Abstract
Chlorate reductase has been isolated from the chlorate-respiring bacterium Ideonella dechloratans, and the genes encoding the enzyme have been sequenced. The enzyme is composed of three different subunits and contains molybdopterin, iron, probably in iron-sulfur clusters, and heme b. The genes (clr) encoding chlorate reductase are arranged as clrABDC, where clrA, clrB, and clrC encode the subunits and clrD encodes a specific chaperone. Judging from the subunit composition, cofactor content, and sequence comparisons, chlorate reductase belongs to class II of the dimethyl sulfoxide reductase family. The clr genes are preceded by a novel insertion sequence (transposase gene surrounded by inverted repeats), denoted ISIde1. Further upstream, we find the previously characterized gene for chlorite dismutase (cld), oriented in the opposite direction. Chlorate metabolism in I. dechloratans starts with the reduction of chlorate, which is followed by the decomposition of the resulting chlorite to chloride and molecular oxygen. The present work reveals that the genes encoding the enzymes catalyzing both these reactions are in close proximity.
In the pulp and paper industry, the substitution of chlorine dioxide for molecular chlorine as a bleaching agent has resulted in a drastic decrease of the release of organically bound chlorine. However, it has also led to substantial amounts of chlorate and smaller amounts of chlorite in the wastewater (17, 33). These toxic by-products are also formed upon disinfection of drinking water or effluent treatment with chlorine dioxide (24). Different approaches to the removal of oxochlorates have been tried, and it has been found that biological treatment is efficient and applicable on an industrial scale (5, 12, 21, 29, 34). Microbial decomposition of oxochlorates has been known since 1928 (4), but until recently only a few organisms able to grow by chlorate and/or perchlorate reduction were described (12, 29, 35, 41, 44, 53). Nevertheless, now it is known that (per)chlorate-reducing bacteria are found in many different environments (13, 31, 40).
(Per)chlorate-reducing bacteria use the highly oxidized chlorate ion as the sole electron acceptor for oxidation of organic matter in a previously unknown mode of respiration (33). As pointed out by Bender et al. (7), significant advances have been made in the last 5 years regarding the biochemistry of chlorate respiration, but the genetic systems involved in the metabolism have not been described earlier. The reduction of chlorate occurs in a two-step reaction: chlorate reduction followed by chlorite decomposition (44). The latter reaction is catalyzed by chlorite dismutase with oxygen and chloride as the end products. In earlier work the isolation and characterization of chlorite dismutase from Ideonella dechloratans (48) and the cloning, sequencing, and expression of its gene (13a) have been described. The present work addresses chlorate reduction. We describe the isolation and characterization of chlorate reductase from I. dechloratans, as well as the sequences for the genes encoding chlorate reductase. To our knowledge, this is the first description of a chlorate reductase at the gene level. The genes for chlorate reductase, and that of chlorite dismutase, are found in close proximity in the genome of I. dechloratans, forming a gene cluster for chlorate metabolism.
MATERIALS AND METHODS
Enzyme assay.
Chlorate reductase activity was measured spectrophotometrically in quartz cuvettes equipped with rubber septa by monitoring the oxidation of reduced methyl viologen with chlorate at 600 nm and 25°C (38). The reaction mixture was 50 mM bis-Tris propane, pH 7.2, 0.3 mM methyl viologen, and an appropriate amount of enzyme. The assay mixture was flushed with nitrogen for 10 min, and a small amount of a dithionite solution was added until an absorbance of 1.0 at 600 nm was obtained. The reaction was initiated by the addition of sodium chlorate (nitrogen flushed) to a final concentration of 1.7 mM. Activities were calculated from the initial rates by using an extinction coefficient of 1.3 × 104 M−1 cm−1 (38) for the methyl viologen radical. Alternative electron acceptors were tested in the same assay system, except that chlorate was replaced by ClO4−, NO3−, IO3−, BrO3−, SeO42, dimethyl sulfoxide (DMSO), and trimethylamine-N-oxide (TMAO) at 1.7 mM.
Purification of chlorate reductase.
Phenyl Sepharose Fast Flow (low substitution) and Sephacryl S-200 HR were obtained from Amersham Biosciences. Tryptone was from Oxoid, and yeast extract was from Difco Laboratories. All other chemicals were of analytical grade. I. dechloratans (35) was obtained from the culture collection of Göteborg University, Göteborg, Sweden. The bacterium was cultured as described previously (35, 48). Enzyme purification was carried out at 4°C. Cells were resuspended in 0.1 M sodium phosphate buffer, pH 7.2 (1 g [wet weight] of cells per ml). The cells were disintegrated by freeze pressing in an X-PRESS (BIOX AB, Göteborg, Sweden) and were homogenized with a blade homogenizer. Cell debris and membranes were removed by ultracentrifugation at 150,600 × g for 1.5 h. Nucleic acids were precipitated by 0.1% (wt/vol) polyethyleneimine (47) followed by centrifugation (18,000 × g, 10 min). This step was followed by ammonium sulfate fractionation. The pellet obtained between 40 and 70% saturation was dissolved in 50 mM sodium phosphate buffer, pH 7.0, containing 0.92 M ammonium sulfate, and was applied to a Phenyl Sepharose column (2.6 by 20 cm). The column was eluted with a decreasing ammonium sulfate gradient (total gradient volume was 0.5 liter). Chlorate reductase activity eluted from a Phenyl Sepharose column as a brown-yellowish band at about 0.12 M ammonium sulfate. The appropriate fractions were pooled, and the protein was precipitated by addition of ammonium sulfate to 70% saturation. The pellet was dissolved in 0.1 M sodium phosphate buffer, pH 7.2, and was applied to a Sephacryl 200 HR column (1.6 by 60 cm) in the same buffer. Fractions containing chlorate reductase activity were pooled and concentrated to 3 ml (Amicon ultrafiltration [YM 30]) and were dialyzed against 25 mM Tris-HCl buffer, pH 8.2, at 4°C. The solution was applied on a Q-Sepharose FF column (1 by 14 cm) equilibrated with the same buffer as in the dialysis. The column was washed with 1 volume of the starting buffer and was eluted with an increasing sodium chloride gradient. The final eluant was 25 mM Tris-HCl, pH 8.2, at 4°C, containing 0.25 M sodium chloride (total gradient volume, 100 ml). Chlorate reductase eluted at 0.1 M NaCl. The fractions containing chlorate reductase activity were pooled, concentrated, and stored at 4°C.
