Abstract
As part of a European research project (FOOD-PCR), we developed a standardized and robust PCR detection assay specific for the three most frequently reported food-borne pathogenic Campylobacter species, C. jejuni, C. coli, and C. lari. Fifteen published and unpublished PCR primers targeting the 16S rRNA gene were tested in all possible pairwise combinations, as well as two published primers targeting the 23S rRNA gene. A panel of 150 strains including target and nontarget strains was used in an in-house validation. Only one primer pair, OT1559 plus 18-1, was found to be selective. The inclusivity and exclusivity were 100 and 97%, respectively. In an attempt to find a thermostable DNA polymerase more resistant than Taq to PCR inhibitors present in chicken samples, three DNA polymerases were evaluated. The DNA polymerase Tth was not inhibited at a concentration of 2% (vol/vol) chicken carcass rinse, unlike both Taq DNA polymerase and DyNAzyme. Based on these results, Tth was selected as the most suitable enzyme for the assay. The standardized PCR test described shows potential for use in large-scale screening programs for food-borne Campylobacter species under the assay conditions specified.
The species Campylobacter jejuni, C. coli, and C. lari are among the most frequently reported food-borne pathogens in humans worldwide (7). They can be found in a wide range of foods, including poultry, pig, beef, and seafood products, with chicken meat considered the most common source of human infection (12, 22). Effective methods for detecting these bacteria in foods are important tools for protecting the public health; however, detection of Campylobacter by standard isolation methods is problematic. In samples such as food, the agent may be present in low numbers, and the organisms are relatively sensitive to environmental factors, such as atmospheric oxygen, low pH, dryness, and temperature (22). Consequently, the number of viable Campylobacter cells can be rapidly and substantially reduced during storage or transportation of food samples to testing laboratories (18). Moreover, antibiotics used to improve the selectivity of culture media may inhibit the growth of certain strains if they are sensitive to one or more of the selective agents (8).
The application of culture-independent detection methods such as PCR may help to overcome the aforementioned problems (15). In addition, PCR in general provides faster results than conventional culture and has the potential for automation (9, 27). The latter is necessary for application of the test in large-scale screening programs in which many samples are examined in a short period of time. Many diagnostic laboratories have developed PCR-based methods for pathogen detection (5, 6, 9, 23, 28, 29), but many variables may affect the efficacy of PCR, and the results of tests developed or published by one laboratory can sometimes be difficult to reproduce by other laboratories (21). Moreover, PCR inhibitors originating from food samples may be difficult to overcome in PCR protocols using conventional enzymes: e.g., Taq polymerases (1). This may include testing different DNA polymerases in the matrices chosen for the study with the aim of identifying a polymerase that best overcomes the present inhibitors and validation of an internal amplification control (IAC) to identify false-negative responses. Proper validation based on consensus criteria is therefore an absolute prerequisite for successful adoption of PCR-based diagnostic methodology (10).
One of the aims of the European FOOD-PCR project (www.pcr.dk) was to evaluate and validate noncommercial PCR assays for the specific detection of C. jejuni, C. coli, and C. lari in foods. The present study evaluated 17 published and unpublished PCR primers targeting various rRNA gene regions. An extensive in-house validation was carried out through a new combination of published primers. Furthermore, in order to find a suitable enzyme resistant to inhibition by chicken samples, three DNA polymerases were investigated.
MATERIALS AND METHODS
Terms.
The terms used in this study refer to conventions described in the MicroVal protocol (3). Selectivity was defined as a measure of the degree of response from target and nontarget microorganisms and comprises inclusivity and exclusivity. Inclusivity describes the ability of a method (here PCR) to specifically detect the target pathogen from a wide range of strains, whereas exclusivity is the lack of response from a relevant range of closely related, nontarget strains (10). According to the new International Organization for Standardization (ISO) standard (3, 17), the terms “inclusivity” and “exclusivity” should replace the traditional terms “specificity” and “sensitivity,” which should only be used to express results from diagnostic samples (10).
Bacterial strains, growth conditions, and DNA extraction.
One hundred fifty strains (mainly Campylobacter spp.) were used in this study (Table 1). These included type, reference, and well-characterized field strains from various sources, including chickens, pigs, and cattle in Denmark, identified by conventional and molecular methods (19). All Campylobacter strains were cultured on 5% calf blood agar plates (CM331; Oxoid, Basingstoke, United Kingdom) under microaerobic conditions (6% O2, 7% CO2, 7% H2, 80% N2). All non-Campylobacter strains were grown in Luria-Bertani (LB) medium prepared from 5 g of sodium chloride, 5 g of yeast extract (L21; Oxoid) and 10 g of tryptone peptone (211705; Difco, Detroit, Mich.) dissolved in 1,000 ml of distilled water. The pH was adjusted to 7.3 to 7.4. The strains were stored as frozen cell suspensions in LB medium-glycerol (1:1) at −80°C. DNA was extracted from 2- to 3-day-old bacterial growth by using protocol no. 3 of the Easy-DNA kit (K1800-01; Invitrogen, Carlsbad, Calif.).
TABLE 1.
