Abstract
The midbrain periaqueductal gray (PAG), and its descending projections to the rostral ventromedial medulla (RVM), provides an essential neural circuit for opioid-produced antinociception. Recent anatomical studies have reported that the projections from the PAG to the RVM are sexually dimorphic and that systemic administration of morphine significantly suppresses pain-induced activation of the PAG in male but not female rats. Given that morphine antinociception is produced in part by disinhibition of PAG output neurons, it is hypothesized that a differential activation of PAG output neurons mediates the sexually dimorphic actions of morphine. The present study examined systemic morphine-induced activation of PAG-RVM neurons in the absence of pain. The retrograde tracer fluorogold (FG) was injected into the RVM to label PAG-RVM output neurons. Activation of PAG neurons was determined by quantifying the number of Fos-positive neurons one hour following systemic morphine administration (4.5 mg/kg). Morphine produced comparable activation of the PAG in both male and female rats, with no significant differences in either the quantitative or qualitative distribution of Fos. While microinjection of FG into the RVM labeled significantly more PAG output neurons in female rats than male rats, very few of these neurons (20%) were activated by systemic morphine administration in comparison to males (50%). The absolute number of PAG-RVM neurons activated by morphine was also greater in males. These data demonstrate widespread disinhibition of PAG neurons following morphine administration. The greater morphine-induced activation of PAG output neurons in male compared to female rats is consistent with the greater morphine-induced antinociception observed in males.
Keywords: pain, morphine analgesia, pain inhibition, opioid, sexual dimorphism endogenous descending pathway
Morphine and other mu-opioid receptor agonists are the most effective pharmacological treatment for the alleviation of persistent pain. However, it is becoming increasingly clear that morphine does not produce the same degree of analgesia in males and females. In the majority of studies reported to date, morphine produced a greater antinociceptive response in males in comparison to females (Bodnar et al., 1988, Craft, 2003b, Craft, 2003a, Cook and Nickerson, 2005, Wang et al., 2006). Sex differences in morphine analgesia have been reported in studies employing orofacial (Okamoto et al., 2005), somatic (Bartok and Craft, 1997, Cicero et al., 1997, Boyer et al., 1998, Kest et al., 1999, Barrett et al., 2001) or visceral (Ji et al., 2006, Ji et al., in press) pain models. To date, the mechanism(s) whereby morphine produces a differential degree of analgesia is unknown.
The midbrain periaqueductal gray (PAG), and its descending projections to the rostral ventromedial medulla (RVM) and spinal cord, comprises an essential neural circuit for both endogenous and exogenous opioid-mediated analgesia (Basbaum et al., 1978, Basbaum and Fields, 1978, Basbaum and Fields, 1984). The PAG contains a high density of mu opioid receptors (Commons et al., 1999, Commons et al., 2000, Wang and Wessendorf, 2002) and microinjection of opioid antagonists into the PAG significantly attenuates systemic morphine-produced analgesia (Zambotti et al., 1982, Randich et al., 1992, Lane et al., 2005, Bernal et al., 2007). Similarly, microinjection of the mu opioid selective neurotoxin dermorphin-saporin (Porreca et al., 2001, Burgess et al., 2002, Vera-Portocarrero et al., 2006a, Vera-Portocarrero et al., 2006b) into the PAG significantly attenuates systemic morphine analgesia (Loyd and Murphy, 2007). Together, these studies indicate that the PAG is a primary neural structure for opioid-mediated analgesia.
Recent studies have reported sex differences in both the anatomical and physiological organization of the PAG-RVM pathway. These sex differences may provide the biological bases for the observed sexually dimorphic actions of morphine. For example, recent anatomical studies have shown that females have a significantly greater number of PAG neurons retrogradely labeled from the RVM in comparison to males. Interestingly, persistent inflammatory pain only activates this pathway in males. Similarly, systemic morphine administration decreases noxious stimulus-induced activation of the PAG in male but not female rats (Loyd and Murphy, 2006). Together, these studies suggest a sexually dimorphic action of morphine within the PAG. This suggestion is further supported by studies showing that microinjection of morphine into the PAG produces greater antinociception in male in comparison to female rats (Krzanowska and Bodnar, 1999, Bernal et al., 2007, Wang et al., submitted).
Electrophysiological studies have reported that morphine, as well as other opioid agonists, inhibit and excite different populations of PAG neurons. In vitro recordings have shown that opioids disinhibit PAG-RVM output neurons by presynaptically inhibiting tonically active GABAergic neurons (Behbehani et al., 1990a, Behbehani et al., 1990b, Chieng and Christie, 1994, Stiller et al., 1995). This GABA-mediated disinhibition results in the activation of PAG-RVM neurons. However, other studies have shown that opioids directly inhibit a subset of PAG-RVM projection neurons (Chieng and Christie, 1994, Osborne et al., 1996), and mu opioid receptors have been localized on approximately 27–50% of PAG neurons retrogradely labeled from the RVM (Commons et al., 2000, Wang and Wessendorf, 2002). If GABA disinhibition is the primary mechanism for morphine action in the PAG, then administration of morphine should preferentially induce Fos in PAG-RVM output neurons. By contrast, if morphine antinociception is primarily mediated via direct inhibition of PAG-RVM output neurons, then systemic administration of morphine should not induce Fos in these neurons as induction Fos induction requires membrane depolarization (Greenberg et al., 1986a, Greenberg et al., 1986b, Bartel et al., 1989).
The present studies were conducted to determine whether morphine administration preferentially activates PAG-RVM output neurons using retrograde tract tracing in combination with Fos immunocytochemistry. Morphine-induced Fos in PAG-RVM output neurons was compared in male and females rats to determine if there were qualitative or quantitative sex differences in activation of this pathway. If sex differences in the PAG-RVM circuit provide that bases for the sexually dimorphic actions of morphine, then activation of this pathway should be greater in male compared to female rats.
1. Experimental Procedures
1.1. Subjects
Adult male and weight-matched (250–350g) cycling female Sprague-Dawley rats (Zivic-Miller; Pittsburg, PA) were used. Rats were housed in same-sex pairs in separate rooms on a 12:12 hour light: dark cycle (lights on at 7:00A.M.). Access to food and water was ad libitum throughout the experiment except during surgery. Vaginal lavages were taken daily to determine if the female rats were cycling normally and to keep daily records on the stages of their cycle in respect to the experimental testing. These studies were performed in compliance with the Institutional Animal Care and Use Committee at Georgia State University.
