Abstract
Retroviral assembly is driven by Gag, and nascent viral particles escape cells by recruiting the machinery that forms intralumenal vesicles of multivesicular bodies. In this study, we show that the clathrin adaptor complex AP-1 is involved in retroviral release. The absence of AP-1μ obtained by genetic knock-out or by RNA interference reduces budding of murine leukemia virus (MLV) and HIV-1, leading to a delay of viral propagation in cell culture. In contrast, overexpression of AP-1μ enhances release of HIV-1 Gag. We show that the AP-1 complex facilitates retroviral budding through a direct interaction between the matrix and AP-1μ. Less MLV Gag is found associated with late endosomes in cells lacking AP-1, and our results suggest that AP-1 and AP-3 could function on the same pathway that leads to Gag release. In addition, we find that AP-1 interacts with Tsg101 and Nedd4.1, two cellular proteins known to be involved in HIV-1 and MLV budding. We propose that AP-1 promotes Gag release by transporting it to intracellular sites of active budding, and/or by facilitating its interactions with other cellular partners.
INTRODUCTION
During assembly of retroviruses, three major components, Gag, envelope, and genomic RNAs, are transported to the plasma membrane to produce virions. Gag is the key player in this process, and it is able to produce viral-like particles (VLPs) when expressed alone (Delchambre et al., 1989; Gheysen et al., 1989). Recently, several links between retroviral biogenesis and endosomes have been discovered. First, Gag buds by hijacking the machinery that forms intralumenal vesicles of the multivesicular body (MVB; Garrus et al., 2001; Martin-Serrano et al., 2003; Strack et al., 2003; von Schwedler et al., 2003); second, preassembled complexes of murine leukemia virus (MLV) Gag, genomic RNA, and envelope are transported on endosomes to reach the plasma membrane (Basyuk et al., 2003); third, MPMV Gag traffics on endosomes during retroviral assembly (Sfakianos and Hunter, 2003; Sfakianos et al., 2003); and fourth, TIP47, a cytosolic and a lipid-droplet–associated protein, suggested to be involved in endosomes-to-Golgi retrograde transport, mediates incorporation of HIV-1 envelope into virions (Blot et al., 2003; Lopez-Verges et al., 2006). Thus, interactions of Gag with cellular cofactors involved in endosomal sorting and trafficking are crucial for retroviral assembly and release. Two major groups of Gag partners have been described: proteins involved in sorting of cargos into multivesicular body and clathrin adaptor complexes (Garrus et al., 2001; Strack et al., 2003; von Schwedler et al., 2003; Batonick et al., 2005; Dong et al., 2005).
MVBs are a subset of late endosomes with multivesicular appearance formed by invagination and budding of vesicles from the limiting membrane of endosomes into the lumen of the compartment (Hurley and Emr, 2006; Slagsvold et al., 2006). Protein sorting into these intralumenal vesicles is critical for diverse cellular functions, including receptor down-regulation, degradation of membrane proteins, and formation of lysosome-related organelles. Sorting of membrane proteins into the intralumenal vesicles of MVBs is often driven by posttranslational attachment of ubiquitin to cargo proteins (for review see Hicke and Dunn, 2003; Marmor and Yarden, 2004), and it proceeds through the action of three soluble complexes, ESCRT I, II, and III (endosomal sorting complex required for transport; Katzmann et al., 2001; Babst et al., 2002a,b). Biochemical analysis in yeast provided a model in which ESCRT-I binds ubiquitinated cargo and activates ESCRT-II, which promotes assembly and recruitment of ESCRT-III. The last complex functions directly in sorting and formation of MVB intralumenal vesicles (Babst et al., 2002a). Finally, the AAA+ ATPase Vps4 catalyzes the disassembly of the complex. Formation of intralumenal MVB vesicles and retroviral budding share several similarities: they follow the same topology, they are dependent on ESCRT components, and both can be inhibited by dominant-negative forms of Vps4 (Garrus et al., 2001; Martin-Serrano et al., 2003; Strack et al., 2003; von Schwedler et al., 2003). However, there are also some differences. For instance, HIV-1 budding does not require ESCRT-II, (Langelier et al., 2006), and MLV can bud without a functional ESCRT-I (Garrus et al., 2001). These findings could reflect the complexity of protein sorting in higher eukaryotes, where several mechanisms of intralumenal vesicle formation may exist (Gullapalli et al., 2006; Theos et al., 2006; White et al., 2006).
To bud, retroviral Gag proteins interact directly with several components of the MVB sorting machinery, through short motifs defined as “late domains.” Mutations in these motifs arrest retroviral budding at late stages (for review see Freed, 2002; Demirov and Freed, 2004; Morita and Sundquist, 2004). Gag of HIV-1, HTLV-1, MPMV, RSV, and MLV interact with a component of ESCRT-I, Tsg101, through a P(T/S)AP motif (Garrus et al., 2001; Martin-Serrano et al., 2001; Bouamr et al., 2003; Gottwein et al., 2003; Segura-Morales et al., 2005). Gag of HIV-1, EIAV, MLV, and RSV recruit AIP1/Alix through LYPLX1–3L motifs (Strack et al., 2003; von Schwedler et al., 2003; Segura-Morales et al., 2005). AIP1/Alix is loosely associated with ESCRT-I and III and was shown to control the formation of internal MVB vesicles (von Schwedler et al., 2003; Matsuo et al., 2004). Finally, several members of the Nedd4 family of ubiquitin-ligases interact with Gag of RSV, MPMV, MLV, and HTLV, by binding to PPXY consensus motifs (Garnier et al., 1996; Strack et al., 2000; Kikonyogo et al., 2001; Yasuda et al., 2002; Bouamr et al., 2003; Gottwein et al., 2003; Heidecker et al., 2004; Martin-Serrano et al., 2005). Nedd-4 like proteins ubiquitinate cellular cargos to target them for endocytosis and subsequent incorporation into intralumenal vesicles of MVB (for review see Ingham et al., 2004; Shearwin-Whyatt et al., 2006).
Another family of Gag partners has recently emerged: the heterotetrameric clathrin adaptor protein (AP) complexes. APs participate in the selection of protein cargo and their incorporation into clathrin-coated transport vesicles (for reviews see McNiven and Thompson, 2006; Ohno, 2006). Four AP complexes have been identified, and they all exhibit a similar organization consisting of two large subunits (γ/β1, α/β2, δ/β3, ε/β4), a medium subunit (μ1-4), and a small subunit (ς1-4). The AP complexes display differences in cellular localization and mediate vesicle formation on distinct membrane compartments. The AP-1 complex is required for both Golgi-to-endosome and endosomes-to-Golgi transport (Meyer et al., 2000). However, some studies suggested that it can also be involved in transport from Golgi or early endosomes to late endosomes, lysosomes, or lysosome-related organelles (Reusch et al., 2002; Kyttala et al., 2005; Theos et al., 2005). AP-2 is involved in endocytosis at the plasma membrane (Traub, 2003). AP-3 mediates endosome-to-lysosome protein sorting (Reusch et al., 2002; Ihrke et al., 2004; Peden et al., 2004) as well as direct sorting from the trans-Golgi network (TGN) to lysosomes for proteins with strong lysosomal targeting signals (Rous et al., 2002; Ihrke et al., 2004).
Recent studies showed that AP-2 and AP-3 interact with HIV-1 Gag and participate in its trafficking and release (Batonick et al., 2005; Dong et al., 2005). HIV-1 Gag binds the δ subunit of AP-3 through the N-terminal portion of the matrix, and this enhances budding by targeting Gag to late endosomal compartments (Dong et al., 2005). In contrast, the AP-2 clathrin adaptor complex inhibits viral egress. HIV-1 Gag interacts with the medium subunit μ2 of this complex through a canonical tyrosine motif localized at the matrix and capsid junction. Disruption of this interaction enhances viral release, while diminishing the infectivity of the virions produced (Batonick et al., 2005).
