Abstract
Epithelial-mesenchymal transition (EMT) events occur during embryonic development and are important for the metastatic spread of epithelial tumors. We show here that spontaneous differentiation of mouse embryonic stem (ES) cells is associated with an E- to N-cadherin switch, up-regulation of E-cadherin repressor molecules (Snail and Slug proteins), gelatinase activity (matrix metalloproteinase [MMP]-2 and -9), and increased cellular motility, all characteristic EMT events. The 5T4 oncofetal antigen, previously shown to be associated with very early ES cell differentiation and altered motility, is also a part of this coordinated process. E- and N-cadherin and 5T4 proteins are independently regulated during ES cell differentiation and are not required for induction of EMT-associated transcripts and proteins, as judged from the study of the respective knockout ES cells. Further, abrogation of E-cadherin–mediated cell–cell contact in undifferentiated ES cells using neutralizing antibody results in a reversible mesenchymal phenotype and actin cytoskeleton rearrangement that is concomitant with translocation of the 5T4 antigen from the cytoplasm to the cell surface in an energy-dependent manner. E-cadherin null ES cells are constitutively cell surface 5T4 positive, and although forced expression of E-cadherin cDNA in these cells is sufficient to restore cell–cell contact, cell surface expression of 5T4 antigen is unchanged. 5T4 and N-cadherin knockout ES cells exhibit significantly decreased motility during EMT, demonstrating a functional role for these proteins in this process. We conclude that E-cadherin protein stabilizes cortical actin cytoskeletal arrangement in ES cells, and this can prevent cell surface localization of the promigratory 5T4 antigen.
INTRODUCTION
Epithelial-mesenchymal transition (EMT) is important for both normal embryo development and tumor cell invasion (Cavallaro and Christofori, 2004a). Typically, EMT involves loss of epithelial cell–cell contact and acquisition of a mesenchymal phenotype, with loss of Cadherin-1 (E-cadherin), a defining characteristic of this process (Cavallaro and Christofori, 2004a; Bates and Mercurio, 2005). E-cadherin is expressed on epithelial cells where its extracellular domain interacts in a homotypic calcium-dependent manner with E-cadherin molecules on neighboring cells to form cell–cell adherens junctions (Cavallaro and Christofori, 2004a). E-cadherin is required for embryogenesis because E-cadherin null embryos fail to develop beyond the blastocyst stage (Larue et al., 1996). The first EMT event during development requires loss of cell surface E-cadherin and gain of Cadherin-2 (N-cadherin) in epiblast cells to facilitate their ingression within the primitive streak (Cavallaro and Christofori, 2004a; Zohn et al., 2006). Similarly, loss of E-cadherin during cancer progression is associated with gain of mesenchymal phenotype and the metastasis of primary tumor cells to secondary sites within the body, the main cause of death in patients (Cavallaro and Christofori, 2004a; Seidel et al., 2004).
Ingression of epiblast cells into the primitive streak is associated with expression of the E-cadherin repressor proteins Snail, Slug, and SIP1 (Nieto et al., 1994; Rhim et al., 1997; Sheng et al., 2003). Similarly, Slug expression has been observed during chick epiblast ingression into the primitive streak and in neural crest cells before their emergence from the neural tube (Nieto et al., 1994). Snail genes have been observed in all EMT processes studied to date (Nieto, 2002; Barrallo-Gimeno and Nieto, 2005) and a common role for Slug, Snail, and SIP1 is binding to the E-cadherin promoter resulting in gene transcript repression (Si et al., 2001; Bolos et al., 2003; Cavallaro and Christofori, 2004a). As well as their function as regulators of cell adhesion, Snail and Slug are potent survival factors. For example, Slug expression is associated with resistance to chemotherapeutic agents in mesotheliomas and induces cell survival in leukemias (Barrallo-Gimeno and Nieto, 2005).
Concomitant with the loss of cell-surface E-cadherin during EMT is increased cellular motility and invasion, which require altered matrix recognition and adhesion, up-regulation of promigratory molecules, and expression of extracellular proteases (Cavallaro and Christofori, 2004a). Altered cellular matrix recognition and adhesion is achieved, in part, by changes in the expression and cellular localization of integrins and members of the cadherin family (Cavallaro and Christofori, 2004a). For example, N-cadherin promotes migration and invasion of cells and is up-regulated on breast, prostate, bladder, thyroid, and squamous cell carcinomas (Derycke and Bracke, 2004). The MMP family, in particular MMP-2 and -9, are known to influence invasion during development and metastasis (Itoh et al., 1999; Paquette et al., 2003; Kim et al., 2004; Samantaray et al., 2004; Bjorklund and Koivunen, 2005). These proteins function by degrading extracellular matrix/cell surface proteins and releasing stored promigratory factors, allowing movement of the cells within tissues. Thus, EMT requires the correct temporal expression, interaction, and modification of a range of cellular and extracellular factors to allow motility and invasion to proceed. However, the precise temporal regulation of EMT events during development and tumor progression are difficult to assess because of the lack of a natural in vitro model system of EMT (Cavallaro and Christofori, 2004a).
Embryonic stem (ES) cells are pluripotent cells derived from the epiblast of preimplantation embryos and are an excellent model system for elucidating mechanisms involved in development and disease (Smith, 2001). Recently, we demonstrated that spontaneous differentiation of mouse and human ES cells is associated with the expression of the 5T4 oncofetal antigen (Ward et al., 2003, 2006), a glycoprotein that correlates with poorer clinical outcome in colorectal, gastric, and ovarian carcinomas (Wrigley et al., 1995; Starzynska et al., 1992, 1994, 1997; Naganuma et al., 2002). Forced expression of 5T4 in epithelial cells results in down-regulation of membranous E-cadherin and alterations in the actin cytoskeleton (Carsberg et al., 1996). Therefore, we hypothesized that upon ES cell differentiation, mechanisms associated with altered cellular motility and invasion would occur, with loss of E-cadherin critical for this event.
In this study we demonstrate that spontaneous differentiation of mouse ES cells is associated with an E- to N-cadherin cell surface protein and transcriptional switch. We have investigated whether this process exhibits characteristics of the EMT process, such as, up-regulation of the E-cadherin repressors, characteristic changes in morphology associated with altered cytoskeletal arrangement, and capacity for invasion through increased matrix metalloproteinase activity and cellular motility. In addition we have assessed the roles of E- and N-cadherin and the 5T4 antigen during the EMT process. We have identified a novel role for E-cadherin in the inhibition of the plasma membrane localization of the promotility 5T4 antigen and show that 5T4 and N-cadherin are required for motility of differentiating ES cells. Furthermore, our data demonstrate that mouse ES cells are a useful in vitro model for investigating the role of EMT events related to both embryogenesis and epithelial tumor metastasis.
MATERIALS AND METHODS
Mouse ES Cell Culture
MESC20, D3, E-cadherin null (Larue et al., 1996), N-cadherin null (Radice et al., 1997), 5T4-GFP (Perez-Campo, Spencer, Stern, and Ward, unpublished data), and 5T4 null mouse ES cells were cultured on gelatin-treated plates in knockout DMEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, nonessential amino acids (100×, 1:100 dilution), 50 μM 2-mercaptoethanol (all from Invitrogen, Paisley, United Kingdom), and 1000 U/ml leukemia inhibitory factor (ESGRO; Chemicon International, Middlesex, United Kingdom; FCS+LIF) at 37°C/5% CO2. The medium was replenished every 24 h, and cells were passaged before confluence. ES cells were differentiated for various periods of time by culture in knockout DMEM supplemented with 10% serum replacement (SR) in the absence of LIF (−LIF; Ward et al., 2003) or fetal bovine serum (FBS)-supplemented medium lacking LIF (motility assays). Gelatin-treated plates were prepared as described previously (Ward et al., 2003). All ES cell lines exhibited a normal karyotype.
Antibody-induced Loss of Cell–Cell E-Cadherin Contacts
E-cadherin–mediated cell–cell contacts were abrogated by culture of D3 or MESC20 ES cells in 5.8 μg/ml and 5T4 null ES cells in 23.2 μg/ml IgG component of rat anti-E-cadherin DECMA-1 ascites solution (Sigma, Dorset, United Kingdom) for various times. Rat anti-Tenascin Ab was used as a control in all experiments at the same concentrations as above (Sigma).
Immunofluorescence Imaging of ES Cells
Mouse ES cells were cultured on 9-cm gelatin-treated tissue culture grade dishes, fixed in 4% paraformaldehyde, and stained in situ, as previously described (Barrow et al., 2006). Primary antibodies were as follows: rat anti-mouse 5T4 mAb9A7 (10 μg/ml; Woods et al., 2002); mouse anti-OCT-4 (1:100 dilution); rabbit anti-OCT-4 (1:100 dilution); rabbit anti-N-cadherin (1:100 dilution), mouse anti-E-cadherin (1:100 dilution); goat anti-Slug (1:100 dilution); and rabbit anti-Snail (1:100 dilution; all Santa Cruz Biotechnology, Santa Cruz, CA). Actin cytoskeleton was detected using phalloidin-Texas red conjugate (1:1000 dilution; Sigma). Secondary antibodies conjugated with Alexa Fluor 488 or 546 were used (1:500 dilution, Molecular Probes, Eugene, OR), and all samples were mounted using DAPI Vector shield (Vector, Peterborough, United Kingdom). The cells were viewed on an Olympus BX51 fluorescence microscope (Lake Success, NY). Images were overlaid using Adobe Photoshop (version 6.0, San Jose, CA).
Fluorescent Flow Cytometry Analysis of ES Cells
ES cells were trypsinized, washed once in 900 μl of phosphate-buffered saline (PBS), and resuspended in 100 μl of 0.2% bovine serum albumin (BSA) in PBS (fluorescence-activated cell sorting [FACS buffer]) containing the primary antibody. Primary antibodies were as follows: rat anti-mouse E-cadherin (DECMA-1; 1:500 dilution; Sigma), rabbit anti-mouse 5T4 (1:500 dilution; for use in DECMA-1–treated cells; Woods et al., 2002); rabbit anti-N-cadherin (1:100 dilution, Santa Cruz), rat anti-mouse 5T4 mAb9A7 (10 μg/ml; Woods et al., 2002), anti-mouse NCAM (1:100 dilution); anti-mouse SSEA-1 (1:100 dilution); anti-mouse β1-integrin (1:100 dilution); anti-FGFR1 (1:100 dilution), or isotype controls (all Santa Cruz) and incubated for 1 h on ice. Cells were washed once in 900 μl of PBS, resuspended in 100 μl of FACS buffer containing the appropriate phycoerythrin-conjugated secondary antibody (all 1:100 dilution; Santa Cruz), and incubated for 30 min on ice. The cells were washed once in 900 μl of PBS and fixed in 400 μl of 1% formaldehyde. Cell fluorescence was analyzed using a Becton Dickinson FACScaliber. Viable cells were gated using forward and side scatter, and all data represent cells from this population. Dual staining of E- and N-cadherin was performed as above except primary antibodies recognizing both of these proteins were added to the cell suspension followed by phycoerythrin (PE)-conjugated anti-N-cadherin and FITC-conjugated anti-E-cadherin primary antibodies.
