Abstract
Despite the recognition that viruses are ubiquitous components of aquatic ecosystems, the number of studies on viral abundance and the ecological role of viruses in sediments is scarce. In this investigation, the interactions between viruses and bacteria were studied in the oxygenated silty sediment layer of a mesotrophic oxbow lake. A long-term study (13 months) and a diel study revealed that viruses are a numerically important and dynamic component of the microbial community. The abundance and decay rates ranged from 4.3 × 109 to 7.2 × 109 particles ml of wet sediment−1 and from undetectable to 22.2 × 107 particles ml−1 h−1, respectively, and on average the values were 2 orders of magnitude higher than the values for the overlying water. In contrast to our expectations, viruses did not contribute significantly to the bacterial mortality in the sediment, since on average only 6% (range, 0 to 25%) of the bacterial secondary production was controlled by viruses. The low impact of viruses on the bacterial community may be associated with the quantitatively low viral burden that benthic bacteria have to cope with compared to the viral burden with which bacterial assemblages in the water column are confronted. The virus-to-bacterium ratio of the sediment varied between 0.9 and 3.2, compared to a range of 5.0 to 12.4 obtained for the water column. We speculate that despite high numbers of potential hosts, the possibility of encountering a host cell is limited by the physical conditions in the sediment, which is therefore not a favorable environment for viral proliferation. Our data suggest that viruses do not play an important role in the processing and transfer of bacterial carbon in the oxygenated sediment layer of the environment investigated.
The discovery, about a decade ago, that viruses are very abundant in natural waters initiated renewed research on the impact of viral infection and lysis on aquatic microorganisms. Pelagic viral populations exhibit extreme variability in abundance, and the concentrations range from less than 104 to more than 108 particles ml−1 in marine and freshwater systems; the highest numbers occur in productive and nutrient-rich environments (49). The abundance of viruses has often been correlated with the number of bacteria, which are the most numerous hosts. On average, virus-induced mortality and flagellate grazing are equally important as causes of bacterial mortality (each causes ca. 25% mortality), but the relative importance of either of these processes may vary in space and time and with environmental characteristics (35). Integration of viral lysis into models of carbon flux through aquatic microbial communities results in diversion of bacterial production away from the higher trophic levels towards the dissolved organic carbon pool. This “viral shunt” may actually augment bacterioplankton production by increasing the amount of labile dissolved organic matter available for bacterioplankton growth (49).
In contrast to the findings for the water column, much less is known about the abundance, distribution, and functional role of viruses in sediments. There have been only a very limited number of investigations of naturally occurring benthic viruses, which revealed that viruses are 10- to 1,000-fold more abundant in the superficial layer of the sediment than in the overlying water column (8, 18, 30, 34, 42). In marine systems, the relative significance of viruses compared to that of bacteria (i.e., the virus-to-bacterium ratio [VBR]) varies widely. Low VBR (0.1 to 5.3) were found in deep-sea sediments of the Mediterranean Sea (5, 6), whereas values ranging from 2 to 65 have been reported for subtropic estuaries of Australia (18) and values ranging from 29 to 85 have been recorded for pore water samples from the Chesapeake Bay in the United States (8). Data from freshwater systems are scarce. Investigations of the sediment in the oligomesotrophic Canadian lake Lac Gilbert revealed a low VBR that varied between 0.5 and 4, compared to a range of 7 to 19 in the overlying water column (30). A similar low VBR was reported for the nearby shallow eutrophic Canadian lake Lac Brome, in which the values were generally near 1 (range, 0.8 to 2.7) (37).
These studies stimulated interest in the role of viruses in benthic microbial processes; however, to date there have still been no reports of the contribution of viruses to bacterial mortality. A first attempt to investigate the effect of viruses on benthic bacteria was made by Hewson et al. (19), who observed that bacterial abundance decreased after enrichment of oligotrophic and eutrophic estuarine sediment pore water with viruses concentrated from seawater collected above the sediment surface. These authors suggested that some viruses in “near-benthic” seawater are bacteriophages specific to hosts on and within the sediments. However, direct measurements of the naturally occurring quantitative impact of viruses on benthic bacteria are still not available. Maranger and Bird (30) speculated that the viral production in sediments might be low relative to that in the water column, because sediments are assumed to be less hospitable physical environments for viruses. This may limit their ability to diffuse and encounter new hosts. These authors suggested that the interaction of sediment particles with bacteria and viruses might reduce viral production considerably and possibly account for the lower VBR compared to water column VBR values in the system investigated. In contrast, because of high numbers of host cells, which may increase the probability of virus-bacterium contact, Danovaro et al. (6) argued that marine sediments could represent the optimal environment for viral development. However, none of these studies provided a database for investigating the potential of viruses to control bacterial secondary production (BSP) and bacterial standing stock in the sediments.
The aim of the present study was to determine whether benthic viruses are a major controlling agent for BSP in the oxygenated sediment layer of a mesotrophic oxbow lake. A recent study showed that heterotrophic nanoflagellates (HNF) and ciliates do not have a regulatory effect on benthic bacterial standing stock and production in this system (48). We therefore hypothesized that the viral compartment could be very important for the control of BSP. During a long-term study (13 months) and a diel study, we monitored variations in viral and bacterial abundances in the same system. We also monitored viral decay, used as a measure of viral production, and BSP to quantify the relative importance of viral lysis as a mechanism of bacterial loss, and we compared our results with available data from the water column of the system used (31). Our data indicated that the role of viruses in the oxygenated sediment layer of the environment investigated is quantitatively not comparable to the role of viruses in the water column.
MATERIALS AND METHODS
Study site and sample collection.
Samples were taken from the Kühwörter Wasser (area, 0.23 km2; average depth, 1 m), a mesotrophic, macrophyte-dominated oxbow lake within the Lobau backwater system of the River Danube (Vienna, Austria). The Lobau system has been separated from the main stream since the 1870s by a series of embankments, and only one downstream connection remains at the lowest part. A thick sediment layer with a mean depth of 39 cm fills the basin of the oxbow lake (23). Because of its shallowness, the Kühwörter Wasser is strongly influenced by wind action. Mixing of the entire water occurs frequently, so no stratification was observed at any time during the study period. The site investigated was characterized by muddy sediments consisting of 5% medium to coarse sand (diameter, ≥250 μm), 14% fine sand (125 to <250 μm), 34% very fine sand (63 to <125 μm), 42% silt (2 to <63 μm), and 5% clay (<2 μm).
Sampling was conducted bimonthly from January 2001 to February 2002 at 9 a.m. to 10 a.m. Additionally, on a calm day at the end of September 2001 a diel study was carried out, in which sampling and measurement of viral and bacterial abundances, BSP, and the viral decay rate (VDR) took place every 6 h. Each viral decay experiment was performed for 24 h. For both the long-term study and the diel study, five replicate sediment samples were collected at each date by hand coring with Plexiglas tubes (inside diameter, 5.8 cm; depth penetration, 15 to 20 cm). The sediment cores were brought to the laboratory within 30 min. The overlying water was removed carefully, and the top 0.5 cm of each core was extruded. Care was taken to sample only the uppermost oxygenated zone as indicated by its light-brown color, in contrast to the dark-brown layer below. The sediment from the five cores was pooled by gentle mixing with a stirring magnet.
