Abstract
The cytoskeleton has a unique property such that changes of conformation result in polymerization into a filamentous form. αB-Crystallin, a small heat shock protein (sHsp), has chaperone activities for various substrates, including proteins constituting the cytoskeleton, such as actin; intermediate filament; and tubulin. However, it is not clear whether the “α-crystallin domain” common to sHsps also has chaperone activity for the protein cytoskeleton. To investigate the possibility that the C-terminal α-crystallin domain of αB-crystallin has the aggregation-preventing ability for tubulin, we constructed an N-terminal domain deletion mutant of αB-crystallin. We characterized its structural properties and chaperone activities. Far-ultraviolet (UV) circular dichroism measurements showed that secondary structure in the α-crystallin domain of the deletion mutant is maintained. Ultracentrifuge analysis of molecular masses indicated that the deletion mutant formed smaller oligomers than did the full-length protein. Chaperone activity assays demonstrated that the N-terminal domain deletion mutant suppressed heat-induced aggregation of tubulin well. Comparison of chaperone activities for 2 other substrates (citrate synthase and alcohol dehydrogenase) showed that it was less effective in the suppression of their aggregation. These results show that αB-crystallin recognizes a variety of substrates and especially that α-crystallin domain binds free cytoskeletal proteins. We suggest that this feature would be advantageous in its functional role of holding or folding multiple proteins denatured simultaneously under stress conditions.
INTRODUCTION
αB-crystallin, which is a member of the family of small heat shock proteins (sHsps), functions as a molecular chaperone and maintains protein homeostasis by preventing substrate aggregation. Some papers have reported the association of αB-crystallin with cytoskeletal elements. For example, α-crystallin modulates the assembly of intermediate filament vimentin and stabilizes actin filaments in a phosphorylation-dependent manner (Nicholl and Quinlan 1994; Wang and Spector 1996). We reported that αB-crystallin associates with tubulin (Arai and Atomi 1997; Fujita et al 2004; Sakurai et al 2005). Previously, we reported that heat-induced denatured tubulin dimer aggregation could be suppressed by αB-crystallin, showing that αB-crystallin may bind to denatured/denatured intermediate forms of tubulins, but not to native tubulin dimers (Arai and Atomi 1997). In addition, αB-crystallin directly associates with the surface of microtubule-associated protein (MAP) microtubules (Fujita et al 2004). Denatured tubulin is reported to inhibit the assembly of native tubulin (Maccioni 1983). It is possible that αB-crystallin binds nonnative tubulin to prevent microtubule disassembly and inhibits formation of abnormal microtubules by avoiding disorder within the denatured tubulin dimer.
The structure of αB-crystallin consists of 3 domains: the N-terminal hydrophobic domain (1–67), the conserved C-terminal “α-crystallin domain” (68–149), and a third domain extending into an exposed flexible C-terminal extension (150–175) (Carver et al 1992; Kim et al 1998; Wistow 1985). Several sites appear to be involved in the chaperone function of αB-crystallin, including the N-terminal domain and the C-terminal conserved α-crystallin domain. For cytoskeletal proteins, a missense mutation in αB-crystallin, R120G, has been shown to cosegregate with a familial desmin-related myopathy (Vicart et al 1998). The R120G mutation of αB-crystallin induces the aggregation of glial fibrillary acidic protein filaments (Perng et al 1999). The conserved β-sheet motif formed by residues 139–148 of αB-crystallin and sHsps are predicted to bind to actin filaments (Mounier and Arrigo 2002). On the other hand, the conserved region F24 through F27 near the N terminus is important for its insulin chaperone activity (Plater et al 1996). Given the diversity of substrates and the number of domains responsible for chaperone activity, the functional regions might be substrate specific. For example, the α-crystallin domain might carry out chaperone functions for cytoskeletal proteins. However, this hypothesis remains to be confirmed because it has not yet been shown that the α-crystallin domain conducts a chaperone function for the cytoskeleton protein tubulin.
In this report, we asked whether the C-terminal α-crystallin domain of αB-crystallin was responsible for inhibiting tubulin aggregation. We constructed a deletion mutant lacking the N-terminal domain of αB-crystallin. The mutant was examined for its chaperone activity for tubulin and for 2 other substrates, alcohol dehydrogenase (ADH) and citrate synthase (CS), frequently chosen for model substrate assays. Furthermore, to exclude possible effects of different buffer conditions, phosphate buffer containing NaCl was commonly used throughout the study. The results of our study demonstrated that the conserved α-crystallin domain is important to prevent tubulin aggregation. Furthermore, the experiments showed that different proteins use different sequences in αB-crystallin to maintain their solubility during heat-induced aggregation.
MATERIALS AND METHODS
Chemicals
CS (EC 4.1.3.7) from pig heart was purchased from Roche (Basel, Switzerland). Yeast ADH, N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acids] (HEPES), piperazine-N,N′-bis[2-ethanesulfonic acid] (PIPES), and guanosine triphosphate (GTP) were from Sigma (St Louis, MO, USA). Bovine serum albumin (BSA) was purchased from Pierce (Rockford, IL, USA). All other reagents used were of the highest grade.