FACS
ES cells were differentiated for 3 d in the absence of LIF in SR-supplemented medium, trypsinized, washed once in 900 μl of PBS, and resuspended in 500 μl of 0.2% BSA in PBS (FACS buffer) containing rat anti-mouse E-cadherin antibody (DECMA-1; 1:500 dilution; Sigma). The cell suspension was incubated on ice for 20 min. Cells were then washed once in 900 μl of PBS and resuspended in 500 μl of FACS buffer containing PE-conjugated anti-rat IgG antibody (1:100 dilution). The cell suspension was incubated on ice for 20 min and washed once in 900 μl of PBS, and the cells were resuspended in 1 ml of culture medium. Viable cells were gated using forward and side scatter on a Becton-Dickinson FACSvantage (Mountain View, CA) and the 20% most E-cadherin–negative and 20% most E-cadherin–positive cells were isolated (a total of 105 cells for each population).
RT-PCR
Total RNA was extracted from cells using RNAzol B according to the manufacturer's instructions (Biogenesis, Poole, Dorset, United Kingdom) and treated with DNase (Promega, Madison, WI), and phenol/chloroform was extracted. Synthesis of cDNA was performed as described previously (Ward et al., 2003). RT-PCR was performed using 1 μl of the cDNA solution and 35 or 45 cycles. Samples were run on 2% agarose gels containing 400 ng/ml ethidium bromide and visualized using an Epi Chemi II Darkroom and Sensicam imager with Labworks 4 software (UVP, San Gabriel, CA). See Table 1 for primer sequences.
Table 1.
List of primer sequences used in this study (all 60°C annealing)
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| β-Tubulin | GGAACATAGCCGTAAACTGC | TCACTGTGCCTGAACTTACC | 317 |
| Oct-4 | AGAAGGAGCTAGAACAGTTTGC | CGGTTACAGAACCATACTCG | 415 |
| E-cadherin | CGAGAGAGTTACCCTACATA | GTGTTGGGGGCATCATCATC | 214 |
| N-cadherin | CCCAAGTCCAACATTTCCATCC | AAAGCCTCCAGCAAGCACG | 781 |
| Snail | CAGCTGGCCAGGCTCTCGGT | GCGAGGGCCTCCGGAGCA | 843 |
| Slug | CACTCCACTCTCCTTTACC | CAGACTCCTCATGTTTATGC | 597 |
| SIP1 | CGTTCAAACACAAACACC | CCAGTCTCTTCCTCATCC | 454 |
| E12/E47 | GAGGAGTGGCCTCACAAGTGG | GTGCGTGGGACCTTCAGGT | 232 |
| MMP-2 | TGGGTGGAAATTCAGAAGGTGC | ATCTACTTGCTGGACATCAGGGGG | 684 |
| MMP-9 | TGCGACCACATCGAACTTCG | CCAGAGAAGAAGAAAACCCTCTTGG | 686 |
| TIMP-1 | CTTGCATCTCTGGCATCTGG | AAGTAGACAGTGTTCAGGC | 652 |
| TIMP-2 | GAGATCAAGCAGATAAAGATG | GACCCAGTCCATCCAGAGGC | 320 |
Western Blotting
Cell lysates were extracted, separated on reduced SDS-PAGE, and transferred onto nitrocellulose membrane as described previously (Ward et al., 2003). The membrane was probed using rat anti-E-cadherin (1:200 dilution; DECMA-1; Sigma) or rabbit anti-N-cadherin (1:200 dilution; Santa Cruz) followed by horseradish peroxidase–conjugated immunoglobulins (DAKO, Cambs, United Kingdom) against the primary antibody. The membrane was developed by enhanced chemiluminescence (Amersham Pharmacia, Newcastle upon Tyne, United Kingdom) and visualized using Kodak film (Eastman Kodak, Rochester, NY). Western blot images were captured using an Epi Chemi II Darkroom and Sensicam imager with Labworks 4 software (UVP).
Zymogram Analysis
MESC20 and 5T4−/− mouse ES cells were cultured in synthetic serum-containing medium in the presence of LIF (undifferentiated) or in the absence of LIF in synthetic serum-containing medium for various times. Protease activity was determined using a precast 10% gelatin gel (Bio-Rad, Richmond, CA) and visualized according to the manufacturer's instructions. In brief, at the desired time points 10 μl of culture medium was removed from the cells and mixed with 40 μl of loading buffer, and the samples were separated on a gelatin zymogram gel at 100 V for 90 min. The gel was renatured for 1 h, developed overnight, and stained with Coomassie R-250 (all reagents from Bio-Rad). Images were captured using an Epi Chemi II Darkroom and Sensicam imager with Labworks 4 software (UVP).
Forced Expression of E-Cadherin cDNA in E-cad−/− ES Cells
E-cadherin null ES cells were cultured as described above, trypsinized, and washed twice in PBS. Cells were electroporated as described in the manufacturer's instructions using an Amaxa Biosystems NucleofectorII and ES cell electroporation kit (Amaxa Biosystems, Köln, Germany). Briefly, 2 × 106 E-cadherin null ES cells were suspended in Amaxa ES cell solution and either pCMVα or pCMVα-E-cadherin vectors (2 μg total plasmid) and electroporated using program A-30 on the Amaxa NucleofectorII. Cells were plated out in a single well of a gelatinized six-well plate in ES cell medium and assessed for cell surface expression of either E-cadherin or 5T4 using fluorescent flow cytometry analysis.
Isolation of 5T4 Null ES Cells
5T4−/− mice were isolated using ES cells in which the entire coding region (exon 2) of a single 5T4 allele was replaced by homologous recombination with a vector containing a LacZ reporter and a neomycin resistance gene (Ward and Stern, unpublished data). 5T4−/− ES cells were isolated from delayed implantation 5T4−/− blastocysts (obtained from homozygous 5T4−/− mouse mating) as previously described (Evans and Hunter, 2002). Briefly, isolated blastocysts were cultured individually on an irradiated mouse embryonic fibroblast feeder layer in defined medium (knockout DMEM, 10% ES-Cult fetal bovine serum, 1% nonessential amino acids, 2 mM l-glutamine, 50 μM β-mercaptoethanol, and LIF at 5.5 × 106 units/500 ml media). Four to 7 d after initial plating, outgrowths were dissociated with 0.25% trypsin/0.2% EDTA and replated on fresh feeder layers to allow the formation of ES cell colonies. Colonies were then passaged on gelatin-treated tissue culture grade dishes for three passages before genotyping and karyotyping.
Genotyping of 5T4 Null ES Cells
DNA was extracted from a near confluent culture of ES cell in a 75-cm2 flask using a Qiagen DNA extraction kit (Crawley, West Sussex, United Kingdom). From a 1:100 dilution of the DNA extract, 1 μl was used to perform PCR as follows: PCR Reddy mix (12.5 μl: ABgene, Surrey, United Kingdom), ddH2O (8.5 μl), 5T4 sense primer (1 μl), 5T4 anti-sense primer (1 μl), and neomycin primer (1 μl). Primer sequences (5′ to 3′) were as follows: 5T4 Sense, GCA TAG GAG CAC CTG CAT CCA; 5T4 Antisense, GAC AAA GTG GCA GCT GTG ACA TG; and Neomycin, TGA TAT TGC TGA AGA GCT TGG. PCR products were separated on a 2% agarose gel containing 400 ng/ml ethidium bromide and visualized using an Epi Chemi II Darkroom and Sensicam imager with Labworks 4 software (UVP). Wild-type 5T4 allele was representative of a band of 590 base pairs, whereas the 5T4 null product was 800 base pairs.
Cell Motility Assay
Costar Transwell 24-well plates exhibiting 5-μm pore size were used for all motility assays (Cambridge, MA). The transwells were immersed in gelatin solution overnight (0.1% in PBS) and rinsed in PBS. Transwells were blocked in fetal calf serum (FCS)-containing ES cell medium for 30 min at 37°C/5% CO2 and washed in PBS. ES cells were cultured on gelatin-treated plates for 3 d in the presence or absence of LIF. These cells were then trypsinized and resuspended in culture medium (1 × 105 cells/ml), and 100 μl of this suspension was added to the transwell plates and incubated overnight at 37°C/5% CO2. The transwell was washed gently in PBS, and cells were removed from within the transwell using a dry cotton bud followed by two washes in PBS. This washing procedure was repeated twice. The transwell was stained with crystal violet for 10 min, washed in water, and allowed to air dry. Cells present on the underside of the transwell (i.e., migrated cells) were counted by microscopy. The number of cells on the bottom of the plate (i.e., cells that had migrated through the pores and become detached from the transwell) was also counted. P values were calculated using unpaired Student's t test.
RESULTS
Mouse ES Cell Differentiation Is Associated with an E- to N-Cadherin Switch
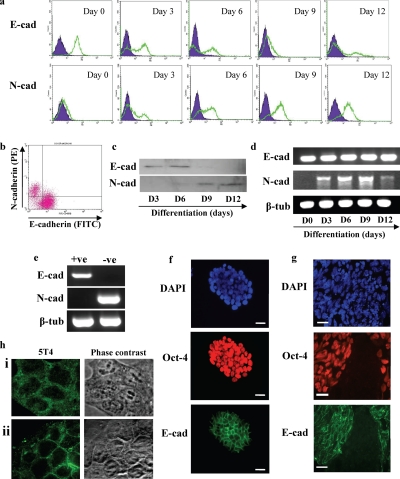
Expression of E-cadherin was assessed in undifferentiated MESC20 ES cells by fluorescent flow cytometry (Figure 1a, E-cad, day 0), with over 95% of the population expressing cell surface protein. In contrast, undifferentiated MESC20 ES cells lacked cell surface N-cadherin expression (Figure 1a, N-cad, day 0). On differentiation of MESC20 ES cells after LIF withdrawal in serum replacement medium, E-cadherin was no longer detected at the cell surface on an increasing proportion of the population labeled after 3, 6, and 9 d (Figure 1A, E-cad), and this correlated reciprocally with up-regulation of cell surface N-cadherin (Figure 1a, N-cad). After 12 d, cell surface E-cadherin was absent from the majority of the population, with increased levels detected on a small proportion of the cells (Figure 1a, E-cad, day 12). This may reflect expansion of differentiated epithelial cells or some residual pluripotent ES cell colonies by this time. Expression of cell surface N-cadherin protein remained high at day 12 (Figure 1a, N-cad, day 12), with the proportion of cells staining positive similar to that observed at day 9.
Figure 1.
