Abstract
Heat shock proteins (Hsp) are families of highly conserved molecules and immunodominant antigens in some infections and in autoimmune diseases. Some reports suggest that different regions of the Hsp60 molecule induce distinct immune responses. However, there are no reports comparing physiological T-cell reactivity to Hsp60 in mice. In this study, we have analyzed T-cell proliferation and cytokine production induced by Hsp60, under physiological conditions, in three mouse strains bearing distinct major histocompatibility complex (MHC) backgrounds. Proliferative response predominantly was found in C57BL/6 mice, mostly induced by N-terminal and intermediate Hsp60 peptides (P < 0.0001). Interferon-γ (IFNγ) production was broadly induced by different regions of Hsp60 in all three mouse strains, although response was focused in different peptide groups in each strain. We did not observe an exclusive Th1 or Th2 cytokine profile induced by any particular region of Hsp60. However, we identified a strain hierarchy in IL-10 production induced by Hsp60 peptides from different regions, mostly detected in C3H/HePas, and in BALB/c, but not in C57BL/6 mice. In contrast, IL-4 production only was induced by the intermediate and C-terminal region peptides in both C3H/HePas and BALB/c mice. Our data give original information on physiological cellular reactivity to Hsp60. We also have identified peptides with the capacity to induce the production of anti-inflammatory cytokines, bringing perspectives for their use in immunotherapy of chronic inflammatory diseases and allograft rejection.
INTRODUCTION
Heat shock proteins (Hsp) are families of highly conserved molecules present in all eukaryotic and prokaryotic species (Hightower and Guidon 1989). These proteins have essential functions as chaperone, taking part in the assembly, stabilization, folding, and translocation of oligomeric proteins, and are classified into several families on the basis of their molecular weight (100, 90, 70, 60, 40 kDA and low molecular weight; Lindquist and Craig 1988).
In addition to their intracellular functions, Hsp are reported to be immunodominant molecules in many infectious diseases (Kaufmann 1990). The phylogenetic similarity between microbial and mammalian Hsp60 and the crossreactivity induced by this protein suggest that Hsp60 may act as a potentially harmful self-antigen under inflammatory conditions (Jones et al 1993). T-cell immunity to Hsp60 has been reported to be predominantly proinflammatory and implicated in different pathological conditions such as arthritis (Gaston et al 1990), type I diabetes (Elias et al 1990), and graft rejection (Moliterno et al 1995), suggesting that reactivity induced by Hsp60 participates in the inflammatory process, activating both innate and adaptative immunity (Prohaszka and Fust 2004).
On the other hand, various studies have shown that T-cell reactivity to Hsp60 may have immunoregulatory activity, indicating that Hsp60 also has the potential to suppress the aggressive immune response elicited in inflammatory diseases both in humans, such as in rheumatoid arthritis (van Roon et al 1997; de Kleer et al 2003), type I diabetes (Cohen 2002), and autoimmune uveitis (Stanford et al 2004), and in animal models of autoimmune adjuvant-induced arthritis (van Eden et al 1988) and diabetes (Elias and Cohen 1994).
In contrast to the vast number of reports on cellular and humoral immunity to Hsp in different pathological contexts, autoreactivity to Hsp60 has not been as explored in physiological conditions. The relevance of studying physiological and potentially beneficial autoimmunity is gaining more strength among different research groups, mostly stimulated by the observations that subpopulations of autoreactive T-cells in fact may develop into regulatory T-cells, which play an important role in keeping self-tolerance (van Eden et al 2005a). This opens a physiological avenue for using beneficial autoimmunity for immunoregulatory therapeutic strategies.
In this study, we have analyzed T-cell proliferation and cytokine production induced by recombinant human Hsp60 molecule and its fragments corresponding to the intermediate and C-terminal regions, and to Hsp60 peptides, in three mouse strains bearing distinct major histocompatibility complex (MHC) backgrounds. Cellular reactivity to Hsp60 was quite diverse in these three mouse strains. Proliferative response predominantly was found in C57BL/6 mice, mostly induced by N-terminal and intermediate Hsp60 peptides, which also induced interferon-γ (IFNγ) production. In contrast, almost no proliferation was detected in BALB/c and C3H/HePas mice. We did not observe an exclusive Th1 or Th2 cytokine profile induced by any particular region of Hsp60. However, IL-4 production only was induced by the intermediate and C-terminal region peptides in both BALB/c and C3H/HePas mice, whereas IL-10 production was induced by peptides from different regions. Our data bring original information on physiological cellular reactivity to Hsp60 in naive mice, indicating the existence of strain and individual variability and suggesting that, in some mouse strains, certain regions of the molecule preferentially may induce some cytokines.
MATERIALS AND METHODS
Animals
We used 6- to 8-week-old male BALB/c (H-2d), C57BL/ 6(H-2b), and C3H/HePas (H-2k) mice. These animals were provided by the animal facility from the Biomedical Sciences Institute from the University of São Paulo, Brazil. Mice were housed in autoclaved microisolator cages (Tecniplast S.p.a, VA, Italy) at the animal facility of the Tropical Medicine Institute, University of São Paulo, Brazil. These animals were fed with autoclaved food (Nuvilab, SP, Brazil) ) and sterilized water. All manipulations were performed under sterile conditions and according to the Brazilian Committee for animal care and use guidelines.
