Abstract
Cells use multiple pathways to internalize and recycle cell surface components. Although Rab11a and Myosin Vb are involved in the recycling of proteins internalized by clathrin-mediated endocytosis, Rab8a has been implicated in nonclathrin-dependent endocytosis and recycling. By yeast two-hybrid assays, we have now demonstrated that Myosin Vb can interact with Rab8a, but not Rab8b. We have confirmed the interaction of Myosin Vb with Rab11a and Rab8a in vivo by using fluorescent resonant energy transfer techniques. Rab8a and Myosin Vb colocalize to a tubular network containing EHD1 and EHD3, which does not contain Rab11a. Myosin Vb tail can cause the accumulation of both Rab11a and Rab8a in collapsed membrane cisternae, whereas dominant-negative Rab11-FIP2(129-512) selectively accumulates Rab11a but not Rab8a. Additionally, dynamic live cell imaging demonstrates distinct pathways for Rab11a and Rab8a vesicle trafficking. These findings indicate that Rab8a and Rab11a define different recycling pathways that both use Myosin Vb.
INTRODUCTION
The organized regulation of cell surface proteins is critical to proper cell physiology. These processes include the movement of newly synthesized lipids and membrane proteins to the plasma membrane, the endocytosis of exogenous material, and the constant recycling of many plasma membrane receptors. Members of the Rab family of small GTPases play critical roles in the regulated trafficking of a variety of membranous vesicles inside eukaryotic cells (for review, see Grosshans et al., 2006). Although there is ample evidence that Rab proteins play an important part in trafficking, far less is understood about how each Rab functions to coordinate this complex process.
Some Rab proteins regulate the cargo binding of unconventional myosin motors, and these motors are necessary for the proper translocation of the Rab-resident membranes (Lapierre et al., 2001, Libby et al., 2004). Rab27a, in association with melanophilin/Slac2-a, is required for the proper localization of Myosin Va to the surface of melanin-filled pigment granules in vertebrates (Fukuda et al., 2002, Provance et al., 2002, Strom et al., 2002). Similarly, Rab11a is known to interact with Myosin Vb (Lapierre et al., 2001), and both of these proteins interact with Rab11-FIP2 (Hales et al., 2002), a member of the Rab11 family interacting proteins (Hales et al., 2001, Junutula et al., 2004). Rab11a, Rab11-FIP2, and Myosin Vb are involved in the recycling of a variety of receptors, including transferrin receptor (Lapierre et al., 2001, Hales et al., 2002, Lindsay and McCaffrey, 2002), the glutamate receptor subunit GluR1 (Lise et al., 2006), the M4-muscarinic acetylcholine receptor (Volpicelli et al., 2002), the chemokine receptor CXCR2 (Fan et al., 2004), the polymeric IgA receptor (Wang et al., 2000; Lapierre et al., 2001), the β2-adrenergic receptor (Moore et al., 2004), and the H+/K+-ATPase of gastric parietal cells (Hales et al., 2001, Lapierre et al., 2001). Myosin Vc, the third member of the vertebrate class V Myosins, localizes with a portion of Rab8a and the transferrin receptor (Rodriguez and Cheney, 2002). Thus, Rab/Myosin V complexes are critical components of the anterograde pathways involved in the transport of cargoes to the plasma membrane.
Another small GTPase, Arf6, regulates the clathrin-independent endocytosis of many plasma membrane proteins, such as the class I major histocompatibility complex (MHC), the complement regulating protein CD59, and the α subunit of the interleukin-2 cytokine receptor (Naslavsky et al., 2004b). Unlike the proteins sorted through the vesicular endocytotic recycling compartment (ERC), these proteins are transported through an Arf6-positive tubular network. EHD1, an Eps-15-homology domain-containing protein, is also involved in the recycling of these clathrin-independent proteins (Caplan et al., 2002). Arf6 regulates the localization of EHD1 to this tubular recycling network. In addition, recent investigations have shown that a guanosine triphosphate (GTP)-locked mutant of Arf6 (Arf6-Q67L) inhibits the tubular localization of Rab8a (Hattula et al., 2006). Rab8a has been implicated in the regulation of plasma membrane recycling and trafficking of class I MHC (Hattula et al., 2006), and expression of the tail of Myosin Vc causes an accumulation of Rab8a in large, round vesicular structures with Myosin Vc tail (Rodriguez and Cheney, 2002).
Although it is known that Myosin Vb interacts with members of the Rab11 family of proteins, we sought to determine whether Myosin Vb could interact with other Rabs. Here, we show that Myosin Vb is also able to interact with Rab8a in a process that is distinct from Rab11a. In addition, we show that Rab8a and Myosin Vb localize to a tubular network containing EHD1 and EHD3 and that Rab11a is not associated with these tubules. These findings indicate that different Rab proteins can use the same myosin motor in two distinct recycling systems.
MATERIALS AND METHODS
Plasmids and DNA
The following DNA constructs have been described previously: pEGFP-Myr6, pEGFP- and pAD-Myosin Va-tail, pEGFP- and pAD-Myosin Vb-tail, pBD-Rab11a, pBD-Rab11a-S20V, pBD-Rab11a-S25N, pBD-Rab25, pBD-Rab25-S21V (Lapierre et al., 2001); pBD-Rab11b (Lapierre et al., 2003); pBD-Rab27a; pBD-Rab11-FIP2, pEGFP-FIP2(129-512) (Hales et al., 2002); pEGFP-Myosin Vc-tail (Rodriguez and Cheney, 2002); myc-EHD1, EGFP-EHD1, myc-EHD3, and EGFP-EHD3 (Caplan et al., 2002); pmCherry (a gift from Roger Tsien, University of California, San Diego). pmCerulean, and pmVenus-Myosin Vb-tail constructs were created by inserting the Myosin Vb-tail coding sequence from pEGFP-Myosin Vb-tail into pmCerulean-C1 (Rizzo et al., 2004), pmVenus-C1 (Nagai et al., 2002), and pmCherry-C1 (Shu et al., 2006) by using the BamHI and SalI restriction sites. The pBD-, pmCerulean, pmVenus, and pmCherry versions of wild-type and mutant Rab8a constructs were created by inserting the Rab8a coding sequences into pBD-GAL4 (Stratagene, La Jolla, CA), mCerulean, mVenus, and mCherry by using the EcoRI and SalI restriction sites. Human EHD1 and EHD3 were subcloned into mCherry by using EcoRI and SalI restriction sites. The species origin and amino acid boundaries of the Myosin V tails used are as follows: Myosin Va tail is from chicken (GenBank accession no. NM_205300), amino acids 1242–1829; Myosin Vb tail is from rabbit (GenBank accession no. AF176517), and it represents the analogous C-terminal 588-amino acid region as the Myosin Va tail; and Myosin Vc tail is from human, amino acids 902-1741 (GenBank accession no. AL133643).