List of strains used for the development and validation of the PCR used in this studya
| No. | Species | Sero/biotype | Strain | No. | Species | Sero/biotype | Strain | |
|---|---|---|---|---|---|---|---|---|
| 1* | C. jejuni | Penner 1 | CCUG 10935 | |||||
| 2* | C. jejuni | Penner 2 | CCUG 10936 | |||||
| 3* | C. jejuni | Penner 3 | CCUG 10937 | |||||
| 4* | C. jejuni | Penner 4 | CCUG 10938 | |||||
| 5* | C. jejuni | Penner 5 | CCUG 10959 | |||||
| 6* | C. coli | Penner 5 | CCUG 10939 | |||||
| 7* | C. jejuni | Penner 6 | CCUG 12778 | |||||
| 8* | C. jejuni | Penner 7 | CCUG 10940 | |||||
| 9* | C. jejuni | Penner 8 | CCUG 16436 | |||||
| 10* | C. jejuni | Penner 9 | CCUG 10942 | |||||
| 11* | C. jejuni | Penner 10 | CCUG 10943 | |||||
| 12* | C. jejuni | Penner 11 | CCUG 10944 | |||||
| 13* | C. jejuni | Penner 12 | CCUG 17625 | |||||
| 14* | C. jejuni | Penner 13 | CCUG 10945 | |||||
| 15* | C. coli | Penner 14 | CCUG 15360 | |||||
| 16* | C. jejuni | Penner 15 | CCUG 10946 | |||||
| 17* | C. jejuni | Penner 16 | CCUG 10947 | |||||
| 18* | C. jejuni | Penner 17 | CCUG 10948 | |||||
| 19* | C. jejuni | Penner 18 | CCUG 10949 | |||||
| 20* | C. jejuni | Penner 19 | CCUG 10950 | |||||
| 21* | C. coli | Penner 20 | CCUG 10951 | |||||
| 22* | C. jejuni | Penner 21 | CCUG 10952 | |||||
| 23* | C. jejuni | Penner 22 | CCUG 10953 | |||||
| 24* | C. jejuni | Penner 23 | CCUG 10954 | |||||
| 25* | C. coli | Penner 24 | CCUG 10955 | |||||
| 26* | C. coli | Penner 25 | CCUG 10956 | |||||
| 27* | C. coli | Penner 26 | CCUG 10957 | |||||
| 28* | C. jejuni | Penner 27 | CCUG 10958 | |||||
| 29* | C. coli | Penner 28 | CCUG 10959 | |||||
| 30* | C. jejuni | Penner 29 | CCUG 15361 | |||||
| 31* | C. coli | Penner 30 | CCUG 10960 | |||||
| 32* | C. jejuni | Penner 31 | CCUG 10961 | |||||
| 33* | C. jejuni | Penner 32 | CCUG 10962 | |||||
| 34* | C. jejuni | Penner 33 | CCUG 10963 | |||||
| 35* | C. coli | Penner 34 | CCUG 10964 | |||||
| 36* | C. jejuni | Penner 35 | CCUG 10965 | |||||
| 37* | C. jejuni | Penner 36 | CCUG 10966 | |||||
| 38* | C. jejuni | Penner 37 | CCUG 10967 | |||||
| 39* | C. jejuni | Penner 38 | CCUG 10968 | |||||
| 40* | C. coli | Penner 39 | CCUG 10969 | |||||
| 41* | C. jejuni | Penner 40 | CCUG 10970 | |||||
| 42* | C. jejuni | Penner 41 | CCUG 10971 | |||||
| 43* | C. jejuni | Penner 42 | CCUG 12782 | |||||
| 44* | C. jejuni | Penner 43 | CCUG 12783 | |||||
| 45* | C. jejuni | Penner 44 | CCUG 14567 | |||||
| 46* | C. jejuni | Penner 45 | CCUG 17753 | |||||
| 47* | C. coli | Penner 46 | CCUG 15362 | |||||
| 48* | C. coli | Penner 47 | CCUG 17715 | |||||
| 49* | C. coli | Penner 48 | CCUG 17754 | |||||
| 50* | C. coli | Penner 49 | CCUG 17755 | |||||
| 51* | C. jejuni | Penner 50 | CCUG 12790 | |||||
| 52* | C. coli | Penner 51 | CCUG 12791 | |||||
| 53* | C. jejuni | Penner 52 | CCUG 12792 | |||||
| 54* | C. jejuni | Penner 53 | CCUG 15013 | |||||
| 55* | C. coli | Penner 54 | CCUG 12794 | |||||
| 56* | C. jejuni | Penner 55 | CCUG 12795 | |||||
| 57* | C. coli | Penner 56 | CCUG 14537 | |||||
| 58* | C. jejuni | Penner 57 | CCUG 14538 | |||||
| 59* | C. jejuni | Penner 58 | CCUG 14539 | |||||
| 60* | C. coli | Penner 59 | CCUG 14540 | |||||
| 61* | C. jejuni | Penner 60 | CCUG 14541 | |||||
| 62* | C. coli | Penner 61 | CCUG 24865 | |||||
| 63* | C. jejuni | Penner 62 | CCUG 24866 | |||||
| 64* | C. jejuni | Penner 63 | CCUG 24867 | |||||
| 65* | C. jejuni | Penner 64 | CCUG 24868 | |||||
| 66* | C. jejuni | Penner 65 | CCUG 24869 | |||||
| 67** | C. jejuni | Type strain | CCUG 11284 | |||||
| 68* | C. jejuni | C. jejunib | CCUG 24567 | |||||
| 69* | C. jejuni | C. jejunib | CCUG 18265 | |||||
| 70* | C. jejuni | C. jejunib | CCUG 18266 | |||||
| 71* | C. jejuni | C. jejunib | CCUG 26155 | |||||
| 72* | C. jejuni | C. jejunib | CCUG 26152 | |||||
| 73* | C. jejuni | C. jejunib | SSI 5384 | |||||
| 74** | C. coli | Type strain | CCUG 11283 | |||||
| 75* | C. lari | Type strain | CCUG 23947 | |||||
| 76** | C. lari | NARTCc | CCUG 15035 | |||||
| 77** | C. lari | NARTC | CCUG 12774 | |||||
| 78** | C. lari | NARTC | CCUG 23949 | |||||
| 79* | C. lari | NARTC | CCUG 19528 | |||||
| 80* | C. lari | NARTC | CCUG 23948 | |||||
| 81* | C. lari | NARTC | SVS 98-40052 | |||||
| 82* | C. lari | UPTCd | CCUG 20707 | |||||