Analyses.
Heme was determined from pyridine hemochrome spectra by using the method of Berry and Trumpower (9). Iron content was determined colorimetrically after release of iron from the protein under oxidizing and acidic conditions by using the method of Fish (14). The fluorescent form A and form B of the pterin cofactor were prepared according to the method described by Hageman and Rajagopalan (19). For quantitation, a standard curve was prepared by using buttermilk xanthine oxidase (Sigma). Total protein was determined using the bicinchoninic acid method as described by the manufacturer (Pierce). Absorbance spectra and measurements were taken on a Shimadzu UV2101 spectrophotometer, and fluorescence spectra were recorded on a Shimadzu RF-5000 spectrofluorimeter. Analytical gel filtration was carried out on a Superose-6 10/30 column (Amersham Biosciences), which was calibrated by using bovine carbonic anhydrase (29 kDa), albumin (60 kDa), yeast alcohol dehydrogenase (150 kDa), β-amylase (200 kDa), and apoferritin (443 kDa). Sodium dodecyl sulfate (SDS)-gel electrophoresis and gel staining with Coomassie brilliant blue were performed by using a Phast system (Amersham Biosciences) with Phast gradient gels (8 to 25%) and SDS buffer strips supplied by the same manufacturer. A low-molecular-weight standard kit (Amersham Biosciences) was used for calibration.
Amino acid sequences.
N-terminal sequencing after separation of the subunits by SDS-polyacrylamide gel electrophoresis (PAGE) was attempted as described in reference 48. For internal sequencing, the separated subunits were transferred from an SDS-PAGE gel to a polyvinyl difluoride membrane (ProBlott; Applied Biosystems). After staining with Ponceau S, appropriate portions were cut from the membrane and treated with trypsin as described in reference 52. The resulting peptides were extracted from the membrane and were separated with reversed-phase high-performance liquid chromatography (52). Two peptide fractions obtained from the α subunit were sent to the Biomolecular Resource Facility (Lund University, Lund, Sweden) for sequencing.
Probe synthesis.
Genomic DNA from I. dechloratans was extracted by using the DNeasy Tissue Kit (Qiagen). Sequencing of tryptic peptides from the α subunit of the chlorate reductase gave two internal sequences, SWAEALTEIA and EQTALPFLV. From these, two degenerate primers were designed, KS1 and KS2 (Table 1), corresponding to the peptides AEALTEIA and TALPFLV, respectively. PCR (95°C for 1 min, 52°C for 1 min, and 72°C for 30 s [30 cycles] with a final extension of 7 min at 72°C) with genomic DNA from I. dechloratans as template, using the Expand High Fidelity PCR System (Roche), resulted in a PCR product that was cloned into the pGEM-T Easy (Promega) vector. The insert was sequenced by using an ABI Prism 310 Genetic Analyzer. A digoxigenin-labeled probe was synthesized from genomic DNA with the PCR DIG probe synthesis kit (Roche). A second labeled probe was synthesized, using KS11 and KS12 (Table 1), under the same cycling conditions as above except that the annealing temperature was 47°C.
TABLE 1.
PCR primers
| Primer | Sequence |
|---|---|
| KS1 | 5′-GCI GAR GCS CTS ACC GAR ATC-3′ |
| KS2 | 5′-ACS AGG AAI GGS AGI GCS GT-3′ |
| KS11 | 5′-CGG CGT CGA CGA GAT GGA GT-3′ |
| KS12 | 5′-CCG CCG GCA GCA CGA TA-3′ |
| GSP1 | 5′-TCT ACG AGT TCT TCG ACG TGC ACC TCA ATC-3′ |
| GSP2 | 5′-CCA ACG CTG GGC GAA CTG GTG GAA ACT CA-3′ |
| JK10 | 5′-GCG CCC GCA GTA GAG TTC CCC GTC AA-3′ |
| JK11 | 5′-GGC AGG CAC CAA TCG CTC CAG TAA CG-3′ |
Cloning and sequencing.
A lambda ZAP genomic library of I. dechloratans was screened with the digoxigenin-labeled probes by using the same conditions as described in reference 13a. Two positive clones (pKS2 and pKS3) were obtained. Primer walking was used to sequence the inserts. To access the part of the sequence not included in these clones, four separate Genome Walker libraries were prepared according to the instructions in the manual for Universal Genome Walker Kit (Clontech) by using genomic DNA from I. dechloratans. Two gene-specific primers (GSP1 and GSP2 in Table 1) were constructed. Primary and secondary PCRs were performed with the Advantage-GC Genomic PCR Kit (Clontech) (94°C for 2 s and 72°C for 3 min for 7 cycles; 94°C for 2 s and 67°C for 3 min for 32 cycles, with a final extension of 4 min at 67°C and 94°C for 2 s; 72°C for 3 min for 5 cycles; and 94°C for 2 s, 67°C for 3 min for 20 cycles, with a final extension of 4 min at 67°C). The secondary PCR product (2 kb) was separated on an agarose gel, and the fragment was extracted by using the Qiagen Gel Extraction Kit. The fragment was cloned into pGEM-T Easy (Promega) vector, and the construct was transformed to chemically competent JM109 (Promega). The resulting plasmid was partially sequenced by using standard pUC/M13 primers. Due to the size of the fragment and difficulties with primer walking, a smaller fragment was amplified by PCR by using a primer pair (JK10 and JK11 in Table 1) complementary to the ends of the previous sequenced regions with the product from the secondary PCR as a template. The PCR (94°C for 20 s and 68°C for 3 min for 25 cycles, with a final extension of 3 min at 68°C) generated a fragment of about 1 kb that was treated in the same way as described above. To achieve sufficient coverage, five additional clones were constructed and sequenced by using similar procedures. For each construct, at least four separate clones were sequenced.