1.2. Retrograde Tracer Injections
Six male and six female rats were deeply anesthetized with ketamine/xylazine (50 mg/kg/10mg/kg; s.c.) and placed in a stereotaxic frame. The position of the skull was adjusted so that bregma and lambda were at the same dorsal-ventral plane. Glass micropipettes (10–20 μM) filled with the retrograde tracer Fluorogold (FG; 2% soln. w/v in saline; Fluorochrome LLC; Denver, CO) were lowered into the RVM using the following coordinates (in mm): AP: −2.0 Lambda; ML: 0.0; DV: −8.5. FG was iontophoresed (50/50 duty cycle, 7.5 μA current) into the RVM for 25 minutes to facilitate neuronal uptake. The current was then turned off, and the pipettes remained in place for an additional 5 minutes to reduce backflow of tracer along the pipette track. Only males and females that had comparable injection sites were used for data collection. Following tracer injections, wounds were sutured closed, the antibiotic Neosporin was applied to the wound, and the animals were placed in clean cages to recover under a heat lamp. Upon complete recovery from the anesthetic, animals were returned to their original housing facilities.
1.3. Morphine Administration
All animals were given a subcutaneous injection of either morphine sulfate (4.5 mg/kg, s.c.; NIDA; Bethesda, MD) or sterile saline as a control. All injections were performed between the hours of 12:00P.M. and 2:00P.M. Morphine sulfate was prepared fresh in a saline vehicle within one hour prior to injection.
1.4. Perfusion fixation
One hour after morphine administration, animals were given a lethal dose of Nembutal (160 mg/kg; i.p.). The animals were transcardially perfused with 150–200 ml of 0.9% sodium chloride containing 2% sodium nitrite as a vasodilator, to remove blood from the brain. Immediately following removal of blood, 300 ml of 4% paraformaldehyde in 0.1M phosphate buffer containing 2.5% acrolein (Polyscience; Niles, IL) was perfused through the brain as a fixative. A final rinse with 150–200 ml of the sodium chloride/sodium nitrate solution was perfused through the brain to remove any residual acrolein. Immediately following perfusion, the brains were carefully removed, placed in a 30% sucrose solution and stored at 4°C for at least one week prior to sectioning. To section the brain, the dura and pia matter were carefully removed and the brains were cut into six series of 25 μm coronal sections with a Leica 2000R freezing microtome and stored free-floating in cryoprotectant-antifreeze solution (Watson et al., 1986) at −20°C until immunocytochemical processing.
1.5. Immunocytochemistry
A 1:6 series through the rostrocaudal axis of each brain was processed for FG and/or Fos immunoreactivity as previously described (Murphy and Hoffman, 2001). Briefly, sections were rinsed extensively in potassium phosphate-buffered saline (KPBS) to remove cryoprotectant solution immediately followed by a 20-minute incubation in 1% sodium borohydride to remove excess aldehydes. The tissue was then incubated in primary antibody solution(s) previously described (Loyd and Murphy, 2006): rabbit anti-Fos (Oncogene; Cambridge, MA, lot no. 4194; 1:50,000) and/or rabbit anti-FG (Chemicon; Billerica, MA, lot no. 25060005; 1:10,000) in KPBS containing 1.0% Triton-X for one hour at room temperature followed by 48 hours at 4°C. After rinsing out the primary antibody with KPBS, the tissue was incubated for one hour in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch; West Grove, PA, 1:200), rinsed with KPBS, followed by a one hour incubation in an avidin-biotin peroxidase complex (1:10; ABC Elite Kit, Vector Labs). After rinsing in KPBS and sodium acetate (0.175 M; pH 6.5), Fos was visualized as a black reaction product using nickel sulfate intensified 3,3′-diaminobenzidine solution containing 0.08% hydrogen peroxide in sodium acetate buffer. FG was visualized as a brown reaction product using 3,3′-diaminobenzidine containing 0.08% hydrogen peroxide in Trizma buffer (pH 7.2). After 15–30 minutes, three rinses in sodium acetate buffer terminated the reaction. Sections were then mounted out of saline onto gelatin-subbed slides, air-dried overnight, dehydrated in a series of graded alcohols, cleared in xylene, and cover-slipped using Permount.
1.6. Data Analysis and Presentation
The number of PAG-RVM output neurons (FG+), the number of activated intrinsic PAG neurons (Fos+), and the number of activated PAG-RVM output neurons (Fos+FG) was determined across six rostrocaudal levels of PAG (Bregma −6.72, −7.04, −7.74, −8.00, −8.30, −8.80). Neurons were counted in two PAG subdivisions: dorsomedial and lateral/ventrolateral; these subdivisions are clearly distinguishable based on the distribution of retrograde labeling from the RVM (van Bockstaele et al., 1991, Bandler and Shipley, 1994). The lateral/ventrolateral region was counted unilaterally as there are no differences in the number of FG+ cells for the left versus right side of PAG (Loyd and Murphy, 2006). There are no cytoarchitectural or anatomical boundaries between the lateral and ventrolateral regions; therefore, these regions were counted together to avoid experimenter bias. The tissue was sectioned at 25 μm so that 125 μm separates each analyzed level of the PAG thus avoiding any bias of counting the same cell twice. Additionally, previous data has shown that there are no sex differences in the mean area (mm2) of PAG between weight-matched male and female Sprague-Dawley rats (Loyd and Murphy, 2006).
Data are expressed as the mean ± standard error of the mean (SEM) from which percentages were calculated. A four-way analysis of variance (ANOVA) was used to test for significant main effects of sex (male, female), PAG subdivision (dorsomedial, lateral/ventrolateral), PAG level (Bregma −6.72 through −8.80) and treatment (morphine, saline). P≤0.05 was considered significant for all analyses. For data presentation, a representative animal from each experimental group was selected and the distribution of FG and Fos within the PAG were plotted using a Nikon Drawing Tube attached to a Nikon Optiphot microscope. Plots were then scanned onto the computer and adjusted to figure format using Adobe Illustrator 10. Photomicrographs were generated using a Synsys digital camera attached to a Nikon Eclipse E800 microscope. Images were captured with IP Spectrum software, adjusted to figure format by adjusting brightness and contrast levels using Adobe Photoshop 7.0.
2. Results
2.1. Systemic Morphine-Induced Activation of the PAG
The objective of the present experiment was to examine if morphine activation of the PAG is quantitatively and/or qualitatively different in male and female rats. Animals were given a systemic injection of either morphine (4.5 mg/kg, s.c.; n=6/sex) or sterile saline (n=5 males and n=6 females) and perfused one hour later. The distribution and number of neurons expressing Fos were determined across six rostrocaudal levels of the PAG (Bregma −6.72, −7.04, −7.74, −8.00, −8.30, −8.80) and within the dorsomedial and lateral/ventrolateral regions.
