Abstract
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins are key components of the fusion machinery in vesicular transport and in homotypic membrane fusion. We previously found that ADP-ribosylation factor GTPase activating proteins (ArfGAPs) promoted a conformational change on SNAREs that allowed recruitment of the small GTPase Arf1p in stoichiometric amounts. Here, we show that the ArfGAP Gcs1p accelerates vesicle (v)-target membrane (t)-SNARE complex formation in vitro, indicating that ArfGAPs may act as folding chaperones. These SNARE complexes were resolved in the presence of ATP by the yeast homologues of α-soluble N-ethylmaleimide-sensitive factor attachment protein and N-ethylmaleimide-sensitive factor, Sec17p and Sec18p, respectively. In addition, Sec18p and Sec17p also recognized the “activated” SNAREs even when they were not engaged in v-t-SNARE complexes. Here again, the induction of a conformational change by ArfGAPs was essential. Surprisingly, recruitment of Sec18p to SNAREs did not require Sec17p or ATP hydrolysis. Moreover, Sec18p displaced prebound Arf1p from SNAREs, indicating that Sec18p may have more than one function: first, to ensure that all vesicle coat proteins are removed from the SNAREs before the engagement in a trans-SNARE complex; and second, to resolve cis-SNARE complexes after fusion has occurred.
INTRODUCTION
Secretion is an essential process for eukaryotic cells. Communication between different organelles in the cell is mostly mediated by vesicles that are formed at a donor compartment and that are consumed by a specific target compartment (Kirchhausen, 2000; Bonifacino and Glick, 2004). Factors involved in these processes show a high degree of conservation between such distantly related species as yeast and mammals. To bud a vesicle, small GTPases of the ADP-ribosylation factor (Arf)-family are first recruited to the donor membrane. Arf1p is activated through interaction with an ARF guanine nucleotide exchange factor (ArfGEF) at the membrane (Donaldson and Jackson, 2000). Subsequently, coatomer is recruited and forms a complex with Arf1p, cargo, or soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and an ARF-GTPase activating protein (ArfGAP), a so-called primer (Springer et al., 1999; Spang, 2002). This interaction does not require the deactivation of Arf1p by a GAP. If more such priming complexes are formed, the coat polymerizes laterally and deforms the membrane until the donor membrane is severed from the nascent vesicle. Thus, ArfGAPs are not only important to stimulate GTP hydrolysis on Arf1p but also are important at a much earlier step in vesicle biogenesis.
Before vesicle fusion the coat has to be shed to expose the vesicle (v)-SNAREs on the vesicle. Four SNARE domains residing in both vesicle and target membrane interact in trans and zipper up into a coiled-coil bundle, pulling the membranes into proximity (Nichols et al., 1997; Ungermann et al., 1998; Weber et al., 1998). Then, a fusion pore is created that opens up, and finally, membrane fusion takes place. Through the fusion event, the trans-SNARE complex becomes a cis-SNARE complex, which needs to be resolved to recycle the SNAREs for the next round of fusion. Therefore, α-soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) and the AAA-ATPase N-ethylmaleimide-sensitive factor (NSF) (Sec17p and Sec18p in Saccharomyces cerevisiae, respectively) are recruited to cis-SNARE complexes and catalytically unwind the helix bundle (Sollner et al., 1993; Morgan et al., 1994; Mayer et al., 1996). The driving force to overcome the strong interaction within the cis-SNARE complex is provided through ATP hydrolysis by NSF (Whiteheart et al., 1994). The involvement of α-SNAP and NSF in the priming step of vesicle fusion with the target membrane has been shown to be essential for all vesicle fusion events examined so far as well as for homotypic vacuole fusion in yeast (Wickner, 2002; Morgan and Burgoyne, 2004; Sollner, 2004). However, it remains unclear whether unwinding the remains of the previous fusion event satisfies the Sec18p/NSF requirement before fusion.
Other factors that intimately interact with SNAREs are the ArfGAPs Gcs1p and Glo3p, which have been shown to catalytically induce a conformational change in SNAREs involved in endoplasmic reticulum (ER)–Golgi trafficking (Rein et al., 2002). SNAREs in this altered conformation were able to recruit Arf1p. This recruitment step required neither other factors nor the activation of the GTPase by a GEF. The interaction between Arf1p and SNAREs might provide a mechanism by which each vesicle carries at least one SNARE protein and would therefore be capable of fusing with the target membrane (Rein et al., 2002). However, membrane-anchored cargo proteins may also provide a platform for Arf1p binding.
Previously, we reported that the ArfGAPs Glo3p and Gcs1p induce a conformational change in v-SNAREs involved in ER–Golgi and post-Golgi trafficking (Rein et al., 2002; Robinson et al., 2006). In this article, we extended these results to t-SNAREs (target-SNAREs on the target membrane) and show that Gcs1p accelerates v-t-SNARE complex formation in vitro. The ArfGAPs may promote the conversion of a high-energy to a low-energy state in SNAREs and thereby help to form SNARE complexes. These v-t-SNARE complexes were not dead-end complexes, because they could be resolved by the action of Sec17p and Sec18p in the presence of ATP. Furthermore, we revealed an additional function of Sec18p as it could displace Arf1p from single SNAREs in vitro. Surprisingly, this binding to single SNAREs was independent from ATP hydrolysis and Sec17p. Thus, Sec18p may have an additional role during the priming step in vesicle fusion: to ensure that the SNAREs are free to engage in v/t-SNARE complexes and that they are not masked by coat proteins bound to the SNAREs.
MATERIALS AND METHODS
Plasmids
C-terminal glutathione S-transferase (GST)-tagged SNAREs were expressed using pETGEXCT (Sharrocks, 1994). GST-Sed5p was expressed from pGEX2T, GST-Snc1p from pGEX4T1, GST-Vam3p and GST-Nyv1p from pGEX4T3, and GST-Tlg2p from pGEX6p-1. His-tagged SNAREs were obtained by expression using vector pET24b (Novagen, Madison, WI). Sec17p and Sec18p were expressed from pQE9 (QIAGEN, Valencia, CA), Sec18E350Qp was expressed from pQE30 (QIAGEN). The vectors for expression of Gcs1p, Glo3p, and Age2p and Arf1ΔN17p-Q71L have been described previously (Poon et al., 1996, 1999, 2001; Rein et al., 2002).