Sequence assembly and gene prediction.
The quality of raw sequence data and sequence assembling and prediction of open reading frames was determined with Lasergene software (DNASTAR Inc. USA). Blast searches (3) were performed with six-frame translations of the contiguous sequence. The protein sequence alignments were done by using the ClustalW alignment algorithm (49). All accession numbers reported refer to the EMBL nucleotide sequence database.
Nucleotide sequence accession number.
The nucleotide sequence data reported herein have been deposited in the EMBL nucleotide sequence database and have received the accession number AJ566363.
RESULTS AND DISCUSSION
Purification and characterization of chlorate reductase.
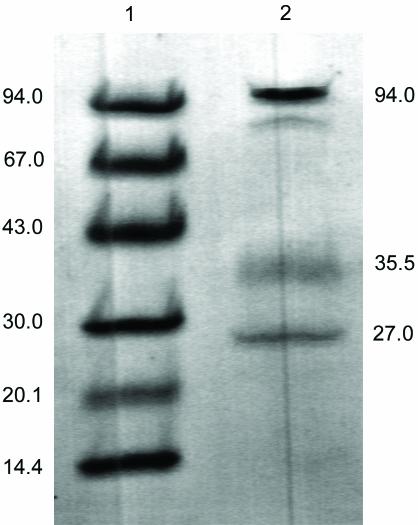
Soluble proteins in chlorate-grown I. dechloratans cells were obtained by ultracentrifugation of cell homogenate. The major part of the chlorate reductase activity was found in the supernatant. Further purification was performed by ammonium sulfate fractionation, hydrophobic chromatography, gel filtration, and ion-exchange chromatography. Substantial activity loss during purification and storage was observed, and all experiments using the purified enzyme were carried out no later than 2 days after the purification. SDS-PAGE of the purified chlorate reductase (Fig. 1) resulted in three major bands with molecular masses of 94, 35.5, and 27 kDa. The molecular mass of the native enzyme was determined by gel filtration, and a value of 160 kDa was found. The molecular masses for the subunits, together with the native molecular mass from gel chromatography, strongly suggest that the chlorate reductase is a complex of three different subunits present in a 1:1:1 stoichiometry.
FIG. 1.
SDS-PAGE (Phast gel [8 to 25%]) of the purified chlorate reductase from I. dechloratans. Lane 1, molecular mass standards in kilodaltons; lane 2, chlorate reductase.
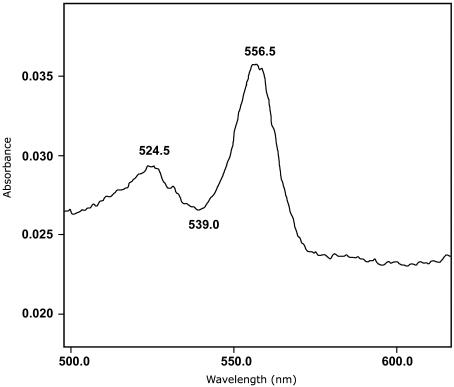
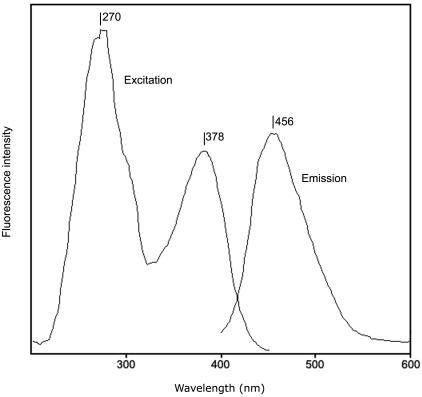
The heme content was determined by using the pyridine hemochrome method as described by Berry and Trumpower (9). Peaks were present at 556.5 and 524.5 nm in the reduced-minus-oxidized difference spectrum (Fig. 2), suggesting the presence of heme b (Fe-protoporphyrin IX). With the extinction coefficient of 24 mM−1 cm−1 (557 minus 540 nm, reduced-minus-oxidized), as given in reference 9, a heme content of 0.6 mol of heme b per mol of enzyme was obtained. The total iron content was determined after release of iron by treatment of the enzyme with acidic permanganate (14). Based on a molecular mass of 160 kDa, a value of 10 mol of iron per mol of enzyme was found. Molybdopterin content in chlorate reductase was determined as described in reference 19. After oxidation of pure chlorate reductase with iodine, a fluorescent compound with excitation peaks at 270 and 378 nm and an emission maximum at 456 nm (Fig. 3) was obtained. This is strongly reminiscent of the fluorescence spectrum reported for form A pterin derivative obtained from xanthine oxidase described in reference 19. These results suggest the presence of a molybdopterin in chlorate reductase. Using xanthine oxidase as standard, the pterin content was estimated to be 1 (± 0.5) mol of pterin per mol of enzyme.
FIG. 2.
Reduced-minus-oxidized difference spectrum of the pyridine hemochrome obtained from purified chlorate reductase.
FIG. 3.
Excitation and emission spectra of the fluorescent form A extracted from the purified chlorate reductase under oxidizing conditions. For the excitation spectrum, emission was detected at 465 nm, whereas the emission spectrum was recorded with 380 nm as the excitation wavelength.
