Abstract
OBJECTIVE
To review evidence on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD), the most common cause of chronic liver disease in human beings.
SOURCES OF INFORMATION
The literature was searched for clinical trials and review articles on NAFLD. Levels I and II evidence indicates the benefit of both lifestyle and pharmacologic interventions for NAFLD and nonalcoholic steatohepatitis (NASH).
MAIN MESSAGE
Scientific evidence does not currently support systematic screening for NAFLD. Both NAFLD and NASH are frequently discovered in overweight and obese patients with asymptomatic elevation of serum aminotransferase levels. Ultrasonography detects the presence of a fatty liver, but is unreliable for detecting and quantifying liver fibrosis. Patients with NAFLD should be monitored for possible progression to NASH, particularly if they have diabetes or metabolic syndrome. Although diet and exercise are the mainstays of treatment, medication might be warranted if an appropriate diet and regular physical activity do not improve biochemical markers and liver morphology. Referral for liver biopsy and further evaluation should be considered for those at higher risk of developing NASH.
CONCLUSION
Although most patients with NAFLD have a benign course, some progress to NASH, liver cirrhosis, and hepatocellular carcinoma. These patients should be carefully monitored for progression of disease and treated for associated metabolic disturbances. An integrated approach to care is essential.
RÉSUMÉ
OBJECTIF
Faire le point sur le diagnostic et le traitement du foie gras non alcoolique (FGNA), la cause la plus fréquente d’affection hépatique chronique chez l’humain.
SOURCE DE L’INFORMATION
On a fait une recension des ouvrages scientifiques à la recherche d’essais cliniques et d’articles de revue portant sur le FGNA. Des preuves de niveaux I et II indiquent que des interventions d’ordre comportemental et pharmacologique sont bénéfiques dans les cas de FGNA et de stéatohépatite non alcoolique (SHNA).
PRINCIPAL MESSAGE
Les données scientifiques actuelles ne sont pas en faveur d’un dépistage systématique du FGNA. On découvre souvent un FGNA ou une SHNA chez un patient souffrant d’excès de poids ou d’obésité qui présente une élévation asymptomatique des aminotransférases sériques. L’échographie est efficace pour identifier un foie gras, mais elle est peu fiable pour détecter et quantifier une fibrose hépatique. Chez les patients atteints de FGNA, on doit surveiller une éventuelle progression vers la SHNA, surtout s’ils ont le diabète ou un syndrome métabolique. Même si le régime alimentaire et la pratique régulière d’exercice sont les piliers du traitement, le recours à une médication peut devenir nécessaire si l’exercice régulier et un régime approprié n’améliorent pas les marqueurs biochimiques et la morphologie hépatique. Chez ceux qui présentent un risque élevé de développer une SHNA, on doit envisager une biopsie et une évaluation plus approfondie par un spécialiste.
CONCLUSION
Quoique la plupart des patients qui ont un FGNA évoluent de façon favorable, certains vont progresser vers une SHNA, une cirrhose ou un carcinome hépatocellulaire. Chez ces derniers, il importe de bien suivre la progression de la maladie et de traiter les anomalies métaboliques associées. Un mode de traitement intégré est essentiel.
Liver steatosis is the most frequently diagnosed chronic liver disease.1 It is often discovered by abdominal ultrasonography during investigations for causes of chronic elevation of liver enzymes or another unrelated condition.
While liver steatosis might look like alcoholic liver disease, it can occur in people who drink little or no alcohol. This condition, described as nonalcoholic fatty liver disease (NAFLD), is an emerging health problem. Erroneously seen in the past as a benign condition, NAFLD has the potential to progress through the inflammatory phase of nonalcoholic steatohepatitis (NASH) to fibrosis, cirrhosis, and hepatocellular carcinoma.2,3 Although there are as yet no diagnostic and therapeutic guidelines for NAFLD, it is important to identify it, to manage risk factors for NASH, and to consider treatment.