Protein Purification
The different SNARE-GST fusion proteins were expressed in Escherichia coli BL21* and purified over glutathione-agarose. Cells from 1-liter culture were resuspended in 20 ml of STE (25% [wt/vol] sucrose, 50 mM Tris-Cl, pH 8.0, and 40 mM EDTA). After 1 mg/ml lysozyme treatment, 8 ml of 50 mM Tris-Cl, pH 8.0, 0.2% Triton X-100, and 100 mM MgCl2 was added, and the suspension was subjected to sonication. The cell lysate was cleared by centrifugation (10,000 × g for 20 min). The supernatant was bound to 1 ml glutathione-agarose beads (Sigma-Aldrich, St. Louis, MO) for 2 h at 4°C. Beads were washed several times in phosphate-buffered saline (PBS), 15% glycerol and transferred into a Poly-Prep column (Bio-Rad, Hercules, CA). Protein was eluted with 50 mM glutathione, 150 mM Tris, pH 8.0, 120 mM NaCl, 5 mM dithiothreitol (DTT), 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Protein-containing fractions were pooled and dialyzed against PBS, 5% glycerol. His6-tagged SNARE proteins and His6-Sec17p were expressed in E. coli BL21* and purified using Ni2+-nitrilotriacetic acid (NTA)-agarose (QIAGEN) according to the manufacturer's instructions. Arf1ΔN17p was purified as described previously (Rein et al., 2002). His6-Sec18p and His6-Sec18E350Qp were purified according to Whiteheart et al., (1994) with the following modifications: Ni2+-NTA-agarose with bound proteins was washed in batch twice with 10 column volumes of wash buffer 20 mM HEPES-KOH, pH 6.8, 400 mM KOAc, 0.5 mM ATP, 1 mM MgCl2, 1 mM 2-mercaptoethanol, and 10% glycerol, and 2 × 10 column volumes of wash buffer20, 2 × 10 column volumes of wash buffer50 (wash buffer containing 20 and 50 mM imidazole, respectively). After the last wash, beads were resuspended in wash buffer100 (wash buffer containing 100 mM imidazole), transferred into a Poly-Prep column, and washed with five column volumes of wash buffer100. Protein was eluted with elution buffer (wash buffer containing 250 mM imidazole). Eluted protein was dialyzed against 20 mM HEPES, pH 6.8, 150 mM KOAc, 5 mM Mg(OAc)2, 15% glycerol, 1 mM ATP, 1 mM DTT, and 1 mM PMSF. ArfGAPs were purified and the GAP activity was determined as described previously (Huber et al., 2001; Rein et al., 2002).
SNARE Binding Assay
SNARE binding assays were performed as described previously (Rein et al., 2002). Arf1ΔN17p-Q71L (5 μM) was used in the reaction. When indicated, the reaction contained 20 nM Gcs1p, Glo3p, or Age2p. Sec17p and Sec18p were used at concentrations of 0.6 μM His6-Sec17p, 0.6 μM His6-Sec18p, or 0.6 μM His6-Sec18(E350Q)p. For the competition assays with Arf1ΔN17p-Q71L, 15 nM to 3.5 μM His6-Sec18p was used. ATP (1 mM) and 0.5 mM GTP were added where indicated. Incubation steps with Arf1ΔN17p-Q71L or ArfGAP were performed for 1 h at 4°C, whereas His6-Sec17p, His6-Sec18p, or His6-Sec18(E350Q)p were incubated for 1 h at room temperature (RT). The recruitment of Arf1ΔN17p-Q71L to SNAREs was visualized by Coomassie blue staining. SDS-polyacrylamide gel electrophoresis (PAGE) containing assays performed with His6-Sec17p or His6-Sec18p were stained with SyproRed (Invitrogen, Carlsbad, CA) or according to Fairbanks et al. (1971), which is 2–4 times more sensitive than conventional Coomassie staining. Images were acquired with a Storm PhosphorImager (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Bands were quantified using the ImageQuant software (GE Healthcare).
Assembly and Disassembly of SNARE Complexes
SNARE complex assembly was performed for indicated times at 4°C as described previously (Peng and Gallwitz, 2002) with minor modifications: 5 μg of Sed5p GST-fusion protein was immobilized on glutathione-agarose beads and was incubated with 10 μg of Bet1p-His6, 20 μg of Bos1p-His6, and 20 μg of Sec22p-His6 in a total assay volume of 200 μl at 4°C, in the presence or absence of 10 nM Gcs1p. The reaction was stopped at indicated time points by centrifugation, followed by three washes in buffer C (25 mM Tris-Cl, pH 7.5, 150 mM KCl, 10% glycerol, 1% Triton X-100, and 2 mM β-mercaptoethanol) and a final wash in 20 mM Tris-Cl, pH 7.5. Bound proteins were dissolved in sample buffer by heating the beads 10 min at 65°C. The proteins were separated by SDS-PAGE and visualized by Fairbanks staining. Band intensities were determined using an Odyssey imaging system (LI-COR, Lincoln, NE). To dissolve SNARE complexes, they were formed as described above for 18–22 h, washed twice in buffer C, and once in disassembly buffer containing 30 mM HEPES-KOH, pH 7.4, 70 mM KCl, 5 mM MgCl2, 2.5 mM EGTA, 0.5 mM DTT, and 2 mM ATP. SNARE complex disassembly was performed by addition of 1.35 μM His6-Sec17p and 1.5 μM His6-Sec18p in an assay volume of 100 μl. The assay was incubated for 1 h at RT, and then it was stopped immediately by the addition of 1 ml of ice-cold disassembly buffer. The beads were settled 30 min on ice. Unbound protein was removed by two washes in disassembly buffer and a final wash in 20 mM HEPES, pH 7.4. Bound protein was analyzed as described above.
Microsome Binding Assay
Preparation of microsomal membranes and the assay for binding to microsomal membranes was carried out as described previously (Rein et al., 2002), with the following modifications: The membranes containing ≈8 μg protein were incubated with 8 nM Gcs1p, 10 nM His6-Sec18p in the presence of 0.5 mM ATP in a total volume of 50 μl with gentle agitation for 30 min at 30°C. Unbound protein was removed by centrifugation. After washing, microsomal membranes were dissolved in sample buffer containing 8 M urea, resolved by SDS-PAGE, and analyzed by immunoblot.
Blue Native Gel Electrophoresis
Fifty micrograms of SNARE-GST or GST was immobilized onto 100 μl of glutathione-agarose beads. After removal of unbound proteins, beads were split evenly. One half was incubated overnight (O/N) at 4°C with 13.3 nM Gcs1p, whereas to the other half an equal volume of buffer was added. The proteins were eluted from the beads in the presence of 100 mM glutathione and loaded on a 10% native gel. Blue native gel electrophoresis was performed according to Schaegger and von Jagow (1991) with minor modifications: 10% minigels were used instead of gradient gels. After the run, proteins were blotted onto polyvinylidene difluoride and analyzed using antibodies against GST.