Mouse ES cell spontaneous differentiation is associated with loss of cell surface E-cadherin protein and gain of cell surface N-cadherin protein. MESC20 ES cells were maintained in an undifferentiated state by culture in ES cell medium containing LIF and differentiated in synthetic serum in the absence of LIF in monolayer culture. (a) Cell surface E-cadherin (E-cad) and N-cadherin (N-cad) proteins were assessed in undifferentiated MESC20 ES cells (day 0) and differentiated cells for 3, 6, 9, and 12 d by fluorescent flow cytometry in a Becton-Dickinson FACScaliber. E- or N-cadherin, open population; isotype control antibodies, closed population. (b) Fluorescent flow cytometry dual staining for E- and N-cadherin on MESC20 ES cells differentiated for 3 d as described above. (c) Western blot was performed to assess total cellular E- (E-cad) or N-cadherin (N-cad) proteins in MESC20 ES cells differentiated for 3, 6, 9, and 12 d as described above. (d) RT-PCR analysis of E- (E-cad) and N-cadherin (N-cad) and β-tubulin (β-tub; control) transcript expression was assessed in undifferentiated MESC20 ES cells (day 0) and in cells differentiated for 3, 6, 9, and 12 d as described above. (e) MESC20 ES cells were differentiated for 3 d as described above and E-cadherin–positive (+ve) and E-cadherin–negative (−ve) cells isolated by FACS. RT-PCR was performed on the samples to assess E- (E-cad) and N-cadherin (N-cad) and β-tubulin (β-tub) transcript expression. (f) Immunofluorescence microscopy analysis of total cells (DAPI) and E-cadherin (E-cad) and OCT-4 protein expression in undifferentiated MESC20 ES cells. Bar, 10 μm. (g) MESC20 ES cells were differentiated for 4 d and assessed for total cells (DAPI) and E-cadherin (E-cad) and OCT-4 protein expression using immunofluorescent microscopy. Bar, 10 μm. (h) ES cells were cultured in FCS+LIF (i) and FCS−LIF (ii) for 2 d and 5T4 antigen expression assessed using fluorescent microscopy and phase-contrast microscopy.
To determine whether cell surface expression of E- and N-cadherin proteins was exclusive, we assessed the presence of these cadherins using flow cytometry dual staining in ES cells differentiated for 3 d as described above (Figure 1b). The majority of cells expressed only E-cadherin or N-cadherin at the cell surface, demonstrating that a cell surface E- to N-cadherin switch occurs during ES cell differentiation. Total E- and N-cadherin protein was assessed by Western blot analysis of total cell lysates (Figure 1c). Total N-cadherin protein was absent from undifferentiated cells and was detected first at 6 d after differentiation, with increased levels observed up to day 12 of differentiation (Figure 1c, N-cad). In contrast, E-cadherin protein was detected in undifferentiated ES cells but was not detected in the differentiating populations by day 9 and day 12 (Figure 1c, E-cad).
Analysis of transcript expression in the undifferentiated and differentiating mouse ES cell populations was assessed by RT-PCR analysis (Figure 1d). N-cadherin transcripts were absent from undifferentiated ES cells and were rapidly up-regulated upon removal of LIF, with levels peaking at day 9 followed by a reduction of the transcripts at day 12. This is in agreement with the up-regulation of cell surface N-cadherin protein shown in Figure 1, a and b, and the Western blot analysis in Figure 1c. Although E-cadherin protein is substantially down-regulated both at the cell surface and intracellularly after removal of LIF from the ES cells this does not correlate with changes in transcript levels detected by RT-PCR from the entire population. To assess whether E- and N-cadherin transcripts were differentially expressed in the differentiating ES cell population, we isolated E-cadherin–positive (+ve) and E-cadherin–negative (−ve) cells by FACS 3 d after induction of differentiation (Figure 1e). The E-cadherin–positive cell population expressed E-cadherin but lacked N-cadherin transcripts. In contrast, E-cadherin–negative cells lacked transcripts for E-cadherin, whereas N-cadherin transcripts were detected. This data demonstrates that a transcriptional E- to N-cadherin switch occurs in a proportion of the differentiating cells within the population.
Indirect immunofluorescent analysis of E-cadherin (green) and OCT-4 (red) protein expression in undifferentiated MESC20 ES cells demonstrated the presence of cell surface E-cadherin in OCT-4–positive cells (Figure 1f). Culture of MESC20 ES cells for 4 d in the absence of LIF resulted in spontaneous differentiation within discrete areas of the colonies, characterized by absence of both E-cadherin and OCT-4 expression (Figure 1g), demonstrating that loss of E-cadherin protein is associated with differentiation of ES cells (as evidenced by transcript analysis shown in Figure 1e). We have previously demonstrated that the 5T4 oncofetal antigen marker is expressed as an early event in ES cell differentiation (Ward et al., 2003; Barrow et al., 2005). In summary, when mouse ES cells are cultured in the absence of a feeder layer in FCS-supplemented medium, the cells exhibit cytoplasmic localization of the 5T4 antigen (Figure 1hi). On withdrawal of LIF, the ES cell population exhibit cell surface 5T4 (Figure 1hii).
ES Cell Differentiation Is Associated with Up-Regulation of E-Cadherin Repressor Proteins, Matrix Metalloproteinase Proteolytic Activity, and Increased Motility
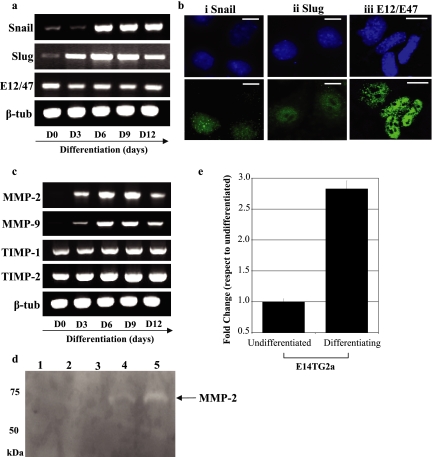
To determine whether down-regulation of cell surface E-cadherin protein upon ES cell differentiation was associated with expression of E-cadherin transcript repressors, we assessed expression of Snail, Slug, and E12/E47 transcripts by RT-PCR (Figure 2a). Mouse ES cells were differentiated in the absence of LIF in serum replacement medium for 12 d as described above, and transcript expression of the E-cadherin repressor molecules was assessed at days 0, 3, 6, 9, and 12 (Figure 2a; 45 PCR cycles). Snail and Slug transcripts were detected at low levels in undifferentiated cells, with Snail and Slug up-regulated after removal of LIF. E12/E47 transcripts were detected at all of the time points assessed during differentiation, with no significant alteration in the levels apparent. Expression of Snail, Slug, and E12/E47 proteins in differentiating mouse ES cells was determined using indirect immunofluorescent microscopy analysis (Figure 2b). Nuclear localization of Snail, Slug, and E12/E47 (Figure 2b, i, ii, and iii, respectively) proteins was observed in a proportion of the differentiating population, demonstrating that these E-cadherin repressor proteins are up-regulated after differentiation of ES cells. Up-regulation of SIP1 transcripts was also detected after differentiation of the cells (data not shown).
Figure 2.
Mouse ES cell differentiation is associated with nuclear localization of the E-cadherin repressor proteins Snail and Slug, matrix metalloproteinase activity, and increased motility. Undifferentiated and differentiating MESC20 ES cells were cultured as described in the legend to Figure 1. (a) Transcript expression of Snail, Slug, E12/E47, and β-tubulin (β-tub; control) was determined by RT-PCR in undifferentiated MESC ES cells (day 0) and in cells differentiated for 3, 6, 9, and 12 d. (b) MESC20 ES cells were differentiated for 6 d and assessed for Snail (i), Slug (ii), and E12/E47 (iii) proteins using immunofluorescent microscopy. DAPI shows the total cells within the field of view. Bar, 5 μm. (c) Transcript expression of matrixmetalloproteinase (MMP)-2 and -9, tissue inhibitor of metalloproteinase (TIMP)-1 and -2 and β-tubulin (β-tub; control) was determined by RT-PCR in undifferentiated and differentiating ES cells. (d) Gelatin zymogram analysis was performed to determine MMP-2 and -9 activity within the culture supernatants of undifferentiated and differentiating MESC20 ES cells. Media control (lane 1); undifferentiated MESC20 ES cells (lane 2); and MESC20 ES cells differentiated for 3 d (lane 3), 5 d (lane 4), and 9 d (lane 5). Arrow shows the size of active MMP-2 (65 kDa). Note that MMP-9 was not detected under these conditions. (e) Cellular motility of undifferentiated and differentiating (3 d in the absence of LIF) wild-type ES cells was assessed using Costar Transwell 5-μm pore size plates. Data represents the fold change in motility compared with undifferentiated cells. Note that cells cultured in the absence of LIF exhibit increased motility compared with undifferentiated cells. Similar results were obtained with D3 and MESC20 ES cells.
To determine whether other EMT-like processes were occurring during ES cell differentiation, we assessed the expression of MMP-2 and -9 and tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 transcripts in MESC20 ES cells (Figure 2c). MMP-2 and -9 transcripts were absent from undifferentiated MESC ES cells and up-regulated 3 d after differentiation. Peak transcript expression of both MMP-2 and -9 occurred at day 9, mirroring the expression of N-cadherin transcripts observed in these cells (see Figure 1d). TIMP-1 and -2 transcripts were detected throughout the differentiation period, and no significant alteration in expression was observed for these transcripts.
To determine MMP proteolytic activity in supernatants from undifferentiated and differentiating mouse ES cells, we utilized gelatin-zymogram analysis (Figure 2d). Gelatin-zymogram analysis provides a simple method to determine gelatinase activity by assessing the degradation of a gelatin substrate within a gel. Gelatinase activity is determined by the absence of staining within the gel compared with MMP control samples. Undifferentiated mouse ES cells lacked any MMP activity when cultured in serum replacement medium in the presence of LIF (Figure 2d, lane 2; lane 1 is medium without ES cells). Serum replacement was used because FBS-supplemented media exhibited significant MMP-2 activity before exposure to the cells (data not shown). Mouse ES cells differentiated using serum replacement medium lacking LIF exhibited no MMP activity at day 3 (lane 3), but proteolytic activity associated with the 65-kDa active form of MMP-2 was detected at days 5 and 9 (lanes 4 and 5, respectively) after induction of differentiation. MMP-9 activity was not observed in differentiating mouse ES cells even though transcripts for this protein were detected. It is possible that TIMP-1 or -2 activity within the supernatant inhibits MMP-9 activity under the conditions described.
The E- to N-cadherin switch during ES cell differentiation, along with up-regulation of E-cadherin repressor proteins and MMP-2, suggested that differentiating ES cells would exhibit increased motility compared with undifferentiated cells. To determine cellular motility during ES cell differentiation, we assessed the ability of undifferentiated and differentiated ES cells to migrate through 5-μm pore size Transwell plates (Figure 2e). Differentiated ES cells exhibited almost threefold increased motility compared with undifferentiated cells, demonstrating that ES cell differentiation is also associated with increased cellular motility.
