Abstract
The genetic heterogeneity of the nomenspecies Enterobacter cloacae is well known. Enterobacter asburiae, Enterobacter cancerogenus, Enterobacter dissolvens, Enterobacter hormaechei, Enterobacter kobei, and Enterobacter nimipressuralis are closely related to it and are subsumed in the so-called E. cloacae complex. DNA-DNA hybridization studies performed previously identified at least five DNA-relatedness groups of this complex. In order to analyze the genetic structure and the phylogenetic relationships between the clusters of the nomenspecies E. cloacae, 206 strains collected from 22 hospitals, a veterinarian, and an agricultural center in 11 countries plus all 13 type strains of the genus and reference strain CDC 1347-71R were examined with a combination of sequence and PCR-restriction fragment length polymorphism (PCR-RFLP) analyses of the three housekeeping genes hsp60, rpoB, and hemB as well as ampC, the gene of a class C β-lactamase. Based on the neighbor-joining tree of the hsp60 sequences, 12 genetic clusters (I to XII) and an unstable sequence crowd (xiii) were identified. The robustness of the genetic clusters was confirmed by analyses of rpoB and hemB sequences and ampC PCR-RFLPs. Sequence crowd xiii split into two groups after rpoB analysis. Only three strains formed a cluster with the type strain of E. cloacae, indicating that the minority of isolates identified as E. cloacae truly belong to the species; 13% of strains grouped with other type strains of the genus, suggesting that the phenotypes of these species seem to be more heterogeneous than so far believed. Three clusters represented 70% of strains, but none of them included a type or reference strain. The genetic clustering presented in this study might serve as a framework for future studies dealing with taxonomic, evolutionary, epidemiological, or pathogenetic characteristics of bacteria belonging to the E. cloacae complex.
Enterobacter cloacae has become increasingly important as a nosocomial pathogen, accounting for up to 5% of hospital-acquired septicemias, 5% of nosocomial pneumonias, 4% of nosocomial urinary tract infections, and 10% of postsurgical peritonitis cases (18, 35, 39). Besides its clinical significance, E. cloacae plays an important role as a pathogen in plants (2, 34) and insects (15) and is ubiquitous in the terrestrial and aquatic environments (22). This diversity of habitats is mirrored by the genetic variety of the nomenspecies E. cloacae, which has been demonstrated in several studies by biotyping and serotyping (16, 47), ribotyping and phage typing (46), pulsed-field gel electrophoresis and enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) (18), and internal transcribed spacer-PCR and random amplification of polymorphic DNA (RAPD)-PCR (6). The 16S ribosomal DNA (rDNA) sequences of E. cloacae did not form a coherent cluster but built a patchy tree, in which the clusters of E. cloacae strains interfused with those of Enterobacter aerogenes, Escherichia coli, Citrobacter species, and Leclercia species. This reflected the genetic heterogeneity of the nomenspecies but did not allow its systematic classification (44).
Over the last few years, five new species (Enterobacter asburiae, Enterobacter cancerogenus, Enterobacter dissolvens, Enterobacter kobei, and Enterobacter nimipressuralis) have been reassigned to or split out of the nomenspecies E. cloacae (4, 31). E. dissolvens is the closest relative to E. cloacae, since the DNA relatedness of the two species was up to 82% in DNA-DNA hybridization experiments and they were phenotypically not differentiable (4, 25). E. nimipressuralis had a DNA relatedness of up to 67% to E. cloacae and differed phenotypically from it by being sucrose negative. E. asburiae had a DNA relatedness of 63% to E. cloacae and differed from it by being Voges-Proskauer, malonate, melibiose, and l-rhamnose negative (4). E. hormaechei also had a DNA relatedness of 63% to E. cloacae and differed from it by being d-sorbitol, melibiose, and esculin negative (31). E. cancerogenus is considered purely phytopathogenic and was transferred from the genus Erwinia to the genus Enterobacter as a senior synonym of Enterobacter taylorae (13). It had a DNA relatedness of 61% to E. cloacae and differed from it by being, inter alia, ornithine decarboxylase negative and d-arabinose positive. E. kobei had a DNA relatedness of 28% to E. cloacae and of 72% to CDC enteric Group 69. It differed from E. cloacae only by being Voges-Proskauer negative (24). Most of these species have a DNA relatedness of over 60% to E. cloacae and form the so-called E. cloacae complex.
Two DNA-DNA hybridization studies have so far partly analyzed the genetic clustering of the E. cloacae complex by examining local isolates with E. cloacae phenotypes (21, 25), delineating the following genogroups. One group contained both the E. cloacae and E. dissolvens type strains. A second formed around reference strain CDC 1347 41R and was not ascribed to a species (42). A third formed around the E. hormaechei type strain and contained seven biogroups. A fourth was found around the E. asburiae type strain and consisted of three biogroups. Finally, one group in the study of Grimont and Grimont (21) and three in that of Lindh and Ursing (25) as well as up to 11 ungroupable strains were not assigned to type or reference strains. However, the hybridization studies of Grimont and Grimont (21) and Lindh and Ursing (25) are difficult to compare. Both authors examined collections of strains recovered at local hospitals in Paris and Copenhagen, respectively. Beside others, this might be the reason for the partly different results of the authors. For example, Grimont and Grimont (21) reported 30% of study strains clustering with the E. asburiae type strain, while none of those of Lindh and Ursing (25) clustered with it. Grimont and Grimont (21) found a genetic group of nitrogen-fixing strains (group 5), whereas Lindh and Ursing (25) did not find such a group but reported almost 10% ungroupable strains. An extensive, population genetic-based classification of E. cloacae remains elusive.
Population genetic studies have proven to be extremely useful for getting insights into the epidemiology and evolution of the pathogenicity of bacterial species, as exemplified in previous studies on the species (3) and on the genus level (14). The objectives of this study were to describe the genetic structure of the nomenspecies E. cloacae and to explore the phylogenetic relationships between its genetic groups and related species. For this purpose, we analyzed 225 strains with E. cloacae phenotypes from 22 European regions and all type strains of the genus Enterobacter with a combination of partial sequencing of three housekeeping genes and PCR-restriction fragment length polymorphism (PCR-RFLP) of a further protein-coding gene.
MATERIALS AND METHODS
Bacterial test collection.
The 241 isolates were sent to us from the United States and from 22 microbiology centers located in 10 European countries from Norway to Mallorca, Spain (north-south axis), and Poland to France (west-east axis). They were recovered from clinical specimen and collected in Austria (Innsbruck 10 strains), Belgium (Brussels 10 strains), France (Marseille 9 strains), Germany (Aachen 12 strains, Berlin 12 strains, Frankfurt 14 strains, Freiburg 9 strains, Gelsenkirchen 11 strains, Hannover 10 strains, Heidelberg 10 strains, Jena 10 strains, Kiel 11 strains, Munich 28 strains, Regensburg 9 strains, and Tübingen 12 strains), Norway (Bergen 4 strains), Poland (Cracow 10 strains), Scotland, United Kingdom (Lanarkshire 6 strains), Spain (Mallorca 6 strains), Switzerland (Lausanne 9 strains), and Sweden (Stockholm 10 strains, Växjö 6 strains). Two isolates were recovered from cucumbers in the United States (34). Eleven isolates from animals were kindly provided by a veterinarian microbiologist of Bavaria, Germany, and were isolated from a monkey, two dogs, a cat, two rabbits, a guinea pig, a wild mouse, a bat, a duck, and a parrot. All isolates were identified as E. cloacae by phenotypic methods with API20E (BioMérieux, Marcy-l'Etoile, France) or Crystals (BBL) plus the typical antibiotic susceptibility pattern of Enterobacter species or by automated systems like Vitek I, Vitek II, or Micronaut. At our laboratory, the API20E code and an extended antibiotic susceptibility pattern was reevaluated for all isolates. Only one isolate per patient, plant and animal was included in the study.
All 13 type strains of the genus Enterobacter were used as points of reference. They are listed in Table 1. Reference strain CDC 1347-71R (42) was included as a reference for DNA relatedness groups 2 of Lindh and Ursing (25) and Grimont and Grimont (21). Type and reference strains were obtained from the American Type Culture Collection and the Collection de l'Institut Pasteur (CIP). In addition, the published hsp60 sequence of Enterobacter aerogenes (accession number AB008141) and the hemB sequence of E. coli (accession number L44595) were downloaded from GenBank for direct comparison.
TABLE 1.