Circular Dichroism (CD) Spectroscopy
Sed5-His6 was mixed with 50 mM potassium phosphate buffer, pH 6.8, in a total volume of 560 μl. The sample was split evenly, and 20 μl of Glo3p or 20 μl of ArfGAP dialysis buffer was added. The final protein concentration of Sed5-His6 was 2.2 μM in the assay and 6.7 nM for Glo3p. For the 2,2,2-trifluorethanol (TFE) treatment, the samples were prepared accordingly, and 150 μl of TFE was added to the reaction mix. The assays were incubated at 4°C for 14–16 h. CD spectra were recorded at 4°C by using a JASCO J720 CD spectrometer. Measurements were performed in Hellma quartz cuvettes with a path length of 0.1 cm. Spectra from 15 consecutive scans (250-190 nm; 50 nm/min scan rate; 1-nm step size; 2-nm bandwidth) were averaged.
RESULTS
The ArfGAPs Glo3p and Gcs1p Recruit Arf1ΔN17p to v- and t-SNAREs
We reported previously that the ArfGAPs Gcs1p and Glo3p catalytically induce a conformational change in v-SNAREs involved in fusion events in the early secretory pathway and in exo- and endocytosis (Rein et al., 2002; Robinson et al., 2006). SNAREs in this primed conformation are able to recruit Arf1p. We aimed to test whether this ArfGAP activity is limited to v-SNAREs or could be extended to t-SNAREs as well. To test this, we used recombinantly expressed ArfGAP and Arf1ΔN17p-Q71L. Arf1ΔN17p lacks the N-terminal 17 amino acids that are strongly hydrophobic and carry the myristoylation site; thus, they would interfere with purification and in vitro assays. The Q71L point mutant reflects the predominantly activated form of Arf1p. We have shown previously that Arf1p does not have to be activated to bind to v-SNAREs (Rein et al., 2002). Despite this finding, we used Arf1ΔN17p-Q71L in most experiments.
We performed GST pull-down assays with SNARE proteins (Table 1) that carried a GST fusion either at the N terminus or which replaced the C-terminal transmembrane domain. Similarly to what we reported previously (Rein et al., 2002), the ArfGAPs Gcs1p and Glo3p were able to induce a conformational change in most SNARE proteins tested, albeit with varying efficiencies (Figure 1). ArfGAPs promoted Arf1ΔN17p-Q71L and Arf1ΔN17p binding to the v-SNAREs Bet1p, Bos1p, Sec22p, Ykt6p, and Snc1p (Rein et al., 2002; Robinson et al., 2006; Figure 1, A and B, and Supplemental Figure S1) without themselves binding in stoichiometric amounts to the SNAREs. Furthermore, ArfGAPs allowed recruitment of the small GTPase on the t-SNARE Sed5p. Arf1ΔN17p-Q71L was also recruited in significant amounts to the GST fusions of the syntaxin t-SNAREs Vam3p, Tlg2p, Sso1p, and Sso2p (Figure 1C; data not shown). In contrast, we did not detect any Arf1ΔN17p-Q71L recruitment to N- or C-terminally tagged Tlg1p or GST-Sso1p. Despite the difference in the level of Arf1pΔN17p-Q71L recruitment by the two ArfGAPs, both GAPs were able to induce a conformational change on most of the SNAREs tested. In contrast, a third ArfGAP, Age2p, did not lead to the recruitment of stoichiometric amounts of Arf1ΔN17p-Q71L to SNAREs (data not shown). The GTPase Arf1p is involved not only in the generation of COPI-coated vesicles at the ER–Golgi interface but also in the formation of several clathrin- and nonclathrin-coated vesicles. Thus, Arf1p recruitment to SNARE proteins in the “primed” conformation occurs throughout the secretory and endocytic pathway. These interactions seem to provide a mechanism to include SNAREs into nascent vesicles and could provide the means by which not only v-SNAREs but also t-SNAREs reach their final compartment. Furthermore, the conformational change induced by ArfGAPs could be important for vesicle fusion at the target membrane.
Table 1.
SNAREs used in this study
| SNARE | v-t-SNARE | Localization |
|---|---|---|
| Bet1p | v | ER–Golgi |
| Bos1p | v | ER–Golgi |
| Nyv1p | v | Vacuole |
| Sec22p | v | ER–Golgi |
| Sed5p | t | ER–Golgi |
| Snc1p | v | Plasma membrane, endosome |
| Sso1p | t | Plasma membrane |
| Sso2p | t | Plasma membrane |
| Tlg2p | t | Endosomes |
| Vam3p | t | Vacuole |
| Ykt6p | v | Golgi |
| Tlg1p | t | Golgi, endosome |
Figure 1.
The ArfGAPs Glo3p and Gcs1p mediate binding of Arf1ΔN17p-Q71L to the cytoplasmic domains of SNAREs. SNARE–GST fusion proteins were immobilized onto glutathione-agarose beads and incubated with Arf1ΔN17p-Q71L. Where indicated Glo3p (A and C) or Gcs1p (B) was added to the reaction. Bound proteins were separated by SDS-PAGE, and bands were visualized by Coomassie blue staining.
ArfGAPs Promote SNARE Complex Formation
Because ArfGAPs induced a conformational change on both v- and t-SNAREs, we wondered whether ArfGAPs could promote v-t-SNARE complex formation in vitro. GST-Sed5p was incubated with His-tagged versions of Bet1p, Bos1p, and Sec22p in the presence or absence of Gcs1p (Figure 2, A–C). The reaction was stopped at the indicated times, and it was followed immediately by extensive washing. On addition of Gcs1p, the formation of SNARE complexes was accelerated, and the number of complexes increased compared with the experiment in which Gcs1p was omitted. A similar result was observed using a C-terminal fusion of GST to Sed5p (Figure 2, D–F). The ArfGAP Age2p served as a control, because Age2p cannot promote recruitment of Arf1pΔN17Q71L to SNAREs. As expected, Age2p did not accelerate SNARE complex formation (Figure 2, G–I). Thus, the conformational change induced by the ArfGAPs Glo3p and Gcs1p catalyzes the zippering process of SNAREs.
Figure 2.
ArfGAP accelerates functional SNARE complex formation in vitro. (A) N-terminally tagged GST-Sed5p was immobilized onto glutathione-agarose beads and incubated with Bet1p-His6, Bos1p-His6 and Sec22p-His6. Ten percent of the input of the different proteins is shown in lanes 11–13. Gcs1p was added to the incubation in lanes 6–10. The reaction was stopped at indicated time points by extensive washing. Bound proteins were eluted in sample buffer, separated by SDS-PAGE and visualized by Fairbanks staining. (B and C) Band intensities from A were determined using an Odyssey imaging system and normalized to the O/N incubation. The ratio of relative staining intensities is plotted on the y-axis. Data for Bos1p-His6 and Sec22p-His6 are shown in B and for Bet1p-His6 in C. (D) C-terminally tagged Sed5p-GST was immobilized and the SNARE complex formation was performed as described in A. (E and F) Quantification of the experiment shown in D. The ratio of relative staining intensities is plotted on the y-axis. (G) SNARE complex formation was performed as described in A. However, Age2p was added instead of Gcs1p where indicated. (H and I) Quantification of the experiment shown in G.