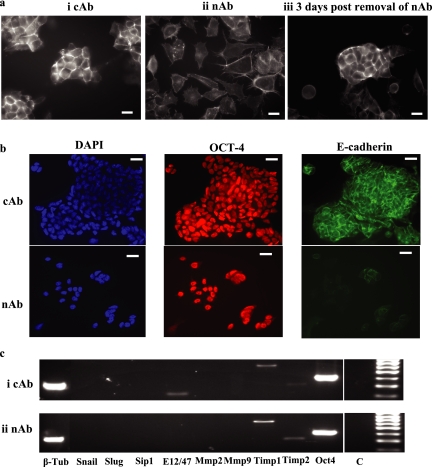
Loss of E-Cadherin–mediated Cell–Cell Contact in ES Cells Induces Reversible Actin Cytoskeleton Rearrangement in the Absence of Up-Regulation of EMT-associated Transcripts
To assess whether loss of E-cadherin–mediated cell–cell contact can induce other associated EMT changes in ES cells, we abrogated cell surface E-cadherin expression in these cells using DECMA-1 neutralizing antibody (nAb) in FCS+LIF medium and assessed actin cytoskeleton arrangement (using Texas Red–conjugated phalloidin) and expression of EMT-associated transcripts (Figure 3). Control antibody (cAb)-treated cells exhibited cortical actin cytoskeleton arrangement, with cell–cell contact clearly evident (Figure 3ai). Treatment of D3 ES cells with nAb resulted in loss of cell–cell contact, an altered actin cytoskeleton arrangement and a mesenchymal phenotype (Figure 3aii). Removal of DECMA-1 from the mouse ES cells restored both cell–cell contact and cortical actin cytoskeleton arrangement within 3 d (Figure 3aiii). Expression of E-cadherin and OCT-4 proteins were assessed in D3 ES cells cultured in FCS+LIF and either cAb or nAb for six passages (∼12 d; Figure 3b). E-cadherin protein expression was significantly reduced in nAb-treated D3 ES cells (Figure 3b; nAb), whereas OCT-4 expression was detected, demonstrating that the cells remained in an undifferentiated state. RT-PCR analysis (35 cycles) of RNA isolated from cAb- or nAb-treated cells demonstrated no difference between the transcript profiles of the cell populations (Figure 3c). For example, both cAb- and nAb-treated (Figure 3c, i and ii, respectively) ES cells expressed E12/E47 (transcripts were detected in nAb-treated cells at 45 cycles), TIMP-1 and -2 and Oct-4 and lacked transcripts encoding Snail, Slug, SIP1, and MMP-2 and -9. Therefore, loss of E-cadherin–mediated cell–cell contacts in mouse ES cells results in reversible actin cytoskeleton rearrangement and mesenchymal phenotype in the absence of induction of EMT-associated transcripts.
Figure 3.
Loss of E-cadherin–mediated cell–cell contact in ES cells induces reversible actin cytoskeleton rearrangement in the absence of up-regulation of EMT-associated transcripts. (a) D3 ES cells cultured in ES cell medium containing FCS+LIF on gelatin-treated plates were treated with either (i) control antibody (cAb) or (ii) E-cadherin–neutralizing antibody (nAb) DECMA-1 for 24 h and actin cytoskeleton arrangement determined using Texas Red–conjugated phalloidin and fluorescent microscopy analysis. (iii) DECMA-1 antibody was removed from the treated cells, and actin cytoskeleton arrangement assessed after 3 d as described above. Bar, 10 μm. (b) D3 ES cells cultured for six passages (∼12 d) in FCS+LIF and either control antibody (cAb) or neutralizing antibody DECMA-1 (nAb) were assessed for DAPI, OCT-4 and E-cadherin protein expression by immunofluorescent microscopy. Bar, 10 μm. (c) Analysis of EMT-associated transcripts was determined by RT-PCR in D3 ES cells treated with either (i) control (cAb) or (ii) DECMA-1 (nAb) antibody for 72 h (C, control; β-tub, β-tubulin). Similar results were also obtained with MESC20 ES cells.
E- and N-Cadherin Proteins Are Independently Regulated after Mouse ES Cell Differentiation, and Neither Proteins Are Required for Up-Regulation of EMT-associated Transcripts
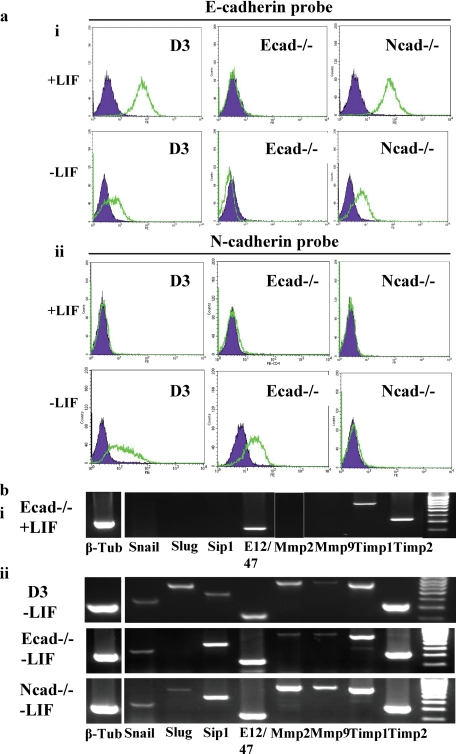
To determine whether up-regulation of N-cadherin after ES cell differentiation induces loss of E-cadherin, and vice versa, we assessed expression of these cell surface proteins in undifferentiated and differentiating (LIF withdrawal for 3 d) wild-type D3, E-cadherin null (Ecad−/−) and N-cadherin null (Ncad−/−) mouse ES cells (Figure 4a). Ncad−/− ES cells exhibit a phenotype similar to wild-type D3 ES cells (data not shown), whereas Ecad−/− ES cells lack cell–cell contacts (see Figure 5ci). D3 and N-cad−/− ES cells expressed E-cadherin when cultured in the presence of LIF whereas, as expected, E-cad−/− cells did not (Figure 4ai). On removal of LIF (i.e., induction of differentiation) both D3 and N-cad−/− ES cells exhibited down-regulation of cell surface E-cadherin, demonstrating that N-cadherin protein is not required for loss of cell surface E-cadherin upon differentiation. Analysis of cell surface N-cadherin expression in undifferentiated (+LIF) ES cells demonstrated absence of the protein from D3, Ecad−/− and Ncad−/− cells (Figure 4aii). On differentiation of the cells by LIF withdrawal for 3 d, both D3 and Ecad−/− ES cells exhibited up-regulation of cell surface N-cadherin. As expected, Ncad−/− ES cells lacked N-cadherin protein after removal of LIF. These results show that E-cadherin protein is not required for up-regulation of N-cadherin during ES cell differentiation and vice versa.
Figure 4.
E- and N-cadherin proteins are independently regulated after ES cell differentiation and neither protein is required for up-regulation of EMT-associated transcripts. (a) (i) D3, E-cadherin null (Ecad−/−) and N-cadherin null (Ncad−/−) ES cells were assessed for expression of cell surface E-cadherin protein in the presence of LIF (+LIF) and in the absence of LIF for 3 d (−LIF) in gelatin-treated plates using fluorescent flow cytometry analysis. E-cadherin (open population); isotype control (closed population). (ii) D3, Ecad−/− and Ncad−/− ES cells were assessed for expression of cell surface N-cadherin protein in the presence of LIF (+LIF) and in the absence of LIF for 3 d (−LIF) as described above. N-cadherin (open population); isotype control (closed population). (b) RT-PCR analysis of EMT-associated transcript expression in (i) undifferentiated ES cells (E-cad−/− only shown) and (ii) D3, Ecad−/− and N-cad−/− ES cells differentiated for 3 d in the absence of LIF in gelatin-treated plates.
Figure 5.
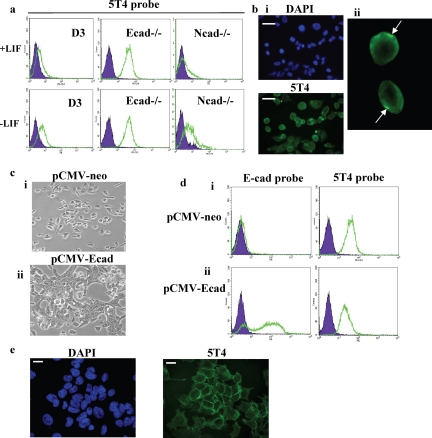
5T4 antigen is localized at the plasma membrane in E-cadherin null ES cells. (a) D3, E-cadherin null (Ecad−/−) and N-cadherin null (Ncad−/−) ES cells were assessed for expression of cell surface 5T4 antigen in the presence of LIF (+LIF) and in the absence of LIF for 3 d (−LIF) in gelatin-treated plates using fluorescent flow cytometry analysis. 5T4 antigen (open population); isotype control (closed population). (b) (i) Immunofluorescence microscopy analysis of 5T4 antigen expression in E-cadherin null ES cells (DAPI shows total nuclei in the field of view). Bar, 10 μm. (ii) Enlarged image of the cells marked in b, pane i, demonstrating polarized expression of 5T4 in E-cadherin null ES cells. (c) Undifferentiated Ecad−/− ES cells were transfected with 2 μg of either (i) control (pCMV-neo) vector or (ii) vector containing full-length E-cadherin cDNA (pCMV-Ecad) using the Amaxa electroporation system and assessed for cellular phenotype by phase-contrast microscopy. (d) Cells transfected with (i) pCMV-neo vector or (ii) pCMV-Ecad were assessed for expression of cell surface E-cadherin (E-cad probe) and 5T4 antigen (5T4 probe) using fluorescent flow cytometry as described above. (e) Immunofluorescence microscopy analysis of 5T4 antigen expression in E-cadherin null ES cells transfected with pCMV-Ecad vector (DAPI shows total nuclei in the field of view). Bar, 5 μm.
To determine whether up-regulation of EMT-associated transcripts during ES cell differentiation is linked to expression of E- or N-cadherin proteins, we assessed expression of EMT-associated transcripts by RT-PCR (35 cycles) in wild-type D3, E-cad−/− and N-cad−/− ES cells cultured in the presence of LIF (+LIF) or the absence of LIF for 3 d (−LIF; Figure 4b). Undifferentiated D3, Ecad−/− and Ncad−/− ES cells expressed transcripts encoding E12/47, TIMP-1 and -2 but lacked Snail, Slug, SIP1, and MMP-2 and -9 (Figure 4bi; Ecad−/− results shown). On removal of LIF for 3 d, D3, Ecad−/− and Ncad−/− ES cells exhibited up-regulation of Snail, Slug, SIP1, and MMP-2 and -9 transcripts (Figure 4bii). Transcripts encoding Slug protein were not detected in Ecad−/− cells at 35 PCR cycles but were observed when reexamined at 40 cycles (data not shown). These results demonstrate that neither N- nor E-cadherin proteins are required for up-regulation of EMT-associated transcripts after differentiation of ES cells.