Cloning of the deletion mutant lacking the N-terminal domain of αB-crystallin
To prepare the expression vector encoding the αB-crystallin deletion mutant lacking the N-terminal domain, αB-ΔN67–expressing clones were amplified by polymerase chain reaction (PCR) with pUC118-αB-crystallin (GenBank accession P23928) (Atomi et al 1991) as a template and custom primers (EcoRI and XbaI restriction sites underlined) synthesized by Espec oligo (Tsukuba, Japan). Primers used were for the αB-ΔN67 mutant: top, 5′-GCGAATTCATGCGTATGGAGAAGGACAG-3′; bottom, 5′-TACTCGAGCTACTTCTTAGGGGCTGCAG-3′. The PCR product was digested with EcoRI and XbaI and cloned into pMAL™-c2 (New England Biolabs, Ipswich, MA, USA), thereby producing pMAL-αB-ΔN67 protein. The DNA constructions were confirmed by DNA sequence analysis.
Expression and purification of the deletion mutant lacking the N-terminal domain of αB-crystallin
The pMAL-αB-ΔN67 expression plasmid was used to transform competent Escherichia coli TB1 (New England Biolabs). The procedures used for expression and purification of protein were described previously (Muchowski et al 1997). Two liters of SB medium containing 50 μg/ mL ampicillin was inoculated with 3 mL of an overnight culture of transformed E. coli cells, and cells were grown with shaking at 37°C. The cells were incubated until the culture reached an optical density of ∼0.6 at absorbance (A) = 600 nm, at which point protein expression was induced by addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 0.3 mM (Takara, Shiga, Japan). Four hours after induction, cells were washed by centrifugation at 6000 × g for 15 minutes at 4°C and resuspended in 50 mL of TEN buffer (10 mM Tris-HCl, pH 8.0, 1 mM ethylenediaminetetraacetic acid [EDTA], and 100 mM NaCl). The cells were harvested by centrifugation at 6000 × g for 15 minutes at 4°C and resuspended in 50 mL of column buffer (20 mM Tris-HCl, pH 7.4, 1 mM EDTA, and 200 mM NaCl). Cells were stored overnight at −30°C. After thawing in cold water with shaking, DNaseI (Takara) was added to the cells, and the cells were incubated at 37°C for 5 minutes. The cells were disrupted by sonication on ice by three 15-second cycles at 80 watts on an Ultrasonic disrupter (TOMY, Tokyo, Japan). Insoluble cellular debris was removed by sedimentation at 9000 × g for 30 minutes at 4°C. Soluble fusion protein present in the supernatant was purified by adsorption to a 20-mL amylose resin affinity column (New England Biolabs) at 4°C. After washing with 10 column volumes of column buffer, bound fusion protein was eluted with column buffer that contained 10 mM maltose. Fusion proteins were concentrated with Vivaspin (Sartorius AG, Goettingen, Germany). The concentration of the purified fusion protein was determined by a protein assay (Bio-Rad, Hercules, CA, USA) with BSA as a standard.
Purification of the protein lacking the N-terminal domain of αB-crystallin
Maltose binding protein (MBP) was cleaved from the purified fusion protein with the use of protease Factor Xa, the recognition sequence of which is encoded in the pMAL-c2-αB-ΔN67 vector within the fourth and fifth codons 5′ from the coding region for αB-crystallin. Deleted αB-crystallin was purified by anion exchange chromatography at 4°C. Cleaved fusion protein was dialyzed against ion exchange buffer (10 mM Tris-HCl, pH 8.5, 20 mM NaCl, and 1 mM EDTA). MBP and Factor Xa were separated from the αB-crystallin deletion mutant protein by absorption to a 1-mL column Resource Q anion exchange resin (GE Healthcare, Bucks, UK). In the presence of ion exchange buffer, deletion mutant protein had no affinity for the Resource Q anion exchange resin and hence could be separated from MBP and Factor Xa. Preparations of mutant deletion protein were dialyzed overnight against 50 mM sodium phosphate, pH 7.0, and 150 mM NaCl and then concentrated on Vivaspin.
Purification of αB-crystallin and tubulin
Human full-length αB-crystallin and bovine tubulin were purified as previously described and stored at liquid nitrogen or −80°C (Arai and Atomi 1997; Sun et al 1997). Purified protein preparations were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with the use of antibodies recognizing the carboxyl-terminal decapeptide (SH) KPAVTAAPKK of rat αB-crystallin and monoclonal anti– α-tubulin antibody (Sigma) to confirm their purity and identity, performed as described previously (Arai and Atomi 1997).
Circular dichroism measurements
Far-UV circular dichroism (CD) spectra were measured with a Jasco J-720 spectropolarimeter. A 1-mm path length cell was used. CD spectra presented here are the average of 8 scans, smoothed by polynomial curve fitting. The protein concentration was 0.3 mg/mL, and the sample was in 50 mM sodium phosphate (pH 7.0), 100 mM NaCl buffer. The circular dichroism data are expressed as molar ellipticity (degrees cm2/dmol).