| 83* | C. lari | UPTC | CCUG 18267 | |||||
| 84* | C. lari | UPTC | CCUG 22395 | |||||
| 85* | C. lari | UPTC | CCUG 18294 | |||||
| 86* | C. lari | UPTC | CCUG 22396 | |||||
| 87* | C. lari | UPTC | LU 6/3BW1 | |||||
| 88* | C. lari | UPTC | LU 21/12OC3 | |||||
| 89* | C. lari | UPTC | LU 16/1BTG4 | |||||
| 90* | C. lari | UPTC | LU 18/3BTG8 | |||||
| 91* | C. lari | UPTC | LU 21/12LW18 | |||||
| 92* | C. lari | UPTC | LU 16/1OC3 | |||||
| 93 | C. jejuni | 2 | 7231127-3 | |||||
| 94 | C. jejuni | 4 complex | 7231127-2 | |||||
| 95 | C. coli | 46 | 9831503-2 | |||||
| 96 | C. coli | 59 | 9831091-1 | |||||
| 97 | C. hyointestinalis | 9930731-1 | ||||||
| 98 | C. jejuni | 35 | 7230701-6 | |||||
| 99 | C. jejuni | 23,36 | 7230127-3 | |||||
| 100 | C. coli | 51 | 7231059-1 | |||||
| 101 | C. coli | 5 | 7231058-1 | |||||
| 102 | C. hyointestinalis | 9930111-1 | ||||||
| 103 | C. jejuni | 11 | 99042253-4 | |||||
| 104 | C. jejuni | 31 | 9904313-14 | |||||
| 105 | C. coli | 5 | 9904602-19 | |||||
| 106 | C. coli | 46 | 9904253-1 | |||||
| 107 | C. jejuni | 29 | 7231125-2 | |||||
| 108 | C. jejuni | 5 | 7231120-5 | |||||
| 109 | C. jejuni | 19 | 723723-2 | |||||
| 110 | C. jejuni | 4 complex | 9904253-5 | |||||
| 111 | C. jejuni | 6,7 | 9930116-6 | |||||
| 112 | C. coli | 30 | 7230141-6 | |||||
| 113 | C. coli | 46 | 7231033-1 | |||||
| 114 | C. coli | NT | 9631023-2 | |||||
| 115 | C. coli | 59 | 7231135-2 | |||||
| 116 | C. coli | 48 | 9631038-4 | |||||
| 117 | C. hyointestinalis | 7230324-3 | ||||||
| 118 | C. hyointestinalis | 9731034-3 | ||||||
| 119 | H. pullorum-like | 9831306-5 | ||||||
| 120 | C. lari | 9831299-3 | ||||||
| 121 | H. pullorum-like | 9831276-8 | ||||||
| 122** | C. upsaliensis | Type strain | CCUG 14913 | |||||
| 123** | C. upsaliensis | CCUG 33890 | ||||||
| 124** | C. upsaliensis | CCUG 20818 | ||||||
| 125** | C. upsaliensis | CCUG 23017 | ||||||
| 126** | C. upsaliensis | CCUG 19559 | ||||||
| 127** | C. helveticus | Type strain | CCUG 30682 | |||||
| 128** | C. helveticus | CCUG 34016 | ||||||
| 129** | C. helveticus | CCUG 30563 | ||||||
| 130** | C. helveticus | CCUG 30564 | ||||||
| 131** | C. helveticus | CCUG 30565 | ||||||
| 132** | C. helveticus | CCUG 30566 | ||||||
| 133** | C. helveticus | CCUG 30683 | ||||||
| 134** | C. helveticus | CCUG 34042 | ||||||
| 135 | C. hyointestinalis | Type strain | CCUG 14169 | |||||
| 136 | C. lanienae | Type strain | NCTC 13004 | |||||
| 137 | C. mucosalis | Type strain | CCUG 6822 | |||||
| 138 | C. fetus | Type strain | CCUG 6823 | |||||
| 139 | C. concisus | Type strain | CCUG 13144 | |||||
| 140 | C. curvus | Type strain | CCUG 13146 | |||||
| 141 | C. showae | Type strain | CCUG 30254 | |||||
| 142 | C. rectus | Type strain | CCUG 20446 | |||||
| 143 | C. gracilis | Type strain | CCUG 27720 | |||||
| 144 | Arcobacter butzleri | Type strain | CCUG 30483 | |||||
| 145 | Helicobacter pylori | Rigsh. 15893 | ||||||
| 146 | H. pullorum | Type strain | CCUG 33837 | |||||
| 147 | Escherichia coli | JEO 908149 | ||||||
| 148 | L. monocytogenes | JEO 2268-179 | ||||||
| 149 | Y. enterocolitica | JH2 O:3 | ||||||
| 150 | S. enterica | CCUG 31969 |
*, strains used for testing the four published PCR methods; **, strains used in the preliminary study for identifying the primer pair with the best selectivity.
C. jejuni, subsp. doylei strain.
NARTC, nalidixic acid-resistant thermophilic Campylobacter.
UPTC, urease-positive thermophilic Campylobacter.
Selection of published primers.
rRNA gene sequences of C. jejuni, C. coli, and C. lari consistently share extensive homology, but are more distinct from other Campylobacter spp. (20). Thus, one probe (25) and three primer sets (Table 2) targeting 16S and one primer pair targeting 23S ribosomal DNA (rDNA) (4, 5, 24, 25) were tested on 105 Campylobacter isolates (Table 1). For testing the published PCR assays, the reaction conditions used, including temperature profile and DNA polymerase, were essentially as described in the original publications. The thermocycler used in this and subsequent studies was a GeneAmp PCR system 9700 (Applied Biosystems, Weiterstadt, Germany). After cycling, the PCR amplicons in this and subsequent studies were detected by electrophoresis in 1.8% agarose gels stained with ethidium bromide.