The time course of methyl viologen radical oxidation with chlorate as electron acceptor was markedly nonlinear, with an increasing rate during the course of the reaction. A similar behavior was observed earlier with DMSO reductase (1) and was attributed to inhibition of the enzyme by excess viologen radical. The Km found for chlorate was 0.85 mM. Alternative electron acceptors were also tested in the assay. Activity was observed with bromate, iodate, nitrate, and selenate. The rates found for the reduction of these substrates were 107, 21, 16, and 7%, respectively, of the rate observed with chlorate. No activity was detected with perchlorate, DMSO, or TMAO. Since the product chlorite is also a potential electron acceptor for chlorate reductase, it was tried in the assay. However, at the chlorite concentration used here (1.7 mM), the methyl viologen radical was rapidly oxidized by chlorite in a nonenzymatic reaction.
Enzymes with chlorate reductase activity have been isolated earlier from Proteus mirabilis (41) and strain GR-1 (25). Unlike chlorate reductase from I. dechloratans, the GR-1 enzyme reduces perchlorate. Other differences include a higher relative activity with iodate and a lower relative activity with nitrate for the I. dechloratans enzyme. Both enzymes reduce chlorate and bromate at comparable relative rates. The Km for chlorate found in the present investigation (0.85 mM) is substantially higher than the value (<5 μM) reported for the GR-1 enzyme.
The subunit composition of the I. dechloratans chlorate reductase (αβγ) is the same as that reported for chlorate reductase from P. mirabilis. In the molybdenum- and iron-containing GR-1 enzyme, on the other hand, an α3β3 composition was observed.
A periplasmic location was observed for GR-1 chlorate reductase (25). In the present work, we observe considerable chlorate reductase activity in whole I. dechloratans cells. Since the electron donor, methyl viologen radical, does not cross the cell membrane (23), this suggests a periplasmic location also for the I. dechloratans enzyme.
N-terminal sequencing of the subunits after their separation by SDS-PAGE was not successful, suggesting N-terminal blockage in all three subunits. To proceed, the α subunit was digested with trypsin and two of the resulting peptides were sequenced. Two internal sequences were obtained, SWAEALTEIA and EQTALPFLV. A search in the SWALL database using the linked peptide option in FASTS3 at the EBI server gave three hits with strong sequence similarity to both peptides. These were the α subunits of selenate reductase from Thauera selenatis (30) and other molybdopterin-containing enzymes.
Cloning and sequencing.
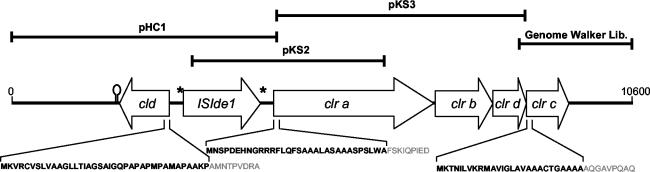
PCR with degenerate primers (Table 1) designed from tryptic peptides in the α subunit of chlorate reductase and genomic DNA from I. dechloratans as template produced a product of approximately 500 bp. PCR was then used to generate digoxigenin-labeled probes to screen the I. dechloratans genomic library (13a). A primer-walking procedure was applied to sequence the inserts of the positive clones. Part of the sequence was cloned from the DraI Genome Walker library and was sequenced with standard pUC/M13 primers as described above. The resulting continuous sequence was found to overlap with the sequence upstream of a previously published (13a) sequence for the chlorite dismutase gene (cld). Figure 4 outlines the contiguous sequence thus obtained. The sequence preceding the cld gene contains an open reading frame. Database searches revealed significant similarities to several transposases belonging to the Pfam family Transposase_12. We found also inverted repeats (* in Fig. 4) on both sides of the transposase gene. The insertion sequence thus identified is given as ISIde1. After the insertion sequence, four adjacent open reading frames that we designate clrA, clrB, clrD, and clrC were found. The gene clrA consists of 2,745 bp and encodes a 914-amino-acid-residue product. clrA is followed by an intergenic region of 10 bp before the start codon of the second gene, clrB. This gene consists of 987 bp encoding a product of 328 amino acids. The start codon for clrD, a 582-bp gene encoding a 193-amino-acid-residue product, overlaps with the stop codon of clrB, and the start codon for clrC overlaps with the stop codon for clrD. The clrC gene consists of 720 bp and encodes a 239-amino-acid sequence.
FIG. 4.
Diagrammatic representation of the gene cluster for chlorate respiration. A putative transcriptional terminator is shown downstream of cld. Derived N-terminal signal sequences of Cld, ClrA, and ClrC are shown in boldface. The cld gene and clrABDC genes are separated by an insertion sequence (ISIde1) with the inverted repeats given as asterisks. The N-terminal 34 amino residues of ClrA are found in the 3′ end of the insert in pHC1.
The arrangement of the genes clrA, clrB, clrC, and clrD (clrABDC) is similar to the disposition of the genes encoding some of the enzymes identified as homologues of chlorate reductase in database searches (see below; Fig. 5 to 8). These are T. selenatis serABDC, encoding selenate reductase (30), and Rhodovulum sulfidophilum ddhABDC, which encode dimethyl sulfide dehydrogenase (37). The ebdABCD genes for ethylbenzene dehydrogenase in the Azoarcus sp.-like strain EbN1 (42) are, however, arranged in a different way. These genes all encode proteins of the DMSO reductase family, a member of the superfamily for molybdopterin-containing enzymes. The DMSO reductase family is composed of prokaryotic molybdenum enzymes participating in anaerobic respiration. The homologues of chlorate reductase found in database searches (Fig. 5 to 8) are all periplasmic, trimeric molybdenum/iron-sulfur/heme enzymes and members of the small subgroup, type II, of DMSO reductases (26, 27, 37, 43, 50). In the type II enzymes the molybdenum cofactor is a bis(molybdopterin guanine dinucleotide)Mo (32, 36).
FIG. 5.