Strains analyzed and their characteristics
| Cluster | Strainsa | No. of strains analyzed |
Amino acid alterationsb |
ampC PCR-RFLP | ||||
|---|---|---|---|---|---|---|---|---|
| hsp60 | rpoB | hemB | Hsp60 (91 aa) | RpoB (336 aa) | HemB (72 aa) | |||
| I | E. asburiae ATCC 35953cT+$, 501R3h⋆+$, E6h⋆+$, EN299c+, EN373a+, EN485c+, EN497b, EN539b+, EN640k$ | 9 | 7 | 4 | E. asburiae ATCC 35953T: A466→T | 501R3: D42→Y | None | g |
| EN373: V28→G | ||||||||
| II | E. kobei ATCC BAA-260aT+$, CDC 1347 71kR+$, EN24d+$, EN326a+, EN330b, EN355b+, EN478a, EN488b, EN499c+, EN529d, EN536d+$, EN549d, EN632b , EN634a | 14 | 7 | 4 | None | EN347: S298→A | None | a |
| III | EN114b+$, EN125c, EN213b, EN261b, EN286b, EN304c+, EN310k, EN313k+, EN317k, EN318k, EN322g+, EN329a+, EN333e, EN336c, EN341b, EN343b+, EN344b, EN346b, EN353g, EN356g, EN362b, EN372a, EN374a, EN376k+, EN378k, EN387b$, EN389g, EN396g, EN397g, EN398g, EN399b, EN401b, EN402b, EN404c, EN467g, EN472g, EN476g, EN482a, EN483g, EN491k, EN492k+, EN494b, EN498b, EN500c, EN521b, EN523a, EN525a$, EN527c, EN528g, EN531e, EN532g, EN535g, EN548b, EN560c, EN563g, EN565e, EN608b , EN611b | 58 | 9 | 3 | EN492: G439→C | EN521: L193→M | None | b |
| EN343: M447→V | EN492: E137→K; V141→L | |||||||
| EN348: V499→A | EN521: L193→M | |||||||
| IV | EN19c+, EN117f+$, EN316k+$, EN345c+, EN347b+, EN496b+, EN519g+, EN533d+$, EN538f+ | 9 | 9 | 3 | All: N468→K | EN19: K2→1; T315→P | None | g |
| EN538: V1→A | ||||||||
| V | EN36a+, EN119c+$, EN124c+, EN128f, EN303c+, EN319g+, EN338c+, EN340b+$, EN342g+, EN479b, EN493k, EN495b, EN517g, EN561g$ | 14 | 8 | 3 | All: N465→M | EN124: K2→1 | None | h |
| VI | EN5b, EN18f+$, EN306c, EN312k, EN314k+, EN332b, EN334c, EN351b, EN360g, EN366g$, EN368b, EN375k, EN386d, EN409g, EN489b, EN508c+$, EN510a, EN514c, EN516g, EN518g, EN526b, EN547c, EN552b, EN556c, EN558b, EN609f , EN631g , EN637k | 28 | 3 | 3 | All: N468→K | None | None | d |
| VII | E. hormaechei ATCC 49162bT+$, EN280b+, EN291b+$ | 3 | 3 | 2 | None | None | None | b |
| VIII | EN30b+$, EN285g, EN288g+, EN290a, EN292g, EN293c, EN305d+, EN311k, EN315k, EN320g, EN323g, EN325c, EN327a, EN331b+$, EN349c, EN352b+, EN359c, EN365a+, EN369a$, EN370a, EN371a, EN380k, EN384c, EN390a, EN391b+, EN395d+, EN400b, EN403g, EN405b, EN407g+$, EN410b, EN466b, EN468e, EN469b, EN474c, EN477c+, EN480g, EN481b, EN484g, EN486a, EN487c, EN490b, EN513c, EN515g, EN530g, EN545c, EN550b, EN551c, EN553c, EN554a, EN559b, EN562g, EN610c , EN613b $, EN628f , EN630g $, EN635k, EN636k, EN638k | 59 | 12 | 6 | None | EN288: L5→M | None | d |
| EN391: V1→A; V84→L | ||||||||
| IX | EN25a+$, EN363g+$, EN364g+, EN520b+$, EN524a+ | 5 | 5 | 3 | None | None | None | c |
| X | E. nimipressuralis ATCC 9912hT⋆+$, EN633g +$ | 2 | 2 | 2 | None | None | None | k |
| XI | E. cloacae ATCC 13047aT+$, EN90b+$, EN287c+$, EN475g+$ | 4 | 4 | 4 | All: T430→S | None | None | No product |
| XII | E. dissolvens ATCC 23373hT⋆+$, EN361g+$, EN408d+$ | 3 | 3 | 3 | All: A468→T | EN408: S168→F | None | e/PICK> |
| XII | E. dissolvens ATCC 23373hT⋆+$, EN361g+$, EN408d+$ | 3 | 3 | 3 | All: A468→T | EN408: S168→F | None | e |
| Xiii | EN28a+, EN385b+, EN509a+, EN512a+, EN612f | 5 | 4 | 0 | All: A466→T | EN28: V1→E | f + i | |
| EN509: E167→Q; T218→S; L219→V | ||||||||
| Type strains | E. amnigenus ATCC 3072kT, E. cancerogenus ATCC 33241hT⋆+, E. cowanii CIP 107300aT, E. gergoviae ATCC 33028cT+, E. intermedius ATCC 33110hT, E. pyrinus ATCC 49851hT⋆, E. sakazakii ATCC 29544bT | 7 | 2 | 0 | ||||
| Total | 220 | 78 | 40 | |||||
All strains for which hsp60 was analyzed. T, type strain. R, reference strain of genomic group 2 (21, 25); + strains for which rpoB and ampC were analyzed in addition to hsp60; $, strains included in the hemB tree of Fig. 3; ⋆, environmental strains isolated from plants; , strains isolated from animals. Footers indicate materials from which strains were isolated: a, blood cultures and primarily sterile fluids like cerebrospinal fluid; b, respiratory tract; c, urogenital tract; d, peritoneal swabs, ascites, gall bladder; e, invasive medical devices; f, stool; g, skin swabs, wounds, abscesses; h, isolates from plants or legumes; k, not specified.
With reference to the majority of strains.
An ERIC-PCR was performed for all isolates (see below). In cases of identical ERIC patterns for two or more isolates, all but one of them was excluded. The remaining study strains are listed in Table 1. Bacteria were routinely grown at 36°C overnight in Luria-Bertani (LB) broth with slow shaking (100 rpm) or on LB-agar plates. For antibiotic susceptibility testing, bacteria were plated onto Mueller Hinton agar. Susceptibility was tested with agar diffusion tests following published guidelines (30).
Biochemical and susceptibility testing.
API20E (BioMérieux, Marcy l'Etoile, France) testing was performed following the manufacturer's instructions. The results were interpreted with the Analytical Profile Index (API) database of the ApiLab Plus software (version 3.3.3; BioMérieux, Marcy l'Etoile, France). Antibiotic susceptibilities to ampicillin, ampicillin plus clavulanic acid, cefoxitin, ceftazidime, and cefotaxime were tested. The expression of the chromosomal AmpC cephalosporinase was interpreted as follows: no expression if the strain was susceptible to all antibiotics tested; basal expression if the strain was resistant to ampicillin, ampicillin-clavulanic acid, and/or cefoxitin, hyperexpression if the strain was resistant to ampicillin, ampicillin-clavulanic acid, cefoxitin, ceftazidime, and cefotaxime.
DNA preparation.
Bacterial DNA was prepared for PCR by quick-heat lyses. Bacteria were grown in LB to an optical density at 600 nm of 1.0. Then 100 μl of the culture was centrifuged (2 min at 10,000 rpm). The pellet was diluted in 1 ml of distilled water and boiled for 10 min.
ERIC-PCR analysis.
ERIC primers annealing to enterobacterial repetitive intergenic consensus regions were used as previously described (18). Amplification reactions were carried out in an Applied Biosystems GeneAmp 2700 PCR thermocycler. Thin-walled PCR tubes were purchased from Applied Biosystems (Branchburg, N.J.). Primers ERIC 1and ERIC 2 (Table 2) were used and synthesized by Metabion, Munich, Germany. Reactions were performed in a total volume of 30 μl under standard conditions (500 pmol of each primer, 200 μM nucleotide mix, 10 mM Tris-Cl [pH 8.3], 5 mM KCl, 1.5 mM MgCl2, and 2.5 U of standard Taq polymerase). After a 10-min heating period at 95°C, 35 amplification cycles were run with denaturation at 95°C for 1 min, annealing at 45°C for 1 min, and extension at 72° for 1 min followed by a final extension time of 15 min at 72°C. ERIC-PCR products were visualized after electrophoresis in 3% agarose gels in 1× TAE buffer, staining with ethidium bromide, and exposure to UV light. In each run, ERIC-PCR was repeated for strain EN114 in order to control the standardization of the conditions. The ERIC patterns of EN114 were identical for all three PCR runs performed. Patterns of different strains were compared by visual inspection. The patterns were interpreted as identical if an identical number of bands of identical sizes was found.
TABLE 2.