The ArfGAPs Glo3p and Gcs1p Change the Conformation of SNAREs by Increasing Their α-Helical Content
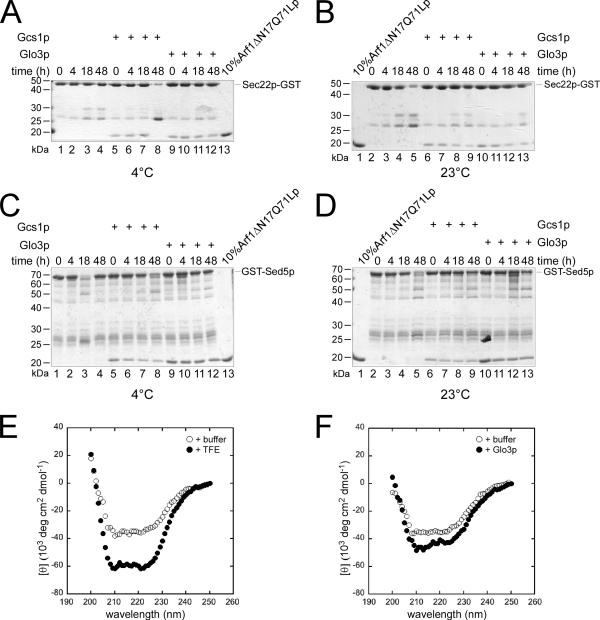
Because this reaction did not require energy, these results also indicate that ArfGAPs may change the conformation of the SNAREs from a high-energy state to a low-energy state, which would enable an efficacious engagement into SNARE complexes in vitro that may reflect the fusion competent SNARE complexes in vivo. If the ArfGAP Glo3p and Gcs1p indeed promote the formation of a low-energy state, this conformation should be stable over a long period. We preincubated Sec22p-GST and GST-Sed5p with either Glo3p or Gcs1p. After the removal of the ArfGAPs, the ability of Arf1ΔN17Q71Lp to bind to the SNAREs was assayed at various times after the preincubation step (Figure 3, A–D). No significant loss of the Arf1ΔN17Q71Lp binding to the SNAREs was observed even after 48 h at 23°C after the preincubation with ArfGAP. This result demonstrates that the conformational change induced by the ArfGAPs Glo3p and Gcs1p is stable and that SNAREs do not revert their conformation spontaneously.
Figure 3.
Glo3p and Gcs1p induce a conformational change in SNAREs. (A–D) Sec22p-GST (A and B) or GST-Sed5p (C and D) were immobilized onto glutathione-agarose beads and preincubated with either Gcs1p or Glo3p for 1 h at 4°C. The ArfGAPs were removed by extensive washing and the samples were left at 4°C (A and C) or at 23°C (B and D) for up to 48 h. At various time points after the preincubation, Arf1ΔN17p-Q71L binding experiments were performed. Even after 48 h at 23°C, no marked decrease in the recruitment of Arf1ΔN17p-Q71L to the SNARE-GST fusion was observed. (E and F) CD spectra of Sed5p-His6 were recorded at 4°C. (E) The maximal α-helicity of Sed5p-His6 was determined by addition of TFE. (F) Glo3p promotes a small but significant increase in α-helical content in Sed5p-His6.
We aimed to determine the nature of this low-energy state of SNAREs. It is conceivable that SNAREs would multimerize upon interaction with Glo3p or Gcs1p and that this process would be favored in our in vitro system by using GST fusion proteins. To test this possibility, we performed gel filtration and blue native gel electrophoresis experiments. Both approaches allowed the same conclusion, namely, that the SNARE–GST fusion proteins as well as GST are already mostly dimers and that the addition of ArfGAP does not change the oligomeric state of the fusion proteins (Supplemental Figure S2; data not shown). Next, we tested whether we could detect a conformational change by CD by using a Sed5p construct in which the transmembrane domain was replaced by a His6-tag. Because Sed5p-His6 possesses already an α-helical content, we first determined the maximal signal we could expect by incubating Sed5p-His6 with the α-helix-stabilizing agent TFE (Figure 3E). Because significant changes were observed with TFE, we recorded CD spectra for Sed5p-His6 in the presence and absence of Glo3p (Figure 3F). For this experiment, we increased the molar ratio of the SNARE over the GAP about another 10-fold to ensure that we would not detected any signal related to Glo3p. Indeed, under these conditions Glo3p did not give a signal over the buffer only control (data not shown). In the presence of Glo3p, Sed5p-His6 showed a small but reproducible increase in α-helical content (Figure 3F), demonstrating that Glo3p induces a conformational change on Sed5p-His6. These data are in agreement with our previous published results showing that SNAREs become more protease-resistant after treatment with Glo3p (Rein et al., 2002).
Sec17p and Sec18p Disassemble SNARE Complexes Formed in the Presence of ArfGAP
We tested next whether the addition of Sec17p (the yeast homologue of α-SNAP) and Sec18p (the yeast homologue of NSF) but not of an ATPase-deficient Sec18p [Sec18(E350Q)p] (Steel et al., 1999) could dissolve the SNARE complexes again, and whether this process requires energy. Sec18(E350Q)p can still bind ATP and thus forms the characteristic hexameric complex; yet, it is deficient in ATP hydrolysis. Indeed, Sec17p, together with Sec18p but not with mutant Sec18(E350Q)p, disassembled SNARE complexes (Figure 4, compare lines 2 and 3 and lines 7 and 8), implying that ArfGAPs may render SNAREs fusion competent already upon inclusion into transport vesicles. Furthermore, these results strongly suggest that ArfGAPs can indeed promote SNARE complex formation, perhaps by acting like a chaperone.
Figure 4.
Sec17p and Sec18p can disassemble the SNARE complexes formed in the presence of ArfGAP. SNARE complexes were formed over night in the absence (lanes 1–5) or presence (lanes 6–10) of Gcs1p were incubated with His6-Sec17p (lanes 2, 3, 7, and 8), His6-Sec18p (lanes 3, 5, 8, and 10), or His6-Sec18E350Qp (lanes 2, 4, 7, and 9). The assay was stopped on ice, and samples were processed as described in Figure 2.
