Abstract
Cadherin-based cell–cell contacts are prominent sites for phosphotyrosine signaling, being enriched in tyrosine-phosphorylated proteins and tyrosine kinases and phosphatases. The functional interplay between cadherin adhesion and tyrosine kinase signaling, however, is complex and incompletely understood. In this report we tested the hypothesis that cadherin adhesion activates c-Src signaling and sought to assess its impact on cadherin function. We identified c-Src as part of a cadherin-activated cell signaling pathway that is stimulated by ligation of the adhesion receptor. However, c-Src has a biphasic impact on cadherin function, exerting a positive supportive role at lower signal strengths, but inhibiting function at high signal strengths. Inhibiting c-Src under circumstances when it is activated by cadherin adhesion decreased several measures of cadherin function. This suggests that the cadherin-activated c-Src signaling pathway serves positively to support cadherin function. Finally, our data implicate PI3-kinase signaling as a target for cadherin-activated c-Src signaling that contributes to its positive impact on cadherin function. We conclude that E-cadherin signaling is an important activator of c-Src at cell–cell contacts, providing a key input into a signaling pathway where quantitative changes in signal strength may result in qualitative differences in functional outcome.
INTRODUCTION
Classical cadherin cell adhesion molecules are fundamental determinants of tissue organization whose biological function is intimately linked to cell signaling. Diverse signaling molecules are found at cell–cell contacts and many are activated in a cadherin-dependent manner when cells adhere to one another (Yap and Kovacs, 2003). Among the relevant signals, it has long been recognized that cadherin adhesions are major sites for protein tyrosine phosphorylation. Many tyrosine-phosphorylated proteins are found at adherens junctions, which are, indeed, conspicuous sites for phosphotyrosine staining in cells (Takata and Singer, 1988; Tsukita et al., 1991; Calautti et al., 1998). Moreover, a wide range of tyrosine kinases are found at cell–cell contacts, including both receptor tyrosine kinases and cytoplasmic kinases (Tsukita et al., 1991; Thomas and Brugge, 1997; Calautti et al., 1998). However, the precise relationship between cadherin adhesion and tyrosine kinase signaling remains poorly understood.
This is exemplified by c-Src and other Src-family kinases (SFKs). Members of this family of cytoplasmic tyrosine kinases are often found at cadherin-based cell–cell contacts (Tsukita et al., 1991; Calautti et al., 1998). A variety of studies further reported that expression of v-Src or constitutively active (CA) Src perturbs the integrity of cell–cell interactions in epithelia (Warren and Nelson, 1987; Volberg et al., 1991). This was accompanied by tyrosine phosphorylation of components of the cadherin/catenin complex, notably β-catenin (Matsuyoshi et al., 1992; Behrens et al., 1993). Together these have led to the common notion that SFK signaling negatively regulates cadherin adhesion. In contrast, other studies that typically utilized loss-of-function approaches reported the capacity for SFKs to positively affect cadherin adhesion. During keratinocyte differentiation components of the cadherin/catenin complex become tyrosine-phosphorylated. However, this fails to occur in fyn-deficient cells, which also display defects in adherens junction assembly (Calautti et al., 1998). Similarly, junctional assembly and dorsal closure were perturbed in Src42A-deficient flies (Takahashi et al., 1996, 2005). The discrepancy between these different sets of studies has yet to be resolved.
What mechanisms might regulate expression of SFK signaling at cell–cell contacts also remain poorly understood. A range of growth factor receptors and adhesion molecules capable of activating SFK are found at cell–cell contacts (McLachlan and Yap, 2007). We recently found that cadherin homophilic ligation with recombinant adhesive ligands could recruit c-Src to adhesions (Pang et al., 2005), suggesting that cadherin adhesion exerted an instructive influence to affect cortical localization of c-Src. Accordingly, in this report we examined the potential for E-cadherin to regulate c-Src signaling. We present evidence that c-Src is an integral part of a cadherin-activated cell signaling pathway that can positively regulate adhesion and cell contact integrity. We further demonstrate that directly modulating c-Src signal intensity has a bimodal impact on cadherin function, where quantitative changes in signal strength can lead to distinctly different functional outcomes.
MATERIALS AND METHODS
Cell Culture, Transfections, and hE/Fc Purification
Parental Chinese hamster ovary (CHO) cells, CHO cells stably expressing full-length human E-cadherin (hE-CHO cells) or hE-Cad 764AAA, and MCF-7 cells were cultured described previously (Kovacs et al., 2002a,b; Goodwin et al., 2003; Paterson et al., 2003). To generate CHO cells stably expressing hE-Cad-PYD, cells were transfected with pcDNA3-ECad-PYD-6MT by lipofection and selected with G418, and clones were screened that expressed cadherin levels comparable to hE-CHO cells and MCF7 cells. hE/Fc was purified as previously described (Kovacs et al., 2002a). For experiments, cells were plated at subconfluent density on plastic dishes or glass coverslips and maintained for 1–2 d before being used.
Plasmids
Plasmids used were: myc-tagged pSG5p110-CAAX (kindly provided by Dr. J. Downward; ICRF); c-Src-mf (K295M, Y527F; Pang et al., 2005); c-Src-251-GFP (green fluorescent protein; Timpson et al., 2001); c-SrcY527F-GFP (a kind gift from Dr. H.-C. Cheng, University of Melbourne, with the permission of Prof. M. Frame, Beatson Institute for Cancer Research). Transient transfections were performed using either Lipofectamine 2000 or Lipofectamine Plus Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and analyzed 24–72 h after transfection. The mutant hE-Cad-PYD-6MT, truncated at amino acid 670 and bearing a C-terminal poly-Myc epitope tag, was made by PCR mutagenesis and was confirmed by sequencing.