In this study, we characterized a new partner of HIV-1 and MLV Gag, the medium subunit μ1A of the AP-1 clathrin adaptor complex, and we show that it is involved in their release.
MATERIALS AND METHODS
Plasmids and Two-Hybrid Assay
AP-1μA was cloned in mammalian expression vectors with the N-terminal glutathione S-transferase (GST) and C-terminal YFP tags, and MA-p12 of MLV was cloned with the N-terminal GST tag using the Gateway cloning system (Invitrogen, Carlsbad, CA). GST-GagHIV-1 was obtained by cloning HIV-1 Gag into pGex4T-1. His-μ1 was obtained by cloning AP-1μA open reading frame (ORF) into pET-32a. Vectors for MLV Gag and Alix were described previously (Segura-Morales et al., 2005). Vectors for WWP2 and Smurf were gifts of Dr. O. Staub (Department of Pharmacology and Toxicology, University of Lausanne, Lausanne, Switzerland) and Dr. X-H. Feng (Baylor College of Medicine, Houston, TX), respectively. The MLV vector encoding green fluorescent protein (GFP) was a gift of J.-L. Battini (CNRS, Montpellier, France). Vector pSG-Flag-μ1 was described previously (Berlioz-Torrent et al., 1999).
Fragments encoding the MA-p12 and CA-NC portions of MLV Gag, the full-length ORF of MLV, HIV-1, RSV, HTLV-1 Gag, and the ORF of μ1, μ2, μ3, β1, β2, β3, γ, σ2, σ3 and Nopp140 were cloned into two-hybrid plasmids (pACT-II and pAS2ΔΔ). Two-hybrid vectors for human Tsg101 and rat Nedd4 were gifts from Dr. W. Sundquist (Department of Biochemistry, University of Utah, Salt Lake City, Utah 84112-5650), Dr. D. Rotin (The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada, M5G 1X8), and Dr. O Staub. The three-hybrid vector pBridge for AP-3δ was a gift of J. Bonifacino (National Institutes of Health, Bethesda, MD 20892) (Janvier et al., 2005). For the two-hybrid assays, plasmids were introduced into the appropriate haploid strains (CG1945 or Y187), which were then crossed. Diploids were plated on double or triple selectable media (−Leu, Trp, or −Leu, Trp, His) and scored visually after 3 d at 30°C.
The in-frame deletion in the MA coding region of HIV-1 HXB2R provirus was generated as follows. Two PCR fragments containing nucleotides 655–833 of HXB2 (corresponds to first eight codons of HIV-1 Gag) and nucleotides 1182–1524 of HXB2 (corresponds to the last six codons of MA and the first 108 codons of CA) were digested with NarI, XhoI, and SpeI and ligated into a NarI-SpeI–digested HXB2R provirus.
Cell Culture and Transfection
HeLa, HeLa P4R5, 293T, HT1080, wild-type (WT) MEFs, and MEFs AP-1−/− cells were grown in DMEM supplemented with glutamine, antibiotics, and 10% decomplemented fetal calf serum (FCS). HeLa P4R5 cells were supplemented with 100 μg/ml geneticin and 1 μg/ml puromycin. HeLa, WT, and AP-1−/− MEFs were transfected using lipofectamine and plus reagent as recommended by the manufacturer (Invitrogen).
293T cells were transfected using the calcium phosphate method. Small interfering RNA (siRNA) transfections were performed with Lipofectamine RNA interference (RNAi) MAX (Invitrogen), using from 5 to 50 nM siRNA concentration. AP-1μ siRNA targeted the AP-1μ mRNA at sequence 5′ GGCAUCAAGUAUCGGAAGATT (positions 594–612), AP-1γ siRNA-targeted AP-1γ at sequence 5′ GCGCCTGTACAAAGCAATT (positions 1694–1713), and AP-3δ siRNA targeted AP-3δ at 5′ CCCTGTCCTTCATTGCCAA (positions 3159–3178). Control siRNAs against luciferase targeted the sequence 5′ CGUACGCGGAAUACUUCGATT (positions 153–171).
GST-Pulldown and Coimmunoprecipitation Assays
GST-μ1 or GST were produced by transiently transfecting 293T cells, purified, and immobilized on glutathione-Sepharose beads. GagMLV-YFP, Tsg101-CFP, or Nedd4.1-YFP were translated in vitro in rabbit reticulocyte lysates, using 35S-Met. They were then incubated with 5 μg of GST-μ1 or GST immobilized on beads, in interaction buffer (20 mM Tris-HCl, pH 8, 150 mM KCl, 1.5 mM MgCl2, 10% NP40). After 2 h at 4°C, beads were washed five times in washing buffer (20 mM Tris-HCl, pH 8, 200 mM KCl, 5 mM MgCl2, 0.1% NP40, 0.5 mM dithiothreitol), and resuspended in 1× Laemmli. Bound proteins were run on 10% SDS-PAGE, and the labeled proteins were detected by autoradiography. In vitro interactions between His-μ1 and GST-GagHIV-1 were performed as described previously, using 3 μg of His-μ1 and 6 μg of immobilized GST fusions (Lopez-Verges et al., 2006).
Coimmunoprecipitation assays with GST-MA-p12, Nedd4.1, and Flag-μ1 were performed as described previously (Segura-Morales et al., 2005; Lopez-Verges et al., 2006). For HIV-1 Gag, 293T cells were transfected with proviral DNAs (HXB2R WT that does not express Nef and Vpu or HXB2R ΔENV that does not express Nef, Vpu, and Env; gift of F. Mammano, INSERM, Paris, France) and treated as described previously (Lopez-Verges et al., 2006).
Purification and Analysis of MLV Virions
Viral particles were purified and analyzed as described previously (Segura-Morales et al., 2005). For monensin treatment, cells were pretreated for 45 min with 5 μM monensin, washed, and then incubated for 5 h with the same concentration of drug.
MLV Entry and Infectivity Tests
For the MLV infectivity tests the supernatants of chronically infected AP-1−/− and WT MEFs were normalized by the level of Gag and used to infect indicator Dunni cells. Two days after, the cells were fixed and labeled with anti-envelope H48 antibodies, and foci of infection were counted.
For the entry test AP-1−/− and WT MEFs were infected with nonreplicating MLV coding for GFP and bearing ecotropic MLV envelope. Three days after infection, cells were fixed, and GFP-positive cells were counted by FACS.
HIV-1 Replication in Cells Lacking or Overexpressing AP-1μ
Viral stocks of HIV-1 HXB2R were prepared as previously described (Lopez-Verges et al., 2006). HeLa P4R5 cells, depleted or not for AP-1μ, were infected with HIV-1. Twenty-four hours after infection, cells were washed and placed in a fresh medium. Supernatants were collected every 24 h and HIV-1 p24 was quantified by ELISA. At the end of the experiment, cells were lysed and extracts were analyzed by Western blotting.
For single-round replication assays, AP-1–depleted HeLa cells were transfected with HIV-1 HXB2R or HXB2ΔEnv proviral DNAs. For overexpression experiments HeLa cells were transfected with HIV-1 HXB2R alone or in combination with pSG-Flag or pSG-Flag-μ1 vector. After 24 h, cells were washed and cultured for two additional days. Viral fractions were collected, quantified for HIV-1 p24 by ELISA, and used to infect CD4+ CCR5+ HeLa P4R5 indicator cells. Cell extracts were also analyzed by Western blotting.
Cell Imaging and Antibodies
Immunofluorescence was performed as described previously (Segura-Morales et al., 2005). Samples were analyzed with a DMRA microscope (Leica, Deerfield, IL). Three-dimensional (3D) image stacks were acquired with a CCD camera (Coolsnap Fx, Roper Scientific, Tucson, AZ), which was controlled by Metamorph (Universal Imaging, West Chester, PA). Image stacks were deconvolved with Huygens (Bitplane, Zurich, Switzerland), and maximal image projections of the 3D stacks were overlayed with Photoshop (Adobe, San Jose, CA).
