Abstract
Utrophin is the autosomal homologue of dystrophin, the protein product of the Duchenne's muscular dystrophy (DMD) locus. Utrophin expression is temporally and spatially regulated being developmentally down-regulated perinatally and enriched at neuromuscular junctions (NMJs) in adult muscle. Synaptic localization of utrophin occurs in part by heregulin-mediated extracellular signal-regulated kinase (ERK)-phosphorylation, leading to binding of GABPα/β to the N-box/EBS and activation of the major utrophin promoter-A expressed in myofibers. However, molecular mechanisms contributing to concurrent extrasynaptic silencing that must occur to achieve NMJ localization are unknown. We demonstrate that the Ets-2 repressor factor (ERF) represses extrasynaptic utrophin-A in muscle. Gel shift and chromatin immunoprecipitation studies demonstrated physical association of ERF with the utrophin-A promoter N-box/EBS site. ERF overexpression repressed utrophin-A promoter activity; conversely, small interfering RNA-mediated ERF knockdown enhanced promoter activity as well as endogenous utrophin mRNA levels in cultured muscle cells in vitro. Laser-capture microscopy of tibialis anterior NMJ and extrasynaptic transcriptomes and gene transfer studies provide spatial and direct evidence, respectively, for ERF-mediated utrophin repression in vivo. Together, these studies suggest “repressing repressors” as a potential strategy for achieving utrophin up-regulation in DMD, and they provide a model for utrophin-A regulation in muscle.
INTRODUCTION
Duchenne's muscular dystrophy (DMD) is a fatal neuromuscular disease caused by gene mutations leading to qualitative or quantitative disturbances in dystrophin expression (Hoffman et al., 1987). Dystrophin-related protein or utrophin is considered the autosomal homologue of dystrophin, because it shares extensive sequence homology as well as its size and abundance in muscle (Love et al., 1989; Khurana et al., 1990; Tinsley et al., 1992). Utrophin and dystrophin also share functional properties such as the ability to associate with the dystroglycan complex and bind F-actin (Matsumura et al., 1992; Winder and Kendrick, 1995; Rybakova et al., 2002). Direct evidence for the ability of utrophin to functionally substitute comes from experiments demonstrating that utrophin overexpression driven either by transgenic means (Tinsley et al., 1996; Rafael et al., 1998; Tinsley et al., 1998; Fisher et al., 2001) or viral vectors (Gilbert et al., 1999; Wakefield et al., 2000; Cerletti et al., 2003) can rescue dystrophin-deficient muscle. Despite these functional similarities, dystrophin and utrophin exhibit distinct localization in normal adult tissues. Perinatally, utrophin is expressed throughout the sarcolemma, however, its expression declines as that of dystrophin increases (Khurana et al., 1991; Clerk et al., 1993), leading to its spatially restricted expression at neuromuscular and myotendinous junctions (NMJs and MTJs, respectively) of adult myofibers. These features and relevance toward a therapy for DMD provide a powerful impetus for better understanding transcriptional control of utrophin expression in myofibers (Jasmin et al., 2002; Khurana and Davies, 2003; Nowak and Davies, 2004).
Although full-length utrophin protein can be generated using independently regulated A (Dennis et al., 1996) and B (Burton et al., 1999) promoters, myofiber utrophin expression is chiefly controlled via the utrophin-A promoter (Weir et al., 2002; Chakkalakal et al., 2003), and it is thus the subject of this investigation. Spatial and temporal expression of utrophin-A at the NMJ parallels that of nicotinic acetylcholine receptor (nACHR) subunits. Indeed, these genes share some features of transcriptional control. For example, the nACHRε subunit and utrophin-A promoter N-box play a critical role in regulating expression levels and more importantly in restricting expression to the NMJ (Schaeffer et al., 2001). We and others (Gramolini et al., 1999a; Khurana et al., 1999) have shown that the nerve-derived growth factor heregulin (HRG) causes a N-box–mediated increase in utrophin-A promoter activity via extracellular signal-regulated kinase-dependent activation of the Ets-related transcription factor complex GABPα/β, in a manner similar to that noted in the nACHRδ and nACHRε subunit promoters (Falls et al., 1993; Koike et al., 1995; Sapru et al., 1998; Schaeffer et al., 1998). Additionally, SP1 (Galvagni et al., 2001; Gyrd-Hansen et al., 2002), NFATc1/calcineurin (Chakkalakal et al., 2003; Chakkalakal et al., 2004), and myogenic factors (Gramolini and Jasmin, 1999; Perkins et al., 2001) can also activate the utrophin-A promoter. Recently, the 5′-untranslated region of utrophin-A promoter has been shown to be important for regulation of utrophin protein levels during regeneration as well (Miura et al., 2005). Indeed, several of these molecules or mechanisms are currently being investigated for development of utrophin up-regulation-based therapeutics for DMD (Chaubourt et al., 1999; Krag et al., 2004; St-Pierre et al., 2004; Segalat et al., 2005).
However, equally important mechanisms controlling concurrent extrasynaptic down-regulation or repression of utrophin-A in myofibers that must occur to achieve expression at the NMJ rather than generalized expression throughout the sarcolemma remain unaddressed. Mechanistic clues arise from studies illustrating that restriction of neuronal sodium channel expression to the nervous system is partly due to RE-1 silencing transcription factor/neuronal-restricted silencing factor binding to a repressor element (Andres et al., 1999; Ballas et al., 2001; Lunyak et al., 2002). Additionally an N-box–mediated silencing mechanism has been suggested for nACHR subunit expression in extrasynaptic regions of muscle (Koike et al., 1995). In silico analysis of the human and murine utrophin-A promoter sequences suggest Ets-2 repressor factor (ERF) as a candidate molecule for N-box–mediated extrasynaptic repression of the utrophin-A promoter (Sgouras et al., 1995). ERF is a novel member of the ets family and encodes a ubiquitously expressed 548-amino acid phospho-protein identified through its ability to repress the Ets-2 promoter via EBS binding (Sgouras et al., 1995; Le Gallic et al., 1999). ERF activity is regulated in part, by ERK-dependent changes in its phosphorylation status and subcellular localization due to nuclear shuttling, with CRM1 suggested to have a role in ERF export (Le Gallic et al., 2004). Here, we use an array of in vitro and in vivo methodologies to delineate the role of ERF in the regulation of the utrophin-A promoter.
MATERIALS AND METHODS
Constructs
The pPUBF utrophin promoter luciferase reporter construct contains 1.3-kb of human utrophin promoter-A sequence (Dennis et al., 1996), and it was a kind gift from Prof. Kay Davies (Oxford University). The pPUBFΔN-box is a pPUBF derivative with a deleted N-box/EBS sequence, and it has been previously described by our laboratory (Khurana et al., 1999). Expression vectors containing human GABPα and -β1 subunits used were GABPα and GABPβ (Rosmarin et al., 1995), and they were kindly gifted by Prof. Alan Rosmarin (Brown University). Expression vectors containing human ERF cDNA used were pSG5-ERF and GFP-ERF (Sgouras et al., 1995; Le Gallic et al., 1999), and they were kindly gifted by Prof. George Mavrothalassitis (IMBB, Greece).