TABLE 2.
Primers used in different combinations to develop the best PCR assay for detection of C. jejuni, C. coli, and C. lari
| Target | Primer (sequence) | Reference |
|---|---|---|
| 23S | Therm1 (5′-TATTCCAATACCAACATTAGT) | 6 |
| Therm4 (5′-CTTCGCTAATGCTAACCC) | ||
| 16S | 6-1 (5′-GTCGAACGATGAAGCTTCTA) | 5 |
| 18-1 (5′-TTCCTTAGGTACCGTCAGAA) | ||
| 16S | CF1 (5′-GGAAGGATGACACTTTTCGGAGCG) | 28 |
| CR2 (5′-TCGCGGTATTGCGTCTCATTGTATATGC) | ||
| 16S | C442 (5′-GGAGGATGACACTTTTCGGAGC) | 29 |
| C490 (5′-ATTACTGAGATGACTAGCACCCC) | ||
| 16S | OT1559 (5′-CTGCTTAACACAAGTTGAGTAGG) | 25 |
| 16S | 18-1rev (5′-TTCTGACGGTACCTAAGGAA) | This study |
| 16S | CF1rev (5′-CGCTCCGAAAAGTGTCATCCTCC) | This study |
| 16S | C490rev (5′-GGGGTGGCTAGCCATCTCAGTATT) | This study |
| 16S | JCL-1 (5′-ATAGTTAATCTGCCCTACACAA) | This study |
| 16S | JCL-2 (5′-TCCTTTTCTTAGGGAAGAATTC) | This study |
| 16S | JCL-3 (5′-CGTCAGAATTCTTCCCTAAG) | This study |
| 16S | JCL-4 (5′-AGTTTAGTATTCCGGCTTCGA) | This study |
| 16S | JCL-5 (5′-GATTCCACTGTGGACGGTAA) | This study |
| IACa | CCL-f (5′-OT1559-GGTTCATGAGGACACCTGAGTT) | This study |
| CCR-r (5′-18-1-TATACACTCTCATCCCTCCAAC) | This study |
IAC, primers used for construction of the internal amplification control.
New primer combinations.
Since none of the published primer sets resulted in the required selectivity (see Results), new primer combinations were tested with 18 strains (Table 1), chosen to identify assays capable of detecting C. jejuni, C. coli, and C. lari, but not the closely related, but not food-borne species C. upsaliensis and C. helveticus. The following PCR mixture (50 μl) was used: 10× PCR buffer, 0.2 mM deoxynucleoside triphosphates (dNTPs) (27-2035-03; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom), 0.2 μM each primer, 0.4 U of DNA polymerase, and 3 mM MgCl2 (N808-0010; Applied Biosystems, Nærum, Denmark), and 1.0 μl of target DNA solution. The thermocycling program comprised an initial denaturation (94°C, 2 min) followed by 35 cycles of denaturation at (94°C, 1 min), annealing (55°C, 1 min), and extension at (72°C, 1 min). A final extension cycle (72°C, 4 min) completed the PCR. For preliminary optimization of the PCR and cycling parameters, the type strain of C. jejuni (CCUG 11284) was used. The optimized PCR mixture in 25 μl contained 10× PCR buffer for Tth DNA polymerase (1480022; Roche Applied Science, Hvidovre, Denmark), 0.2 mM dNTP, 0.22 μM primer OT1559, 0.24 μM primer 18-1, 1 U of Tth DNA polymerase (14800322; Roche Applied Science), 5 μg of bovine serum albumin (20 mg/ml) (711454; Roche Applied Science), 2 mM MgCl2, 0.25 μl (≅103 copies) of an internal control DNA (described below), and 1 μl of target sample DNA solution (≅100 pg ≅ 5 × 104 copies). All PCRs were made in triplicate in 0.2-ml PCR tubes.
Final standard PCR.
The most selective new combination of primers OT1559 and 18-1 (see Results) was chosen as the final standard test. The final thermocycling program was as follows: initial denaturation 94°C at 2 min; then 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 15 s, and extension at 72°C for 30 s; and finally an extension at 72°C for 4 min.
Sequencing.
The 16S rRNA gene sequence from strain CCUG 19559 was determined by a sequencing method as described previously (2). Alignment and numerical comparison of this sequence with GenBank database sequences of the type strains of all 16 Campylobacter species were performed with the program BioNumerics v2.5 (Applied Maths, Kortrijk, Belgium) using both default parameters and those described previously (20).
Construction of internal amplification control.
A 124-bp internal amplification control (IAC) amplicon was constructed based on DNA from the viral hemorrhagic septicema virus (GenBank accession no. X66134). This DNA was chosen since it is not found in food samples and has shown to work well previously (11). The IAC was produced in 50-μl reaction mixtures comprising 10 mM Tris (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.2 mM dNTP, 0.1 μM each primer, 0.5 U of Taq polymerase (1146165; Roche Applied Science), and 2 μl of target DNA sample. The thermocycling program was as follows: 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. The PCR products were purified from the agarose gel by using the QIAquick gel extraction kit (28704; Qiagen, Hilden, Germany) and finally eluated in 50 μl of sterile water. The fragment was furthermore cloned in plasmid pCR2.1 by using the TA cloning kit and One Shot TOP10 competent cells as recommended by the supplier (K2040-01; Invitrogen, Carlsbad, Calif.). Plasmids were recovered with the QIAprep Spin Miniprep kit (27104; Qiagen).
DNA polymerase and PCR inhibition test.