Lysotracker (Molecular Probes, Eugene, OR) was used to label late endosomes. Anti-CA antibody (monoclonal R187 against the capsid part of MLV Gag) and monoclonal and polyclonal anti-MLV αEnv (H48 and 805-24) were kind gifts of B. Chesebro (Laboratory of Persistent Viral Diseases, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases/NIH, Hamilton, MT 59840). Anti-μ1 antibody was a kind gift of L. M. Traub (Department of Cell Biology and Physiology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261). Anti-AP-1γ (100/3) and anti-tubulin (DM1A) antibodies were from Sigma (St. Louis, MO). HIV-1 Gag was detected with rabbit anti-CAp24 (from National Institutes of Health), and Flag-AP-1μ with mouse anti-Flag antibody (FLAG M2 HRP, Sigma). Anti CD-81 antibody was a kind gift of E. Rubinstein (INSERM, Villejuif, France).
To quantify colocalization of Gag MLV with endosomal marker, a series of 3D, deconvolved images of Gag in fixed cells were taken together with a marker of lysosomes (lysotracker). The images of the marker were then used to create 3D masks, and the masks were used to count the fraction of Gag associated with the marker. The percent of Gag on the lysosomal compartment was then calculated by dividing the fluorescence of Gag associated with lysotracker by the total amount of Gag fluorescence.
Electron Microscopy
Cells were fixed in 3.5% glutaraldehyde, phosphate buffer (0.1 M, pH 7.4) overnight at 4°C. Next day cells were washed in phosphate buffer and postfixed in 1% osmic acid, 0.8% potassium ferrocyanide for 1 h at room temperature, then washed twice with phosphate buffer, dehydrated in a graded series of ethanol, and embedded in epon resin. Sections at 85 nm were cut with Leica-Reichert Ultracut E and collected at different levels of each block. The sections were counterstained with uranyl acetate and lead citrate and observed using a Hitachi 7100 transmission electron microscope (Pleasanton, CA).
RESULTS
MLV and HIV-1 Gag Interact with AP-1μ
To find new partners of retroviral Gag proteins, we performed systematic two-hybrid assays in yeast. The entire coding region of HIV-1 Gag was fused to the Gal4 DNA-binding domain and was used in directed tests against proteins of the endocytic machinery (Figure 1A). We found that the μ1A medium chain of the AP-1 clathrin-adaptor complex (AP-1μ) interacted with HIV-1 Gag. This interaction was specific for AP-1μ because HIV-1 Gag did not interact with the homologous subunits μ2 and μ3 of AP-2 and AP-3 complexes, respectively (Figure 1B). We did not detect a two-hybrid interaction with AP-2μ and AP-3δ (data not shown), although these interactions have been found by other methods and with other two-hybrid vectors (Batonick et al., 2005; Dong et al., 2005). To verify that the yeast two-hybrid data reflected physiological interactions that occur in mammalian cells, we performed pulldown and coimmunoprecipitation experiments. AP-1μ purified from bacteria (His-μ1) interacted in vitro with recombinant HIV-1 Gag fused to GST, but not with GST alone (Figure 2A, lanes 3 and 2, respectively). These in vitro results were confirmed by coimmunoprecipitation assays between AP-1 and HIV-1 Gag and in an infectious proviral expression context, as well as with a provirus that did not express the envelope glycoprotein. Indeed, in extracts of infected cells, the γ subunit of AP-1 efficiently coprecipitated the Pr55Gag precursor as well as the p41 maturation product (an intermediate cleavage product corresponding to the MA domain linked to CA; Figure 2B, lanes 5 and 6). Interestingly, the CA domain did not coprecipitate with AP-1γ (Figure 2B, lanes 5 and 6). These data correlated with our in vitro binding assay showing that the MA domain of HIV-1 Gag was sufficient for the interaction with AP-1μ (Figure 2A, lane 5).
Figure 1.
Two-hybrid interactions of AP-1μ with MLV and HIV-1 Gag. (A) Schematic representation of retroviral Gag proteins. Matrix (MA), capsid (CA), and nucleocapsid (NC) domains are shown respectively in gray, light gray, and dark gray, respectively. (B) MLV and HIV-1 Gag interact with AP-1μ in two-hybrid assays. HIV-1 and MLV Gag were fused to the Gal4 DNA-binding domain and tested against μ1, μ2, μ3 fused to the Gal4 activation domain. Nopp140 was used as a negative control. Strains producing the hybrid proteins were analyzed for histidine auxotrophy. -L-W, mating control; -L-W-H, growth only if the tested proteins interact. (C) MLV MA-p12 mediates the interaction of Gag with AP-1μ. The indicated portions of MLV Gag were fused to the Gal4 DNA-binding domain and tested as in B.
Figure 2.
MLV and HIV-1 Gag interact with the AP-1 complex in vitro and in vivo. (A) In vitro binding of AP-1μ to HIV-1 Gag. His-μ1–purified from bacteria was incubated with equal amounts of immobilized GST, GST-Gag, GST-MA, or GST-CA. Top panel, bound material was analyzed by Western blot using anti-His antibodies. Bottom panel corresponds to the ponceau red staining of the membrane; 20% of input was loaded in lane 1. (B) Coimmunoprecipitation of HIV-1 Gag with the AP-1 complex. 293T cells were transfected with HIV-1 HXB2R (HIV) or HXB2RΔEnv (HIVΔEnv) proviral DNAs. The endogenous AP-1 complex was immunoprecipitated with anti-AP-1γ or with anti-α-tubulin antibodies as a control. Bound material was analyzed by Western blot with anti-AP-1γ (top panel) and anti-CAp24 antibodies (bottom panel). Left panel, 10% of input. (C) In vitro binding of AP-1μ to MLV Gag. 35S-labeled MLV Gag was translated in vitro and incubated with GST or GST-μ1 immobilized on beads. Top panel, the autoradiogram of bound material; the bottom panel corresponds to the Coomassie blue staining of purified GST and GST-μ1. Asterisk shows a nonspecific protein that copurified; 5% of input was loaded in lane 1. (D) Interaction of the endogenous AP-1 complex with MLV MA-p12. GST-MA-p12 and GST were expressed in 293T cells and purified on glutathione-Sepharose beads, and bound proteins were analyzed by Western blotting using anti-AP-1γ antibodies.
Many proteins involved in the budding of HIV-1 also interact with the Gag proteins of other retroviruses, and this conservation likely reflects a selective advantage conferred by the interaction. To test whether this was the case for AP-1μ, we investigated whether it could bind MLV Gag. Remarkably, we found that AP-1μ also interacted with MLV Gag in two-hybrid assays (Figure 1B). Similar to HIV-1, the matrix-p12 region of MLV Gag was necessary and sufficient for this interaction (Figure 1C), and the interaction was specific because MLV Gag did not interact with other subunits of AP complexes tested in our screen, including β1, σ1, γ of AP-1; μ2, β2, σ2 of AP-2; and μ3, β3, σ3 of AP-3 (Figure 1B and data not shown). The interaction between MLV Gag and AP-1μ could also be confirmed in vitro: 35S-labeled, in vitro–translated MLV Gag interacted with purified GST-AP-1μ, but not with GST alone or with control beads (Figure 2C). Finally the MA-p12 portion of MLV Gag recruited the γ subunit of the AP-1 complex in vivo, as shown in coimmunoprecipitation experiments using 293T cell extracts (Figure 2D).
Taken together, these results demonstrate that Gag proteins of two different viruses interact with AP-1μ in vitro and are able to recruit the whole AP-1 complex in cells.