Case
A 48-year-old man with chronically abnormal results from liver function tests (aspartate aminotransferase, 94 IU/L [normal value <31 IU/L], alanine aminotransferase, 124 IU/L [normal value <35 IU/ L]) comes to an outpatient department with newly diagnosed high levels of fasting serum glucose and triglycerides. He is obese (body mass index, 31) and hypertensive (blood pressure, 160/90 mm Hg). Abdominal ultrasonography suggests he has a fatty liver. He is a truck driver and has not exercised regularly for more than 15 years. He drinks little alcohol. Hepatitis serology and history of hepatotoxic medications are negative. His family history is positive for major cardiovascular events.
Sources of information
The information in this article is based on a synthesis of both basic and clinical scientific studies discovered through exhaustive literature searches for articles on NAFLD published in peer-reviewed journals from 1985 to 2006. The term “nonalcoholic fatty liver disease” was cross-matched with “obesity,” “metabolic syndrome,” and “insulin resistance” and with the MeSH headings “natural history,” “management,” and “pathophysiology.” There is levels I and II evidence for both lifestyle and pharmacologic interventions for NAFLD and NASH.
Prevalence
The estimated prevalence of NAFLD in the general population in western countries is 20% to 40%. Prevalence is higher among obese and diabetic people4,5 (Table 15). Nonalcoholic steatohepatitis accounts for about 20% of NAFLD (estimated prevalence of NASH in western countries is 2% to 3%) and might be the cause of approximately 80% of cryptogenic cirrhosis.6 For every 1000 patients they see, family physicians are likely to encounter more than 300 cases of NAFLD and 20 to 30 cases of NASH.
Table 1.
Prevalence of nonalcoholic fatty liver disease and related conditions
| CONDITION | IN THE GENERAL POPULATION % | AMONG OBESE PEOPLE % | AMONG DIABETICS % |
|---|---|---|---|
| Nonalcoholic fatty liver disease | 20–40 (children: 2.6) | 50–90 (children : 53) | |
| Nonalcoholic steatohepatitis | 2–3 (undergone liver biopsy and histology: 7–9) | 19 | |
| Steatosis | 48 | 100 | |
| Steatohepatitis | 26 | 50 | |
| Steatosis and fibrosis | 8 | 33 | |
| Steatosis and cirrhosis | 8 | 19 |
Adapted from Clark et al.5
Demographics
Patient characteristics vary according to region and race. In the United States, NAFLD is 3 to 5 times more common in men than in women. Hispanic (28%) and Asian (18%) people are at higher risk of NAFLD. Clinical correlates of NAFLD are similar among racial and ethnic groups.7 Among adolescents, NAFLD is more prevalent among obese boys than among obese girls (44% vs 7%) and among Hispanics (36%) than among whites (22%) and blacks (14%).8 In the Mediterranean, most patients with NAFLD are men (70%) in the third and fourth decade of life.9,10 More than 80% of NAFLD patients are overweight. More than 30% are obese, 20% have type 2 diabetes, 80% have hyperlipidemia, and 30% to 70% are hypertensive.
Risk factors
Frequent association with glucose and lipid metabolism disturbances often renders NAFLD a “satellite” element of metabolic syndrome.11,12 The prevalence of metabolic syndrome in patients with NAFLD is more than 40%. Metabolic syndrome is a strong predictor of NAFLD, particularly among those of Japanese descent.13
Factors linked with severity of disease include age >50 years, body mass index >30, and chronic elevation of transaminase levels (twice as high as normal).6 Diabetes and obesity are risk factors for progression to hepatic fibrosis.14,15 Diabetes is also a risk factor for death among patients with NAFLD.16
Pathogenesis
In NAFLD, fatty infiltration is classified as mild if fat involves <30% of hepatocytes, moderate if it involves up to 60%, and severe if it involves >60%.17 Accumulation of neutral fat (triglycerides) in hepatocytes can regress once the cause is eliminated. Nonalcoholic fatty liver disease arises mainly from insulin resistance, abnormal secretion of some hormones governing glucose and lipid metabolism (leptin, adiponectin), and increased release of inflammatory cytokines (tumour necrosis factor-α, interleukins).18,19
Increased flow of free fatty acids from visceral fat to the liver via portal veins further contributes to impaired intracellular lipid metabolism.20,21 In hepatocytes, both insulin resistance and excess free fatty acids impair mitochondrial oxidation of fatty acids, which accumulate and contribute to generation of free radicals by activating the metabolic pathways of peroxisomes and microsomes.22 These events are pathogenic factors for NASH.