To identify a DNA polymerase resistant to PCR inhibitors present in chicken samples, a previously standardized PCR assay specific for pathogenic Yersinia enterocolitica was used (14). Different concentrations (from 1 fg/ml to 1 mg/ml) of DNA isolated from Y. enterocolitica Y79 (14) were added to the amplification mixture containing different percentage dilutions (vol/vol) of the chicken rinse sample (Table 3). All chicken samples had been certified free from naturally occurring pathogenic Y. enterocolitica by PCR. Whole chicken carcasses or neck skins were obtained from slaughterhouses or retailers in Denmark or Sweden. The chicken rinse samples comprised whole chickens washed in 500 ml of buffered peptone water (BPW) or sterile saline as described previously (13). Chicken neck skin samples were prepared by adding 10 g of neck skin to 100 ml of BPW or saline, homogenizing it in a stomacher for 30 s, and removing the skin sample. To test the PCR inhibitory effect of these samples, aliquots were added to the PCR mixture in a final concentration of 20% (vol/vol). Also, the inhibition of 10- and 100-fold-diluted chicken carcass rinse samples (respectively, 2 and 0.2% in the PCR mixture) were tested. Two potentially resistant enzymes, along with Taq, were tested (1). DyNAzyme (F501L; Finnzymes, Espoo, Finland), Taq and Tth DNA polymerases and accompanying buffer systems were evaluated for resistance to the inhibitory effect of chicken carcass rinse matrix. For real-time PCR, a LightCycler instrument (Roche Diagnostics) and a real-time assay based on the same Y. enterocolitica primer pair were used. The PCR mix contained 10× buffer supplied with the appropriate DNA polymerase (Taq, Tth, or DyNAzyme), 2.5 U of enzyme, 4 mM MgCl2, 0.44 μM each primer, 0.2 mM each dNTP, 10,000-times-diluted SYBR Green I (1988131; Roche Applied Science), and 4 μl of sample. The total volume was 20 μl. The amplification conditions included a denaturation step of 1 min at 95°C, followed by 40 cycles of 0.1 s of denaturation at 95°C, 5 s of annealing at 60°C, and 15 s of elongation at 72°C, followed by a single fluorescent measurement and finally 25 s of final elongation. Amplification was followed by a melting curve analysis between 65 and 95°C and finally a cooling step for 1 min at 40°C. During amplification, the fluorescence was measured by using gain setting F1:1 with display mode F1.
TABLE 3.
Effect of inhibition by carcass rinse from chicken on PCR amplification with different DNA polymerases in a Y. enterocolitica PCR assay
| DNA polymerase | Rinse sample (%)a | Result with
Y. enterocolitica concen (CFU/25-μl reaction
tube)d
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Conventional
PCRb
|
Real-time
PCRc
|
||||||||
| 5 × 103 | 5 × 102 | 5 × 101 | 5 × 100 | 5 × 103 | 5 × 102 | 5 × 101 | 5 × 100 | ||
| Taq polymerase | Water | ++ | ++ | ++ | +− | ++ | ++ | ++ | −− |
| 20 | −− | −− | −− | −− | −− | −− | −− | −− | |
| 2 | +− | −− | −− | −− | ++ | −− | −− | −− | |
| 0.2 | +− | ++ | +− | −− | ++ | ++ | ++ | −− | |
| Tth polymerase | 20 | −− | −− | −− | −− | −− | −− | −− | −− |
| 2 | ++ | ++ | ++ | −− | ++ | ++ | ++ | −− | |
| 0.2 | ++ | ++ | ++ | −− | ++ | ++ | ++ | −− | |
| DyNAzyme II | 20 | −− | −− | −− | −− | −− | −− | −− | −− |
| 2 | −− | ++ | −− | −− | ++ | ++ | −− | −− | |
| 0.2 | ++ | ++ | ++ | +− | ++ | ++ | ++ | −− | |
Carcass-rinse sample from neck skin of chicken in physiological saline.
Two independent PCR results confirmed by gel electrophoresis.
Two independent PCR results confirmed by melting-curve analysis in a LightCycler instrument.
Overnight culture of Y. enterocolitica Y79 in Tris-buffered saline with CFU determination by plating on TGE plates. Dilutions of the cell suspensions were made in physiological saline and added to the PCR mixture to a final amount of 20% (vol/vol).
RESULTS
Selectivity.
The results of the inclusivity and exclusivity tests are presented in Table 4. Only one primer pair, OT1559 plus 18-1, showed adequate selectivity. This primer pair was then tested in PCR against all 150 strains to verify its selectivity. The results showed that the inclusivity was 100%, whereas the exclusivity was 97%; only C. upsaliensis strain CCUG 19559 resulted in a positive PCR amplification. A comparison of the 16S rDNA sequence of strain CCUG 19559 and those of C. upsaliensis, C. jejuni, C. coli, and C. lari showed that the two primer annealing sites in strain CCUG 19559 were identical to sequences of the latter group of species. However, the 16S rRNA sequences of CCUG 19559 differed by 6 and 2 bp, respectively, in the primer-binding region compared to seven published C. upsaliensis 16S rRNA sequences. Nonetheless, the ca. 1,500-bp segment of the CCUG 19559 16S rDNA gene sequence was found to be 98.4% similar to C. upsaliensis 16S rDNA, compared with a corresponding value of 96.5% similarity for other C. jejuni strains.
TABLE 4.