Water temperature was measured about 5 cm above the sediment surface. The actual water level was read from a fixed gauge at the lower end of the Kühwörter Wasser. Depth measurements at the sampling site in the middle of the oxbow lake were obtained with a portable depth gauge. Sediment parameters (bulk density, water content, and organic matter content) were determined as described by Kirschner and Velimirov (23). Porosity was calculated from the bulk density and water content of the sediment.
Viral counts and decay experiments.
The decay (i.e., the decrease in viral concentration over time) was recorded after the production of new viruses was inhibited by addition of potassium cyanide (KCN; final concentration, 2 mM) to 40-g sediment slurries in 100-ml glass bottles. The pH of the KCN stock solution was adjusted to the in situ pH. The bottles were incubated at the in situ temperature and under constant-light conditions (corresponding to average in situ light intensity of 50 W m−2) in a glass container filled with a 10-cm sediment layer and an overlying 30-cm water column. To ensure that the concentration of KCN usually used for water column experiments (2 mM) (17) was also sufficient to inhibit the metabolism of the high number of benthic bacteria, we measured BSP after addition of KCN. The BSP was always below the detection limit in KCN-treated samples, indicating that the KCN concentration used in the water column experiments could also be used for sediment investigations. Samples (1 g) were taken at intervals of 30 min to 9 h, diluted, and fixed with electron microscopy grade glutaraldehyde (final concentration, 3%). Prior to the removal of samples, the sediment was gently mixed with a stirring magnet. Samples were kept at 4°C in the dark until they were processed, which always occurred within 24 h. After treatment with filtered (pore size, 0.02 μm) sodium tetrapyrophosphate (final concentration, 5 mM) for at least 20 min, samples were sonicated three times for 20 s at 70 W by using a Branson Sonifier 450 (Branson Ultrasonics Corporation, Danbury, Conn.) (6, 30). They were diluted 200-fold, and triplicate aliquots (1 ml) were filtered through 0.02-μm-pore-size Al2O3 Anodisc membrane filters backed by a 0.2-μm-pore-size cellulose nitrate filter with an approximately 20-kPa vacuum. After they were stained with SYBR Gold (Molecular Probes, Eugene, Oreg.; final dilution of the stock solution, 2.5 × 10−3) for 15 min in the dark, the filters were mounted in a solution containing 50% glycerol, 50% phosphate-buffered saline (120 mM NaCl, 10 mM NaH2PO4; pH 7.5), and 0.1% p-phenylenediamine (4, 32). The filters were examined at a magnification of ×1,250 with a Leitz-Diaplan microscope (Leica, Wetzlar, Germany) equipped with an HBO 50-W mercury lamp (excitation wavelength, 450 to 490 nm; cutoff filter wavelength, 515 nm). To estimate the concentration of viruses, at least 200 viruses were counted per subsample. The viruses from bulk sediment included particle-adsorbed viruses, as well as viruses in pore water. VDR were calculated with data from the initial 9-h periods of the experiments (10, 31). We expressed the viral parameters in particles per milliliter of wet sediment (30, 37). Methodological aspects of the determination of benthic viral abundance and VDR, especially in silty freshwater sediments, will be discussed in detail elsewhere (U. R. Fischer, W. Weisz, C. Wieltschnig, A. K. T. Kirschner, and B. Velimirov, unpublished data).
DNase test.
In order to eliminate uncertainties in virus counting due to extracellular DNA interference, we tested the effect of DNase treatment on sediment samples. Samples (1 g) of the sediment slurry were diluted, treated with sodium tetrapyrophosphate, and sonicated; 250 Kunitz units of DNase I from bovine pancreas were added to 1-ml aliquots, which were incubated for 30 min at room temperature (44). Additional aliquots (1 ml) without DNase were incubated under the same conditions and served as controls. After fixation with glutaraldehyde and further dilution, the viruses in triplicate samples containing DNase and in untreated samples were counted by epifluorescence microscopy. The numbers of viruses obtained from DNase-treated samples did not differ significantly from the numbers obtained from untreated samples (P > 0.60, as determined by a Mann-Whitney U test; n = 12) (data not shown).
Bacterial parameters.
Numbers of bacteria and cell volumes were determined by using acridine orange fluorescence microscopy as described by Kirschner and Velimirov (23). Filters were observed at a magnification of ×1,250 with a Leitz Diaplan microscope equipped with an HBO 50-W mercury lamp (see above). The cellular carbon content (C) (in femtograms of C per cell) was calculated from the estimated cell volume (V) (in cubic micrometers) by assuming the allometric relationship C = 120 × V0.72, as described by Norland (33). To ensure accurate estimates of BSP, we used two independent methods. BSP was measured at the beginning of each decay experiment by determining the incorporation of [3H]thymidine (TdR) and [14C]leucine [LEU] by the protocol of Kirschner and Velimirov (23) and Wieltschnig et al. (48). Leucine incorporation rates were converted to carbon production values by using a conversion factor of 1.82 g of C mmol of leucine incorporated−1 (40). For TdR a conversion factor of 2 × 106 cells pmol of TdR incorporated−1 was used (23).
Calculation of η.
The single collector efficiency (η) (i.e., the rate at which particles strike a single porous medium grain divided by the rate at which particles move toward the grain) (16) was calculated by using the Smoluchowski-Levich approximation (38):
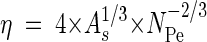 |
where NPe = (dc × n × v)/DBM, a Péclet number (dimensionless), accounts for diffusion; dc is the average collector size (8.3 × 10−5 m in the system investigated); n is the porosity (dimensionless); v is the pore water velocity (in meters per second); DBM = KB × T/(3π × dp × μ) is the diffusion coefficient (in square meters per second); KB is the Boltzmann constant (1.38 × 10−23 J K−1); T is the water temperature (in Kelvin); dp is the virus particle size (6 × 10−8 m in our study, based on preliminary observations by transmission electron microscopy that icosahedral viruses that are approximately 60 nm in diameter dominated the benthic viral community of the Kühwörter Wasser [unpublished data]); μ is the dynamic viscosity of water (in kilograms per meter per second), as calculated from values for different water temperatures in Schwörbel (39); and As = [2 × (1 − γ5)]/[2 − (3 × γ) + (3 × γ5) − (2 × γ6)], which is Happel's porosity-dependent parameter, with γ = (1 − n)1/3.
Pore water velocity was calculated as described by Freeze and Cherry (11):
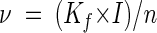 |
where Kf is the hydraulic conductivity (10−7 m s−1 for silty sand) (11) and I is the hydraulic gradient (30 m m−1 for the top 0.5-cm sediment layer in the system investigated, average for 11 years of record, from 1989 to 2000) (F. S. Seebacher, personal communication).
Statistical analysis.
Data were analyzed as described by Zar (52). A P value of ≤0.05 was considered significant in all statistical analyses. We used the SPSS 10.0 (2001) software.
RESULTS
Environmental parameters.
The water level at the lower part of the Kühwörter Wasser varied between 1.80 and 2.85 m (mean, 2.30 m). The lowest values usually occurred in winter and early spring, while the maximum values were recorded after the snow melt in late spring and again in summer. At the sampling site in the middle part of the oxbow lake, water levels between 0.7 and 1.6 m (mean, 1 m) were recorded. From December 2001 to the end of January 2002 the Kühwörter Wasser was covered with ice. The water column was generally well mixed, and the superficial layer of the sediment (from 0.0 to 0.5 cm) was well oxygenated throughout the study period. Sediment characteristics are shown in Table 1.