Yeast Two-Hybrid Assays
Yeast two-hybrid assays were performed essentially as described previously (Hales et al., 2002). Briefly, the yeast strain Y109 was cotransformed with various Rab baits in the pBD-GAL4 plasmid and either pAD-GAL4-Myosin Vb-tail or pAD-GAL4-Myosin Va-tail. Transformed yeast were plated on dual-deficient synthetic dropout media (SD/−Leu/−Trp), and they were allowed to grow at 30°C for 72 h. Surviving colonies were transferred onto filter paper discs (VWR, Westchester, PA), lysed by freezing twice in liquid N2, and incubated in 300 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) for up to 4 h to test for lacZ expression.
Live Imaging and Immunocytochemistry
HeLa cells grown on MatTek dishes (MatTek, Ashland, MA) or on glass coverslips were transiently transfected with plasmids encoding mCerulean- or mVenus-tagged Rab8a and Rab11a constructs by using Effectene transfection reagent (QIAGEN, Chatsworth, CA) according to the manufacturer's recommendations. After transfection, cells were incubated at 37°C in 5% CO2 for 18 h. For live cell imaging, chambers were rinsed twice in Opti-MEM (Invitrogen, Carlsbad, CA) medium at room temperature. Live imaging was then performed at room temperature by using confocal microscopy on an LSM510 (Carl Zeiss, San Jose, CA). Images were captured at roughly 7-s intervals.
For immunocytochemistry, 18 h after transfection, coverslips were rinsed once with phosphate-buffered saline (PBS), and then they were fixed by incubation in 4% paraformaldehyde for 10 min at room temperature. Cells were both permeablized and blocked by incubation in blocking buffer (PBS with 1% bovine serum albumin and 0.1% Triton X-100) for at least 1 h. Blocking buffer was used for all subsequent steps. Cells were incubated with primary antibodies for 1 h, washed three times for 10 min, incubated with secondary antibodies for 1 h, washed three times for 10 min, and then mounted onto glass slides with Prolong Antifade reagent with 4,6-diamidino-2-phenylindole (Invitrogen). The antibodies used in this study have been described previously: anti-Rab8a polyclonal (Hattula et al., 2006); VU57, anti-Rab11a polyclonal (Lapierre et al., 2007); 8H10, anti-Rab11a monoclonal (Goldenring et al., 1996); and 9E10, anti-myc monoclonal (Covance, Berkeley, CA). Confocal images with captured on an LSM510 (Carl Zeiss).
Fluorescent Resonant Energy Transfer (FRET)
FRET microscopy was performed essentially as described previously (Kenworthy, 2001). HeLa cells grown on glass coverslips were cotransfected with mCerulean-tagged Myosin Vb tail and mVenus-tagged Rab8a-WT, Rab8a-Q67L, Rab11a-WT, or Rab11a-S20V as described above. Eighteen hours after transfection, cells were washed with PBS and fixed by incubation in 4% paraformaldehyde for 10 min at room temperature. After fixation, cells were washed twice with PBS and stored at 4°C until used. For microscopy, coverslips were inverted onto a silicone membrane sealed to a glass slide. Digital images were captured on an LSM510 (Carl Zeiss) equipped with a 40-mW argon laser. Three prebleach images were taken of both mCerulean (excited at 458 nm) and mVenus (excited at 514 nm) fluorescence. Acceptor photobleaching was achieved by exciting with 100 bursts of 514-nm wavelength light at 100% transmission. This was followed by the collection of three postbleach images. The fluorescence intensity of the photobleached region of interest (ROI) was measured, and the average of the three prebleach images was compared with the average of the three postbleach images after background subtraction. The FRET efficiency was calculated as follows: E = 100 (mCeruleanpost − mCeruleanpre)/mCeruleanpost, where mCeruleanpre is the average fluorescence intensity before photobleaching, and mCeruleanpost is the average after photobleaching. FRET data were collected for 10 cells per experimental condition, and each experiment was repeated three times. As a control, the fluorescence intensity of mCerulean-Myosin Vb tail expressed alone was measured both before and after photobleaching.