Antibodies
Primary Antibodies were: 1) mouse mAb HECD-1 against the ectodomain of human E-cadherin for immunofluorescence and immunoblotting (a kind gift from Dr. Peggy Wheelock (University of Nebraska Medical Center, Omaha, NE) with the permission of Dr. M. Takeichi, University of Kyoto); 2) mouse mAb Ab-1 raised against the SH3 domain of c-Src (Calbiochem, La Jolla, CA) for immunofluorescence and immunoblotting; 3) rabbit pAb raised against the phosphorylated-tyrosine-419 residue in c-Src (pY419; Cell Signaling Technologies, Beverly, MA) for immunofluorescence and immunoblotting; 4) mouse mAb raised against p120 (Transduction Laboratories, Lexington, KY); 5) mouse mAb against β-catenin (Transduction Laboratories); 6) mouse mAb against α-catenin (Transduction Laboratories); 7) Akt and 8) phospho-Akt (Cell signaling Technologies); 9) rabbit pAb raised against GAPDH (Bioscientific, Sydney, Australia); 10) mouse mAb raised against β-tubulin (Sigma, St. Louis, MO); 11) Texas Red–conjugated phalloidin or phaloidin-594 were for F-actin staining (Molecular Probes, Eugene, OR). Secondary antibodies were species-specific antibodies conjugated with Alexa-488, Alexa-594, or Texas Red (Molecular Probes). The mouse mAb SHE 78-7 (Zymed, San Francisco, CA) raised against the ectodomain of human E-cadherin was used in the cadherin blocking antibody experiments.
Immunofluorescence Microscopy, Quantification, and Curve Fitting
Cells were fixed with 4% paraformaldehyde in CSK buffer (100 mM KCl, 300 mM sucrose, 2 mM EGTA, 2 mM MgCl2, 10 mM PIPES) at room temperature for 30 min, permeabilized in 0.25% Triton X-100 in phosphate-buffered saline (PBS) at room temperature for 5 min, washed in PBS, and blocked overnight at 4°C with 5% (wt/vol) nonfat dried milk in PBS or 0.5% (wt/vol) fish gelatin in tris-buffered saline (TBS) for phosphotyrosine detection. Immunolabeled cells were mounted in 1% N-propyl-gallate in 50% glycerol:PBS for immunofluorescence microscopy.
Fixed specimens were examined using IX81 microscopes fitted with X100, 1.40 NA objectives. Images were acquired with Hamamatsu Orca-1 ER cameras (Bridgewater, NJ) driven by Metamorph imaging software (Version 6.2, Universal Imaging, West Chester, PA). Figures were assembled for presentation in Adobe Photoshop (Version 9; San Jose, CA).
Planar spreading assays (Kovacs et al., 2002a), hE/Fc adhesion assays (Yap et al., 1997), and calcium manipulation assays (Helwani et al., 2004; Stehbens et al., 2006) were all performed as previously described. Quantification of fixed immunofluorescence data were performed using ImageJ Version 1.37. The intensity of E-cadherin at cell–cell contacts was measured using the region of interest (ROI) tool. After an initial background correction, the ROI tool was used to trace around the entire cell–cell contact allowing the “mean gray value” (mean pixel intensity) to be measured. For planar spreading assays, the ROI tool was used to measure the surface area of cells adherent to hE/Fc as described previously (Pang et al., 2005). All graphs were compiled using Prism Version 4 (GraphPad Software, San Diego, CA).
To analyze the relationship between CA c-Src transgene expression and cell spreading, we plotted the cell surface area versus cell fluorescence intensity. The relationship between the conditional mean of surface area (y) given fluorescence intensity (x) was obtained by taking the conditional distribution of y given x to be a mixture of two normal distributions in proportions p and (1 − p), where the logarithm of the ratio of p to (1 − p) was taken to be a quadratic function of x. The mean of the first normal component was assumed to be independent of x, whereas the mean of the second component was modeled by a cubic function of x.
Biochemical Assays
For the bead assays, cells were plated to form subconfluent cultures of isolated cells. hE/Fc- or poly-l-lysine (PLL)-coated latex beads (6-μm-diam) were incubated with the cells for various times. In the pY419 c-Src bead assay, cells were treated with 10 μM sodium orthovanodate for 5 min before the end of each time point. Lysates were then separated by SDS-PAGE and analyzed for total c-Src, pY419 c-Src, or GAPDH (loading control).
To determine Akt activation, cell fractionation was performed at the end of the bead assay. Serum-starved isolated cell cultures were incubated with either hE/FC or PLL-coated beads, with 10 μM PP2 or PP3 being added to cells incubated with hE/Fc-coated beads. At the end of each time point, lysates were washed twice in cold HES buffer (20 mM HEPES, pH 7.4, 1 mM EDTA, 250 mM sucrose) and homogenized in the same buffer supplemented with Complete Protease Inhibitors (Roche, Indianapolis, IN) and phosphatase inhibitors (2 mM sodium orthovanodate, 10 mM sodium fluoride). After an initial preclearing spin of 2000 × g for 10 min, the resulting supernatant was spun at 100,000 × g for 30 min to give a pelleted membrane fraction (P100) and the cytosolic fraction (S100) as the supernatant. The P100 fraction was probed for Akt, phospho-Akt, and transferrin receptor (loading control).
For the E-cadherin coimmunoprecipitation analysis, cells were lysed in 1 ml of cold lysis buffer (0.5% NP-40, 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 mM CaCl2, 2 mM sodium vanodate, and Complete Protease Inhibitors [Roche]). Protein complexes were immunoprecipitated with a polyclonal E-cadherin antibody or a preimmune serum control antibody bound to protein A-Sepharose beads and were separated using SDS-PAGE. Immune complexes were blotted for E-cadherin, β-catenin, α-catenin, or p120. The trypsin protection assay used to measure surface E-cadherin levels was performed as described previously (Verma et al., 2004; Shewan et al., 2005). All blots were scanned and compiled using Adobe Photoshop Version 9.
RESULTS
E-Cadherin Adhesion Activates c-Src Signaling
We began by using indirect immunofluorescence (IF) microscopy to confirm that c-Src localized with E-cadherin at cell–cell contacts between MCF-7 mammary epithelial cells (Figure 1A), consistent with what has been observed in other epithelia (Tsukita et al., 1991; Calautti et al., 1998; Calautti et al., 2002). In addition, using a phospho-specific antibody against pY419 as a marker of Src activity, we also identified active c-Src at established E-cadherin–based cell–cell contacts (Figure 1A).
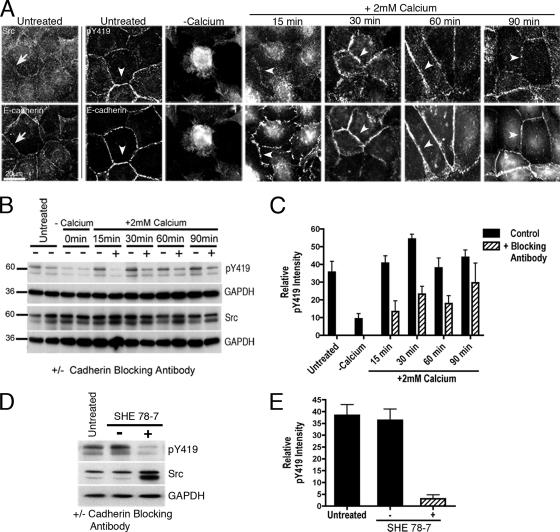
Figure 1.
