Abstract
OBJECTIVE
To provide family physicians and pharmacists with practical, evidence- and expertise-based guidance on choosing the safest approach to using analgesics to manage patients with musculoskeletal pain.
SOURCES OF INFORMATION
Health care providers from family practice, rheumatology, gastroenterology, hepatology, internal medicine, and pharmacy participated in an educational needs assessment regarding the management of pain and the safety of commonly used analgesics. Feedback from one-on-one interviews was compiled and distributed to participants who selected key topics. Topics chosen formed the basis for the discussions of this multidisciplinary panel that reviewed data on the safety of analgesics, particularly in regard to comorbidity and concurrent use with other therapies.
MAIN MESSAGE
Treatment should begin with an effective analgesic with the best safety profile at the lowest dose and escalate to higher doses and different analgesics as required. Acetaminophen is a safe medication that should be considered first-line therapy. Nonsteroidal anti-inflammatory drugs (NSAIDs) are associated with potential adverse gastrointestinal, renal, hepatic, and cardiovascular effects. Physicians should not prescribe NSAIDs before taking a careful history and doing a physical examination so they have the information they need to weigh the risks (adverse effects and potential drug interactions) and benefits for individual patients.
CONCLUSION
Taking a complete and accurate history and doing a physical examination are essential for choosing the safest analgesic for a particular patient.
RÉSUMÉ
OBJECTIF
À partir de données probantes et d’opinions d’experts, fournir au médecin de famille et au pharmacien des directives sur la façon la plus sécuritaire d’utiliser les analgésiques pour traiter la douleur musculo-squelettique.
SOURCE DE L’INFORMATION
Divers membres du personnel soignant, médecins de famille, pharmaciens, spécialistes en rhumatologie, gastro-entérologie, hépatologie et médecine interne ont participé à une évaluation des besoins de formation sur le traitement de la douleur et l’innocuité des analgésiques d’usage courant. Les commentaires exprimés lors d’entrevues individuelles ont été compilés et distribués aux participants qui ont ensuite choisi les sujets les plus importants. Les sujets retenus ont alors été soumis pour discussion à ce groupe d’étude multidisciplinaire qui a fait une revue des données sur l’innocuité des analgésiques, notamment en relation avec la comorbidité et l’usage concomitant d’autres types de traitement.
PRINCIPAL MESSAGE
On devrait commencer par un analgésique efficace ayant le meilleur profil d’innocuité à la dose la plus faible et passer à des doses plus fortes ou à un autre analgésique si nécessaire. L’acétaminophène est un médicament sécuritaire qui devrait être envisagé comme traitement de première intention. Les anti-inflammatoires non stéroïdiens (AINS) sont susceptibles d’entraîner des effets indésirables aux niveaux gastrointestinal, rénal, hépatique et cardiovasculaire et le médecin ne devrait en prescrire qu’après une histoire et un examen physique complet de façon à posséder l’informationnécessaire pour en évaluer les avantages et risques (effets indésirables et possibilité d’interaction médicamenteuse) pour chaque patient.
CONCLUSION
Une histoire complète et précise et un examen physique sont requis pour choisir l’analgésique le plus sécuritaire pour un patient donné.
Pain is among the most common reasons for visits to primary care physicians,1,2 and the proportion of Canadians seeking treatment for pain is expected to rise as the population ages and people develop chronic conditions, such as osteoarthritis. Each year in Canada, more than 19 million prescriptions are written for analgesics,3 and 4.5 billion nonprescription doses of pain medication4 are purchased.
Case
Mrs R.N., who is 66 years old, comes to you for treatment of pain related to osteoarthritis in her knees and hips. Her history includes myocardial infarction at age 58, controlled hypertension, and occasional heartburn. Current medications include hydrochlorothiazide, ramipril, atenolol, and low-dose acetylsalicyclic acid. How would you manage her pain?