In animals treated with systemic morphine, Fos+ cells were primarily distributed throughout the dorsomedial, lateral and ventrolateral regions of the PAG with very little Fos in the dorsolateral or ventromedial regions (see Figure 1). The quantity and distribution of Fos labeled neurons was relatively consistent across the rostrocaudal axis of the PAG (Bregma −6.72 to −8.80 mm) and was almost two-fold greater in morphine treated animals in comparison to saline control [F(1,252) = 106.7, p < 0.0001]. In animals treated with morphine, the mean number of Fos+ cells in the rostral PAG was 77.5 ± 13.4 in comparison to 25 ± 8 in saline treated animals. Caudally (Bregma −8.30), the mean number of Fos+ cells in morphine treated animals was 78 ± 3 versus 33 ± 7 for saline control. Morphine administration induced comparable Fos expression in the PAG in both male and female rats [F (1,252) = 1.60, p > 0.05]. For example, at rostral levels of PAG (Bregma −7.04), the mean number of Fos+ cells was 73 ± 16 for males, in comparison to 77 ± 13 for females. More caudally (Bregma −8.30), the mean number of Fos+ cells was 78.3 ± 14 in males in comparison to 81 ± 12 in females.
Figure 1.
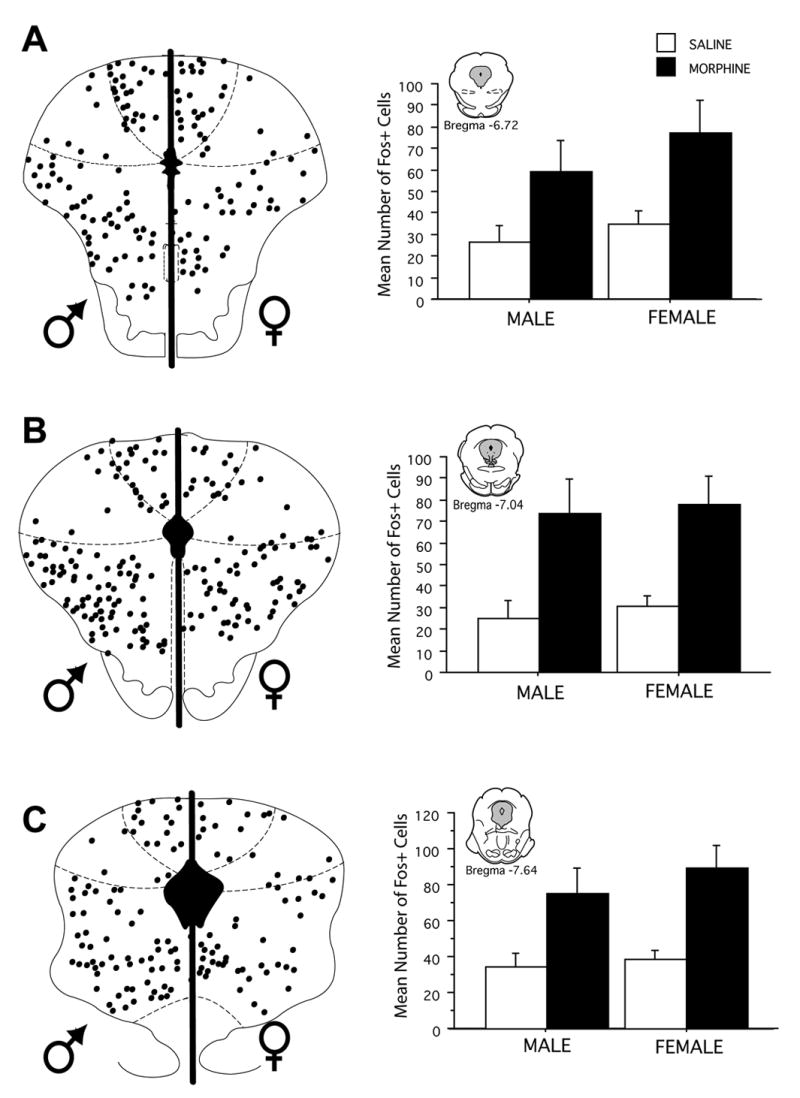
Distribution of morphine-induced Fos in male and female rats at six rostrocaudal levels of the PAG. Each black circle represents one Fos-immunoreactive cell. Bar graphs compare the mean number (± S.E.M.) of Fos-immunoreactive cells in male and female rats 1 hr after administration of morphine or saline. Morphine administration induced a significant increase in the number of Fos labeled neurons compared to saline. This increase was comparable in male and female rats.
2.2. Sexually Dimorphic Activation of the PAG-RVM Circuit by Morphine
The results from the first set of experiments demonstrate that acute morphine administration induces greater activation of the PAG compared to acute saline administration, and that this activation is comparable in male and female rats. The objectives of the next experiment were to determine (1) whether morphine preferentially activates PAG-RVM output neurons, and (2) if this activation is sexually dimorphic. Animals received Fluorogold injections into the RVM; ten days later they were injected with either systemic morphine (4.5 mg/kg; n=5 per sex) or sterile saline (n=5 males and n=6 females) and perfused one hour later.
Figure 2 shows an example of a typical iontophoretic injection of FG into the RVM of a male (top) and female (bottom). All injections were located on the midline and dorsal to the pyramidal tract, at the level of the caudal pole of the facial nucleus (lambda –2.0mm). Injections outside of the RVM were not included for analysis.
Figure 2.