TABLE 1.
Bulk density, porosity, pore water velocity, organic matter content, and pH of the sediment measured during a long-term study, and averages for these parameters for a diel studya
| Date | Bulk density (g ml−1) | Porosity | Pore water velocity (10−6 m s−1) | Organic matter content (mg ml−1) | pH |
|---|---|---|---|---|---|
| January 2001 | 1.378 | 0.64 | 4.7 | 25.1 | 7.91 |
| March 2001 | 1.199 | 0.79 | 3.8 | 26.0 | 7.64 |
| May 2001 | 1.143 | 0.76 | 4.0 | 27.0 | 7.87 |
| July 2001 | 1.258 | 0.72 | 4.2 | 39.5 | 7.77 |
| September 2001 | 1.198 | 0.75 | 4.0 | 24.0 | 7.82 |
| November 2001 | 1.214 | 0.77 | 3.9 | 22.3 | 8.31 |
| February 2002 | 1.155 | 0.80 | 3.8 | 21.1 | 8.23 |
| Avg of long-term study | 1.221 (0.073) | 0.75 (0.10) | 4.0 (0.03) | 26.4 (5.7) | 7.94 (0.23) |
| Avg of diel study | 1.187 (0.022) | 0.76 (0.01) | 3.9 (0.1) | 22.6 (0.8) | 8.00 (0.10) |
The values in parentheses are standard deviations. The long-term study run from January 2001 to February 2002 (samples were taken from 9 a.m. to 10 a.m.), while the diel study took place in September 2001 (from 9 a.m. one day to 9 a.m. on the following day).
Viral abundance and bacterial abundance.
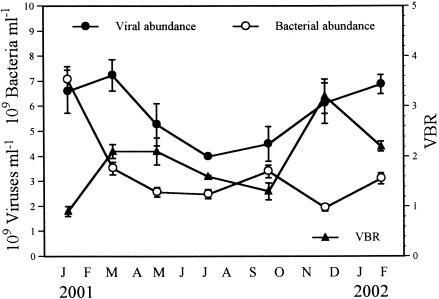
The numbers of viruses exhibited a dynamic pattern of fluctuation during the investigation period in 2001 (Fig. 1). The values ranged from 4.3 × 109 to 7.2 × 109 particles ml of wet sediment−1 (mean, 5.8 × 109 particles ml−1). The highest numbers of viruses were found at the beginning of the study in winter and spring 2001, and after this the numbers decreased continuously until July, when the minimum level was recorded. From July to the end of the investigation period in February 2002 the viral level increased steadily, reaching values similar to those observed in January 2001. The numbers of viruses were negatively correlated with water temperature (P < 0.05, as determined by Spearman rank correlation; n = 11). The numbers of bacteria varied from 1.9 × 109 to 7.1 × 109 cells ml−1 (mean, 3.4 × 109 cells ml−1) (Fig. 1). The highest values were recorded in January 2001. Thereafter, the cell numbers decreased and showed little fluctuation throughout the rest of the investigation period (range, 1.9 × 109 to 3.5 × 109 cells ml−1). The VBR was very low, varying between 0.9 and 3.2 (mean, 1.9) (Fig. 1). The η ranged from 0.45 to 0.71 (mean, 0.56) (Table 2). The values for η were negatively correlated with viral production (P < 0.05, as determined by Spearman rank correlation; n = 10).
FIG. 1.
Variations in benthic viral and bacterial counts and VBR in the Kühwörter Wasser from January 2001 to February 2002. The error bars indicate standard deviations for triplicate samples.
TABLE 2.
Benthic VDR, percentage of initial viral abundance remaining after 9 h in the decay experiments, benthic viral turnover, and ηa
| Date | VDR (h−1) | % of initial viral abundance | Viral turnover (days) | η |
|---|---|---|---|---|
| January 2001 | 0.023 (0.004) | 82 (2) | 2.0 (0.2) | 0.56 |
| March 2001 | 0.036 (0.004) | 72 (3) | 1.4 (0.1) | 0.52 |
| May 2001 | 0.010 (0.002) | 91 (1) | 4.4 (0.6) | 0.65 |
| July 2001 | 0.025 (0.001) | 80 (1) | 1.9 (0.0) | 0.71 |
| September 2001 | BDb | 100 (3) | —c | 0.60 |
| November 2001 | 0.031 (0.005) | 76 (3) | 1.6 (0.2) | 0.46 |
| February 2002 | 0.031 (0.003) | 76 (2) | 1.5 (0.1) | 0.45 |
| Avg | 0.022 (0.012) | 82 (9) | 2.1 (1.0) | 0.56 (0.09) |
The values in parentheses are standard errors for triplicate samples for the VDR and standard deviations for triplicate samples for the percentage of initial viral abundance and viral turnover.
BD, below the detection limit.
—, Viral production below the detection limit gave a meaningless high turnover rate.
Viral control of bacterial production.
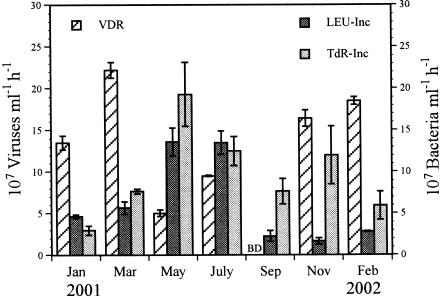
BSP ranged from 1.7 × 107 to 13.6 × 107 cells ml−1 h−1 (mean, 6.3 × 107 cells ml−1 h−1) and from 3.0 × 107 to 19.4 × 107 cells ml−1 h−1 (mean, 9.8 × 107 cells ml−1 h−1) as determined by LEU incorporation and TdR incorporation, respectively (Fig. 2). These values corresponded to growth rates of 0.007 to 0.054 h−1 (mean, 0.026 h−1) for the LEU data and 0.004 to 0.076 h−1 (mean, 0.034 h−1) for the TdR data. The values for BSP determined by the TdR method were on average twice the values determined by the LEU method (range, 0.47 to 7.23 times). However, no significant difference (P > 0.40, as determined by a Mann-Whitney U test; n = 10) between the two methods was detected. When the value obtained in November was excluded, the results obtained with the two approaches were significantly correlated (P < 0.05, as determined by Spearman rank correlation; n = 9).
FIG. 2.
Variations in benthic VDR and BSP as estimated by incorporation of [14C]leucine (LEU-Inc) and [3H]TdR (TdR-Inc) in the Kühwörter Wasser from January 2001 to February 2002. The error bars indicate standard errors for triplicate samples for the VDR and for four subsamples for the BSP. BD, below the detection limit.