Expression and purification of recombinant Hsp60 and its fragments
Human Hsp60 and its intermediate ([I-Hsp60]; Hsp60 amino acid residues 195–391) and C-terminal ([C-Hsp60]; Hsp60 amino acid residues 392–573) fragments were expressed in Escherichia coli BL21 pLysS (DE3) and purified as described previously (Caldas et al 2006). Briefly, transformed cells were grown in 2YT/ampicillin/cloranphenicol medium and induced with isopropyl-thio-2-D galactopyranoside (IPTG). French Press (Thermospectronic, Waltham, MA, USA) disrupted the cells and these proteins were purified in a Ni+2-charged Sepharose column (Chelating Sepharose Fast Flow, Amersham Pharmacia Biotech, Uppsala, Sweden). Contaminant lipopolysacharide (LPS) was removed using Triton X-114, as described previously (Aida and Pabst 1990). Quantification of endotoxin contamination in protein preparations was performed with the Lymulus amoebocyte lysate kit (QCL-1000, Biowhittaker, Cambrex Inc, Walkerville, MD, USA). The endotoxin concentrations in our proteins were <0.01 EU/μg of protein. Quantification of protein was performed by bicinchoninic acid (BCA) kit assay (Pierce Biotechnology Inc, Rockford, IL, USA) and resolved in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Peptide synthesis
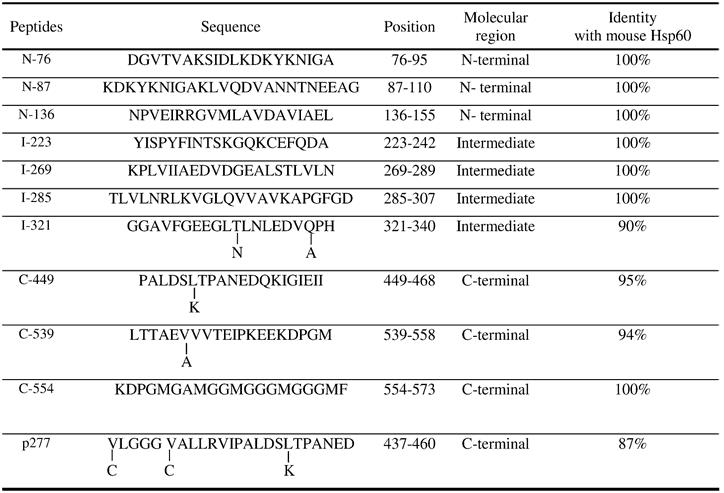
Eleven peptides from different regions of human Hsp60 were designed previously according to human leukocyte antigen (HLA)-DR interaction using a computer algorithm called TEPITOPE, for the T-cell studies we have been carrying out in the human system (Caldas et al 2006). These peptides were synthesized in an automated multiple peptide synthesizer (PSSM8 Shimadzu Co, Tokyo, Japan) using N-α-fluorenylmehoxycarbonyl (Fmoc) solid-phase strategy (Wellings and Atherton 1997; Table 1). The valine-substituted version of the p277 peptide was synthesized as described by Birk et al 1996. Peptides were analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Tof-Spec E, Micromass, Manchester, UK) and by analytical reverse-phase high profile liquid chromatography (HPLC) (Shimadzu Inc., Tokyo, Japan).
Table 1.
Peptides from human Hsp60
T-cell proliferation
T-cell proliferative response was evaluated using spleen cells from naive mice. Briefly, 2.5 × 105 cells in 200 μl/ well were cultured on RPMI 1640 culture medium (Gibco, Grand Island, NY, USA) supplemented with 2 nM of L-glutamine, 1 mM sodium piruvate, 40 μg/ml of gentamycin, 0.1 ng/ml of cephalosporin, and 5% of inactivated fetal calf serum (Imunoquimica, Rio de Janeiro, Brazil). Cells were incubated in triplicate into 96 culture U-bottom well plates (Costar, Corning, NY, USA). The antigens used were: Hsp60 peptides, recombinant I-Hsp60, C-Hsp60, and Hsp60 proteins, at 10 μg/ml and Concanavalin A (ConA) at 4 μg/ml as positive control. Cultures were kept at 37°C and 5% CO2 for 4 d. Methyl-[3H]thymidine (0.5 μCi/well Amersham Biosciences, Uppsala, Sweden) was added in the last 18 h of culture when cells were harvested and proliferation measured by thymidine incorporation into DNA, in liquid scintillation beta plate beta counter (Wallac, Turku, Finland). The stimulation index (SI) was calculated as the ratio of the mean counts per minute of culture with Ag or mitogen by the spontaneous control of cells without antigen. SI ≥ 2 was considered positive.
Cytokine enzyme-linked immunosorbent assay
Culture supernatants from 106 spleen cells/well were harvested for 48 h in the same culture conditions as described for proliferation assays and tested for IFNγ, IL-4, and IL-10 by sandwich-linked immunosorbent assay (ELISA). Briefly, 96-well plates (model 3369, High Binding, Costar, Corning, NY, USA) were coated with 50 μl of capture antibodies (Pharmigen, BD-Biosciences, San Jose, CA, USA), anti-IFNγ (R4-6A2), anti-IL-4 (BVD4-1D11), and anti-IL-10 (JES5-2A5) in sodium carbonate– bicarbonate buffer (pH = 9.6) and incubated overnight at 4°C. Plates then were blocked for 2 h at room temperature with 200 μ/well phosphate-buffered saline (PBS) containing 1% of bovine serum albumin ([BSA], Sigma Aldrich, St Louis, MO, USA) and washed with PBS containing 0.05% Tween 20. Standard curve (2000–15.6 pg/ml) and supernatant samples were added in duplicate and incubated overnight at 4°C. Biotinylated secondary antibodies anti-IFNγ (XMG1.2), anti-IL-4 (BVD6-24G2), and anti-IL-10 (SXC-1) were added and incubated for 60 min, and avidin–peroxidase (Pharmigen) was added. Assays were developed with o-phenylenediamine ([OPD], Sigma Aldrich), and absorbance was measured at 490 nm using ELISA microplate reader (model 1550, Bio-Rad Laboratories Inc, Hercules, CA, USA). Analysis was performed using Microplate Manager 4.0 Software (Bio-Rad Laboratories Inc) based on the standard concentration curve. Cytokine production was considered positive over the detection limit of each cytokine: 62.5 pg/ml for IFNγ and 31 pg/ml for IL-4 and IL-10. Cytokine production induced by antigens or ConA was considered over the spontaneous production of cells alone without antigen. When no spontaneous production was observed, antigen-induced production was calculated over the detection limit of the assay.