Natural history
Interest in NAFLD began after the observation that liver steatosis was the cause of 70% of “primary non-function” of the liver and of many rejections of grafted livers.23 The natural history of NAFLD in community settings is poorly defined. Most studies report data on highly selected patients referred to tertiary care centres.24–26 Among hospitalized patients, morbidity and mortality rates have been found to be 5 times higher among patients with NAFLD than among patients in the general community.27 Cirrhosis was found in 20% of NAFLD patients, and these patients had an overall death rate of 9% over a mean follow-up period of 8.3 years. A retrospective community-based analysis over 20 years concluded that the absolute risk of liver-related death is associated with older age, impaired fasting glucose levels, and cirrhosis.28
Having NASH is a risk factor for increased mortality, and about 1 in 30 patients with NASH develops cirrhosis. Data on long-term outcomes of NASH-associated cirrhosis suggest that liver failure is the main cause of morbidity and mortality.29 The likelihood that someone with NAFLD will develop liver dysfunction in 15 to 20 years is 1% to 2%.30
Risk of hepatocellular carcinoma is doubled among diabetic men with NAFLD.31 Patients with diabetes are at higher relative risk of death from liver cirrhosis (odds ratio, 4.3) than from ischemic heart disease (odds ratio, 1.8).32
Diagnosis
Nonalcoholic fatty liver disease has no definitive biochemical markers or peculiar clinical signs. A simple and effective screening approach for NAFLD should include inquiry into other common causes of fatty liver (alcohol, drugs, hepatitis C virus–related chronic hepatitis, hemochromatosis), an ultrasound scan of the liver, and assessment of serum transaminase levels.
The disease can be associated with mild elevation of serum aminotransaminase (usually aspartate-to-alanine aminotranferase ratio <1) and increased γ-glutamyl-transpeptidase. High fasting serum glucose levels, elevated basal serum insulin, insulin resistance (Table 2), or diabetes might also be present. Many patients have normal results of liver tests even when they are in the advanced stages of liver disease.33
Table 2.
Assessment of insulin resistance by the HOmeostasis Model Assessment (HOMA) formula
| Although the criterion standard for quantifying insulin resistance is the euglycemic clamp, a technique not feasible in primary care settings, the HOMA formula* can be used as a reliable substitute. Patients are classified as insulin resistant if the value determined by the formula exceeds 1.64. |
HOMA—(fasting glycemia [mmol/L] x fasting insulinemia [μIU/L])/22.5.
Liver ultrasonography has high sensitivity (89%) and specificity (93%) for diagnosis of steatosis but not of fibrosis.34 This is important for at least 2 reasons: ultrasonography should not be used to search for liver fibrosis, and other techniques are needed to assess the presence and extent of liver fibrosis.
Liver biopsy and histology is the best way to confirm NAFLD and to assess the presence of liver inflammation and fibrosis.35 Liver biopsy should not be done in all patients likely to have NAFLD, but should be done in patients with suspected NASH. Patients’ age, duration and type of biochemical changes, and presence of obesity or diabetes point to more aggressive forms of disease that should be evaluated histologically (Table 3).
Table 3.