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed analgesics even though dyspepsia5 and gastrointestinal (GI) complications6 are well documented among patients taking them. Among patients taking NSAIDs for at least 2 months, 1 in 5 will have an endoscopically seen ulcer, 1 in 70 will have a symptomatic ulcer, 1 in 150 will have GI bleeding, and 1 in 1200 will die from a bleeding ulcer.7
Cyclooxygenase-2 selective NSAIDs (COX-2 inhibitors, or coxibs) were introduced as safer than traditional NSAIDs based on their better GI safety profile, but were subsequently found to be associated with an increase in cardiovascular (CV) adverse events.8,9 The withdrawal of rofecoxib and valdecoxib and the discovery that most, if not all, traditional NSAIDs carry a risk of CV adverse events10 has left physicians questioning how best to manage pain.
Low-dose ASA is commonly used for prevention of myocardial infarction and thrombotic stroke. While this is appropriate therapy for patients with coronary artery disease (as secondary prevention),11 ASA is increasingly being taken by people with no or few CV risk factors (as primary prevention) where the possibility of serious GI bleeding or hemorrhagic stroke12 outweighs any potential benefit.13
Prescribing appropriate analgesics is complicated by concomitant treatments and underlying conditions. Clopidogrel, an important antithrombotic agent, is commonly used with ASA for patients at high risk of CV events,14 but combination therapy carries a heightened risk of GI bleeding.14 Selective serotonin reuptake inhibitors might also cause GI bleeding, and risk increases with concomitant ASA or NSAID use.15 Common comorbidity, such as CV disease, diabetes, and GI problems, further complicate appropriate management.
Because of the prominence of pain-related conditions, the complexities of their clinical management, and the changing therapeutic landscape, a panel was convened to explore the issues surrounding management of pain. The aim was to provide practical, evidence- and expertise-based guidance for family physicians and pharmacists because they are frequently asked for advice on nonprescription medications and should be prepared to give appropriate answers.
Sources of information
Given the hypothesis that health care providers could be confused regarding the safety of commonly used analgesics given the known risks of traditional NSAIDs, new concerns over the CV safety of COX-2 inhibitors and traditional NSAIDs, and the added complexity of commonly encountered comorbidity and adjunct therapies, we set out to clarify the issues. A third-party consultant and the Chair of this multidisciplinary panel (R.H.H.) conducted 1-on-1 interviews with family physicians, a gastroenterologist, a hepatologist, an internist, a rheumatologist, and a pharmacist to solicit unprompted feedback on the particular issues that each felt to be important. This feedback was summarized into a comprehensive list that was distributed to all panel members who were then asked to select the topics they thought were most important. These topics then formed the basis for discussion during a day-and-a-half meeting of the panel in January 2006.
During the meeting, each specialist summarized data on the safety of pain medication in use in his or her area. Their summaries were based on key references and, where available, meta-analyses. Following each review of data in a particular therapeutic area (rheumatology, gastroenterology, hepatology, and internal medicine and cardiology), a family physician presented 1 or more cases that illustrated the challenges of choosing an analgesic in light of patients’ conditions and concomitant medications. The entire process of conducting the needs assessment, summarizing feedback, and organizing the meeting was facilitated by the consultant with direction from R.H.H. and was supported by an unrestricted educational grant from McNeil Consumer Healthcare. This article outlines the main messages from the meeting and lists important, more recent references in lieu of a full systematic review.
Main message
Common pain conditions
Pain-related conditions that most frequently result in primary care visits include back pain (17.63 cases/1000 visits), headaches (16.10/1000), knee pain (8.51/1000), low back pain (8.42/1000), shoulder pain (6.97/1000), and neck pain (6.50/1000).1 Complaints can be divided into acute pain, which implies a shorter, discrete treatment period with potentially reduced risk of adverse events from analgesics, and chronic pain (Table 1).
Table 1.
Pain conditions commonly encountered by family physicians
| ACUTE PAIN CONDITIONS |
| Musculoskeletal injury |
| Headaches (non-migraine) |
| Migraine headaches |
| Renal pain |
| Dysmenorrhea |
| CHRONIC PAIN CONDITIONS |
| Musculoskeletal injury |
| Osteoarthritis |
| Rheumatoid arthritis |
| Fibromyalgia |
The nature of the pain is an important determinant of the approach to treatment and of prognosis, and even in the absence of an identifiable cause, pain must still be managed effectively. We review the safety of various analgesics with a particular focus on treatment of musculoskeletal pain.