The AP-1 Complex Is Important for the Dissemination of HIV-1 and MLV
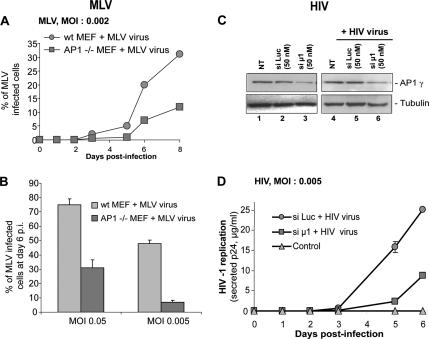
To establish whether the AP-1μ/Gag interaction had a functional role, cells lacking AP-1μ were challenged with MLV and HIV-1 viruses. In the case of MLV, we used AP-1−/− mouse embryonic fibroblasts (MEFs) that contained a targeted disruption of the μ1A gene. In these cells, the lack of μ1A leads to an absence of the AP-1 complex on membranes, provoking defects in mannose-6-phosphate receptors recycling and mis-sorting of lysosomal enzymes (Meyer et al., 2000). AP-1−/− and WT MEFs were infected with MLV at a low multiplicity of infection (MOI of 0.002), cells were harvested every 2 days and labeled with anti-envelope antibodies, and the number of infected cells was counted by fluorescence-activated cell sorting (FACS). A defect in viral spreading was observed in AP-1−/− cells, because the percentage of infected cells was fivefold and threefold lower at days 5 and 8 after infection, respectively (Figure 3A). At higher MOIs of 0.005 and 0.05, the percentage of infected AP-1−/− cells at days 6 and 7 after infection was 7- and 2.4-fold lower, respectively, compared with WT cells (Figure 3B).
Figure 3.
MLV and HIV-1 replication requires AP-1. (A and B) Kinetic of MLV replication in AP-1 knockout cells. (A) WT and AP-1−/− MEFs cells were infected with MLV (strain F57) at an MOI of 0.002. Infected cells were labeled with anti-envelope antibodies and counted by FACS (mean of two experiments, ±SD). (B) WT and AP-1−/− MEFs cells were infected with MLV (strain F57) at MOIs of 0.05 or 0.005. Infected cells were counted by FACS at day 6 after infection (mean of five experiments, ±SD). (C and D) Kinetic of HIV-1 replication in AP-1μ–depleted cells. HeLa P4R5 cells were depleted with siRNAs against luciferase (si Luc) or AP-1μ (si μ1) and infected with HIV-1 HXB2R at an MOI of 0.005. (C) Western blots demonstrate a decrease in AP-1γ levels, both at day 0 (lanes 1–3) and day 6 after infection (lanes 4–6). (D) Culture supernatants were collected daily for 6 d after infection and quantified for HIV Gag p24 (mean of two experiments, ±SD). Controls: nontransfected, and noninfected cells.
We next studied HIV-1 replication in HeLa P4R5 cells, which stably expressed CD4 and CCR5. Cells were treated with siRNAs against AP-1μ or luciferase (as a negative control) and infected with HIV-1 at an MOI of 0.005. Previous studies have reported that the silencing of AP-1μ destabilizes the AP-1 complex (Meyer et al., 2000; Janvier and Bonifacino, 2005), and we thus followed the effect of the siRNAs by controlling expression of the AP-1γ subunit (Figure 3C). We observed a significant replication defect in AP-1–depleted cells: the release of p24 was decreased by 80% at day 5 after infection (Figure 3D).
Taken together, these results demonstrate that the AP-1 complex is required to obtain an optimal replication of HIV-1 and MLV in cell culture.
Gag Release Is Affected by the Absence of AP-1μ
The observed delay in viral propagation could be due either to an entry defect or to a decreased production of infectious particles. We first assessed whether viral entry was affected. For this purpose, AP-1−/− and WT MEFs were infected with nonreplicating MLV particles encoding GFP, and GFP-positive cells were counted by FACS. These experiments showed similar percentages of infected AP-1−/− and WT cells (Supplementary Figure 1D). Similarly, the entry of HIV-1 was unchanged in cells treated with the AP-1μ siRNAs (data not shown). Thus, viral entry was not affected by the absence of AP-1.
The AP-1 complex is involved in the bidirectional trafficking of cellular proteins between the TGN and endosomes. Thus, we hypothesized that AP-1 could be involved in the proper trafficking of Gag and other viral components before they form virions and leave the cells. To address this issue, we monitored whether the release of Gag was affected by the absence of AP-1. A fusion between MLV Gag and yellow fluorescent protein (YFP), which allows budding defects to be easily detected (Segura-Morales et al., 2005), was transfected into AP-1−/− and WT MEFs, and its ability to bud was evaluated by measuring its levels in the viral and cellular fractions. We observed that AP-1−/− cells released 7 ± 2.4-fold less of GagMLV-YFP than WT cells, whereas intracellular levels were similar (Figure 4A, lane 2 vs. 1, respectively). We next measured viral release in the context of the entire virus. The amount of Gag released by chronically infected WT and AP-1−/− cells was quantified, and we found that AP-1−/− cells secreted 4.1 ± 2.3-fold less Gag in the extracellular medium, suggesting that viral release was affected even in the presence of other viral components (Figure 4B). To confirm that the defects in Gag release were due to the absence of AP-1μ, we attempted to restore its egress by introducing an exogenous AP-1μ. AP-1−/− MEFs were cotransfected with GagMLV-YFP and increasing amounts of AP-1μ-YFP. As expected, introduction of AP-1μ-YFP led to an increase of γ-adaptin expression and its recruitment to the Golgi apparatus and endosomes (data not shown). We observed that the release of GagMLV-YFP increased when AP-1μ was present, and this increase correlated with the amount of AP-1μ expressed within the cells (Figure 4A, lanes 2–5).
Figure 4.
Production of MLV and HIV-1 viral particles is inhibited by the absence of AP-1. (A) Release of MLV Gag by WT and AP-1−/− MEFs. WT MEFs were transfected with MLV Gag fused to YFP, and AP-1−/− cells were transfected with either Gag-YFP alone or with Gag-YFP plus increasing amounts of μ1-YFP. Top panel, expression of μ1-YFP detected by Western blotting with anti-μ1 antibodies, and the numbers indicate the amount of transfected μ1-YFP plasmid (μg). Middle and the bottom panels, the levels of Gag-YFP in the cellular and viral fractions (detected with anti-capsid antibodies), respectively. Left panel, WT MEFs, right panel, AP-1−/− MEFs. (B) MLV Gag release by chronically infected WT and AP-1−/− MEFs. Cell lysates and virions produced by WT and AP-1−/− cells chronically infected with MLV were analyzed by Western blot using anti-capsid antibodies. (C and D) AP-1 depletion decreases the release of HIV-1 particles. HeLa cells treated with siRNAs against AP-1μ (si μ1) or luciferase (si Luc) were transfected with HIV-1 proviral DNA. (C) Depletion of AP-1 and the intracellular expression of HIV-1 Gag were analyzed by Western blotting with anti-AP-1γ and anti-CAp24 antibodies. (D) Secreted HIV-1 CAp-24 was quantified by ELISA (mean of four experiments, ±SD). NT, nontransfected cells. (E and F) AP-1μ overexpression has a dose-dependent effect on HIV-1 release. HeLa cells were cotransfected with HIV-1 proviral DNA and increasing amounts of Flag-μ1. (E) Expression of Flag-μ1, AP-1γ, and Gag (p55Gag, p41, CAp24) were analyzed by Western blot using anti-FLAG M2, anti-AP-1γ, and anti-CAp24γ antibodies, respectively. (F) Secreted HIV-1 CAp24 was quantified by ELISA (mean of two experiments performed in duplicate). NT, nontransfected cells.
Similarly, the release of HIV-1 Gag was studied in AP-1–depleted cells. HeLa cells were treated with siRNAs against AP-1μ or luciferase and then transfected with an HIV provirus. Silencing of AP-1μ inhibited the release of viral particles by 57%, whereas the amount of intracellular Gag was not modified (Figure 4, C and D). Interestingly, this defect was also observed in absence of the retroviral envelope (Supplementary Figure 1, A and B). To confirm these results, we investigated the effect of depletion of AP-1γ, and we found that the release of viral particles was inhibited by 70% (see below). To further document the role of AP-1 in HIV-1 release, we investigated the effect of AP-1μ overexpression in nondepleted cells. An HIV-1 provirus was cotransfected with increasing amounts of Flag-AP-1μ in HeLa cells. Flag-AP-1μ was incorporated in the AP-1 complex (Supplementary Figure 2), and its overexpression led to an increased expression of AP-1γ (Figure 4E). Remarkably, we observed that viral release was enhanced by Flag-AP-1μ in a dose-dependant manner (Figure 4, E and F). Taken together, these data suggested that the AP-1 complex is involved in the release of MLV and HIV-1 Gag.