Recycling of MHC Class I Molecules
MHC class I recycling was performed essentially as described previously (Naslavsky et al., 2003). Briefly, HeLa cells grown on glass coverslips were transiently transfected with EGFP-Myosin Vb tail. Eighteen hours after transfection, cells were cooled to 4°C in complete medium with 25 mM HEPES, pH 7.6, added. Cells were incubated with a 1 μg/ml monoclonal antibody (mAb) against human MHC class I, W6/32 (Abcam, Cambridge, MA) for 30 min at 4°C. Cells were then washed three times with 4°C PBS to remove unbound antibody, and then they were incubated in prewarmed (37°C) complete medium for varying times to allow MHC/antibody internalization. After internalization, cells were washed with cold PBS, and surface-bound antibodies were removed by a 30-s incubation with 0.5% acetic acid, 0.5 M NaCl, pH 3.0, rinsed with PBS, and fixed in 2% paraformaldehyde for 15 min. Internalized MHC class I was visualized by staining cells permeablized in 0.1% Triton X-100 with Cy3-labeled anti-mouse antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
RESULTS
Myosin Vb Interacts with Rab8a in Yeast Two-Hybrid Assays
Evidence from a variety of biological systems shows that class V myosin motors require association with Rab GTPases for proper association with target membranes (for review, see Seabra and Coudrier, 2004). Previously, our laboratory identified the members of the Rab11 family of small GTPases, Rab11a, Rab11b, and Rab25, as proteins capable of interacting with the C-terminal tail of Myosin Vb (Lapierre et al., 2001). To identify additional regulators of Myosin Vb, we tested the ability of Myosin Vb tail constructs to interact with a variety of rab proteins in yeast two-hybrid assays. In addition to positive interactions with wild-type Rab11a, Rab11b, and Rab25 as well as GTP-locked mutants of Rab11a (S20V) and Rab25 (S21V) in a β-galactosidase assay, Myosin Vb tail interacted with Rab8a (Table 1). Both wild-type Rab8a and the GTP-locked mutant (Q67L) reacted strongly with the tail of Myosin Vb, whereas the guanosine diphosphate (GDP)-locked mutant (T22N) did not. No interactions were observed between Myosin Vb tail and Rab8b, indicating that the interaction with Rab8a was specific. Furthermore, any truncation of the Myosin Vb tail target inhibited the interaction with Rab8a (Supplemental Table S1), whereas the entire first coil of Myosin Vb tail needed to be removed to disrupt Rab11a interaction (Lapierre et al., 2001), suggesting that the two Rab proteins bind in distinct manners. Because previous studies have shown that Rab8a partially colocalizes with Myosin Vc (Rodriguez and Cheney, 2002), we also tested the ability of Myosin Vc tail to interact with Rab8a. We found that Myosin Vc tail does interact with both wild-type Rab8a and Rab8a-Q67L but not with Rab8a-T22N, Rab8b, Rab11a, or any other Rab tested (Table 1). Neither Rab11a nor Rab8a interacted with Myosin Va tail.
Table 1.
Rab8a interacts with Myosin Vb tail and Myosin Vc tail in a yeast two-hybrid assay
| pBD- | pAD-Myosin Va-tail | pAD-Myosin Vb-tail | pAD-Myosin Vc-tail |
|---|---|---|---|
| Rab11a | − | + | − |
| Rab11a-S20V | − | + | − |
| Rab11a-S25N | − | − | − |
| Rab11b | − | + | − |
| Rab25 | − | + | − |
| Rab25-S21V | − | + | − |
| Rab8a | − | + | + |
| Rab8a-Q67L | − | + | + |
| Rab8a-T22N | − | − | − |
| Rab8b | − | − | − |
| Rab11-FIP2 | − | + | − |
Yeast (Y190 strain of Saccharomyces cerevisiae) expressing both a pBD-Rab construct and a pAD-Myosin V tail construct were selected by growth on leucine- and tryptophan-deficient synthetic dropout agar. Colonies were transferred to filter paper, lysed by freezing and thawing, and incubated in an X-Gal solution. Each two-hybrid assay was considered a positive result only if X-Gal conversion (blue color) was visible in <4 h. The chart illustrates that Myosin Va tail was unable to react with any of the Rab proteins used as bait in this experiment, whereas Myosin Vb tail was able to interact with Rab11a, Rab11a-S20V, Rab11b, Rab25, Rab25-S21V, Rab8a, Rab8a-Q67L, and Rab11-FIP2. Myosin Vc tail was only able to interact with Rab8a and the GTP-locked mutant Q67L and not with any member of the Rab11 family of proteins (Rab11a, Rab11b, and Rab25) or with Rab11-FIP2.
Myosin Vb Tail Alters Endogenous Rab8a Distribution In Vivo
Exogenous expression of the cargo binding domain of many molecular motors results in a dominant-negative phenotype specific to that motor (Wu et al., 1998, Lapierre et al., 2001, Rodriguez and Cheney, 2002). This approach is frequently used to confirm in vitro interactions between a motor and its putative cargoes or associated proteins. In Myosin Vb, expression of the C-terminal 588 amino acids of mammalian Myosin Vb results in an inhibition of the Rab11a-based recycling of receptors such as CXCR2 and GluR1 (Fan et al., 2004, Lise et al., 2006). To confirm our yeast two-hybrid observations in vivo, we transfected HeLa cells with fluorescent protein-tagged Myosin Vb tail constructs, and we probed for endogenous Rab8a with a specific rabbit polyclonal antibody. In nontransfected cells, Rab8a was localized to a system of long tubules and small vesicles (Figure 1, A–C) that were not associated with markers of the Golgi apparatus or endoplasmic reticulum (Supplemental Figure S1) as described previously (Hattula et al., 2006). As reported previously, in a typical HeLa cell culture, ∼10% of cells showed the tubular Rab8a pattern at any time (Hattula et al., 2006). As seen in Figure 1E, expression of dominant-negative Myosin Vb tail constructs dramatically altered the distribution of endogenous Rab8a. In Myosin Vb tail-expressing cells, Rab8a lost its tubular localization and instead relocated to a cluster of perinuclear puncta that colocalized with Myosin Vb tail. This pattern was similar to the effect that expression of Myosin Vb tail has on the localization of Rab11a (Lapierre et al., 2001) and known recycling cargoes (Brock et al., 2003, Hobdy-Henderson et al., 2003, Fan et al., 2004, Lise et al., 2006). As reported previously (Rodriguez and Cheney, 2002), expressed Myosin Vc tail also colocalized with Rab8a and altered its distribution (Figure 1F). In contrast, expression of Myosin Va tail did not perturb Rab8a tubular localization (Figure 1D).
Figure 1.
Myosin Vb disrupts Rab8a tubular localization. (A–C) HeLa cells stained with anti-Rab8a polyclonal primary and Cy3-labeled secondary antibodies were imaged by confocal microscopy. Three examples are shown. Bar, 10 μm (A and B) and 5 μm (C). (D) HeLa cells transfected with pEGFP-Myosin Va tail (MVa-tail) and stained for Rab8a as in described in A. Myosin Va tail does not colocalize with endogenous Rab8a. (E) HeLa cells transfected with pEGFP-Myosin Vb tail (MVb-tail) and stained for Rab8a. Myosin Vb tail localizes to a perinuclear cisterna and causes Rab8a to localize to the same cisterna. (F) HeLa cells transfected with pEGFP-Myosin Vc tail (MVc-tail) and stained for Rab8a show a similar result as Myosin Vb tail, although in this case, Myosin Vc tail labels round vesicular structures. Bar, 10 μm (D–F).