Risk factors for evolution toward more advanced forms of disease (nonalcoholic steatohepatitis)
| Obesity |
|---|
| Diabetes |
| Elevated serum aminotransferase levels |
| Age >50 y |
| Family history of liver disease |
Validation of novel, noninvasive methods of revealing progression of NAFLD is actively being pursued. Combinations of several different serologic markers of liver function and fibrosis appear to predict progression.36,37 Breath test analysis using stable carbon isotope (13C) labeled substrates that assess specific microsomal and mitochondrial functions in the liver appears promising.38 Such approaches still require validation and are currently not applicable to daily practice.
Further evaluation
Once a diagnosis of NAFLD is made, patients should be assessed for presence of other frequently associated conditions, such as metabolic syndrome and cardiovascular disease. Stratification of cardiovascular risk with commonly used risk assessment tools is important when metabolic abnormalities coexist. Metabolic syndrome can be diagnosed if 3 of the following features are present39:
waist circumference ≥ 102 cm for men and ≥ 88 cm for women,
fasting glucose level ≥ 6.1 mmol/L,
triglyceridemia ≥ 1.7 mmol/L,
high-density lipoprotein cholesterol level <1.3 mmol/L in women and <1.03 mmol/L in men, and
hypertension ≥ 135/80 mm Hg.
Having NAFLD worsens the prognosis of other chronic liver diseases. In patients with chronic hepatitis C, having NAFLD is associated with worsening of the hepatitis and resistance to treatment.40 The NAFLD should be treated before starting antiviral therapy for hepatitis C.
General recommendations
A major concern in treating NAFLD is to prevent progression to cirrhosis and liver failure. No general consensus exists on the effectiveness of any therapeutic agent for treating NAFLD. Treatment strategies for NAFLD include identification and treatment of associated metabolic disturbances, including lifestyle modifications to reduce the likelihood that patients will develop metabolic syndrome.
Recommendations for lifestyle modifications should be chosen according to patients’ general health. If a patient is obese, he or she should be encouraged to start with regular and gradually increasing aerobic activity. Dietary restriction of about 25 to 30 kcal/kg daily is reasonable, with a target weight loss of about 10% of body weight over 6 months.41
Too much rapid weight loss (using a very low calorie diet or bariatric surgery) can expose patients to severe metabolic and chemical imbalances and increase risk of steatohepatitis or liver failure.42,43 Diets with a low glycemic index are indicated if patients have elevated triglyceridemia or fasting hyperglycemia; complex carbohydrates are recommended. For patients with high serum cholesterol, the diet should be limited in lipids and especially in saturated fats. Patients should be encouraged to eat a diet rich in vegetables and fruit that contain high levels of fibre and antioxidants. Consumption of alcoholic beverages should be strongly discouraged.
Beyond steatogenic drugs (eg, corticosteroids, amiodarone, valproate),44 all potential hepatotoxic drugs that undergo first-pass liver metabolism and result in toxic metabolites should be avoided or administered cautiously (Table 4). Medications for concomitant diseases should be adjusted accordingly. For example, statins should be selected from among those that do not interfere with cytochrome P-450, such as pravastatin and rosuvastatin.
Table 4.
Commonly used drugs that should be administered with caution to patients with nonalcoholic fatty liver disease
| Acetaminophen |
|---|
| Amiodarone |
| Valproic acid |
| Tamoxifen |
| Statins (lovastatin, simvastatin, fluvastatin) |
| Antiretroviral drugs |
Pharmacologic treatments
Although many agents have had promising results in preliminary pilot studies, few treatments have been examined in rigorous randomized controlled trials. Any pharmacologic approach for NAFLD must take into the account the strong links with overnutrition, underactivity, genetic factors, and insulin resistance. Despite these complications, the current criterion standard for treatment is weight reduction and reversal of insulin resistance. Although levels I and II evidence indicates that diet, lifestyle adjustment, and weight loss improve liver histology (including steatohepatitis and fibrosis) in patients with NAFLD,45 the long-term implications of remission (normalization of serum transaminases, disappearance of liver brightness on ultrasonography, reduction to <5% of fat content on liver histology) are unclear.