E-cadherin is required for cell–cell contact to activate c-Src. MCF-7 monolayers were studied at confluence (untreated), immediately after cell–cell contacts were broken by chelation of extracellular calcium with 4 mM EDTA (−Calcium), or 15–90 min after replenishing extracellular calcium to allow contacts to reassemble (+2 mM Calcium). (A) Active c-Src is present at established and reassembling cell–cell contacts. Fixed cells were immunostained for E-cadherin and either c-Src (Src, arrows) or activated c-Src (pY419, arrowheads). (B and C) E-cadherin activity is necessary for stimulation of c-Src as cells reassemble contacts with one another. Cells were allowed to reassemble contacts after chelation of extracellular calcium (+2 mM Calcium) either in the presence (+) or absence (−) of an E-cadherin function-blocking antibody (SHE 78-7). Western blots from cell lysates were probed for c-Src, pY419 c-Src, and GAPDH as a loading control; a representative blot is shown in B. pY419 c-Src levels were quantified by densitometry and expressed relative to total c-Src levels (C). Data are means ± SEM (n = 3; p < 0.0001 for cells treated with SHE78-7 for 15, 30 and 60 min). (D and E) E-cadherin is necessary to maintain active c-Src at established cell–cell contacts. Confluent MCF-7 monolayers treated with a cadherin function-blocking antibody (+; SHE 78-7), or with a control Mouse IgG antibody (−), were compared with untreated established monolayers (untreated). Cells were incubated with antibodies for 5 min and then lysed, and Western blots were probed for pY419 c-Src, c-Src, and GAPDH (D). pY419 c-Src levels expressed relative to total c-Src levels (E). Data are means ± SEM (n = 3; p < 0.0001 for SHE 78-7-treated cells compared with control Ab cells).
To analyze the relationship between cadherin adhesion and c-Src signaling, we then used the pY419 Ab to assess c-Src activity as the integrity of cell–cell contacts was manipulated. Breaking apart cell contacts by depletion of extracellular calcium decreased the amount of cellular pY419-Src detectable by immunoblotting (Figure 1B). pY419-Src levels then increased acutely after restoration of calcium (Figure 1, B and C), and IF analysis demonstrated that pY419-Src rapidly reaccumulated with E-cadherin as cells reassembled cell–cell contacts with one another (Figure 1A). The change in pY419-Src levels occurred without any associated change in the total levels of c-Src in these cells (Figure 1B). Importantly, addition of an E-cadherin function-blocking antibody (SHE 78-7) as cells were forming contacts significantly retarded the increase in pY419-Src (Figure 1, B and C). Thus, E-cadherin adhesion appeared to be necessary to stimulate Src signaling as cells formed contacts with one another.
Function-blocking antibodies were next used to test whether E-cadherin adhesion was required for the basal c-Src signaling observed at cell–cell contacts in already-established monolayers (Figure 1, D and E). Incubation of MCF-7 cells for 5 min with SHE 78-7 clearly reduced the cellular levels of pY419-Src detectable by immunoblotting (Figure 1, D and E) and reduced the amount of pY419-Src staining detected at contacts by IF before the integrity of the cadherin contacts was overtly disrupted (not shown). A lower MW c-Src band that became more prominent after treatment with SHE 78-7, and which was recognized in c-Src immune complexes (not shown), may represent a different post-translational modification or a degradation product. Nonetheless, quantitation confirmed that after SHE 78-7 treatment pY419-Src levels were clearly reduced relative to the higher c-Src band whose MW corresponded to that detected by the pY419 Ab (Figure 1E). Therefore E-cadherin activity was necessary for the basal Src signaling detected at established cell–cell contacts.
E-Cadherin Homophilic Ligation Activates c-Src Signaling
We then sought to test whether homophilic ligation of E-cadherin alone could activate c-Src signaling (Figure 2, A and B). MCF-7 cells were plated at low density to prevent cell–cell interactions and then incubated with latex beads coated with either a recombinant cadherin ligand (hE/Fc) or PLL. As reported earlier (Kovacs et al., 2002a), hE/Fc comprises the E-cadherin ectodomain fused to the Fc portion of human IgG and supports E-cadherin adhesion and signaling. pY419-Src was undetectable in control cells not exposed to beads (Figure 2A) or in cells incubated with uncoated beads (Figure 2A). In contrast, pY419-Src levels rose rapidly after addition of hE/Fc-coated beads without any detectable change in total cellular levels of c-Src (Figure 2A). Although PLL-coated beads induced some degree of c-Src activation, it was consistently less than hE/Fc (Figure 2B). Together, these data suggest that the Src signaling detected at E-cadherin–based cell–cell contacts may arise in response to adhesive ligation of E-cadherin itself.
Figure 2.
c-Src is activated by E-cadherin homophilic ligation. (A and B) Isolated cultures of MCF-7 cells were incubated for 15–60 min with latex beads coated with either hE/Fc or PLL. These samples were compared with control cell cultures not exposed to beads (−Beads) or control cultures incubated with beads that had been blocked but not coated with any adhesive ligand (+Beads). Western blots from the samples were probed for activated c-Src (pY419 c-Src), total c-Src, and GAPDH (loading control). A representative blot is shown in A. (B) pY419 c-Src levels were measured by densitometry and expressed relative to total c-Src levels. Data are means ± SEM (n = 3; p < 0.0001 at each time point comparing hE/Fc-beads and PLL beads). (C and D) CHO cells expressing wild-type E-cadherin (hE), hE-Cad-764AAA (764), or hE-Cad-PYD-6MT (PYD) were incubated for 15 min with latex beads coated with hE/Fc. In control experiments hE-CHO cells were also incubated with poly-l-lysine–coated beads (PLL). Western blots from cell lysates were probed for pY419 c-Src, c-Src and GAPDH (C). (D) pY419 c-Src levels quantified by densitometry were expressed relative to total c-Src levels. Data are means ± SEM (n = 3).