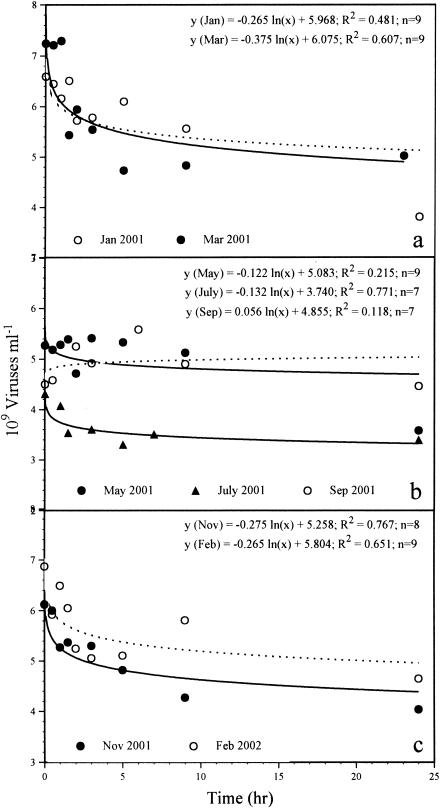
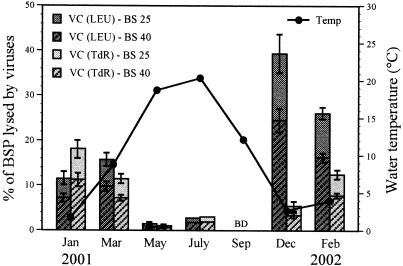
The time courses of the decay experiments are shown in Fig. 3. The VDR varied from undetectable to 0.036 h−1 (mean, 0.022 h−1), corresponding to no particle production to a production of 22.2 × 107 particles ml−1 h−1 (mean, 12.2 × 107 particles ml−1 h−1) (Table 2 and Fig. 2). No obvious loss of viral particles was detected during the decay experiment carried out in September 2001. Between 72 and 100% (mean, 82%) of the initial viral population remained 9 h after the beginning of the experiments. The VDR followed a temperature pattern, with the lowest values occurring at temperatures above 12°C and the highest values below 10°C. The water temperature exhibited a seasonal pattern, with values usually below 5°C for the winter season and up to 20.5°C in the summer (Fig. 4). However, the VDR did not correlate with water temperature (P > 0.08, as determined by Spearman rank correlation; n = 10), but they correlated positively with viral abundance (P < 0.05). The benthic viral turnover varied between 1.4 and 4.4 days (mean, 2.1 days, excluding September) (Table 2). To estimate the number of lysed bacteria, we divided the VDR by assumed burst sizes of 25 and 40 (see below). Calculating the number of lysed bacteria with a burst size of 25 revealed that 0.0 to 8.9 × 106 cells ml−1 h−1 (mean, 4.9 × 106 cells ml−1 h−1) had to be lysed to maintain viral production. Hence, viruses controlled 0.0 to 39.4% (mean, 13.9%) of BSP as determined via the LEU method and 0.0 to 18.1% (mean, 7.4%) of BSP as determined by incorporation of TdR (Fig. 4). Using a burst size of 40 resulted in virus-induced bacterial mortality values between 0.0 and 5.6 × 106 cells ml−1 h−1 (mean, 3.0 × 106 cells ml−1 h−1). Values for viral control of BSP ranged from 0.0 to 24.6% (mean, 8.7%) for LEU data and 0.0 to 11.4% (mean, 4.6%) for TdR data (Fig. 4). Viral control of BSP followed a distinct temperature pattern, with the lowest values occurring at temperatures above 12°C and maximum control below 10°C. However, only the viral control of BSP measured by LEU incorporation was negatively correlated with water temperature (P < 0.05, as determined by Spearman rank correlation; n = 10).
FIG. 3.
Changes in benthic viral counts as a function of time after inhibition of production of new viruses by addition of KCN in January (dotted line) and March 2001 (a), in May, July, and September (dotted line) 2001 (b), and in November 2001 and February 2002 (dotted line) (c).
FIG. 4.
Variations in viral control (VC) of benthic BSP as estimated by incorporation of [14C]leucine (LEU) and [3H]TdR and water temperature in the Kühwörter Wasser from January 2001 to February 2002. The error bars indicate standard deviations for triplicate samples. In July, the standard deviation was less than 0.05. BD, below the detection limit. BS, burst size.
Diel cycle.
Results for the diel cycle are presented in Tables 1 and 3. All environmental parameters varied little during the 24-h period. The daily averages for all chemophysical parameters were close to the mean values obtained in the long-term study that lasted for 13 months. The water temperature increased from 12.3°C at 9 a.m. to a maximum of 14.9°C at 9 p.m. and then decreased to 13.1°C at 9 a.m. the following day. Viral and bacterial abundances showed statistically insignificant fluctuations during the investigation period (P > 0.05, as determined by a Kruskal-Wallis H test; n = 15). The number of viruses steadily increased from 4.5 × 109 particles ml−1 at 9 a.m. to 5.8 × 109 and 5.6 × 109 particles ml−1 at 3 p.m. and 9 a.m. the next day, respectively (mean, 5.2 × 109 particles ml−1). Minor variations were observed in the bacterial concentration, which ranged from 2.6 × 109 to 3.4 × 109 cells ml−1 (mean, 3.0 × 109 cells ml−1). Because there was little fluctuation in the numbers of viruses and bacteria, the VBR also varied only slightly, from 1.3 to 2.1 (mean, 1.8). No daily pattern was detected for bacterial abundance and the VBR. In contrast to the bacterial and viral standing stocks, VDR and BSP exhibited great variability during the day. At 9 a.m., as well as at 9 p.m., the VDR was below the detection limit. The highest VDR was observed at night (0.024 h−1 at 3 a.m., corresponding to 12.4 × 107 particles ml−1 h−1), and the value was about twice the rate measured in the afternoon (0.015 h−1 at 3 p.m., corresponding to 6.7 × 107 particles ml−1 h−1). The average VDR was 0.010 h−1 (corresponding to 4.8 × 107 viruses ml−1 h−1). Variations in the VDR over the investigation period were statistically significant (P < 0.02, as determined by the Kruskal-Wallis H test; n = 12). BSP showed a clear daily pattern, with an increase during the day, a maximum at 9 p.m., and a decrease at night. The values varied from 2.3 × 107 to 11.6 × 107 cells ml−1 h−1 (mean, 7.8 × 107 cells ml−1 h−1) as determined by the LEU method and from 4.1 × 107 to 11.3 × 107 cells ml−1 h−1 (mean, 7.9 × 107 cells ml−1 h−1) as determined by the TdR method. These values corresponded to growth rates of 0.007 to 0.038 h−1 (mean, 0.027 h−1) for the LEU data and 0.015 to 0.037 h−1 (mean, 0.027 h−1) for the TdR data. The variations in BSP during the investigation period were significant only when BSP was measured by the LEU method (P < 0.05 for LEU values and P > 0.08 for TdR values, as determined by the Kruskal-Wallis H test; n = 16). The patterns of BSP corresponded to the daily variations in water temperature. The level of viral control of BSP was always low, ranging from 0.0 to 5.8% (mean, 2.2%) as determined by the LEU method and from 0.0 to 12.2% (mean, 3.8%) as determined by the TdR method, when calculations were made by using a burst size of 25. Using a burst size of 40 led to values for virus-induced control of BSP of 0.0 to 3.4% (mean, 1.4%) for the LEU method and of 0.0 to 7.6% (mean, 2.4%) for the TdR method.
TABLE 3.