Gel Shift Assay
Double-stranded probes encompassing the EBS and N-box of the human utrophin-A promoter (5′-GCTGATCTTCCGGAACAAAGTTGCT-3′; N-box underlined; EBS bold) or probes containing a mutated N-box/EBS site (5′-GCTGATCTTCCATCTACAAGTTGCT-3′) were end labeled with [γ-32P]ATP by using polynucleotide kinase. Unlabeled probes (10- and 100-fold excess) were used in cold competitor assays as outlined in Figure 1B. Recombinant human ERF (rhERF) was produced using a coupled in vitro transcription/translation system (Promega, Madison, WI), by using 0.5 μg (GFP)-ERF construct and unlabeled methionine. Additionally, we used nuclear extracts from C2C12 cell line (Active Motif, Carlsbad, CA). Gel shift analysis was performed using the Gel Shift Assay system (Promega). For supershifts, 5 μl of ERF-specific rabbit polyclonal antibody (S17C), and as controls, a nonspecific polyclonal antibody against the transcription factor Twist (Santa Cruz Biotechnology, Santa Cruz, CA), were added to the reaction mix for 1 h at room temperature before probe addition. Protein–DNA complexes were resolved on 4% polyacrylamide gels by using 0.25× Tris-glycine buffer at 200 V for 2 h. Gels were dried and autoradiographed on a Typhoon 8600 Imager (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
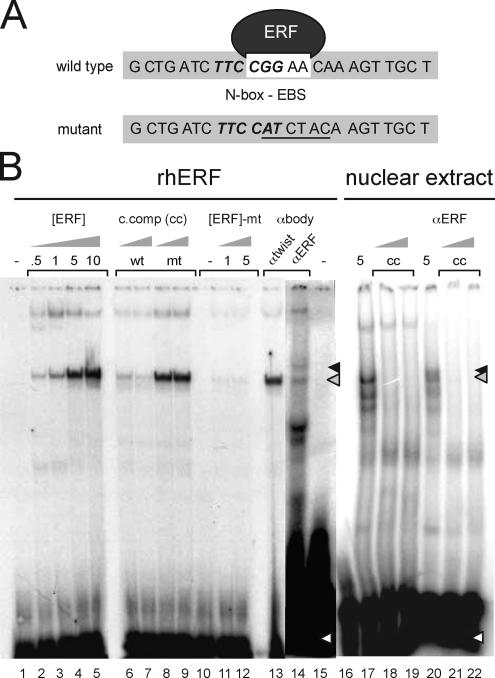
Figure 1.
ERF binds to the utrophin-A promoter N-box/EBS element. (A) Sequence of the human utrophin-A promoter probes containing the N-box/EBS motif used in gel shifts. The N-box is in bold italics; the EBS is in a white box. Mutant refers to the gel shift probe used that contains a mutant N-box/EBS site. (B) The N-box/EBS site in the human utrophin A promoter binds ERF. rhERF was in vitro synthesized and 0.5–10 μl (0.5–10; lanes 2–5) was incubated with the labeled oligonucleotide probe. Five microliters each of C2C12 extract was used for analysis of endogenous ERF in lanes 17–22. Formation of a single specific DNA–protein complex (gray arrow) was observed with rhERF (lanes 2–5), which could be specifically competed away with 10- and 100-fold excess of wild-type cold competitor oligonucleotide (lanes 6 and 7) but not the mutant probe (lanes 8 and 9). rhERF did not bind a double-stranded probe of the same region with a mutated N-box/EBS site (lanes 10–12). The black arrow indicates ERF–DNA complex supershifted in the presence of 5 μl of ERF-specific antibodies (lane 14) but not in the presence of a nonspecific antibody against the transcription factor Twist (lane 13). White arrows represent free radiolabeled probe. Wt, wild-type probe; mt, N-box/EBS mutant probe; [ERF], amount of rhERF protein in microliters; c, comp, unlabeled N-box/EBS oligonucleotide; αERF, ERF S17C rabbit polyclonal antibody.
Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR was performed on RNA obtained from ∼100 mg of freshly dissected dystrophin-deficient (mdx) hindlimb muscle from different developmental stages, as described previously (Khurana et al., 1999). Murine ERF and GABPα primers were designed to amplify a 490-base pair fragment of ERF spanning exons 1–4 (ERF1F, 5′-GATTGGCCTACAAACCGGAGTCATCC-3′) and ERF2R, 5′-GTC GGGCAACCACAGGAGAGAAGAG-3′) and a 409-bp fragment of GABPα spanning exons 8–10 (GABPα2F, 5′-CAGCTAAAGTGCAACGGTCCCCAAG-3′ and GABPα1R, 5′-CCGTGCCAGTTTCTTCTGTTCACACTC-3′). Primers used for MyoD were M_MyoD_1483F (5′-CTCCACATCCTTTTGTTTGT-3′) and M_MyoD_1825R (5′-AGCGTCTTTATTTCCAACAC-3′) and for Myogenin were M_Myogenin_924F (5′-CAATACACAAAGCACTGGAA-3′) and M_Myogenin_1222R (5′-TCTGAGGAGAGAAAGATGGA-3′). Utrophin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were as described previously (Khurana et al., 1999). PCR products were resolved on 4% acrylamide gels, dried, and radioactively incorporated products quantified using a Typhoon 8600 Imager and ImageQuant IQ Tools version 2.2 (GE Healthcare).
Tissue Culture and Transfection
Murine C2C12 muscle cells and Drosophila S2 embryonic cells were maintained and transfected as described previously (Gyrd-Hansen et al., 2002). For cotransfection of human GABPα, GABPβ, and pSG5-ERF expression vectors, 1 μg of vector(s) and 1 ng of Renilla control plasmid (pRL-TK; Promega) were used. The mitogen-activated protein kinase kinase (MEK) inhibitor UO126 (10 μM; Promega) was added concurrent with transfection to block Drosophila (Gobert Gosse et al., 2005) and mammalian MEK activity. Cells were allowed to express fusion genes for 12–24 h before analysis of cell extracts for luciferase activity by using the Dual Luciferase Assay kit (Promega) with a 20/20 luminometer (Turner Designs, Sunnyvale, CA). All assays were performed in triplicate with the transfection/assay process repeated using separate cultures for particular experiments as outlined in the figure legends.
MEK-dependent translocation of ERF from nucleus to cytoplasm was studied using C2C12 muscle cells that were incubated with MEK inhibitor U0126 for different times and analyzed using immunofluorescence of cells and immunoblotting of subcellular fractions as described previously for Ref1 cells (Le Gallic et al., 1999, 2004). For immunofluorescent analysis, cells were fixed with ice-cold methanol for 10 min, incubated with anti-ERF antibodies (Santa Cruz Biotechnology), and detected using Alexa Fluor 546 donkey anti-goat secondary antibodies (Invitrogen, Carlsbad, CA). Images were acquired using a Radiance 2000 confocal laser imaging system (Bio-Rad, Hercules, CA). Nuclear and cytoplasmic fractions for immunoblotting were obtained by lysing the cells with 35 strokes in a glass Dounce homogenizer (pestle B) in buffer A (10 mM Tris-HCl, pH 7.5, 300 mM sucrose, and 1 mM EDTA). Nuclei were pelleted at 2000 × g for 5 min and washed with buffer B (10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 1 mM CaCl2, 0.5 mM MgCl2, and 0.1% Nonidet P-40). All buffers were supplemented with 1 mM orthovanadate and protease inhibitor complete (Roche Diagnostics, Basel, Switzerland). To control for fractionation of nuclear and cytoplasmic compartments, aliquots of each of the fractions were monitored using fluorescent microscopy and DNA binding dyes. To control for loading equivalent amounts of proteins, the concentration of proteins was measured using a Bradford assay (Bio-Rad), and equal concentration (50 μg) of total protein from cytoplasmic and nuclear fractions were loaded and resolved using 3–8% Tris-acetate gradient SDS-polyacrylamide gel electrophoresis, electrotransferred onto polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA), and membranes were probed using anti-ERF antibodies (Santa Cruz Biotechnology). Blots were washed thoroughly, incubated with horseradish peroxidase-conjugated donkey anti-goat secondary antibodies (Promega), and enhanced chemiluminescence was performed as described by manufacturer (Pierce Chemical, Rockford, IL), by using X-Omat Blue XB-1 films (Eastman Kodak, Rochester, NY).