Analytical ultracentrifugation
Sedimentation equilibrium was carried out to determine the weight-averaged molecular mass (MW) distributions of the αB-ΔN67 and full-length αB-crystallin. Sedimentation equilibrium experiments were performed in a Beckman Optima XL-I analytical ultracentrifuge (Beckman Coulter, Fullerton, CA, USA). Protein solutions were dialyzed extensively against 50 mM sodium phosphate (pH 7.0) and 100 mM NaCl at 4°C. Aliquots (120 μL) of αB-ΔN67 and full-length αB-crystallin were loaded into 2-channel, 12-mm centerpieces, and 140 μL of dialysate was loaded into the corresponding reference channels. Centrifugation was conducted in a 60-Ti rotor at 3000 rpm, 4000 rpm, and 5000 rpm for full-length αB-crystallin and 13 000 rpm, 19 000 rpm, and 24 000 rpm for the αB-ΔN67 protein to produce a sedimentation equilibrium at 20°C. Radial absorbance scans were collected in continuous scan mode at 230 nm every 2 hours with 20 replicates and a step size of 0.001 cm.
Sedimentation velocity was performed in a Beckman XL-I analytical ultracentrifuge. Sample volumes of 400 μL were centrifuged at 50 000 rpm for both αB-ΔN67 and full-length αB-crystallin at 20°C. Radial scans of absorbance at 230 nm were recorded. Data were analyzed with the SEDFIT program (National Institutes of Health, Bethesda, MD, USA). The partial specific volumes (v) at 20°C, were calculated from the amino acid compositions, and the solvent density was 1.005 g/cm3 by “sednterp” (http://www.jphilo.mailway.com/download.htm) (Laue et al 1992). The average number of subunits per assembly for full-length αB-crystallin and the N-terminal domain deletion mutant (αB-ΔN67) listed in Table 1 were calculated with the formula: number of subunits per oligomer = (average molecular mass calculated from analytical centrifugation)/(molecular mass of a single full-length αB-crystallin or N-terminal domain–deleted mutant [αB-ΔN67] subunit).
Table 1.
Analysis of oligomer size of full-length αB-crystallin and the N-terminal domain deletion mutant (αB-ΔN67)
Assay for chaperone activity
Assays to measure chaperone activity of αB-crystallin were performed with the use of previously established methods with minor modifications (Arai and Atomi 1997; Kamradt et al 2001; Kelley and Abraham 2003). Tubulin, CS, and ADH were used as chaperone target proteins. For ADH or CS aggregation, the assay was performed in sodium phosphate buffer containing NaCl (50 mM sodium phosphate, 150 mM NaCl, pH 7.0) or HEPES buffer (40 mM HEPES, pH 7.5), respectively. For tubulin aggregation, the assay was performed in PME buffer (80 mM PIPES, pH 6.8, 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid [EGTA], 1 mM GTP). Alternatively, sodium phosphate buffer containing NaCl was used. Thermally induced protein aggregation was monitored by measuring the turbidity of the solution on a spectrophotometer (Beckman DU-65), with the temperature regulated by a water bath. Molar ratio was represented as (moles of chaperone as a monomer):(moles of substrate as a monomer). All aggregation experiments were normalized to control experiments, in which aggregation with the substrate alone was defined as being 100% of the total substrate aggregation.
RESULTS
Construction of the deletion mutant lacking the N-terminal domain of αB-crystallin
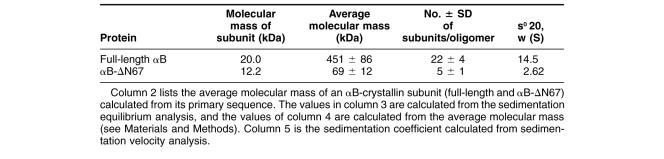
To investigate whether the conserved C-terminal α-crystallin domain of αB-crystallin includes the functional region for tubulin, we constructed a deletion mutant lacking the N-terminal domain of αB-crystallin (αB-ΔN67). We chose the cleavage site on the basis of the conserved α-crystallin domain (68–149 amino acids; Fig 1A) (Wistow 1985; Carver et al 1992; Kim et al 1998).
Fig 1.
Construction of the N-terminal domain deletion mutant of αB-crystallin (αB-ΔN67). (A) We chose the 67th amino acid as the cleavage site on the basis of the location of the α-crystallin domain (amino acids 68–149). The deletion mutant is named αB-ΔN67. (B) Expression and purification of αB-ΔN67 in Escherichia coli. Full-length αB-crystallin (lane 1) and purified αB-ΔN67 (lane 2) were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Immunoblotting of full-length αB-crystallin (lane 3) and αB-ΔN67 (lane 4) with anti–αB-crystallin C-terminal peptide antibody
After proteolytic cleavage of the MBP-tagged fusion protein, αB-ΔN67 included an additional 4 N-terminal, vector-derived amino acids (isoleucine, serine, glutamic acid, and phenylalanine). The untagged form of the αB-crystallin C-terminal domain eluted with >98% purity in the flow-through from the Resource Q anion exchange column. The peak fractions containing αB-ΔN67 were pooled and stored at −80°C. To confirm the purification of these proteins, we conducted SDS-PAGE and Western blot analysis with an anti–αB-crystallin C-terminal antibody (Fig 1B). The stained gel showed the anticipated bands with expected gel mobilities of 22 kDa for full-length αB-crystallin and 12 kDa for αB-ΔN67. Densitometry revealed >95% purity (data not shown).