5T4 Antigen Is Localized at the Plasma Membrane in E-Cadherin Null ES Cells
5T4 oncofetal antigen expression was also determined in undifferentiated (FCS+LIF) and differentiating (−LIF) D3, Ecad−/−, and Ncad−/− ES cells (Figure 5a). 5T4 antigen was either absent or expressed at low levels in undifferentiated D3 and Ncad−/− ES cells. By contrast, undifferentiated E-cad−/− ES cells constitutively expressed high levels of the 5T4 antigen at the cell surface. On removal of LIF, cell surface 5T4 antigen was detected at higher levels in D3 and Ncad−/− ES cells but remained unchanged in Ecad−/− ES cells (Figure 5a; −LIF). Immunofluorescent microscopy analysis of 5T4 antigen in E-cad−/− ES cells revealed both cytoplasmic and cell surface expression (Figure 5bi). In addition, there was evidence of polarized cell surface expression of 5T4 antigen (Figure 5bii), although this was not observed in all of the cells within the population. It is unlikely that polarization of 5T4 antigen is a result of antibody induced redistribution because the cells were fixed before addition of the primary and secondary antibodies.
To determine whether forced expression of E-cadherin protein in Ecad−/− ES cells could reverse cell surface localization of the 5T4 antigen, Ecad−/− ES cells were transfected with full-length E-cadherin cDNA and cell surface expression of E-cadherin and 5T4 proteins assessed (Figure 5, c and d). Ecad−/− cells transfected with control plasmid for 24 h did not exhibit restoration of cell–cell contact (Figure 5ci; pCMV-neo), nor was E-cadherin protein detected at the cell surface (Figure 5di, pCMV-neo; E-cadherin probe). As expected, 5T4 antigen was detected at the cell surface in pCMV-neo–transfected cells (Figure 5di, pCMV-neo; 5T4 probe). Ecad−/− cells transfected with full-length E-cadherin cDNA exhibited restoration of cell–cell contacts (Figure 5cii, pCMV-Ecad) and high levels of cell surface E-cadherin expression was detected in the majority of the population (Figure 5dii, pCMV-Ecad; E-cadherin probe). However, cell surface 5T4 antigen expression was not reversed after E-cadherin cDNA expression in Ecad−/− ES cells (Figure 5dii, pCMV-Ecad; 5T4 probe). Fluorescent microscopy analysis of Ecad−/− ES cells transfected with pCMV-Ecad vector demonstrated cell surface and cytoplasmic expression of the 5T4 antigen (Figure 5e). Interestingly, polarized 5T4 antigen expression was not observed in Ecad−/− ES cells transfected with pCMV-Ecad vector, suggesting that E-cadherin may alter the distribution of the 5T4 antigen at the cell surface. It is unlikely that alteration in the localization of 5T4 antigen is a result of antibody induced redistribution because the cells were fixed before addition of the primary and secondary antibodies. We conclude that forced expression of E-cadherin in Ecad−/− ES cells restores cell–cell contact but does not reverse the 5T4 cell surface phenotype in these cells over the time course of the experiment. This could reflect the relatively long half-life of the 5T4 protein at the cell membrane (Stern, unpublished data).
Loss of E-Cadherin–mediated Cell–Cell Contacts in Wild-type ES Cells Results in Transcriptional- and Translational-independent Localization of the 5T4 Antigen at the Cell Surface
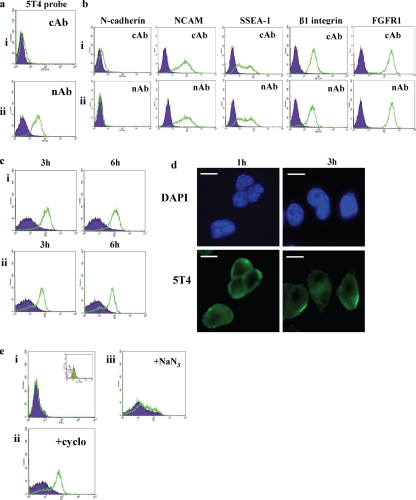
To determine whether 5T4 antigen localization is altered after loss of E-cadherin–mediated cell–cell contacts in undifferentiated wild-type mouse ES cells, we cultured D3 ES cells in FCS+LIF in the presence of either cAb or E-cadherin nAb (DECMA-1) and assessed cell surface expression of the 5T4 antigen by fluorescent flow cytometry (Figure 6a). As described previously, ES cells cultured in the presence of FCS and LIF exhibit cytoplasmic localization of the 5T4 antigen but lack cell surface antigen (Barrow et al., 2005). On ablation of E-cadherin–mediated cell–cell contacts, 5T4 antigen was detected at the cell surface of ES cells cultured in FCS+LIF (Figure 6Aii, nAb), whereas cell surface 5T4 antigen was absent in cAb-treated cells (Figure 6ai, cAb). To assess whether 5T4 localization at the cell membrane in nAb-treated cells represented nonspecific alterations at the cell surface, we assessed expression of N-cadherin, NCAM, SSEA-1, β1-integrin, and FGFR1 expression in ES cells treated with cAb or E-cadherin nAb (Figure 6b). No significant changes in cell surface expression of these proteins were observed after treatment with nAb antibody compared with the control antibody. These results demonstrate that loss of E-cadherin–mediated cell–cell contacts in ES cells results in localization of 5T4 at the cell membrane and that this is unlikely to be due to nonspecific alterations at the plasma membrane.
Figure 6.
Loss of E-cadherin–mediated cell–cell contacts in wild-type ES cells results in transcriptional- and translational-independent localization of the 5T4 antigen at the cell surface. Undifferentiated D3 or MESC20 ES cells were cultured on gelatin-treated plates in FCS+LIF. (a) D3 ES cells were cultured in the presence of (i) control antibody (cAb) or (ii) E-cadherin–neutralizing antibody DECMA-1 (nAb) and 5T4 antigen expression assessed by fluorescent flow cytometry analysis. 5T4 antigen (open population); isotype control (closed population). (b) Undifferentiated D3 cells were cultured in FCS+LIF and the presence of (i) cAb or (ii) nAb and assessed for expression of the cell surface proteins N-cadherin, NCAM, SSEA-1, β1-integrin, and FGFR1. Note that no significant differences were observed for these antigens in the two antibody treatments. Similar results were obtained with MESC20 ES cells and E-cadherin null ES cells (data not shown). (c) (i) Undifferentiated MESC20 ES cells were cultured in the presence of cAb (filled population) or nAb (open population) for 3 and 6 h, and cell surface 5T4 antigen was assessed by fluorescent flow cytometry. (ii) Cell surface 5T4 antigen expression was determined in MESC20 ES cells after removal of cAb or nAb from the cells for 3 and 6 h using fluorescent flow cytometry. (d) Undifferentiated MESC20 ES cells were cultured in the presence of nAb for 1 and 3 h and 5T4 antigen (5T4) assessed by fluorescent microscopy. DAPI shows all cell nuclei within the field of view. Bar, 5 μm. (e) (i) ES cells expressing GFP under control of the 5T4 promoter were cultured in the presence of cAb (closed population) or nAb (open population) for 3 h, and GFP expression was assessed using fluorescent flow cytometry. Inset shows GFP expression in these cells after removal of LIF for 3 d. (ii) MESC20 ES cells were cultured in the presence of cAb (closed population) or nAb (open population) and 10 μg/ml cyclohexamide to inhibit total protein synthesis, and cell surface 5T4 antigen was assessed using fluorescent flow cytometry after 3 h. (iii) MESC20 ES cells were cultured in the presence of cAb (closed population) or nAb (open population) and 10 μM sodium azide to inhibit energy (ATP)-dependent processes and cell surface 5T4 antigen assessed using fluorescent flow cytometry after 3 h. Similar results were also obtained with D3 ES cells (data not shown).
The kinetics of 5T4 presentation at the cell membrane was assessed by addition of DECMA-1 antibody to MESC20 ES cells cultured in the presence of FCS+LIF and cell surface 5T4 antigen determined by fluorescent flow cytometry after 3 and 6 h (Figure 6ci, 3 and 6 h). Cell surface 5T4 antigen was detected at 3 h after addition of DECMA-1, and the levels were unchanged at 6 h. Cell surface 5T4 antigen localization was also assessed at 3 and 6 h after removal of DECMA-1 from nAb-treated MESC ES cells (Figure 6cii, 3 and 6 h). 5T4 antigen was detected at the cell surface at both 3 and 6 h after removal of DECMA-1, demonstrating that the kinetics of loss of 5T4 from the cell membrane are not as rapid as its presentation upon loss of cell surface E-cadherin. The long half-life of 5T4 antigen at the cell membrane after removal of DECMA-1 may explain why transient transfection of E-cadherin cDNA does not reverse cell surface localization of the 5T4 antigen (Figure 5d). Immunofluorescent microscopy analysis of 5T4 antigen expression in D3 cells treated with DECMA-1 antibody for 1 h demonstrated mostly cytoplasmic localization of the antigen (Figure 6d, 1 h). At 3 h after addition of DECMA-1, the cells exhibited both cytoplasmic and polarized cell surface expression of the antigen (Figure 6d, 3 h), similar to that observed for E-cadherin null ES cells (Figure 5b).
The rapid detection of 5T4 at the cell membrane suggested that transcriptional and translational regulation of the antigen were not responsible for its presentation at the cell surface. We determined transcriptional up-regulation of 5T4 transcripts by utilizing an ES cell line expressing green fluorescent protein (GFP) under control of a single 5T4 allele (5T4-GFP ES cells; Figure 6ei; Perez-Campo, Spencer, Stern, and Ward, unpublished data). Addition of DECMA-1 to the cells cultured in FCS+LIF for 3 h did not result in up-regulation of GFP (5T4 transcripts), demonstrating that transcriptional up-regulation is not a factor in the presentation of 5T4 at the cell membrane. The inset to Figure 6ei shows GFP expression in the 5T4-GFP ES cell line after removal of LIF for 3 d, demonstrating up-regulation of 5T4 (GFP) transcripts. To assess whether protein synthesis was involved in 5T4 presentation at the cell membrane, MESC20 ES cells were cultured in the presence of DECMA-1 and 10 μg/ml cyclohexamide to inhibit de novo protein synthesis (Figure 6eii). Inhibition of protein synthesis did not affect cell surface localization of the 5T4 antigen after addition of DECMA-1 for 3 h, suggesting that upon loss of E-cadherin–mediated cell–cell contacts, cytoplasmic 5T4 is translocated to the cell surface. To determine whether 5T4 antigen presentation at the cell membrane is a result of energy (ATP)-dependent mechanisms, we incubated MESC20 ES cells in the presence of 10 μM sodium azide for 15 min before the addition of DECMA-1 antibody and sodium azide for 3 h (Figure 6eiii). Compared with control antibody-treated cells in the presence of sodium azide, 5T4 antigen was not detected at the cell surface, demonstrating that translocation of 5T4 antigen to the cell surface upon loss of E-cadherin is an energy-dependent process.