To gain further mechanistic insights, we then used well-characterized cadherin mutants to examine which region of the cytoplasmic tail was necessary for E-cadherin to stimulate c-Src (Figure 2, C and D). We analyzed a cadherin mutant bearing point mutations that abolish p120-ctn binding (hE-Cad-764AAA) as well as a C-terminal truncation that ablates the β-catenin–binding site (hE-Cad-PYD; Yap et al., 1997; Thoreson et al., 2000). To isolate the impact of the cadherin mutants themselves, we stably expressed them in CHO cells, which do not express classical cadherins, thereby providing an effective null background (Kovacs et al., 2002b). Wild-type and mutant cadherins were expressed to similar levels (not shown) and coimmunoprecipitation analysis confirmed that hE-Cad764AAA did not bind p120-ctn, whereas hE-Cad-PYD failed to bind β-catenin (not shown).
CHO cells stably expressing wild-type E-cadherin (hE-CHOs) showed higher activated c-Src levels within 15 min of binding hE/Fc beads compared with PLL beads (Figure 2, C and D). This confirmed that E-cadherin retained the ability to stimulate c-Src in a CHO background as it did in MCF7 cells. Cadherin-coated beads similarly stimulated c-Src activity in hE764AAA-CHO cells, but failed to do so above background in hE-Cad-PYD-CHO cells (Figure 2, C and D). Therefore the distal C-terminus, which binds β-catenin, is necessary for E-cadherin to activate c-Src.
c-Src Signaling Is Necessary for the Integrity of E-Cadherin Cell–Cell Contacts
To investigate the functional significance of c-Src signaling for E-cadherin, we first tested whether dominant-negative c-Src mutants affected the integrity of E-cadherin–based cell– cell contacts (Figure 3, A and B). We transiently expressed two well-validated c-Src mutants in MCF7 monolayers. Dominant negative (DN) c-Src-251-GFP lacks the catalytic domain and C-terminus, whereas DN c-Src-mf (K295M Y527F) is a catalytically inactive mutant in the “open” configuration (Nada et al., 1991; Timpson et al., 2001). Cells expressing c-Src-251-GFP were identified using the GFP tag, whereas the DN c-Src-mf mutant was coexpressed with GFP using an internal ribosomal entry site (IRES) vector. Although control cells expressing GFP alone were indistinguishable from untransfected cells (Figure 3A), both DN c-Src mutants significantly perturbed the distribution of E-cadherin at cell–cell contacts (Figure 3A). In both cases, E-cadherin staining was significantly reduced at contacts between cells expressing the DN c-Src mutants compared with either untransfected cells in the same monolayer or GFP-transfected controls. Quantitation of fluorescence intensity indicated that cadherin accumulation was reduced by ∼65% in DN-c-Src–expressing cells compared with GFP controls (Figure 3B).
Figure 3.
c-Src signaling is required for the integrity of E-cadherin–based cell–cell contacts. (A) MCF-7 cell monolayers transiently expressing dominant-negative (DN) c-Src-251-GFP or DN c-Src-mf (K295M Y527F) were compared with control cells transfected with pEGFP-N1 alone. After fixation, cells were immunostained for E-cadherin and GFP (Tag). Expression of dominant-negative c-Src mutants reduced the intensity of E-cadherin staining found at contacts between two transfected cells (arrows) compared with contacts between either pEGFP-N1 transfected cells or between untransfected cells (arrowheads). (B) Dominant-negative c-Src expression reduces E-cadherin accumulation at epithelial cell–cell contacts. The intensity of E-cadherin staining at transfected cell–cell contacts was measured and expressed as a ratio of the fluorescence intensity at untransfected cell–cell contacts. Data are means ± SEM (n = 30; p < 0.0001 for DN mutants). (C) Inhibition of c-Src with PP2 significantly affects the integrity of epithelial cell–cell contacts. MCF-7 monolayers treated with PP2 (5–10 μM) or PP3 (10 μM) were compared with untreated monolayers. The fluorescence intensity of E-cadherin staining at cell–cell contacts was quantified (Cadherin Intensity). Data are means ± SEM (n = 50; p < 0.0001 for 5–10 μM PP2). (D) Inhibition of c-Src does not affect the total or surface expression of E-cadherin. MCF-7 monolayers trypsinized in the presence (+Ca2+) or absence of extracellular calcium (−Ca2+) were compared with untreated cell monolayers (WCL). Western blots from cell lysates were probed for E-cadherin and β-tubulin as a loading control. (E) The stoichiometry of the cadherin–catenin complex is not significantly affected by c-Src inhibition. Untreated MCF-7 cell monolayers were compared with monolayers treated with either 10 μM PP2 or 10 μM PP3. E-cadherin immunoprecipitates from cell lysates were separated by SDS-PAGE and probed for E-cadherin, β-catenin, α-catenin, and p120.
We corroborated these findings using the Src inhibitor, PP2 (10 μM), which reduced E-cadherin fluorescence intensity at cell–cell contacts by ∼40% compared with the inactive analog, PP3, which had no effect on cadherin accumulation (Figure 3C). Trypsin protection assays demonstrated that neither the total or surface pools of E-cadherin were affected by PP2 (Figure 3D), nor was there any significant change in the amount of β-catenin, α-catenin, or p120-ctn that coimmunoprecipitated with E-cadherin in PP2-treated cells compared with PP3-treated cells (Figure 3E). Together, these findings suggested that c-Src signaling supports the integrity of E-cadherin cell–cell contacts, perhaps by regulating the local accumulation and retention of cadherin at contacts.
Src Signaling Supports E-Cadherin Homophilic Adhesion
We then asked what effect Src signaling might have on E-cadherin adhesive activity, using laminar flow assays that measure cell resistance to detachment from hE/Fc-coated substrata (Yap et al., 1997). For these experiments we used hE-CHO cells because they allowed us to also compare adhesiveness conferred by expressing E-cadherin with any background adhesiveness as determined with the parental, cadherin-deficient, CHO cell line. As expected, parental CHO cells failed to adhere to hE/Fc-coated substrata, whereas hE-CHO cells incubated with PP3 robustly resisted detachment (Figure 4A). Adhesion to hE/Fc, however, was reduced by ∼40% in hE-CHO cells treated with PP2, indicating that inhibiting Src signaling compromised E-cadherin adhesiveness.