To investigate in more details the role of AP-1 in retroviral budding, we examined HIV-1– and MLV–producing cells by thin-section electron microscopy. HeLa cells infected with HIV-1 revealed clusters of budding viral particles at the plasma membrane. In contrast, in AP-1–depleted cells, only isolated budding profiles were detected (Figure 5A). WT MEFs chronically infected with MLV displayed numerous free and budding virions, opposite to AP-1−/− MEFs, where few free virions and isolated budding profiles were observed (Figure 5B). Thus, AP-1 depletion did not lead to a “late phenotype,” but to a decrease of the number of budding virions.
Figure 5.
AP-1 depletion leads to a decrease of budding profiles. (A) Thin-section electron microscopy analysis of HeLa cells infected with HIV-1 and treated either with siRNA luc or with siRNA AP-1μ. (B) Thin-section electron microscopy analysis of WT and AP-1−/− MEFs chronically infected with MLV.
An HIV-1 Gag Mutant Lacking the Matrix Is Insensitive to AP-1μ Depletion
To confirm that the role of AP-1 was mediated by a direct interaction between the matrix and AP-1μ, we used an HIV-1 provirus lacking most of the matrix domain (HIV-1-ΔMA). In this virus, only the first eight amino acids of the matrix were retained, and this was sufficient to promote its efficient release in the extracellular medium (Reil et al., 1998). The release of HIV-1 Gag-ΔMA was studied in AP-1γ–depleted cells. HeLa cells were treated with siRNAs against AP-1γ or luciferase and then transfected with the HIV-ΔMA provirus. Although siRNA against AP-1γ efficiently inhibited the release of WT virus (Figure 6B), the production of ΔMA particles was not significantly diminished (Figure 6A). These data suggest that AP-1 participates in HIV-1 release through a direct interaction with the MA domain of HIV-1 Gag.
Figure 6.
AP-1 mediates its effect through a direct interaction with matrix and acts in concert with AP-3 to facilitate Gag release. (A) Release of HIV-1 ΔMA is not inhibited by AP-1 and AP-3 depletion. HeLa cells were treated with siRNAs against: AP-1γ (si γ1), AP-3δ (si δ3), AP-1γ/AP-3δ (si γ1δ3), and siRNA luciferase (si luc). Cells were then transfected with an HIV-1ΔMA provirus. Top panel, secreted HIV-1 CAp-24 was quantified by ELISA (mean of four experiments, ±SD). NT, nontransfected cells. Bottom panel, intracellular expression of AP-1 and AP-3, and HIV-1 Gag were analyzed by Western blotting with anti-AP-1γ, anti-AP-3δ, and anti-CAp24 antibodies. (B) AP-1 and AP-3 function on the same pathway of HIV-1 Gag release. HeLa cells were treated with siRNAs against the following: AP-1μ (si μ1), AP-1γ (si γ1), both AP-1μ and AP-1γ (si μ1γ1), AP-3δ (si δ3), AP-1γ, and AP-3δ (si γ1δ3) and siRNA luciferase (si luc) and then transfected with HIV-1 provirus. Top panel, secreted HIV-1 CAp-24 was quantified by ELISA (mean of four experiments, ±SD). NT, nontransfected cells. Bottom panel, depletion of AP-1 and AP-3 and the intracellular expression of HIV-1 Gag were analyzed by Western blotting.
Retroviral Infectivity Is Not Affected by the Absence of AP-1μ
The cytoplasmic domains of HIV-1 and MLV envelope glycoproteins interact with AP-1μ, and the lack of this factor could thus affect envelope incorporation and the infectivity of viral particles (Berlioz-Torrent et al., 1999; Blot et al., 2006). To test this hypothesis, we purified the virions produced by AP-1−/− and WT fibroblasts chronically infected with MLV, and we measured the amount of incorporated envelope. Quantification of Western blots showed that WT and AP-1−/− cells produced viruses with similar amounts of envelope (data not shown). Furthermore, when the viral supernatants were normalized to the Gag level and were used to infect permissive cells, the infectivity of virions produced by WT and AP-1−/− cells was equivalent (Supplementary Figure 1E).
We next studied the infectivity of HIV-1 virions produced in the absence of AP-1μ. Viral particles produced by control and AP-1μ–depleted HeLa cells were purified, normalized to p24 levels and used to infect indicator cells. The virions produced by AP-1μ–depleted and control cells had similar titers (Supplementary Figure 1C). Altogether, these results indicate that the absence of AP-1μ does not affect infectivity of MLV or HIV-1 virions.
AP-1 and AP-3 Act on the Same Pathway That Leads to Gag Release
It has been previously shown that the AP-3 clathrin adaptor complex interacts directly with HIV-1 Gag through AP-3δ subunit, and that it enhances Gag release (Dong et al., 2005). Because AP-1 and AP-3 can cooperate to transport cellular proteins, we were interested to find out if AP-1 and AP-3 complexes were acting on the same pathway leading to Gag release. To answer this question, the release of HIV-1 Gag was compared in AP-1γ– and AP-3δ–depleted cells, as well as in cells where both were depleted simultaneously. HeLa cells were treated with siRNAs against AP-1γ, AP-3δ, or both and then transfected with a WT or HIV ΔMA provirus. Depletion of AP-1γ and AP-3δ was very efficient as these proteins could no longer be detected by Western blot (Figure 6B). The release of HIVΔMA was not significantly diminished by silencing of AP-1 alone or in combination with AP-3 (Figure 6A). In contrast, silencing of AP-1γ inhibited the release of WT viral particles by 70% (Figure 6B), whereas silencing of AP-3δ led to a 75% inhibition (Figure 6B). Interestingly, when the AP-1 and AP-3 complexes were silenced simultaneously, the release of WT viral particles was inhibited by 85%, which was similar to the inhibition observed upon the simultaneous depletion of AP-1μ and AP-1γ (Figure 6B).
Altogether, these data showed that the effect of AP-1 and AP-3 depletion on HIV-1 Gag release was not additive, suggesting that these two adaptors do not compensate for each other to promote Gag release. The most plausible interpretation is that AP-1 and AP-3 could be involved in two different steps of the same pathway that leads to Gag budding.
Intracellular Trafficking of MLV Gag Is Altered by the Absence of AP-1μ
To evaluate a possible role of AP-1 in the trafficking of MLV Gag, we studied its intracellular localization, with a particular interest for late endosomes. To label this compartment, we used lysotracker, which colocalized with the tetraspanin CD81, known to be present in late endosomal compartments in several cell types (Pelchen-Matthews et al., 2003; Supplementary Figure 3). It appeared that less Gag was accumulated in late endosomes in AP-1−/− cells compared with WT (Figure 7A). The amount of Gag colocalized with late endosomes was then quantified, and we found that in WT MEFs, 35 ± 10% of intracellular Gag colocalized with lysotracker, whereas in AP-1−/− cells, only 12 ± 7% was associated with this marker.
Figure 7.
Intracellular trafficking of MLV Gag is affected in the absence of AP-1. (A) Localization of MLV Gag in WT and AP-1−/− cells chronically infected with MLV. WT and AP-1−/− MEFs were labeled with anti-capsid antibodies (green) and lysotracker (a marker for late endosomes, red). The numbers on the right indicate the proportion of Gag associated with late endosomes; 30 cells were analyzed in each case. (B) Monensin differentially affects the production of MLV virions in WT and AP1−/− cells. The level of MLV Gag in cell lysates (top) and viral supernatants (bottom) of WT and AP1−/− chronically infected cells treated with 5 μM monensin for 5 h, and control was analyzed by Western blot with anti-CA antibodies.