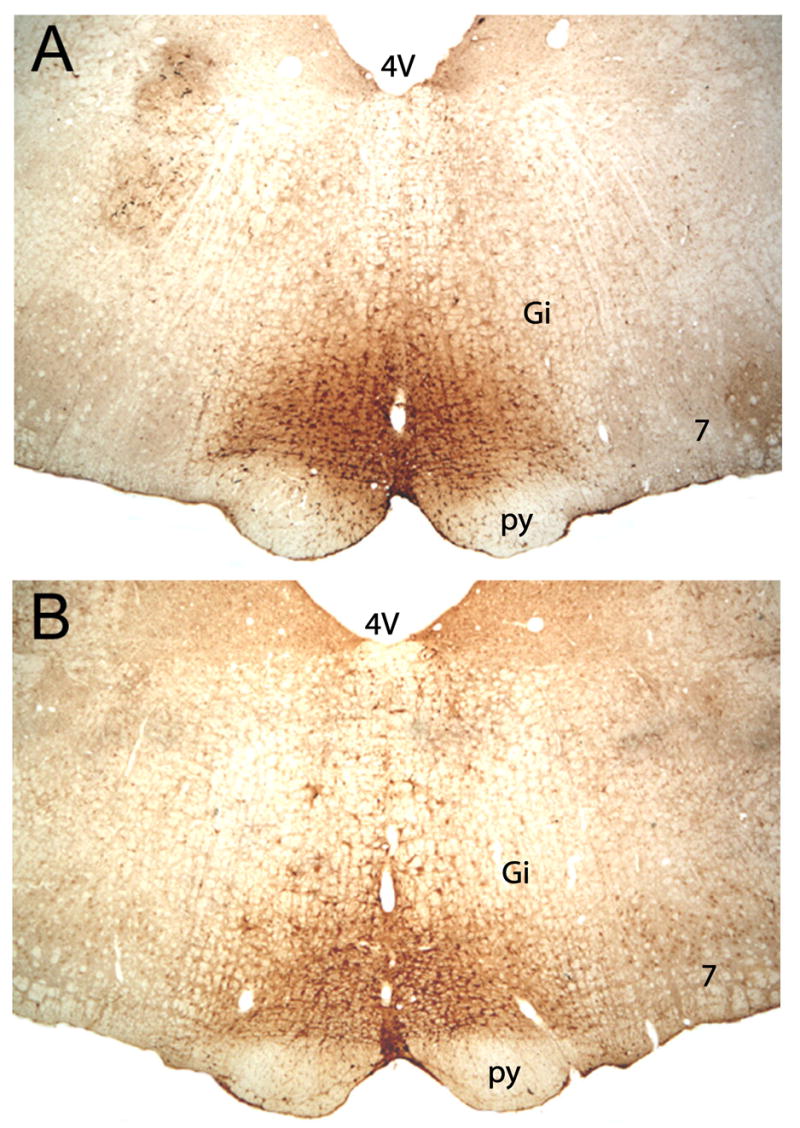
Representative examples of FG injection into the RVM of a male (top) and female (bottom) rat. All injections were along the midline and in the bottom third of the medulla. Gi, gigantocellularis; 7, facial nucleus; py, pyramidal tract; 4V, fourth ventricle.
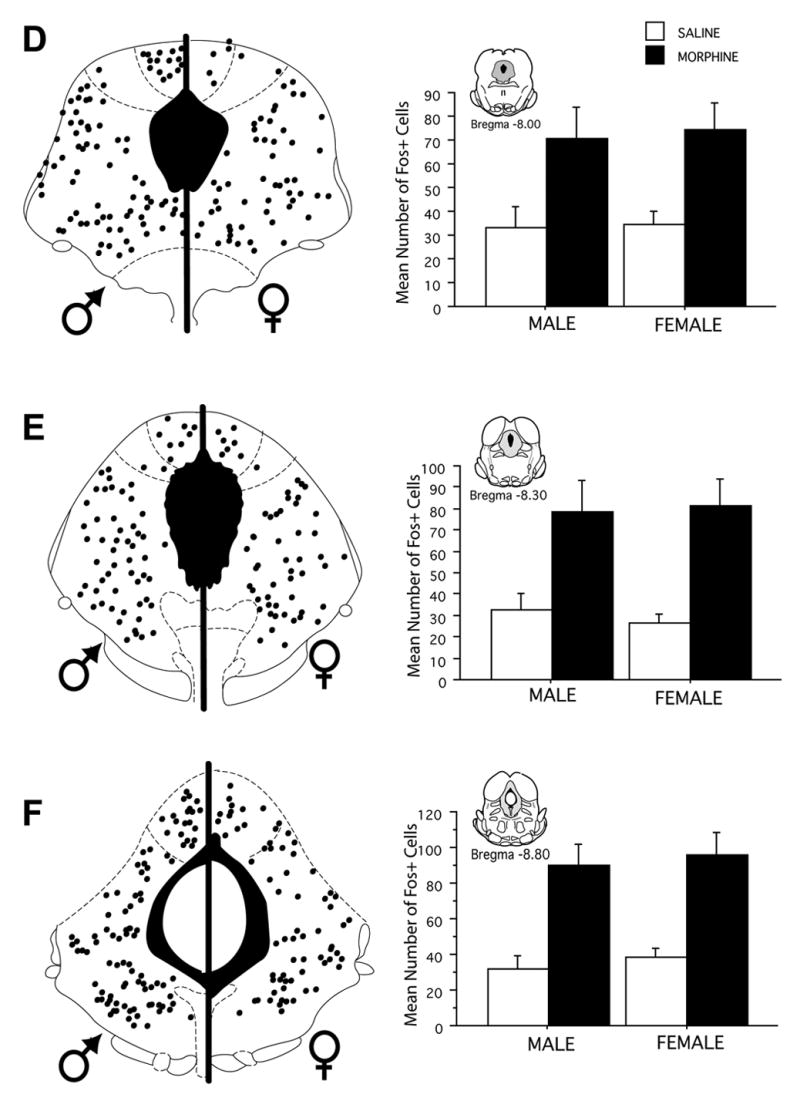
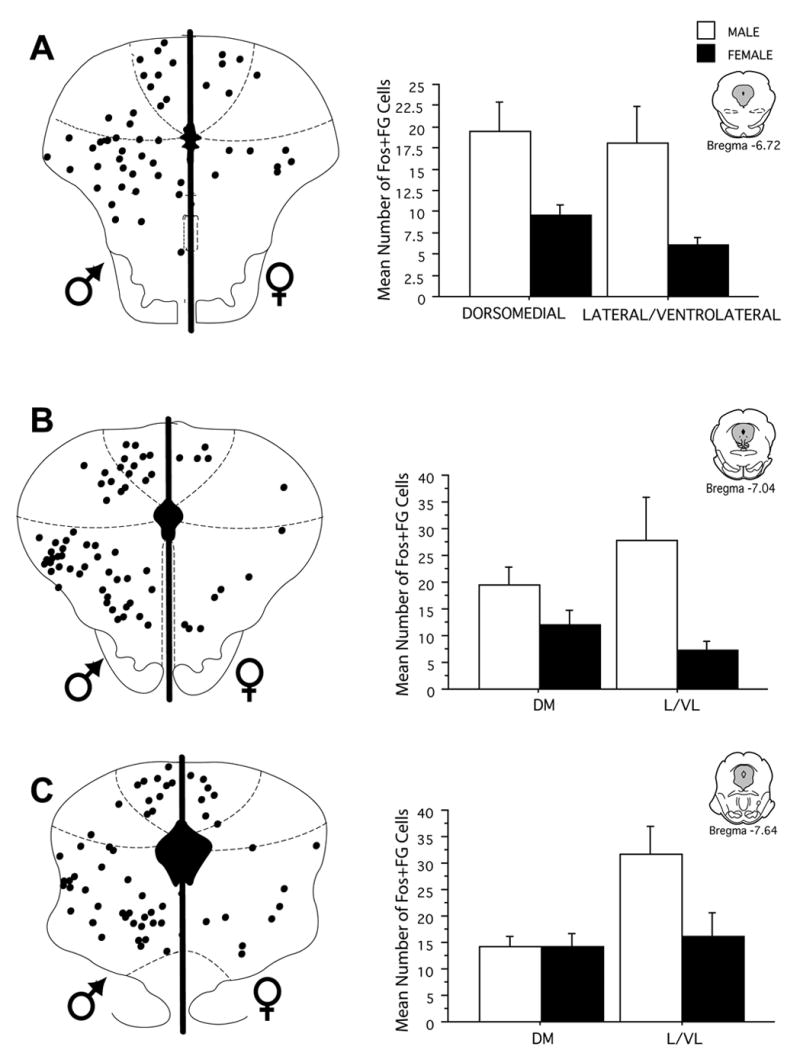
Injection of FG into the RVM produced dense retrograde labeling across the rostrocaudal axis of the PAG in both male and female rats. As reported previously (Loyd & Murphy, 2006), there were significantly more retrogradely labeled neurons across all rostrocaudal levels of the PAG in female as compared to male rats [F (1,216) = 48.2, p < 0.0001]. Acute systemic administration of morphine induced Fos labeling in a subset of PAG output neurons in both male and female rats. The number of activated PAG output neurons was consistently higher in the PAG of male compared to female rats (Figure 3). An example of morphine induced Fos within FG+ cells is shown in Figure 4. At rostral levels of PAG (Bregma −6.72), the mean number of double-labeled cells in the lateral/ventrolateral subdivision of the PAG was 18 ± 4 for males, in comparison to 6 ± 1 for females. More caudally (Bregma −8.30), the mean number of double-labeled