Benthic viral and bacterial parameters measured during a diel cycle (September 2001)a
| Time | Viral abundance (109 particles ml−1) | Bacterial abundance (109 cells ml−1) | VDR (107 particles ml−1 h−1) | VDR (h−1) | η | BSP-LEU (107 cells ml−1 h−1)b | BSP-TdR (107 cells ml−1 h−1)b | Viral control
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of BSP-LEU
|
% of BSP-TdR
|
||||||||||
| BS 25c | BS 40d | BS 25c | BS 40d | ||||||||
| 9 a.m. | 4.5 (0.7) | 3.4 (0.3) | BDe | BD | 0.60 | 2.3 (0.7) | 7.7 (1.5) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| 3 p.m. | 4.9 (0.7) | 2.6 (0.2) | 6.7 (0.5) | 0.015 (0.001) | 0.60 | 8.6 (2.3) | 8.6 (2.1) | 3.1 (0.4) | 1.9 (0.2) | 3.1 (0.4) | 1.9 (0.2) |
| 9 p.m. | 5.1 (0.2) | 3.0 (0.2) | BD | BD | 0.61 | 11.6 (0.6) | 11.3 (1.9) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| 3 a.m. | 5.8 (0.8) | 2.8 (0.2) | 12.4 (0.8) | 0.024 (0.003) | 0.62 | 8.6 (1.1) | 4.1 (0.8) | 5.8 (0.6) | 3.6 (0.4) | 12.2 (1.4) | 7.6 (0.8) |
| 9 a.m. | 5.6 (0.1) | 3.0 (0.2) | NDf | ND | 0.58 | ND | ND | ||||
| Avg | 5.2 (0.5) | 3.0 (0.3) | 4.8 (5.2) | 0.010 (0.010) | 0.60 (0.02) | 7.8 (3.4) | 7.9 (2.6) | 2.2 (2.4) | 1.4 (1.5) | 3.8 (5.0) | 2.4 (3.1) |
The values in parentheses are standard deviations for triplicate samples for viral and bacterial abundance and for viral control of BSP, as well as standard errors for triplicate samples for the VDR, and for four subsamples for LEU and TdR incorporation.
BSP was measured at the beginning of the decay experiments by determining incorporation of [14C]leucine (BSP-LEU) and [3H]thymidine (BSP-TdR).
Calculated with a burst size of 25 (BS 25).
Calculated with a burst size of 40 (BS 40).
BD, below the detection limit.
ND, not determined.
DISCUSSION
Viral abundance and decay.
Viruses in the Kühwörter Wasser were more abundant in the sediment than in the overlying water (31), a result anticipated from a synthesis of previously published ecological data (5, 8, 18, 30, 34, 42) and public health data (1, 12, 13, 24). The ranges of numbers of benthic viruses and VBR of the system investigated were on the same order of magnitude as the ranges previously reported for marine sediments, including deep-sea sediments (5, 6, 18), and the values were similar to values reported for freshwater sediments (30, 37) (Table 4). The only exception is the data in a study of Hewson et al. (19), who reported numbers of viruses of up to 2.2 × 1011 particles cm−3 at a eutrophic site (Brisbane River, Australia). However, none of the previous studies dealing with viral abundance in aquatic sediments provided a database for examining the potential of benthic viruses to control bacterial production and standing stock. We used the approach of Heldal and Bratbak (17) to assess viral production, adopting the conservative assumptions that the viral abundance in the system is maintained and that viruses from lysed bacterial cells replace viruses lost by decay. To our knowledge, this was the first attempt to determine benthic viral production by the decay method, which has been well established for the water columns of aquatic systems. However, there are several problems with this method. It has been suggested that the rate of pelagic viral production and decay may follow a diel periodicity (3, 17, 21, 43, 47), and we also observed variations in viral proliferation in the sediment layer of the Kühwörter Wasser during a 24-h period (Table 3). Since our decay experiments were performed with sediment samples taken in the morning, the assumption that our viral decay values are representative of the daily average is rather conservative. Assuming that bacteria play an active role in viral decay via ecto- and extracellular enzymes, the production of new enzymes is inhibited by KCN that inactivates bacterial metabolism (17, 31). However, it is not known yet to what extent bacteria produce enzymes capable of contributing to the degradation of viral protein capsids. Therefore, it is difficult to determine the importance of the processes mentioned above in viral decay. Moreover, it has yet to be determined if wall effects during bottle incubation may influence viral production in sediments. We cannot claim that all potential loss factors leading to viral decay were functional in our experimental setup, nor could we control whether some of these factors had an enhanced effect on viral decay compared to the decay under in situ conditions. Thus, a definite conclusion concerning the direction of the influence cannot be drawn with the present state of knowledge. Nevertheless, we favor the method described here over other methods, such as estimation of the frequency of infected bacterial cells (36) as determined by transmission electron microscopy, because a decay experiment provides direct access to the dynamic aspect of viral production, while other methods are extrapolations of assumptions. Assessment of the frequency of infected bacterial cells by transmission electron microscopy provides only a snapshot and does not take into account the dynamic nature of viral production and bacterial mortality in a microbial system.
TABLE 4.
Comparison of viral counts and VBR in freshwater and marine sediments from different locations
| Study area | Depth (m) | Viral abundance (109 viruses) | VBR | Methoda | Reference |
|---|---|---|---|---|---|
| Freshwater sediments | |||||
| Lac Gilbert (Canada) | 2-13 | 0.7-2.9 ml of sediment−1b | 0.5-4.0 | TEM | 30 |
| Lac Brome (Canada) | 3-12 | 3.2-13.0 ml of sediment−1 | 0.8-2.7 | YO-PRO | 37 |
| Kühwörter Wasser (Austria) | 0.8-1.7 | 4.3-7.2 ml of sediment−1 | 0.9-3.2 | SYBR Go | This study |
| Marine sediments | |||||
| Key Largo (United States) | 1-10 | 0.14-0.53 cm of sediment−3 | NDc | TEM | 34 |
| Chukchi Sea (United States) | ND | 0.027 ml of pore water−1 | 12.9 | TEM | 42 |
| Chesapeake Bay (United States) | 10-22 | 0.22-0.44 ml of pore water−1 | 29.3-84.7 | YO-PRO | 8 |
| Aegean Sea (eastern Mediterranean Sea) | 1,221-4,263 | 1.21-2.38 g of sediment−1d | 2.2-5.3 | SYBR Gr | 5 |
| Eastern and western Mediterranean Sea | 1,290-4,000 | 0.036-0.12 g of sediment−1d | 0.1-0.5 | SYBR Gr | 6 |
| Brisbane River, Moreton Bay estuary, and Noosa River estuary (Australia) | ND | 0.2-4.8 cm of sediment−3 | 2-65 | SYBR Gr | 18 |
| Brisbane River, Moreton Bay estuary | ND | 0.24-220 cm of sediment−3 | ND | SYBR Gr | 19 |
TEM, transmission electron microscopy; YO-PRO, epifluorescence microscopy of samples stained with YO-PRO fluorochrome stain; SYBR Gr, epifluorescence microscopy of samples stained with SYBR Green I fluorochrome stain; SYBR Go, epifluorescence microscopy of samples stained with SYBR Gold fluorochrome stain.
The value obtained at a depth of 4 m was not included.
ND, not determined.
Viral abundance was normalized to sediment dry weight after desiccation (60°C, 24 h).