5T4 Null ES Cells Exhibit EMT-associated Events after Differentiation
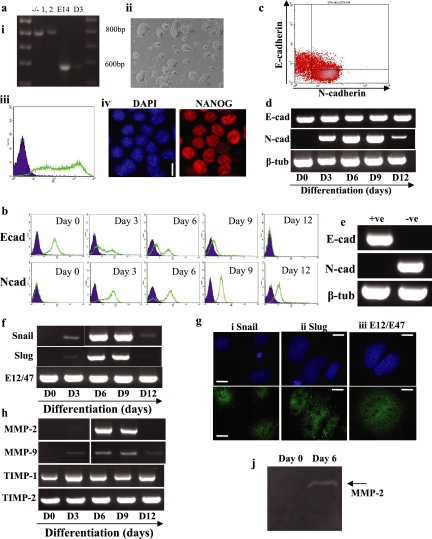
To assess the role of 5T4 during ES cell differentiation, we isolated 5T4 null ES cells from homozygous 5T4−/− mouse crosses (Figure 7ai). The phenotype of the 5T4−/− mouse is still under investigation; however, the animals are fertile and no gross abnormalities during embryonic development have yet been observed. 5T4−/− ES cells exhibit normal colony morphology (Figure 7aii) and express the pluripotent markers SSEA-1 (Figure 7aiii) and NANOG (Figure 7aiv).
Figure 7.
5T4 null ES cells exhibit EMT-associated events after differentiation. 5T4 null ES cells were isolated as described in Materials and Methods. (a) (i) Genotyping of 5T4 null ES cells (clones 1 and 2) compared with wild-type E14 and D3 ES cells was assessed by PCR. Wild-type ES cells exhibited a 600-bp product, whereas 5T4−/− ES cells exhibited an 800-bp product. (ii) Phase-contrast microscopy image of undifferentiated 5T4−/− ES cells cultured in ES cell medium (FCS+LIF). (iii) SSEA-1 expression was determined in undifferentiated 5T4−/− ES cells by fluorescent flow cytometry analysis. (iv) Expression of NANOG protein in undifferentiated 5T4−/− ES cells was assessed using fluorescent microscopy. Bar, 5 μm. (b) Cell surface E-cadherin (E-cad) and N-cadherin (N-cad) proteins were assessed in undifferentiated 5T4−/− ES cells (day 0) and differentiated cells for 3, 6, 9, and 12 d by fluorescent flow cytometry in a Becton-Dickinson FACScaliber. E- or N-cadherin, open population; isotype control antibodies, closed population. (c) Fluorescent flow cytometry dual staining for E- and N-cadherin on 5T4−/− ES cells differentiated for 3 d as described above. (d) RT-PCR analysis of E- (E-cad) and N-cadherin (N-cad) and β-tubulin (β-tub; control) transcript expression was assessed in undifferentiated 5T4−/− ES cells (day 0) and in cells differentiated for 3, 6, 9, and 12 d as described above. (e) 5T4−/− ES cells were differentiated for 3 d as described above, and E-cadherin–positive (+ve) and E-cadherin–negative (−ve) cells were isolated by FACS. RT-PCR was performed on the samples to assess E- (E-cad) and N-cadherin (N-cad) and β-tubulin (β-tub) transcript expression. (f) Transcript expression of Snail, Slug, and E12/E47 was determined by RT-PCR in undifferentiated 5T4−/− ES cells (day 0) and in cells differentiated for 3, 6, 9, and 12 d. (g) 5T4−/− ES cells were differentiated for 6 d and assessed for Snail (i), Slug and (ii), E12/E47 (iii) proteins using immunofluorescent microscopy. DAPI shows the total cells within the field of view. Bar, 5 μm. (h) Transcript expression of matrixmetalloproteinase (MMP)-2 and -9 and tissue inhibitor of metalloproteinase (TIMP)-1 and -2 was determined by RT-PCR in undifferentiated and differentiating 5T4−/− ES cells as described above. (j) Gelatin zymogram analysis was performed to determine MMP-2 and -9 activity within the culture supernatants of undifferentiated (day 0) and 5T4−/− ES cells differentiated for 6 d (day 6). Arrow shows the size of active MMP-2 (65 kDa). Note that MMP-9 was not detected under these conditions.
Expression of E-cadherin was assessed in undifferentiated 5T4−/− ES cells by fluorescent flow cytometry (Figure 7b, E-cad, day 0), with over 95% of the population expressing cell surface protein. In contrast, undifferentiated 5T4−/− ES cells lacked cell surface N-cadherin expression (Figure 7b, Ncad, day 0). On differentiation of 5T4−/− ES cells after LIF withdrawal in serum replacement medium, E-cadherin was no longer detected at the cell surface on an increasing proportion of the population labeled after 3, 6, 9, and 12 d (Figure 7b, Ecad), and this correlated reciprocally with up-regulation of cell surface N-cadherin (Figure 7b, N-cad). To determine whether cell surface expression of E- and N-cadherin was exclusive in 5T4−/− ES cells, we assessed the presence of these proteins using flow cytometry dual staining in these cells differentiated for 3 d (Figure 7c). The majority of cells expressed only E- or N-cadherin at the cell surface, demonstrating that a cell surface E- to N-cadherin switch occurs during 5T4−/− ES cell differentiation.
Analysis of E- and N-cadherin transcript expression in the undifferentiated and differentiating 5T4−/− ES cell population was assessed by RT-PCR analysis (Figure 7d). N-cadherin transcripts were absent from undifferentiated ES cells and rapidly up-regulated upon removal of LIF, with levels peaking at day 9 followed by a reduction of the transcripts at day 12. This is in agreement with the up-regulation of cell surface N-cadherin protein shown in Figure 7b. Expression of E-cadherin transcripts was similar to that observed in wild-type ES cells, with transcripts detected throughout the differentiation period. To assess whether E- and N-cadherin transcripts were differentially expressed in differentiating 5T4−/− ES cell, we isolated E-cadherin–positive (+ve) and E-cadherin–negative (−ve) cells by FACS 3 d after induction of differentiation (Figure 7e). The E-cadherin–positive cell population expressed E-cadherin but lacked any N-cadherin transcripts. In contrast, E-cadherin–negative cells lacked transcripts for E-cadherin, whereas N-cadherin transcripts were detected. This data demonstrates that a transcriptional E- to N-cadherin switch occurs in a proportion of the differentiating cells within the population.
To further investigate EMT events associated with differentiation of 5T4−/− ES cells, we assessed transcript and protein expression of the E-cadherin repressor molecules Snail, Slug, and E12/E47 (Figure 7f). Undifferentiated 5T4−/− ES cells lacked transcripts encoding Snail and Slug, and these were detected at days 3, 6, 9, and 12 after induction of differentiation, with peak transcript expression occurring at days 6 and 9 for both genes. E12/E47 transcripts were detected in undifferentiated and differentiating 5T4−/− ES cells at all time points. Expression of Snail, Slug, and E12/E47 proteins was also assessed using fluorescent microscopy in 5T4−/− ES cells differentiated as described above for 6 d (Figure 7g). Nuclear localization of Snail, Slug, and E12/E47 (Figure 7g, i–iii, respectively) was observed in a proportion of the differentiating population of 5T4−/− ES cells (with E12/47 also detected within the cytoplasm), demonstrating that these proteins are likely to be unaffected by lack of 5T4 antigen.
Transcript expression of MMP-2 and -9 and TIMP-1 and -2 in undifferentiated and differentiating 5T4−/− ES cells was also determined by RT-PCR analysis (Figure 7h). Undifferentiated 5T4−/− ES cells lacked MMP-2 and -9 transcripts, and these were both up-regulated after differentiation of the cells, similar to that observed in wild-type ES cells. TIMP-1 and -2 transcripts were detected in undifferentiated 5T4−/− ES cells and at all time points after differentiation of the cells. Although both MMP-2 and -9 transcripts were detected during 5T4−/− ES cell differentiation, only MMP-2 activity was observed using zymogram analysis after differentiation of the cells for 6 d (Figure 7j). This data demonstrates that EMT-associated events in differentiating 5T4−/− ES cells are similar to those observed in wild-type ES cells.
5T4−/− ES Cells Exhibit Altered Phenotype after Treatment with DECMA-1 Antibody
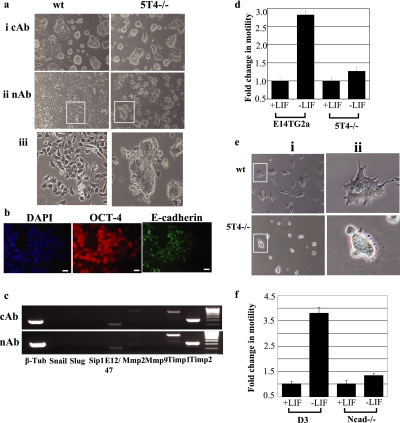
Forced expression of the 5T4 oncofetal antigen in mouse ES cells leads to loss of cell–cell contact and a mesenchymal phenotype (Ward et al., 2003); therefore, we hypothesized that 5T4 antigen presentation at the cell surface could be responsible for the phenotype observed in DECMA-1–treated ES cells (i.e., loss of cell–cell contact and acquisition of mesenchymal phenotype). To address this, we cultured 5T4 null (5T4−/−) ES cells in the presence of LIF and an excess of either cAb or E-cadherin nAb (Figure 8a, i or ii, respectively; 23.2 μg/ml IgG component of DECMA-1) and assessed the cellular phenotype after 24 h using phase-contrast microscopy (Figure 8a). Wild-type E14TG2a and 5T4−/− ES cells treated with cAb maintained cell–cell contacts and typical ES cell colony morphology. Wild-type ES cells treated with DECMA-1 for 24 h exhibited a mesenchymal phenotype and loss of characteristic ES cell colony morphology (Figure 8aii, wt). By contrast, 5T4−/− ES cells treated with DECMA-1 for 24 h resulted in very few cells exhibiting a mesenchymal phenotype, and the majority of the cells exhibited cell–cell contact and characteristic ES cell colony morphology (Figure 8aii, 5T4−/−). We believe that this effect reflects the incomplete loss of cell surface E-cadherin after DECMA-1 treatment because 5T4−/− ES cells treated with nAb exhibited cell surface E-cadherin protein (Figure 8b). In addition, treatment of 5T4−/− ES cells with control (cAb) or DECMA-1 (nAb) antibody did not affect the expression of EMT-associated transcripts (Figure 8c). For example, both cAb- and nAb-treated 5T4 ES cells expressed transcripts encoding E12/47, low levels of MMP-2 and TIMP-1 and -2 but lacked Snail, Slug, SIP1, and MMP-9.