Structural characterization of the deletion mutant lacking the N-terminal domain of αB-crystallin
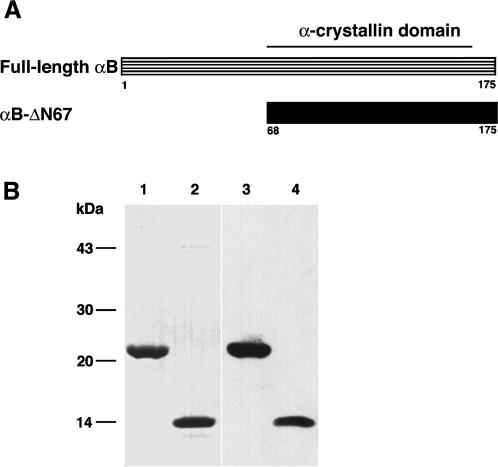
Next, to characterize the structure of the deletion protein (αB-ΔN67), far-UV CD spectroscopy was performed. The spectrum of αB-ΔN67 showed negative ellipticity with a minimum at 215 nm, which was typical for a β-sheet conformation (Fig 2). The spectrum was in agreement with the predicted secondary structure for the αB-ΔN67 region of αB-crystallin and the α-crystallin domain of αB-crystallin (Siezen and Argos 1983; Feil et al 2001). The secondary structure indicated by the far-UV CD spectrum of full-length αB-crystallin was consistent with a previous study (Muchowski et al 1997). Thus, analysis of the secondary structure of αB-ΔN67 shows that the anti–parallel β-sheet that is the main structure in the α-crystallin domain is maintained. Thus, it appears that the N-terminal domain deletion mutant, αB-ΔN67, retained the functional domain.
Fig 2.
Analysis of secondary structure of the N-terminal domain deletion mutant (αB-ΔN67) by far-ultraviolet circular dichroism. The circular dichroism spectra shown here represent the average of 8 scans, smoothed by polynomial curve fitting. The circular dichroism data are expressed as molar ellipticity (degrees cm2/dmol)
Next, for the purpose of investigating the oligomeric structure of αB-ΔN67, the average molecular masses of αB-ΔN67 and full-length αB-crystallin were determined by sedimentation equilibrium to be 69 ± 12 kDa and 451 ± 86 kDa, respectively (Table 1). The number of subunits per assembly and its standard deviation calculated from the average molecular mass of αB-ΔN67 and full-length αB-crystallin were determined to be 5 ± 1 and 22 ± 4, respectively (Table 1). On the basis of the results of the sedimentation velocity, the s-values of αB-ΔN67 and full-length αB-crystallin were found to be 2.62 S and 14.5 S, respectively. The peaks of both samples were broad, suggesting that the protein exists not as a single species but in a dynamic equilibrium between oligomeric states. These results suggested that the N-terminal domain deletion mutant of αB-crystallin cannot form large oligomers.
Tubulin chaperone activity of the deletion mutant lacking the N-terminal domain of αB-crystallin
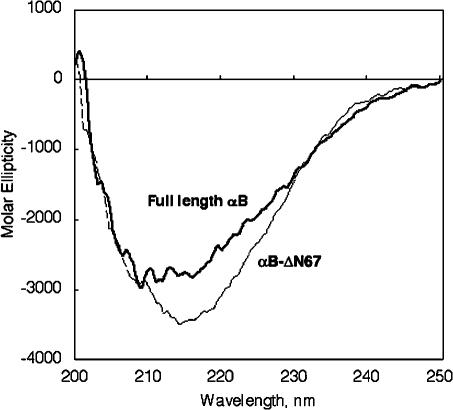
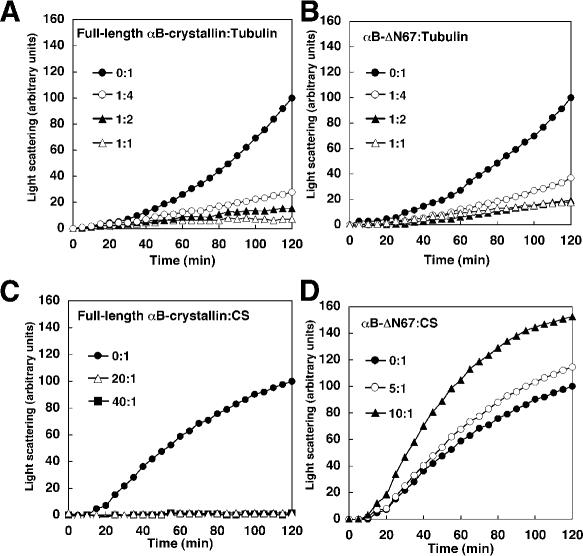
To investigate the functionality of αB-ΔN67 for tubulin, we asked whether the mutant could prevent tubulin aggregation. Tubulin was incubated at 42°C for 120 minutes, either alone or in the presence of the full-length αB-crystallin. As observed in Figure 3A, at a 1:4 (full-length αB-crystallin:tubulin) molar ratio, the aggregation of tubulin was reduced from 100 to 40 arbitrary units (a.u.). At a 1:2 (full-length αB-crystallin:tubulin) molar ratio, the aggregation of tubulin decreased from 100 to 50 a.u. in this buffer condition. Higher concentrations of αB-crystallin enhanced turbidity when PME buffer was used in the assay (Fig 3A).