Statistical analysis
All statistical analyses and graphics were performed using GraphPad InStat version 3.00, GraphPad Software Inc, and GraphPad Prism version 3.02 software (San Diego, CA). Kruskall Wallis and two-tailed Mann Whitney U-test were used for median comparison between stimulation index and cytokine production. These median values were calculated using individual stimulus. We also calculated median level of cytokine production for each cytokine per mouse strain. In addition, for comparison of responses induced by different Hsp60 regions, we calculated the median level of response to N-terminal and intermediate and C-terminal Hsp60 peptides grouping the positive responses or cytokine production induced by peptides from these regions. The P value <0.05 was considered statistically significant.
RESULTS
Hsp60 peptides from N-terminal and intermediate regions induced significantly higher proliferation in C57BL/6 mice
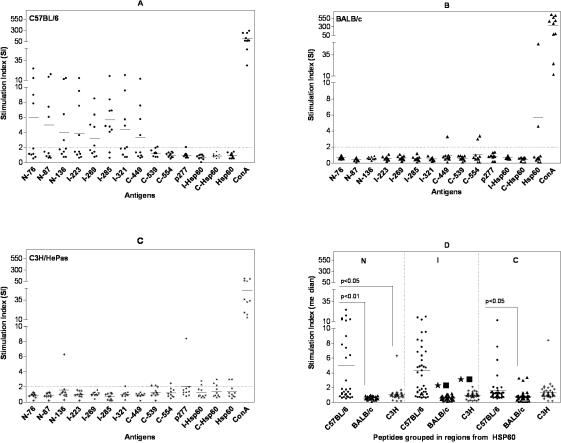
Comparing the proliferative response in the three strains of mice, C57BL/6 presented the most frequent and intense proliferative response induced mainly by peptides from N-terminal and intermediate regions of Hsp60 (Fig 1A). In this mouse strain, 8 out of 10 animals presented spleen cell proliferation induced by peptide I-285 from the intermediate region (median of SI = 4.7), which was recognized preferentially by C57BL/6 mice (Table 2). The proliferative response induced by I-285 peptide in this mouse strain was significantly higher than in both BALB/c and C3H/HePas mice (P < 0.01). Additionally, peptides N-87, N-136, I-269, and I-321 induced proliferative response in 40% of C57BL/6 mice, but C-554 and p277 peptides did not elicit proliferation.
Fig 1.
Proliferative response to Hsp60, I-Hsp60, and C-Hsp60 fragments and Hsp60 peptides, using splenocytes (2.5 × 105 cells/well) from 10 naive male mice of the following strains: (A) C57BL/6 mice, (B) BALB/c, and (C) C3H/HePas. Cells were stimulated with Hsp60, its fragments, and 11 Hsp60-derived peptides at 10 μg/ml and Concanavalin A (ConA) 4 μg/ml as control. Results are expressed in stimulation index. Stimulation index was calculated as (SI) = mean counts per minute (cpm) of cells cultured with antigens/mean cpm of cells without antigens. SI ≥ 2 were considered positive. (A) C57BL/6 mice showed high prevalence of proliferative response to Hsp60 antigens and significantly stronger proliferation induced by N-terminal and intermediate (I) region peptides than the other two mouse strains (P < 0.0001). (B) BALB/c mice showed low proliferative response induced by C-terminal peptides (C-449, C-539) and Hsp60. (C) C3H/HePas mice presented proliferative response induced by C-terminal peptides (C-539, C-554), Hsp60, and I-Hsp60 and C-Hsp60 fragments. (D) Comparison of cumulative proliferative response to Hsp60 peptides grouped according to N-, I-, and C-regions measured by median of SI. These results are from two independent experiments
Table 2.
Percentage of mice presenting cellular response induced by Hsp60, fragments and peptides
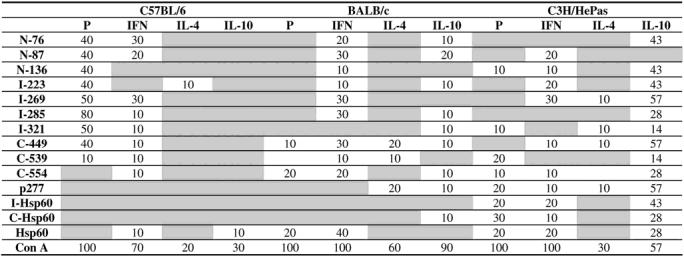
In contrast with C57BL/6 mice, we observed almost no proliferative response induced by Hsp60, peptides, or fragments in BALB/c (Fig 1B). Only Hsp60 and C-terminal peptides C-449 and C-554 induced proliferation in 2 out of 10 BALB/c mice (Fig 1B; Table 2). Similarly, in C3H/HePas mice, proliferation was infrequent and preferentially directed to peptides form the C-terminal region, namely C-539, C-554, and p277, in 10 to 30% of mice, as well as to Hsp60 and its fragments (Fig 1C; Table 2).
Both intra- and interstrain differences were analyzed for proliferation induced by Hsp60 peptides, grouped according to N-, I-, and C- regions. C57BL/6 mice showed significantly higher proliferation induced by both N-terminal and intermediate peptides in comparison with C-terminal peptides (P = 0.0014; Fig 1D). The comparative analysis of the 3 mouse strains (Fig 1D) showed strong statistically significant differences of the median proliferative response (P < 0.0001). C57BL/6 mice presented significantly higher proliferative response to N-terminal and intermediate region peptides (P < 0.001) and to C-terminal peptides (P < 0.05) than BALB/c mice. Moreover, C57BL/6 mice also displayed significantly higher response to peptides from the intermediate region (P < 0.001), and N-terminal (P < 0.05) than C3H/HePas mice, but not to C-terminal peptides (P > 0.05). In contrast, we did not observe significant difference when proliferation to N-terminal and C-terminal peptides was compared between BALB/c and C3H/HePas groups (P > 0.05).