RNA Interference (Small Interfering RNA) Studies
Duplexed stealth RNA oligomers to the murine ERF sequence were designed using the BLOCK-iT RNA interference system (Invitrogen). Proliferating C2C12 cells (50% confluent) were transfected using LipofectAMINE 2000 (Invitrogen) with 25 pmol each of ERF-292 (5′-GGUUCACCUACAAG UUCAACUUCAA-3′), ERF-326 (5′-GCUGGUCAAUUACCCUUUCAUCGAU-3′), ERF-937 (5′-CCCACACCCAAAGCGUCUACAACUA-3′), and ERF-1268 (5′-GAUUAAGGUGGAGCCCA UCUCAGAA-3′) or 100 pmol of an unrelated, scrambled control “egg” oligomer (5′-GCUUACUC AUCCAUGCAUCGGUAUG-3′). Transcript levels of utrophin, GAPDH, and ERF postoligomer addition were determined using semiquantitative RT-PCR. Analysis of ERF knockdown effects on utrophin promoter activity used 1 μg of pPUBF construct and transfection of oligomers after 24 h. Cells were incubated an additional 24 h before assaying for luciferase activity.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed with goat polyclonal anti-ERF antibodies (Santa Cruz Biotechnology) according to the manufacturer's protocol (Upstate Biotechnology, Lake Placid, NY). Briefly, cells from one 100-mm plate were treated with or without 2 nM heregulin for 15 min after overnight serum starvation, and they were cross-linked with 0.37% final concentration of formaldehyde. For U0126 treatment, cells were grown in presence of serum and treated with 10 μM U0126 for 15 min. Cells were washed twice with ice-cold phosphate-buffered saline (PBS). Cell pellets were lysed in 200 μl of lysis buffer and sonicated. Cell lysate was diluted 10-fold in ChIP dilution buffer and precleared with 120 μl of protein A-agarose and 120 μl of protein G-agarose. The precleared set was incubated with or without antibody at 4°C overnight with constant rotation. The antibody–chromatin complex was then collected with 60 μl of protein A-agarose and 60 μl of protein G-agarose, incubating 1 h at 4°C with constant rotation. The agarose beads were washed with wash buffers, and finally, chromatin was eluted with 500 μl of elution buffer (0.1 M NaHCO3 and 1% SDS) at room temperature. Beads were reverse cross-linked at 65°C overnight with 20 μl of 5 M NaCl. One percent of input reserved before immunoprecipitation was reverse cross-linked in parallel. All solutions were supplemented with protease inhibitor complete (Roche Diagnostics), 1 mM Na3VO4 and 1 mM NaF. DNA was extracted with PCR purification kit (QIAGEN, Valencia, CA). Presence of utrophin-A promoter was detected in different sets by PCR in the presence of [α-32P]dCTP with primers 5′-CCCAAACTCAACAACCTCAGTAAAC-3′ and 5′-CAAATTGTCCGAAAATGTGTGTCA-3′ designed to amplify 151 bp of utrophin-A promoter (NCBI accession no. X95524). Primers did not amplify products without appropriate templates. The products were separated using 12% acrylamide gel, and they were imaged using a Typhoon 8600 (GE Healthcare).
Laser Capture Microdissection (LCM) and Quantitative (q)PCR
Microscopy was performed using a PALM laser capture microscope with sections of adult rat tibialis anterior (TA) muscle (200–300 μm2 and 10 μm in thickness) by using methods similar to those used for regional expression profiling of muscle (Budak et al., 2004; Nazarian et al., 2005). Extracted total RNA (yield typically between 50 and 150 ng) was used for one cycle linear amplification by using the Affymetrix two-cycle labeling kit (Affymetrix, Santa Clara, CA). Subsequently, amplified RNA (aRNA; yield typically 1000–5000 ng) was converted to cDNA by using an Invitrogen SuperScript RT kit and used as a template for qPCR. Taqman qPCR cholinergic receptor, nicotinic, α polypeptide 1 (Chrna1) (assay ID Rn00577938_m1), Utrn (utrophin) (assay ID Rn00565137_m1, murine Ets-2 repressor factor (Erf) (mouse, assay ID Mm00468761_m1) and β actin primers (part 4352931E; Applied Biosystems, Foster City, CA). Rat v-ets erythroblastosis virus E26 oncogene homologue 1 (Ets1) (forward, 5′-TTGTGTGTGACCCGGAAGC-3′ and reverse, 5′-GCTGATTATCCGGAAAGGCC-3′) primers were designed by primer expressed software version 2 for SYBR Green chemistry qPCR. An ABI 7500 qPCR machine (Applied Biosystems) was used for Taqman qPCR and ABI 7900HT qPCR machine (Applied Biosystems) was used for SYBR Green chemistry. cDNA (RNA) template (an aRNA equivalent of 3–5 ng/well cDNA used) of same animal was run in duplicate on the plate, and results were analyzed using the delta count method for relative gene expression analysis by using ABI SDS software version 1.3.1 (Applied Biosystems).
Direct Gene Transfer
All animal care and experimental procedures were performed in accordance with the guidelines established by the Canadian Council of Animal Care. These procedures were approved by the University of Ottawa Animal Care and Use Committee. Three- to 4-wk-old C57BL/6 mice were anesthetized, and anterior portions of both lower hindlimbs were shaved and disinfected. The underlying TA muscles were then directly injected with 25 μl of DNA solution as described previously (Gramolini et al., 1997; Chakkalakal et al., 2003). The DNA solution contained equal amounts of the 1.3-kb human utrophin-A promoter-reporter (LacZ) construct (see Gramolini et al., 1997; Chakkalakal et al., 2003), the pPUBFΔN-box construct (luciferase), a constitutively expressed chloramphenicol acetyl transferase (CAT) plasmid (to control for transduction efficiency), and either the ERF expression plasmid or its control (pSG5). Plasmid DNA was prepared using the Mega-Prep kit (QIAGEN, Mississauga, Ontario, Canada). DNA pellets were resuspended in sterile PBS at a final concentration of 1–2 μg/μl. Five days postinjection, TA muscles were quickly excised, immediately frozen in liquid nitrogen, and stored at −80 C until processed for RT-PCR analysis. Total RNA was extracted by using TriPure (Roche Diagnostics, Indianapolis, IN) as recommended by the manufacturer. Quantitative RT-PCR was performed to determine the relative abundance of LacZ, luciferase, and CAT transcripts. Before RT-PCR, samples were digested using DNase1 to eliminate possible plasmid contamination (Gramolini et al., 2001). RT-PCR assays were performed using previously described protocols and primers (Gramolini et al., 1999b, 2001; Chakkalakal et al., 2003). Cycle numbers varied depending on the primers used and they were all within the linear range of amplification. In all assays, negative controls consisted of RT mixtures in which total RNA was replaced with RNase-free water. PCR products were first visualized on 1.5% agarose gels containing ethidium bromide. Labeling intensity of the PCR products that is linearly related to the abundance of cDNAs was then quantified using Digital Science 1D Image Analysis software (Eastman Kodak). Values were standardized relative to the amount of CAT present in the same sample. The Student's t tests were used to analyze the data.
RESULTS
ERF Can Physically Associate with the Utrophin-A N-Box/EBS Site
To determine whether the utrophin-A promoter N-box/EBS sequence is recognized by ERF, a double-stranded probe spanning the region (Figure 1A) was incubated with recombinant ERF (Sgouras et al., 1995) for gel shift assays. The protein–DNA complex could be competed with excess unlabeled N-box/EBS probe and supershifted using ERF-specific antibodies (Figure 1B). The protein–DNA complex could not be competed using a probe containing mutations in the N-box/EBS. The complex was not supershifted using an irrelevant antibody. Additionally, a mutant probe was not retarded when incubated with ERF (Figure 1B). To demonstrate the existence of ERF and show its ability to form the complex in the context of muscle, we performed the gel shift and supershift by using nuclear extracts obtained from C2C12 muscle cell lines. As shown in Figure 1B, a protein–DNA complex was formed that was able to retard probe migration and able to be competed with excess unlabeled probe as well as supershifted using ERF-specific antibodies in muscle cell lines, suggesting the relevance of this mechanism for muscle in vivo.