Figure 8.
5T4−/− ES cells exhibit altered phenotype after treatment with DECMA-1 antibody and decreased motility after differentiation. (a) E14 and 5T4−/− ES cells were cultured on gelatin-treated plates in the presence of LIF and an excess (23.2 μg/ml IgG component) of either (i) control antibody (cAb) or (ii) E-cadherin neutralizing antibody DECMA-1 (nAb) and colony phenotype assessed after 24 h using phase contrast microscopy. (iii) Enlarged image of the marked areas in panel ii. Note that the cellular phenotype of 5T4−/− ES cells is altered compared with wild-type ES cells. (b) 5T4−/− ES cells cultured for 24 h in FCS+LIF and neutralizing antibody DECMA-1 (nAb) and assessed for DAPI, OCT-4, and E-cadherin protein expression by immunofluorescent microscopy. Bar, 5 μm. (c) Analysis of EMT-associated transcripts was determined by RT-PCR in 5T4−/− ES cells treated with either (i) control (cAb) or (ii) DECMA-1 (nAb) antibody for 72 h (C, control; β-tub, β-tubulin). (d) Cellular motility of undifferentiated and differentiating (3 d in the absence of LIF) wild-type and 5T4−/− ES cells was assessed using Costar Transwell 5-μm pore size plates. Data represents the fold change in motility compared with undifferentiated cells. Note that 5T4−/− cells cultured in the absence of LIF exhibit decreased motility compared with control cells. (e) (i) Phase-contrast microscopy images showing wild-type (wt) and 5T4−/− ES cell colony morphology 24 h after induction of differentiation. (ii) Enlarged images of the marked areas shown in panel i. (f) Cellular motility of undifferentiated and differentiating (3 d in the absence of LIF) wild-type (D3) and N-cadherin−/− ES cells was assessed using Costar Transwell 5-μm pore size plates. Data represents the fold change in motility compared with undifferentiated cells. Note that Ncad−/− cells cultured in the absence of LIF exhibit decreased motility compared with control cells.
5T4 Null and N-Cadherin Null ES Cells Exhibit Decreased Motility after Differentiation
To determine whether 5T4−/− ES cells exhibit altered motility after induction of EMT by differentiation, we assessed the ability of undifferentiated and differentiated wild-type (wt; E14TG2a) and 5T4−/− ES cells to migrate through 5-μm pore size Transwell plates (Figure 8d). Wild-type ES cells differentiated for 3 d in the absence of LIF exhibited almost a threefold increase in migrating cells compared with undifferentiated cells. By contrast, 5T4−/− ES cells differentiated for 3 d in the absence of LIF exhibited only a 1.25-fold increase in migrating cells compared with undifferentiated cells (p < 0.001, wt vs. 5T4−/− differentiating cells). Phase-contrast microscopy of differentiating wild-type and 5T4−/− ES cells demonstrated that the latter lacked a motile phenotype (Figure 8e, i and ii; differentiated for 1 d). For example, the majority of wild-type ES cell colonies exhibited projections consistent with increased motility, whereas 5T4−/− colonies lacked such morphological appearance. We also assessed the ability of N-cadherin−/− ES cells to migrate after differentiation (Figure 8f), as described above for 5T4−/− ES cells. Differentiating wild-type D3 ES cells exhibited ∼3.75-fold increased motility compared with undifferentiated cells. By contrast, differentiating N-cadherin−/− ES cells exhibited only 1.13-fold increased motility compared with undifferentiated cells (p < 0.001 for wt and Ncad−/− differentiating populations). We conclude that the function of 5T4 and N-cadherin proteins during ES cell differentiation is to enable increased cellular motility.
DISCUSSION
EMT events occur during embryonic development and are important for the metastatic spread of epithelial tumors (Cavallaro and Christofori, 2004a,b). However, the study of EMT is currently impeded by lack of a naturally regulated in vitro model system (Cavallaro and Christofori, 2004b). We show here that spontaneous differentiation of mouse ES cells is associated with an E- to N-cadherin switch, up-regulation of E-cadherin repressor molecules, increased gelatinase activity, and cellular motility, all characteristic EMT events. E- and N-cadherin/5T4 are reciprocally coregulated at the plasma membrane, and upon ES differentiation their altered expression provides for the loss of cell–cell contacts, acquisition of the mesenchymal phenotype, and increased motility. Thus, the 5T4 oncofetal antigen is a novel component of EMT, as found in ES cells, and may contribute similar properties in cancer. An EMT event has recently been described in rhesus monkey (Behr et al., 2005) and human ES cells (Ullmann et al., 2006), where up-regulation of Slug and Snail were shown to correspond with epithelial-to-mesenchymal cellular events within individual ES cell colonies. Although the method used by Behr et al. and Ullmann et al. to induce EMT differs from our study, together these data show that ES cells are a useful in vitro model system with which to elucidate mechanisms associated with EMT during development and may be useful to model molecular and biochemical events that occur during metastasis.
Snail genes have been observed in all EMT processes studied to date (Nieto, 2002; Barrallo-Gimeno and Nieto, 2005) and a common role for Slug and Snail is repression of E-cadherin transcripts by binding to the proximal region of the E-cadherin promoter (Peinado et al., 2004; Barrallo-Gimeno and Nieto, 2005; De Craene et al., 2005; Saito et al., 2006). Therefore, one possible function of Snail proteins during ES cell differentiation is the regulation of E-cadherin expression at the transcript level, particularly in light of the observation that cell surface E-cadherin–negative cells do not express transcripts for this protein. However, the mechanism of action of Snail and Slug may be more complex. For example, as well as functioning as regulators of E-cadherin, Snail and Slug can induce cell survival in the absence of EMT (Barrallo-Gimeno and Nieto, 2005), and this may counteract the significantly increased apoptosis that occurs during ES cell differentiation. Other mechanisms of E-cadherin regulation may also play a role during ES cell differentiation. For example, p38 and p38-interacting protein have been shown to be critical for the loss of E-cadherin expression during mouse gastrulation (Zohn et al., 2006), and these proteins may also exert an effect in regulating E-cadherin during ES cell EMT. Alternatively, MMP cleavage of cell surface E-cadherin may be a further mechanism by which E-cadherin may be lost from the cell membrane during EMT (Cavallaro and Christofori, 2004a,b). However, preliminary studies using an MMP-2/-9 chemical inhibitor during ES cell differentiation did not inhibit loss of E-cadherin from the cell surface (Ward, unpublished data). Because of the heterogeneous nature of the differentiating ES cell population, we believe that multiple mechanisms of cell surface E-cadherin regulation are occurring during ES cell EMT.
The gelatinases, MMP-2 and -9, are required for invasive processes during development and have been extensively studied in cancer (Bjorklund and Koivunen, 2005). Up-regulation of the gelatinases has been demonstrated in breast, colon, lung, skin, ovarian, and prostate cancers and both MMP-2 and -9 are critical for tumor metastasis in mouse models (Itoh et al., 1998, 1999; Bjorklund and Koivunen, 2005). The gelatinases degrade a wide variety of proteins, including those within the extracellular matrix, growth factors, cell surface receptors, and adhesion molecules (Bjorklund and Koivunen, 2005), allowing movement of the cells within their microenvironment. Our observation that both MMP-2 and -9 transcripts are up-regulated upon ES cell differentiation is in contrast to the current evidence that MMP-2 transcription is constitutively active and MMP-9 activity is stimulated in response to factors such as epidermal growth factor (EGF), human growth factor, and transforming growth factor beta (TGFβ; Bjorklund and Koivunen, 2005). The ES cell model therefore provides a useful in vitro system with which to study transcriptional regulation of these genes. Up-regulation of MMPs during ES cell differentiation could be due to a down-stream effect of Snail expression, which can promote MMP transcriptional activity (Yokoyama et al., 2003; Barrallo-Gimeno and Nieto, 2005; Miyoshi et al., 2005; Taki et al., 2006). Furthermore, integrin-matrix interactions, including β1, have been shown to induce selective expression of MMPs (Bjorklund and Koivunen, 2005), and it is known that integrin-matrix interactions play an important role during ES cell differentiation (Czyz and Wobus, 2001).
Alteration of the dynamics of the actin cytoskeleton has been shown to induce MMP activation (Bjorklund and Koivunen, 2005), although we did not observe any up-regulation of MMP-2 or-9 transcripts in undifferentiated ES cells treated with E-cadherin nAb. Neither were Snail, Slug, or SIP1 transcripts up-regulated under these conditions, demonstrating that a mesenchymal cellular phenotype is not dependent on up-regulation of the EMT-associated transcripts in ES cells. Thus, loss of E-cadherin itself cannot account for the onset of EMT in ES cells, and it appears that LIF acts as a negative regulator to the onset of EMT. However, this may be an oversimplification because EMT can be triggered by many signaling processes, including EGF, FGF, and TGFβ1 and 2, which may be affected by withdrawal of LIF. Many of these pathways lead to the up-regulation of Snail and/or Slug and direct or indirect repression of cell surface E-cadherin (Cavallaro and Christofori, 2004b). It is possible that multiple mechanisms exist for the activation of EMT in mouse ES cells and this requires further investigation.
The 5T4 oncofetal antigen is associated with increased cellular motility and decreased adhesion (Carsberg et al., 1996). Furthermore, forced expression of 5T4 in mouse ES cells induces loss of characteristic colony morphology and increased cell spread (Ward et al., 2003). In murine epithelial cells, forced expression of 5T4 cDNA can result in loss of cell–cell contacts through down-regulation of E-cadherin and reorganization of the actin cytoskeleton (Carsberg et al., 1996). The observation that DECMA-1–treated ES cells exhibit 5T4 antigen at the cell surface via a transcriptional- and translational-independent process leads to our proposal of a new function for E-cadherin in inhibiting translocation of 5T4 antigen from the cytoplasm to the cell surface in these cells. Because DECMA-1– and cyclohexamide-treated mouse ES cells resulted in localization of 5T4 antigen at the cell membrane, it is likely that cofactors required for relocalization of 5T4 to the plasma membrane are present within OCT-4–positive, intracellular 5T4–positive ES cells. However, forced expression of E-cadherin cDNA in Ecad−/− ES cells failed to reverse the 5T4 cell surface phenotype, although it does appear to reverse polarized 5T4 antigen expression at the cell membrane. It is possible that the presence of 5T4 at the cell surface in Ecad−/− ES cells results in incorrect localization of E-cadherin at the cell membrane after forced expression of E-cadherin cDNA. It is clear that membranous 5T4 antigen exhibits a prolonged half-life because wild-type ES cells treated with DECMA-1 exhibited 5T4 antigen localization at the cell membrane for at least 72 h. Therefore, we believe that the relatively slow turnover of membranous 5T4 antigen reflects the inability to reverse the phenotype in Ecad−/− ES cells after forced expression of E-cadherin cDNA.