PCR assays and primers used
| PCR assay | PCR primer | Primer sequence, 5′→3′ | Tm (°C) | PCR product (nt) | Reference |
|---|---|---|---|---|---|
| ERIC | ERIC-1 | ATG TAA GCT CCT GGG GAT TCA C | 60.3 | Random | 18 |
| ERIC-2 | AAG TAA GTG ACT GGG GTG AGC G | 62.1 | |||
| RpoB | RpoB-F | AAC CAG TTC CGC GTT GGC CTG G | 65.8 | 1,088 | 29 |
| RpoB-R | CCT GAA CAA CAC GCT CGG A | 58.8 | |||
| Hsp60 | Hsp60-F | GGT AGA AGA AGG CGT GGT TGC | 61.8 | 341 | |
| Hsp60-R | ATG CAT TCG GTG GTG ATC ATC AG | 60.6 | |||
| HemB | HemB-F | GGC AGA CCA TGA CAG ACT TAA T | 64 | 570 | |
| HemB-R | ACC TGC AGC AGC TGC AAC CA | 60 | |||
| HemBu | HemBu-F | ACT TCT CAC GGT CAC TGC GGT | 61.8 | 459 | |
| HemBu-R | CAT SGC ATA CTC ACC GCT CAC | 61.8 | |||
| HemB_all | HemB_all-F | CG RCG GTT VAG CGG GTT CAT CTG | 66.3 | 237 | |
| HemB_all-R | TGA AYC TBG GCA AGC AGG CBG T | 63.7 | |||
| AmpC | AmpC-F | TCT CTT GCT CTG CTC GCC | 61.4 | 647 | |
| AmpC-R | ACC GCT TTA CCG TCA CGA TAG | 59.8 | |||
| AmpCu | AmpCu-F | AAA TCC CTT TGC TGT GCC CTG | 59.8 | 657 | |
| AmpCu-R | CCA GGC GTA ATG CGC CTC TTC | 63.7 |
PCR assays.
The following PCR assays were used: Hsp60, amplifying the groEL homologue coding for the 60-kDa heat shock protein; RpoB, amplifying the RNA polymerase beta subunit (rpoB) gene; HemB, amplifying the porphobilinogen synthase gene (hemB), which codes for a single-pathway enzyme in the early heme biosynthesis cascade; and AmpC, amplifying the chromosomal gene of the Bush type 1 cephalosporinase of E. cloacae, which has recently been crystallized for E. cloacae strain 99 (26). The PCR primers listed in Table 2 were designed based on conserved regions of the alignment of gene homologues of different Enterobacteriaceae. Since only very few sequence data are available for Enterobacter species, the sequences used belonged to species of other genera, too. The sequences of the following GenBank accession numbers were used for the design of the PCR primers: for Hsp60, Escherichia coli M11294, E. amnigenus AB008140, E. asburiae AB008137, E. gergoviae AB008139, and Yersinia enterocolitica X82212; for HemB, Escherichia coli L44595, Salmonella enterica serovar Typhimurium AE008712, and Shigella flexneri AE016978; and for AmpC, E. asburiae AJ311172, E. cloacae AB016611, E. cloacae AF411144, E. cloacae D44479, and E. cloacae X08081. The RpoB primers were taken over as previously published (29).
Single sets of primers could be used for Hsp60 and RpoB PCRs. Different sets of primers were needed for HemB PCR (sets HemB, HemBu, and HemB_all) and AmpC PCR (sets AmpC and AmpCu) because conserved regions were not identical in all sequences and for some clusters PCR products could not be achieved with one of the primer sets alone. PCR products of HemB, HemBu, AmpC, and AmpCu were sequenced unidirectionally with the forward primers for representatives of each strain cluster in order to verify that the correct target gene had been amplified. The hemB sequences obtained in this first step were aligned. Universal HemB_all primers were designed corresponding to their most homologous regions. PCR products were purified with the Nucleospin PCR cleaning kit (Macherey and Nagel, Dueren, Germany) before further processing.
PCR assays were performed with a Perkin-Elmer Gene Amp PCR System 2400 or an Applied Biosystems GeneAmp PCR System 2700 and Taq Gold DNA polymerase (Perkin-Elmer, Branchburg, N.J.) under standard conditions (see ERIC-PCR). Reactions were carried out in a total volume of 50 μl and started after a preheating time of 10 min at 95°C. Samples were subjected to 30 cycles of 30 s at 95°C, 30 s at annealing temperature, and 30 min of extension at 72°C. Cycles were followed by a final elongation step of 15 min at 72°C. Annealing temperatures were optimized by increasing the annealing temperature stepwise by 2°C, starting at 6°C below the lowest Tm of the primer sets used, until specific, single-banded PCR products were obtained. Due to the relatively high variability of the genes, PCRs of the ampC and hemB genes were only applicable at annealing temperatures of 50°C. Otherwise, the following annealing temperatures were chosen: 57.5°C for hsp60 and 59°C for rpoB. PCR products were visualized in 2.0% agarose gels after electrophoresis and ethidium bromide staining.
DNA sequencing.
PCR-amplified DNA was sequenced with the dye terminator method in both directions. To prepare cycle sequencing reactions, 0.2 μg of purified DNA was added to a 20-μl reaction solution containing 4 μl of BigDye dye terminator sequencing mix and 0.7 μl of a 5-pmol primer solution. Cycle sequencing was performed in the Applied Biosystems GeneAmp PCR System 2700. Cycle parameters were 25 cycles with an initial 96°C denaturation step of 10 s, followed by an annealing step at 57°C of 10 s and an extension step of 4 min at 60°C. Sequences were determined by electrophoresis with the ABI Prism 377 DNA sequencer. About 1% of base pairs sequenced resulted in a nontypeable character. They have been corrected, based on the reverse sequences, with the SeqMan (DNAStar) program.
PCR-RFLP.
In order to determine group-specific restriction patterns of ampC, PCR products were subjected to analysis of the PCR-RFLP patterns after restriction with HaeIII (Invitrogen, Groningen, The Netherlands). This restriction enzyme was chosen after computer analysis of the different ampC sequences of representative strains (EN30, EN117, EN124, E. asburiae type strain, and E. dissolvens type strain) with MapDraw (DNAStar Inc., 1999). Restriction was performed with 1 μg of DNA in a total volume of 20 μl over 4 h at 37°C. DNA was electrophoresed in 2% or 3% agarose gels, stained with ethidium bromide, and visualized with UV light.
Data analysis.
Sequence data were analyzed with MegAlign (DNAStar Inc., 1999) and PAUP version 4.0b10 (43) on a Macintosh G4 personal computer. Multiple alignment was performed with Clustal V, included in the MegAlign program. With MegAlign, pairwise percent sequence divergence was calculated as 100 times the sum of the residue distances from one strain to the other along the tree divided by the sum of all branch lengths. With Microsoft Excel, mean percent sequence divergences between clusters of sequences and the distinctness parameters k were calculated as previously described (32).
With PAUP 4.0b10, neighbor-joining trees (38) were estimated based on pairwise genetic distances on the basis of all substitutions with the Jukes-Cantor distance parameter. A group of strains was called a genetic cluster if the mean distinctness parameter k was above 2.0; otherwise it was called a sequence crowd. The significance of branchings was evaluated by bootstrap analysis of 100 replicates. Only groups with frequencies above 50% were kept. Bootstrap values above 80% were considered significant, and the cluster was designated as being strongly supported.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences reported in this paper are: for hsp60, AJ417108 to AJ417143, AJ543761 to AJ543910, and AJ567846 to AJ567902; for rpoB, AJ543656 to AJ543728 and AJ566948 to AJ566941; for hemB, AJ426490 to AJ426522 and AJ567697 to AJ567724.
RESULTS
Strain collection.
In order to establish a representative population genetic study, we aimed to examine strains with great genetic variety. Therefore, we collected 241 isolates with E. cloacae phenotypes from 22 European medical and veterinarian centers and an agricultural center in the United States. All isolates were twice identified as E. cloacae, at the respective centers and at our laboratory by biochemical and susceptibility analyses. Although the isolates derived from different patients, animals, or plants, there was still a theoretical chance that clonal isolates were recovered in the frame of epidemic outbreaks at the centers. Their inclusion would jeopardize the representativeness of the population genetics because pseudoclusters of clonal strains would form.
To eliminate this risk, ERIC-PCRs were performed for all isolates. The good specificity of ERIC-PCR for the detection of identical isolates has recently been demonstrated (18). Comparing the ERIC patterns obtained by visual inspection, 35 isolates showed patterns which were identical to those of other strains (data not shown). They were excluded. In addition to the remaining 206 study strains, all 13 type strains of the genus Enterobacter and reference strain CDC 134771R, which represents genetic group 2 of Grimont and Grimont (21), were included. All 220 strains and their characteristics are listed in Table 1. They were isolated from blood cultures (26 strains), the respiratory tract (59 strains), urogenital tract (38 strains), abdomen (9 strains), superficial swabs (44 strains), artificial devices (7 strains), stool (7 strains), plants (7 strains), and unknown origins (23 strains).
Molecular systematics analysis.
Despite their public health significance, only very few sequence data for housekeeping genes were available for Enterobacter species at public databases. Hence, we tried oligonucleotides for the amplification of the gyrase A gene (gyrA), gyrase B gene (gyrB), or subunit C of topoisomerase IV (parC) published for other species (5, 10), but PCR products were not obtained with any of them for all of the study strains. However, we obtained PCR products for all strains by amplifying hsp60, rpoB, and hemB.
Molecular phylogeny based on hsp60 sequence analysis.