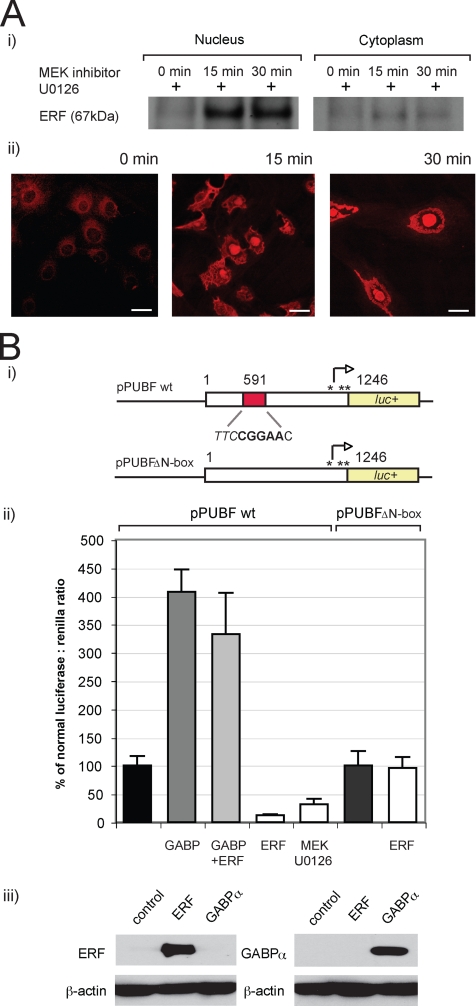
Having demonstrated the ability of ERF to associate with the N-box/EBS site of the utrophin-A promoter using gel shift assays, we asked whether the ERK-dependent nuclear shuttling mechanisms previously characterized in Ref-1 cells (Le Gallic et al., 2004) are functional in C2C12 muscle cell lines. To test this, we performed subcellular fractionation experiments to determine the levels of ERF in the nuclear and cytoplasmic fractions of C2C12 muscle cell lines after incubation with U0126, an inhibitor for MEK, the upstream kinase for ERK1/2 at different times. As shown in Figure 2Ai, incubation with U0126 led to increased ERF levels in the nuclear compared with cytoplasmic fractions. Next we used anti-ERF antibodies for indirect immunofluorescent confocal microscopy to independently validate the MEK-dependent nuclear shuttling by visualizing changes in subcellular localization of ERF in C2C12 cells when incubated with U0126. As shown in Figure 2Aii, incubation with U0126 caused ERF to preferentially localize in the nucleus. To demonstrate the effect of ERF on utrophin-A promoter activity, we used the pPUBF utrophin-A luciferase reporter construct containing the entire human utrophin promoter (Dennis et al., 1996), as well as the pUBFΔN-box reporter construct that contains a deletion mutation of the N-box region (Khurana et al., 1999) (Figure 2Bi). Drosophila S2 cells were cotransfected with pPUBF and 1) expression constructs encoding GABPα/β (Rosmarin et al., 1995) as a positive control or 2) ERF expression vector pSG5-ERF. In agreement with previous studies (Khurana et al., 1999; Gyrd-Hansen et al., 2002), GABPα/β trans-activated the utrophin A promoter fourfold (407 ± 39.2%) (Figure 2Bii). In contrast, ERF trans-repressed the promoter by ∼88% (12.3 ± 2.2%). Use of the MEK inhibitor U0126 and the subsequent nuclear export of ERF also resulted in an approximately threefold decrease in promoter activity (32.1 ± 9.3%). Utrophin promoter reporter assays were also performed using cotransfection of ERF and GABPα/β expression constructs together in Drosophila S2 cells (Figure 2B). To determine whether the N-box was required for ERF-mediated repression, we transfected cells using the pUBFΔN-box construct. As shown in Figure 2Bii, ERF was unable to repress the mutant utrophin promoter lacking the N-box motif. Additionally, immunoblotting by using anti-ERF and anti-GABP antibodies was performed on cells that had been transfected with the ERF and GABP constructs, respectively, to verify that the expression constructs were functional (Figure 2Biii). Together, these assays demonstrated that 1) ERF-mediated trans-repression could be successfully competed by GABPα/β-mediated promoter activation and 2) that the N-box motif is required for ERF-mediated trans-repression of the utrophin promoter.
Figure 2.
The human utrophin-A promoter is trans-repressed by ERF overexpression and MEK inhibition, which may be alleviated by GABPα/β. (A) Changes in subcellular fractions and subcellular localization of ERF protein in C2C12 cells caused by inhibition of MEK by using U1026. Nuclear shuttling of ERF noted over a 30-min treatment period as demonstrated by i) immunoblotting or ii) immunofluorescence by using anti-ERF antibodies, as described in Materials and Methods. Bar, 25 μm. (B) The human utrophin-A promoter is trans-repressed by ERF overexpression and MEK inhibition, which may be alleviated by GABPα/β. (i) The 1.3-kb human utrophin-A promoter-luciferase construct pPUBF showing location of the N-box/EBS according to X95523. The pPUBFΔN-box construct has a deletion that removes the N-box/EBS. (ii) pPUBF or pPUBFΔN-box derived firefly luciferase activities were normalized to pRL-TK-derived Renilla luciferase activity and expressed as a percentage of normalized luciferase activity (black column). Utrophin promoter-reporter constructs and a transfection control pRL-TK vector were cotransfected into S2 cells along with equimolar combinations of the following: GABPα/β expression vectors (GABP, gray columns), ERF expression vector pSG5-ERF (ERF, white columns), GABPα/β and ERF vectors (light gray column), or a 10 μM final concentration of MEK inhibitor UO126 (white column), and luciferase activity was assayed after a 24-h incubation. GABPα/β trans-activated the utrophin promoter construct almost fourfold (gray column), whereas decreases to 12 and 33% of normalized promoter activity was noted with ERF overexpression and MEK inhibition, respectively. However, trans-repression by ERF was completely competed by GABPα/β trans-activation (light gray column). No difference in pPUBFΔN-box reporter activity was observed upon the addition of ERF (96%). Luciferase values are the means of triplicate wells in nine separate experiments (n = 27) for pPUBF and ERF and in three separate experiments (n = 9) for MEK inhibition and GABPα/β. Error bars are SEM. (iii) Immunoblot controls showing that transfection of GABP and ERF expression vectors causes an increase in protein levels in transfected C2C12 cells.