N-terminal and intermediate Hsp60 peptides were the major IFNγ inducers
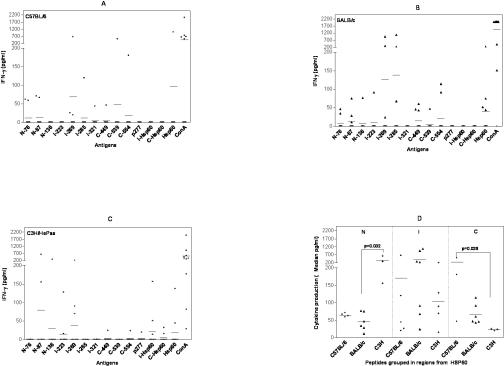
Although, IFNγ was the most frequently detected cytokine in response to different Hsp60 peptides in BALB/c and C57BL/6 mice, it was only detected in 3 out of 10 mice of each strain. C3H/HePas also presented IFNγ production induced by different Hsp60 peptides. However, this mouse strain had a much higher frequency of IL-10 production. Median levels of overall IFNγ production essentially were the same in the 3 mouse strains 67.9 pg/ ml, 60.4 pg/ml, and 60.8 pg/ml, for C57BL/6, BALB/c, and C3H/HePas, respectively (data not shown).
We observed IFNγ production induced by N-76 and I-269 peptides in 3 out of 10 C57BL/6 mice (Fig 2A; Table 2). On the other hand, peptides N-136, I-223, and p277 did not induce IFNγ production in this mouse strain, and peptide I-285, which was immunodominant in the proliferative response, only induced IFNγ in one animal.
Fig 2.
Interferon-γ production (pg/ml) induced by Hsp60, peptides, and fragments in 48-h culture supernatants of spleen cells (106 cells/well) from 10 naive male mice from the following strains: (A) C57BL/6 mice, (B) BALB/c, and (C) C3H/HePas by enzyme-linked immunosorbent assay (ELISA). Cells were stimulated with Hsp60, its fragments, and 11 Hsp60-derived peptides at 10 μg/ml and Concanavalin A (ConA) 4 μg/ml as control. Detection limit was 62.5 pg/ml. Cytokine production induced by antigen was calculated over spontaneous production of cells without antigen or over the detection limit. (A) In C57BL/6 mice peptides N-76 and I-269 induced IFNγ production. (B) In BALB/c mice peptides Hsp60 and N-87, I-269, I-285, and C-449 induced IFNγ production. (C) In C3H/HePas mice peptides N-87, I-223, I-269 and I-Hsp60, C-Hsp60, and Hsp60 mostly induced IFNγ production. (D) Hsp60 peptides grouped by Hsp60 regions showed that N-terminal–derived Hsp60 peptides elicited more IFNγ production (median pg/ml) in C3H/HePas mice than in BALB/c mice (P = 0.032). Also, C-terminal–derived Hsp60 peptides showed more cytokine production in C57BL/6 than in C3H/HePas mice (P = 0.029). These results are from two independent experiments
In BALB/c mice, peptides N-87, I-269, I-285, and C-449 induced a higher frequency of IFNγ production, in 3 out of 10 each (Fig 2B; Table 2). On the other hand, peptides p277 and I-321 did not induce IFNγ production. In C3H/HePas, the most frequent inducers of IFNγ production were peptides N-87, I-223, and I-269 in 2 to 3 out of 10 mice (Fig 2C; Table 2). Peptides N-76, I-285, I-321, and C-539 did not induce IFNγ production in this mouse strain.
Significant differences were detected (P < 0.05) in IFNγ production when Hsp60 peptides were grouped in N, I, and C regions (Fig 2D). N-terminal peptides induced higher IFNγ production when compared to C-terminal peptides in the C3H/HePas mouse strain (Fig 2D; P < 0.05). We also observed significant interstrain differences (P < 0.05). N-terminal peptides were higher inducers of IFNγ production in C3H/HePas mice when compared with BALB/c mice (P = 0.03; Fig 2D). C-terminal peptides induced more IFNγ production in C57BL/6 mice in comparison with C3H/HePas mice (P = 0.029).
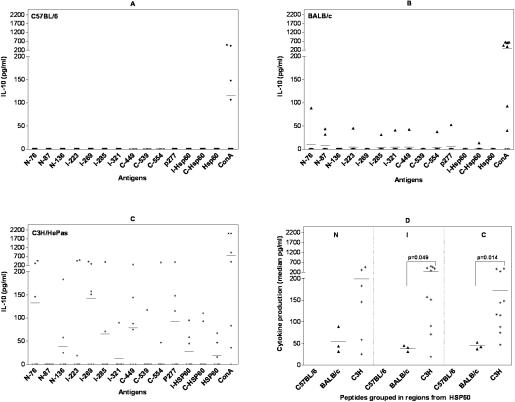
IL-10 production induced by Hsp60 peptides was predominant in C3H/HePas mice
IL-10 was the most predominant cytokine induced by Hsp60 peptides in C3H/HePas mouse strain, with 57% of mice presenting IL-10 production induced by at least one peptide (Table 2). Almost all peptides were capable of inducing IL-10 production, with a median of 115 pg/ ml (Fig 3C). Some peptides induced higher levels of IL-10, namely, I-269 and C-449 with median of 151 pg/ml and 75 pg/ml, respectively, of cytokine production. The most frequent inducers of IL-10 were peptides I-223, I-269, C-449, and p277, which induced the production of this cytokine in 4 out of 7 tested mice (Fig 3C; Table 2).
Fig 3.