The altered localization of Rab8a seen in Myosin Vb tail-expressing cells indicates that disruption of normal Myosin Vb function affects Rab8a, but it does not directly demonstrate that the two proteins colocalize in the absence of such a disruption. To determine whether Rab8a does indeed localize with wild-type Myosin Vb in vivo, HeLa cells were transfected with an enhanced green fluorescent protein (EGFP)-tagged full-length rat Myosin Vb (Myr6) construct, and they were probed for endogenous Rab8a. Overexpressed EGFP-Myr6 was predominantly observed on tubules that often costained for Rab8a; not all Rab8a-positive tubules were EGFP-Myr6 positive (Figure 2, A and B). In contrast, endogenous Rab11a is only localized to tubules in the presence of EGFP-Myr6 (Figure 2C).
Figure 2.
Full-length Myosin Vb and endogenous Rab8a colocalize. (A and B) Endogenous Rab8a was labeled by immunohistochemical techniques in HeLa cells expressing EGFP-tagged full-length Myosin Vb (GFP-Myr6). Both Rab8a and full-length Myosin Vb localize to a system of long tubules, similar to what is observed for Rab8a alone in nonexpressing cells. Bar, 10 μm (A) and 5 μm (B). (C) Endogenous Rab11a was labeled in HeLa cells expressing pEGFP-Myr6. Rab11a and full-length Myosin Vb also colocalize. Bar, 10 μm.
The observation that Myosin Vb tail altered Rab8a localization in vivo suggested that Rab8a might reside within the Rab11a-regulated recycling system. However, immunostaining for both endogenous Rab proteins revealed that they have limited overlapping localization, whereas the majority of the staining for each Rab is distinct (Figure 3A). Overexpression of either Rab protein did not alter the distribution of the other protein (Figure 3, B and C). In agreement with previous data (Hattula et al., 2006), overexpression of either Rab4a or Rab5a, two other Rabs involved in recycling (Zerial and McBride, 2001), did not alter Rab8a tubular localization either (Supplemental Figure S2). Importantly, and in contrast to previous studies with Rab11a, expression of dominant-negative Rab11-FIP2(129-512), a protein that binds both Rab11a and Myosin Vb (Hales et al., 2002), did not alter the distribution of Rab8a (Figure 3D). All of these results suggest that the Rab8a and Rab11a interact with Myosin Vb at different steps in the recycling system or in distinct recycling pathways.
Figure 3.
Rab8a and Rab11a have distinct localizations. (A) HeLa cells were labeled with an anti-Rab8a rabbit polyclonal antibody and an anti-Rab11a mouse mAb (8H10) as well as Cy3-labeled anti-rabbit and Alexa 488-labeled anti-mouse secondary antibodies, and they were imaged by confocal microscopy. Staining for both Rab proteins reveals that endogenous Rab8a and Rab11a have distinct localizations with little or no overlapping localization. (B) Overexpression of EGFP-tagged Rab11a (GFP-Rab11a) does not colocalize with endogenous Rab8a, nor does expressed EGFP-Rab11a disrupt Rab8a tubular staining pattern. (C) Overexpression of mCherry-tagged Rab8a (mCherry-Rab8a) in HeLa cells does not overlap with or alter endogenous Rab11a staining. Rab11a was labeled with an anti-Rab11a rabbit polyclonal antibody (VU57) and Alexa 488-labeled anti-rabbit secondary antibodies. (D) Endogenous Rab8a tubular localization is not perturbed in HeLa cells overexpressing monomeric red fluorescent protein-tagged dominant-negative Rab11-FIP2(129-512). Bars, 10 μm.
Rab8a and Rab11a Interact with Myosin Vb Tail In Situ
Although yeast two-hybrid and colocalization all indicate that Rab8a and Myosin Vb interact, neither of these assays can show conclusively that the two proteins are in direct contact in vivo. In addition, as is the case with Rab11a, we were unable to coimmunoprecipitate Rab8a and full-length Myosin Vb from detergent-solubilized cells (Lapierre et al., 2001). For this reason, we sought to evaluate protein–protein interactions with Myosin Vb tail by FRET. Specifically, we used the method of acceptor photobleaching FRET (Kenworthy, 2001) between Rab8a or Rab11a and Myosin Vb tail. In this method, FRET was measured by analyzing the increase in the donor tag's fluorescent signal before and after the acceptor tag is photobleached. Target proteins were tagged with either the monomeric cyan fluorescent protein derivative mCerulean (Rizzo et al., 2004) or the monomeric yellow fluorescent protein derivative mVenus (Nagai et al., 2002). HeLa cells were cotransfected with mCerulean-tagged Myosin Vb-tail and either mVenus-tagged Rab8a or Rab11a constructs. Figure 4 represents the results of 30 averaged FRET measurements for each condition listed. Only wild-type and GTP-locked mutants were able to be analyzed by FRET, because the GDP-locked mutants do not colocalize with Myosin Vb tail. Our data show that both Rab8a and Rab11a produced a positive FRET signal with Myosin Vb tail after acceptor photobleaching, whereas mCerulean-Myosin Vb tail alone did not. The energy transfer efficiencies measured for wild-type and GTP-locked Rab8a were 15.1 ± 1.6 and 10.1% ± 1.1, respectively. Similarly, the energy transfer efficiencies for wild-type and GTP-locked Rab11a were 15.6 ± 1.0 and 13.9 ± 1.1%, respectively. In addition to these FRET pairs, Rab8b and Myosin Vb tail, or Rab8a and Myosin Va tail, were coexpressed to control for specificity. Neither of these two combinations colocalized or produced a positive FRET measurement (Supplemental Figure S3), indicating that the FRET observed between Rab8a or Rab11a and Myosin Vb tail was specific.
Figure 4.