ERF Knockdown Enhances Endogenous Utrophin mRNA Levels and Utrophin-A Promoter Activity
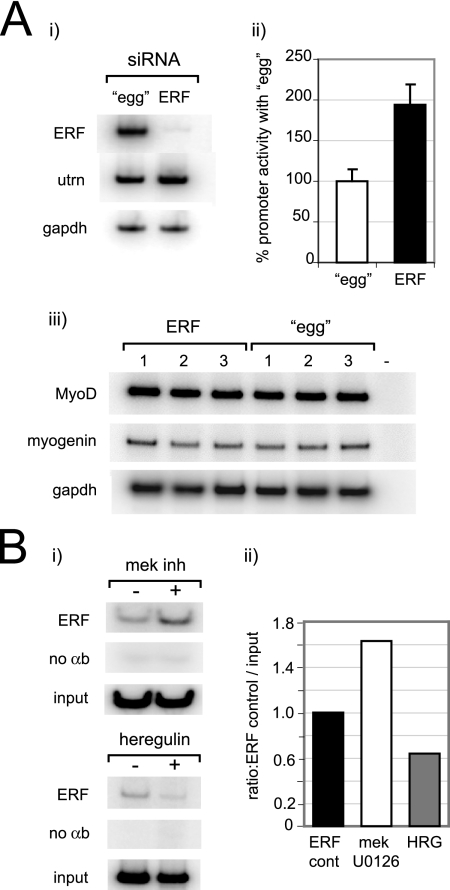
Because ERF is able to bind and repress promoter activity, we sought to delineate the effect of siRNA-mediated silencing of ERF on 1) endogenous utrophin mRNA levels and 2) utrophin-A promoter activity. To ensure selective knockdown, four siRNA oligomers corresponding to regions of ERF outside the conserved N-box/EBS binding domain with no homology to other known gene were transfected into C2C12 muscle cells, and semiquantitative RT-PCR was performed after 24 h. ERF siRNA decreased endogenous ERF levels to 2%, and it enhanced endogenous utrophin levels (1.4-fold) compared with an unrelated scrambled oligomer control (egg), after adjustment to GAPDH levels (Figure 3Ai). We postulated the increase in utrophin mRNA was due to increased promoter activity as a result of decreased ERF levels; therefore, pPUBF was transfected 24 h postoligomer addition, and C2C12 cells were assayed for luciferase activity after an additional 24-h period (Figure 3Bii). In concordance with our hypothesis, a twofold elevation of promoter activity (199.1 ± 24%) was observed for ERF oligomers in comparison with the egg control. Additional transfection experiments were performed to address whether siRNA-mediated silencing of ERF altered the program of myogenic differentiation in the cultured myotubes and hence secondarily effected utrophin expression via altered expression of myogenic factors (Gramolini and Jasmin, 1999; Perkins et al., 2001). As shown in Figure 3Aiii, no alterations in expression levels of the myogenic factors MyoD and myogenin were detected using semiquantitative RT-PCR after transfecting with ERF siRNA oligomers or scrambled egg control, making it highly unlikely that the alterations of utrophin promoter activity were due to changes in expression of myogenic factors. Rather, the increase in utrophin promoter activity noted in Figure 3A was indeed a direct result of decreased ERF levels due to a siRNA-mediated mechanism, as postulated.
Figure 3.
Inhibition of ERF gene expression enhances utrophin mRNA levels and utrophin-A promoter activity in C2C12 cells. (Ai) Semiquantitative RT-PCR of ERF, utrophin, and GAPDH transcript levels in murine C2C12 cells after treatment with either 100 pmol (left lane) of an unrelated, scrambled control egg oligomer, or 25 nmol each of four siRNA complementary to ERF. ERF siRNA oligomers caused a specific decrease in ERF transcript to ∼2% and an ∼1.4-fold increase in utrophin mRNA after adjustment to GAPDH levels. (ii) ERF knockdown causes an increase in utrophin promoter activity. The pPUBF utrophin promoter-luciferase construct was transfected into C2C12 cells 24 h after either egg or ERF siRNA oligomer transfection, and luciferase activity was assayed after an additional 24-h incubation. An approximate twofold elevation of utrophin promoter activity in C2C12 cells was observed with the ERF oligomer mix (1.99 ± 0.24) in comparison with the egg scrambled control. Luciferase values are the means of four separate experiments performed in triplicate (n = 12). Error bars are SEM. (iii) Semiquantitative RT-PCR of MyoD, myogenin, and GAPDH transcript levels in murine C2C12 cells after treatment with either 25 nmol each of four siRNA complementary to ERF (left lanes, 1–3) or 100 pmol (right lanes, 1–3) of an unrelated, scrambled control egg oligomer and assayed after 24 h. The − marked lane shows a negative control for RT-PCR where reaction was performed without template. No significant changes in MyoD or myogenin transcript levels were noted. Gels show results of three separate experiments. (B) Chromatin immunoprecipitation analysis of the utrophin-A promoter with ERF antibodies in C2C12 cells, demonstrating changes in ERF occupancy of the utrophin-A promoter upon treatment with MEK inhibitor U0126 and HRG treatment for 15 min. (i) RT-PCR of utrophin-A promoter from ERF-precipitated chromatin from C2C12 cells. (ii) Quantification of changes in ERF occupancy compared with the untreated ERF controls shows increased (1.63-fold) and decreased (0.64-fold) ERF levels with MEK inhibitor U0126 and HRG, respectively. Controls incubated without antibodies are shown below.
ChIP Analysis Demonstrates ERF Association with the Utrophin-A Promoter
To independently validate the interaction between ERF and the utrophin-A promoter and the role of MEK and heregulin in modulating the promoter (Gramolini et al., 1999a; Khurana et al., 1999), we used anti-ERF antibodies to perform ChIP analysis of the utrophin-A promoter in C2C12 cells that had been treated with MEK inhibitor U0126 or heregulin for 15 min or left untreated. As shown in Figure 3B, ERF was associated with the utrophin-A promoter, and its levels were modulated by U0126 and heregulin. Consistent with the gel shift assays, subcellular localization and reporter assays (Figure 2) as well as previous literature on heregulin-mediated utrophin promoter activation (Gramolini et al., 1999a; Khurana et al., 1999), MEK inhibitor U0126 increased, and conversely heregulin decreased, the levels of ERF associated with the utrophin-A promoter, respectively.
Utrophin, GABPα, and ERF Expression during Development
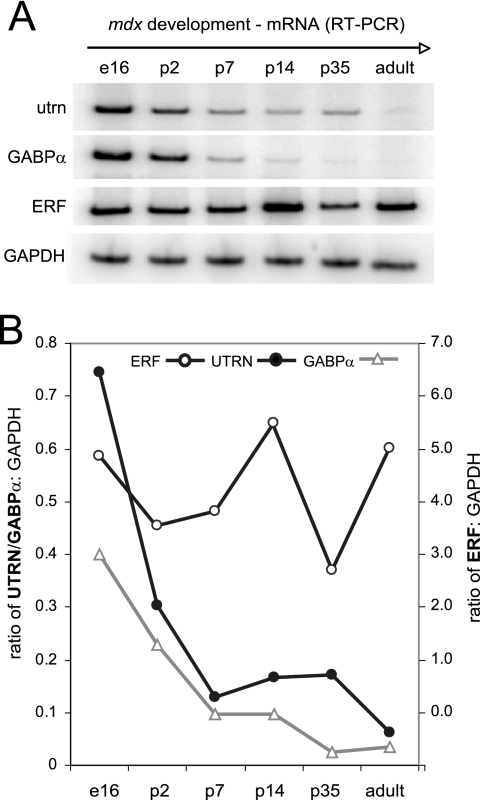
To address the approximately eightfold perinatal reduction of utrophin levels that leads to relatively low levels (typically 0.01% of message) in the adult compared with embryonic muscle (Khurana et al., 1990, 1991), we analyzed utrophin, GABPα, and ERF transcript levels by using semiquantitative RT-PCR at six times from embryonic day 16- (e16) to adult (1-yr)-old mdx mouse hindlimb muscle (Figure 4). Utrophin and GABPα transcripts were most abundant during embryogenesis (e16), and they showed a decline of 2.5- and 1.7-fold, respectively, by perinatal day 2 (p2). Analysis of adult muscle indicated that transcripts had decreased to 11.6-fold for utrophin and 12.1 for GABPα from values obtained during embryogenesis (Figure 4B). Interestingly, ERF transcript levels remained high from late embryogenesis to adult muscle similar (cf. 4.9 and 5.1, respectively), with the highest level detected at p14 (5.74 arbitrary units standardized to GAPDH).
Figure 4.
Transcript levels of GABPα, utrophin, and ERF in mdx mouse muscle. Semiquantitative RT-PCR of utrophin, GABPα, ERF, and control GAPDH transcript levels at various times from embryonic day 16 to adult (12-mo-old) muscle. Utrophin and GABPα transcripts are shown after a 48-h exposure, and GAPDH and ERF are shown after 24-h exposure. The graph illustrates arbitrary units of each PCR product measured by ImageQuant Tool software and expressed as a ratio to GAPDH at each time point after 24-h exposure. Left y-axis refers to ratios to GAPDH obtained for utrophin and GABPα, and right y-axis refers to ERF:GAPDH ratios. E, embryonic; p, perinatal.