IL-10 production (pg/ml) induced by different Hsp60 antigens in 48-h culture supernatants of spleen cells (106 cell/well) from 7 to 10 male naive mice from the following strains: (A) C57BL/6 mice, (B) BALB/c, and (C) C3H/HePas by enzyme-linked immunosorbent assay (ELISA). Cells were stimulated with Hsp60, its fragments, and 11 Hsp60-derived peptides at 10 μg/ml and Concanavalin A (ConA) 4 μg/ml as control. Detection limit was 31.5 pg/ml. Cytokine production induced by antigen was calculated over spontaneous production of cells without antigen or over the detection limit. (A) We did not observe IL-10 production induced by Hsp60 peptides in C57BL/6 mice. (B) In BALB/c mice, IL-10 production was low and induced by peptides from different regions of Hsp60. (C) IL-10 production in C3H/HePas mice was higher than in BALB/c and C57BL/6 mice and was induced by peptides and proteins from different regions of Hsp60 molecule, predominantly (I-223, I-269, C-449, p277, I-Hsp60, and Hsp60). (D) Hsp60 peptides grouped by Hsp60 regions showed that both I- and C-terminal– derived Hsp60 peptides elicited more IL-10 production (median pg/ml) in C3H/HePas than in BALB/c mice, with P = 0.049 and P = 0.014, respectively. These results are from two independent experiments
In contrast with C3H/HePas, C57BL/6 mice presented no IL-10 production induced by any peptide and, in BALB/c mice, the IL-10 production was lower (median of 40.8 pg/ml) and induced by 7 peptides from different regions of Hsp60 in only 1 to 2 out of 10 mice (Fig 3A; Table 2).
Analyzing the median production of cytokine induced by Hsp60 peptides grouped by regions (Fig 3D), peptides from the intermediate and C-terminal regions induced significantly more IL-10 production in C3H/HePas when compared with BALB/c mice (P = 0.014 and P = 0.049, respectively).
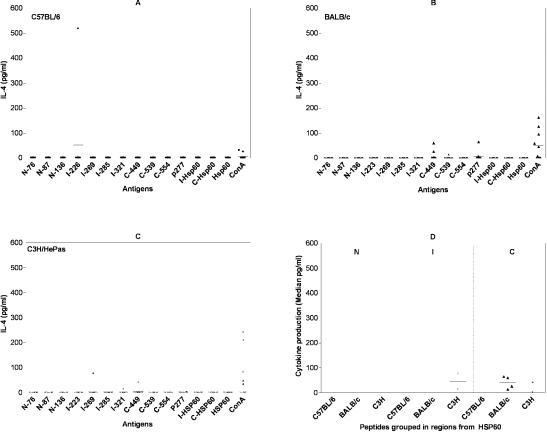
IL-4 production was the least-frequent cytokine induced by Hsp60 peptides and exclusively induced by C-terminal peptides (C-449, C-539, p277) in BALB/c mice, with median of 76.9 pg/ml (Fig 4B, Table 2) and by C-terminal (C-449, p277) and the intermediate region peptides (I-269, I-321) in C3H/HePas mice, with a median of 29.4 pg/ml (Fig 4C), and in one C57BL/6 mouse (Fig 4A).
Fig 4.
IL-4 production (pg/ml) induced by Hsp60, peptides, and fragments in 48-h culture supernatants of spleen cells (106 cells/well) from 10 naive male mice from the following strains: (A) C57BL/6, (B) BALB/c, and (C) C3H/HePas mice by enzyme-linked immunosorbent assay (ELISA). Cells were stimulated with Hsp60, its fragments, and 11 Hsp60-derived peptides at 10 μg/ml and Concanavalin A (ConA) 4 μg/ml as control. Detection limit was 31.5 pg/ml. Cytokine production induced by antigen was calculated over spontaneous production of cells without antigen or over the detection limit. (A) C57BL/6: IL-4 production was only detected in one mouse, induced by I-223 peptide. (B) BALB/ c: IL-4 production was induced by C-terminal peptides (C-449, C-539, p277). (C) C3H/HePas: IL-4 production was induced by intermediate (I-269, I-321) and C-terminal (C-449, p277) peptides. (D) Hsp60 peptides grouped by Hsp60 regions (N-terminal, intermediate, and C-terminal regions) (median of pg/ml). We did not detect any statistically significant differences between these different mouse strains. These results are from two independent experiments
Hsp60 and I-Hsp60, C-Hsp60 fragments induced both IFN and IL-10 production
Hsp60 induced IFNγ production in all three mouse strains (Fig 2; Table 2), though BALB/c and C3H/HePas displayed higher frequency, with 4 out of 10 mice and 2 out of 7 mice, respectively (Table 2).
On the other hand, IFNγ production induced by I-Hsp60 and C-Hsp60 fragments was detected in only 1 and 2 out of 10 C3H/HePas mice, respectively, but not in BALB/c or C57BL/6 (Fig 2A,B,C; Table 2).
IL-10 production was induced by I-Hsp60, C-Hsp60, and Hsp60 in up to 3 out of 7 C3H/Hepas mice (Fig 3C; Table 2) and by C-Hsp60 in one BALB/c mouse (Table 2). Hsp60 fragments did not induce IL-4 production in any of the 3 mouse strains.
DISCUSSION
Besides its potential involvement in autoimmune diseases, autoreactivity to Hsp60 is considered to play an important role in immunoregulation, and peptides from Hsp60 have been shown to present immunoregulatory properties both in animal models and in humans (Pockley and Muthana 2005; van Eden et al 2005b) However, the discrimination between pathological and beneficial autoimmunity to Hsp60 (as well as to other self-antigens) still is a challenge, and it is not clear whether or not different regions of the Hsp60 molecule may determine these opposing functional activities. Therefore, the better understanding of autoimmunity to Hsp60 under physiological conditions should contribute to exploring its potential immunoregulatory properties and future development of new immunotherapeutic agents. To our knowledge, this is the first report on cellular reactivity to Hsp60 in different strains of naive mice.
The main observation in this work is that cellular reactivity to Hsp60 (including fragments and peptides) is not homogeneous among different mouse strains, though we did find some strain-dominance in relation to either proliferation or to Th1/Th2 cytokine production. We identified an immunodominant peptide from the intermediate region, peptide I-285 (285-307), which elicited proliferative response in C57BL/6 mice, but not in the two other mouse strains. It is interesting to point that a peptide (p20) sharing sequence homology with I-285 was reported as one of at least 7 peptides from human Hsp60 molecule to induced proliferation in peripheral blood mononuclear cells (PBMC) from type I diabetes mellitus patients and nonobese diabetic (NOD) mice, suggesting that it may be immunodominant in other contexts (Abulafia-Lapid et al 1999). On the other hand, the reactivity induced by this peptide could not be detected when the intermediate Hsp60 fragment (I-Hsp60) was used in the assay, though it contains this peptide's sequence. One possible explanation is that the intermediate region contains some regulatory sequences capable of inhibiting T-cell proliferation. No reports are published on the anti-inflammatory activity of I-Hsp60 recombinant protein. However, there is a report in which a peptide from Hsp60 intermediate region induced anti-inflammatory cytokine production and prevented the development of autoimmune Uveitis in murine models (Lehner et al 2003). Further studies are now being carried out to clarify the potential immunomodulatory activity of different regions Hsp60 regions.