Rab8a and Rab11a FRET with Myosin Vb tail. (A and B) FRET microscopy was performed on HeLa cells grown on glass coverslips, cotransfected with mCerulean-tagged Myosin Vb tail and mVenus-tagged Rab8a-WT, Rab8a-Q67L, Rab11a-WT, or Rab11a-S20V. The panels show representative examples of cells used for FRET measurements collected by confocal microscopy by using a Zeiss LSM510. mCerulean is pseudocolored with light blue and mVenus with yellow; overlap is represented by green. The fluorescence intensity of a photobleached ROI, indicated by a dashed line circle on the example cells, was measured before (pre) and after (post) photobleaching, and the average of prebleach images was compared with the average of the postbleach images after background subtraction. (C) The FRET efficiency was calculated as E = 100(mCeruleanpost − mCeruleanpre)/mCeruleanpost. FRET data were collected for 10 cells per experimental condition, and each experiment was repeated three times. As a control, the fluorescence intensity of mCerulean-Myosin Vb tail expressed alone (MVb-tail alone) was measured both before and after photobleaching. Error bars indicate the SEM. The energy transfer efficiency between mCerulean-Myosin Vb tail and each of the mVenus-tagged Rab proteins was as follows: mVenus-Rab8a-WT, 15.1 ± 1.6%; mVenus-Rab8a-Q67L, 10.1 ± 1.1%; mVenus-Rab11a-WT, 15.6 ± 1.0%; and mVenus-Rab11a-S20V, 13.9 ± 1.1%. All of these values are significantly more positive then the measured value for mCerulean-Myosin Vb tail by itself (0.6 ± 1.0%), indicating that both Rab8a and Rab11a are able to produce a positive FRET signal with Myosin Vb tail.
Rab11a- and Rab8a-positive Vesicles and Tubules Define Distinct Trafficking Pathways In Vivo
Myosin Vb is known to interact with members of the Rab11 family (Lapierre et al., 2001), yet our immunocytochemical data showed only minimal colocalization between Rab11a and Rab8a. Furthermore, there was no evidence that Rab11a was localized to large Rab8a-containing tubules. These results indicate that two separate Myosin Vb/Rab complexes may exist and that each may regulate distinct trafficking systems. To elucidate the relationship between Rab8a and Rab11a in vivo, we studied the movements of the two proteins in live cells. HeLa cells expressing Rab8a and Rab11a, tagged with either mCerulean or mVenus, were imaged in live cells by confocal microscopy. As we have observed with the endogenous proteins, Rab8a was localized to long tubules and small vesicles, whereas Rab11a resides strictly in fast-moving, small vesicles. We observed Rab11a-positive vesicles (Figure 5, red) seeming to dock with and travel along Rab8a-positive tubules (Figure 5, green, and Supplemental Video 1). However, we did not observe discrete fusion or budding events. Our data indicate that Rab8a and Rab11a may not function in the same recycling pathway, but rather each is associated with distinct trafficking pathways.
Figure 5.
Rab11a vesicles are observed traveling along Rab8a tubules in vivo. HeLa cells expressing mCerulean-tagged Rab8a and mVenus-tagged Rab11a were analyzed by live cell confocal microscopy. Sixty images were captured at roughly 7-s intervals, and three of those images are shown here. The panels on the right show a zoomed-in view of the area boxed in each panel on the left. Arrowheads indicate the position of a Rab11a-positive vesicle (red) as it travels along a Rab8a-positive tubule (green). Bar, 10 μm. These still images are representative of the full-length movie that is available as a Supplemental Video.
Rab8a and Myosin Vb-Tail Localize to an EHD1/EHD3-positive Tubular Network
The system of tubules with in which endogenous and expressed Rab8a are localized strongly resembles the tubular recycling networks observed in EHD1- and EHD3-expressing cells (Caplan et al., 2002, Galperin et al., 2002). To determine whether Rab8a colocalizes to the same network of tubules as these EHD proteins, HeLa cells were transfected with myc-tagged forms of either EHD1 or EHD3. As reported previously (Caplan et al., 2002, Galperin et al., 2002), these proteins associated with a large tubular network (Figure 6, A–D). Rab8a was localized to these same tubules by using a specific rabbit polyclonal antibody (Figure 6, A and C). In addition, coexpression of fluorescently tagged-Rab8a and either EHD1 or EHD3 confirmed that all three colocalized in the same tubular network (Supplemental Figure S4). Nevertheless, although endogenous Rab11a was often observed in proximity to these tubules, it rarely colocalized with them (Figure 6, B and D). However, overexpression of EGFP-tagged Rab11a did partially colocalize with EHD1 and EHD3 as reported previously by Blume et al. (2007) (Supplemental Figure S4).
Figure 6.
Rab8a localizes to a tubular network containing EHD1 and EHD3. (A) HeLa cells expressing myc-tagged EHD1 were labeled with an anti-myc mAb (9E10) and an anti-Rab8a rabbit polyclonal antibody followed by Cy3-labeled rabbit and Alexa 488-labeled mouse secondary antibodies. Confocal microscopy revels that the tubular localization of endogenous Rab8a exactly matches that of myc-EHD1. Bar, 5 μm. (B) In contrast to Rab8a, endogenous Rab11a (labeled with VU57) does not colocalize with myc-EHD1 in transfected cells. Bar, 5 μm. (C) Similar to what is observed for myc-EHD1, endogenous Rab8a colocalizes with myc-tagged EHD3 on a network of long tubules in expressing cells. Bar, 10 μm. (D) Endogenous Rab11a and myc-EHD3 do not colocalize in expressing cells. Bar, 5 μm.