Pharmacologic therapy is recommended when an appropriate diet and regular physical activity do not successfully improve biochemical markers and liver morphology (Table 546). If obesity is the target, only 2 medications, sibutramine and orlistat, are currently approved for long-term use, but new drugs (rimonabant) will be soon available.47 Sibutramine, a serotonin-norepinephrine reuptake inhibitor, is superior to placebo in reducing both biochemical markers and ultrasonographic findings of liver steatosis.48 Orlistat, an inhibitor of pancreatic lipases, improves hepatic steatosis and fibrosis.49
Table 5.
Therapeutic approaches proven effective in patients with nonalcoholic fatty liver disease or in animal models of nonalcoholic fatty liver
Gradual weight reduction
|
Insulin sensitizers
|
Lipid-lowering agents
|
Antioxidants
|
Adapted from Portincasa et al.46
Insulin sensitizers, such as biguanides (500 to 1000 mg of metformin twice daily) and thiazolidinediones (rosiglitazone and pioglitazone), induce substantial histologic improvement in NASH patients with or without diabetes (levels I and II evidence).50 As oxidative stress plays an important role in pathogenesis of NASH, antioxidant supplementation has been considered as a therapeutic option, and interesting results have been reported in animal studies testing vitamin E, N-acetyl-cysteine, and S-adenosyl-L-methionine.
Medications with detoxifying and antioxidant properties (eg, silybin–vitamin E complex) seem to ameliorate the dyspeptic symptoms that often accompany NAFLD and to reduce hepatic disturbances (level II evidence). As bacterial overgrowth can have a role in NASH by delivering endogenous hepatotoxins,51 probiotics could have some benefit in patients with NAFLD who have “bowel contamination syndrome.” Finally, lipid-lowering agents might be indicated for patients with alterations in blood-lipid profile not corrected by the aforesaid remedies.
Integrated approach
To improve adherence to diet and lifestyle regimens and to reach therapeutic targets, a validated chronic care model can be helpful. An integrated intervention includes education to increase patients’ knowledge of both the disease and related risk factors.52 An important part of such an intervention is empowering patients to take responsibility for their own care. They need to understand the problem and learn how to manage it themselves, including taking steps of increasing complexity.53,54 Physicians’ role is to help patients identify their objectives and goals and develop management plans by making informed choices.
Follow-up
As with other chronic noncirrhotic liver diseases, follow-up of NAFLD patients should consist of monitoring biochemical, metabolic, and anthropometric parameters every 6 months and performing abdominal ultrasonography yearly (Table 6). Patients with NAFLD can usually be managed in primary care. Referral to consultants might be indicated to assess the need for liver biopsy or before bariatric surgery, however, if patients have other concurrent illnesses.
Table 6.
Recommended follow-up of patients with nonalcoholic fatty liver disease
Every 6 months:
|
Every year:
|
Case resolution
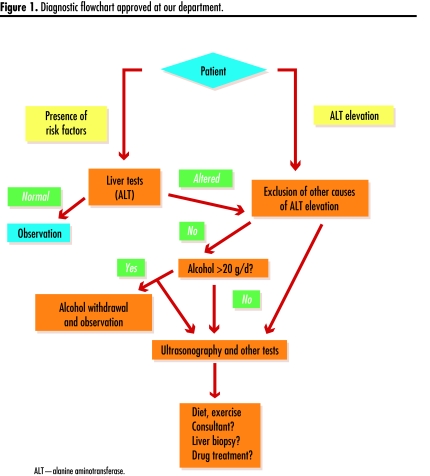
Once the major causes of elevated serum transaminases and liver steatosis are excluded, the most likely diagnosis is NAFLD (Figure 1). Clinical suspicion is strengthened by this patient’s lifestyle and clinical and biochemical features (metabolic syndrome). Given his age and associated factors, he will most likely require referral for consideration of liver biopsy if 6 months of diet and exercise fail to help him reach therapeutic targets.
Figure 1.
Diagnostic flowchart approved at our department.