Results from the preliminary screening of 26 primer combinations against DNA from 18 isolates and strains
| Primer pair | Screening results for:
|
||||||
|---|---|---|---|---|---|---|---|
| Inclusivity (no. of
strains)
|
Exclusivity (no. of strains)
|
||||||
| C. jejuni (n = 1) | C. coli (n = 1) | C. lari (n = 3) | No. of positive target strains/total true positive (%) | C. upsaliensis (n = 5) | C. helveticus (n = 8) | No. of negative nontarget strains/total true negative (%) | |
| 6-1 + 18-1 | 1 | 1 | 3 | 5/5 (100) | 5 | 8 | 0/13 (0) |
| CF1 + CR2 | 1 | 0 | 3 | 4/5 (80) | 4 | 8 | 1/13 (8) |
| C442 + C490 | 1 | 0 | 3 | 4/5 (80) | 5 | 8 | 0/13 (0) |
| 18-1rev + CR2 | 1 | 0 | 3 | 4/5 (80) | 5 | 8 | 0/13 (0) |
| 18-1rev + C490 | 1 | 0 | 3 | 4/5 (80) | 5 | 8 | 0/13 (0) |
| OT1559 + 18-1a | 1 | 1 | 3 | 5/5 (100) | 1 | 0 | 12/13 (92) |
| OT1559 + C490 | 1 | 0 | 3 | 4/5 (80) | 1 | 1 | 11/13 (85) |
| OT1559 + CR2 | 1 | 0 | 3 | 4/5 (80) | 1 | 1 | 11/13 (85) |
| 6-1 + CR2 | 1 | 0 | 3 | 4/5 (80) | 5 | 8 | 0/13 (0) |
| JCL-1 + JCL-3 | 1 | 1 | 3 | 5/5 (100) | 2 | 6 | 5/13 (39) |
| JCL-1 + JCL-4 | 0 | 0 | 0 | 0/5 (0) | 0 | 0 | 13/13 (100) |
| JCL-1 + JCL-5 | 0 | 0 | 0 | 0/5 (0) | 0 | 0 | 13/13 (100) |
| JCL-2 + JCL-4 | 1 | 0 | 2 | 3/5 (60) | 0 | 1 | 12/13 (92) |
| JCL-2 + JCL-5 | 0 | 0 | 0 | 0/5 (0) | 0 | 1 | 12/13 (92) |
| OT1559 + JCL3 | 1 | 1 | 3 | 5/5 (100) | 2 | 5 | 6/13 (46) |
| OT1559 + JCL4 | 0 | 0 | 0 | 0/5 (0) | 0 | 0 | 13/13 (100) |
| OT1559 + JCL5 | 0 | 0 | 0 | 0/5 (0) | 0 | 1 | 12/13 (92) |
| JCL-1 + 18-1 | 1 | 1 | 3 | 5/5 (100) | 2 | 6 | 5/13 (39) |
| C490rev + JCL-4 | 1 | 0 | 3 | 4/5 (80) | 4 | 7 | 2/13 (15) |
| C490rev + JCL-5 | 1 | 0 | 3 | 4/5 (80) | 2 | 2 | 9/13 (69) |
| 18-1rev + JCL-4 | 0 | 0 | 0 | 0/5 (0) | 1 | 1 | 11/13 (85) |
| 18-1rev + JCL-5 | 0 | 0 | 1 | 1/5 (20) | 1 | 2 | 10/13 (77) |
| 6-1 + CF1rev | 1 | 0 | 0 | 1/5 (20) | 1 | 8 | 4/13 (31) |
| JCL-1 + CF1rev | 1 | 1 | 3 | 5/5 (100) | 4 | 8 | 1/13 (8) |
| OT1559 + CF1rev | 1 | 1 | 3 | 5/5 (100) | 2 | 4 | 7/13 (54) |
| C490 + CR2 | 0 | 0 | 0 | 0/5 (0) | 0 | 0 | 13/13 (100) |
Note that the OT1559 + 18-1 primer combination was the most selective new combination and was chosen as the final standard test.
IAC and detection limit.
A dilution series of the purified IAC fragment was made to determine the detection level in the final PCR. The IAC was coamplified with the target DNA (C. jejuni CCUG 11284) at 287 bp (Fig. 1). The detection limits for the IAC were 2.2 × 10−17 g (50 to 100 copies) when it was amplified alone and 2.2 × 10 15 g (5 × 103 copies) when it was amplified together with 10 pg of target DNA by using 35 amplification cycles. The detection limit for the target DNA (C. jejuni CCUG 11284) was 3.1 × 10−14 g (17 copies, assuming a genome size of 1.64 × 106 bp) when it was amplified without IAC.
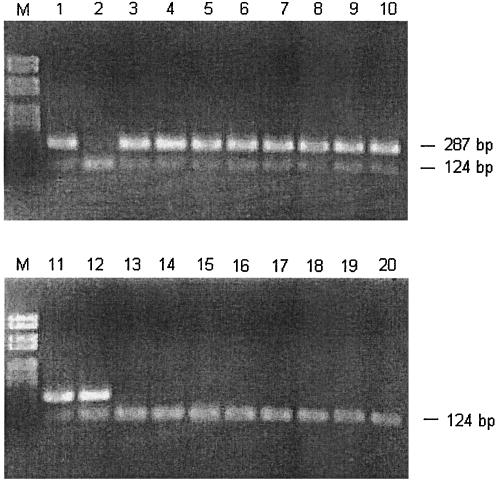
FIG. 1.
Result of the optimized PCR protocol detecting C. jejuni, C. coli, and C. lari (upper band), including the internal control (lower band). Lanes: M, molecular size marker; 1, positive control (CCUG 11284); 2, negative control (only internal control is added); 3 to 12, different strains of C. jejuni, C. coli, and C. lari; 13 to 20, other Campylobacter and non-Campylobacter species (strains marked with double asterisks in Table 1).
Evaluation of thermostable DNA polymerases.
Undiluted chicken rinse 20% (vol/vol) was found to completely inhibit PCR independent of the concentration of the target or the DNA polymerase was used. Taq and DyNAzyme polymerases were not inhibited when the chicken rinse was added to the PCR at a concentration of 0.2% (vol/vol), while Tth DNA polymerase showed no inhibition at a concentration of 2% (vol/vol) chicken rinse. Similar results were obtained for conventional and real-time PCR. Based on these results, Tth was selected as the most suitable DNA polymerase for the final Campylobacter PCR assay.