ArfGAPs Glo3p and Gcs1p Recruit Sec17p and Sec18p to SNAREs Involved in ER–Golgi and Post-Golgi Trafficking
If our hypothesis that ArfGAPs could render v-SNAREs fusion competent upon inclusion into transport vesicles was correct, then Sec17p and Sec18p may already bind to the single SNARE proteins, which are not engaged in SNARE complexes. Therefore, SNARE-GST fusions were pretreated with the ArfGAP Gcs1p as described above, and after extensive washing, equimolar amounts of Sec17p and Sec18p, in the presence or absence of ATP and GTP, were added to the reaction. We found that both Sec17p and Sec18p bound independently of the presence of nucleotides in the reaction mix but rather required the preincubation of SNAREs with ArfGAP (Figure 5A). Again, no binding of Gcs1p was detected on the gel. More Sec17p (Figure 5B) and Sec18p (Figure 5C) bound to the v-SNARE Bet1p compared with the t-SNARE Sed5p. Thus, the Sec17p/Sec18p binding mode to SNARE tails might be similar to that to SNARE complexes. Therefore, we could use this much simpler model to investigate the binding of Sec17p/Sec18p to SNAREs.
Figure 5.
Sec17p and Sec18p binding to SNARE proteins depends on ArfGAP. (A) Bet1p-GST (lanes 1–4), GST-Sed5p (lanes 5–8), and GST (lanes 9–12) were immobilized onto glutathione-agarose beads, and they were incubated with Gcs1p. After washing, 0.6 μM His6-Sec17p and 0.6 μM His6-Sec18p were added to each sample, and nucleotides were added as indicated. Lane 13 and 14 show 25 and 10% of the input of His6-Sec17p and His6-Sec18p, respectively. The reactions were stopped by extensive washing. Bound proteins were eluted in sample buffer and resolved by SDS-PAGE followed by SyproRed staining. (B and C) Quantification of A for His6-Sec17p and His6-Sec18p, respectively. The ratio of relative staining intensities is plotted on the y-axis. Binding of 0.6 μM His6-Sec17p (D) or 0.6 μM His6-Sec18p (E) to a variety of single SNAREs is dependent on pretreatment with ArfGAP Glo3p. The experiment was performed as in described in A except that Glo3p instead of Gcs1p was used to induce the conformational change.
Next, we explored whether Sec17p and Sec18p require each other for binding to SNAREs. Sec17p-bound SNAREs involved in ER–Golgi and post-Golgi trafficking almost exclusively in the primed conformation that is induced by the ArfGAPs Gcs1p or Glo3p (Figure 5D; data not shown). Strong Sec17p binding occurred on Bet1p, Bos1p, and Sed5p, whereas binding to Sec22p, Snc1p, and Ykt6p was much weaker. Surprisingly, even in the absence of Sec17p, Sec18p was recruited to SNAREs in the primed SNARE conformation (Figure 5E). However, in contrast to Sec17p binding to SNAREs, Sed5p recruited much less Sec18p compared with Bet1p or Bos1p. Similarly to the binding to the entire SNARE complex, Sec18p binding occurred in absence of additional ATP in the reaction. Thus, Sec18p and Sec17p can bind to single SNAREs. Furthermore, our data demonstrate that Sec18p does not require Sec17p for binding individual SNAREs, which may point to a function of Sec18p unrelated to unwinding of SNARE complexes.
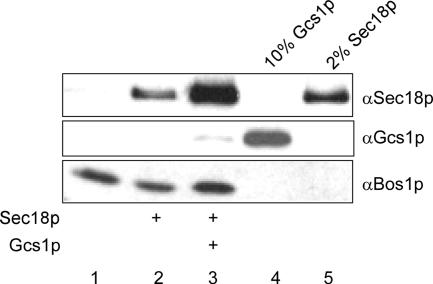
Gcs1p Enhances Sec18p Binding to Microsomal Membranes
We wanted to extend our in vitro findings that Sec18p bound to SNAREs in an ArfGAP-dependent manner, to conditions that would better reflect the in vivo situation. Therefore, we tested whether Sec18p would be recruited to microsomal membranes in an ArfGAP-dependent manner. Microsomal membranes were incubated with Sec18p and ATP in the presence or absence of Gcs1p. After incubation at 30°C, the membrane fraction was isolated and analyzed by immunoblot (Figure 6). Although Sec18p binding to membranes did not necessitate the presence of ArfGAP, Gcs1p strongly enhanced the interaction of Sec18p with SNAREs on microsomal membranes. Sec17p is still present on the microsomal membranes and may recruit Sec18p in the absence of ArfGAP. It is very unlikely that SNARE complex formation was induced by Gcs1p, especially because there are no transport vesicles formed under these conditions. Therefore, it seems more likely that Gcs1p permits binding of Sec18p to single SNAREs. Together, our data demonstrate an ArfGAP-dependent Sec18p recruitment to SNAREs and that this recruitment might not correlate to the Sec18p function to resolve SNARE complexes.
Figure 6.
Gcs1p mediates Sec18p recruitment to microsomal membranes. Buffer-washed microsomal membranes were incubated with His6-Sec18p and ATP in the absence (lane 2) or presence (lane 3) of Gcs1p. After extensive washing, the membrane fraction was solubilized and analyzed by immunoblot. Lane 1 contains the microsomal membranes incubated with buffer. Ten percent of the Gcs1p and 2% of Sec18p present in the incubation are shown in lanes 4 and 5, respectively.
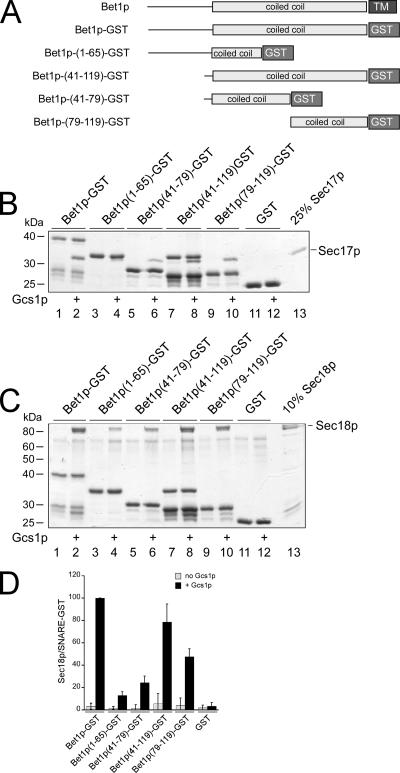
Sec17p and Sec18p Bind to the SNARE Domain
We have shown previously that Arf1p is recruited to the membrane-proximal region of the SNARE domain of Bet1p (Rein et al., 2002). We next asked where Sec17p and Sec18p might bind to Bet1p. Fragments of the cytoplasmic domain of Bet1p (Figure 7A) were used to determine the binding sites of Sec17p and Sec18p. Sec17p bound most efficiently to the coiled-coil SNARE domain made up of Bet1p(residues 41-119) and to a smaller fragment of the membrane-proximal region, Bet1p(79-119) (Figure 7B). However, strong binding also occurred to the membrane-distal region of the SNARE domain [Bet1p(41-79)]. Because Sec17p migrates at the same position on the gel as the N-terminal fragment Bet1p(1-65), Sec17p binding to Bet1p(1-65), was determined instead by semiquantitative immunoblot and was found to be close to background levels (data not shown). Thus, Sec17p binds to the SNARE domain of Bet1p. Sec18p also bound to the SNARE domain, although a preference for the membrane-proximal part was detectable (Figure 7, C and D).