The VDR was calculated with data from the initial 9-h period of the experiments, because after this no distinct changes in the numbers of viruses occurred in most cases and the VDR tended to be zero. Only during the experiment performed in January 2001 did viral abundance decrease markedly from 9 to 24 h. When we used decay data corresponding to time zero to 24 h and to 9 to 24 h, we obtained VDR of 11.6 × 107 and 11.7 × 107 particles ml−1 h−1, respectively. These rates are close to the rate for 0 to 9 h (namely, 13.5 × 107 particles ml−1 h−1) (Fig. 2), which was higher by a factor of only 1.16. The decrease in the number of viruses during the experiment performed in May 2001 is not statistically significant (R = 0.464; P > 0.1; n = 9), implying that the decay tended towards undetectable. When we calculated the VDR for this month, it turned out that the value obtained was comparable to the overall trend for benthic viral control of BSP, which was low for the 13-month investigation period. Therefore, the VDR for May was integrated into our calculations. Generally, the VDR was on average 2 orders of magnitude higher than the rate of decay in the water column, and variations in the benthic VDR were more distinct (31). The benthic viral turnover was similar to that in the water column, with values varying between 1.4 and 4.4 days (excluding September) (Table 2).
However, it is still not clear whether viruses found in the sediments were actually produced in this environment or originated from the overlying water column. There are no routine techniques that could be used to distinguish between the viruses that were locally produced in the sediments and those that accumulated after sedimentation out of the water column. Correlations of viral abundance and solids suggest that viruses adsorb to suspended material in the water column and thus may settle out and contribute to the benthic viral population (18). In contrast, Simon et al. (41) argued that viruses are readily decomposed in aggregates and only infected bacteria may bring viruses to deep-water layers and the bottom sediment. Since the Kühwörter Wasser has an average depth of only 1 m, it seems reasonable to propose that the viruses in the sediment are, at least partially, supplied by particle fluxes. The benthic viruses observed in this study could, therefore, have originated either from the sediment or the overlying water.
Viral control of bacterial production.
By compiling information about VDR and BSP and assuming burst sizes ranging from 25 to 40 (see below), we attempted to estimate the impact of virus-induced bacterial cell lysis on control of the bacterial compartment. Using a burst size of 25, we obtained values for BSP control by viruses ranging from undetectable to 39.4% (averages, 13.9% [BSP measured by incorporation of LEU] and 7.4% [BSP measured by incorporation of TdR]). When a burst size of 40 was used, the values for virus-induced control of BSP ranged from undetectable to 24.6% (averages, 8.7% [LEU values] and 4.6% [TdR values]). In contrast to the viral control in the oxygenated sediment layer, the level of viral control in the water column of the same system ranged from 15 to 30%, with a mean of 20% (31).
For determinations of the viral impact on BSP, the burst size is a crucial parameter. We chose a burst size range from 25 to 40 based on the fact that the lower value is the minimum burst size (i.e., the average number of phages contained in all visibly infected cells) of pelagic bacteria in the Kühwörter Wasser (31), and the higher value was determined by measuring the average number of phages released from visibly infected pelagic bacterial cells in a eutrophic freshwater environment, which were completely filled with phages (maximum burst size) (10). To us, a burst size of 25 for benthic bacteria in the Kühwörter Wasser appears to be an unrealistically low value because this value represents the average minimum burst size; it may be more appropriate to use the maximum burst size for calculations of bacterium-virus interactions because the burst size of the virus-infected bacterial cells that are not entirely filled with viruses may increase until the cells are actually lysed (45). We therefore suppose that calculations made with a burst size of 40 give a more accurate assessment of the viral control of the benthic bacterial population. Discussions of the virus-bacterium interactions in this paper are therefore based on calculations obtained with a burst size of 40.
One may argue that the burst size of benthic bacteria differs from that of pelagic bacteria because of obvious differences in bacterial cell size. Weinbauer and Peduzzi (46) reported that burst sizes of pelagic bacteria correlate positively with cell size. Since the bacteria in the sediment of the Kühwörter Wasser are larger (average size, 0.167 μm3 cell−1 [data not shown]) than those found in the overlying water (average size, 0.065 μm3 cell−1) (22) and in the water column of the eutrophic freshwater environment mentioned above (average size, 0.079 μm3 cell−1) (7), we suggest that an even higher number of mature phages are released during lysis of virus-infected benthic bacterial cells than during lysis of pelagic bacterial cells. We calculated a potential burst size of 85 ± 18 viruses per cell, taking into account the variations in cell volumes for the whole investigation period. In this case, the number of lysed bacterial cells (estimated by using a burst size of 40) would have been overestimated, and the level of viral control of BSP would be even lower (undetectable to 14.0%; mean, 3.3%) than the values presented above. Moreover, the considerations regarding the coupling of bacterial cell size with burst size further support the hypothesis that a burst size of 25 might be an unrealistically low value for benthic bacteria.
We are aware that estimation of viral control of BSP depends on the method used to measure BSP. In our calculations of BSP based on LEU or TdR incorporation a number of conversion factors and assumptions were included. If we assumed that there was twofold isotope dilution (LEU approach) and a conversion factor of >2 × 106 cells pmol of TdR incorporated−1, on average the benthic viral impact would be reduced to 4.4 and 2.4% of the BSP (range, undetectable to 12.3%), respectively. Assuming a conversion factor of 1 × 106 cells pmol of TdR incorporated−1, which has been used for sediment bacteria by other authors (14), the maximum level of viral control would be about 22.8% of the BSP, with an average of 9.5%. Thus, the general trend of low viral impact on benthic bacteria in the Kühwörter Wasser was unchanged irrespective of the calculation method used.
Factors influencing virus-bacterium interactions.
Despite generally high numbers of viruses and high viral production in the oxygenated sediment layer of the Kühwörter Wasser, the relative significance of viruses compared to the significance of bacteria was much lower than that in the overlying water. We suggest that the physical conditions of the sediment may affect the host-virus interactions and could partly explain the low VBR and viral control of BSP estimated in the system investigated. The transport of viruses in the immediate vicinity of the bacterial surfaces, even if it is induced by bioturbation, is dominated by Brownian diffusion. Since this process may be limited by the high tortuosity of the sediment, the possibility of a virus-host encounter (8) and consequently viral production may be reduced. Moreover, bacterial attachment to sediment particles may provide a spatial refuge that decreases viral attack. Bacterial cell attachment is known to have a strong but unpredictable impact on cell physiology (27), and it is not known whether this results in changes in the receptor-mediated viral adsorption and infection capacity. In this context, one has to consider that viruses can proliferate only in active bacteria and that there seem to be large differences in the fraction of active bacteria in aquatic sediments. Luna et al. (29) reported that only 4% of bacteria in coastal marine sediments were actively growing, whereas Haglund et al. (15) found that 46% ± 10% of the total bacterial population was active in the sediment of a mesotrophic lake. At this stage of our investigation we cannot decide on a value to correct for dead and/or inactive cells. Also, we have little information about the proportion of lysogenic bacteria in sediment systems. If we adopt the findings of Lammers (26), who showed that more than 80% of the bacteria isolated from river sediments contained lysogenic bacteriophages, the process of bacterial infection which does not lead to sustained viral production could explain the lack of correlation between viral and bacterial abundances in the sediment of the Kühwörter Wasser (P > 0.17, as determined by Spearman rank correlation; n = 11).