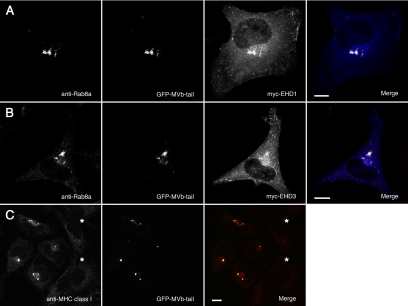
To determine whether Myosin Vb tail can affect either EHD1 or EHD3 distribution, we transfected HeLa cells with the EGFP-tagged Myosin Vb tail construct. Both myc-EHD1 and myc-EHD3 distribution was altered in cells coexpressing Myosin Vb tail (Figure 7, A and B). A perinuclear cisterna and fragmented tubular structures were observed for cells expressing both myc-EHD1 and EGFP-Myosin Vb tail (Figure 7A). Similarly, Rab8a was redistributed to the Myosin Vb tail-containing perinuclear cisterna. Although myc-EHD3 distribution is also affected by Myosin Vb tail expression, it did not colocalize exactly with Myosin Vb tail, but rather it surrounded the perinuclear cisterna (Figure 7B), indicating that Myosin Vb may not interact directly with this protein but rather regulate the transport or proper positioning of the tubular structures to which it localizes.
Figure 7.
Myosin Vb tail colocalizes with Rab8a, EHD1, EHD3, and MHC class I molecules. (A) Coexpression of EGFP-tagged Myosin Vb tail (GFP-MVb-tail) and myc-EHD1 in HeLa cells labeled with an anti-Rab8a polyclonal antibody. Myosin Vb tail expression caused the relocalization of endogenous Rab8a and expressed myc-EHD1 to a perinuclear cisterna. The far right panel is a merged overlay of all three images on the left: EGFP-Myosin Vb tail (green), Rab8a (red), and myc-EHD1 (blue). (B) Coexpression of EGFP-Myosin Vb tail with myc-EHD3 resulted in colocalization of Rab8a and Myosin Vb tail in a perinuclear cisterna, but a portion of both Myosin Vb and Rab8a colocalize with myc-EHD3 on tubules. As in A, the far right panel is a merge of all three single channel images except that blue represents myc-EHD3. (C) MHC class I recycling was assayed in HeLa cells expressing EGFP-tagged Myosin Vb tail. MHC class I molecules were internalized in the presence of a specific mAb, W6/32, and visualized by counterstaining with Cy3-labeled anti-mouse antibodies. MHC class I molecules were trapped in a perinuclear cisterna along with Myosin Vb tail in transfected cells, in contrast to neighboring nontransfected cells (*). Bars, 10 μm.
Previous work has shown a role for EHD1 in the recycling of class I MHC molecules (Caplan et al., 2002) and that Rab8a colocalizes with MHC class I (Hattula et al., 2006). Because Myosin Vb tail alters the distribution of both Rab8a and EHD1, we sought to determine whether Myosin Vb tail could also disrupt class I MHC recycling. We observed class I MHC molecules colocalized with EGFP-Myosin Vb tail in transfected cells in an MHC internalization and recycling assay (Figure 7C). Figure 7C illustrates that MHC class I molecules remained colocalized with Myosin Vb tail 120 min after the initialization of MHC internalization, compared with the weak, dispersed staining pattern seen in nontransfected cells. These results indicate that Myosin Vb participates in the recycling of MHC class I molecules.
DISCUSSION
The constant recycling of proteins between the plasma membrane and endocytic vesicles is a critical step in maintaining homeostasis of constituents of the cell surface. Investigations over the past two decades have detailed multiple pathways for endocytosis, including clathrin-dependent receptor-mediated endocytosis, nonclathrin-dependent endocytosis, and caveolar endocytosis. In particular, although transferrin receptor seems to use a clathrin-dependent pathway for internalization (Johnson et al., 1993, Maxfield and McGraw, 2004), MHC class I protein and β1-integrin are internalized through a nonclathrin-dependent pathway (Radhakrishna and Donaldson, 1997, Brown et al., 2001, Naslavsky et al., 2004b). Far less is understood about the number of pathways for recycling of endocytosed proteins back to the plasma membrane. Many studies over the past several years have noted the association in nonpolarized cells of Rab11a with the regulation of transferrin receptor recycling (Ullrich et al., 1996, Green et al., 1997, Wang et al., 2000). In contrast, recent studies have suggested an association of Rab8a with MHC class I recycling (Hattula et al., 2006). Previous studies have not considered the relationship of critical regulators of these two recycling systems to each other. The present investigations demonstrate that Myosin Vb associates with both Rab11a and Rab8a. Nevertheless, the preponderance of data indicates that the trafficking pathways defined by these two Rab proteins are substantially distinct.
Myosin motors, especially class V motors, play critical roles in the proper trafficking and positioning of many intracellular cargos. Increasing evidence suggests that myosin motors are used in multiple locations within cells dependent on cell-specific differentiated functions or stages of the cell cycle. The present investigations demonstrate that Myosin Vb is used in the same cell for regulation of two distinct trafficking pathways. Although previous studies had established the role of Myosin Vb in Rab11a-mediated recycling (Lapierre et al., 2001, Hales et al., 2002), yeast two-hybrid studies demonstrated that Rab8a could also interact with the tail region of Myosin Vb. This interaction was dependent on association of Rab8a with GTP and showed structural specificity, because Rab8b did not demonstrate any association with Myosin Vb. Most importantly, yeast-two hybrid studies demonstrated that the structural requirements for Rab8a binding were significantly different from Rab11a. Indeed, the binding requirements were most similar to those previously reported for Rab11-FIP2 association with Myosin Vb (Lapierre et al., 2001). Therefore, association of Myosin Vb with either Rab11a- or Rab8a-containing membranes is not mediated exclusively by competition for a common binding site. Our studies have demonstrated that Myosin Vc can also interact with Rab8a but not Rab11a. As reported by Cheney and colleagues (Rodriguez and Cheney, 2002), expression of the tail of Myosin Vc caused accumulation of a subpopulation of Rab8a and transferrin receptor. The morphology of the Myosin Vc tail-containing membranes is markedly different from that observed for Myosin Vb tail. It is therefore possible that Myosin Vc defines a further subspecialization of trafficking.