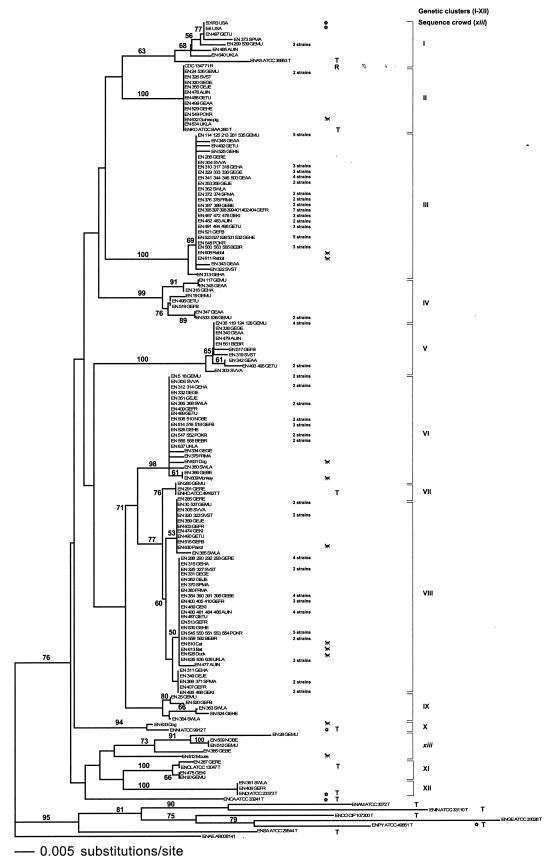
The sequences of a portion of 273 nucleotides of the hsp60 gene were determined for the study set of 220 strains by bidirectional sequencing. All sequences were pairwise aligned; 91 characters (33%) were variable (defined as different in at least one strain). A neighbor-joining tree was constructed with all 220 study strains (Fig. 1). Since E. aerogenes is a homotypic synonym to Klebsiella mobilis and belongs to another genus, its hsp60 sequence was used to root the tree; 13 groups of strains were found.
FIG.1.
Neighbor-joining tree after analyzing 273 nucleotides (91 variable) of the hsp60 gene of 206 study strains and 14 type and reference strains of the genus Enterobacter. The tree was rooted with the hsp60 sequence of E. aerogenes downloaded from GenBank (ENAE, accession number indicated). Type strains are labeled with T, reference strains with R, isolates from plants with a flower symbol, and isolates from animals with a picture of a dog. The animals from which strains were isolated are indicated after strain denominations, as are the abbreviations of the centers (see below) from which the strains originated. If several strains from one center had identical sequences, they were pooled at one spot of the tree, and the number of pooled strains is indicated after the center abbreviation. The scale gives the Jukes-Cantor distance along the branches. Numbers at the nodes of the tree indicate bootstrap values obtained after 100 replicates. A group of strains were considered a genetic cluster (I to XII) if the mean distinctness parameter k was above 2.0 (Table 3); otherwise it was called a sequence crowd (xiii). Cluster and sequence crowd denominations are indicated at the right side of the figure. Species abbreviations: ENAM, E. amnigenus; ENAS, E. asburiae; ENCA, E. cancerogenus; ENCL, E. cloacae; ENCO, E. cowanii; ENDI, E. dissolvens; ENGE, E. gergoviae; ENHO, E. hormaechei; ENIN, E. intermedius; ENKO, E. kobei; ENNI, E. nimipressuralis; ENPY, E. pyrinus; ENSA, E. sakazakii. Center abbreviations: AUIN, Innsbruck, Austria; BEBR, Brussels, Belgium; FRMA, Marseille, France; GEAA, Aachen, Germany; GEBE, Berlin, Germany; GEFB, Freiburg, Germany; GEFR, Frankfurt, Germany; GEGE, Gelsenkirchen, Germany; GEHA, Hannover, Germany; GEHE, Heidelberg, Germany; GEJE, Jena, Germany; GEKI, Kiel, Germany; GEMU, Munich, Germany; GERE, Regensburg, Germany; GETU, Tuebingen, Germany; NOBE, Bergen, Norway; POKR, Cracow, Poland; SPMA, Mallorca, Spain; SVST, Stockholm, Sweden; SVVA, Vaexjoe, Sweden; SWLA, Lausanne, Switzerland; UKLA, Lanarkshire, Scotland, United Kingdom.
A group of strains was considered a genetic cluster when its mean distinctness parameter k, which represents a quantitative measure of distinctness (32), was above 2.0 (Table 3). A k parameter above 2.0 indicates that sequence similarity groups can be considered distinct genetic clusters. Otherwise the group was called a sequence crowd. Based on the k parameters, 12 clusters (I to XII) and one sequence crowd (xiii) could be distinguished.
TABLE 3.
Mean percent sequence divergence within and between the sequence clusters and k parameters (given as ratio of between-group divergence to the mean of within-group divergence)a
| Gene | Cluster | Data for cluster |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | xiii | ||
| hsp60 | I | 3.8 ± 2.3 | 2.5 | 2.9 | 1.7 | 3.7 | 3.4 | 3.1 | 3.2 | 1.9 | 3.0 | 2.8 | 4.3 | 1.2 |
| II | 4.8 ± 0.5 | 0 ± 0 | 31.0 | 3.4 | 47.0 | >99 | >99 | 18.0 | 8.6 | 8.7 | 17.7 | >99 | 1.8 | |
| III | 6.0 ± 0.4 | 4.6 ± 0.2 | 0.3 ± 0.2 | 3.7 | 25.1 | 36.2 | 39.5 | 13.4 | 6.8 | 10 | 14.7 | 54.6 | 1.8 | |
| IV | 5.2 ± 1.8 | 4.5 ± 0.3 | 5.4 ± 0.3 | 1.0 ± 0.6 | 4.1 | 3.8 | 4.0 | 3.4 | 2.4 | 6.4 | 3.5 | 5.6 | 1.3 | |
| V | 7.5 ± 0.7 | 7.1 ± 0.2 | 7.5 ± 0.3 | 6.1 ± 0.3 | 0.3 ± 0.2 | 38.3 | 38.0 | 12.7 | 10.9 | 11.6 | 19.2 | 60.3 | 2.2 | |
| VI | 6.4 ± 0.5 | 4.9 ± 0.0 | 5.4 ± 0.2 | 5.0 ± 0.3 | 5.8 ± 0.2 | 0 ± 0 | >99 | 6.4 | 6.0 | 15.4 | 21.7 | >99 | 1.9 | |
| VII | 6.0 ± 0.5 | 5.3 ± 0.0 | 5.9 ± 0.2 | 5.2 ± 0.3 | 5.7 ± 0.2 | 1.9 ± 0.0 | 0 ± 0 | 2.9 | 6.8 | 13.1 | 20.3 | >99 | 1.9 | |
| VIII | 7.0 ± 0.5 | 5.4 ± 0.3 | 6.0 ± 0.3 | 5.5 ± 0.4 | 5.7 ± 0.3 | 1.9 ± 0.3 | 0.9 ± 0.3 | 0.6 ± 0.4 | 4.3 | 8.3 | 10.4 | 23.6 | 1.9 | |
| IX | 4.6 ± 0.7 | 3.9 ± 0.6 | 4.1 ± 0.4 | 4.3 ± 0.5 | 6.5 ± 0.6 | 2.7 ± 0.5 | 3.1 ± 0.5 | 3.2 ± 0.5 | 0.9 ± 0.4 | 6.3 | 7.0 | 15.0 | 1.4 | |
| X | 6.6 ± 0.3 | 6.1 ± 0.4 | 5.0 ± 0.2 | 5.4 ± 0.6 | 5.8 ± 0.3 | 5.4 ± 0.4 | 4.6 ± 0.4 | 5.4 ± 1 | 5.0 ± 0.3 | 0.7 ± 0 | 12 | 20.3 | 1.5 | |
| XI | 6.1 ± 1.2 | 5.3 ± 0.0 | 6.6 ± 0.2 | 5.6 ± 0.5 | 8.6 ± 0.2 | 6.5 ± 0.0 | 6.1 ± 0.0 | 6.3 ± 0.3 | 5.2 ± 0.6 | 7.8 ± 0.1 | 0.6 ± 0.3 | 16.7 | 1.9 | |
| XII | 8.2 ± 1.7 | 6.9 ± 0.0 | 8.2 ± 0.2 | 7.3 ± 1.0 | 9.1 ± 0.1 | 8.5 ± 0.0 | 7.3 ± 0.0 | 7.1 ± 0.6 | 6.7 ± 0.4 | 7.1 ± 0.2 | 5.0 ± 0.2 | 0 ± 0 | 2.0 | |
| xiii | 6.7 ± 1.6 | 6.0 ± 1.2 | 6.4 ± 0.9 | 6.0 ± 2.0 | 7.7 ± 1.5 | 6.5 ± 1.6 | 6.4 ± 1.3 | 6.9 ± 1.6 | 5.3 ± 0.9 | 6.1 ± 1.1 | 6.9 ± 0.7 | 6.8 ± 0.7 | 6.7 ± 1.3 | |
| rpoB | I | 0.9 ± 0.3 | 4.0 | 4.9 | 2.8 | 3.7 | 6.5 | 5.9 | 4.6 | 3.8 | 8.4 | 3.8 | 4.5 | 1.4 |
| II | 2.4 ± 0.2 | 0.3 ± 0.2 | 6.0 | 5.0 | 9.1 | 13.7 | 11.2 | 5.1 | 4.17 | 14.9 | 5.1 | 6.6 | 1.4 | |
| III | 2.7 ± 0.1 | 1.5 ± 0.1 | 0.2 ± 0.1 | 6.7 | 11.7 | 9.6 | 5.6 | 5.4 | 2.2 | 16.5 | 5.6 | 8.4 | 1.8 | |
| IV | 2.0 ± 0.2 | 2.0 ± 0.2 | 2.4 ± 0.1 | 0.5 ± 0.2 | 5.0 | 11.3 | 9.3 | 6.3 | 4.6 | 12.2 | 5.6 | 6.4 | 1.5 | |
| V | 2.2 ± 0.2 | 2.7 ± 0.2 | 2.9 ± 0.1 | 2.0 ± 0.2 | 0.3 ± 0.1 | 19.7 | 19.3 | 8.5 | 7.5 | 14.2 | 7.1 | 10.9 | 1.9 | |