Figure 4.
c-Src is required for E-cadherin adhesive interactions. (A) Src signaling is necessary for E-cadherin adhesion. Adhesion of cells to hE/Fc-coated substrata was assessed using a laminar flow assay that measures the ability of cells to resist detachment by laminar flow (Flow rate). CHO cells stably expressing human E-cadherin (hE-CHO cells) were studied after pre-treatment with either PP2 (10 μM) or PP3 (10 μM). Negative controls were parental CHO cells that lack E-cadherin (CHO φ) and hE-CHO cells incubated with uncoated capillary tubes (No hE/Fc). PP2 significantly reduced the adhesiveness of cells compared with PP3-treated cells. Data are means ± SEM (n = 3; p < 0.0001). (B) c-Src is required for cells to spread on cadherin-coated substrata. hE-CHO cells were allowed to adhere and spread on hE/Fc-coated substrata. Cells transiently transfected with dominant-negative (DN) c-Src-251 or DN c-Src-mf (K295M Y527F) were compared with cells expressing pEGFP-N1 alone (GFP) or with untransfected cells. The ability of the cells to form and extend cadherin-based adhesions was quantified by measuring cell surface area. Representative images are shown in insets. Data are means ± SEM (n = 35; p < 0.0001 for DN mutants compared with untransfected controls). (C) PP2 prevents cells from spreading on cadherin-coated substrata. hE-CHO cells incubated with either PP2 (10 μM) or PP3 (10 μM) were allowed to adhere to hE/Fc-coated substrata, and spreading was assessed by measuring cell surface area. Representative images are shown in insets. Withdrawal of drug for 1 h was sufficient to restore spreading in PP2-treated cells (Drug + Washout). Data are means ± SEM (n = 35; p < 0.0001 for PP2 vs. PP3 and p = 0.8 for PP2 + Washout vs. PP3 + Washout).
The ability of cells to spread on cadherin-coated substrata is another index of cellular responsiveness to cadherin homophilic ligation (Kovacs et al., 2002a). This process entails changes in cell signaling and cytoskeletal regulation that arise in response to productive ligation of the cadherin adhesion receptor. Cells transiently expressing GFP alone rapidly spread when plated onto hE/Fc, as determined by measuring the surface area of phalloidin-stained cells (Figure 4B). In contrast, cells expressing either DN c-Src mutant failed to spread, retaining rounded morphologies similar to those of cadherin-deficient CHO cells (not shown) and surface areas reduced by ∼85% compared with GFP-transfected controls (Figure 4B). Similarly, PP2 reduced cell surface area by ∼70% (Figure 4C), but spreading was activated when the drug was washed out, indicating that cell spreading was not inhibited by irreversible drug toxicity.
Constitutively-Active Src Perturbs Cadherin Activity
Together, these data indicated that c-Src signaling could contribute positively to cadherin–based adhesion and cell–cell interactions. This contrasted, however, with a range of studies demonstrating that expression of v-Src or CA Src mutants perturbs the integrity of cell–cell interactions (Warren and Nelson, 1987; Volberg et al., 1991; Avizienyte et al., 2002). To compare our results, we transiently expressed a constitutively-active c-Src mutant (c-SrcY527F-GFP) in MCF7 cells. As expected, EGFP alone had no effect on the morphology of E-cadherin–based epithelial cell–cell contacts (Figure 5A). In contrast, intense expression of CA c-Src perturbed the morphology of MCF-7 cells, with the cells becoming more mesenchymal in appearance (Figure 5A). Furthermore, transfected cells appeared to grow over neighboring untransfected cells, with concomitant loss of E-cadherin staining at cell–cell contacts.
Figure 5.
Constitutively active c-Src disrupts E-cadherin function. (A) c-SrcY527F-GFP expression perturbs the morphology of E-cadherin–based cell–cell contacts. MCF-7 cells expressing c-SrcY527F-GFP or EGFP were allowed to form cellular contacts. After fixation, cells were immunostained for E-cadherin and GFP (Tag). In comparison to untransfected or pEGFP-N1 cell–cell contacts (arrowheads), c-SrcY527F-GFP–expressing cells failed to form E-cadherin–based cell–cell contacts (arrows). (B) Constitutively active c-Src prevents the formation and extension of E-cadherin–based contacts. hE-CHO cells expressing c-SrcY527F-GFP or EGFP were allowed to adhere to hE/Fc-coated substrata, and their ability to spread was assessed by measuring the surface area of the cells. Representative images are shown in insets. Data are means ± SEM (n = 30; p < 0.0001).
To further assess its impact on cadherin activity, we tested the ability of cells expressing CA c-Src to spread on cadherin-coated substrata. Although EGFP did not affect cadherin-based cell spreading, cells with strong c-SrcY527F-GFP expression were unable to spread (Figure 5B). Together, these data indicated that overstimulation of c-Src activity perturbed cadherin function in our assay systems comparable to previous reports.
Src Signal Strength Affects Cadherin Function in a Bimodal Manner
These results then suggested that the impact of Src signaling on cadherin function might depend critically on signal strength: positively supporting cadherin function at lower signal strengths, but perturbing function at higher levels. We sought direct evidence for this hypothesis by transiently expressing c-SrcY527F-GFP in hE-CHO cells and testing their ability to spread on cadherin-coated substrata (Figure 6). We assessed the full range of transgene expression and also serum-starved cells beforehand to reduce background SFK signaling. The impact of CA c-Src was then quantified by comparing surface area to transgene expression measured by GFP fluorescence intensity for each cell.
Figure 6.
Increasing CA c-Src levels have a bimodal effect on cadherin-based cell spreading. Serum-starved hE-CHO cells transiently transfected with c-Src Y525F-GFP were allowed to adhere to hE/Fc-coated substrata for 90 min. Cell spreading (pixels) was then compared with expression of c-Src Y525F-GFP, assessed by fluorescence intensity of GFP (arbitrary units, au). The curve represents average cell surface area as a function of GFP intensity. Representative images of cells expressing low, medium, or high levels of c-Src Y525F-GFP are shown in insets.
As shown in Figure 6, there was a bell-shaped relationship between transgene expression and cell spreading. Untransfected serum-starved control cells attached, but failed to spread and remained rounded. Surface area increased progressively with CA c-Src at lower levels of expression, but then fell progressively at higher levels. Thus c-Src signaling can have either positive or negative effects on this parameter of cadherin function, depending on signal intensity. It should be noted, however, that CA-Src–induced cell spreading was morphologically different from that shown by control cells, typically displaying spiky peripheral protrusions rather than the broad lamellipodia seen in control cells (compare insets in Figure 6 with Figure 5B). Modulation of c-Src signaling is then unlikely to be the only signal that couples cadherin homophilic ligation to cell spreading in this assay.
c-Src Supports E-Cadherin Integrity through PI3-Kinase
One potential pathway for c-Src to influence cadherin function is through PI3-kinase. PI3-kinase signaling is active at cadherin cell–cell contacts and is capable of supporting cell–cell adhesion (Pece et al., 1999; Watton and Downward, 1999; Kovacs et al., 2002a). Moreover, c-Src participates as an upstream element in several signaling pathways that activate PI3-kinase, including growth factor receptor pathways (Wymann and Pirola, 1998; Cantrell, 2001). Recently we reported that an upstream tyrosine kinase activity was necessary for E-cadherin to activate PI3-kinase and, interestingly, recruitment of PI3-kinase to cadherin adhesions required c-Src activity (Pang et al., 2005). This therefore suggested that c-Src might act upstream of PI3-kinase to support cadherin function.