Figure 7.
Binding sites of Sec17p and Sec18p on Bet1p-GST. (A) Schematic depiction of Bet1p and GST fusions of Bet1p. (B and C) GST fusions were immobilized onto glutathione-agarose beads and incubated with Gcs1p. After washing, His6-Sec17p (B) or His6-Sec18p (C) and 1 mM ATP were added. Unbound proteins were removed, and bound proteins were analyzed as described in Figure 5. (D) Quantification of the experiment in C. The ratio of relative staining intensities is plotted on the y-axis.
We also confirmed these results by determining the binding of Sec17p and Sec18p to truncations of the vacuolar SNAREs Vam3p and Nyv1p. Xu et al. (1998) demonstrated that not only Sec17p and Sec18p bind to a SNARE complex containing Vam3p and Nyv1p but also that there is a Sec17p-independent function of Sec18p after unwinding of the complex. The Vam3p(Δ1-144)-GST construct contained the SNARE domain, which was deleted in the Nyv1p(1-136)-GST construct. Although Sec18p and Sec17p bound the full-length Vam3p-GST and Nvy1p-GST as well as to the SNARE domain of Vam3p(Δ1-144)-GST alone, no binding was observed to the Nyv1p(1-136)-GST construct lacking the SNARE domain (data not shown). Thus, both Sec17p and Sec18p bind to the SNARE domains of different SNAREs.
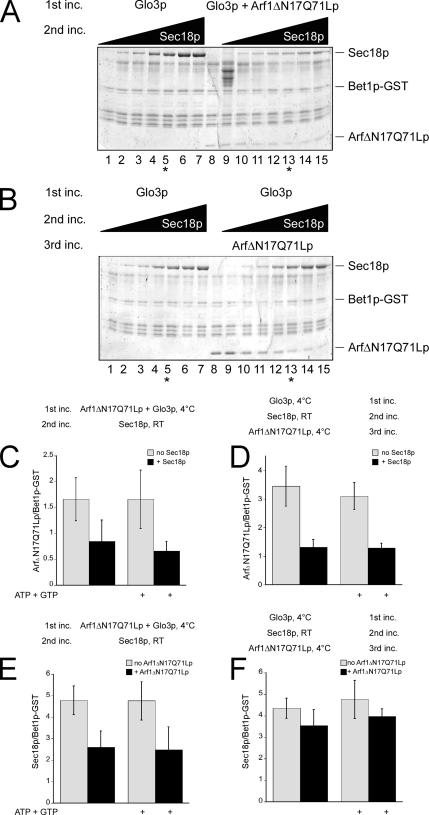
Sec18p Competes with Arf1ΔN17p-Q71L for Binding Sites on SNARE Domains
We have reported previously that Arf1p also binds to the SNARE domain, preferentially to the membrane-proximal region. Because of the overlap of the binding sites of Sec18p, Sec17p, and Arf1p, we wanted to test whether these proteins would compete with each other for binding sites on SNAREs. Therefore, we first formed a complex of Arf1ΔN17p-Q71L and Bet1p-GST with the help of Glo3p. After extensive washing, this complex was then incubated with increasing concentrations of Sec18p. Arf1ΔN17p-Q71L was partially displaced from the SNARE at high Sec18p concentrations (Figure 8A). However, the amount of Sec18p recruited to the Arf1ΔN17p–Q71L–SNARE complex was reduced compared with primed SNAREs alone. When we reversed the order of the addition of Arf1ΔN17p-Q71L and Sec18p, binding of Arf1ΔN17p-Q71L was strongly reduced in the presence of stoichiometric amounts of Sec18p on the SNAREs (Figure 8B). We repeated the assays with an intermediate Sec18p concentration (Figure 8, C–F). Under these conditions, Sec18p displaced ∼50% of Arf1ΔN17p-Q71L from Bet1p-GST, whereas in the converse experiment, Arf1ΔN17p-Q71L could not compete with Sec18p for binding sites on Bet1p (Figure 8, compare C with F). Yet, not all binding sites could be exchanged; preincubation of Bet1p with Arf1ΔN17p-Q71L reduced Sec18p binding by ∼50%, and a similar effect of Sec18p on Arf1ΔN17p-Q71L was observed when the order of addition was inverted (Figure 8, D and E). Furthermore, Arf1ΔN17p-Q71L was unable to chase Sec18p off Bet1p-GST (Figure 8F). The Sec18p-mediated loss of Arf1ΔN17p-Q71L from the SNARE was independent of nucleotide in the reaction mix for all conditions tested. Together, Sec18p could partially displace prebound Arf1ΔN17p-Q71L from Bet1p-GST, and this displacement did not require energy.
Figure 8.
Sec18p and Arf1ΔN17p-Q71L compete for binding sites on Bet1p-GST. (A) Bet1p-GST was immobilized on glutathione-agarose beads. The immobilized SNARE was incubated with Glo3p and Arf1ΔN17p-Q71L (lanes 8–15). After removal of unbound proteins, increasing amounts of His6-Sec18p (15 nM–3.6 μM), 1 mM ATP, and 0.5 mM GTP were added in lanes 1–7 and 9–15. After the incubation, unbound proteins were removed, and bound proteins were analyzed as described above. Binding of Arf1ΔN17p and His6-Sec18p (0.6 μM) to Bet1p-GST was quantified as before and is shown in C and E. (B) Immobilization and induction of the conformational change were performed as described in A. In the second incubation step, increasing amounts of His6-Sec18p (15 nM–3.6 μM) were added. After incubation, the unbound Sec18p was removed, and Arf1ΔN17p was added. Binding of Arf1ΔN17p and His6-Sec18p (0.6 μM) to Bet1p-GST was quantified as before and is shown in D and F. The ratio of relative staining intensities is plotted on the y-axis.
Displacement of Arf1p by Sec18p Is Independent of ATP Hydrolysis
Because recombinant Sec18p is in the ATP-bound state on both the catalytic and the structural ATP binding sites, Sec18p could potentially undergo at least one round of ATP hydrolysis even in the absence of ATP from the reaction mixture. To exclude effects of ATP hydrolysis in the competition experiments, we used the ATPase-deficient mutant Sec18(E350Q)p. Sec18(E350Q)p bound the SNARE Bet1p even stronger than wild type (Figure 9A). Addition of equal amounts of Sec18p or Sec18(E350Q)p led in both cases to a loss of 50% of the Arf1ΔN17p-Q71L or Arf1ΔN17p bound to Bet1p-GST (Figure 9B). Furthermore, the ability of Sec18(E350Q)p to bind to Bet1p-GST prebound to Arf1ΔN17p or Arf1ΔN17p-Q71L was similar to that of Sec18p (Figure 9C). Therefore, the competition of Arf1ΔN17p or Arf1ΔN17p-Q71L and Sec18p for binding sites on the SNARE protein is most likely not due to unwinding of SNARE complexes but rather involves steric hindrance by Sec18p.