Fig 3.
Tubulin chaperone activity of deletion mutant αB-ΔN67 and full-length αB-crystallin. Kinetics of thermal aggregation with 10 μM tubulin at 42°C alone (closed circle), or in the presence of full-length αB-crystallin (A) or αB-ΔN67 (B) at concentrations of 1.25 μM (open circle), 2.5 μM (closed triangle), and 5 μM (open triangle). Protein aggregation was determined by light scattering at 350 nm. These assays were performed in PME buffer (80 mM PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 1 mM GTP)
Next, we determined whether the deletion of the N-terminal domain of αB-crystallin influenced the chaperone activity for heat-induced tubulin aggregation. At a 1:4 (αB-ΔN67:tubulin) molar ratio, the aggregation of tubulin was reduced from 100 to 14 a.u. (Fig 3B). Thus, αB-crystallin lacking the N-terminal domain was quite efficient in preventing heat-induced aggregation of tubulin.
Characterization of the chaperone activity of the N-terminal domain deletion mutant for CS and ADH
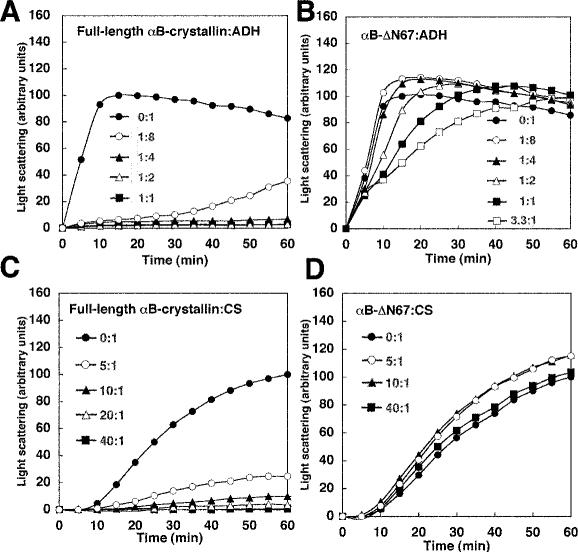
To compare the ability of the α-crystallin domain to function with different substrates, chaperone activity was also performed with ADH and CS instead of tubulin. Because ADH and CS are sensitive to heat, the aggregation time was reduced to 60 minutes. At a 1:4 (full-length αB-crystallin:ADH) molar ratio, ADH aggregation decreased from 100 to 5 a.u. at maximum aggregation (Fig 4A). In the ADH assay, αB-ΔN67 was less effective for ADH than for tubulin in preventing heat-induced aggregation. Specifically, at a 1:4 (αB-ΔN67:ADH) molar ratio, ADH aggregation increased from 100 to 110 a.u. (Fig 4B). Higher molar ratios of αB-ΔN67 did not prevent aggregation of ADH. At a 3.3:1 (αB-ΔN67:ADH) molar ratio, ADH aggregation increased from 100 to 122 a.u. However, at the 60-minute time point, αB-ΔN67 delayed the increase of ADH aggregation. Thus, αB-ΔN67 has little inhibitory effect on ADH compared with its effect on tubulin.
Fig 4.
Chaperone activities of deletion mutant αB-ΔN67 and full-length αB-crystallin for ADH and CS. Kinetics of thermal aggregation with 20 μM ADH at 42°C alone (closed circle) or in the presence of full-length αB-crystallin (A) or αB-ΔN67 (B) at concentrations of 2.5 μM (open circle), 5 μM (closed triangle), 10 μM (open triangle), 20 μM (closed square), and 66 μM (open square). These assays were performed in a buffer of 50 mM sodium phosphate, 150 mM NaCl (pH 7.0), and 2 mM EDTA. Kinetics of thermal aggregation of 0.5 μM CS at 42°C alone (closed circle) or in the presence of full-length αB-crystallin (C) or αB-ΔN67 (D) at concentrations of 2.5 μM (open circle), 5 μM (closed triangle), 10 μM (open triangle), and 20 μM (closed square). These assays were performed in 40 mM HEPES (pH 7.5). In each case, protein aggregation was determined as in Figure 3
Incubation of CS in the presence of full-length αB-crystallin resulted in decreased CS aggregation to 10 a.u. (10:1 full-length αB-crystallin:CS; Fig 4C). A 25-fold excess of full-length αB-crystallin was necessary to prevent CS aggregation compared with the inhibition of tubulin or ADH. αB-ΔN67 for CS was unable to prevent heat-induced aggregation (Fig 4D). At a 10:1 (αB-ΔN67:CS) molar ratio, CS aggregation increased from 100 to 115 a.u. Excessive amounts of αB-ΔN67 did not prevent aggregation of CS. At a 40:1 (αB-ΔN67:CS) molar ratio, CS aggregation increased from 100 to 103 a.u. Thus, αB-ΔN67 cannot prevent heat-induced CS aggregation.