| VI | 2.9 ± 0.2 | 2.1 ± 0.1 | 1.9 ± 0.0 | 2.8 ± 0.1 | 3.0 ± 0.1 | 0 ± 0 | >99 | 5.5 | 9.6 | 23 | 10.9 | 19.1 | 1.9 | |
| VII | 2.6 ± 0.2 | 1.7 ± 0.1 | 1.1 ± 0.0 | 2.3 ± 0.1 | 2.9 ± 0.1 | 1.7 ± 0.0 | 0 ± 0 | 8.5 | 5.6 | 23.7 | 7.7 | 12.9 | 1.9 | |
| VIII | 3.0 ± 0.2 | 1.8 ± 0.2 | 1.6 ± 0.1 | 2.8 ± 0.2 | 3.0 ± 0.1 | 1.1 ± 0.1 | 1.7 ± 0.1 | 0.4 ± 0.2 | 4.3 | 14 | 5.5 | 7.5 | 1.7 | |
| IX | 2.5 ± 0.2 | 1.5 ± 0.2 | 0.8 ± 0.2 | 2.1 ± 0.2 | 2.6 ± 0.2 | 1.9 ± 0.2 | 1.1 ± 0.2 | 1.7 ± 0.2 | 0.4 ± 0.2 | 13.6 | 3.7 | 5.5 | 0.7 | |
| X | 6.3 ± 0.2 | 6.7 ± 0.9 | 6.6 ± 0.1 | 6.7 ± 0.2 | 6.4 ± 0.1 | 6.9 ± 0.0 | 7.1 ± 0.1 | 7.0 ± 0.1 | 6.8 ± 0.2 | 0.6 ± 0.0 | 12.7 | 16.2 | 3.8 | |
| XI | 2.6 ± 0.3 | 2.0 ± 0.3 | 1.9 ± 0.2 | 2.8 ± 0.2 | 2.9 ± 0.2 | 2.7 ± 0.2 | 1.9 ± 0.2 | 2.5 ± 0.3 | 1.7 ± 0.3 | 7.0 ± 0.1 | 0.5 ± 0.2 | 3.2 | 1.8 | |
| XII | 2.7 ± 0.2 | 2.0 ± 0.4 | 2.1 ± 0.1 | 2.6 ± 0.2 | 3.3 ± 0.2 | 2.9 ± 0.1 | 1.9 ± 0.1 | 2.6 ± 0.2 | 1.9 ± 0.2 | 7.3 ± 0.2 | 1.3 ± 0.3 | 0.3 ± 0.2 | 1.9 | |
| xiii | 2.6 ± 0.4 | 2.2 ± 0.2 | 2.7 ± 0.7 | 2.4 ± 0.4 | 2.9 ± 0.3 | 2.7 ± 0.6 | 2.6 ± 0.8 | 2.8 ± 0.6 | 2.4 ± 0.7 | 6.6 ± 0.5 | 2.9 ± 0.7 | 3.0 ± 0.7 | 2.8 ± 0.8 | |
Data in the left lower portions indicate mean percent sequence divergence [arithmetic mean ± standard deviation of percent sequence divergence (100 × pairwise nucleotide distances divided by total distance) within and between sequence clusters based on all pairwise comparisons of strains from the respective clusters]. Data in the right upper portions indicate the k parameter [ratio of the between-group divergence to the mean of the within-group divergence]. A ratio of more than 2 indicates that the groups can be considered separate sequence similarity clusters (26).
Three prominent clusters were found, together representing more than two thirds of the study strains (cluster III, 58 strains; cluster VI, 28 strains; and cluster VIII, 59 strains). None of these clusters included a type or reference strain. Separate clusters formed around the type strains of E. asburiae, E. cloacae, E. dissolvens, E. hormaechei, E. kobei, and E. nimipressuralis. However, these clusters contained only one (0.1%) to 12 (6%) study strains. The results of the bootstrap analysis strongly supported the majority of the clusters with the exceptions of clusters I (63%), containing the E. asburiae type strain, VII (76%), VIII (60%) and IX (<50%). Taking clusters VII and VIII together, the resulting metacluster was strongly supported (82%). Sequence crowd xiii had only poor bootstrap support (24%).
In order to get a better understanding of the distances of the clusters, the mean numbers of nucleotide differences along the tree were calculated as parameters of distance (Table 3). Mean percent sequence divergences within the clusters were equal to or below 1.0 (0 to 1.0 ± 0.6), with the exception of cluster I (3.8 ± 2.3), which was due to a relatively high divergence of the E. asburiae type strain to the other strains of the cluster. Sequence crowd xiii had a within group divergence of 6.7 ± 1.3. Divergences between the clusters were above 2.5 (2.7 ± 0.5 to 9.1 ± 0.1) with the exceptions of cluster pairs VI-VII, VI-VIII, and VII-VIII.
Some clusters showed specific amino acid alterations in the 60-kDa heat shock protein coded by the hsp60 gene (Table 1). For example, cluster V had a specific amino acid alteration at position 465, clusters IV and VI at position 468, cluster XI, including the E. cloacae type strain, at positions 430 and 466, and cluster XII, including the E. dissolvens type strain, at position 430 [E. coli amino acid numbering system (M11294)]. Interestingly, the E. asburiae type strain differed from the other strains of cluster I by an alteration at position 466.
Summarizing, hsp60 analysis suggested the existence of at least 12 genetic clusters and one heterogeneous sequence crowd within the E. cloacae complex. Eleven clusters (II to XII) were supported by bootstraps, and eight of them had strong bootstrap support. Clusters VII and VIII were well supported at a higher node.
Molecular phylogeny based on rpoB sequence analysis.
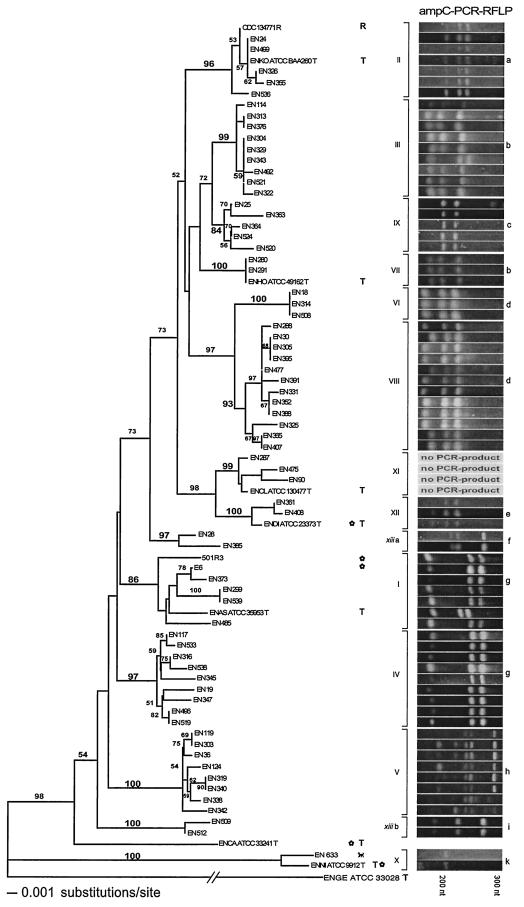
The sequences of a second housekeeping gene were investigated in order to reconfirm the clustering observed after hsp60 analysis. For this purpose, a representative subset of 78 strains was chosen, including all type and reference strains of the E. cloacae complex and the type strain of E. gergoviae. Sequences of a portion of 1,008 nucleotides of the rpoB gene were determined for these strains by bidirectional sequencing. About 700 nucleotides overlapped from both directions, leaving about 150 bp at each end sequenced unidirectionally. Multiple alignment by Clustal V revealed a total of 218 (22%) variable characters. The neighbor-joining relationships of the sequences are presented in Fig. 2 (left side). With the only exception being sequence crowd xiii, the rpoB tree was in strong agreement with the hsp60 tree. All strains were assigned to the same clusters. Moreover, much sharper delineations were observed, and bootstrap values were higher for clusters I, VIII, and IX. All k parameters but those of sequence crowd xiii were well above 2.0 (2.8 to 99), indicating that the rpoB sequence clusters could be considered distinct genetic groups (Table 2). However, in contrast to the hsp60 results, sequence crowd xiii was split into two groups (xiii.a and xiii.b). Correspondingly, its mean k parameter was well below 2.0 (1.6 ± 0.4). Recapitulating, the same genetic clusters observed for hsp60 appeared for rpoB. Sequence crowd xiii was unstable and fell apart.