Figure 9.
Displacement of Arf1ΔN17p-Q71L by Sec18p does not require ATP hydrolysis by Sec18p. (A) Bet1p-GST was immobilized on glutathione-agarose beads. The beads were incubated with Gcs1p in the presence of 1 mM ATP and 0.5 mM GTP. Arf1ΔN17p-Q71L was present in lanes 1, 5, and 6 and Arf1ΔN17p in lanes 2, 7, and 8. After removal of unbound proteins, His6-Sec18p (0.6 μM; lanes 3, 5, and 7) or His6-Sec18E350Qp (0.6 μM; lanes 4, 6, and 8) were added in the presence of 1 mM ATP and 0.5 mM GTP. After the incubation, unbound proteins were removed, and the bound proteins were analyzed as described above. Ten percent of the input of Arf1ΔN17p-Q71L (lane 9), His6-Sec18p (lane 11), and His6-Sec18E350Qp (lane 12) are shown. (B) Quantification of the loss of Arf1ΔN17p-Q71L from Bet1p-GST in the presence of His6-Sec18p and His6-Sec18E350Qp. (C) Quantification of the Arf1ΔN17p-Q71L effect on subsequent His6-Sec18p and His6-Sec18E350Qp binding.
DISCUSSION
In this article, we show that the transient interaction of ArfGAPs with v- and t-SNAREs not only allows the recruitment of Arf1p and therefore promotes the inclusion of SNAREs into vesicles but also accelerates the formation of SNARE complexes. This led us to propose that ArfGAP might have a chaperone-like function to help the SNAREs to assume a low-energy conformation. Furthermore, these findings indicate that the v-SNAREs might adopt a conformation that will allow rapid engagement in trans-SNARE complexes, which might play a role in determining the velocity of vesicle fusion.
We tested three different ArfGAPs for their ability to recruit Arf1ΔN17p-Q71L to SNARE proteins as indication for the conformational change. Two of them, Glo3p and Gcs1p, but not Age2p, promoted Arf1ΔN17p-Q71L binding. We have previously demonstrated that the transient interaction Glo3p with the v-SNAREs Sec22p and Bet1p renders the SNAREs more protease resistant (Rein et al., 2002). This conformational change may promote the inclusion of SNAREs into vesicles, and they might even serve as primers for vesicle formation (Springer et al., 1999; Spang, 2002).
Gcs1p and Glo3p are the orthologues of mammalian ARFGAP1 and ARFGAP3, respectively. At least for ARFGAP1, a similar role in the inclusion of the v-SNARE membrin into vesicles has been reported previously (Honda et al., 2005). ARFGAP1 contains a domain that senses membrane curvature via an amphiphatic helix (Bigay et al., 2003). This domain could be as well responsible for the catalytic change on SNAREs. This exact domain is not conserved in Glo3p. However, Glo3p contains a coiled-coil region, which could fulfill a similar function. Age2p contains neither an amphiphatic helix nor a coiled-coil domain. The difference in the structural organization of the ArfGAPs may explain the variation in the ability to act as chaperone on SNAREs. Whether this chaperone-like function is limited to SNAREs or whether ArfGAPs are generally involved in cargo recruitment remains to be established. The KDEL receptor Erd2 and members of the p24 family of proteins, however, require ARFGAP1 for uptake into COPI-coated vesicles (Aoe et al., 1999; Lanoix et al., 2001).
Both v- and t-SNAREs were positively affected by the interaction with ArfGAPs. One explanation for this observation is that this interaction provides the mechanism by which t-SNAREs reach their final compartment and that the conformational change serves as switch form a high- to a low-energy state, which promotes efficient incorporation of also t-SNAREs into transport vesicles. v-SNAREs seem to use this pathway for efficient inclusion into at least COPI-coated vesicles (Rein et al., 2002; Robinson et al., 2006). Alternatively, the conformational change on t-SNAREs may only take place on the target membrane and may accelerate trans-SNARE complex formation.
We found that ArfGAP accelerated the formation of SNARE complexes and that these complexes were resolved by the yeast homologues of NSF and α-SNAP, Sec18p and Sec17p, respectively. Surprisingly, Sec18p and Sec17p also bound independently of each other to SNAREs. This interaction was strictly dependent on the induced conformational switch on SNAREs by ArfGAPs. Moreover, Sec18p competed with Arf1ΔN17p for binding sites on SNAREs. Because this interaction did not require nucleotide hydrolysis, this function of Sec18p is most likely unrelated to the well-established unwinding activity of the AAA-ATPase required to dissolve the four-helix bundle of cis-SNARE complexes. Why would Sec18p bind to SNAREs and displace Arf1p? A transport vesicle arrives at the target membrane, and although it is generally thought that all coat has been shed at this point, experimental proof is lacking. The SNAREs on the vesicles must be exposed to engage in trans-SNARE complexes with the SNAREs on the target membrane. Sec18p could bind to SNAREs and displace coat proteins, especially Arf1p. We have shown previously that binding of Arf1ΔN17p binds to SNAREs in a nucleotide-independent manner. Therefore, it is conceivable that Arf1p-GDP might still stick to SNARE proteins after GTP hydrolysis has occurred and most of the coat proteins have left the transport vesicle. Displacement of Arf1p by Sec18p would provide a mechanism, which would ensure the availability of the SNAREs for complex formation. Because Sec17p is not required at this step, Sec18p is unable to unwind the four-helix bundle and efficient membrane fusion could proceed. This function of Sec18p would also be independent of the action of tethering complexes. Tethering complexes may actually require the presence of residual coat components as determinants for the tethering activity (Malsam et al., 2005).
This is the first report demonstrating binding of Sec18p/NSF in stoichiometric amounts to SNAREs in the absence of Sec17p/α-SNAP. Supposedly, Sec18p/NSF only binds to SNAREs after Sec17p/α-SNAP recruitment to cis-SNARE complexes (Sollner et al., 1993; Morgan et al., 1994). We used, however, single SNARE molecules; therefore, we likely provide a different molecular environment compared with the four-helix bundle of the cis-SNARE complex. The binding of Sec17p to single SNARE molecules has been shown previously, and this binding was independent of ArfGAP (Rossi et al., 1997). However, in the experimental setup of Rossi et al. (1997), only substiochiometric amounts of Sec17p were recruited, which were detected by immunoblot. ArfGAP-mediated binding of Sec17p to SNAREs was stoichiometric and detectable by Coomassie blue staining, despite an at least fivefold lower concentration of Sec17p in the assay. Therefore, the ArfGAP-induced conformational change greatly enhances the binding of Sec17p to SNAREs. Furthermore, Sec18p binding to single SNAREs was probably undetected because of the low affinity of Sec18p to the single SNARE in the high-energy state.