cells in the lateral/ventrolateral subdivision of the PAG was 42 ± 11 in males, in comparison to 20 ± 3 in females. This activation of output neurons was significantly greater in male compared to female rats whether analyzed as number of double labeled neurons [F(1,192) = 43.3, p < 0.0001] (Figure 3), percent of FG labeled neurons expressing Fos [F(1,192) = 223.2, p < 0.0001] or percent Fos that was expressed in FG neurons [F(1,192) = 65.379, p < 0.0001] (Figure 5). The greater mean number of Fos-positive PAG-RVM neurons in male rats is particularly noteworthy given that the total number of projecting neurons is greater in female rats. The greater number of double labeled neurons in male rats is evident in both dorsomedial and lateral/ventrolateral regions across all rostrocaudal levels of the PAG (Figure 3).
Figure 3.
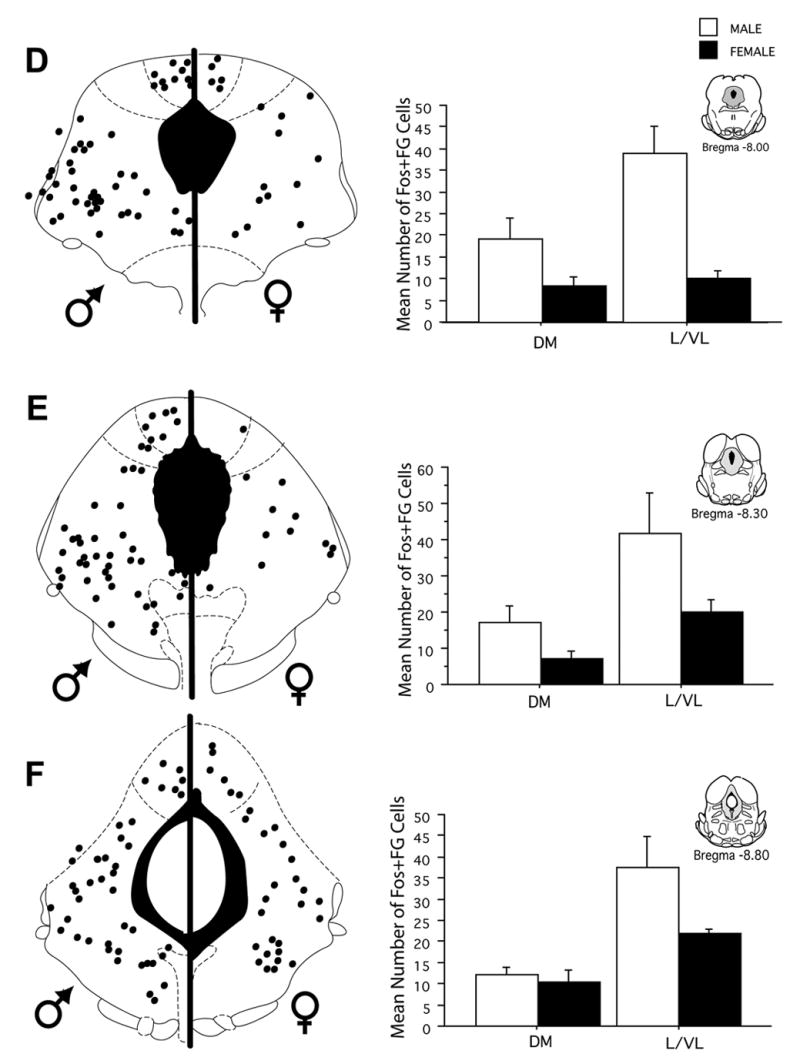
Distribution of PAG-RVM output neurons that expressed morphine-induced Fos in male and female rats across six rostrocaudal levels of the PAG. Each black circle represents one double-labeled (Fos+FG) cell. Bar graphs display the mean number of Fos+FG cells (± S.E.M.) for each level of the dorsomedial and lateral/ventrolateral regions of the PAG. Fos labeling was greater in male than female rats in all PAG regions measured.
Figure 4.