Safety of commonly used analgesics
Acetaminophen
Acetaminophen is the most widely used nonprescription pain medication, presumably because it is effective, is well tolerated, and has a good safety profile.16 Nonprescription products with acetamin-ophen as the sole medicinal ingredient account for 35% of sales of analgesics; if acetaminophen-codeine combination products are also considered, acetaminophen sales reach 51%.4
The GI safety of acetaminophen has been demonstrated in controlled clinical trials, meta-analyses, and epidemiologic studies involving acetaminophen doses up to 2 g/d.16 The possible association of higher doses of acetaminophen with increased upper GI bleeding might be biased by selection of more serious cases because patients at higher risk of NSAID gastropathy are more likely to be prescribed acetaminophen as a safer alternative to NSAIDs.16
Acute renal failure has been reported with overdoses of acetaminophen, but not with therapeutic doses of acetaminophen.16 Epidemiologic data suggesting an association between acetaminophen and chronicrenal disease are also subject to the potential bias noted above.16
Acetaminophen overdose is a well-known cause of acute liver failure. Hepatotoxicity from therapeutic doses of acetaminophen is very unusual, and recent critical reviews point to overdose as the major cause of toxicity.16 Acetaminophen can be taken safely in doses up to 4 g/d,16,17 even by patients with stable liver disease (level I evidence).18 Hepatotoxicity might develop following ingestion of >150 mg/kg over 8 hours or less,17 but might occur with lower doses in patients at risk.19 Overdose is often related to suicide attempts,19 but given patients’ ignorance of acetaminophen dose and possible toxicity and the wide range of products containing acetaminophen,20 unintentional overdose sometimes occurs.19
In moderate-to-heavy drinkers, liver damage from therapeutic doses of acetaminophen (alcohol-acetaminophen syndrome) has been reported.21 Critical evaluation of these reports suggests that overdoses rather than therapeutic doses of acetaminophen were more commonly involved.16 Prospective, controlled short-term studies have not found hepatic damage in alcoholics administered therapeutic doses of acetaminophen.22 Similar findings in long-term trials would provide reassuring evidence of the safety of acetaminophen for moderate-to-heavy drinkers.
Traditional and COX-2 NSAIDs
Both types of NSAIDs are widely used as analgesics. In the United States, about 70% of people 65 years old or older take NSAIDs weekly and about 50% take them daily.23 Unfortunately, NSAIDs are associated with potential adverse effects on the liver, kidneys, and most notably, the GI tract.
Traditional and COX-2 NSAIDs cause some GI toxicity; dyspepsia is the most common reason patients discontinue NSAIDs. While COX-2 inhibitors are associated with less dyspepsia than traditional NSAIDs (level I evidence), GI upset still occurs more frequently than in patients taking placebo.24 Of more concern is the risk of serious upper GI complications, such as perforations, ulcers, and bleeding, posed by traditional NSAIDs, including ASA.6,25 Risk increases with dose,26,27 and mucosal damage can be caused by both local and systemic effects. Treatment with NSAID suppositories is not associated with less risk of complications,28 and there are no conclusive safety data on topical NSAID creams, although systemic absorption and serum concentration are substantially lower with creams. Cyclooxygenase-2 inhibitors are associated with significantly fewer GI complications (level I evidence).29,30
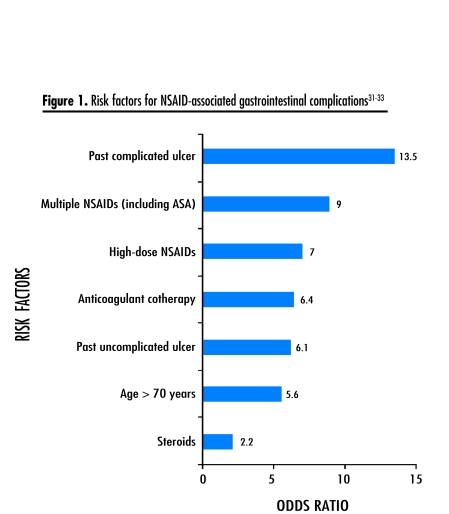
Risk factors for NSAID-related GI complications31–33 need to be carefully considered before initiating therapy (Figure 1). Gastroprotective cotherapy with a proton pump inhibitor or misoprostol might be appropriate for patients at increased risk of complications (level I evidence),30 but will not prevent bleeding more distally in the GI tract, such as in the small or large intestine.34 Helicobacter pylori infection increases the risk of NSAID-associated ulcers and bleeding (level I evidence).35 Patients considered more likely to harbour H pylori infection (eg, those older than 50) should be tested and the infection, if found, eradicated before initiating NSAID therapy.35
Figure 1.