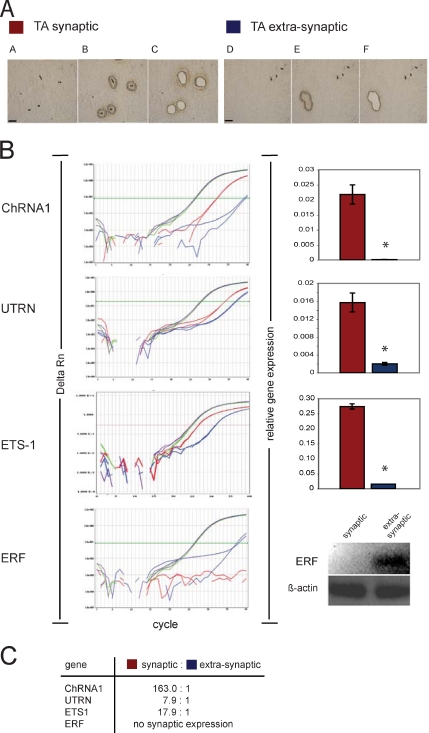
ERF Expression Is Localized Extrasynaptically in Adult Limb Muscle
To understand the mechanisms behind the spatially restricted expression of utrophin at NMJs in adult myofibers, we used LCM to selectively study gene expression levels in synaptic versus extrasynaptic regions in adult rat TA muscle (Figure 5). Demonstrating the sensitivity and specificity of this approach, we found evidence for highly enriched transcription at the synapse for the well-known synaptic markers α subunit of nACHR or Chrna1 (163-fold), utrophin (7.9-fold), and Ets1 (17.9-fold). Interestingly, ERF expression was not detected at the synapse; however, it was exclusively expressed extrasynaptically in TA muscle in vivo (Figure 5B).
Figure 5.
ERF expression is restricted exclusively to extrasynaptic regions in muscle, suggesting a role in utrophin repression. (A) Microdissection steps of synaptic and extrasynaptic regions of rat TA muscle. Cryosections of 10 μm (TA) are stained with Karnowsky and Root's method to show NMJ localization by using PEN membrane glass slides, and they are visualized using PALM light microscopy at 20× power. (A and D) Visualization of TA tissue before LCM. (B and E) Same slide cut by laser for synaptic and nonsynaptic tissue parts intending to capture. (C and F) Section visualized post-LCM, showing removal of synaptic and nonsynaptic material by catapulting to the extraction buffer. Bars, 100 μm. (B) Taqman qRT-PCR amplification plots of Chrna1 (muscle), Utrn, Ets1, Erf, and β-actin are shown on the left side of Figure 5B. The x-axis shows PCR cycle numbers up to 40, and the y-axis illustrates the Δ of normalized reporter fluorescence dye (ΔRn), which refers to new product formation in each cycle. Color codes used refer to β-actin in TA-synaptic (purple) and TA-nonsynaptic (green) and the gene of interest in TA-synaptic (red) and TA-nonsynaptic (blue) in PCR analysis. Relative gene expression of analysis data results for duplicate wells of each gene are shown as bar graphs with standard deviations, with a star indicating statistically significant differences. Because ERF (blue lines) did not reach threshold levels in this analysis, its expression level is illustrated as an electrophoretic agarose (1%) gel product at the end of 40 cycles. (C) Summary of the relative expression synaptic:nonsynaptic ratio for all genes is illustrated.
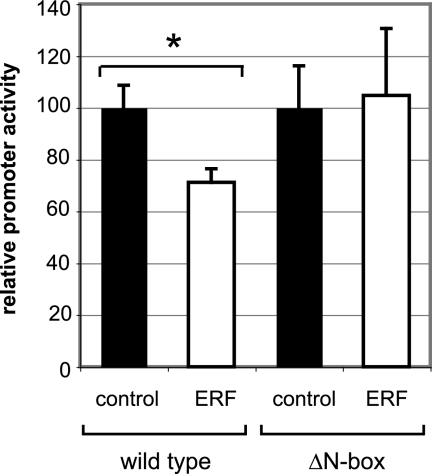
Direct Gene Transfer Confirms N-Box–mediated ERF Repression of the Utrophin-A Promoter In Vivo
Finally, we confirmed the ability of ERF to repress the wild-type utrophin-A promoter in vivo by coinjecting the wild-type utrophin promoter-A reporter plasmids with a CAT control plasmid to monitor transduction efficiency into TA muscles of 4-wk-old control mice with control or ERF plasmids. Five days later, muscles were harvested, RNA was extracted, and RT-PCR analysis for LacZ and CAT was performed. In comparison with the control plasmid, promoter-A activation was significantly impaired by cotransfection with the ERF expression vector (31.5%; p < 0.01; Figure 6). To determine whether the N-box was required for ERF-mediated repression, we performed additional experiments by using the pUBFΔN-box construct as a reporter plasmid. As shown in Figure 6, ERF was unable to repress the mutant utrophin promoter lacking the N-box motif in vivo. These results support and extend our findings from cultured muscle cells to skeletal muscle in vivo.
Figure 6.
Direct gene transfer demonstrates N-box–mediated utrophin-A promoter repression by ERF in vivo. Utrophin promoter-A constructs (wild type; with N-box) were coinjected with CAT (to monitor transduction efficiency) into TA muscles of 4-wk-old control mice with control (pSG5 vector) or ERF (pSG5-ERF) expression plasmid. A different cohort was injected using the mutant utrophin promoter constructs (ΔN-box; without N-box). Five days later, muscles were harvested, and then RNA extracted and qPCR analysis was performed. Values obtained for LacZ or luciferase are standardized relative to the amount of CAT present in the same sample, with the ERF-injected samples compared with their respective control (normalized to 100%). Student's t tests were used to analyze the data. The asterisk denotes the statistically significant (p < 0.02) decrease in promoter-A activation (∼30%) observed with the ERF plasmid (black bar) versus the control injected plasmid (white bar). No significant differences were observed using the ΔN-box construct. Error bars represent SEM; sample size for wild type, n = 5; ΔN-box, n = 10 for both ERF and control experiments.
DISCUSSION
In this study, we used gel shift (Figure 1), utrophin-A promoter reporter assays and monitoring of ERF subcellular translocation studies (Figure 2), siRNA-mediated knockdown studies and ERF-utrophin promoter ChIP analysis (Figure 3), in vitro expression/developmental time course (Figure 4) qPCR expression/localization in muscle (Figure 5), and direct gene transfer analysis (Figure 6) to identify and characterize ERF-mediated repression as a novel mechanism for regulation of the utrophin-A promoter at a transcriptional level.
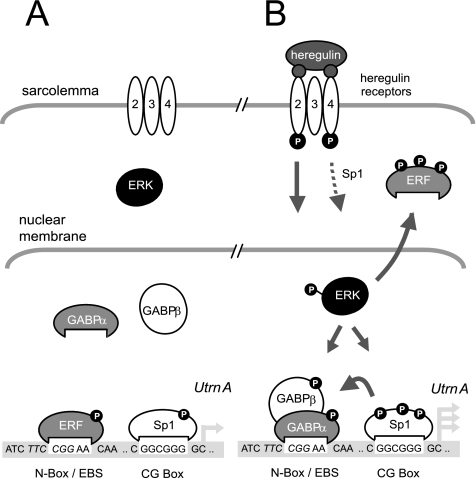
This study addresses a fundamental question in molecular and cellular biology—how to achieve selective localization of genes/gene products such as ACHR or utrophin at synapses. In skeletal muscle, the problem is more extreme (than in neurons), because muscle is composed of elongated multinucleated cells, and the muscle synapse or NMJs occupy only an extremely small portion of the cell membrane; yet, synaptic localization is achieved with considerable fidelity. Although subsynaptic transcription plays an important role in enriching genes such as AChR and utrophin at the NMJ (Schaeffer et al., 2001), we suggest that the converse mechanisms of extrasynaptic silencing may play an important role as well. ERF-mediated repression provides a novel molecular mechanism contributing to concurrent extrasynaptic silencing that must occur to achieve NMJ localization of utrophin-A in muscle. Because ERF expression seems to be exclusively extrasynaptic in TA rat muscle (Figure 5), we postulate that within these regions (or in unstimulated muscle), low levels of utrophin transcription exist, in part, due to occupation of the N-box/EBS site by ERF that may serve as a constitutive damper of expression (Figure 7). However, the repression is repressed at synaptic regions (or when stimulated by neurite-associated HRG). On binding of HRG to the HER 2,3,4 receptors at the NMJ, ERK is activated by MEK-dependent phosphorylation, leading to nuclear localization and facilitating two events, each of which favor increased utrophin transcription to occur: 1) multiple serine/threonine residue phosphorylation of ERF, causing MEK-dependent nuclear export and a loss of repressor activity, hence derepressing the promoter (or making it more likely to be activated); and 2) activation of GABPα/β, leading to greater N-box/EBS binding as well as synergistic activation of the utrophin promoter with Sp1. Consistent with this, ERK immunoprecipitated from HRG-treated C2C12 cells phosphorylated ERF in a MEK-dependent manner by using in vitro kinase assays (unpublished observations).
