. [DOI] [PubMed] [Google Scholar]
- 121.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338(13):867–72. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 122.Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three auto-immune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81(6):1267–75. doi: 10.1562/2005-02-15-IR-441. [DOI] [PubMed] [Google Scholar]
- 123.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holick MF. Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92(1):49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 125.Gross MD. Vitamin D and calcium in the prevention of prostate and colon cancer: new approaches for the identification of needs. J Nutr. 2005;135(2):326–31. doi: 10.1093/jn/135.2.326. [DOI] [PubMed] [Google Scholar]
- 126.Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147(2):197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- 127.Corona R. Another paradigm lost or just a paradox? Arch Dermatol. 2005;141(12):1587–8. doi: 10.1001/archderm.141.12.1587. [DOI] [PubMed] [Google Scholar]
- 128.Kricker A, Armstrong B. Does sunlight have a beneficial influence on certain cancers? Prog Biophys Mol Biol. 2006;92(1):132–9. doi: 10.1016/j.pbiomolbio.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 129.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215(1–2):31–8. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 130.Melanson SF, Lewandrowski EL, Flood JG, Lewandrowski KB. Measurement of organochlorines in commercial over-the-counter fish oil preparations: implications for dietary and therapeutic recommendations for omega-3 fatty acids and a review of the literature. Arch Pathol Lab Med. 2005;129(1):74–7. doi: 10.5858/2005-129-74-MOOICO. [DOI] [PubMed] [Google Scholar]
- 131.Foran SE, Flood JG, Lewandrowski KB. Measurement of mercury levels in concentrated over-the-counter fish oil preparations: is fish oil healthier than fish? Arch Pathol Lab Med. 2003;127(12):1603–5. doi: 10.5858/2003-127-1603-MOMLIC. [DOI] [PubMed] [Google Scholar]
- 132.Bouillon R, Verstuyf A, Mathieu C, Van Cromphaut S, Masuyama R, Dehaes P, et al. Vitamin D resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):627–45. doi: 10.1016/j.beem.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006;174(9):1273–7. doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64(6):430–5. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 135.Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165(11):1246–52. doi: 10.1001/archinte.165.11.1246. [DOI] [PubMed] [Google Scholar]
- 136.Gorham ED, Garland CF, Grant WB, Morh SB, Lipkin M, Newmark HL, et al. Optimal vitamin D status for colorectal cancer prevention; a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Slattery ML, Neuhausen SL, Hoffman M, Caan B, Curtin K, Ma KN, et al. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer. 2004;111(5):750–6. doi: 10.1002/ijc.20330. [DOI] [PubMed] [Google Scholar]
- 138.Garland CF, Garland FC, Gorham ED. Can colon cancer incidence and death rates be reduced with calcium and vitamin D? Am J Clin Nutr. 1991;54(1 Suppl):193S–201S. doi: 10.1093/ajcn/54.1.193S. [DOI] [PubMed] [Google Scholar]
- 139.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94(6):437–46. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 140.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108(1):104–8. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 141.Vieth R. Enzyme kinetics hypothesis to explain the U-shaped risk curve for prostate cancer vs. 25-hydroxyvitamin D in Nordic countries. Int J Cancer. 2004;111(3):468. doi: 10.1002/ijc.20218. author reply 469. [DOI] [PubMed] [Google Scholar]
- 142.Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1688–95. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 143.Colston KW, Lowe LC, Mansi JL, Campbell MJ. Vitamin D status and breast cancer risk. Anticancer Res. 2006;26(4A):2573–80. [PubMed] [Google Scholar]
- 144.Freedman DM, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002;59(4):257–62. doi: 10.1136/oem.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23(6):1133–6. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 146.Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89–90(1–5):575–9. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 147.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 148.Sharma OP. Vitamin D, calcium, and sarcoidosis. Chest. 1996;109(2):535–9. doi: 10.1378/chest.109.2.535. [DOI] [PubMed] [Google Scholar]
- 149.Tuohy KA, Steinman TI. Hypercalcemia due to excess 1,25-dihydroxyvitamin D in Crohn’s disease. Am J Kidney Dis. 2005;45(1):3–6. doi: 10.1053/j.ajkd.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 150.Pronicka E, Rowinska E, Kulczycka H, Lukaszkiewicz J, Lorenc R, Janas R. Persistent hypercalciuria and elevated 25-hydroxyvitamin D3 in children with infantile hypercalcaemia. Pediatr Nephrol. 1997;11(1):2–6. doi: 10.1007/s004670050221. [DOI] [PubMed] [Google Scholar]
- 151.Better OS, Shabtai M, Kedar S, Melamud A, Berenheim J, Chaimovitz C. Increased incidence of nephrolithiasis (N) in lifeguards (LG) in Israel. Adv Exp Med Biol. 1980;128:467–72. doi: 10.1007/978-1-4615-9167-2_51. [DOI] [PubMed] [Google Scholar]
- 152.Misselwitz J, Hesse CF, Markestad T. Nephrocalcinosis, hypercalciuria and elevated serum levels of 1,25-dihydroxyvitamin D in children. Possible link to vitamin D toxicity. Acta Paediatr Scand. 1990;79(6–7):637–43. doi: 10.1111/j.1651-2227.1990.tb11528.x. [DOI] [PubMed] [Google Scholar]
- 153.Adams JS, Lee G. Gains in bone mineral density with resolution of vitamin D intoxication. Ann Intern Med. 1997;127(3):203–6. doi: 10.7326/0003-4819-127-3-199708010-00004. [DOI] [PubMed] [Google Scholar]