Risk factors for NSAID-associated gastrointestinal complications31–33
Liver damage is rare (1–10/100 000)36 with traditional and COX-2 NSAIDs and is largely associated with diclofenac and sulindac.37–40 Cyclooxygenase-2 inhibitors might be less likely to cause hepatic injury.36,41,42 Ibuprofen has been reported to cause hepatotoxicity in patients with hepatitis C and should not be used by them.43 It is important to rule out viral causes and other liver diseases when investigating suspected NSAID-induced hepatotoxicity. Patients with compensated liver disease can use NSAIDs, but liver function tests should be performed and NSAIDs discontinued if liver enzymes increase (level III evidence). In high-risk patients, alanine aminotransferase levels should be monitored within the first month of therapy and every 3 to 6 months after that (level III evidence).44 Patients with cirrhosis should not take NSAIDs (level III evidence).45
In susceptible patients and patients with congestive heart failure, existing renal failure, or transplanted kidneys, NSAIDs can compromise renal blood flow and glomerular filtration, which can lead to acute renal failure. Renal clearance diminishes considerably with age. Creatinine clearance (estimated) is a more effective measure of renal function than creatinine levels and is easily calculated using the Cockcroft-Gault formula.46
Cockcroft-Gault formula: For men, multiply by 1.2
Creatinine clearance should be evaluated at the start of NSAID therapy and shortly thereafter in patients at risk of renal problems (level II evidence).44,46 It is wise to be cautious if creatinine clearance is 30 to 60 mL/min; NSAID therapy should be avoided if clearance is <30 mL/min (level III evidence).
Traditional and COX-2 NSAIDs also have adverse CV effects. They raise blood pressure, which might be of particular concern in hypertensive patients. Understanding the CV risks of NSAIDs is an evolving issue, with blood pressure being only 1 factor that must be considered in the context of increased risk of thrombotic complications. Cyclooxygenase-2 inhibitors and traditional NSAIDs (with the possible exception of naproxen, although it also carries a class label warning) both appear to confer increased risk of myocardial infarction (level I evidence).8–10,47
Acetylsalicylic acid
Low-dose ASA is commonly prescribed for cardioprotection rather than for analgesia. Atherosclerotic disease is a major cause of death; coronary artery disease is responsible for 1 in 5 deaths and stroke for 1 in 14 deaths.48 Peripheral arterial disease, which affects 12% to 20% of Americans, is associated with a 4- to 5-fold increased risk of dying from CV disease.48 Taking ASA puts patients at risk of GI complications and dyspepsia, similar to the complications and dyspepsia associated with NSAIDs, even in its enteric-coated or buffered form.49 Even the lower doses (75 to 325 mg) commonly taken for cardioprotection put patients at risk of ulcers and GI bleeding12,50; higher doses further increase the risk of bleeding without increasing cardioprotection.51 Therefore, low-dose ASA should be used for cardioprotection and reserved for patients with established CV risk (secondary prevention) (level I evidence).