DISCUSSION
Selectivity was the principal criterion used to identify a PCR test for international validation as a tool for rapid and effective detection of C. jejuni, C. coli, and C. lari in foods. We aimed to identify an assay that included all strains of these three predominant food-borne Campylobacter species, but excluded all other species. The four published PCR assays evaluated based upon 16S and 23S rRNA gene sequences lacked the accepted selectivity to food-borne campylobacters, since they also yielded amplicons for C. upsaliensis and C. helveticus. This was considered disadvantageous, since domestic pets are the only known animal reservoir for these taxa, and C. helveticus has not yet been reported from human disease (19). Similar results have been reported with 23S rRNA gene sequence-derived PCR tests first proposed as selective for the identification of C. jejuni and C. coli in a study that emphasized the need for appropriate strain selection in the validation process (21). Subsequently, new combinations of existing primers, together with new primers were tested to improve the selectivity for C. jejuni, C. coli, and C. lari (Table 1). The primer pair with a 100% inclusivity score and the best exclusivity score was then tested in PCR against all 150 strains (Table 1) to assess its overall selectivity (Table 4). However, it was observed that two strains of C. helveticus appeared with a faint, nearly invisible band when an annealing temperature of 55°C was used, but these amplicons were not obtained with the final optimized cycling parameters (30 s at 94°C, 15 s at 58°C, and 30 s at 72°C for 35 cycles). After the change to these conditions, only one C. upsaliensis strain (CCUG 19559) was still detected by the assay. Given that both C. upsaliensis and C. helveticus are highly related to the food-borne species C. jejuni, C. coli, and C. lari (19, 20), this result is not altogether unexpected. A recent publication (4) showed that 28 of 29 hipO-negative Campylobacter isolates possessed 16S rRNA genes that were indistinguishable from those of C. jejuni type strains (based on 16S rRNA restriction fragment length polymorphism data). These hipO-negative isolates were found to be C. coli by the cumulative evidence of six published PCR-based assays, suggesting that speciation data based solely on this gene should be interpreted with caution. Furthermore, four 16S rRNA genes from hipO-negative strains were sequenced, which showed that they were almost identical to C. jejuni type strain 16S rRNA sequences deposited in GenBank. This observation is important given that others have reported problems with phylogenetic analyses of bacterial species based solely on 16S rRNA gene sequence comparison (26).
Moreover, the specific characteristics of the 16S rDNA sequence of CCUG 19559 infer that up to one-third of the gene may have been acquired from C. jejuni in a horizontal gene transfer event, a phenomenon that has attracted substantial credence in recent years (30). However, the fact that most of the C. upsaliensis and C. helveticus strains tested did not give an amplicon in the assay described indicates that the selectivity is acceptable.
Since the assay may be considered as an ISO or European international standard for detection of thermotolerant Campylobacter in food, it was of considerable importance to find the best DNA polymerase enzyme for the assay: i.e., that most resistant to PCR inhibitors naturally occurring in foods and chicken samples in particular. We evaluated DyNAyme, Tth, and Taq for their ability to withstand inhibitors from chicken rinse. To facilitate the evaluation of the effect of the sample matrix only, regardless of the specificity of the selected Campylobacter primers, an already validated PCR assay for detection of pathogenic Y. enterocolitica (14) was used as a test model.
The results indicated Tth to be the DNA polymerase of choice when examining chicken wash samples, since the PCR was substantially less inhibited when this enzyme was used compared with the Taq and DynaZyme polymerases. The improved performance of the PCR assay by use of Tth polymerase was observed in both the conventional and real-time PCR assays studied, which we consider to be an important observation. Real-time PCR assays are becoming of increasing importance in food quality matters, since they assess the level of contamination (and not simply the presence or absence) with a given pathogen. Based on the results obtained in chicken rinse, Tth DNA polymerase was chosen for international validation of the selected PCR assay with the highest specificity. This assay employs a novel combination of two previously published primers, the forward primer OT1559 (5) and the reverse primer 18-1 (25), and amplifies a 287-bp sequence of the 16S gene.
We conclude that the PCR test designed in the present study could form the basis of an accurate, standardized, and robust high-throughput, screening tool for enteropathogenic campylobacters in foods. Results from an international collaborative trial are described elsewhere (16).
Acknowledgments
The work was supported by EU project no. QLK1-CT-1999-00226 (FOOD-PCR).
We thank Jeanette Knudsen, Lise Christensen, Kirsten Vestergaard, and Penny Jordan for excellent technical assistance; Eva Møller Nielsen for providing us with the field strains; Mathilde H. Josefsen and Nigel Cook for critical reading of the manuscript; and Stefan Jensen for editorial work.
REFERENCES
- 1.Abu Al-Soud, W., and P. Rådström. 1998. Capacity of nine thermostable DNA polymerases to mediate DNA amplification in the presence of PCR-inhibiting samples. Appl. Environ. Microbiol. 64:3748-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angen, Ø., P. Ahrens, and C. Tegtmeier. 1998. Development of a species-specific PCR test for identification of Haemophilus somnus in pure and mixed cultures. Vet. Microbiol. 63:39-48. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2002. Microbiology of food and animal feeding stuffs—protocol for the validation of alternative methods (EN ISO/FIDS 16140). European Committee for Standardization, AFNOR, Paris, France.
- 4.Burnett, T. A., M. A. Hornitzky, P. Kuhnert, and S. P. Djordjevic. 2002. Speciating Campylobacter jejuni and Campylobacter coli isolates from poultry and humans using six PCR-based assays. FEMS Microbiol. Lett. 216:201-209. [DOI] [PubMed] [Google Scholar]
- 5.Docherty, L., M. R. Adams, P. Patel, and J. McFadden. 1996. The magnetic immuno-polymerase chain reaction assay for the detection of Campylobacter in milk and poultry. Lett. Appl. Microbiol. 22:288-292. [DOI] [PubMed] [Google Scholar]
- 6.Fermér, C., and E. O. Engvall. 1999. Specific PCR identification and differentiation of the thermophilic campylobacters, Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 37:3370-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freidman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 8.Goossens, H., and J.-P. Butzler. 1992. Isolation and identification of Campylobacter spp., p. 93-109. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni. Current status and future trends. ASM Press, Washington, D.C.