Conclusion
Scientific evidence does not currently support systematic screening for NAFLD. Patients with NAFLD should be monitored for possible progression to NASH, particularly patients with diabetes and metabolic syndrome. Although diet and exercise are the mainstays of treatment for NAFLD, medications might be warranted if an appropriate diet and regular physical activity do not successfully improve biochemical markers and liver morphology. Referral for liver biopsy and further evaluation should be considered for those at high risk of developing NASH.
Levels of evidence
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
EDITOR’S KEY POINTS
Investigation of suspected nonalcoholic fatty liver disease includes assessing liver transaminases, doing an ultrasound scan, and evaluating risk factors for nonalcoholic fatty liver disease and other causes of liver abnormalities.
Nonalcoholic fatty liver disease can progress to nonalcoholic steatohepatitis, fibrosis, and end-stage liver disease. Evidence of this progression can be determined only by liver biopsy.
Mainstays of treatment are weight loss for obese patients and careful metabolic control for patients with diabetes and hyperlipidemias.
POINTS DE REPèRE DU RÉDACTEUR
L’investigation d’un patient soupçonné de foie gras non alcoolique comprend un dosage des transaminases hépatiques, une échographie et une évaluation des facteurs de risque pour cette maladie et pour d’autres causes d’affections hépatiques.
Le foie gras non alcoolique peut évoluer vers une stéatohépatite non alcoolique, une fibrose et une maladie hépatique terminale. La biopsie hépatique est le seul examen qui peut identifier ces stades.
Le traitement est basé principalement sur la perte de poids chez les obèses et un contrôle métabolique serré en cas de diabète et d’hyperlipidémie.
Footnotes
This article has been peer reviewed.
Contributors
Dr Grattagliano conceived and wrote the article. Dr Portincasa, Dr Palmieri, and Dr Palasciano made substantial contributions to its content. All the authors critically revised the article and gave final approval for publication.
Competing interests
None declared
References
- 1.Bellentani S, Tiribelli C, Saccoccio G, Sodde M, Fratti N, De Martin C, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20(6):1442–9. doi: 10.1002/hep.1840200611. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40(4):578–84. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12(5):1106–10. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 6.Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35(6):1485–93. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 7.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–9. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. [Accessed 2007 April 9];Pediatrics. 2005 115(5):e561–5. doi: 10.1542/peds.2004-1832. Available from: http://pediatrics.aappublications.org/cgi/content/full/115/5/e561. [DOI] [PubMed] [Google Scholar]
- 9.Bahcecioglu IH, Koruk M, Yilmaz O, Bolukbas C, Bolukbas F, Tuncer I, et al. Demographic and clinicopathological characteristics of nonalcoholic fatty liver disease in the east-southeastern Anatolia regions in Turkey. Med Princ Pract. 2006;15(1):62–8. doi: 10.1159/000089388. [DOI] [PubMed] [Google Scholar]
- 10.Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, et al. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001;35(5):568–74. doi: 10.1016/s0168-8278(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 12.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 13.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–8. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42(1):132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40(4):820–6. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2(3):262–5. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 17.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation—a multivariate analysis. Transplantation. 1993;55(4):807–13. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 19.Marra F. NASH: are genes blowing the hits? J Hepatol. 2004;40(5):853–6. doi: 10.1016/j.jhep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, et al. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J Biol Chem. 1999;274(27):19, 228–36. doi: 10.1074/jbc.274.27.19228. [DOI] [PubMed] [Google Scholar]
- 21.Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall’Agata M, et al. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology. 2001;33(4):808–15. doi: 10.1053/jhep.2001.23060. [DOI] [PubMed] [Google Scholar]
- 22.Grattagliano I, Caraceni P, Portincasa P, Domenicali M, Palmieri VO, Trevisani F, et al. Adaptation of subcellular glutathione detoxification system to stress conditions in choline-deficient diet induced rat fatty liver. Cell Biol Toxicol. 2003;19(6):355–66. doi: 10.1023/b:cbto.0000013341.73139.fc. [DOI] [PubMed] [Google Scholar]