Alternatives for pain management
Use of corticosteroids for arthritis decreased with the introduction of NSAIDs that effectively controlled inflammation. Extended use of systemic corticosteroids is limited by the many known adverse effects including, but not limited to, hyperglycemia, hypertension, osteoporosis, thinning of the skin, muscle weakness, acne, dyspepsia, adrenal suppression, hypertriglyceridemia, dyslipidemia, and proatherogenic effects.52 Localized treatment of inflammation by injecting a joint up to 4 times over 2 years is, however, a reasonable approach (level I evidence).53
Although still controversial, use of opioids for chronic non-cancer pain is accepted practice and might be appropriate for carefully selected patients with long-lasting or recurrent nociceptive and neuropathic pain who have not obtained adequate analgesia from other therapies.54,55 Amitriptyline alters pain thresholds and might be effective as an adjunct to therapy or for specific conditions, such as fibromyalgia.56,57 There are many nonpharma-cologic approaches to managing chronic pain, such as therapeutic exercise and stretching, stress management, biofeedback, physical modalities, cognitive-behavioural approaches, and alternative approaches.54 Hypnosis has been used to manage cancer- and childbirth-related pain and might be useful for other pain syndromes.58,59
Cotherapies of special concern
Clopidogrel and ASA
Patients at high risk of ath-erothrombosis and after coronary vessel stenting11 are increasingly taking ASA in combination with the antiplatelet drug clopidogrel. While the combination is effective for preventing thrombosis, it increases the risk of GI bleeding,14 particularly in patients taking higher doses of ASA.60
Combined NSAID and ASA
While use of multiple NSAIDs for analgesia should generally be avoided, the requirement for ASA and NSAID cotherapy for cardioprotection and analgesia is a clinical reality. Cyclooxygenase-2 inhibitors and ASA cotherapy is associated with fewer GI ulcers and serious upper GI complications than treatment with traditional NSAIDs and ASA (level I evidence).61–63 Regular use of some NSAIDs, including ibuprofen, might interfere with the antiplatelet effect of ASA,64,65 so ASA should be taken an hour before the NSAID (level I evidence).64
Drug interactions with NSAIDs
Given the prevalence of prescription and nonprescription NSAID use, physicians should recognize contraindications to other drugs, such as anticoagulants, in patients taking NSAIDs. Certain NSAIDs might be a safer option for cotherapy, depending on concomitant medications,66 so physicians should review product monographs carefully. Higher-risk patients taking combined drugs should be monitored closely.66
Patient considerations
Many patients are reluctant to admit that they use alternative medicines, and these medicines might have adverse effects or interact with other medications. Physicians need to encourage patients to mention their use of alternative approaches and to reassure them that disclosure will not incur disapproval.
Knowledge of patients provides an important context for understanding their pain and tolerance levels. Physicians who know patients well can tailor medications and messages to individual patients, although physicians in emergency departments or walk-in clinics are not able to do this. Patients’ concerns, for example, that pain indicates life-threatening or serious conditions, are important to recognize and address. Reassurance can be helpful, as can specialist consultation and testing, if appropriate.
Given patients’ confusion about nonprescription pain medications,30 physicians and pharmacists should alert patients to maximum daily analgesic doses and the potential for overmedicating with multiple products. Patients should be advised that, if pain is not adequately controlled on the recommended treatment, they should return to their physicians or clinics rather than take additional nonprescription products that might increase their risk of adverse events.
Patients might be reluctant to take a particular drug but be willing to consider an alternative. Awareness of patients’ preferences can prevent their returning with symptoms without having filled their prescriptions. Physicians should explain what the prescribed drug is for, the benefits and risks of therapy, the length of treatment, and when to return for re-assessment.
Case resolution
Following the guideline of “safest analgesic, lowest dose,” Mrs R.N. was started on 1000 mg of acetaminophen twice daily. When little relief was noted after 24 hours, her dose was increased to 1000 mg 4 times daily, which was moderately effective. She received steroid injections in an effort to treat locally and to avoid NSAID use given her hypertension, GI symptoms, and advanced age.
Conclusion
This case and discussion highlight the challenges of choosing appropriate analgesic treatment in light of the evolving literature on the safety of NSAIDs and the complexity of comorbidity and cotherapies. Acetaminophen is a safe, well tolerated, and appropriate first-line treatment for managing pain. Treatment with NSAIDs should be initiated only after a full evaluation of risks and anticipated benefits. Important points to consider in selecting an analgesic are outlined in Table 2.
Table 2.
General guide to analgesic use
Take a thorough history
|
Use the lowest dose of the safest analgesic
|
Avoid use of multiple NSAIDs and ASA
|
| Test and treat for Helicobacter pylori before initiating NSAID therapy: The breath test is preferred to serology because it detects active rather than past infection |
| Treat locally rather than systemically where feasible: Use a local injection rather than an oral steroid |
| Balance risks and benefits: Consider the benefits of analgesia in light of the risks associated with NSAIDs for particular patients |
| Recognize and address patients’ concerns: Address patients’ misconceptions (for example, that pain equals life-threatening disease) |
Encourage effective communication
|
Monitor and reevaluate patients as needed
|
ASA—acetylsalicylic acid, NSAID—nonsteroidal anti-inflammatory drug, OTC—over-the-counter.