Together, these results indicated that full-length αB-crystallin inhibits aggregation of tubulin, ADH, and CS. In contrast, an equivalent molar ratio of αB-ΔN67 did not prevent CS and ADH aggregation with similar efficiency, yet efficiently inhibited heat-induced tubulin. Hence, the chaperone activity of the N-terminal domain deletion mutant αB-ΔN67 seems to depend on the nature of the substrate.
Buffer effect on chaperone activity
We performed the chaperone activity assay with the use of previously reported conditions (Horwitz 1992; Arai and Atomi 1997; Buchner et al 1998; Figs 3 and 4). However, the results for any one substrate might be affected by the buffer employed. The buffer was limited to sodium phosphate buffer (50 mM sodium phosphate, pH 7.0, 150 mM NaCl), which was used in ADH aggregation to exclude the effects derived from different conditions. With that buffer, the raw light scattering of tubulin alone or CS alone was lower than that using PME buffer or HEPES buffer (data not shown). Figure 5 shows that αB-ΔN67 prevents tubulin aggregation but not CS aggregation in sodium phosphate buffer containing NaCl. At a 1:4 (full-length αB-crystallin:tubulin) molar ratio, the aggregation of tubulin was reduced from 100 to 28 a.u. (Fig 5A), whereas at the same 1:4 ratio of αB-ΔN67:tubulin, we observed reduced aggregation of tubulin (from 100 to 37 a.u.; Fig 5B). Higher concentrations of αB-crystallin decreased aggregation of tubulin, as far as we investigated, when sodium phosphate buffer was used. Incubation of CS in the presence of full-length αB-crystallin resulted in decreased CS aggregation to 2 a.u. (20:1 full-length αB-crystallin:CS; Fig 5C). At a 10:1 (αB-ΔN67:CS) molar ratio, CS aggregation increased from 100 to 152 a.u. Because more αB-crystallin is needed to prevent CS aggregation compared with tubulin or ADH, αB-crystallin seems to function as a CS chaperone in an oligomeric form. In terms of oligomer molar ratios, both are the same molar ratio of 2:1 at 10:1 αB-ΔN67:CS and at 40:1 full-length αB-crystallin:CS from Table 1. Hence, mutant αB-ΔN67 was less effective for CS than for an equal molar ratio of full-length αB-crystallin. Thus, these results suggested that the N-terminal domain is not needed for tubulin chaperone activity but is required for ADH and CS, in spite of the difference of buffer conditions.
Fig 5.
Chaperone activities of the deletion mutant αB-ΔN67 and full-length αB-crystallin in a buffer of 50 mM sodium phosphate and 150 mM NaCl (pH 7.0). Kinetics of thermal aggregation with 10 μM tubulin at 42°C alone (closed circle) or in the presence of full-length αB-crystallin (A) or αB-ΔN67 (B) at concentrations of 2.5 μM (open circle), 5 μM (closed triangle), and 10 μM (open triangle). Kinetics of thermal aggregation at 0.5 μM CS at 42°C alone (closed circle) or in the presence of full-length αB-crystallin (C) or αB-ΔN67 (D) at concentrations of 2.5 μM (open circle), 5 μM (closed triangle), 10 μM (open triangle), and 20 μM (closed square). These assays were performed in 50 mM sodium phosphate (pH 7.0), 150 mM NaCl
DISCUSSION
In this report, we asked whether the α-crystallin domain has chaperone activity for the cytoskeletal protein tubulin and compared the results with those obtained with CS and ADH. We constructed a deletion mutant of αB-crystallin with a truncated N-terminal domain, αB-ΔN67, and characterized its secondary structure and molecular mass. We showed that the hydrophobic N-terminal domain of αB-crystallin is not necessary for chaperone activity for denatured tubulin. Instead, the remainder of the α-crystallin domain is able to inhibit tubulin aggregation.
Analysis of the secondary structure of mutant αB-ΔN67 showed that the anti–parallel β-sheet conformation that is the main structure in the α-crystallin domain is maintained. Furthermore, it appears that the mutant retains functionality. Because the α-crystallin domain retained its structural integrity, deletion of the N-terminal domain of αB-crystallin did not dramatically affect its conformation.
The average molecular mass of the α-crystallin domain (αB-ΔN67) and full-length αB-crystallin determined by sedimentation equilibrium suggests that the α-crystallin domain (αB-ΔN67) cannot form large oligomers. It has been reported that wild-type αB-crystallin forms large oligomers and that the N-terminal region plays a role in the oligomerization. The results in this study are consistent with the previous report, as the truncation of the N-terminal domain of αB-crystallin inhibited the formation of oligomers (Feil et al 2001). Thus, the N-terminal domain of αB-crystallin seems to be responsible for oligomer formation.
The α-crystallin domain (αB-ΔN67) has been reported to associate with the cytoskeletal protein molecules actin and the intermediate filament vimentin (Vicart et al 1998; Perng et al 1999; Mounier and Arrigo 2002). According to Vicart et al (1998), the R120G mutation of αB-crystallin causes desmin aggregation in a desmin-related myopathy. Polar residues might contribute to the chaperone activity for cytoskeletal protein. In this report, we showed for the first time that the α-crystallin domain efficiently blocked heat-induced aggregation of tubulin. An important role of αB-crystallin might be to protect microtubules from stress by maintaining native tubulin amounts. Because the tubulin molecule is rich in polar amino acids, the polar regions of the α-crystallin domain might be involved in tubulin recognition. Given the requirement for the α-crystallin domain, the capacity of αB-crystallin to block tubulin aggregation could well require polar amino acids.