To pursue this, we first tested whether c-Src was necessary for E-cadherin to activate PI3-kinase signaling, measured using activation-specific phospho-Akt (pAkt) antibodies (Figure 7). Akt is phosphorylated by E-cadherin adhesion, either when cells make contacts with one another or when they bind to recombinant cadherin ligands (Pece et al., 1999; Kovacs et al., 2002a). Accordingly, cells were incubated with hE/Fc-coated beads to ligate cellular cadherin and pAkt levels measured in membrane fractions. Binding of hE/Fc beads, but not PLL-coated beads, increased membrane pAkt and Akt levels (Figure 7) in a wortmannin-dependent manner (not shown), confirming that these changes reflected responses to PI3-kinase. PP2 (10 μM) prevented this rise in plasma membrane pAkt, with the levels of pAkt being comparable to those found in cells incubated with PLL-coated beads (Figure 7, A and C). Furthermore, there was less Akt recruited to the plasma membrane fraction in cells treated with PP2 compared with PP3-treated cells (Figure 7, A and B). Together, these data suggest that c-Src is necessary for E-cadherin ligation to activate PI3-kinase signaling.
Figure 7.
c-Src signaling is necessary for E-cadherin to activate PI3-kinase signaling. Isolated MCF-7 cell cultures were incubated for 30–90 min with either hE/Fc coated latex beads (hE/Fc) or PLL-coated beads (PLL). Cells exposed to cadherin-coated beads were incubated with either PP2 (10 μM) or PP3 (10 μM). At each time point, cell lysates were subjected to subcellular fractionation and the membrane-enriched (P100) fraction was probed for Akt, pAkt, and transferrin receptor (TfR) by Western analysis. Additionally, total lysates were probed for Akt and GAPDH as a loading control. A representative of three independent experiments is shown in A. (B) Recruitment of Akt to membranes was quantitated relative to total cellular levels of Akt in the cells. (C) pAkt levels in membranes were quantitated relative to TfR levels in membranes. Data are means ± SEM (n = 3). PP2 reduced the amounts of both Akt (A and B) and pAkt (C) found in membranes in response to cadherin homophilic ligation.
If PI3-kinase acts downstream of c-Src to support cadherin function, this further predicts that restoration of PI3-kinase activity should overcome the functional effects of c-Src inhibition. To test this notion, we transiently expressed a membrane-tethered form of the p110 catalytic subunit (p110-CAAX) that has been shown to activate PI3-kinase signaling in earlier studies (Pang et al., 2005). We found that transient expression of p110-CAAX increased the E-cadherin detectable at cell–cell contacts in PP2-treated cells (Figure 8A). Contacts between p110-CAAX–transfected cells had an approximately threefold increase in E-cadherin staining compared with contacts between untransfected cells in the same cultures (Figure 8B). Transient expression of p110-CAAX also affected the ability of cells to spread on cadherin-coated substrata (Figure 8, C and D). Although PP2-treated cells expressing EGFP alone did not spread upon binding to hE/Fc, p110-CAAX restored the ability of cells to spread despite treatment with PP2 (Figure 8, C and D). Only cells expressing p110-CAAX displayed broad actin-rich lamellipodia similar to those found in control untreated and PP3-treated cells (Figure 8C). Together these data suggested that PI3-kinase signaling could restore the functional defects that occur when c-Src signaling is blocked.
Figure 8.
PI3-kinase signaling can restore E-cadherin function in c-Src inhibited cells. (A and B) Expression of the constitutively active (CA) PI3-kinase (p110-CAAX) rescues the integrity of c-Src-inhibited cadherin-based contacts. p110-CAAX was transiently expressed in untreated, PP3- and PP2-treated MCF-7 monolayers. After fixation, the monolayers were probed for E-cadherin and anti-myc (Tag) to identify p110-CAAX–expressing cells (A). E-cadherin fluorescence intensity at contacts between untransfected cells (U-U) and cells expressing p110-CAAX (arrowheads, T-T) was quantitated by digital image analysis (B). Data are means ± SEM (n = 30). (C and D) Constitutively active p110-CAAX restores the ability of PP2-treated cells to spread on hE/Fc. Cells transiently transfected with either p110-CAAX or GFP alone were incubated with either PP2 (10 μM) or PP3 (10 μM) and allowed to adhere to hE/Fc-coated substrata. Cells were then stained for either F-actin (Actin) or GFP/Myc (Tag) (C). Cell spreading was quantitated by measuring the surface area of adherent cells (D). Note that expression of p110-CAAX restored the ability of PP2-treated cells to spread on hE/Fc to levels comparable to negative controls (Untreated, GFP) as well as after PP2 was withdrawn (PP2+Washout). Data are means ± SEM (n = 30).
DISCUSSION
It has long been recognized that c-Src and other SFKs prominently accumulate at cell–cell adhesions. The signaling pathways in which these participate, and what their functional consequences might be, remain to be fully understood. Three lines of evidence in our current study now identify c-Src as the target of a cadherin-activated cell signaling pathway that is stimulated by ligation of the adhesion receptor. Using phospho-specific antibodies directed against a key tyrosine residue in the active site (Y419 in human c-Src, Y416 in mouse c-Src) that is necessary for catalytic activation of the enzyme (Cooper et al., 1986; Parsons and Weber, 1989), we found that c-Src is activated as cells make contacts with one another. This was effectively reduced by E-cadherin function-blocking antibodies, indicating that E-cadherin adhesion is necessary for c-Src to be activated as cells form contacts with one another. Cadherin function-blocking antibodies also reduced the level of activated Src detectable at contacts in established monolayers. Productive cadherin adhesion was therefore necessary for the maintenance, as well as the induction, of active c-Src at cell–cell contacts.