In our study, IFNγ production was induced preferentially by N-terminal peptides in C3H/HePas mice, intermediate region peptides in BALB/c, and C-terminal peptides in C57BL/6 mice, indicating the existence of strain variability in cytokine production. In addition, Hsp60 induced IFNγ production in BALB/c and C3H/HePas mice. Taking IFNγ as a predominantly inflammatory cytokine, these data denote Hsp60 as inducer of T-cell proinflammatory repertoire. Accordingly, Hsp60 has been described as a danger signal (Matzinger 1994), activating innate immunity, tumor necrosis factor alpha (TNFα), IL;ch12, and nitric oxide (NO) production (Chen et al 1999) and inducing maturation of dendritic cells (Flohe et al 2003) through toll-like receptor-4 (TLR4) (Ohashi et al 2000) and TLR2 (Zanin-Zhorov et al 2003) activation.
An interesting result in our work was the induction of potentially regulatory cytokines, IL-4 and IL-10, mostly by the intermediate and C-terminal Hsp60 peptides in BALB/c and C3H/HePas mice. This result suggests a Th2 or T-regulatory profile associated with both regions of Hsp60. Also, C-449 and p277 peptides induced a mixed production of cytokines in both mouse strains. These peptides share a common sequence (PALDSLTPANED), which could be involved in inducing this pattern of cytokine production. The p277 peptide was described by Elias et al (1997) as a peptide with immunoregulatory activity, capable of inducing IL-4 and IL-10 and protecting NOD mouse from insulitis both by direct peptide infusion and by passive splenocyte transfer from tolerant mice (Ablamunits et al 1999). In the clinic, p277 was used in a phase II clinical trial of type I diabetes patients, showing reduction in need of insulin intake and induction of anti-inflammatory IL-10 and IL-13 T-cell subpopulations (Raz et al 2001). In the adjuvant-induced arthritis model (AA) in Lewis rats, Paul et al (2000) reported a peptide (256-270) from the intermediate region of self Hsp60 capable of inducing a predominant IL-10 T-cell production, upregulating CTLA-4 expression, and leading to arthritis resolution. These results also suggest the capacity of mammalian Hsp60 to induce the production of a major anti-inflammatory cytokine, though not in all mouse strains. Accordingly, some investigators observed that Hsp60 could induce tolerance with monocytes exposed to repeated stimulation with Hsp60 and crosstolerance with LPS, inducing both proinflammatory (IL-1 and TNFα) and anti-inflammatory (IL-10) cytokines (Kilmartin and Reen 2004). Further supporting the immunoregulatory activity of Hsp60, this molecule has been shown to upregulate the Th2-cell promoting GATA3 transcription factor in T-cells, increasing the secretion of IL-10 (Zanin-Zhorov et al 2005). In line with these data, Kamphuis et al (2005) described that peripheral blood mononuclear cells from patients with juvenile arthritis showed IL-10 production induced by Hsp60 peptides, reinforcing the role of Hsp60 epitopes as potentially anti-inflammatory agents in the prevention and treatment of chronic inflammatory diseases.
We used recombinant Hsp60 and two fragments corresponding to its intermediate (I-Hsp60), C-terminal regions (C-Hsp60), as well as 11 Hsp60 peptides, to study whether different Hsp60 regions induced distinct functional patterns of cellular reactivity in different mouse strains. Seven of these peptides are identical to the mouse sequence and 4 have 90 to 95% of homology (Table 1). Even though these human Hsp60 peptides have a high homology with mice Hsp60 counterparts, we cannot exclude the possibility that xeno-sequence differences have lead to the lower reactivity described. Comparative studies using both sequences will be developed. We used the valine-substituted version of p277 peptide, as described by others, to present more stability (Birk et al 1996). The N-terminal fragment (N-Hsp60) was not used in this study due to insufficient production.
In the past years, cumulative data have highlighted the importance of using endotoxin-free Hsp60 for cellular studies because LPS contamination induces the production of several inflammatory cytokines. Therefore, it is noteworthy that the recombinant proteins used in our study were submitted to endotoxin removal with Triton X114, as previously described (Aida and Pabst 1990), and proteins used had LPS contamination <0.01 EU/μg of protein (Gao and Tsan 2004).
Given that H-2 background is relevant to antigen presentation to T-cells and recognition via T-cell receptor (TCR), it probably influenced the different patterns of proliferative response induced by different Hsp60 regions, in different mouse strains. Accordingly, Moudgil et al (1997) described a particular pattern of intramolecular determinant spreading process (rat C-terminal diversification), in which two different inbred rat strains recognized distinct regions of Hsp65 and had differential susceptibility to develop and cure adjuvant arthritis. However, it is also possible that the interaction of Hsp60 peptides with TLR in T-cells may contribute to the outcome of T-cell activation and proliferation, as described by others (Cohen-Sfady et al 2005).
One debated issue yet to be clarified is whether different Hsp60 regions elicit distinct functional activities. It was reported recently that a C-terminal region of Hsp60 (354-366aa) is involved in LPS binding and innate immune activation (Habich et al 2005), suggesting a potential proinflammatory role induced by this C-terminal region. On the other hand, p277 (437-460), which is also from the C-terminal region, has been reported to have immunoregulatory capacity in animal models of autoimmune disease and in type I diabetes mellitus in humans (Cohen 2002). In addition, both a DNAHsp60 vaccine encoding 31-50aa from the N-terminal region of mammalian Hsp60 (Quintana et al 2003) and peptides from the C-terminal region of rat Hsp60 (Durai et al 2004) also have been reported to display immunoregulatory activity in adjuvant-induced arthritis models (AA). It is more likely then that different Hsp60 regions may induce multiple functional activities.