Usually, chaperone effects gradually increase depending on the concentration of chaperones; however, chaperone effect of full-length αB-crystallin is different. We observed that higher concentrations of full-length αB-crystallin increased turbidity in PIPES buffer in this study. This phenomenon was previously observed (Arai and Atomi 1997). When sodium phosphate buffer is used instead of PME buffer in this study, prevention of aggregation is dependent on the concentration of full-length αB-crystallin (Fig 4). Therefore, PME buffer might affect this phenomenon. Because PME buffer has usually been used in the polymerization of microtubules, some specific interactions might occur between the conformational change of tubulin to polymerize and full-length αB-crystallin. Interestingly in PIPES buffer, which might facilitate tubulin polymerization compared with PBS, mutant αB-ΔN67 (mainly α-crystallin domain) effectively inhibited tubulin aggregation in a concentration-dependent manner (Fig 2). More precise experiments will be needed for characterization of these polymerization-directed conformational changes of the cytoskeletal proteins.
By comparing the activities of the α-crystallin domain on several chaperone substrates, the results clearly show that the region of αB-crystallin that inhibits aggregation of heat-denatured tubulin differed from that effective for CS and ADH. Recently, Ghosh et al (2006) reported that selectivity for unfolded target proteins varied with the specific amino acid composition in αB-crystallin. Hence, different proteins use different sequences in αB-crystallin to maintain their solubility during heat-induced aggregation.
Assay conditions used in the measurement of the chaperone activity, such as temperature, reaction buffers, and the denaturing procedures, were not uniform because the optimal conditions for denaturation differed between substrates. However, temperature is known to have different effects on secondary, tertiary, and oligomeric structures (Das and Surewicz 1995) and on chaperone activity (Raman et al 1995). Therefore, we used the same buffers in the chaperone assay for all 3 substrates, to investigate whether the functional region in the deletion mutant showed different efficiencies with different substrates. We used sodium phosphate buffer, which mimics physiological conditions. By comparing the activities of the mutant on several chaperone substrates, the results clearly showed that the functional region of αB-crystallin for heat-denatured tubulin differed from that for CS and ADH and that different proteins use different sequences in αB-crystallin to maintain their solubility during heat-induced aggregation. Thus, these results suggest that the N-terminal domain is not needed for tubulin chaperone activity but is required for ADH and CS activities, in spite of the difference of buffer condition.
Moreover, our results in this study are complementary to these previous established roles of αB-crystallin. The intrinsic role of the cytoskeleton in the cell is to keep “structure homeostasis,” which is accomplished through “protein homeostasis.” Although sHsps are constitutively expressed in heart and slow muscles, the underlying reason has not been elucidated. Unlike α-crystallin in the lens, we suggest that the αB-crystallin domain of sHsps maintains a dynamic cytoskeleton within heart and skeletal muscles. On the basis of our results, we suggest that the very properties of the cytoskeleton (“treadmilling” for actin/actin filaments, and dynamic instability of the tubulin/microtubule system) require sHsps (chaperones) to possess an α-crystallin domain. It was previously reported that sHsps might recognize an early change of protein conformation. We do not call the conformational changes through the polymerization “denaturation”; however, the intrinsic property is similar. The cytoskeletal dynamics must be supported by sHsps.
We showed that the region of αB-crystallin that blocks tubulin aggregation is not located at the N-terminal domain but at the conserved C-terminal α-crystallin domain and is different from that for other substrates, such as CS and ADH. We suggest that multiple chaperone functional regions within the αB-crystallin molecule, each capable of recognizing a variety of specific substrates, would be an advantage in its functional role of holding or folding multiple proteins denatured simultaneously under stress conditions.
Acknowledgments
We are greatly appreciative of Dr Richard I. Morimoto for helpful discussions and for critical reading of the manuscript, Dr Fumio Arisaka for assistance with analytical ultracentrifugation and helpful discussions, and Dr Munehito Arai for assistance with CD measurements. This study was supported in part by a Grant-in-Aid for Scientific Research (B:15300219) and (A:17200039) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT); by JST (Japan Science and Technology Corporation)/RISTEX (Research Institute of Science and Technology for Society); by the Fund for Basic Experiments Oriented to Space Station Utilization from ISAS; by “Ground Research Announcement for Space Utilization” promoted by the Japan Space Forum; and by the Fund for Scientific Experiments Oriented to Mechanical Stimulus in Biology from JPBSI.