To further elucidate the role of AP-1μ in the trafficking of MLV Gag, we studied the effect of endosomal poisons. Monensin is a monovalent ion-selective ionophore that facilitates exchange of sodium ions for protons and blocks the acidification of Golgi, endosomes, and lysosomes. It does not inhibit internalization, but it affects intracellular vesicular trafficking (Mollenhauer et al., 1990). We have previously shown that monensin inhibits the release of MLV Gag, but only when coexpressed with envelope. This suggested that MLV Gag follows an endosomal pathway when the envelope is present, but that it exits cells in an endosome-independent manner when expressed alone (Basyuk et al., 2003). Chronically infected WT and AP-1−/− cells were treated with monensin for 5 h, and the VLPs were purified and analyzed by Western blot. As expected, the release of MLV Gag was inhibited by monensin. However, this inhibition was 6 ± 1.2-fold lower in the absence of AP-1 (Figure 7B). This result suggests that in AP-1−/− cells, MLV Gag transits via a different intracellular route, which is less sensitive to monensin.
AP-1μ Interacts with Proteins Involved in MVB Sorting Nedd4.1 and Tsg101
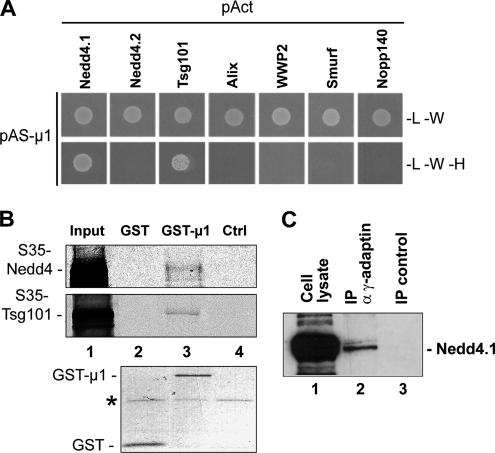
To obtain more insights into the function of AP-1 in Gag budding, we tested whether there was any connection between AP-1 and the formation of intralumenal vesicles of MVBs. For this purpose, we checked yeast two-hybrid interactions between AP-1 and endosomal proteins involved in this pathway: Tsg101, Alix, and Nedd4-family members (Nedd4.1, Nedd4.2, WWP2, and Smurf). Remarkably, AP-1μ interacted with Nedd4.1 and Tsg101 in this assay (Figure 8A and Supplementary Figure 4A). In addition, we have found that AP-1γ also interacted with Tsg101 (Supplementary Figure 4A). To confirm these results, we performed GST pulldown experiments. We found that in vitro–transcribed Nedd4.1-YFP and Tsg101-CFP interacted specifically with GST-μ1, but not with GST alone or with control beads (Figure 8B). In addition, we found that endogenous Nedd4.1 coimmunoprecipitated with AP-1 in cell extracts (Figure 8C). These results suggested that AP-1μ interacted with Nedd4.1 and Tsg101. Importantly, both proteins are involved in MLV budding, while Tsg101 is essential for HIV-1 budding.
Figure 8.
AP-1 interacts with Nedd4.1 and Tsg101. (A) Interaction of AP-1μ with Nedd4.1 and Tsg101 in yeast two-hybrid assays. AP-1μ was fused to the N-terminus of the Gal4 DNA-binding domain, and tested against Tsg101, Smurf, Alix, WWP2, Nedd4.1, Nedd4.2, and Nopp140. Legend as in Figure 1B. (B) In vitro binding of AP-1μ to Nedd4.1 and Tsg101. 35S-labeled Nedd4.1-YFP and Tsg101-YFP were translated in vitro and mixed with immobilized GST or GST-μ1. Top and middle panels, the autoradiograms; bottom panel, corresponding Coomassie blue staining of the purified GST proteins. Asterisk shows a nonspecific protein that copurified; 5% of input was loaded in lane 1. (C) Coimmunoprecipitation of Nedd4.1 with the AP-1 complex. AP-1 was immunoprecipitated from extracts of HT1080 cells, using anti-γ-adaptin antibodies, and bound proteins were analyzed by Western blots with anti-Nedd4.1 antibodies. The control corresponds to materials precipitated without antibodies; 5% of input was loaded in lane 1.
DISCUSSION
In this study, we report that two retroviral Gag proteins interact with the clathrin adaptor complex AP-1 and require its function for optimal budding. Indeed, both MLV and HIV-1 Gag bind AP-1μ in yeast two-hybrid, GST pulldown, and coimmunoprecipitation assays. It has been previously demonstrated that HIV-1 Gag binds to AP-2 and AP-3 clathrin adaptor complexes. Binding to the AP-2μ subunit involves a tyrosine motif, which is located at the matrix-capsid junction (Batonick et al., 2005), whereas binding to AP-3 occurs between the H1 helix of the matrix and the large subunit AP-3δ (Dong et al., 2005). Here, we demonstrate that the binding of AP-1 to Gag requires the matrix of HIV-1 Gag and the MA-p12 portion of MLV Gag. We show that the binding of Gag to AP-1 occurs via the AP-1μ chain, but the exact motif involved in this interaction was not identified. Two putative tyrosine-based motifs and one di-leucine motif are present in the HIV-1 matrix, whereas MA-p12 of MLV contains one tyrosine motif and three di-leucine motifs. These sequences could act as sorting motifs recognized by the AP-1 complex. Further study will be necessary to fully elucidate the motifs implicated in this interaction.
We have shown that the adaptor complex AP-1 is required for HIV-1 and MLV release. Knockout of AP-1μ in MEFs or silencing of the AP-1 complex by AP-1μ or -1γ siRNA in HeLa cells reduced egress of MLV and HIV-1 viruses. This defect could be restored by re-expression of AP-1μ in AP-1−/− cells. Similarly, overexpression of AP-1μ stimulated HIV-1 release by HeLa cells. AP-1 interacts with HIV-1 and MLV envelope glycoproteins (Ohno et al., 1997; Wyss et al., 2001; Blot et al., 2006), as well as with Nef (Bresnahan et al., 1998; Greenberg et al., 1998; Le Gall et al., 1998; Piguet et al., 1998). However, the defect of particle production was observed in the absence of these proteins, suggesting that the lack of AP-1 affects Gag. Furthermore, the release of an HIV-1 Gag mutant lacking the matrix was insensitive to the AP-1 depletion. Taken together, our results suggest that AP-1μ facilitates particle production through a direct interaction with the MA domain of Gag.
The AP-1 adaptor complex is involved in the transport of cargos from the TGN to the early endosomal compartments (Doray et al., 2002; Puertollano et al., 2003; Waguri et al., 2003) and in the retrograde transport between early endosomes and the TGN (Meyer et al., 2000). In addition, there is some evidence that AP-1 can drive cargos into MVBs and late endosomes. In many cases, AP-1 does this by cooperating with AP-3 (Reusch et al., 2002; Kyttala et al., 2005). For instance, mCMV protein gp48 directs MHC-I to lysosomes, which involves AP-1–mediated TGN-to-endosome sorting, followed by AP-3–mediated endosome-to-lysosome sorting (Reusch et al., 2002). However, recent studies suggest that in specialized cell types, AP-1 could be involved in the transport from early endosomes to specialized lysosome-related organelles. Indeed, in melanocytes AP-1 and AP-3 function in partially redundant sorting pathways (Theos et al., 2005).
It was shown previously that AP-3 binds HIV-1 Gag and is involved in its release (Dong et al., 2005). Our data show that the release of HIV-1 Gag was inhibited to a similar extent either by the absence of AP-1 or AP-3 alone, or by both adaptors simultaneously. This indicates that AP-1 and AP-3 cannot compensate for each other and suggests that both complexes are probably involved in sequential steps of the same pathway leading to Gag release. Interestingly, electron microscopy analysis showed that AP-1 silencing does not lead to a late phenotype, but to a decrease of budding profiles. Similar phenotypes were described in the AP-3–depleted cells (Dong et al., 2005), indicating that AP-1 and AP-3 act upstream of ESCRT machineries.