We mapped the binding sites of Sec18p and Sec17p on the SNAREs. Sec17p and Sec18p bound to the SNARE domain and Sec18p had within this domain a slight preference for the membrane proximal part. These data seem to somewhat disagree with previously published reports, where Sec18p/NSF and Sec17p/α-SNAP bind to the N-terminal (membrane distal) part of cis-SNARE complexes (Hanson et al., 1997; Hohl et al., 1998). However, our mapping data were performed on single SNAREs, and the binding of Sec18p was determined in the absence of Sec17p. Sec18p binding to cis-SNARE complexes is strictly dependent on Sec17p. Therefore, the mode of interaction of Sec18p with single SNAREs and with SNAREs complexes could be different. However, one could probably expect a high-affinity of Sec18p to SNARE domains and that is exactly what we observe. These data also support our model of dual function of Sec18p in membrane traffic: first, Sec18p ensures that the single SNAREs can engage in trans-SNARE complexes by displacing residual coat components; and second, Sec18p, together with Sec17p, unwinds cis-SNARE complexes to free the SNAREs for another round of transport.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Banfield, P. Brennwald, R. Duden, A. Mayer, A. Morgan, P. Poon, J. Rizo, R. Schekman, C. Ungermann, and W. Wickner for reagents and I. G. Macara for comments on the manuscript. We are indebted to D. Klostermeier and her laboratory members for help with the biophysical experiments. We thank the members of the Spang laboratory for discussions. V. Olik and F. Seiler are acknowledged for expert assistance. This work was supported by the Minna James Heineman Fund, the Deutsche Forschungsgemeinschaft SFB 446, and the Schweizer Nationalfond.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0756) on May 23, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Aoe T., Huber I., Vasudevan C., Watkins S. C., Romero G., Cassel D., Hsu V. W. The KDEL receptor regulates a GTPase-activating protein for ADP-ribosylation factor 1 by interacting with its non-catalytic domain. J. Biol. Chem. 1999;274:20545–20549. doi: 10.1074/jbc.274.29.20545. [DOI] [PubMed] [Google Scholar]
- Bigay J., Gounon P., Robineau S., Antonny B., Schekman R., Orci L. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Self-assembly of minimal COPII cages. Nature. 2003;426:563–566. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Jackson C. L. Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Roth R., Morisaki H., Jahn R., Heuser J. E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hohl T. M., Parlati F., Wimmer C., Rothman J. E., Sollner T. H., Engelhardt H. Arrangement of subunits in 20 S particles consisting of NSF, SNAPs, and SNARE complexes. Mol Cell. 1998;2:539–548. doi: 10.1016/s1097-2765(00)80153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A., Al-Awar O. S., Hay J. C., Donaldson J. G. Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE. J. Cell Biol. 2005;168:1039–1051. doi: 10.1083/jcb.200409138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber I., Rotman M., Pick E., Makler V., Rothem L., Cukierman E., Cassel D. Expression, purification, and properties of ADP-ribosylation factor (ARF) GTPase activating protein-1. Methods Enzymol. 2001;329:307–316. doi: 10.1016/s0076-6879(01)29092-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Ouwendijk J., Stark A., Szafer E., Cassel D., Dejgaard K., Weiss M., Nilsson T. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol. 2001;155:1199–1212. doi: 10.1083/jcb.200108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsam J., Satoh A., Pelletier L., Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner W., Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Membrane traffic: controlling membrane fusion by modifying NSF. Curr. Biol. 2004;14:R968–R970. doi: 10.1016/j.cub.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Morgan A., Dimaline R., Burgoyne R. D. The ATPase activity of N-ethylmaleimide-sensitive fusion protein (NSF) is regulated by soluble NSF attachment proteins. J. Biol. Chem. 1994;269:29347–29350. [PubMed] [Google Scholar]
- Nichols B. J., Ungermann C., Pelham H. R., Wickner W. T., Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Peng R., Gallwitz D. Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol. 2002;157:645–655. doi: 10.1083/jcb.200202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon P. P., Cassel D., Spang A., Rotman M., Pick E., Singer R. A., Johnston G. C. Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 1999;18:555–564. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon P. P., Nothwehr S. F., Singer R. A., Johnston G. C. The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network. J. Cell Biol. 2001;155:1239–1250. doi: 10.1083/jcb.200108075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon P. P., Wang X., Rotman M., Huber I., Cukierman E., Cassel D., Singer R. A., Johnston G. C. Saccharomyces cerevisiae Gcs1 is an ADP-ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA. 1996;93:10074–10077. doi: 10.1073/pnas.93.19.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein U., Andag U., Duden R., Schmitt H. D., Spang A. ARF-GAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J. Cell Biol. 2002;157:395–404. doi: 10.1083/jcb.200112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M., Poon P. P., Schindler C., Murray L. E., Kama R., Gabriely G., Singer R. A., Spang A., Johnston G. C., Gerst J. E. The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast. Mol. Biol. Cell. 2006;1:1. doi: 10.1091/mbc.E05-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., Salminen A., Rice L. M., Brunger A. T., Brennwald P. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J. Biol. Chem. 1997;272:16610–16617. doi: 10.1074/jbc.272.26.16610. [DOI] [PubMed] [Google Scholar]
- Schaegger H., von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- Sharrocks A. D. A T7 expression vector for producing N- and C-terminal fusion proteins with glutathione S-transferase. Gene. 1994;138:105–108. doi: 10.1016/0378-1119(94)90789-7. [DOI] [PubMed] [Google Scholar]
- Sollner T. H. Intracellular and viral membrane fusion: a uniting mechanism. Curr. Opin. Cell Biol. 2004;16:429–435. doi: 10.1016/j.ceb.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Spang A. ARF1 regulatory factors and COPI vesicle formation. Curr. Opin. Cell Biol. 2002;14:423. doi: 10.1016/s0955-0674(02)00346-0. [DOI] [PubMed] [Google Scholar]
- Springer S., Spang A., Schekman R. A primer on vesicle budding. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Steel G. J., Laude A. J., Boojawan A., Harvey D. J., Morgan A. Biochemical analysis of the Saccharomyces cerevisiae SEC18 gene product: implications for the molecular mechanism of membrane fusion. Biochemistry. 1999;38:7764–7772. doi: 10.1021/bi990315v. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Nichols B. J., Pelham H. R., Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Sollner T. H., Rothman J. E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Rossnagel K., Buhrow S. A., Brunner M., Jaenicke R., Rothman J. E. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Yeast vacuoles and membrane fusion pathways. EMBO J. 2002;21:1241–1247. doi: 10.1093/emboj/21.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Sato K., Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.