Although MHC class I molecules are trafficked through a nonclathrin-dependent pathway, proteins such as the transferrin receptor (TrFR) are primarily endocytosed by way of AP-2 and clathrin-coated pits (Johnson et al., 1993). Several recent investigations have demonstrated that regulators of Rab11a, including Rab11-FIP2 and Myosin Vb, are required for the recycling of TrFR back to the plasma membrane through an endocytotic recycling compartment (Lapierre et al., 2001, Hales et al., 2002, Lindsay and McCaffrey, 2002). Overexpression of dominant-negative truncations of either Myosin Vb or Rab11-FIP2 elicits a prominent inhibition of transferrin recycling (Lapierre et al., 2001, Hales et al., 2002, Lindsay and McCaffrey, 2002). The association of Rab8a with transferrin recycling has been controversial. Some investigations have seen some colocalization of Rab8a with either internalized transferrin or Rab11a (Rodriguez and Cheney, 2002, Ang et al., 2003, Hattula et al., 2006). We have not observed any effect of expression of mCherry-chimeric forms of Rab8a WT or its GDP-bound or GTP-bound mutants on transferrin trafficking by using a flow cytometry-based assay (data not shown). In contrast, in this same assay, expression of either Myosin Vb tail or truncations of Rab11-FIP proteins elicits significant decrements in transferrin recycling (Jin and Goldenring, 2006). Indeed, the present investigations using both high-resolution imaging and manipulations with multiple transfected chimeras suggest that Rab11a and Rab8a define distinct trafficking compartments. Importantly, although the expression of Myosin Vb tail caused accumulation of both Rab11a and Rab8a in a perinuclear tubular cisterna, expression of Rab11-FIP2(129-512) only caused accumulation of Rab11a, and it had no effect on the localization of Rab8a. In addition, Myosin Vb tail caused the accumulation of class I MHC molecules into this same perinuclear tubular cisterna, supporting the idea that this motor is involved in two distinct recycling pathways. Nevertheless, because Myosin Vc can cause a clear alteration of the localization of a subset of Rab8a and the transferrin receptor, it is possible that the effects of Rab8a on transferrin trafficking are mediated through Myosin Vc rather than Myosin Vb.
In contrast to the findings on Rab11a, Rab8a seems to define a geographically and functionally separate recycling system. Rab8a localized to vesicular and tubular elements that were distinct from Rab11a-containing vesicles. Although various manipulations can cause tubulation of the Rab11-containing recycling membranes (Wang et al., 2001), these tubules have a substantially different structure from the tubules demonstrated with Rab8a. In addition, although EHD1 and EHD3 both colocalized extensively with Rab8a, we only rarely observed Rab11a association with Rab8a and with either EHD protein, although EHD1 and EHD3 interact with the Rab-binding proteins Rabenosyn-5 (Naslavsky et al., 2004a) and Rab11-FIP2 (Naslavsky et al., 2006). Nevertheless, one clear possibility is that Rab11a and Rab8a define different stages in series along a common recycling pathway. Such a possibility would be consistent with the common association of Myosin Vb with both Rab11a and Rab8a. Live cell studies did not reveal any clear fusion or budding events between Rab8a and Rab11a. Thus, it seems less likely that Rab8a-containing cisternae are obligate precursors for Rab11a-containing recycling vesicles. Even so, these dynamic studies do demonstrate that Rab11a-containing vesicles can travel through the cell in contiguity with Rab8a-containing elements. This associated movement may reflect a common use of microtubule highways for movement and tubulation. Still, it remains possible that transient interactions may occur. These interactions may be critical in providing alternative pathways for trafficking in the case of pathway inhibition. Thus, transferrin receptor molecules whose Rab11a-mediated recycling path is blocked may be able to shunt into another recycling pathway containing either Rab8a or Rab4 (Provance et al., 2004).
In summary, the present studies demonstrate that Myosin V motors can influence multiple membrane recycling pathways through their interactions with specific Rab proteins. Thus, Myosin Vb molecules may serve as either multifunctional motor proteins, or they could subsume distinct roles in different vesicle trafficking pathways as either motors or cycling anchors to the actin cytoskeleton. Although we have previously used the Myosin Vb tail as a probe for trafficking of plasma membrane proteins through Rab11a-containing recycling systems, the present studies suggest this mutant is a more general inhibitor of multiple recycling pathways. The integration of different Myosin V motors with Rab11a and Rab8a again suggests that Rab protein interactions with Myosin V motors likely define the assembly of multiprotein trafficking complexes regulating specific aspects of membrane trafficking.
Supplementary Material
ACKNOWLEDGMENTS
Confocal images were generated through the use of the VUMC Cell Imaging Shared Resource (supported by National Institutes of Health [NIH] grants CA-68485, DK-20593, DK-58404, HD-15052, DK-59637, and EY-08126). This work was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK-070856 and R01DK-48370 (to J.R.G.) and F32DK-072789 (to J.T.R.), and National Institute of General Medical Science grants R01GM-073846 (to A.K.K.) and R01GM-074877 (to S.C.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0169) on May 16, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Ang A. L., Folsch H., Koivisto U. M., Pypaert M., Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J. Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume J. J., Halbach A., Behrendt D., Paulsson M., Plomann M. EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp. Cell Res. 2007;313:219–231. doi: 10.1016/j.yexcr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Brock S. C., Goldenring J. R., Crowe J. E., Jr Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells. Proc. Natl. Acad. Sci. USA. 2003;100:15143–15148. doi: 10.1073/pnas.2434327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. D., Rozelle A. L., Yin H. L., Balla T., Donaldson J. G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan S., Naslavsky N., Hartnell L. M., Lodge R., Polishchuk R. S., Donaldson J. G., Bonifacino J. S. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G. H., Lapierre L. A., Goldenring J. R., Sai J., Richmond A. Rab11-family interacting protein 2 and myosin Vb are required for CXCR2 recycling and receptor-mediated chemotaxis. Mol. Biol. Cell. 2004;15:2456–2469. doi: 10.1091/mbc.E03-09-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Kuroda T. S., Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- Galperin E., Benjamin S., Rapaport D., Rotem-Yehudar R., Tolchinsky S., Horowitz M. EHD 3, a protein that resides in recycling tubular and vesicular membrane structures and interacts with EHD1. Traffic. 2002;3:575–589. doi: 10.1034/j.1600-0854.2002.30807.x. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Smith J., Vaughan H. D., Cameron P., Hawkins W., Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am. J. Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- Green E. G., Ramm E., Riley N. M., Spiro D. J., Goldenring J. R., Wessling-Resnick M. Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem. Biophys. Res. Commun. 1997;239:612–616. doi: 10.1006/bbrc.1997.7520. [DOI] [PubMed] [Google Scholar]