It has been proposed that the localization of HIV-1 Gag to late endosomes is important to promote budding, and AP-1 could be thus involved in the transport of Gag to this compartment. HIV-1 Gag is associated with the plasma membrane and is also found in late endocytic compartment in macrophages and in several cell lines including HeLa, 293, and Mel JuSo (Raposo et al., 2002; Nydegger et al., 2003; Pelchen-Matthews et al., 2003; Sherer et al., 2003; Ono and Freed, 2004; Grigorov et al., 2006). Recently, Resh and coworkers have reported that, early after its synthesis, HIV-1 Gag accumulates in perinuclear cluster and then travels through the late endocytic compartment on its way to the plasma membrane (Perlman and Resh, 2006). Furthermore, HIV-1 Gag was depleted from the late endosomes upon disruption the Gag-AP-3 interaction, and this correlated with a decrease of retroviral release (Dong et al., 2005). Interestingly, the ubiquitin ligase POSH, involved in HIV-1 release, is localized to TGN. Moreover, HIV-1 Gag has been found associated with this compartment upon inhibition of vesicular trafficking, suggesting that it may transit through the Golgi on its way to plasma membrane (Alroy et al., 2005). Thus, one possibility could be that AP-1 drives Gag from the Golgi to early endosomes, where AP-3 subsequently directs it to late endosomes. Consistently with this hypothesis, we observed that less MLV Gag was associated with late endosomes in AP-1−/− fibroblasts.
However, the role of late endosomes in HIV-1 release has been questioned recently (Jouvenet et al., 2006; Welsch et al., 2007). It is thus possible that AP-1 may be directly involved in early steps of the budding process and in the biogenesis of virions. Indeed, we have shown that AP-1μ binds Tsg101 and Nedd4.1, and it could thus facilitate the recruitment of ESCRT machineries to Gag. For HIV-1, the simultaneous binding of AP-1 and Gag to Tsg101 could also help to localize the ESCRT machinery to the sites of budding. We could also imagine that the property of the clathrin adaptors to form coats and to recruit simultaneously clathrin, cargoes, and accessory proteins may be used by Gag to create a scaffold facilitating its assembly and interactions with budding partners. In this respect, it is worth to mention that AP-1 and clathrin form a stabilizing scaffold that is essential for the morphogenesis of WPBs (Weibel Palade Bodies), a specialized organelle of endothelial cells, and this role is independent of the function of AP-1 in vesicular trafficking (Lui-Roberts et al., 2005).
It is important to keep in mind that retroviral budding is very complex. In this regard, cell-type variations in the role of AP complexes are known to exist and could account for some of this complexity. For instance, AP-1 and AP-3 may function in parallel pathways in some cells (Theos et al., 2005), and Gag may thus use preferentially AP-1 or AP-3 for its targeting to the viral budding site (the plasma membrane or an intracellular site, depending on the cell type). It is also possible that viruses may use AP complexes in a nonconventional way, for instance, to bring early Gag complexes from the cytosol to cellular membranes.
Remarkably, the interaction of Gag with AP-1μ is conserved among different retroviruses. Gag of RSV and HTLV interact with AP-1μ in two-hybrid assays (Supplementary Figure 4B), suggesting that AP-1 could also play a role in the assembly of these retroviruses. Interestingly, the hepatitis B virus needs both a clathrin adaptor γ2-adaptin and a ubiquitin ligase Nedd4 for its assembly (Rost et al., 2006), raising the more general possibility that enveloped viruses can use a combination of clathrin adaptors and ubiquitin-ligases or ESCRT components for their egress.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Benarous for his constant support, helpful discussions and precious advices. We thank K. Janvier, A. Benmerah, P. Benaroch, and H. Wodrich for helpful discussions and critical reading of the manuscript; N. Taylor and I. Robbins for critical reading of the manuscript; M. Thali (University of Vermont), A. Benmerah (INSERM, Paris, France), the National Institute for Biological Standards and Control (NIBSC, Hertfordshire, United Kingdom) and the National Institutes of Health (Bethesda, MD) for kindly providing reagents; and R. Beauvoir for technical assistance. G.C., C.S-M. and E.B. have fellowships from ANRS and S.L.V. from FRM. This work was supported by grants from the ANRS, SIDACTION, the Fondation pour la Recherche Médicale, and from Fondation de France.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1147) on May 30, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Alroy I., et al. The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production. Proc. Natl. Acad. Sci. USA. 2005;102:1478–1483. doi: 10.1073/pnas.0408717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002a;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002b;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Basyuk E., Galli T., Mougel M., Blanchard J., Sitbon M., Bertrand E. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell. 2003;5:161–174. doi: 10.1016/s1534-5807(03)00188-6. [DOI] [PubMed] [Google Scholar]
- Batonick M., Favre M., Boge M., Spearman P., Honing S., Thali M. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology. 2005;342:190–200. doi: 10.1016/j.virol.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Berlioz-Torrent C., Shacklett B. L., Erdtmann L., Delamarre L., Bouchaert I., Sonigo P., Dokhelar M. C., Benarous R. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 1999;73:1350–1361. doi: 10.1128/jvi.73.2.1350-1361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot G., Janvier K., Le Panse S., Benarous R., Berlioz-Torrent C. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity. J. Virol. 2003;77:6931–6945. doi: 10.1128/JVI.77.12.6931-6945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot V., Lopez-Verges S., Breton M., Pique C., Berlioz-Torrent C., Grange M. P. The conserved dileucine- and tyrosine-based motifs in MLV and MPMV envelope glycoproteins are both important to regulate a common Env intracellular trafficking. Retrovirology. 2006;3:62. doi: 10.1186/1742-4690-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouamr F., Melillo J. A., Wang M. Q., Nagashima K., de Los Santos M., Rein A., Goff S. P. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101 [corrected] J. Virol. 2003;77:11882–11895. doi: 10.1128/JVI.77.22.11882-11895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan P. A., Yonemoto W., Ferrell S., Williams-Herman D., Geleziunas R., Greene W. C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989;8:2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirov D. G., Freed E. O. Retrovirus budding. Virus Res. 2004;106:87–102. doi: 10.1016/j.virusres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Dong X., et al. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in particle assembly. Cell. 2005;120:663–674. doi: 10.1016/j.cell.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Doray B., Ghosh P., Griffith J., Geuze H. J., Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- Freed E. O. Viral late domains. J. Virol. 2002;76:4679–4687. doi: 10.1128/JVI.76.10.4679-4687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier L., Wills J. W., Verderame M. F., Sudol M. WW domains and retrovirus budding. Nature. 1996;381:744–745. doi: 10.1038/381744a0. [DOI] [PubMed] [Google Scholar]
- Garrus J. E., et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Gottwein E., Bodem J., Muller B., Schmechel A., Zentgraf H., Krausslich H. G. The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release. J. Virol. 2003;77:9474–9485. doi: 10.1128/JVI.77.17.9474-9485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M., DeTulleo L., Rapoport I., Skowronski J., Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- Grigorov B., Arcanger F., Roingeard P., Darlix J. L., Muriaux D. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines. J. Mol. Biol. 2006;359:848–862. doi: 10.1016/j.jmb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Gullapalli A., Wolfe B. L., Griffin C. T., Magnuson T., Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1, evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol. Biol. Cell. 2006;17:1228–1238. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Lloyd P. A., Fox K., Nagashima K., Derse D. Late assembly motifs of human T-cell leukemia virus type 1 and their relative roles in particle release. J. Virol. 2004;78:6636–6648. doi: 10.1128/JVI.78.12.6636-6648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Emr S. D. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrke G., Kyttala A., Russell M. R., Rous B. A., Luzio J. P. Differential use of two AP-3-mediated pathways by lysosomal membrane proteins. Traffic. 2004;5:946–962. doi: 10.1111/j.1600-0854.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Ingham R. J., Gish G., Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B-Y., Venkatesan S., Bonifacino J. S. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 γ–ó1 and AP-3 δ–ó3 hemicomplexes. J. Cell Biol. 2005;163:1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K., Bonifacino J. S. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell. 2005;16:4231–4242. doi: 10.1091/mbc.E05-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N., Neil S. J., Bess C., Johnson M. C., Virgen C. A., Simon S. M., Bieniasz P. D. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 2006;4:e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D. J., Babst M., Emr S. D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Kikonyogo A., Bouamr F., Vana M. L., Xiang Y., Aiyar A., Carter C., Leis J. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA. 2001;98:11199–11204. doi: 10.1073/pnas.201268998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A., Yliannala K., Schu P., Jalanko A., Luzio J. P. AP-1 and AP-3 facilitate lysosomal targeting of Batten disease protein CLN3 via its dileucine motif. J. Biol. Chem. 2005;280:10277–10283. doi: 10.1074/jbc.M411862200. [DOI] [PubMed] [Google Scholar]