Benthic viruses themselves may also adsorb to sediment particles. In studies of collection of colloidal particles in porous media, it is convenient to use a dimensionless deposition/capture rate, η, known as the single collector efficiency (9). η is the rate at which particles strike a single porous medium grain divided by the rate at which particles move towards the grain, and it represents the physical factors determining particle collision (16). Since viral transport is dominated by Brownian diffusion, the fraction of viruses that collide with the sediment surface is given by the Smoluchowski-Levich approximation (38). The lowest values for η coincided with high levels of viral production in March and November 2001, as well as February 2002. Since every encounter with a collector particle reduces the chance of contacting a host bacterium and vice versa, the data indicate that viral transport to the specific hosts was facilitated in these months, resulting in enhanced viral proliferation. This conclusion was corroborated by the results obtained in January 2001, when we observed relatively low levels of production compared to other winter values, and η reached the highest winter level. Despite high viral and bacterial abundances, the virus-host encounter probability seemed to be a limiting factor for viral proliferation in this month. Moreover, the highest η values coincided with the lowest levels of viral production in the summer. In this context, we have to point out that the reduced proliferation of viruses in this period could also have been caused in part by enhanced inactivation of viruses due to high water temperatures. Several authors have reported that temperature is the most important factor that influences virus inactivation (20, 50, 51) and that the rate of inactivation increases with the increasing temperatures that naturally occur in aquatic systems (2, 25, 28). We therefore speculate that a fraction of the small viral population in the summer was inactivated by high temperatures and could therefore not contribute to the production of new viruses. This hypothesis is supported by the estimates of high numbers of viruses and high levels of viral production at low temperatures in the winter and early spring.
Generally, the enhancing and attenuating effects of biotic and abiotic factors on adsorption, persistence, and production of benthic viruses make quantification of virus-bacterium interactions very difficult. The complexity of these processes in the sediment is also illustrated by the results obtained for a diel cycle. Although the chemophysical conditions, as well as the numbers of viruses and bacteria, were nearly constant, viral and bacterial production varied distinctly over the 24-h investigation period. However, the general trend observed during the long-term study, that viruses play only a minor role in the regulation of benthic BSP in the oxygenated sediment layer of the oxbow lake investigated, is also reflected in the results obtained for the diel cycle.
Conclusion.
Viruses are numerically important components of the microbial community in the oxygenated layer of a silty freshwater sediment, and the number of viruses exceeds the number in the overlying water column by 2 orders of magnitude. However, the significance of viruses is low compared to the significance of bacteria, and the viral impact is not a controlling force for bacterial production. At best, virus-induced cell lysis may significantly affect small subpopulations of bacteria. The effect of the low viral burden that benthic bacteria have to cope with compared to the viral burden that bacterial assemblages in the water column are confronted with is even reduced by the physical conditions in the sediment, which decrease the probability of virus-host encounters and consequently limit viral proliferation. Our data suggest that viruses play only a minor role in the processing and transfer of bacterial carbon in the sediment investigated. A recent long-term study carried out in the same ecosystem during the same investigation period showed that HNF and ciliates also do not have a regulatory effect on bacterial standing stock and production (48). Consumption by HNF and ciliates together accounted for only 0 to 14% of the BSP. If we add the bacterial mortality due to viral lysis and the contribution of HNF and ciliates, the two factors together are responsible for degradation of only 1% to at most 38% of the BSP. Thus, the fate of bacterial production and biomass in the oxygenated sediment layer of the freshwater environment investigated remains unclear.
Acknowledgments
This study was financed by the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (project no. P14220-BIO).
We are very grateful to F. S. Seebacher (Donau Consult, Zottl & Erber, Vienna, Austria) for providing hydrological data and to D. Danielopol (Institute of Limnology, Austrian Academy of Sciences, Mondsee, Austria) for stimulating discussions. We thank W. Weisz (VCPC, Institute for Software Science, University of Vienna, Vienna, Austria) for help with the mathematical analysis and two anonymous reviewers for constructive comments on the manuscript.
REFERENCES
- 1.Bitton, G. 1980. Fate of viruses in the aquatic environment, p. 67-90. In Introduction to environmental virology. Wiley, Inc., New York, N.Y.
- 2.Blanc, R., and A. Nasser. 1996. Effect of effluent quality and temperature on the persistence of viruses in soil. Water Sci. Technol. 33:237-242. [Google Scholar]
- 3.Bratbak, G., M. Heldal, T. F. Thingstad, B. Riemann, and O. H. Haslund. 1992. Incorporation of viruses into the budget of microbial C-transfer. A first approach. Mar. Ecol. Prog. Ser. 83:273-280. [Google Scholar]
- 4.Chen, F., J. Lu, B. J. Binder, Y. Liu, and R. E. Hodson. 2001. Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR Gold. Appl. Environ. Microbiol. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danovaro, R., and M. Serresi. 2000. Viral density and virus-to-bacterium ratio in deep-sea sediments of the eastern Mediterranean. Appl. Environ. Microbiol. 66:1857-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danovaro, R., E. Manini, and A. Dell'Anno. 2002. Higher abundance of bacteria than of viruses in deep Mediterranean sediments. Appl. Environ. Microbiol. 68:1468-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokulil, M., K. Donabaum, M. Schagerl, S. Brozek, S. Jirsa, W. Kabas, R. Mutschlechner, G. Pfister, B. Pritz, P. Riedler, M. Salbrechter, T. Schuh, J. Zika, H. Müller, B. Velimirov, A. K. T. Kirschner, A. Steitz, T. Ulbricht, C. Wieltschnig, and P. Wihlidal. 1997. Limnologische Untersuchung zur Sanierung der Alten Donau: Zustandsanalyse des freien Wassers und des Sedimentes in den Jahren 1995 und 1996. Abschlußbericht, Tabellenanhang, Vienna, Austria.
- 8.Drake, L. A., K.-H. Choi, A. G. E. Haskell, and F. C. Dobbs. 1998. Vertical profiles of virus-like particles and bacteria in the water column and sediments of Chesapeake Bay, USA. Aquat. Microb. Ecol. 16:17-25. [Google Scholar]
- 9.Elimelech, M. 1994. Particle deposition on ideal collectors from dilute flowing suspensions: mathematical formulation, numerical solution, and simulations. Sep. Technol. 4:186-212. [Google Scholar]
- 10.Fischer, U. R., and B. Velimirov. 2002. High control of bacterial production by viruses in a eutrophic oxbow lake. Aquat. Microb. Ecol. 27:1-12. [Google Scholar]
- 11.Freeze, R. A., and J. A. Cherry. 1979. Groundwater. Prentice-Hall, Inc., Englewood Cliffs, N.J.