Figure 7.
Model for transcriptional regulation of utrophin promoter- A via the N-box/EBS site in muscle. Transcriptional model of the utrophin-A promoter pre- and post-ERK nuclear localization by HRG stimulation at the NMJ. Multiple arrows represent increased transcription. P, phosphorylated protein; dotted and full arrowed lines represent potential and defined signaling cascades, respectively. For more information, please refer to the text.
In conclusion, we have identified promoter repression as a novel mechanism that leads to utrophin down-regulation in extrasynaptic regions of skeletal muscles. We think that derepressing the promoter by blocking the activity of repressor molecules (such as ERF or ERF-like molecules) either alone or in concurrence with positively trans-activating ETS family members may be a functional means to synergistically enhance utrophin promoter activation as a therapeutic strategy for DMD. However, it is important to test these hypotheses in vivo before assigning these important roles to the ERF transcription factors.
ACKNOWLEDGMENTS
We thank Dr. G. Mavrothalassitis, Prof. K. Davies, and Prof. A. Rosmarin for providing constructs and antibodies used in this study. We also thank Drs. C. Bonnemann, S. Bogdanovich, and T.O.B. Krag (University of Pennsylvania) for experimental and manuscript advice. This study was supported by grants from the Muscular Dystrophy Association, National Institutes of Health (AR-048871), and the Canadian Institute of Health Research.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1069) on May 16, 2007.
REFERENCES
- Andres M. E., Burger C., Peral-Rubio M. J., Battaglioli E., Anderson M. E., Grimes J., Dallman J., Ballas N., Mandel G. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc. Natl. Acad. Sci. USA. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N., et al. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- Budak M. T., Bogdanovich S., Wiesen M. H., Lozynska O., Khurana T. S., Rubinstein N. A. Layer-specific differences of gene expression in extraocular muscles identified by laser-capture microscopy. Physiol. Genomics. 2004;20:55–65. doi: 10.1152/physiolgenomics.00191.2004. [DOI] [PubMed] [Google Scholar]
- Burton E. A., Tinsley J. M., Holzfeind P. J., Rodrigues N. R., Davies K. E. A second promoter provides an alternative target for therapeutic up-regulation of utrophin in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA. 1999;96:14025–14030. doi: 10.1073/pnas.96.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M., et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- Chakkalakal J. V., Harrison M. A., Carbonetto S., Chin E., Michel R. N., Jasmin B. J. Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice. Hum. Mol. Genet. 2004;13:379–388. doi: 10.1093/hmg/ddh037. [DOI] [PubMed] [Google Scholar]
- Chakkalakal J. V., Stocksley M. A., Harrison M. A., Angus L. M., Deschenes-Furry J., St-Pierre S., Megeney L. A., Chin E. R., Michel R. N., Jasmin B. J. Expression of utrophin A mRNA correlates with the oxidative capacity of skeletal muscle fiber types and is regulated by calcineurin/NFAT signaling. Proc. Natl. Acad. Sci. USA. 2003;100:7791–7796. doi: 10.1073/pnas.0932671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubourt E., Fossier P., Baux G., Leprince C., Israel M., De La Porte S. Nitric oxide and l-arginine cause an accumulation of utrophin at the sarcolemma: a possible compensation for dystrophin loss in Duchenne muscular dystrophy. Neurobiol. Dis. 1999;6:499–507. doi: 10.1006/nbdi.1999.0256. [DOI] [PubMed] [Google Scholar]
- Clerk A., Morris G. E., Dubowitz V., Davies K. E., Sewry C. A. Dystrophin-related protein, utrophin, in normal and dystrophic human fetal skeletal muscle. Histochem. J. 1993;25:554–561. [PubMed] [Google Scholar]
- Dennis C. L., Tinsley J. M., Deconinck A. E., Davies K. E. Molecular and functional analysis of the utrophin promoter. Nucleic Acids Res. 1996;24:1646–1652. doi: 10.1093/nar/24.9.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls D. L., Rosen K. M., Corfas G., Lane W. S., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fisher R., Tinsley J. M., Phelps S. R., Squire S. E., Townsend E. R., Martin J. E., Davies K. E. Non-toxic ubiquitous over-expression of utrophin in the mdx mouse. Neuromuscul. Disord. 2001;11:713–721. doi: 10.1016/s0960-8966(01)00220-6. [DOI] [PubMed] [Google Scholar]
- Galvagni F., Capo S., Oliviero S. Sp1 and Sp3 physically interact and co-operate with GABP for the activation of the utrophin promoter. J. Mol. Biol. 2001;306:985–996. doi: 10.1006/jmbi.2000.4335. [DOI] [PubMed] [Google Scholar]
- Gilbert R., Nalbantoglu J., Petrof B. J., Ebihara S., Guibinga G. H., Tinsley J. M., Kamen A., Massie B., Davies K. E., Karpati G. Adenovirus-mediated utrophin gene transfer mitigates the dystrophic phenotype of mdx mouse muscles. Hum. Gene Ther. 1999;10:1299–1310. doi: 10.1089/10430349950017987. [DOI] [PubMed] [Google Scholar]
- Gobert Gosse S., Bourgin C., Liu W. Q., Garbay C., Mouchiroud G. M-CSF stimulated differentiation requires persistent MEK activity and MAPK phosphorylation independent of Grb2-Sos association and phosphatidylinositol 3-kinase activity. Cell Signal. 2005;17:1352–1362. doi: 10.1016/j.cellsig.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Gramolini A. O., Angus L. M., Schaeffer L., Burton E. A., Tinsley J. M., Davies K. E., Changeux J. P., Jasmin B. J. Induction of utrophin gene expression by heregulin in skeletal muscle cells: role of the N-box motif and GA binding protein. Proc. Natl. Acad. Sci. USA. 1999a;96:3223–3227. doi: 10.1073/pnas.96.6.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramolini A. O., Belanger G., Thompson J. M., Chakkalakal J. V., Jasmin B. J. Increased expression of utrophin in a slow vs. a fast muscle involves posttranscriptional events. Am. J. Physiol. 2001;281:C1300–C1309. doi: 10.1152/ajpcell.2001.281.4.C1300. [DOI] [PubMed] [Google Scholar]
- Gramolini A. O., Dennis C. L., Tinsley J. M., Robertson G. S., Cartaud J., Davies K. E., Jasmin B. J. Local transcriptional control of utrophin expression at the neuromuscular synapse. J. Biol. Chem. 1997;272:8117–8120. doi: 10.1074/jbc.272.13.8117. [DOI] [PubMed] [Google Scholar]
- Gramolini A. O., Jasmin B. J. Expression of the utrophin gene during myogenic differentiation. Nucleic Acids Res. 1999;27:3603–3609. doi: 10.1093/nar/27.17.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramolini A. O., Karpati G., Jasmin B. J. Discordant expression of utrophin and its transcript in human and mouse skeletal muscles. J. Neuropathol. Exp. Neurol. 1999b;58:235–244. doi: 10.1097/00005072-199903000-00003. [DOI] [PubMed] [Google Scholar]
- Gyrd-Hansen M., Krag T. O., Rosmarin A. G., Khurana T. S. Sp1 and the ets-related transcription factor complex GABP alpha/beta functionally cooperate to activate the utrophin promoter. J. Neurol. Sci. 2002;197:27–35. doi: 10.1016/s0022-510x(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Kunkel L. M. Dystrophin: the protein product of the Duchene muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Jasmin B. J., Angus L. M., Belanger G., Chakkalakal J. V., Gramolini A. O., Lunde J. A., Stocksley M. A., Thompson J. Multiple regulatory events controlling the expression and localization of utrophin in skeletal muscle fibers: insights into a therapeutic strategy for Duchenne muscular dystrophy. J. Physiol. 2002;96:31–42. doi: 10.1016/s0928-4257(01)00078-x. [DOI] [PubMed] [Google Scholar]