It should be mentioned that we did not study the response of other self-antigens, which could be interesting to better dissect the functional diversity of autoreactivity to multiple antigens. It is indeed possible that other self-antigens also display a regulatory role. In the present study, we focused on Hsp60, which has been reported by several investigators to display regulatory activity.
Overall, in the present work, we observed that many peptides induced mostly a mixed production of IFNγ and IL-10, irrespective of the location on the Hsp60 molecule. Nevertheless, in the context of human renal transplantation, our group recently has reported IFNγ and IL-10 production induced by Hsp60 peptides in different time points after human kidney transplantation, with IL-10 production mainly induced by I-intermediate and C-terminal peptides in late periods post-transplantation, when patients presented no rejection (Caldas et al 2006). It is therefore possible that, under certain inflammatory conditions, peptides derived from particular regions of Hsp60 may assume a predominant regulatory activity, though this most likely is to show specie, strain, and interindividual variations. We have identified peptides with the capacity to induce the production of anti-inflammatory cytokines, bringing perspectives for use in immunotherapy of chronic inflammatory diseases. The use of Hsp as agents of immunoregulation may offer opportunities for novel therapies and preventive vaccination in autoimmune diseases (Maron et al 2002; Puga Yung et al 2003; Cohen 2004; van Eden 2006) and in allotransplantation (Birk et al 1999).
Acknowledgments
This work was supported by grants from The State of São Paulo Research Foundation ([FAPESP] 02/06495-7) and The National Council for Scientific and Technological Development (CNPq), Brazil. We thank Dr. Kamal Moudgil for critical reading of the manuscript and helpful suggestions and Maisa Takenaka and Adalberto Socorro for technical support. Dr Luna attended in the PhD program in Allergy and Immunopathology at the Division of Clinical Immunology and Allergy, University of Sao Paulo Medical School, Sao Paulo, Brazil and was supported by FAPESP (01/11795-7).
REFERENCES
- Ablamunits V, Elias D, Cohen IR. The pathogenicity of islet-infiltrating lymphocytes in the nonobese diabetic (NOD) mouse. Clin Exp Immunol. 1999;115:260–267. doi: 10.1046/j.1365-2249.1999.00802.x.0009-9104(1999)115[0260:TPOILI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulafia-Lapid R, Elias D, Raz I, Keren-Zur Y, Atlan H, Cohen IR. T-cell proliferative responses of type 1 diabetes patients and healthy individuals to human Hsp60 and its peptides. J Autoimmun. 1999;12:121–129. doi: 10.1006/jaut.1998.0262.0896-8411(1999)012[0121:TPROTD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u.0022-1759(1990)132[0191:ROEFPS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Birk OS, Elias D, and Weiss AS. et al. 1996 NOD mouse diabetes: the ubiquitous mouse Hsp60 is a beta-cell target antigen of autoimmune T-cells. J Autoimmun. 9:159–166. [DOI] [PubMed] [Google Scholar]
- Birk OS, Gur SL, and Elias D. et al. 1999 The 60-kDa heat shock protein modulates allograft rejection. Proc Natl Acad Sci U S A. 96:5159–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas C, Luna E, and Spadafora-Ferreira M. et al. 2006 Cellular autoreactivity against heat shock protein 60 in renal transplant patients: peripheral and graft-infiltrating responses. Clin Exp Immunol. 146:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219.0022-1767(1999)162[3212:HKHSPA]2.0.CO;2 [PubMed] [Google Scholar]
- Cohen IR. Peptide therapy for type I diabetes: the immunological homunculus and the rationale for vaccination. Diabetologia. 2002;45:1468–1474. doi: 10.1007/s00125-002-0937-z.0012-186X(2002)045[1468:PTFTID]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cohen IR. Thoughts on the pathogenesis of type 1 diabetes and on the arrest of autoimmune beta-cell destruction by peptide p277 vaccination. Isr Med Assoc J. 2004;6:260–261.0017-7768(2004)006[0260:TOTPOT]2.0.CO;2 [PubMed] [Google Scholar]
- Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594.0022-1767(2005)175[3594:HSPABC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- de Kleer IM, Kamphuis SM, and Rijkers GT. et al. 2003 The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T-cells directed to human heat shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. 48:2001–2010. [DOI] [PubMed] [Google Scholar]
- Durai MR, Gupta S, Moudgil KD. The T-cells specific for the carboxyl-terminal determinants of self (rat) heat shock protein 65 escape tolerance induction and are involved in regulation of autoimmune arthritis. J Immunol. 2004;172:2795–2802. doi: 10.4049/jimmunol.172.5.2795.0022-1767(2004)172[2795:TTSFTC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Elias D, Cohen IR. Peptide therapy for diabetes in NOD mice. Lancet. 1994;343:704–706. doi: 10.1016/s0140-6736(94)91582-2.0140-6736(1994)343[0704:PTFDIN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Elias D, Markovits D, Reshef T, van der Zee R, Cohen IR. Induction and therapy of autoimmune diabetes in the nonobese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990;87:1576–1580. doi: 10.1073/pnas.87.4.1576.1091-6490(1990)087[1576:IATOAD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D, Meilin A, and Ablamunits V. et al. 1997 Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various beta-cell antigens. Diabetes. 46:758–764. [DOI] [PubMed] [Google Scholar]