Many questions remain regarding the safety of analgesics, including the GI and CV risk posed by various NSAIDs, the safety of topical NSAIDs, and the future of COX-2 inhibitors based on emerging safety data and the newest COX-2 drugs. We encourage readers to watch the literature for developments in these areas.
Acknowledgment
This project was supported through an unrestricted educational grant from McNeil Consumer Healthcare Canada.
EDITOR’S KEY POINTS
Musculoskeletal pain can be difficult to manage, particularly in patients with comorbidity. Treatment should begin with the safest analgesic (generally acetaminophen) at the lowest dose.
Use of multiple nonsteroidal anti-inflammatory drugs should be avoided. Low-dose acetylsalicylic acid counts as a nonsteroidal anti-inflammatory drug.
Many treatment modalities might be required to achieve appropriate pain control. It is preferable to treat locally (eg, local steroid injection) rather than systemically, where feasible.
POINTS DE REPèRE DU RéDACTEUR
La douleur musculo-squelettique peut être difficile à traiter, surtout en présence de comorbidité. On devrait commencer par l’analgésique le plus sécuritaire (habituellement l’acétaminophène) à la dose la plus faible.
On devrait éviter d’utiliser plusieurs anti-inflammatoires non stéroïdiens (AINS). L’acide acétylsalicylique à faible dose équivaut à un AINS.
On doit parfois recourir à plusieurs modalités de traitement pour bien contrôler la douleur. Un traitement local (par ex., une infiltration stéroïdienne) est préférable à un traitement systémique.
Footnotes
This article has been peer reviewed.
Competing interests
Dr Hunt has acted as consultant, investigator, or speaker for the following companies: Allergan, Altana Pharma, AstraZeneca, Axcan Pharma, Merck Frosst, NEGMA Laboratories, Novartis Pharmaceuticals, Tap Pharmaceuticals, and Santarus. He is Chief Executive Officer of Strategic Consultants International.
References
- 1.Green LA, Phillips RL, Fryer GE. The nature of primary medical care. In: Jones R, Britten N, Culpepper L, Gass D, Grol R, Mant D, et al., editors. Oxford textbook of primary medical care. London, Engl: Oxford University Press; 2003. pp. 3–10. [Google Scholar]
- 2.Mäntyselkä P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89:175–80. doi: 10.1016/s0304-3959(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 3.IMS Health Canada. Canadian disease and therapeutic index, March 2005. Norwalk, Conn: IMS Health; 2006. [Google Scholar]
- 4.A.C. Nielsen MarketTrack. National drug+GB+MM, latest 52 weeks ending March 31, 2006. Markham, Ont: A.C. Nielsen Canada; 2006. [Google Scholar]
- 5.Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, et al. Meta-analysis of dyspepsia and nonsteroidal anti-inflammatory drugs. Arthritis Rheum. 2003;49:508–18. doi: 10.1002/art.11192. [DOI] [PubMed] [Google Scholar]
- 6.Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, et al. A meta-analysis of severe upper gastrointestinal complications of nonsteroidal anti-inflammatory drugs. J Rheumatol. 2002;29:804–12. [PubMed] [Google Scholar]
- 7.Tramèr MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000;85:169–82. doi: 10.1016/s0304-3959(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 8.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 10.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) Circulation. 2002;106(14):1893–900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 12.García Rodríguez LA, Hernandez-Diaz S, de Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol. 2001;52:563–71. doi: 10.1046/j.0306-5251.2001.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med. 2002;136(2):161–72. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 14.The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 15.Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Graham GG, Scott KF, Day RO. Tolerability of paracetamol. Drug Saf. 2005;28:227–40. doi: 10.2165/00002018-200528030-00004. [DOI] [PubMed] [Google Scholar]
- 17.McNeil Consumer Healthcare. Tylenol. In: Repchinsky C, editor. Compendium of pharmaceuticals and specialties. The Canadian drug reference for health professionals. Ottawa, Ont: Canadian Pharmacists Association; 2006. pp. 2290–2. [Google Scholar]