- 23.Trevisani F, Colantoni A, Caraceni P, Van Thiel DH. The use of donor fatty liver for liver transplantation: a challenge or a quagmire? J Hepatol. 1996;24(1):114–21. doi: 10.1016/s0168-8278(96)80195-4. [DOI] [PubMed] [Google Scholar]
- 24.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sorensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53(5):750–5. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20(6):594–8. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 26.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22(6):1714–9. [PubMed] [Google Scholar]
- 27.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 28.Adams LA, Lymp JF, St Sauver SJ, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Day CP. The potential role of genes in nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8(3):673–91. xi. doi: 10.1016/j.cld.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129(1):375–8. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 31.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 32.De Marco MR, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22(5):756–61. doi: 10.2337/diacare.22.5.756. [DOI] [PubMed] [Google Scholar]
- 33.Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40(2):475–83. doi: 10.1002/hep.20323. [DOI] [PubMed] [Google Scholar]
- 34.Davies RJ, Saverymuttu SH, Fallowfield M, Joseph AE. Paradoxical lack of ultrasound attenuation with gross fatty change in the liver. Clin Radiol. 1991;43(6):393–6. doi: 10.1016/s0009-9260(05)80567-7. [DOI] [PubMed] [Google Scholar]
- 35.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98(9):2042–7. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127(6):1704–13. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25(4):779–86. doi: 10.1111/j.1478-3231.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 38.Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin Biochem. 2005;38(3):203–17. doi: 10.1016/j.clinbiochem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–7. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 40.Ramesh S, Sanyal AJ. Hepatitis C and nonalcoholic fatty liver disease. Semin Liver Dis. 2004;24(4):399–413. doi: 10.1055/s-2004-860869. [DOI] [PubMed] [Google Scholar]
- 41.Okita M, Hayashi M, Sasagawa T, Takagi K, Suzuki K, Kinoyama S, et al. Effect of a moderately energy-restricted diet on obese patients with fatty liver. Nutrition. 2001;17(7–8):542–7. doi: 10.1016/s0899-9007(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 42.Grattagliano I, Vendemiale G, Caraceni P, Domenicali M, Nardo B, Cavallari A, et al. Starvation impairs antioxidant defense in fatty livers of rats fed a choline-deficient diet. J Nutr. 2000;130(9):2131–6. doi: 10.1093/jn/130.9.2131. [DOI] [PubMed] [Google Scholar]
- 43.James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29(3):495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- 44.Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21(1):57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 45.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 46.Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. The emerging problem of nonalcoholic steatohepatitis (NASH) Rom J Gastroenterol. 2005;14(1):43–51. [PubMed] [Google Scholar]
- 47.Kushner RF. Medical management of obesity. Semin Gastrointest Dis. 2002;13(3):123–32. [PubMed] [Google Scholar]
- 48.Sabuncu T, Nazligul Y, Karaoglanoglu M, Ucar E, Kilic FB. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol. 2003;12(3):189–92. [PubMed] [Google Scholar]
- 49.Harrison SA, Fincke C, Helinski D, Torgerson S, Hayashi P. A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Aliment Pharmacol Ther. 2004;20(6):623–8. doi: 10.1111/j.1365-2036.2004.02153.x. [DOI] [PubMed] [Google Scholar]
- 50.Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): treatment. Best Pract Res Clin Gastroenterol. 2004;18(6):1105–16. doi: 10.1016/j.bpg.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 51.Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38(5):681–7. doi: 10.1016/s0168-8278(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 52.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 53.Baker M. Patient care (empowerment): the view from a national society. BMJ. 2000;320(7250):1660–2. doi: 10.1136/bmj.320.7250.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Werner A, Malterud K. “The pain isn’t as disabling as it used to be”: how can the patient experience empowerment instead of vulnerability in the consultation?”. Scand J Public Health Suppl. 2005;66:41–6. doi: 10.1080/14034950510033363. [DOI] [PubMed] [Google Scholar]