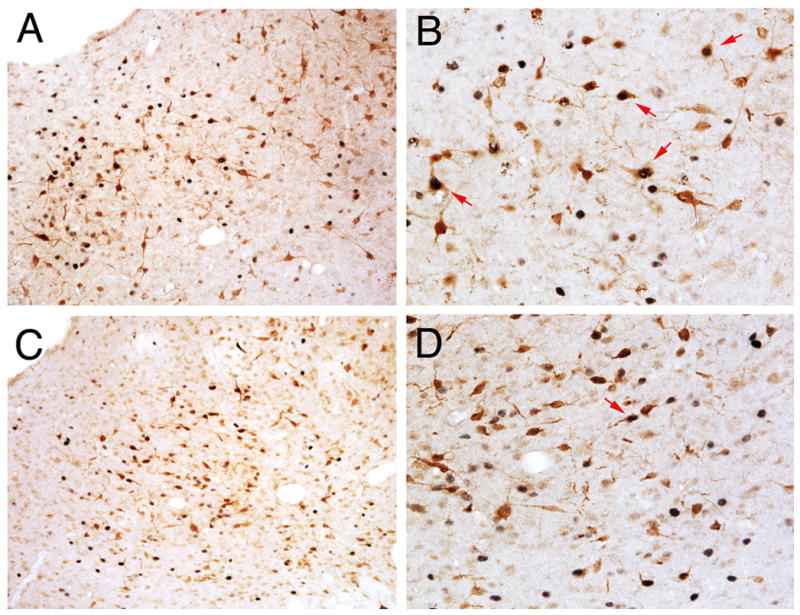
Photomicrograph showing morphine-induced Fos in FG+ cells for males (A, 10X; B, 20X) and females (C, 10X; D, 20X). Note that while females have a greater number of FG+ cells than males, very few of these cells expressed morphine-induced Fos (indicated with red arrows). No sex differences were noted in the total number of Fos+ cells.
Figure 5.
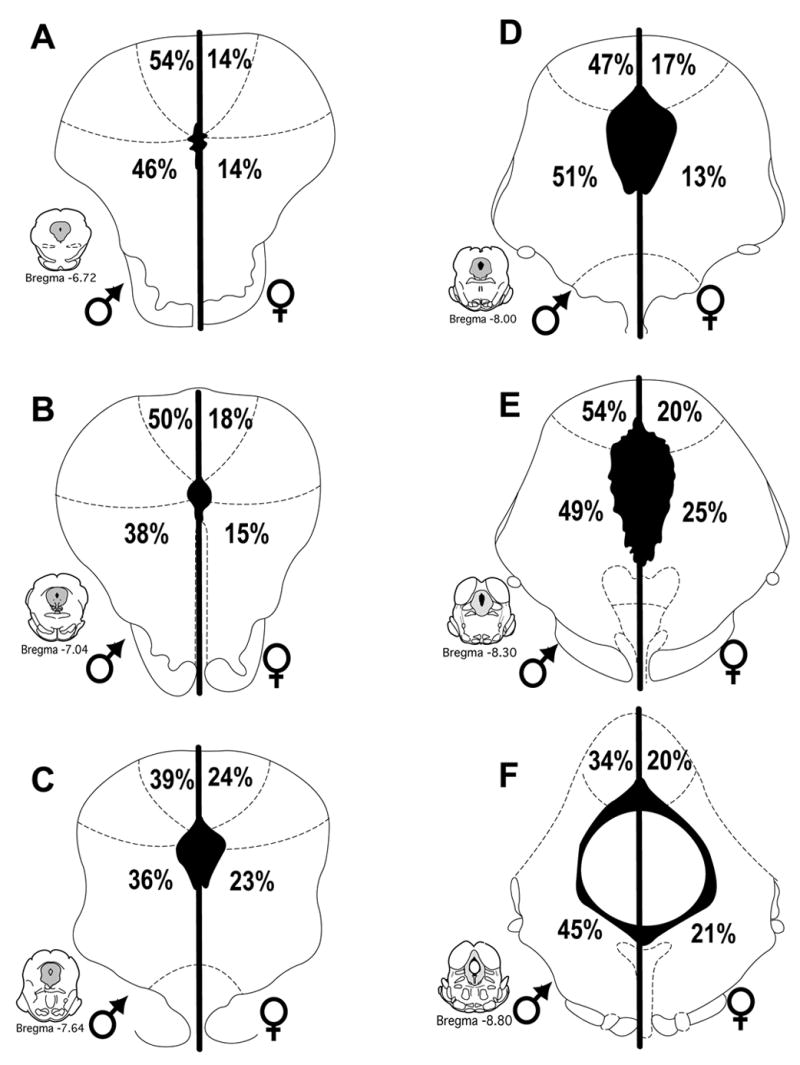
Percentage of morphine-induced Fos+ PAG neurons that were retrogradely labeled from the RVM (Fos+FG) in male (left) and female (right) rats in the dorsomedial and lateral/ventrolateral regions of the PAG at six representative levels. The proportion of Fos positive neurons projecting to the RVM was greater in male than female rats across all PAG regions.
The number of double-labeled cells also varied across PAG levels with caudal regions showing greater label in the lateral/ventrolateral compared to dorsomedial subdivision and rostral regions showing comparable labeling in the two subdivisions. An ANOVA on the number of Fos+ neurons revealed a significant main effect of levels-by-subdivision interaction [F(5,252) = 2.97, p < 0.05]; no other interactions reached significance (p > 0.05).
3. Discussion
It is becoming increasingly evident that morphine produces a greater degree of analgesia in males in comparison to females. To date, the mechanisms underlying sex differences in morphine analgesia remain unknown. The PAG and its descending projections to the RVM provide the primary circuit for opioid-produced analgesia, and sex differences in the activation of this circuit by morphine would be predicted to contribute to the reported sex differences in morphine analgesia. The results of our studies demonstrate that systemic morphine administration induces extensive Fos expression within the PAG of male and female rats. Interestingly, while no sex differences were noted in the overall number or distribution of morphine-induced Fos, morphine preferentially activated the PAG-RVM circuit in males. Indeed, at most rostral-caudal levels of PAG, the percentage of PAG-RVM neurons expressing morphine induced Fos was two-fold higher in males in comparison to females.