- 18.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12:133–41. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 19.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multi-center, prospective study. Hepatology. 2005;42(6):1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Schneider S, Wax P. Knowledge about acetaminophen toxicity among emergency department visitors. Vet Hum Toxicol. 2002;44:370–3. [PubMed] [Google Scholar]
- 21.Draganov P, Durrence H, Cox C, Reuben A. Alcohol-acetaminophen syndrome. Even moderate social drinkers are at risk. Postgrad Med. 2000;107:189–95. doi: 10.3810/pgm.2000.01.831. [DOI] [PubMed] [Google Scholar]
- 22.Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients. Arch Intern Med. 2001;161:2247–52. doi: 10.1001/archinte.161.18.2247. [DOI] [PubMed] [Google Scholar]
- 23.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JL, Eisen GM, Burke TA, Pena BM, Lefkowith J, Geis GS. Dyspepsia tolerability from the patients’ perspective: a comparison of celecoxib with diclofenac. Aliment Pharmacol Ther. 2002;16:819–27. doi: 10.1046/j.1365-2036.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- 25.Henry D, McGettigan P. Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. Int J Clin Pract Suppl. 2003;135:43–9. [PubMed] [Google Scholar]
- 26.Blot WJ, McLaughlin JK. Over the counter non-steroidal anti-inflammatory drugs and risk of gastrointestinal bleeding. J Epidemiol Biostat. 2000;5:137–42. [PubMed] [Google Scholar]
- 27.Stack WA, Atherton JC, Hawkey GM, Logan RF, Hawkey CJ. Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment Pharmacol Ther. 2002;16:497–506. doi: 10.1046/j.1365-2036.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- 28.Hansen TM, Matzen P, Madsen P. Endoscopic evaluation of the effect of indomethacin capsules and suppositories on the gastric mucosa in rheumatic patients. J Rheumatol. 1984;11(4):484–7. [PubMed] [Google Scholar]
- 29.Bombardier C. An evidence-based evaluation of the gastrointestinal safety of coxibs. Am J Cardiol. 2002;89(6A):3–9D. doi: 10.1016/s0002-9149(02)02231-2. [DOI] [PubMed] [Google Scholar]
- 30.Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. [Accessed 2007 April 27];BMJ. 2004 329:948. doi: 10.1136/bmj.38232.680567.EB. Epub. Available from: http://www.bmj.com/cgi/content/full/329/7472/948. [DOI] [PMC free article] [PubMed]
- 31.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–96. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 32.García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769–72. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
- 33.Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:241–9. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 34.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–9. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 35.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in pepticulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 36.Teoh NC, Farrell GC. Hepatotoxicity associated with non-steroidal anti-inflammatory drugs. Clin Liver Dis. 2003;7:401–13. doi: 10.1016/s1089-3261(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. Q J Med. 2003;96:787–91. doi: 10.1093/qjmed/hcg138. [DOI] [PubMed] [Google Scholar]
- 38.Boelsterli UA. Diclofenac-induced liver injury: a paradigm of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. 2003;192:307–22. doi: 10.1016/s0041-008x(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 39.Rubenstein JH, Laine L. The hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2004;20:373–80. doi: 10.1111/j.1365-2036.2004.02092.x. [DOI] [PubMed] [Google Scholar]
- 40.Banks AT, Zimmerman HJ, Ishak KG, Harter JG. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology. 1995;22:820–7. [PubMed] [Google Scholar]
- 41.Maddrey WC, Maurath CJ, Verburg KM, Geis GS. The hepatic safety and tolerability of the novel cyclooxygenase-2 inhibitor celecoxib. Am J Ther. 2000;7:153–8. doi: 10.1097/00045391-200007030-00003. [DOI] [PubMed] [Google Scholar]
- 42.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS Study: a randomized controlled trial. JAMA. 2000;284:1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 43.Riley TR, Smith JP. Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol. 1998;93:1563–5. doi: 10.1111/j.1572-0241.1998.00484.x. [DOI] [PubMed] [Google Scholar]
- 44.Bush TM, Shlotzhauer TL, Imai K. Nonsteroidal anti-inflammatory drugs. Proposed guidelines for monitoring toxicity. West J Med. 1991;155:39–42. [PMC free article] [PubMed] [Google Scholar]