REFERENCES
- Arai H, Atomi Y. Chaperone activity of αB-crystallin suppresses tubulin aggregation through complex formation. Cell Struct Funct. 1997;22:539–544. doi: 10.1247/csf.22.539.0386-7196(1997)022[0539:CAOBST]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Atomi Y, Yamada S, Nishida T. Early changes of alpha B-crystallin mRNA in rat skeletal muscle to mechanical tension and denervation. Biochem Biophys Res Commun. 1991;181:1323–1330. doi: 10.1016/0006-291x(91)92083-v.0006-291X(1991)181[1323:ECOABM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Buchner J, Grallert H, Jakob U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 1998;290:323–338. doi: 10.1016/s0076-6879(98)90029-5.0076-6879(1998)290[0323:AOCFUC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carver JA, Aquilina JA, Truscott RJ, Ralston GB. Identification by 1H NMR spectroscopy of flexible C-terminal extensions in bovine lens alpha-crystallin. FEBS Lett. 1992;311:143–149. doi: 10.1016/0014-5793(92)81386-z.0014-5793(1992)311[0143:IBHNSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Das KP, Surewicz WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5.0014-5793(1995)369[0321:TEOHSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feil IK, Malfois M, Hendle J, van Der Zandt H, Svergun DI. A novel quaternary structure of the dimeric alpha-crystallin domain with chaperone-like activity. J Biol Chem. 2001;276:12024–12029. doi: 10.1074/jbc.M010856200.0021-9258(2001)276[12024:ANQSOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Ohto E, Katayama E, Atomi Y. AlphaB-crystallin– coated MAP microtubule resists nocodazole and calcium-induced disassembly. J Cell Sci. 2004;117:1719–1726. doi: 10.1242/jcs.01021.0021-9533(2004)117[1719:ACMMRN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Houck SA, Clark JI. The function of the beta3 interactive domain in the small heat shock protein and molecular chaperone, human alphaB crystallin. Cell Stress Chaperones. 2006;11:187–197. doi: 10.1379/CSC-186.1.1466-1268(2006)011[0187:TFOTBI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449.1091-6490(1992)089[10449:ACFAAM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c– and caspase-8–dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200.0021-9258(2001)276[16059:TSHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kelley PB, Abraham EC. Thermally induced disintegration of the oligomeric structure of alphaB-crystallin mutant F28S is associated with diminished chaperone activity. Mol Cell Biochem. 2003;252:273–278. doi: 10.1023/a:1025568417000.0300-8177(2003)252[0273:TIDOTO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim S-H. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106.1476-4687(1998)394[0595:CSOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Laue TM, Shah BD, Ridgeway TM, and Pelletier SL 1992 Computer-aided interpretation of analytical sedimentation data for proteins. In: Analytical Ultracentrifugation in Biochemistry and Polymer Science, ed Harding SE, Rowe AJ, Horton JC. Royal Society of Chemistry, Cambridge, UK, 90–125. [Google Scholar]
- Maccioni RB. Microtubule assembly affected by the presence of denatured tubulin. Biochem Biophys Res Commun. 1983;110:463–469. doi: 10.1016/0006-291x(83)91172-5.0006-291X(1983)110[0463:MAABTP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2.1466-1268(2002)007[0167:ACASHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Bassuk JA, Lubsen NH, Clark JI. Human alphaB-crystallin. Small heat shock protein and molecular chaperone. J Biol Chem. 1997;272:2578–2582. doi: 10.1074/jbc.272.4.2578.0021-9258(1997)272[2578:HASHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nicholl ID, Quinlan RA. Chaperone activity of α-crystallins modulates intermediate filament assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x.1460-2075(1994)013[0945:CAOCMI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng MD, Muchowski PJ, van Den IJssel P, Wu GJ, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in αB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274:33235–33243. doi: 10.1074/jbc.274.47.33235.0021-9258(1999)274[33235:TCALCM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Plater ML, Goode D, Crabbe MJ. Effects of site-directed mutations on the chaperone-like activity of αB-crystallin. J Biol Chem. 1996;271:28558–28566. doi: 10.1074/jbc.271.45.28558.0021-9258(1996)271[28558:EOSMOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Raman B, Ramakrishna T, Rao CM. Temperature dependent chaperone-like activity of alpha-crystallin. FEBS Lett. 1995;365:133–136. doi: 10.1016/0014-5793(95)00440-k.0014-5793(1995)365[0133:TDCAOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Fujita Y, Ohto E, Oguro A, Atomi Y. The decrease of the cytoskeleton tubulin follows the decrease of the associating molecular chaperone alphaB-crystallin in unloaded soleus muscle atrophy without stretch. FASEB J. 2005;19:1199–1201. doi: 10.1096/fj.04-3060fje.0892-6638(2005)019[1199:TDOTCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Siezen RJ, Argos P. Structural homology of lens crystallins. III. Secondary structure estimation from circular dichroism and prediction from amino acid sequences. Biochim Biophys Acta. 1983;748:56–67. doi: 10.1016/0167-4838(83)90027-4.0006-3002(1983)748[0056:SHOLCI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sun TX, Das BK, Liang JJ. Conformational and functional differences between recombinant human lens alphaA- and alphaB-crystallin. J Biol Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220.0021-9258(1997)272[6220:CAFDBR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, and Guicheney P. et al. 1998 A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 20:92–95. [DOI] [PubMed] [Google Scholar]
- Wang K, Spector A. Alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x.0014-2956(1996)242[0056:ASAFAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wistow G. Domain structure and evolution in alpha-crystallin and small heat-shock proteins. FEBS Lett. 1985;181:1–6. doi: 10.1016/0014-5793(85)81102-9.0014-5793(1985)181[0001:DSAEIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]