FIG.2.
rpoB tree and ampC PCR-RFLP. Left side: Neighbor-joining tree based on the analysis of 1,008 nucleotides (142 nucleotides variable) of the rpoB gene of 78 study strains. The tree was rooted with the rpoB sequence of the E. gergoviae type strain. Bootstrap values after 100 replicates are indicated at the nodes of the tree. Cluster denominations, labeling, and abbreviations correspond to those of Fig. 1. Sequence crowd xiii was split into groups xiiia and xiiib. Right side: For PCR-RFLP analysis. PCR products of partial ampC genes were restricted with HaeIII, electrophoresed in 3% agarose, and strained with ethidium bromide. Resulting clusters were designated a to k.
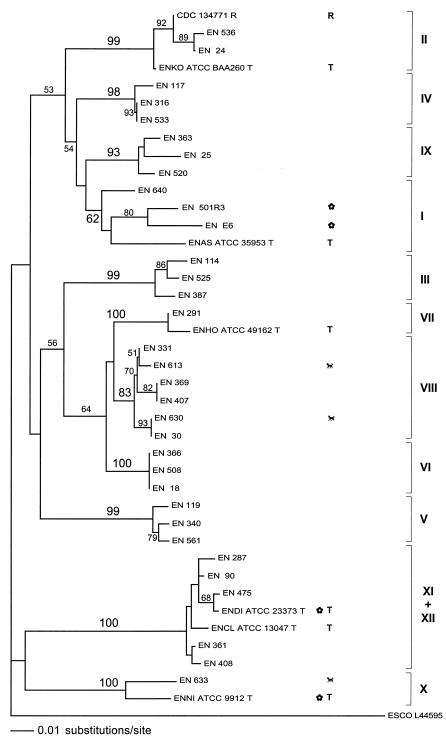
Molecular phylogeny based on hemB sequence analysis.
The sequences of a third protein-coding gene were analyzed in order to substantiate the robustness of the genetic clustering observed. Therefore, the partial hemB sequences of a subset of 40 study strains were analyzed. Only the stable genetic clusters I to XII were considered. Of the 214 characters determined, 68 (32%) were variable. The neighbor-joining relationships are presented in Fig. 3. Genetic clusters I to X reemerged as in the hsp60 and rpoB trees. Bootstrap supports were strong for all these clusters except for cluster I, containing the E. asburiae type strain (62%). Similar to the hsp60 tree, clusters VI, VII, and VIII formed a metacluster, which was strongly (98%) supported at a higher node. In contrast to the hsp60 and rpoB analyses, clusters XI and XII, containing the type strains of E. cloacae and E. dissolvens, were not separable. Strains of both clusters were mixed in a common cluster (XI plus XII), which however had strong bootstrap support (100%).
FIG. 3.
Neighbor-joining tree based on the analysis of 214 nucleotides (68 nucleotides variable) of the hemB gene of 40 study strains. Only the genetic clusters (not the sequence crowd) were considered. The tree was rooted with the hemB sequence of Escherichia coli (GenBank accession number L44595). Cluster denominations, labeling, and abbreviations correspond to those of Fig. 1.
PCR-RFLP analysis of ampC and susceptibility testing.
The gene products of housekeeping genes interact at best weakly with the environment (32). In order to include a gene coding for a protein specialized on the interaction with the environment, the ampC gene was subjected to PCR-RFLP with HaeIII. Based on antibiotic susceptibility testing, AmpC was expressed in 203 of the 206 (98%) study strains examined. Strains EN304, EN313, and EN633, which did not express AmpC, belonged to clusters III, IV, and X, respectively. However, ampC PCR was positive for all of them.
The ampC gene was amplified with one of the two PCR assays presented and restricted with HaeIII for all strains included in the rpoB analysis (Fig. 2). By visual inspection of the resulting PCR-RFLP patterns, 10 different clusters were delineable, designated a to k. Three pairs of sequence clusters showed similar restriction patterns (I and IV, III and VII, and VI and VIII). Four strains of cluster VIII showed patterns aberrant from the others, demonstrating a little within-group variability. The similarity of the restriction patterns of clusters III and VII was striking: whereas the distance of cluster VII to cluster III was much higher than that to clusters VI and VIII in the hsp60 and hemB trees, the distances were just the other way around in the ampC PCR-RFLP and rpoB tree. The E. asburiae type strain differed from the other strains of cluster I by an additional restriction site in the ampC pattern, underlining the relatively high within-group divergences of cluster I due to the distance of the E. asburiae type strain from the other members of the group (Fig. 1, Fig. 2, Fig. 3, and Table 3).
Whereas the PCR-RFLP patterns were similar for all strains of cluster XII, no PCR products were obtained for the strains of sequence cluster XI, including the E. cloacae type strain, with both PCR assays used. This supported the clustering of the hsp60 and rpoB trees, in which E. cloacae and E. dissolvens represented two distinct phylogenetic lineages, whereas they were mixed in the hemB tree.
Do different genetic clusters show specific distributions to clinical materials?
To some extent, genetic clusters were not randomly distributed to the different groups of clinical materials from which the strains were recovered. Some features should be outlined. First, E. nimipressuralis (cluster X) was not isolated from a human specimen. Second, 15% of strains of cluster VIII (59 strains) were recovered from blood cultures representing more than a third of all blood culture isolates. In contrast, only 3% of strains of the closely related cluster VI (28 strains) were recovered from blood cultures. Third, whereas none of the 58 strains of cluster III were recovered from abdominal specimens, 40% of studied strains of cluster II were recovered from abdominal specimens, representing almost half of all abdominal isolates. Fourth, whereas about 20% of strains of all clusters derived from superficial skin or wound swabs, none of the strains of clusters I and II did so. These data suggest that the different clusters could have different virulence-associated properties. However, since only strains with E. cloacae phenotypes were considered in this study, the other species might have been underrepresented. This might have distorted the picture. More appropriately designed studies are needed in order to evaluate the different clinical relevances of the clusters.
API20E analysis.
Twenty-one different API20E codes were generated by the 206 study strains included. The predominating codes were 3.305.573 (58%) and 3.305.773 (19%). Only a few group-specific characteristics could be drawn out of the biochemical information from the API20E analysis. First, cluster IX was 100% myoinositol positive, whereas clusters VI and VII were 100% negative. Second, cluster VII was 100% sorbitol negative, but sorbitol-negative strains were also found in clusters I, III, VI, and VIII. Third, cluster IV was 100% rhamnose negative, which was otherwise found only in the E. asburiae type strain. This short analysis suggested that at least some of the clusters could have specific biochemical properties.
DISCUSSION
In spite of the considerable clinical relevance and the genetic heterogeneity of the nomenspecies E. cloacae, its population genetics have not attracted much attention. Previous studies demonstrated the genetic diversity of E. cloacae (21, 25, 46). However, none of them gave good insight into the phylogenetic relationships between the genetic groups delineated. The present study focused on this issue. We examined 220 strains out of a collection of 241 isolates with E. cloacae phenotypes, originating from 23 regions in 11 countries, plus all type strains and a reference strain of the genus Enterobacter; 12 robust genetic clusters and an unstable sequence crowd were delineated by the use of sequence and PCR-RFLP analysis of three housekeeping genes and the chromosomal ampC gene, coding for an Ambler class C β-lactamase.
Six species (E. asburiae, E. cancerogenus, E. dissolvens, E. hormaechei, E. kobei, and E. nimipressuralis) which are closely related to E. cloacae are subsumed in the so-called E. cloacae complex; 13% of our study strains clustered around their type strains, whereas 84% formed genetic clusters which were not associated with any type or reference strain. Only 3% of our study strains clustered with the type strain of E. cloacae. In DNA-DNA hybridization studies, Grimont and Grimont (21), who examined 49 clinical isolates, and Lindh and Ursing (25), who examined 123 clinical isolates, reported similarly low frequencies of true E. cloacae strains. It has been known for years that the choice of the type strain of E. cloacae was unfortunate, since its genotype does not represent the majority of what are generally considered E. cloacae strains (21).
For this reason, many authors preferred to use reference strain CDC 1347-71R as representative of E. cloacae for their DNA-DNA hybridization experiments (4, 31, 37); 5% of our study strains grouped around CDC 1347-71R which complied with the rate reported by Lindh and Ursing (25). In our study, the type strain of E. kobei fell into the same cluster. E. kobei was described based on a small group of clinical isolates selected as being Voges-Proskauer negative (24). The species showed close relatedness to CDC enteric group 69 (16). Data on the relatedness of strain CDC 1347-71R and CDC enteric group 69 are not available in the literature. CDC 1347-71R and our study strains clustering with the E. kobei type strain were all Voges-Proskauer positive. The following two problems are raised by these findings and need to be addressed in further DNA-DNA hybridization studies. First, if reference strain CDC 1347-71R in fact represents E. kobei and not E. cloacae, the relatedness of the species E. hormaechei, E. asburiae, E. dissolvens, and E. nimipressuralis to E. cloacae needs to be reevaluated. Second, if CDC1347-71R, CDC enteric group 69, and E. kobei represent the same relatedness group, the species description of E. kobei needs to be emended. Forty percent of our strains of the E. kobei cluster were isolated from intra-abdominal sites, and 45% of all intra-abdominal isolates belonged to the E. kobei cluster. Whether this is just an odd coincidence needs to be investigated in an appropriately designed epidemiological study including more clinical strains.