- 45.Westphal JF, Brogard JM. Drug administration in chronic liver disease. Drug Saf. 1997;17:47–73. doi: 10.2165/00002018-199717010-00004. [DOI] [PubMed] [Google Scholar]
- 46.Tannenbaum H, Bombardier C, Davis P, Russell AS Third Canadian Consensus Conference Group. An evidence-based approach to prescribing nonsteroidal antiinflammatory drugs. Third Canadian Consensus Conference. J Rheumatol. 2006;33(1):140–57. [PubMed] [Google Scholar]
- 47.Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Norgard B, Friis S, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med. 2005;165:978–84. doi: 10.1001/archinte.165.9.978. [DOI] [PubMed] [Google Scholar]
- 48.American Heart Association. Heart disease and stroke statistics—2006 update. Dallas, Tex: American Heart Association; 2007. [Accessed 2006 March 11]. Available from: http://www.americanheart.org/downloadable/heart/113535864858055-1026_HS_Stats06book.pdf. [Google Scholar]
- 49.Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet. 1996;348:1413–6. doi: 10.1016/S0140-6736(96)01254-8. [DOI] [PubMed] [Google Scholar]
- 50.Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson B, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005;22:795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- 51.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomized trials of anti-platelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Vollenhoven RF. Corticosteroids in rheumatic disease. Understanding their effects is key to their use. Postgrad Med. 1998;103:137–42. doi: 10.3810/pgm.1998.02.349. [DOI] [PubMed] [Google Scholar]
- 53.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intra-articular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 54.Jovey RD, Ennis J, Gardner-Nix J, Goldman B, Hays H, Lynch M, et al. Use of opioid analgesics for the treatment of chronic noncancer pain. A consensus statement and guidelines from the Canadian Pain Society, 2002. Pain Res Manage. 2003;8(Suppl A):3–14A. doi: 10.1155/2003/436716. [DOI] [PubMed] [Google Scholar]
- 55.Breivik H. Opioids in chronic non-cancer pain, indications and controversies. Eur J Pain. 2005;9:127–30. doi: 10.1016/j.ejpain.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Bryson HM, Wilde MI. Amitriptyline. A review of its pharmacological properties and therapeutic use in chronic pain states. Drugs Aging. 1996;8:459–76. doi: 10.2165/00002512-199608060-00008. [DOI] [PubMed] [Google Scholar]
- 57.Godfrey RG. A guide to the understanding and use of tricyclic antidepres-sants in the overall management of fibromyalgia and other chronic pain syndromes. Arch Intern Med. 1996;156:1047–52. [PubMed] [Google Scholar]
- 58.Cyna AM, McAuliffe GL, Andrew MI. Hypnosis for pain relief in labour and childbirth: a systematic review. Br J Anaesth. 2004;93:505–11. doi: 10.1093/bja/aeh225. [DOI] [PubMed] [Google Scholar]
- 59.Montgomery GH, David D, Winkel G, Silverstein JH, Bovbjerg DH. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth Analg. 2002;94:1639–45. doi: 10.1097/00000539-200206000-00052. [DOI] [PubMed] [Google Scholar]
- 60.Sanofi-Synthelabo, Bristol-Myers Squibb. Plavix. In: Repchinsky C, editor. Compendium of pharmaceuticals and specialties. The Canadian drug reference for health professionals. Ottawa, Ont: Canadian Pharmacists Association; 2006. pp. 1680–3. [Google Scholar]
- 61.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ. 2002;325:619–26. doi: 10.1136/bmj.325.7365.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. [Accessed 2007 April 27];Arthritis Res Ther. 2005 7:R644–65. doi: 10.1186/ar1704. Epub 2005 Mar 24. Available from: http://arthritis-research.com/content/7/3/R644. [DOI] [PMC free article] [PubMed]
- 63.Singh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS, Bello AE, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med. 2006;119:255–66. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 64.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclo-oxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 65.Kurth T, Glynn RJ, Walker AM, Chan KA, Buring JE, Hennekens CH, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by non-steroidal antiinflammatory drugs. Circulation. 2003;108:1191–5. doi: 10.1161/01.CIR.0000087593.07533.9B. [DOI] [PubMed] [Google Scholar]
- 66.Johnson AG, Seidemann P, Day RO. NSAID-related adverse drug interactions with clinical relevance. An update. Int J Clin Pharmacol Ther. 1994;32:509–32. [PubMed] [Google Scholar]