- Grosshans B. L., Ortiz D., Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. M., Griner R., Hobdy-Henderson K. C., Dorn M. C., Hardy D., Kumar R., Navarre J., Chan E. K., Lapierre L. A., Goldenring J. R. Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- Hales C. M., Vaerman J. P., Goldenring J. R. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- Hattula K., Furuhjelm J., Tikkanen J., Tanhuanpaa K., Laakkonen P., Peranen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- Hobdy-Henderson K. C., Hales C. M., Lapierre L. A., Cheney R. E., Goldenring J. R. Dynamics of the apical plasma membrane recycling system during cell division. Traffic. 2003;4:681–693. doi: 10.1034/j.1600-0854.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- Jin M., Goldenring J. R. The Rab11-FIP1/RCP gene codes for multiple protein transcripts related to the plasma membrane recycling system. Biochim. Biophys. Acta. 2006;1759:281–295. doi: 10.1016/j.bbaexp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Johnson L. S., Dunn K. W., Pytowski B., McGraw T. E. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol. Biol. Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junutula J. R., Schonteich E., Wilson G. M., Peden A. A., Scheller R. H., Prekeris R. Molecular characterization of Rab11 interactions with members of the family of Rab11-interacting proteins. J. Biol. Chem. 2004;279:33430–33437. doi: 10.1074/jbc.M404633200. [DOI] [PubMed] [Google Scholar]
- Kenworthy A. K. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 2001;24:289–296. doi: 10.1006/meth.2001.1189. [DOI] [PubMed] [Google Scholar]
- Lapierre L. A., Avant K. M., Caldwell C. M., Ham A. J., Hill S., Williams J. A., Smolka A. J., Goldenring J. R. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1249–G1262. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]
- Lapierre L. A., Dorn M. C., Zimmerman C. F., Navarre J., Burnette J. O., Goldenring J. R. Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp. Cell Res. 2003;290:322–331. doi: 10.1016/s0014-4827(03)00340-9. [DOI] [PubMed] [Google Scholar]
- Lapierre L. A., Kumar R., Hales C. M., Navarre J., Bhartur S. G., Burnette J. O., Provance D. W., Jr, Mercer J. A., Bahler M., Goldenring J. R. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby R. T., Lillo C., Kitamoto J., Williams D. S., Steel K. P. Myosin Va is required for normal photoreceptor synaptic activity. J. Cell Sci. 2004;117:4509–4515. doi: 10.1242/jcs.01316. [DOI] [PubMed] [Google Scholar]
- Lindsay A. J., McCaffrey M. W. Rab11-FIP2 functions in transferrin recycling and associates with endosomal membranes via its COOH-terminal domain. J. Biol. Chem. 2002;277:27193–27199. doi: 10.1074/jbc.M200757200. [DOI] [PubMed] [Google Scholar]
- Lise M. F., Wong T. P., Trinh A., Hines R. M., Liu L., Kang R., Hines D. J., Lu J., Goldenring J. R., Wang Y. T., El-Husseini A. Involvement of myosin Vb in glutamate receptor trafficking. J. Biol. Chem. 2006;281:3669–3678. doi: 10.1074/jbc.M511725200. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Moore R. H., Millman E. E., Alpizar-Foster E., Dai W., Knoll B. J. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J. Cell Sci. 2004;117:3107–3117. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Naslavsky N., Boehm M., Backlund P.S.J., Caplan S. Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Mol. Biol. Cell. 2004a;15:2410–2422. doi: 10.1091/mbc.E03-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N., Rahajeng J., Sharma M., Jovic M., Caplan S. Interactions between EHD proteins and Rab11-FIP 2, a role for EHD3 in early endosomal transport. Mol. Biol. Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N., Weigert R., Donaldson J. G. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell. 2003;14:417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N., Weigert R., Donaldson J. G. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004b;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance D. W., Jr, Gourley C. R., Silan C. M., Cameron L. C., Shokat K. M., Goldenring J. R., Shah K., Gillespie P. G., Mercer J. A. Chemical-genetic inhibition of a sensitized mutant myosin Vb demonstrates a role in peripheral-pericentriolar membrane traffic. Proc. Natl. Acad. Sci. USA. 2004;101:1868–1873. doi: 10.1073/pnas.0305895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance D. W., James T. L., Mercer J. A. Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic. 2002;3:124–132. doi: 10.1034/j.1600-0854.2002.030205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Donaldson J. G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M. A., Springer G. H., Granada B., Piston D. W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- Rodriguez O. C., Cheney R. E. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 2002;115:991–1004. doi: 10.1242/jcs.115.5.991. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Shu X., Shaner N. C., Yarbrough C. A., Tsien R. Y., Remington S. J. Novel chromophores and buried charges control color in mFruits. Biochemistry. 2006;45:9639–9647. doi: 10.1021/bi060773l. [DOI] [PubMed] [Google Scholar]
- Strom M., Hume A. N., Tarafder A. K., Barkagianni E., Seabra M. C. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli L. A., Lah J. J., Fang G., Goldenring J. R., Levey A. I. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 2002;22:9776–9784. doi: 10.1523/JNEUROSCI.22-22-09776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Pennington J. G., Goldenring J. R., Hunziker W., Dunn K. W. Brefeldin A rapidly disrupts plasma membrane polarity by blocking polar sorting in common endosomes of MDCK cells. J. Cell Sci. 2001;114:3309–3321. doi: 10.1242/jcs.114.18.3309. [DOI] [PubMed] [Google Scholar]
- Wang X., Kumar R., Navarre J., Casanova J. E., Goldenring J. R. Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- Wu X., Kocher B., Wei Q., Hammer J. A., 3rd Myosin Va associates with microtubule-rich domains in both interphase and dividing cells. Cell Motil. Cytoskeleton. 1998;40:286–303. doi: 10.1002/(SICI)1097-0169(1998)40:3<286::AID-CM7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.