- 12.Gerba, C. P., S. M. Goyal, E. M. Smith, and J. L. Melnick. 1977. Distribution of viral and bacterial pathogens in a coastal canal community. Mar. Pollut. Bull. 8:279-282. [Google Scholar]
- 13.Goyal, S. M., C. P. Gerba, and J. L. Melnick. 1978. Prevalence of human enteric viruses in coastal canal communities. J. Water Pollut. Control Fed. 50:2247-2256. [Google Scholar]
- 14.Gsell, T. C., W. E. Holben, and R. M. Ventullo. 1997. Characterization of the sediment bacterial community in groundwater discharge zones of an alkaline fen: a seasonal study. Appl. Environ. Microbiol. 63:3111-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haglund, A.-L., E. Törnblom, B. Boström, and L. Tranvik. 2002. Large differences in the fraction of active bacteria in plankton, sediments, and biofilm. Microb. Ecol. 43:232-241. [DOI] [PubMed] [Google Scholar]
- 16.Harvey, R. W., and S. P. Garabedian. 1991. Use of colloid filtration theory in modeling movement of bacteria through a contaminated sandy aquifer. Environ. Sci. Technol. 25:178-185. [Google Scholar]
- 17.Heldal, M., and G. Bratbak. 1991. Production and decay of viruses in aquatic environments. Mar. Ecol. Prog. Ser. 72:205-212. [Google Scholar]
- 18.Hewson, I., J. M. O'Neil, J. A. Fuhrman, and W. C. Dennison. 2001. Virus-like particle distribution and abundance in sediments and overlying waters along eutrophication gradients in two subtropical estuaries. Limnol. Oceanogr. 46:1734-1746. [Google Scholar]
- 19.Hewson, I., J. M. O'Neil, C. A. Heil, G. Bratbak, and W. C. Dennison. 2001. Effects of concentrated viral communities on photosynthesis and community composition of co-occurring benthic microalgae and phytoplankton. Aquat. Microb. Ecol. 25:1-10. [Google Scholar]
- 20.Hurst, C. J., C. P. Gerba, and I. Cech. 1980. Effects of environmental variables and soil characteristics on virus survival in soil. Appl. Environ. Microbiol. 40:1067-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, S. C., and J. H. Paul. 1994. Seasonal and diel abundances of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar. Ecol. Prog. Ser. 104:163-172. [Google Scholar]
- 22.Kirschner, A. K. T., and B. Velimirov. 1997. A seasonal study of bacterial community succession in a temperate backwater system, indicated by variation in morphotype numbers, biomass, and secondary production. Microb. Ecol. 34:27-38. [DOI] [PubMed] [Google Scholar]
- 23.Kirschner, A. K. T., and B. Velimirov. 1999. Benthic bacterial secondary production measured via simultaneous 3H-thymidine and 14C-leucine incorporation, and its implication for the carbon cycle of a shallow macrophyte-dominated backwater system. Limnol. Oceanogr. 44:1871-1881. [Google Scholar]
- 24.LaBelle, R. L., C. P. Gerba, S. M. Goyal, J. L. Melnick, I. Cech, and G. F. Bogdan. 1980. Relationship between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl. Environ. Microbiol. 39:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaBelle, R. L., and C. P. Gerba. 1982. Investigations into the protective effect of estuarine sediment on virus survival. Water Res. 16:469-478. [Google Scholar]
- 26.Lammers, W. T. 1988. Yearly flux of virus-like particles and humic acid in river sediment. Verh. Interact. Ver. Limnol. 23:1219-1223. [Google Scholar]
- 27.Lehman, R. M., and S. P. O'Connell. 2002. Comparison of extracellular enzyme activities and community composition of attached and free-living bacteria in porous medium columns. Appl. Environ. Microbiol. 68:1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liew, P.-F., and C. P. Gerba. 1980. Thermostabilization of enteroviruses by estuarine sediment. Appl. Environ. Microbiol. 40:305-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luna, G. M., E. Manini, and R. Danovaro. 2002. Large fraction of dead and inactive bacteria in coastal marine sediments: comparison of protocols for determination and ecological significance. Appl. Environ. Microbiol. 68:3509-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maranger, R., and D. F. Bird. 1996. High concentrations of viruses in the sediments of Lac Gilbert, Quebec. Microb. Ecol. 31:141-151. [DOI] [PubMed] [Google Scholar]
- 31.Mathias, C. B., A. K. T. Kirschner, and B. Velimirov. 1995. Seasonal variations of virus abundance and viral control of the bacterial production in a backwater system of the Danube River. Appl. Environ. Microbiol. 61:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 33.Norland, S. 1993. The relatioship between biomass and volume of bacteria, p. 303-307. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 34.Paul, J. H., J. B. Rose, S. C. Jiang, C. A. Kellogg, and L. Dickson. 1993. Distribution of viral abundance in the reef environment of Key Largo, Florida. Appl. Environ. Microbiol. 59:718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul, J. H., and C. A. Kellogg. 2000. Ecology of bacteriophages in nature, p. 211-246. In C. J. Hurst (ed.), Viral ecology. Academic Press, San Diego, Calif.
- 36.Proctor, L. M., A. Okubo, and J. A. Fuhrman. 1993. Calibrating estimates of phage-induced mortality in marine bacteria: ultrastructural studies of marine bacteriophage development from one-step growth experiments. Microb. Ecol. 25:161-182. [DOI] [PubMed] [Google Scholar]
- 37.Ricciardi-Rigault, M., Bird, D. F., and Y. T. Prairie. 2000. Changes in sediment viral and bacterial abundances with hypolimnetic oxygen depletion in a shallow eutrophic Lac Brome (Quebec, Canada). Can. J. Fish. Aquat. Sci. 57:1284-1290. [Google Scholar]
- 38.Schijven, J. F., and S. M. Hassanizadeh. 2000. Removal of viruses by soil passage: overview of modeling, processes, and parameters. Crit. Rev. Environ. Sci. Technol. 30:49-127. [Google Scholar]
- 39.Schwörbel, J. 1987. Einführung in die Limnologie. Gustav Fischer Verlag, Stuttgart, Germany.
- 40.Simon, M., and F. Azam. 1989. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 51:201-213. [Google Scholar]
- 41.Simon, M., H. P. Grossart, B. Schweitzer, and H. Ploug. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 28:175-211. [Google Scholar]
- 42.Steward, G. F., D. C. Smith, and F. Azam. 1996. Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar. Ecol. Prog. Ser. 131:287-300. [Google Scholar]
- 43.Suttle, C. A., and F. Chen. 1992. Mechanisms and rates of decay of marine viruses in seawater. Appl. Environ. Microbiol. 58:3721-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suttle, C. A. 1993. Enumeration and isolation of viruses, p. 121-134. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 45.Weinbauer, M. G., D. Fuks, and P. Peduzzi. 1993. Distribution of viruses and dissolved DNA along a coastal trophic gradient in the northern Adriatic Sea. Appl. Environ. Microbiol. 59:4075-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinbauer, M. G., and P. Peduzzi. 1994. Frequency, size and distribution of bacteriophages in different marine bacterial morphotypes. Mar. Ecol. Prog. Ser. 108:11-20. [Google Scholar]
- 47.Weinbauer, M. G., D. Fuks, S. Puskaric, and P. Peduzzi. 1995. Diel, seasonal, and depth-related variability of viruses and dissolved DNA in the northern Adriatic Sea. Microb. Ecol. 30:25-41. [DOI] [PubMed] [Google Scholar]
- 48.Wieltschnig, C., U. R. Fischer, A. K. T. Kirschner, and B. Velimirov. Benthic bacterial production and protozoan predation in a silty freshwater environment. Microb. Ecol., in press. [DOI] [PubMed]
- 49.Wommack, K. E., and R. R. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yates, M. V., C. P. Gerba, and L. M. Kelley. 1985. Virus persistence in groundwater. Appl. Environ. Microbiol. 49:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yates, M. V., S. R. Yates, J. Wagner, and C. P. Gerba. 1987. Modelling virus survival and transport in the subsurface. J. Contam. Hydrol. 1:329-345. [Google Scholar]
- 52.Zar, J. H. 1974. Biostatistical analysis. Prentice-Hall, Inc., Englewood Cliffs, N.J.