Such cadherin-dependent Src signaling might arise either as a response to E-cadherin adhesion itself or via juxtacrine mechanisms that require adhesion to bring cell surfaces together, but where other receptors are responsible for activating c-Src (Yap and Kovacs, 2003). It was therefore noteworthy that ligation of cellular E-cadherin alone could stimulate c-Src activity, both in MCF7 cells and hE-CHO cells, as demonstrated by a clear rise in pY416/419 c-Src levels when cells bound to hE/Fc-beads. Similarly, Fukuyama et al. (2006) observed that pY416-Src readily accumulated at contacts between cells and cadherin-coated beads. This implies that ligation of the adhesion receptor is sufficient to activate c-Src and identifies c-Src as one of several membrane-localized signals that can respond to the E-cadherin adhesion receptor. The precise molecular mechanism has yet to be characterized, although our findings indicate that the β-catenin–binding region of the cadherin cytoplasmic tail is necessary for c-Src activation. Although not necessarily excluding possible contributions from other receptors found at native cell–cell contacts (McLachlan and Yap, 2007), our findings further imply that the active c-Src readily detectable at cell–cell contacts may arise in response to E-cadherin adhesion itself.
What then may be the functional consequences of such cadherin-activated c-Src signaling? Here we found that inhibiting Src consistently reduced cadherin function, as measured by several parameters. Thus, blocking c-Src signaling in established monolayers reduced the accumulation of E-cadherin at cell–cell contacts. Consistent with this, adhesion of cells to cadherin-coated substrata was substantially weakened by PP2. Furthermore, the ability of cells to spread on cadherin-coated substrata, a process that entails cytoskeletal reorganization and cadherin redistribution in response to cadherin ligation, was reduced by blocking c-Src signaling. Because these are all contexts where we also observed cadherin-dependent c-Src signaling, this implies that cadherin-activated c-Src signaling makes a positive contribution to cadherin function.
Although consistent with several earlier studies that identified a positive contribution of SFK signaling to cadherin adhesion and cell–cell integrity (Takahashi et al., 1996; Calautti et al., 1998), these data contrast with many other reports that clearly documented a negative effect of Src signaling on cadherin function. Thus, overexpression of viral (v)-Src (pp60v-Src) in cells completely perturbed intercellular adherens junctions in cultured chick lens epithelial cells (Volberg et al., 1991) and uncoupled junctional complexes between Madin-Darby canine kidney (MDCK) cells (Warren and Nelson, 1987). Moreover, transformation with v-Src increases tyrosine phosphorylation of the cadherin–catenin complex (Matsuyoshi et al., 1992; Behrens et al., 1993), a process that was accompanied by E-cadherin becoming diffusely distributed throughout the cells, instead of concentrating at cell–cell contacts (Behrens et al., 1993). Indeed, such observations led to the suggestion that increased tyrosine phosphorylation of the cadherin–catenin complex by Src might cause cell–cell adhesive contacts to become unstable. We found that stimulation of Src signaling produced similar effects in our experimental system. Thus, expression of the constitutively active c-Src-Y527F mutant disrupted the integrity of MCF7 cell–cell contacts and caused cells to acquire a spindle-like mesenchymal morphology, changes similar to those reported with this and other constitutively active SFK mutants (Frame, 2004). Moreover, we found that expression of c-SrcY527F-GFP significantly perturbed the ability of these cells to form and extend adhesive contacts on cadherin substrata. Clearly, then, SFK signaling can both positively and negatively affect cadherin function.
How might this disparity be resolved? One attractive possibility is that SFK signals may have either positive or negative effects on cadherin function, depending on the strength of the signal. This might be inferred from earlier studies, but they are not directly comparable. Reports documenting a positive impact of SFK signaling on cadherin function generally used loss-of-function approaches (Takahashi et al., 1996, 2005; Calautti et al., 1998), whereas negative effects of SFK signaling have generally been identified when constitutively active SFK mutants were overexpressed in cells, as a gain-of-function approach (Warren and Nelson, 1987; Volberg et al., 1991; Matsuyoshi et al., 1992; Behrens et al., 1993).
Our current study provides direct evidence for this hypothesis as we found that the ability of serum-starved cells to spread on cadherin-coated substrata varied in a bell-shaped pattern with the level of CA c-Src. Cell spreading first increased with the level of CA c-Src, then progressively fell. Therefore modulating the single parameter of transgene expression has a biphasic impact on cadherin-based cell spreading. It implies that quantitative changes in signal strength may have qualitatively different effects on cadherin function. The positive contribution of SFK signaling to cadherin function may then correspond to the lower range of SFK signal strength, whereas supraphysiologic Src signaling may be principally responsible for inhibiting cadherin function. We hasten to add, however, that these test-of-principle experiments measured only one parameter of cadherin function and cannot be directly extrapolated to measures of Src signaling in native cell–cell contacts, as they do not distinguish between the impact of signal intensity and signal duration on cadherin function. Moreover, it is possible that the inhibitory effect of high CA c-Src may reflect differences in the target proteins phosphorylated at high signal strength (Vojtechova et al., 2004). These are clearly important issues for future work.
We therefore propose that c-Src serves as one of several key cellular signals that are recruited to, and activated at, cell–cell adhesions in response to ligation of the E-cadherin receptor. Activated c-Src may then support cadherin function through a signaling cascade that ultimately controls processes such as cadherin accumulation and cytoskeletal reorganization. Several observations suggest that PI3-kinase is a downstream component of this signaling cascade. Not only does inhibiting PI3-kinase affect cadherin function in a manner similar to blocking SFK signaling (Kovacs et al., 2002a), but in the current study we found that restoring PI3-kinase could overcome the functional effects of SFK blockade. This latter observation does not, in itself, exclude the possibility that PI3-kinase and c-Src may operate in parallel pathways. However, we also found that inhibitors of SFK signaling blocked the ability of E-cadherin adhesion to activate PI3-kinase. Taken with our earlier observation that c-Src is necessary for homophilic adhesive ligation to recruit PI3-kinase into cadherin adhesions (Pang et al., 2005), this indicates that c-Src is necessary both for E-cadherin to recruit PI3-kinase to the membrane and also for the subsequent activation of a well-characterized downstream signaling pathway. This is consistent with the more general paradigm that SFK signaling can critically link cell surface receptors into signaling pathways with PI3-kinase (Wymann and Pirola, 1998; Cantrell, 2001). Overall, then, our findings suggest strongly that PI3-kinase is functionally downstream of SFK in an E-cadherin–activated signaling pathway necessary for cadherin function. Whether this reflects a role for SFK in regulating binding of PI3-kinase to the cadherin/catenin complex and/or catalytic activation of PI3-kinase by SFK remains to be determined.