- Flohe SB, Bruggemann J, Lendemans S, Nikulina M, Meierhoff G, Flohe S, Kolb H. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol. 2003;170:2340–2348. doi: 10.4049/jimmunol.170.5.2340.0022-1767(2003)170[2340:HHSPIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Induction of cytokines by heat shock proteins and endotoxin in murine macrophages. Biochem Biophys Res Commun. 2004;317:1149–1154. doi: 10.1016/j.bbrc.2004.03.160.0006-291X(2004)317[1149:IOCBHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gaston JS, Life PF, Jenner PJ, Colston MJ, Bacon PA. Recognition of a mycobacteria-specific epitope in the 65-kD heat shock protein by synovial fluid-derived T-cell clones. J Exp Med. 1990;171:831–841. doi: 10.1084/jem.171.3.831.0022-1007(1990)171[0831:ROAMEI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habich C, Kempe K, and van Der Zee R. et al. 2005 Heat shock protein 60: specific binding of lipopolysaccharide. J Immunol. 17:1298–1305. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT Jr.. Selective release from cultured mammalian cells of heat shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206.0021-9541(1989)138[0257:SRFCMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jones DB, Coulson AF, Duff GW. Sequence homologies between Hsp60 and autoantigens. Immunol Today. 1993;14:115–118. doi: 10.1016/0167-5699(93)90210-C.0167-5699(1993)014[0115:SHBHAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamphuis S, Kuis W, and de Jager W. et al. 2005 Tolerogenic immune responses to novel T-cell epitopes from heat shock protein 60 in juvenile idiopathic arthritis. Lancet. 366:50–56. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j.0167-5699(1990)011[0129:HSPATI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kilmartin B, Reen DJ. Hsp60 induces self-tolerance to repeated Hsp60 stimulation and crosstolerance to other proinflammatory stimuli. Eur J Immunol. 2004;34:2041–2051. doi: 10.1002/eji.200425108.0014-2980(2004)034[2041:HISTRH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lehner T, Stanford MR, and Phipps PA. et al. 2003 Immunopathogenesis and prevention of uveitis with the Behcet's disease-specific peptide linked to cholera toxin B. Adv Exp Med Biol. 528:173–180. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022[0631:THSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Maron R, Sukhova G, and Faria AM. et al. 2002 Mucosal administration of heat shock protein 65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 106:1708–1715. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015.0732-0582(1994)012[0991:TDATEF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moliterno R, Valdivia L, Pan F, Duquesnoy RJ. Heat shock protein reactivity of lymphocytes isolated from heterotopic rat cardiac allografts. Transplantation. 1995;59:598–604.0041-1337(1995)059[0598:HSPROL]2.0.CO;2 [PubMed] [Google Scholar]
- Moudgil KD, Chang TT, and Eradat H. et al. 1997 Diversification of T-cell responses to carboxy-terminal determinants within the 65-kD heat shock protein is involved in regulation of autoimmune arthritis. J Exp Med. 185:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558.0022-1767(2000)164[0558:CEHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Paul AG, van Der Zee R, Tams LS, van Eden W. A self–Hsp60 peptide acts as a partial agonist inducing expression of B7-2 on mycobacterial Hsp60-specific T-cells: a possible mechanism for inhibitory T-cell regulation of adjuvant arthritis? Int Immunol. 2000;12:1041–1050. doi: 10.1093/intimm/12.7.1041.0953-8178(2000)012[1041:ASPAAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Paul AG, van Kooten PJ, van Eden W, van Der Zee R. Highly autoproliferative T-cells specific for 60-kDa heat shock protein produce IL-4/IL-10 and IFN-gamma and are protective in adjuvant arthritis. J Immunol. 2000;165:7270–7277. doi: 10.4049/jimmunol.165.12.7270.0022-1767(2000)165[7270:HATSFK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M. Heat shock proteins and allograft rejection. Contrib Nephrol. 2005;148:122–134. doi: 10.1159/000086057.0302-5144(2005)148[0122:HSPAAR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Fust G. Immunological aspects of heat shock proteins—the optimum stress of life. Mol Immunol. 2004;41:29–44. doi: 10.1016/j.molimm.2004.02.001.0161-5890(2004)041[0029:IAOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Puga Yung GL, Le TD, Roord S, Prakken B, Albani S. Heat shock proteins (Hsp) for immunotherapy of rheumatoid arthritis (RA) Inflamm Res. 2003;52:443–451. doi: 10.1007/s00011-003-1204-6.1023-3830(2003)052[0443:HSPHFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Carmi P, Mor F, Cohen IR. DNA fragments of the human 60-kDa heat shock protein (Hsp60) vaccinate against adjuvant arthritis: identification of a regulatory Hsp60 peptide. J Immunol. 2003;171:3533–3541. doi: 10.4049/jimmunol.171.7.3533.0022-1767(2003)171[3533:DFOTHK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzer M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat shock protein peptide (DiaPep277): a randomized, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5.0140-6736(2001)358[1749:BFINTD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stanford M, Whittall T, and Bregmeier LA. et al. 2004 Oral tolerization with peptide 336–351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet's disease. Clin Exp Immunol. 137:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W. Immunoregulation of autoimmune diseases. Hum Immunol. 2006;67:446–453. doi: 10.1016/j.humimm.2006.03.010.0198-8859(2006)067[0446:IOAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- van Eden W, Hauet-Broere F, and Berlo S. et al. 2005a Stress proteins as inducers and targets of regulatory T-cells in arthritis. Int Rev Immunol. 24:181–197. [DOI] [PubMed] [Google Scholar]
- van Eden W, Thole JE, and van Der Zee R. et al. 1988 Cloning of the mycobacterial epitope recognized by T-lymphocytes in adjuvant arthritis. Nature. 331:171–173. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Prakken B. Heat shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005b;5:318–330. doi: 10.1038/nri1593.1474-1733(2005)005[0318:HSPITR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- van Roon JA, van Eden W, van Roy JL, Lafeber FJ, Bijlsma JW. Stimulation of suppressive T-cell responses by human but not bacterial 60-kD heat shock protein in synovial fluid of patients with rheumatoid arthritis. J Clin Invest. 1997;100:459–463. doi: 10.1172/JCI119553.0021-9738(1997)100[0459:SOSTRB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings DA, Atherton E. Standard Fmoc protocols. Methods Enzymol. 1997;289:44–67. doi: 10.1016/s0076-6879(97)89043-x.0076-6879(1997)289[0044:SFP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Bruck R, and Tal G. et al. 2005 Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J Immunol. 174:3227–3336. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T-cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. Faseb J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje.0892-6638(2003)017[1567:TRTHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]