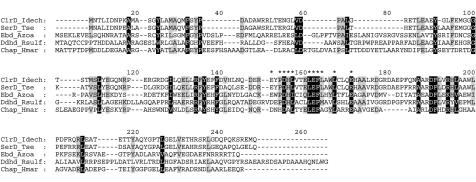
Alignment of ClrA (I. dechloratans), SerA (T. selenatis; AJ007744), DdhA (R. sulfidophilum; AF453479), Nar1 (NarG; H. marismortui) (AJ277440), and EbdA (Azoarcus sp.-like strain EB1; AF337952). Asterisks represent the twin-arginine motif of the predicted signal peptides and conserved residues of the predicted H/C-X3-C-X3-C-X34-C motif described as characteristic for the N terminus in enzymes belonging to type II of the DMSO reductase family.
FIG. 8.
Alignment of ClrD (I. dechloratans), SerD (T. selenatis; AJ007744), EbdD (Azoarcus sp.-like strain EbN1; CAD58338), DdhD (R. sulfidophilum; AF453479), and a chaperone protein (H. marismortui; AJ429077). Asterisks represent a motif suggested by Rabus et al. (42) as a domain characteristic of putative enzyme-specific chaperones for molybdenum.
The presence of the insertion sequence (ISIde1) close to the chlorate reductase and chlorite dismutase genes raises the intriguing possibility that these genes are part of a mobile genetic element. In that case, another copy of the transposase gene would be required further downstream of the chlorate reductase genes or upstream of the chlorite dismutase gene (16).
The first of the four adjacent open reading frames encode the chlorate reductase α subunit. Database searches with ClrA resulted in four sequences with substantial similarity. These are α-subunit SerA from T. selenatis (30), the α-subunit DdhA from R. sulfidophilum (37), Nar1 (NarG) from Haloarcula marismortui (54), and the α-subunit EbdA from Azoarcus sp.-like strain EB1 (22). The sequences were aligned as shown in Fig. 5. Major parts of the ClrA sequence match pfam00384 (molybdopterin oxidoreductase) and pfam01568 (molybdopterin dinucleotide binding) (6). A 32-amino-acid-long signal peptide is predicted by Signal P (39). The sequence GRRRFLQ in the signal peptide is strongly reminiscent of the twin-arginine motif (S/T)RRXFLK (8, 45), which specifies export to the periplasm through the Tat pathway (11, 51). Here proteins are folded in the cytosol and are exported without unfolding. Signal peptides are found in all the aligned sequences (30, 37, 42, 54), and the twin-arginine motif is conserved in the multiple-sequence alignment in Fig. 5. The mature ClrA subunit, without the leader peptide, is predicted to contain 881 amino acid residues with a calculated molecular mass of 99.7 kDa. The molecular mass determined by SDS-PAGE was found to be 94 kDa (Fig. 1). Many of the catalytic subunits of the bacterial molybdoenzymes show conserved cysteine residues in their N termini arranged similar to the cysteines involved in [4Fe-4S] cluster coordination (10). This is the case also in ClrA, but the first cysteine residue is replaced by a histidine, as also observed in SerA, DdhA, NarI, and EbdA (Fig. 5) and in the Escherichia coli NarG protein. The conserved histidine residue and cysteine residues in the alignment (Fig. 5) correspond to the motif H/C-X3-C-X3-C-X34-C, which is described as characteristic for the N terminus in enzymes belonging to type II of the DMSO reductase family (37, 50).
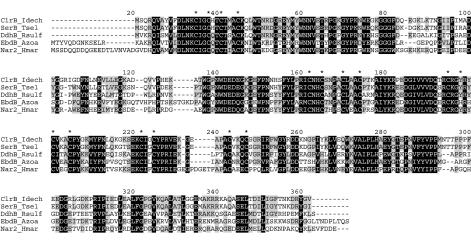
Analysis of the predicted ClrB protein revealed strong similarity to the β-subunit SerB of T. selenatis (30), the β-subunit DdhB of R. sulfidophilum (37), the β-subunit EbdB of Azoarcus sp.-like strain EB1 (22), and the nitrate reductase subunit 2 (Nar2) of H. marismortui (54). Although chlorate reductase probably is a periplasmic enzyme, the clrB gene does not contain any sequence coding for a signal peptide. It is therefore likely that both ClrA and ClrB fold in the cytosol and that ClrB is translocated together with ClrA by the Tat system as a fully folded protein complex by using the “hitchhiker” mechanism of Rodrigue et al. (45). This mechanism has also been proposed for the transportation of SerB (30) and DdhB (37). The deduced amino acid sequence of ClrB consists of 328 amino acids with a calculated molecular mass of 36.8 kDa. The molecular mass was determined by SDS-PAGE to be 35.5 kDa. ClrB and its homologues (Fig. 6) contain four groups of conserved cysteine residues similar to those binding Fe-S clusters. Pfam identifies the first and the third cysteine clusters in ClrB as members of the ferredoxin-like proteins (Pfam PF00037) where the clusters are 4Fe-4S binding domains. For ClrB, as well as for SerB, DdhB, and Nar2 (NarH), the second cysteine residue in the third cluster is replaced by a tyrosine. In EbdB the cysteine is instead replaced by a histidine (Fig. 6). The presence of iron-sulfur clusters has been shown by electron paramagnetic resonance in dimethyl sulfide dehydrogenase from R. sulfidophilum (36) and in H. marismortui nitrate reductase (54). In dimethyl sulfide dehydrogenase, one of the clusters is a 3Fe-4S cluster (36), and in H. marismortui nitrate reductase, one 3Fe-4S cluster and three 4Fe-4S clusters are found (54). Iron-sulfur clusters have also been found in GR-1 chlorate reductase by electron paramagnetic resonance spectroscopy (25). In addition to the two groups in ClrB, recognized by Pfam, there are also two other groups of four conserved cysteines in the sequence. The cysteine pattern (C-X2-C-X11-C-X3-C) in the fourth group is conserved in the alignment (Fig. 6). In nitrate reductases this pattern is found for the fourth group, which coordinates a 4Fe-4S cluster (10, 18). Based on sequence similarities with the iron-sulfur proteins above, it appears likely that ClrB harbors two to four Fe-S clusters. This suggests that the enzyme contains 10 to 15 mol of Fe, indicating that our finding of 10 mol of Fe/mol of enzyme might be an underestimate.