In the present study, the observed morphine-induced Fos may be due to direct actions of morphine at the mu opioid receptor (MOR) or alternatively, due to secondary effects associated with morphine administration, including changes in blood pressure, respiration and cardiac output. These autonomic changes have been shown previously to induce Fos expression within the PAG of the rat (Murphy et al., 1995). However, as pharmacological or neurotoxic blockade of MOR in the PAG significantly attenuates the analgesic effects of systemic morphine, this suggests that the Fos observed in the present study was primarily due to direct actions of morphine in the PAG. In support, Fos expression was primarily localized within the dorsomedial and lateral/ventrolateral PAG; these regions contain high levels of mu opioid receptor (Commons et al., 2000, Wang and Wessendorf, 2002, Wang et al., submitted). Interestingly, mu opioid receptors are virtually absent in the dorsolateral PAG; similarly, this region contained very little, if any, morphine-induced Fos.
3.1. The PAG-RVM Circuit is Preferentially Activated in Male Rats
Fluorogold injection into the RVM produced dense retrograde labeling within the dorsomedial, lateral and ventrolateral divisions of the PAG, consistent with previous anatomical studies in the rat (Beitz et al., 1983, van Bockstaele et al., 1991, Rizvi et al., 1996). Interestingly, while the number of PAG neurons retrogradely labeled from the RVM is almost two-fold higher in females in comparison to males, very few of these PAG-RVM cells were activated by morphine (FG/Fos; overall between 14–25% of FG+ cells). Similarly, although morphine induced comparable levels of Fos within the PAG of males and females, the percentage of Fos present in FG+ cells (Fos/FG) was only between 14–25%. By contrast, in males, the percentage of FG+ cells co-expressing Fos ranged between 48–77%, while the percentage of Fos+ cells labeled with FG was between 34–54%. The results of the present study indicating that between 48–77% of PAG-RVM neurons in males express morphine induced Fos suggest that administration of morphine results primarily in the net excitation of PAG-RVM neurons, most likely through removal of tonic GABA inhibition (Moreau and Fields, 1986, Behbehani et al., 1990b, Vaughan and Christie, 1997, Commons et al., 2000). Direct inhibition of PAG-RVM neurons by morphine may also contribute to morphine analgesia and cannot be ruled out in the present study.
Interestingly, while females had extensive Fos present in the PAG, very little Fos was present in PAG output neurons. As activation of the PAG-RVM pathway is essential for morphine-induced analgesia, these results provide a likely mechanism for sex differences in morphine analgesia. It is not clear why females had such extensive Fos expression overall in the PAG, but very little in RVM projection neurons. Unfortunately, to date, no in vitro recordings have been conducted from female PAG slices so the effects of morphine on the physiological properties of PAG neurons can only be inferred from male data. Clearly, this needs to be addressed in the future. One possibility is that in females, morphine activates a pain facilitation network. A PAG-RVM pain facilitatory system has been shown to contribute to hyperalgesia produced by opiate withdrawal (Bederson et al., 1990), chronic inflammation (Terayama et al., 2000, Guan et al., 2002), and morphine tolerance (Vanderah et al., 2001). Interestingly, morphine-induced hyperalgesia is significantly more pronounced in females in comparison to males (Holtman and Wala, 2005).
Alternatively, there may be a sex difference in the organization or function of the GABAergic system inhibiting the PAG cells involved in modulating nociceptive transmission. Intra-PAG administration of GABA antagonists block pain behaviors (Budai and Fields, 1998; Moreau and Fields, 1986) and lead to depolarization, increased firing frequency, and increased excitatory post-synaptic potentials in PAG neurons responsive to GABA (Behbehani et al., 1990b). Intra-PAG GABA agonists (Moreau and Fields, 1986) and mu opioid receptor antagonists (Budai and Fields, 1998) reverse the analgesic effects of the GABA antagonists and of morphine. While the ventrolateral PAG contains a high density of mu opioid receptors, very few of the neurons that project to the RVM are directly inhibited by opioid agonists (Osborne et al., 1996). In the present study, males had greater activation of the PAG-RVM pathway by systemic morphine, suggesting that morphine is acting through local GABA neurons (Bellchambers et al., 1998), whereas females potentially have a different organization or function of this GABAergic system.
The present study used gonadally intact, cycling females and a number of studies have suggested that gonadal steroids may influence morphine potency. For example, treatment of female rat pups with androgen on the day of birth results in a leftward shift of the morphine dose response curve (Krzanowska and Bodnar, 1999, Cicero et al., 2002, Cataldo et al., 2005). In adulthood, ovariectomy increases morphine potency in female rats and this effect is blocked by exogenous estradiol replacement (Negus and Mello, 1999, Stoffel et al., 2005, Ji et al., in press). Similarly, in males, castration decreases and testosterone replacement restores morphine potency (Stoffel et al., 2005). The rat PAG contains a dense population of estrogen (ERα) and androgen (AR) receptor containing neurons (Murphy and Hoffman, 1999, Murphy and Hoffman, 2001, Marson and Murphy, 2006). These receptors are localized in similar regions of the PAG as the mu opioid receptor and in our preliminary studies, we noted that a large proportion of PAG-RVM neurons also contain ERα. In hypothalamic slices, estrogen has been shown to rapidly uncouple the mu opioid receptor from G protein-gated inwardly rectifying potassium channels resulting in a reduced hyperpolarization by MOR agonists (Kelly et al., 2003). Estrogen-mediated hyperpolarization of PAG-RVM neurons would provide a direct mechanism whereby morphine failed to elicit Fos in PAG-RVM neurons. Alternatively, as the gene encoding Fos contains an estrogen response element (Loose-Mitchell et al., 1988, Wang et al., 2003), changes in cycle status may have potentially influenced the ability of morphine to induce Fos in PAG-RVM neurons in females. Lastly, estradiol has also been shown to induce MOR internalization (Eckersell et al., 1998, Sinchak and Micevych, 2001, Micevych et al., 2003, Mills et al., 2004); this would limit the amount of receptor available for ligand binding and would thereby potentially decrease morphine’s ability to induce Fos in PAG-RVM neurons. Future studies are necessary to further test these potential mechanisms.
3.2. Sexually Dimorphic PAG-RVM Pathway
The results of the present study indicate that morphine preferentially activates the PAG-RVM pathway in males. These results concur with our previous study demonstrating that persistent inflammatory pain selectively activates the PAG-RVM pathway in males, but not females. In that study, we also demonstrated that morphine preferentially suppressed persistent pain induced Fos in males, but not females. Unfortunately, the effects of morphine on persistent pain induced Fos in PAG-RVM output neurons was quite variable, and it was unknown whether the effects we were observing were due to morphine acting directly on PAG-RVM neurons and/or if morphine was decreasing pain-induced drive to the PAG. In the present study, morphine was given in the absence of pain and was shown to preferentially activate the PAG-RVM circuit in males. Indeed, at most rostral-caudal levels of the PAG, the percentage of PAG-RVM neurons expressing morphine induced Fos was two-fold higher in males in comparison to females. As the PAG-RVM circuit is essential for morphine analgesia, sex differences in the activation of this pathway may provide the biological bases for the sexually dimorphic actions of morphine.