PI3-kinase may not, however, be the only target for cadherin-activated Src signaling as other potential SFK substrates, such as p120-ctn, β-catenin, and cortactin are found at cell–cell adhesions (Behrens et al., 1993; Mariner et al., 2001; Helwani et al., 2004). Whether these are tyrosine phosphorylated under physiological circumstances, and what effect this has on cadherin function, remain to be determined. More generally, our findings add to earlier observations that highlight how important stringent regulation of SFK signaling is in controlling cadherin adhesion and cell–cell interactions. The duration, intensity, and location of signaling by c-Src and its relatives will determine whether SFK positively or negatively regulate cadherin function. It will thus be essential to elucidate the upstream mechanisms that allow cadherin adhesion to activate c-Src and to identify their interplay with other cell signaling pathways, in order to understand how this central signaling molecule determines the fate of cell–cell interactions.
ACKNOWLEDGMENTS
We thank all our colleagues who provided reagents for this work and Prof. Geoff McLachlan for assistance in curve-fitting. As always, our laboratory friends were unstinting in their help, support, and good advice. This work was funded by the National Health and Medical Council (NHMRC) of Australia. A.K. was also funded by a fellowship from the Deutsche Forschungsgemeinschaft, R.McL. holds a Queensland Cancer Fund PhD Scholarship, F.M.H. received an Australian Post-graduate Award, and A.S.Y. is an NHMRC Senior Research Fellow and a Research Affiliate of the Australian Research Council Special Research Centre for Functional and Applied Genomics, which provided infrastructure support for this work.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1154) on June 6, 2007.
REFERENCES
- Avizienyte E., Wyke A. W., Jones R. J., McLean G. W., Westhoff M. A., Brunton V. G., Frame M. C. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E., Cabodi S., Stein P. L., Hatzfeld M., Kedersha N., Paolo Dotto G. Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion. J. Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E., Grossi M., Mammucari C., Aoyama Y., Pirro M., Ono Y., Li J., Dotto G. P. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J. Cell Biol. 2002;156:137–148. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A. Phosphoinositide 3-kinase signalling pathways. J. Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Frame M. C. Newest findings on the oldest oncogene; how activated src does it. J. Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- Fukuyama T., Ogita H., Kawakatsu T., Inagaki M., Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25:8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- Goodwin M., Kovacs E. M., Thoreson M. A., Reynolds A. B., Yap A. S. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J. Biol. Chem. 2003;278:20533–20539. doi: 10.1074/jbc.M213171200. [DOI] [PubMed] [Google Scholar]
- Helwani F. M., Kovacs E. M., Paterson A. D., Verma S., Ali R. G., Fanning A. S., Weed S. A., Yap A. S. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. M., Ali R. G., McCormack A. J., Yap A. S. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 2002a;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- Kovacs E. M., Goodwin M., Ali R. G., Paterson A. D., Yap A. S. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 2002b;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- Mariner D. J., Anastasiadis P. Z., Keilhack H., Bohmer F.-D., Wang J., Reynolds A. B. Identification of Src phosphorylation sites in the catenin p120ctn. J. Biol. Chem. 2001;276:28006–28013. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- Matsuyoshi N., Hamaguchi M., Taniguchi S., Nagafuchi A., Tsukita S., Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J. Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan R. W., Yap A. S. Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J. Mol. Med. 2007;85:545–554. doi: 10.1007/s00109-007-0198-x. [DOI] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Pang J. H., Kraemer A., Stehbens S. J., Frame M. C., Yap A. S. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signaling to E-cadherin function. J. Biol. Chem. 2005;280:3043–3050. doi: 10.1074/jbc.M412148200. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr. Top. Microbiol. Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Paterson A. D., Parton R. G., Ferguson C., Stow J. L., Yap A. S. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- Pece S., Chiariello M., Murga C., Gutkind J. S. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J. Biol. Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Shewan A. M., Maddugoda M., Kraemer A., Stehbens S. J., Verma S., Kovacs E. M., Yap A. S. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehbens S. J., Paterson A. D., Crampton M. S., Shewan A. M., Ferguson C., Akhmanova A., Parton R. G., Yap A. S. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J. Cell Sci. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- Takahashi F., Endo S., Kojima T., Saigo K. Regulation of cell-cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev. 1996;10:1645–1656. doi: 10.1101/gad.10.13.1645. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Takahashi F., Ui-Tei K., Kojima T., Saigo K. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development. 2005;132:2547–2559. doi: 10.1242/dev.01850. [DOI] [PubMed] [Google Scholar]
- Takata K., Singer S. J. Phosphotyrosine-modified proteins are concentrated at the membranes of epithelial and endothelial cells during tissue development in chick embryos. J. Cell Biol. 1988;106:1757–1764. doi: 10.1083/jcb.106.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Thoreson M. A., Anastasiadis P. Z., Daniel J. M., Ireton R. C., Wheelock M. J., Johnson K. R., Hummingbird D. K., Reynolds A. B. Selective uncoupling of p120ctn from E-cadherin disrupts strong adhesion. J. Cell Biol. 2000;148:189–201. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson P., Jones G. E., Frame M. C., Brunton V. G. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Akiyama T., Yamanashi Y., Yamamoto T., Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J. Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Shewan A. M., Scott J. A., Helwani F. M., den Elzen N. R., Miki H., Takenawa T., Yap A. S. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 2004;279:34062–34070. doi: 10.1074/jbc.M404814200. [DOI] [PubMed] [Google Scholar]
- Vojtechova M., Tuhackova Z., Hlavacek J., Velek J., Sovova V. The v-Src and c-Src tyrosine kinases immunoprecipitated from Rous sarcoma virus-transformed cells display different peptide substrate specificities. Arch. Biochem. Biophys. 2004;421:277–282. doi: 10.1016/j.abb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Volberg T., Geiger B., Dror R., Zick Y. Modulation of intercellular adherens-type junctions and tyrosine phosphorylation of their components in RSV-transformed cultured chick lens cells. Cell Regul. 1991;2:105–120. doi: 10.1091/mbc.2.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. L., Nelson W. J. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol. Cell. Biol. 1987;7:1326–1337. doi: 10.1128/mcb.7.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watton S. J., Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 1999;9:433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- Wymann M. P., Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- Yap A. S., Brieher W. M., Pruschy M., Gumbiner B. M. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr. Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Yap A. S., Kovacs E. M. Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]