FIG. 6.
Alignment of ClrB (I. dechloratans), SerB (T. selenatis; AJ007744), DdhB (R. sulfidophilum; AF453479), EbdB (Azoarcus sp.-like strain EB1; AF337952), and Nar2 (H. marismortui; AJ277440). Asterisks represent the four groups of conserved cysteine residues.
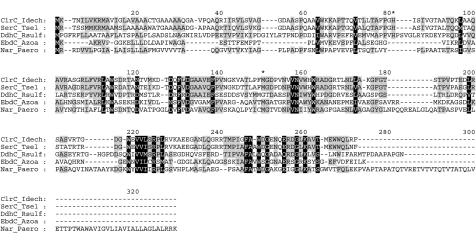
The mature ClrC protein consists of 212 amino acids with a calculated molecular mass of 23 kDa. A value of 27 kDa was determined by SDS-PAGE analysis of the protein. Database searches with this subunit resulted in hits on subunits belonging to the same enzymes as found with database searches with ClrA and ClrB (with the exception of the sequence from Pyrobaculum aerophilum, which is from whole-genome sequencing [15] and probably encodes the γ subunit in the Nar enzyme [2]). A ClustalW alignment (Fig. 7) of the proteins shows that the γ subunits are less conserved than the α and β subunits (Fig. 5 and 6). SignalP (39) predicts a 27-amino-residue-long leader peptide in ClrC, analogous to the Sec-dependent leader peptides found in SerC (30) and DdhC (37). SignalP also predicts a 28-amino-residue-long signal peptide in the γ subunit from P. aerophilum, whereas no signal peptide can be predicted in the EbdC sequence. A b-type heme is found in chlorate reductase from I. dechloratans (Fig. 2). A single heme b has also been found in selenate reductase (46), DMSO reductase (20), ethylbenzene dehydrogenase (EbN1) (28) and nitrate reductase from P. aerophilum (2). From magnetic circular dichroism results, McDevitt et al. (36) suggest that the axial ligands to the heme iron in dimethyl sulfide dehydrogenase are histidine and methionine. In the alignment shown in Fig. 7, these residues are not completely conserved. However, details in the alignment at this level of sequence similarity are uncertain, and we note that histidine and methionine residues are present in the vicinity (* in Fig. 7) in all the aligned sequences, except in the sequence from P. aerophilum. Heme is not found in the α3β3 GR-1 chlorate reductase (25), which lacks the γ subunit. The location of heme in the smallest subunit is consistent with the absence of both heme and a third subunit in the GR-1 enzyme.
FIG. 7.
Alignment of ClrC (I. dechloratans), SerC (T. selenatis; AJ007744), DdhC (R. sulfidophilum; AF453479), EbdC (Azoarcus sp.-like strain EB1; AF337952), and a Nar subunit (P. aerophilum; NP_560862). Asterisks represent the heme ligands suggested for DdhC by McDevitt et al. (36).
The clrD gene encodes a protein that is not a part of the mature chlorate reductase. The deduced polypeptide contains 193 amino acid residues and has a calculated molecular mass of 22 kDa. The clrD gene does not contain any sequence coding for a signal peptide. A Blastp search reveals that ClrD is similar to SerD, EbdD, DdhD, and Chap-Hmar (54). The Pfam search results indicates a function similar to that of the δ subunit of nitrate reductases, which is proposed to be a specific chaperone protein needed for the assembly of the αβ complex. Blasco et al. (10) suggest that the role of the NarJ protein is to hold apo-NarGH in a molybdenum-cofactor insertion-competent conformation. This is also the proposed function of SerD (30), DdhD (37), and EbdD (42). The motif E/D-X-P/H-D-H/A-L/I-X3-L-E-F-L-X2-L-X3-E is suggested by Rabus et al. (42) as characteristic of chaperones for molybdenum enzymes. This motif is conserved in the alignment of the δ subunits (* in Fig. 8).
In conclusion, a gene cluster for chlorate respiration has been identified in I. dechloratans. The gene cluster is composed of the gene encoding chlorite dismutase (13a), an insertion sequence, and the genes encoding chlorate reductase. The latter enzyme is a novel molybdenum-, iron-, and heme-containing protein of 160 kDa that consists of three subunits (94, 35.5, and 27 kDa) in an αβγ structure. The I. dechloratans chlorate reductase shows strong sequence similarity to the T. selenatis selenate reductase (84% identity). Selenate is, however, a poor substrate for the chlorate reductase, and chlorate is not reduced at all by selenate reductase (46). Based on sequence similarities and cofactor contents, chlorate reductase is most closely related to T. selenatis selenate reductase (30, 46), R. sulfidophilum dimethyl sulfide dehydrogenase (36, 37), and Azoarcus ethylbenzene dehydrogenase (22, 28, 42). These enzymes all belong to the DMSO reductase family, class II, of the molybdenum cofactor enzymes (26, 27, 37, 50). Hence, chlorate reductase from I. dechloratans is a new member of this subgroup.
Acknowledgments
This work was supported by the Carl Trygger Foundation.
REFERENCES
- 1.Adams, B., A. T. Smith, S. Bailey, A. G. McEwan, and R. C. Bray. 1999. Reactions of dimethylsulfoxide reductase from Rhodobacter capsulatus with dimethyl sulfide and with dimethyl sulfoxide: complexities revealed by conventional and stopped-flow spectrophotometry. Biochemistry 38:8501-8511. [DOI] [PubMed] [Google Scholar]
- 2.Afshar, S., E. Johnson, S. de Vries, and I. Schroder. 2001. Properties of a thermostable nitrate reductase from the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Bacteriol. 183:5491-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Åslander, A. 1928. Experiments on the eradication of Canada thistle, Cirsum arvense, with chlorates and other herbicides. J. Agric. Res. 36:915-934. [Google Scholar]
- 5.Axegård, P., L. Gunnarsson, and Å. Malmqvist. 1989. Biologisk metod att reducera kloratutsläpp. Sven. Papperstidn. 1989:50-52.