- 9.Hanai, K., M. Satake, H. Nakanishi, and K. Venkateswaran. 1997. Comparison of commercially available kits with standard methods for detection of Salmonella strains in foods. Appl. Environ. Microbiol. 63:775-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoorfar, J., and N. Cook. 2002. Critical steps in standardization of PCR. Methods Mol. Biol. 216:51-64. [DOI] [PubMed] [Google Scholar]
- 11.Hoorfar, J., P. Ahrens, and P. Rådström. 2000. Automated 5′ nuclease PCR assay for identification of Salmonella enterica. J. Clin. Microbiol. 38:3429-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs-Reitsma, W. 2000. Campylobacter in the food supply, p. 467-481. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 13.Josefsen, M., P. S. Lübeck, B. Aalbæk, and J. Hoorfar. 2002. Preston and Park-Sanders protocols adapted for semi-quantitative isolation of thermotolerant Campylobacter from chicken rinse. Int. J. Food Microbiol. 80:177-183. [DOI] [PubMed] [Google Scholar]
- 14.Lantz, P. G., R. Knutsson, Y. Blixt, W. Abu Al-Soud, E. Borch, and P. Rådström. 1998. Detection of pathogenic Yersinia enterocolitica in enrichment media and pork by a multiplex PCR: a study of sample preparation and PCR-inhibitory components. Int. J. Food Microbiol. 45:93-105. [DOI] [PubMed] [Google Scholar]
- 15.Lübeck, P. S. and J. Hoorfar. 2002. PCR technology and applications to zoonotic food-borne bacterial pathogens. Methods Mol. Biol. 216:65-83. [DOI] [PubMed] [Google Scholar]
- 16.Lübeck, P. S., N. Cook, M. Wagner, P. Fach, and J. Hoorfar. 2003. Towards an international standard for PCR-based detection of foodborne thermotolerant campylobacters: validation in multi-center collaborative trials. Appl. Environ. Microbiol. 69:5670-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malorny, B., P. T. Tassios, P. Rådström, N. Cook, M. Wagner, and J. Hoorfar. 2003. Standardization of diagnostic PCR for the detection of foodborne pathogens. Int. J. Food. Microbiol. 83:39-48. [DOI] [PubMed] [Google Scholar]
- 18.Moorhead, S. M., and G. A. Dykes. 2002. Survival of Campylobacter jejuni on beef trimmings during freezing and frozen storage. Lett. Appl. Microbiol. 34:72-76. [DOI] [PubMed] [Google Scholar]
- 19.On, S. L. W. 1996. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 9:405-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.On, S. L. W. 2001. Taxonomy of Campylobacter, Arcobacter, Helicobacter, and related bacteria: current status, future prospects, and immediate concerns. Symp. Suppl. Soc. Appl. Microbiol. 90:1S-15S. [DOI] [PubMed] [Google Scholar]
- 21.On, S. L. W., and P. J. Jordan. 2003. Evaluation of 11 PCR assays for species-level identification of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 41:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, R. W. A., P. L. Griffiths, and G. S. Moreno. 1991. Sources and survival of campylobacters: relevance to enteritis and the food industry. Symp. Suppl. J. Appl. Bacteriol. 70:97S-106S. [PubMed] [Google Scholar]
- 23.Scheu, P. M., K. Berghof, and U. Stahl. 1998. Detection of pathogenic and spoilage microorganisms in food with the polymerase chain reaction. Food Microbiol. 15:13-31. [Google Scholar]
- 24.Trust, T. J., S. M. Logan, C. E. Gustafson, P. J. Romaniuk, N. W. Kim, V. L. Chan, M. A. Ragan, P. Guerry, and R. R. Gutell. 1994. Phylogenetic and molecular characterization of a 23S rRNA gene positions the genus Campylobacter in the epsilon subdivision of the Proteobacteria and shows that the presence of transcribed spacers is common in Campylobacter spp. J. Bacteriol. 176:4597-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uyttendaele, M., R. Schukkink, B. van Gemen, and J. Debevere. 1994. Identification of Campylobacter jejuni, Campylobacter coli and Campylobacter lari by the nucleic acid amplification system NASBA. J. Appl. Bacteriol. 77:694-701. [DOI] [PubMed] [Google Scholar]
- 26.Van Berkum, P., Z. Terefework, L. Paulin, S. Suomalainen, K. Lindström, and B. D. Eardly. 2003. Discordant phylogenies within the rrn loci of rhizobia. J. Bacteriol. 185:2988-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaneechoutte, M., and J. Van Eldere. 1997. The possibilities and limitations of nucleic acid amplification technology in diagnostic microbiology. J. Med. Microbiol. 46:188-194. [DOI] [PubMed] [Google Scholar]
- 28.Vanniasinkam, T., J. A. Lanser, and M. D. Barton. 1999. PCR for the detection of Campylobacter spp. in clinical specimens. Lett. Appl. Microbiol. 28:52-56. [DOI] [PubMed] [Google Scholar]
- 29.Waller, D. F., and S. A. Ogata. 2000. Quantitative immunocapture PCR assay for detection of Campylobacter jejuni in foods. Appl. Environ. Microbiol. 66:4115-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young, J. M. 2001. Implications of alternative classifications and horizontal gene transfer for bacterial taxonomy. Int. J. Sys. Evol. Microbiol. 51:945-953. [DOI] [PubMed] [Google Scholar]