- Khurana T. S., Davies K. E. Pharmacological strategies for muscular dystrophy. Nat. Rev. Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- Khurana T. S., Hoffman E. P., Kunkel L. M. Identification of a chromosome 6-encoded dystrophin-related protein. J. Biol. Chem. 1990;265:16717–16720. [PubMed] [Google Scholar]
- Khurana T. S., Rosmarin A. G., Shang J., Krag T. O., Das S., Gammeltoft S. Activation of utrophin promoter by heregulin via the ets-related transcription factor complex GA-binding protein alpha/beta. Mol. Biol. Cell. 1999;10:2075–2086. doi: 10.1091/mbc.10.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana T. S., Watkins S. C., Chafey P., Chelly J., Tome F. M., Fardeau M., Kaplan J. C., Kunkel L. M. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul. Dis. 1991;1:185–194. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Koike S., Schaeffer L., Changeux J. P. Identification of a DNA element determining synaptic expression of the mouse acetylcholine receptor delta-subunit gene. Proc. Natl. Acad. Sci. USA. 1995;92:10624–10628. doi: 10.1073/pnas.92.23.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag T. O., Bogdanovich S., Jensen C. J., Fischer M. D., Hansen-Schwartz J., Javazon E. H., Flake A. W., Edvinsson L., Khurana T. S. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc. Natl. Acad. Sci. USA. 2004;101:13856–13860. doi: 10.1073/pnas.0405972101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gallic L., Sgouras D., Beal G., Jr, Mavrothalassitis G. Transcriptional repressor ERF is a Ras/mitogen-activated protein kinase target that regulates cellular proliferation. Mol. Cell. Biol. 1999;19:4121–4133. doi: 10.1128/mcb.19.6.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gallic L., Virgilio L., Cohen P., Biteau B., Mavrothalassitis G. ERF nuclear shuttling, a continuous monitor of Erk activity that links it to cell cycle progression. Mol. Cell. Biol. 2004;24:1206–1218. doi: 10.1128/MCB.24.3.1206-1218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. R., Hill D. F., Dickson G., Spurr N. K., Byth B. C., Marsden R. F., Walsh F. S., Edwards Y. H., Davies K. E. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989;339:55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- Lunyak V. V., et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298:1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992;360:588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Miura P., Thompson J., Chakkalakal J. V., Holcik M., Jasmin B. J. The utrophin A 5′-untranslated region confers internal ribosome entry site-mediated translational control during regeneration of skeletal muscle fibers. J. Biol. Chem. 2005;280:32997–33005. doi: 10.1074/jbc.M503994200. [DOI] [PubMed] [Google Scholar]
- Nazarian J., Bouri K., Hoffman E. P. Intracellular expression profiling by laser capture microdissection: three novel components of the neuromuscular junction. Physiol. Genomics. 2005;21:70–80. doi: 10.1152/physiolgenomics.00227.2004. [DOI] [PubMed] [Google Scholar]
- Nowak K. J., Davies K. E. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep. 2004;5:872–876. doi: 10.1038/sj.embor.7400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K. J., Burton E. A., Davies K. E. The role of basal and myogenic factors in the transcriptional activation of utrophin promoter A: implications for therapeutic up-regulation in Duchenne muscular dystrophy. Nucleic Acids Res. 2001;29:4843–4850. doi: 10.1093/nar/29.23.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafael J. A., Tinsley J. M., Potter A. C., Deconinck A. E., Davies K. E. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat. Genet. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- Rosmarin A. G., Caprio D. G., Kirsch D. G., Handa H., Simkevich C. P. GABP and PU. 1 compete for binding, yet cooperate to increase CD18(β2 Integrin) transcription. J. Biol. Chem. 1995;270:23627–23633. doi: 10.1074/jbc.270.40.23627. [DOI] [PubMed] [Google Scholar]
- Rybakova I. N., Patel J. R., Davies K. E., Yurchenco P. D., Ervasti J. M. Utrophin binds laterally along actin filaments and can couple costameric actin with sarcolemma when overexpressed in dystrophin-deficient muscle. Mol. Biol. Cell. 2002;13:1512–1521. doi: 10.1091/mbc.01-09-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapru M. K., Florance S. K., Kirk C., Goldman D. Identification of a neuregulin and protein-tyrosine phosphatase response element in the nicotinic acetylcholine receptor e subunit gene: regulatory role of an ets transcription factor. Proc. Natl. Acad. Sci. USA. 1998;95:1289–1294. doi: 10.1073/pnas.95.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., de Kerchove d'Exaerde A., Changeux J. P. Targeting transcription to the neuromuscular synapse. Neuron. 2001;31:15–22. doi: 10.1016/s0896-6273(01)00353-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Duclert N., Dymanus M. H., Changeux J. P. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 1998;17:3078–3090. doi: 10.1093/emboj/17.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalat L., Grisoni K., Archer J., Vargas C., Bertrand A., Anderson J. E. CAPON expression in skeletal muscle is regulated by position, repair, NOS activity, and dystrophy. Exp. Cell Res. 2005;302:170–179. doi: 10.1016/j.yexcr.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Sgouras D. N., Athanasiou M. A., Beal G. J., Fisher R. J., Blair D. G., Mavrothalassitis G. J. ERF: an ets domain protein with strong transcriptional repressor activity, can suppress ets-associated tumorigenesis and is regulated by phosphorylation during cell cycle and mitogenic stimulation. EMBO J. 1995;14:4781–4793. doi: 10.1002/j.1460-2075.1995.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre S. J., Chakkalakal J. V., Kolodziejczyk S. M., Knudson J. C., Jasmin B. J., Megeney L. A. Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 2004;18:1937–1939. doi: 10.1096/fj.04-1859fje. [DOI] [PubMed] [Google Scholar]
- Tinsley J., Deconinck N., Fisher R., Kahn D., Phelps S., Gillis J. M., Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- Tinsley J. M., et al. Primary structure of dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Tinsley J. M., Potter A. C., Phelps S. R., Fisher R., Trickett J. I., Davies K. E. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- Wakefield P. M., Tinsley J. M., Wood M. J., Gilbert R., Karpati G., Davies K. E. Prevention of the dystrophic phenotype in dystrophin/utrophin-deficient muscle following adenovirus-mediated transfer of a utrophin minigene. Gene Ther. 2000;7:201–204. doi: 10.1038/sj.gt.3301066. [DOI] [PubMed] [Google Scholar]
- Weir A. P., Burton E. A., Harrod G., Davies K. E. A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J. Biol. Chem. 2002;277:45285–45290. doi: 10.1074/jbc.M205177200. [DOI] [PubMed] [Google Scholar]
- Winder S. J., Kendrick J. J. Calcium/calmodulin-dependent regulation of the NH2-terminal F-actin binding domain of utrophin. FEBS Lett. 1995;357:125–128. doi: 10.1016/0014-5793(94)01347-4. [DOI] [PubMed] [Google Scholar]