We found that 2% of our study strains clustered with the E. dissolvens type strain. The species E. dissolvens was reassigned from the genus Erwinia to the genus Enterobacter with the reservation that it might in fact be identical to E. cloacae (4). Strains of E. dissolvens and E. cloacae were 60 to 82% related and had ΔTm values far below 5°C in reciprocal DNA. hybridization studies (21, 25). In our study, E. cloacae and E. dissolvens strains were closely related to each other but still represented two distinct phylogenetic lineages in three of the four genes analyzed. Extended multilocus sequencing and DNA-DNA hybridization studies with larger sets of strains are needed in order to finally decide whether both species denominations should be maintained or whether one of them should be abandoned.
We found that 3% of our study strains clustered around the E. hormaechei type strain. The description of the species E. hormaechei was based on 23 strains which were selected on the criterions that they were similar to E. cloacae but did not produce acid from d-sorbitol and melibiose. They formed a distinct DNA-DNA hybridization group and had 63% DNA relatedness to reference strain CDC 1347-71R (31). In the hybridization study of Gimont and Grimont (21), the largest cluster formed around the E. hormaechei type strain and consisted of seven biotypes with only one (3a, one strain) matching the original description of the species. Later, Davin-Regli et al. (11) reported outbreaks with E. hormaechei strains that were d-sorbitol and melibiose positive, suggesting that additional biotypes might exist.
In our study, two study strains together with the E. hormaechei type strain formed the small genetic cluster VII. As far as possible with the information from the API20E system, the biochemical properties of this cluster largely met the species definition of E. hormaechei (31). These data suggest that the species E. hormaechei is actually a valid description (31) and represents only a small phylogenetic lineage within a larger genogroup (21). Our clusters VI and VIII were closely related to E. hormaechei cluster VII. DNA-DNA hybridization studies are needed to verify whether these clusters form a common DNA relatedness group allowing emending and broadening of the species description of E. hormaechei.
We found that 4% of our strains clustered with the type strain of E. asburiae. The species E. asburiae was deduced from the former enteric group 17, which formed a single DNA relatedness group and consisted of Enterobacter strains which were Voges-Proskauer, melibiose, and l-rhamnose negative (4). In previous studies, this biotype represented only one of at least three different biotypes found in the DNA-DNA hybridization group around the E. asburiae type strain (21). In our hsp60 analysis, cluster I was only poorly supported by bootstraps due to the E. asburiae type strain, which grouped slightly apart the cluster. In the ampC PCR-RFLP, the E. asburiae type strain had an additional restriction site. None of the strains of cluster I except the E. asburiae type strain was Voges-Proskauer, melibiose, or l-rhamnose negative. These data reflect the geno- and phenotypic heterogeneity of E. asburiae. In former studies, it played only a minor role as a clinical pathogen (9, 12, 40). In our study, one strain of the E. asburiae cluster (EN373) was isolated from the blood culture of a neonate (18). Many more strains need to be analyzed in order to get a better understanding of the species E. asburiae.
E. nimipressuralis was isolated from elm trees with a disease called wetwood. The species was transferred from the genus Erwinia to the genus Enterobacter and was 52 to 67% related to E. cloacae in reciprocal DNA hybridization reactions (4). Until now, it has never been described as a human pathogen. In our study, only one isolate from a dog's footpad clustered with the E. nimipressuralis type strain, reconfirming the suggested low clinical relevance of this species. Little is known about the species E. cancerogenus. First ascribed to the genus Erwinia, it has recently been transferred to the genus Enterobacter (13) as a senior synonym of Enterobacter taylorae, which was 61% related to E. cloacae reference strain CDC 1371-71R in reciprocal DNA hybridization reactions (17). Human infections with E. cancerogenus are incidental (1), which was also reflected by the fact that none of our strains clustered with the E. cancerogenus type strain.
All our study strains were identified as E. cloacae by their phenotypic characteristics. Nonetheless, 13% of them belonged to other species of the genus. The species of the E. cloacae complex were without exception described based on phenotypically selected strain collections, of which the genetic relatedness was proven in a second step. From our findings, it follows that the species descriptions of presumably all species of the E. cloacae complex need to be revised and potentially emended.
The value of sequence data in population genetic studies has been more and more acknowledged (27). Since its advent, the outstanding role of the analysis of 16S rDNA sequences for the determination of phylogenetic relationships between prokaryotes is indisputable. However, for closely related species such as Enterobacteriaceae, the role of the 16S rDNA sequence analysis in population genetic studies has been questioned (29, 32). For the E. cloacae complex in particular, its limitations have been demonstrated (44). We analyzed the genetic relationships of 16S rDNA sequences of E. cloacae strains in a limited prestudy (data not shown). Only vague genetic clusters were generated. In view of this restricted discriminatory power of the 16S rDNA, we decided to focus on analysis of protein coding genes (45).
The analyses of four protein coding genes, hsp60, rpoB, hemB, and ampC, were included in the study. Sequencing of partial hsp60, also known as groEL or cpn60, has been successfully applied for the classification of many bacteria and turned out to be useful for the phylogenetic analysis of Enterobacter in the present study (19, 20, 28, 33, 45). Mollet et al. (29) demonstrated that phylogenetic trees generated on the bases of rpoB sequences were well compatible with the currently accepted classification of Enterobacteriaceae. In this aspect, it was far superior to 16S rDNA, a fact that we could reproduce in our study. Anyway, housekeeping genes—as most of the genes typically sequenced by bacterial systematists—are not likely to be involved in the ecological delimitation of bacterial populations (32). β-Lactamases evolved as defense mechanisms against competitors. They directly affect the competitive superiority of the bacterial strains in their ecological niches (8). ampC, coding for an Ambler class C cephalosporinase, is coded on the chromosome and is universally present in the genus Enterobacter. The clonality of ampC genes on the species level (including E. asburiae and E. hormaechei) has recently been shown (36). Following our PCR-RFLP results, ampC genes seemed to be clonal even on the level of genetic groups.
The population genetics presented provide a framework of the genetic structure of the E. cloacae complex. This may be of value for further studies examining the epidemiological, phenotypic, and virulence-associated properties of clones of the E. cloacae complex. In Escherichia coli, it has been demonstrated that factors associated with virulence are not randomly distributed among genetic groups (3, 7). Some virulence-associated properties have been described for strains of the E. cloacae complex (23). Clonality of virulence factors might cause the unequal distribution of the genetic clusters to the clinical materials which we noticed in our study. DNA-DNA hybridization experiments and biotyping of our strain collection will help determine to what extent our sequence clusters correspond to distinct species. Additionally, this will allow us to correlate sequence data for protein coding genes with DNA reassociation data, as exemplified in previous studies for 16S rDNA (41).
Acknowledgments
This study was funded by a grant from the German Research Association (DFG) in the frame of a special research field (SFB no. 576) and in part by the Friedrich Bauer Foundation.
We thank Kleinhuber and ESCMID for the publication of our call for strains. Special thanks to all colleagues who collected and sent strains for this study—S. Alberti, Palma de Mallorca; F. Allerberger, Innsbruck; J. Bille, Lausanne; D. Bitter-Suermann, Hannover; K. Bottolfsen, Bergen; M. Breuer-Wera, Aachen; A. Dierkes-Kersting and P. Breuer, Gelsenkirchen; H. Erichsen, Kiel; H. K. Geiss, Heidelberg; P. B. Heczko, Cracow; A. Leanord, Lanarkshire, United Kingdom; S. Lukas, Regensburg; C. E. Nord, Stockholm; W. Pfister, Jena; D. Pierard, Brussels; K. Poschinger, Munich; D. P. Roberts, New Brunswick; V. Schäfer, Frankfurt; R. Smyth, Växjö; M. Stark and I. Authenrieth, Tübingen; J. Wagner, Berlin; A. Wenger, Lausanne; Zwilling; and M. Kist, Freiburg—and all other colleagues who have not specified their names but provided us with strains. We are grateful to Gudrun Maindok for excellent technical assistance in laboratory work.