Acknowledgments
This work was supported by NIH grants DA16272 and P50 AR49555 awarded to Anne Z. Murphy, Ph.D. and NIH grant DA015498 awarded to Michael M. Morgan, Ph.D.
Grant Support: NIH grants DA16272 and AR49555 to AZM and DA015498 to MMM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? TINS. 1994;19:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl) 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Bartel DP, Sheng M, Lau LF, Greenberg ME. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989;3:304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Jiang M, Chandler SD. The effect of [Met]enkephalin on the periaqueductal gray neurons of the rat: an in vitro study. Neuroscience. 1990a;38:373–380. doi: 10.1016/0306-4522(90)90035-3. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Jiang MR, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain. 1990b;40:195–204. doi: 10.1016/0304-3959(90)90070-T. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. Reticular formation, central gray and related tegmental nuclei. In: Paxinos G, editor. The Rat Nervous System. Academic Press Australia; Sydney: 1985a. pp. 1–28. [Google Scholar]
- Beitz AJ. The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell number, density, orientation, and regional subdivisions. J Comp Neurol. 1985b;237:445–459. doi: 10.1002/cne.902370403. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983;261:132–137. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- Bellchambers CE, Chieng B, Keay KA, Christie MJ. Swin-stress but not opioid withdrawal increases expression of c-Fos immunoreactivity in rat periaqueductal gray neurons which project to the rostral ventromedial medulla. Neurosci. 1998;83(2):517–524. doi: 10.1016/s0306-4522(97)00399-0. [DOI] [PubMed] [Google Scholar]
- Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav Brain Res. 2007;177:126–133. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Romero MT, Kramer E. Organismic variables and pain inhibition: roles of gender and aging. Brain Res Bull. 1988;21:947–953. doi: 10.1016/0361-9230(88)90032-9. [DOI] [PubMed] [Google Scholar]
- Boyer JS, Morgan MM, Craft RM. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 1998;796:315–318. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- Budai D, Fields HL. Endogenous opioid peptides acting at mu opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol. 1998;79:677–687. doi: 10.1152/jn.1998.79.2.677. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo G, Bernal S, Markowitz A, Ogawa S, Ragnauth A, Pfaff DW, Bodnar RJ. Organizational manipulation of gonadal hormones and systemic morphine analgesia in female rats: effects of adult ovariectomy and estradiol replacement. Brain Res. 2005;1059:13–19. doi: 10.1016/j.brainres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Commons KG, van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999;408:549–559. [PubMed] [Google Scholar]
- Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund’s adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in drug- and non-drug-induced analgesia. Life Sci. 2003a;72:2675–2688. doi: 10.1016/s0024-3205(03)00178-4. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003b;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Hermanowski AL, Ziff EB. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986a;6:1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986b;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Wala EP. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain. doi: 10.1016/j.jpain.2007.01.006. in press. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther. 1999;289:1370–1375. [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicted from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell DS, Chiappetta C, Stancel GM. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol. 1988;2:946–951. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Stezhka VV. Neurones in the dorsolateral periaqueductal grey matter in coronal slices of rat midbrain: electrophysiological and morphological characteristics. Exp Brain Res. 1999;124:53–58. doi: 10.1007/s002210050599. [DOI] [PubMed] [Google Scholar]
- Loyd D, Murphy A. Selective lesions of the mu opioid receptor in the periaqueductal gray significantly attenuates systemic morphine analgesia in male rats. Soc Neurosci Abstr 2007 [Google Scholar]
- Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R419–428. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Ennis M, Rizvi TA, Behbehani MM, Shipley MT. Fos expression induced by changes in arterial pressure is localized in distinct, longitudinally organized columns of neurons in the rat midbrain periaqueductal gray. J Comp Neurol. 1995;360:286–300. doi: 10.1002/cne.903600207. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of androgen and estrogen receptor containing neurons in the male rat periaqueductal gray. Horm Beh. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290:1132–1140. [PubMed] [Google Scholar]
- Okamoto K, Tashiro A, Hirata H, Bereiter DA. Differential modulation of TMJ neurons in superficial laminae of trigeminal subnucleus caudalis/upper cervical cord junction region of male and cycling female rats by morphine. Pain. 2005;114:203–211. doi: 10.1016/j.pain.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to the rostral ventromedial medulla in vitro. J Physiol (London) 1996;490:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Thurston C, Ludwig P, Robertson J, Tasmussen C. Intravenous morphine-induced activation of vagal afferents: peripheral, spinal, and CNS substrates mediating inhibition of spinal nociception and cardiovascular responses. J Neurophysiol. 1992;68:1027–1045. doi: 10.1152/jn.1992.68.4.1027. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Murphy AZ, Ennis M, Behbehani MM, Shipley MT. Medial preoptic area afferents to periaqueductal gray medullo-output neurons: A combined Fos and tract tracing study. J Neurosci. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller CO, Linderoth B, O’Connor WT, Franck J, Falkenberg T, Ungerstedt U, Brodin E. Repeated spinal cord stimulation decreases the extracellular level of gamma-aminobutyric acid in the periaqueductal gray matter of freely moving rats. Brain Res. 1995;699:231–241. doi: 10.1016/0006-8993(95)00911-9. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1991;309:305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006a;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006b;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109:619–634. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Wang LH, Yang XY, Zhang X, Mihalic K, Xiao W, Farrar WL. The cis decoy against the estrogen response element suppresses breast cancer cells via target disrupting c-fos not mitogen-activated protein kinase activity. Cancer Res. 2003;63:2046–2051. [PubMed] [Google Scholar]
- Wang X, Smith G, Murphy AZ. Morphine injection into the periaqueductal gray produces a sexually dimorphic response in a model of persistent inflammatory pain: Role of differential mu opioid receptor expression. Pain submitted. [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain longterm peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Zambotti F, Zonta N, Parenti M, Tommasi R, Vicentini L, Conci F, Mantegazza P. Periaqueductal gray matter involvement in the muscimol-induced decrease of morphine antinociception. Naunyn Schmiedebergs Arch Pharmacol. 1982;318:368–369. doi: 10.1007/BF00501180. [DOI] [PubMed] [Google Scholar]