- 6.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, K. L. Howe, and E. L. Sonnhammer. 2000. The Pfam protein families database. Nucleic Acids Res. 28:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender, K. S., S. M. O'Connor, R. Chakraborty, J. D. Coates, and L. A. Achenbach. 2002. Sequencing and transcriptional analysis of the chlorite dismutase gene of Dechloromonas agitata and its use as a metabolic probe. Appl. Environ. Microbiol. 68:4820-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berks, B. C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393-404. [DOI] [PubMed] [Google Scholar]
- 9.Berry, E. A., and B. L. Trumpower. 1987. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 161:1-15. [DOI] [PubMed] [Google Scholar]
- 10.Blasco, F., B. Guigliarelli, A. Magalon, M. Asso, G. Giordano, and R. A. Rothery. 2001. The coordination and function of the redox centres of the membrane-bound nitrate reductases. Cell. Mol. Life Sci. 58:179-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolhuis, A., J. E. Mathers, J. D. Thomas, C. M. Barrett, and C. Robinson. 2001. TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J. Biol. Chem. 276:20213-20219. [DOI] [PubMed] [Google Scholar]
- 12.Bruce, R. A., L. A. Achenbach, and J. D. Coates. 1999. Reduction of (per)chlorate by a novel organism isolated from paper mill waste. Environ. Microbiol. 1:319-329. [DOI] [PubMed] [Google Scholar]
- 13.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Danielsson Thorell, H., J. Karlsson, E. Portelius, and T. Nilsson. 2002. Cloning, characterisation, and expression of a novel gene encoding chlorite dismutase from Ideonella dechloratans. Biochim. Biophys. Acta 1577:445. [DOI] [PubMed] [Google Scholar]
- 14.Fish, W. W. 1988. Rapid colorimetric micromethod for quantitation of complexed iron in biological samples. Methods Enzymol. 158:357-364. [DOI] [PubMed] [Google Scholar]
- 15.Fitz-Gibbon, S. T., H. Ladner, U. J. Kim, K. O. Stetter, M. I. Simon, and J. H. Miller. 2002. Genome sequence of the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Proc. Natl. Acad. Sci. USA 99:984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galas, D. J., and M. Chandler. 1996. Mobile DNA. American Society for Microbiology, Washington, D.C.
- 17.Germgård, U., A. Teder, and D. Tormund. 1981. Chlorate formation during chlorine dioxide bleaching of softwood kraft pulp. Pap. Puu 63:127-133.
- 18.Guigliarelli, B., A. Magalon, M. Asso, P. Bertrand, C. Frixon, G. Giordano, and F. Blasco. 1996. Complete coordination of the four Fe-S centers of the beta subunit from Escherichia coli nitrate reductase. Physiological, biochemical, and EPR characterization of site-directed mutants lacking the highest or lowest potential [4Fe-4S] clusters. Biochemistry 35:4828-4836. [DOI] [PubMed] [Google Scholar]
- 19.Hageman, R. V., and K. V. Rajagopalan. 1986. Assay and detection of the molybdenum cofactor. Methods Enzymol. 122:399-412. [DOI] [PubMed] [Google Scholar]
- 20.Hanlon, S. P., T. H. Toh, P. S. Solomon, R. A. Holt, and A. G. McEwan. 1996. Dimethylsulfide:acceptor oxidoreductase from Rhodobacter sulfidophilus. The purified enzyme contains b-type haem and a pterin molybdenum cofactor. Eur. J. Biochem. 239:391-396. [DOI] [PubMed] [Google Scholar]
- 21.Herman, D. C., and W. T. J. Frankenberger. 1999. Bacterial reduction of perchlorate and nitrate in water. J. Environ. Qual. 28:1018-1024. [Google Scholar]
- 22.Johnson, H. A., D. A. Pelletier, and A. M. Spormann. 2001. Isolation and characterization of anaerobic ethylbenzene dehydrogenase, a novel Mo-Fe-S enzyme. J. Bacteriol. 183:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, R. W., and P. B. Garland. 1977. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Biochem. J. 164:199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz, A., and N. Narkis. 2001. Removal of chlorine dioxide disinfection by-products by ferrous salts. Water Res. 35:101-108. [DOI] [PubMed] [Google Scholar]
- 25.Kengen, S. W., G. B. Rikken, W. R. Hagen, C. G. van Ginkel, and A. J. Stams. 1999. Purification and characterization of (per)chlorate reductase from the chlorate-respiring strain GR-1. J. Bacteriol. 181:6706-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisker, C., H. Schindelin, D. Baas, J. Retey, R. U. Meckenstock, and P. M. Kroneck. 1998. A structural comparison of molybdenum cofactor-containing enzymes. FEMS Microbiol. Rev. 22:503-521. [DOI] [PubMed] [Google Scholar]
- 27.Kisker, C., H. Schindelin, and D. C. Rees. 1997. Molybdenum-cofactor-containing enzymes: structure and mechanism. Annu. Rev. Biochem. 66:233-267. [DOI] [PubMed] [Google Scholar]
- 28.Kniemeyer, O., and J. Heider. 2001. Ethylbenzene dehydrogenase, a novel hydrocarbon-oxidizing molybdenum/iron-sulfur/heme enzyme. J. Biol. Chem. 276:21381-21386. [DOI] [PubMed] [Google Scholar]
- 29.Korenkov, V. N., V. I. Romanenko, S. I. Kuznetsov, and J. V. Voronov. March1976. Process for purification of industrial waste waters from perchlorates and chlorates. U.S. patent US3943055.