REFERENCES
- 1.Abbott, S. L., and J. M. Janda. 1997. Enterobacter cancerogenus (Enterobacter taylorae) infections associated with severe trauma or crush injuries. Am. J. Clin. Pathol. 107:359-361. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, W. L., J. Marcon, W. Maccheroni, Jr., J. D. Van Elsas, J. W. Van Vuurde, and J. L. Azevedo. 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol. 68:4906-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd, E. F., and D. L. Hartl. 1998. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J. Bacteriol. 180:1159-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner, D. J., A. C. McWhorter, A. Kai, A. G. Steigerwalt, and J. J. Farmer, 3rd. 1986. Enterobacter asburiae sp. nov., a new species found in clinical specimens, and reassignment of Erwinia dissolvens and Erwinia nimipressuralis to the genus Enterobacter as Enterobacter dissolvens comb. nov. and Enterobacter nimipressuralis comb. nov. J. Clin. Microbiol. 23:1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisse, S., and J. Verhoef. 2001. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC gene sequencing, and automated ribotyping. Int. J. Syst. Evol. Microbiol. 51:915-924. [DOI] [PubMed] [Google Scholar]
- 6.Clementino, M. M., I. de Filippis, C. R. Nascimento, R. Branquinho, C. L. Rocha, and O. B. Martins. 2001. PCR analyses of tRNA intergenic spacer, 16S-23S internal transcribed spacer, and randomly amplified polymorphic DNA reveal inter- and intraspecific relationships of Enterobacter cloacae strains. J. Clin. Microbiol. 39:3865-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohan, F. M. 1994. Genetic exchange and evolutionary divergence in procaryotes. Am. Nat. 143:965-986. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulous, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 10.Dauga, C. 2002. Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematic studies. Int. J. Syst. Evol. Microbiol. 52:531-547. [DOI] [PubMed] [Google Scholar]
- 11.Davin-Regli, A., C. Bosi, R. Charrel, E. Ageron, L. Papazian, P. A. Grimont, A. Cremieux, and C. Bollet. 1997. A nosocomial outbreak due to Enterobacter cloacae strains with the E. hormaechei genotype in patients treated with fluoroquinolones. J. Clin. Microbiol. 35:1008-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Man, P., E. van Der Veeke, M. Leemreijze, W. van Leeuwen, G. Vos, J. van Den Anker, H. Verbrugh, and A. van Belkum. 2001. Enterobacter species in a pediatric hospital: horizontal transfer or selection in individual patients? J. Infect. Dis. 184:211-214. [DOI] [PubMed] [Google Scholar]
- 13.Dickey, R. S., and C. H. Zumoff. 1988. Emended description of Enterobacter cancerogenus comb. nov. (formerly Erwinia cancerogena). Int. J. Syst. Bacteriol. 38:371-374. [Google Scholar]
- 14.Dolina, M., and R. Peduzzi. 1993. Population genetics of human, animal, and environmental Yersinia strains. Appl. Environ. Microbiol. 59:442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dushay, M. S., B. Asling, and D. Hultmark. 1996. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl. Acad. Sci. USA 93:10343-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farmer, J. J., 3rd, B. R. Davis, F. W. Hickman-Brenner, A. McWhorter, G. P. Huntley-Carter, M. A. Asbury, C. Riddle, H. G. Wathen-Grady, C. Elias, G. R. Fanning, et al. 1985. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J. Clin. Microbiol. 21:46-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer, J. J., 3rd, G. R. Fanning, B. R. Davis, C. M. O'Hara, C. Riddle, F. W. Hickman-Brenner, M. A. Asbury, V. A. Lowery 3rd, and D. J. Brenner. 1985. Escherichia fergusonii and Enterobacter taylorae, two new species of Enterobacteriaceae isolated from clinical specimens. J. Clin. Microbiol. 21:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Baca, V., F. Ballesteros, J. A. Hervas, P. Villalon, M. A. Dominguez, V. J. Benedi, and S. Alberti. 2001. Molecular epidemiological typing of Enterobacter cloacae isolates from a neonatal intensive care unit: three-year prospective study. J. Hosp. Infect. 49:173-182. [DOI] [PubMed] [Google Scholar]
- 19.Goh, S. H., R. R. Facklam, M. Chang, J. E. Hill, G. J. Tyrrell, E. C. Burns, D. Chan, C. He, T. Rahim, C. Shaw, and S. M. Hemmingsen. 2000. Identification of Enterococcus species and phenotypically similar Lactococcus and Vagococcus species by reverse checkerboard hybridization to chaperonin 60 gene sequences. J. Clin. Microbiol. 38:3953-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh, S. H., S. Potter, J. O. Wood, S. M. Hemmingsen, R. P. Reynolds, and A. W. Chow. 1996. HSP60 gene sequences as universal targets for microbial species identification: studies with coagulase-negative staphylococci. J. Clin. Microbiol. 34:818-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimont, F., and P. A. Grimont. 1992. The genus Enterobacter, p. 2797-2815. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The procaryotes, 2nd ed. Springer Verlag, New York, N.Y.
- 22.Halda-Alija, L., S. P. Hendricks, and T. C. Johnston. 2001. Spatial and temporal variation of Enterobacter genotypes in sediments and the underlying hyporheic zone of an agricultural stream. Microb. Ecol. 42:286-294. [DOI] [PubMed] [Google Scholar]
- 23.Keller, R., M. Z. Pedroso, R. Ritchmann, and R. M. Silva. 1998. Occurrence of virulence-associated properties in Enterobacter cloacae. Infect. Immun. 66:645-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosako, Y., K. Tamura, R. Sakazaki, and K. Miki. 1996. Enterobacter kobei sp. nov., a new species of the family Enterobacteriaceae resembling Enterobacter cloacae. Curr. Microbiol. 33:261-265. [DOI] [PubMed] [Google Scholar]
- 25.Lindh, E., and J. Ursing. 1991. Genomic groups and biochemical profiles of clinical isolates of Enterobacter cloacae. APMIS 99:507-514. [DOI] [PubMed] [Google Scholar]
- 26.Lobkovsky, E., P. C. Moews, H. Liu, H. Zhao, J. M. Frere, and J. R. Knox. 1993. Evolution of an enzyme activity: crystallographic structure at 2-A resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc. Natl. Acad. Sci. USA 90:11257-11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marston, E. L., J. W. Sumner, and R. L. Regnery. 1999. Evaluation of intraspecies genetic variation within the 60-kDa heat-shock protein gene (groEL) of Bartonella species. Int. J. Syst. Bacteriol. 49:1015-1023. [DOI] [PubMed] [Google Scholar]
- 29.Mollet, C., M. Drancourt, and D. Raoult. 1997. rpoB sequence analysis as a novel basis for bacterial identification. Mol. Microbiol. 26:1005-1011. [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing; approved standard, 7th ed., vol. 20, no. 1. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 31.O'Hara, C. M., A. G. Steigerwalt, B. C. Hill, J. J. Farmer, 3rd, G. R. Fanning, and D. J. Brenner. 1989. Enterobacter hormaechei, a new species of the family Enterobacteriaceae formerly known as enteric group 75. J. Clin. Microbiol. 27:2046-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palys, T., L. K. Nakamura, and F. M. Cohan. 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Bacteriol. 47:1145-1156. [DOI] [PubMed] [Google Scholar]
- 33.Rastogi, N., K. S. Goh, and M. Berchel. 1999. Species-specific identification of Mycobacterium leprae by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J. Clin. Microbiol. 37:2016-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts, D. P., P. D. Dery, I. Yucel, J. Buyer, M. A. Holtman, and D. Y. Kobayashi. 1999. Role of pfkA and general carbohydrate catabolism in seed colonization by Enterobacter cloacae. Appl. Environ. Microbiol. 65:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roehrborn, A., L. Thomas, O. Potreck, C. Ebener, C. Ohmann, P. E. Goretzki, and H. D. Roher. 2001. The microbiology of postoperative peritonitis. Clin. Infect. Dis. 33:1513-1519. [DOI] [PubMed] [Google Scholar]
- 36.Rottman, M., Y. Benzerara, B. Hanau-Bercot, C. Bizet, A. Philippon, and G. Arlet. 2002. Chromosomal ampC genes in Enterobacter species other than Enterobacter cloacae, and ancestral association of the ACT-1 plasmid-encoded cephalosporinase to Enterobacter asburiae. FEMS Microbiol Lett. 210:87-92. [DOI] [PubMed] [Google Scholar]
- 37.Ryun Chung, Y., D. J. Brenner, A. G. Steigerwalt, B. Sup Kim, H. Tae Kim, and K. Yun Cho. 1993. Enterobacter pyrinus sp. nov., an organism associated with brown leaf spot disease of pear trees. Int. J. Syst. Bacteriol. 43:157-161. [Google Scholar]
- 38.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 39.Sanders, W. E., Jr., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders, W. E., Jr., J. H. Tenney, and R. E. Kessler. 1996. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin. Infect. Dis. 23:454-461. [DOI] [PubMed] [Google Scholar]
- 41.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: A place for DNA-DNA reassociation and 16S-rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 42.Steigerwalt, A. G., G. R. Fanning, M. A. Fife-Asbury, and D. J. Brenner. 1976. DNA relatedness among species of Enterobacter and Serratia. Can. J. Microbiol. 22:121-137. [DOI] [PubMed] [Google Scholar]
- 43.Swofford, D. L. 1998. PAUP: phylogenetic analysis with parsimony (and other methods), version 4.0b10. Sinauer Associates, Sunderland, Mass..
- 44.Tang, Y. W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 36:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng, L. J., P. R. Hsueh, J. C. Tsai, P. W. Chen, J. C. Hsu, H. C. Lai, C. N. Lee, and S. W. Ho. 2002. groESL sequence determination, phylogenetic analysis, and species differentiation for viridans group streptococci. J. Clin. Microbiol. 40:3172-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weischer, M., and H. J. Kolmos. 1993. Ribotyping of selected isolates of Enterobacter cloacae and clinical data related to biotype, phage type, O-serotype, and ribotype. APMIS 101:879-886. [DOI] [PubMed] [Google Scholar]
- 47.Weischer, M., H. J. Kolmos, M. E. Kaufmann, and V. T. Rosdahl. 1993. Biotyping, phage typing, and O-serotyping of clinical isolates of Enterobacter cloacae. APMIS 101:838-844. [DOI] [PubMed] [Google Scholar]