- Langelier C., von Schwedler U. K., Fisher R. D., De Domenico I., White P. L., Hill C. P., Kaplan J., Ward D., Sundquist W. I. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol. 2006;80:9465–9480. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S., Erdtmann L., Benichou S., Berlioz-Torrent C., Liu L., Benarous R., Heard J. M., Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Verges S., Camus G., Blot G., Beauvoir R., Benarous R., Berlioz-Torrent C. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc. Natl. Acad. Sci. USA. 2006;103:14947–14952. doi: 10.1073/pnas.0602941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui-Roberts W. W., Collinson L. M., Hewlett L. J., Michaux G., Cutler D. F. An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J. Cell Biol. 2005;170:627–636. doi: 10.1083/jcb.200503054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. D., Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J., Eastman S. W., Chung W., Bieniasz P. D. HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J. Cell Biol. 2005;168:89–101. doi: 10.1083/jcb.200408155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J., Yarovoy A., Perez-Caballero D., Bieniasz P. D. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA. 2003;100:12414–12419. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J., Zang T., Bieniasz P. D. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- Matsuo H., et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- McNiven M. A., Thompson H. M. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/science.1118133. [DOI] [PubMed] [Google Scholar]
- Meyer C., Zizioli D., Lausmann S., Eskelinen E., Hamann J., Saftig P., von Figura K., Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer H., Morre D., Rowe L. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta. 1990:224–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E., Sundquist W. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Nydegger S., Foti M., Derdowski A., Spearman P., Thali M. HIV-1 egress is gated through late endosomal membranes. Traffic. 2003;4:902–910. doi: 10.1046/j.1600-0854.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- Ohno H. Clathrin-associated adaptor protein complexes. J. Cell Sci. 2006;119:3719–3721. doi: 10.1242/jcs.03085. [DOI] [PubMed] [Google Scholar]
- Ohno H., Aguilar R. C., Fournier M. C., Hennecke S., Cosson P., Bonifacino J. S. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology. 1997;238:305–315. doi: 10.1006/viro.1997.8839. [DOI] [PubMed] [Google Scholar]
- Ono A., Freed E. O. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J. Virol. 2004;78:1552–1563. doi: 10.1128/JVI.78.3.1552-1563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden A. A., Oorschot V., Hesser B. A., Austin C. D., Scheller R. H., Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Kramer B., Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 2003;162:443–455. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman M., Resh M. D. Identification of an intracellular trafficking and assembly pathway for HIV-1 gag. Traffic. 2006;7:731–745. doi: 10.1111/j.1398-9219.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- Piguet V., Chen Y. L., Mangasarian A., Foti M., Carpentier J. L., Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R., van der Wel N. N., Greene L. E., Eisenberg E., Peters P. J., Bonifacino J. S. Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network. Mol. Biol. Cell. 2003;14:1545–1557. doi: 10.1091/mbc.02-07-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Moore M., Innes D., Leijendekker R., Leigh-Brown A., Benaroch P., Geuze H. Human macrophages accumulate HIV-1 particles in MHCII compartments. Traffic. 2002;3:718–729. doi: 10.1034/j.1600-0854.2002.31004.x. [DOI] [PubMed] [Google Scholar]
- Reil H., Bukovsky A. A., Gelderblom H. R., Gottlinger H. G. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 1998;17:2699–2708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch U., Bernhard O., Koszinowski U., Schu P. AP-1A and AP-3A lysosomal sorting functions. Traffic. 2002;3:752–761. doi: 10.1034/j.1600-0854.2002.31007.x. [DOI] [PubMed] [Google Scholar]
- Rost M., Mann S., Lambert C., Doring T., Thome N., Prange R. Gamma-adaptin, a novel ubiquitin-interacting adaptor, and Nedd4 ubiquitin ligase control hepatitis B virus maturation. J. Biol. Chem. 2006;281:29297–29308. doi: 10.1074/jbc.M603517200. [DOI] [PubMed] [Google Scholar]
- Rous B. A., Reaves B. J., Ihrke G., Briggs J. A., Gray S. R., Stephens D. J., Banting G., Luzio J. P. Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol. Biol. Cell. 2002;13:1071–1082. doi: 10.1091/mbc.01-08-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Morales C., Pescia C., Chatellard-Causse C., Sadoul R., Bertrand E., Basyuk E. Tsg101 and Alix interact with murine leukemia virus Gag and cooperate with Nedd4 ubiquitin ligases during budding. J. Biol. Chem. 2005;280:27004–27012. doi: 10.1074/jbc.M413735200. [DOI] [PubMed] [Google Scholar]
- Sfakianos J. N., Hunter E. M-PMV capsid transport is mediated by Env/Gag interactions at the pericentriolar recycling endosome. Traffic. 2003;4:671–680. doi: 10.1034/j.1600-0854.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Sfakianos J. N., LaCasse R. A., Hunter E. The M-PMV cytoplasmic targeting-retention signal directs nascent Gag polypeptides to a pericentriolar region of the cell. Traffic. 2003;4:660–670. doi: 10.1034/j.1600-0854.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- Shearwin-Whyatt L., Dalton H. E., Foot N., Kumar S. Regulation of functional diversity within the Nedd4 family by accessory and adaptor proteins. Bioessays. 2006;28:617–628. doi: 10.1002/bies.20422. [DOI] [PubMed] [Google Scholar]
- Sherer N. M., Lehmann M. J., Jimenez-Soto L. F., Ingmundson A., Horner S. M., Cicchetti G., Allen P. G., Pypaert M., Cunningham J. M., Mothes W. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. doi: 10.1034/j.1600-0854.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Slagsvold T., Pattni K., Malerod L., Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Strack B., Calistri A., Accola M. A., Palu G., Gottlinger H. G. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack B., Calistri A., Craig S., Popova E., Gottlinger H. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:686–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- Theos A. C., et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A. C., Truschel S. T., Tenza D., Hurbain I., Harper D. C., Berson J. F., Thomas P. C., Raposo G., Marks M. S. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler U., et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Waguri S., Dewitte F., Le Borgne R., Rouille Y., Uchiyama Y., Dubremetz J. F., Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol. Biol. Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch S., Keppler O. T., Habermann A., Allespach I., Krijnse-Locker J., Krausslich H. G. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3:e36. doi: 10.1371/journal.ppat.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. J., Bailey L. M., Aghakhani M. R., Moss S. E., Futter C. E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss S., Berlioz-Torrent C., Boge M., Blot G., Honing S., Benarous R., Thali M. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adaptor [correction of adapter] J. Virol. 2001;75:2982–2992. doi: 10.1128/JVI.75.6.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda J., Hunter E., Nakao M., Shida H. Functional involvement of a novel Nedd4-like ubiquitin ligase on retrovirus budding. EMBO Rep. 2002;3:636–640. doi: 10.1093/embo-reports/kvf132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.