- 30.Krafft, T., A. Bowen, F. Theis, and J. M. Macy. 2000. Cloning and sequencing of the genes encoding the periplasmic-cytochrome b-containing selenate reductase of Thauera selenatis. DNA Seq. 10:365-377. [DOI] [PubMed] [Google Scholar]
- 31.Lovley, D. R., and J. D. Coates. 2000. Novel forms of anaerobic respiration of environmental relevance. Curr. Opin. Microbiol. 3:252-256. [DOI] [PubMed] [Google Scholar]
- 32.Maher, M. J., and J. M. Macy. 2002. Crystallization and preliminary X-ray analysis of the selenate reductase from Thauera selenatis. Acta Crystallogr. Sect. D 58:706-708. [DOI] [PubMed] [Google Scholar]
- 33.Malmqvist, Å., and T. Welander. 1992. Anaerobic removal of chlorate from bleach effluents. Water Sci. Technol. 25:237-242. [Google Scholar]
- 34.Malmqvist, Å., T. Welander, and L. Gunnarsson. 1991. Anaerobic growth of microorganisms with chlorate as an electron acceptor. Appl. Environ. Microbiol. 57:2229-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmqvist, Å., T. Welander, E. Moore, A. Ternström, G. Molin, and I. Stenström. 1994. Ideonella dechloratans gen. nov., sp. nov., a new bacterium capable of growing anaerobically with chlorate as an electron acceptor. Syst. Appl. Microbiol. 17:58-64. [Google Scholar]
- 36.McDevitt, C. A., G. R. Hanson, C. J. Noble, M. R. Cheesman, and A. G. McEwan. 2002. Characterization of the redox centers in dimethyl sulfide dehydrogenase from Rhodovulum sulfidophilum. Biochemistry 41:15234-15244. [DOI] [PubMed] [Google Scholar]
- 37.McDevitt, C. A., P. Hugenholtz, G. R. Hanson, and A. G. McEwan. 2002. Molecular analysis of dimethyl sulphide dehydrogenase from Rhodovulum sulfidophilum: its place in the dimethyl sulphoxide reductase family of microbial molybdopterin-containing enzymes. Mol. Microbiol. 44:1575-1587. [DOI] [PubMed] [Google Scholar]
- 38.McEwan, A. G., H. G. Wetzstein, S. J. Ferguson, and J. B. Jackson. 1985. Periplasmic location of the terminal reductase in trimethylamine N-oxide and dimethylsulfoxide respiration in the photosynthetic bacterium Rhodopseudomonas capsulata. Biochim. Biophys. Acta 806:410-417. [Google Scholar]
- 39.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor, S. M., and J. D. Coates. 2002. Universal immunoprobe for (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 68:3108-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oltmann, L. F., W. N. Reijnders, and A. H. Stouthamer. 1976. Characterization of purified nitrate reductase A and chlorate reductase C from Proteus mirabilis. Arch. Microbiol. 111:25-35. [DOI] [PubMed] [Google Scholar]
- 42.Rabus, R., M. Kube, A. Beck, F. Widdel, and R. Reinhardt. 2002. Genes involved in the anaerobic degradation of ethylbenzene in a denitrifying bacterium, strain EbN1. Arch. Microbiol. 178:506-516. [DOI] [PubMed] [Google Scholar]
- 43.Rajagopalan, K. V., J. L. Johnson, and B. E. Hainline. 1982. The pterin of the molybdenum cofactor. Fed. Proc. 41:2608-2612. [PubMed] [Google Scholar]
- 44.Rikken, G. B., A. G. Kroon, and C. G. van Ginkel. 1996. Transformation of (per)chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl. Microbiol. Biotechnol. 45:420-426. [Google Scholar]
- 45.Rodrigue, A., A. Chanal, K. Beck, M. Muller, and L. F. Wu. 1999. Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial tat pathway. J. Biol. Chem. 274:13223-13228. [DOI] [PubMed] [Google Scholar]
- 46.Schroder, I., S. Rech, T. Krafft, and J. M. Macy. 1997. Purification and characterization of the selenate reductase from Thauera selenatis. J. Biol. Chem. 272:23765-23768. [DOI] [PubMed] [Google Scholar]
- 47.Scopes, R. K. 1994. Protein purification: principles and practice, 3rd ed. Springer-Verlag, New York, N.Y.
- 48.Stenklo, K., H. Danielsson Thorell, H. Bergius, R. Aasa, and T. Nilsson. 2001. Chlorite dismutase from Ideonella dechloratans. J. Biol. Inorg. Chem. 6:601-607. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trieber, C. A., R. A. Rothery, and J. H. Weiner. 1996. Engineering a novel iron-sulfur cluster into the catalytic subunit of Escherichia coli dimethyl-sulfoxide reductase. J. Biol. Chem. 271:4620-4626. [DOI] [PubMed] [Google Scholar]
- 51.Voordouw, G. 2000. A universal system for the transport of redox proteins: early roots and latest developments. Biophys. Chem. 86:131-140. [DOI] [PubMed] [Google Scholar]
- 52.Walker, J. M. (ed.). 1998. Protein protocols on CD-ROM. Humana Press, Totowa, N.J.
- 53.Wallace, W., T. Ward, A. Breen, and H. Attaway. 1996. Identification of an anaerobic bacterium which reduces perchlorate and chlorate as Wolinella succinogenes. J. Ind. Microbiol. 16:68-72. [Google Scholar]
- 54.Yoshimatsu, K., T. Iwasaki, and T. Fujiwara. 2002. Sequence and electron paramagnetic resonance analyses of nitrate reductase NarGH from a denitrifying halophilic euryarchaeote Haloarcula marismortui. FEBS Lett. 516:145-150. [DOI] [PubMed] [Google Scholar]