The 5T4 antigen is a putative antiadhesion molecule, and we hypothesized that 5T4 null ES cells would exhibit a less mesenchymal phenotype after loss of E-cadherin using DECMA-1–neutralizing antibody. Although 5T4 null ES cells exhibited decreased mesenchymal phenotype compared with wild-type cells treated with DECMA-1–neutralizing antibody, this result is likely to reflect inefficient loss of cell surface E-cadherin in 5T4 null ES cells. This suggests that 5T4 plays an active role in the loss of E-cadherin after treatment with DECMA-1, perhaps by potentiating internalization of E-cadherin upon its binding with DECMA-1. Because 5T4 is translocated from the cytoplasm to the cell membrane in wild-type ES cells after treatment with DECMA-1, it is possible that the absence of cytoplasmic 5T4 in the 5T4 null cells affects localization of cytoplasmic proteins involved in membrane trafficking. However, the observation that 5T4−/− and N-cadherin−/− ES cells exhibit loss of E-cadherin after differentiation demonstrates that these proteins do not have an obligate role in loss of cell surface E-cadherin. Instead it appears that 5T4 and N-cadherin proteins function during ES cell differentiation to allow increased cellular motility, a critical component of EMT.
Our results provide an insight into the early events associated with differentiation of mouse ES cells. EMT is difficult to study in vivo and ES cells provide an ideal system to investigate events associated with this process in vitro. Furthermore, the similarities between the EMT event during ES cell differentiation and those associated with cancer metastasis are striking. Epithelial tumors account for over 80% of all cancers, and patient 5-year survival is significantly decreased upon metastatic spread of the disease. Therefore, the ES cell model system may allow the study of EMT events in vitro and the subsequent comparison with tumor biopsies/cell lines to elucidate critical events associated with the onset of tumor cell spread.
ACKNOWLEDGMENTS
C.M.W. is supported by grants from the Association for International Cancer Research, the Biotechnology and Biological Sciences Research Council, and the Royal Society. P.L.S. is funded by Cancer Research UK. A.M.E. is supported by the Biotechnology and Biological Sciences Research Council and previously by Cancer Research UK. H.S. is supported by the Medical Research Council.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0875) on May 16, 2007.
References
- Barrallo-Gimeno A., Nieto M. A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Barrow K. M., Perez-Campo F., Ward C. M. Use of the cytomegalovirus promoter for transient and stable transgene expression in mouse embryonic stem cells. Methods Mol. Biol. 2006;329:283–294. doi: 10.1385/1-59745-037-5:283. [DOI] [PubMed] [Google Scholar]
- Barrow K. M., Ward C. M., Rutter J., Ali S., Stern P. L. Embryonic expression of murine 5T4 oncofoetal antigen is associated with morphogenetic events at implantation and in developing epithelia. Dev. Dynam. 2005;233:1535–1545. doi: 10.1002/dvdy.20482. [DOI] [PubMed] [Google Scholar]
- Bates R. C., Mercurio A. M. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol. Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- Behr R., Heneweer C., Viebahn C., Denker H. W., Thie M. Epithelial-mesenchymal transition in colonies of rhesus monkey embryonic stem cells: a model for processes involved in gastrulation. Stem Cells. 2005;23:805–816. doi: 10.1634/stemcells.2004-0234. [DOI] [PubMed] [Google Scholar]
- Bjorklund M., Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta Rev. Cancer. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Bolos V., Peinado H., Perez-Moreno M. A., Fraga M. F., Esteller M., Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Carsberg C. J., Myers K. A., Stern P. L. Metastasis-associated 5T4 antigen disrupts cell-cell contacts and induces cellular motility in epithelial cells. Int. J. Cancer. 1996;68:84–92. doi: 10.1002/(SICI)1097-0215(19960927)68:1<84::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 2004a;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Christofori G. Multitasking in tumor progression—signaling functions of cell adhesion molecules. Gastroenteropancreatic Neuroendocrine Tumor Disease: Molecular and Cell Biological Aspects. 2004b;Vol. 1014:58–66. doi: 10.1196/annals.1294.006. [DOI] [PubMed] [Google Scholar]
- Czyz J., Wobus A. M. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–174. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- De Craene B., Gilbert B., Stove C., Bruyneel E., van Roy F., Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 2005;65:6237–6244. doi: 10.1158/0008-5472.CAN-04-3545. [DOI] [PubMed] [Google Scholar]
- Derycke L.D.M., Bracke M. E. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- Evans M., Hunter S. Source and nature of embryonic stem cells. Comptes Rendus Biologies. 2002;325:1003–1007. doi: 10.1016/s1631-0691(02)01527-5. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tanioka M., Matsuda H., Nishimoto H., Yoshioka T., Suzuki R., Uehira M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin. Exp. Metastasis. 1999;17:177–181. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tanioka M., Yoshida H., Yoshioka T., Nishimoto H., Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Kim E. S., Kim M. S., Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int. J. Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- Larue L., Antos C., Butz S., Huber O., Delmas V., Dominis M., Kemler R. A role for cadherins in tissue formation. Development. 1996;122:3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- Miyoshi A., Kitajima Y., Kido S., Shimonishi T., Matsuyama S., Kitahara K., Miyazaki K. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br. J. Cancer. 2005;92:252–258. doi: 10.1038/sj.bjc.6602266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma H., Kono K., Mori Y., Takayosh S., Stern P. L., Tasaka K., Matsumoto Y. Oncofetal antigen 5T4 expression as a prognostic factor in patients with gastric cancer. Anticancer Res. 2002;22:1033–1038. [PubMed] [Google Scholar]
- Nieto M. A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc-finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Paquette B., Bisson M., Therriault H., Lemay R., Pare M., Banville P., Cantin A. M. Activation of matrix metalloproteinase-2 and -9 by 2- and 4-hydroxyestradiol. J. Steroid Biochem. Mol. Biol. 2003;87:65–73. doi: 10.1016/s0960-0760(03)00386-8. [DOI] [PubMed] [Google Scholar]
- Peinado H., Ballestar E., Esteller M., Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell. Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice G. L., Rayburn H., Matsunami H., Knudsen K. A., Takeichi M., Hynes R. O. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Rhim H., Savagner P., Thibaudeau G., Thiery J. P., Pavan W. J. Localization of a neural crest transcription factor, Slug, to mouse Chromosome 16 and human Chromosome 8. Mamm. Genome. 1997;8:872–873. doi: 10.1007/s003359900601. [DOI] [PubMed] [Google Scholar]
- Saito T., Nagai M., Ladanyi M. SYT-SSX1 and SYT-SSX2 interfere with repression of E-cadherin by snail and slug: a potential mechanism for aberrant mesenchymal to epithelial transition in human synovial sarcoma. Cancer Res. 2006;66:6919–6927. doi: 10.1158/0008-5472.CAN-05-3697. [DOI] [PubMed] [Google Scholar]
- Samantaray S., Sharma R., Chattopadhyaya T. K., Gupta S. D., Ralhan R. Increased expression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:37–44. doi: 10.1007/s00432-003-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel B., Braeg S., Adler G., Wedlich D., Menke A. E- and N-cadherin differ with respect to their associated p120(ctn) isoforms and their ability to suppress invasive growth in pancreatic cancer cells. Oncogene. 2004;23:5532–5542. doi: 10.1038/sj.onc.1207718. [DOI] [PubMed] [Google Scholar]
- Sheng G. J., dos Reis M., Stern C. D. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- Si H. X., Tsao S. W., Lam K. Y., Srivastava G., Liu Y., Wong Y. C., Shen Z. Y., Cheung A.L.M. E-cadherin expression is commonly downregulated by CpG island hypermethylation in esophageal carcinoma cells. Cancer Lett. 2001;173:71–78. doi: 10.1016/s0304-3835(01)00646-2. [DOI] [PubMed] [Google Scholar]
- Smith A. G. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- Starzynska T., Bromley M., Marlicz K., Roberts S. A., Ucinski M., Stern P. L. Accumulation of p53 in relation to long-term prognosis in colorectal carcinoma. Eur. J. Gastroenterol. Hepatol. 1997;9:183–186. doi: 10.1097/00042737-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Starzynska T., Marsh P. J., Schofield P. F., Roberts S. A., Myers K. A., Stern P. L. Prognostic-Significance of 5t4 oncofetal antigen expression in colorectal-carcinoma. Br. J. Cancer. 1994;69:899–902. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzynska T., Rahi V., Stern P. L. The Expression of 5t4 antigen in colorectal and gastric-carcinoma. Br. J. Cancer. 1992;66:867–869. doi: 10.1038/bjc.1992.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki M., Verschueren K., Yokoyama K., Nagayama M., Kamata N. Involvement of Ets-1 transcription factor in inducing matrix metalloproteinase-2 expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Int. J. Oncol. 2006;28:487–496. [PubMed] [Google Scholar]
- Ullmann U., Veld P. I., Gilles C., de Velde H. V., Sermon K., De Rycke M., Van Steirteghem A., Liebaers I. Epithelial-mesenchymal transition as spontaneous differentiation process in feeder-free human embryonic stem cells culture. Hum. Reprod. 2006;13:21–32. doi: 10.1093/molehr/gal091. [DOI] [PubMed] [Google Scholar]
- Ward C. M., Barrow K., Woods A. M., Stern P. L. The 5T4 oncofoetal antigen is an early differentiation marker of mouse ES cells and its absence is a useful means to assess pluripotency. J. Cell Sci. 2003;116:4533–4542. doi: 10.1242/jcs.00767. [DOI] [PubMed] [Google Scholar]
- Ward C. M., Eastham A. M., Stern P. L. Cell surface 5T4 antigen is transiently upregulated during early human embryonic stem cell differentiation: effect of 5T4 phenotype on neural lineage formation. Exp. Cell Res. 2006;312:1713–1726. doi: 10.1016/j.yexcr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Woods A. M., Wang W. W., Shaw D. M., Ward C. M., Carroll M. W., Rees B. R., Stern P. L. Characterization of the murine 5T4 oncofoetal antigen: a target for immunotherapy in cancer. Biochem. J. 2002;366:353–365. doi: 10.1042/BJ20020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley E., McGown A. T., Rennison J., Swindell R., Crowther D., Starzynska T., Stern P. L. 5t4 oncofetal antigen expression in ovarian-carcinoma. Int. J. Gynecol. Cancer. 1995;5:269–274. doi: 10.1046/j.1525-1438.1995.05040269.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Kamata N., Fujimoto R., Tsutsumi S., Tomonari M., Taki M., Hosokawa H., Nagayama M. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int. J. Oncol. 2003;22:891–898. [PubMed] [Google Scholar]
- Zohn I. E., Li Y. Q., Skolnik E. Y., Anderson K. V., Han J. H., Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]