Abstract
Background
The epidemiology of E. ruminantium infection in extensively managed young animals is not adequately understood. Thus in this study, we monitored the onset (age at first infection) and kinetics of E. ruminantium infection and antibody response in extensively managed newborn lambs and kids at three sites in The Gambia.
Methods
We used a nested pCS20 PCR and MAP1-B ELISA in a longitudinal study to monitor the onset (age at first infection) and kinetics of E. ruminantium infection and antibody response respectively, in 77 newborn lambs and kids under a traditional husbandry system at three sites (Kerr Seringe, Keneba, Bansang) in The Gambia where heartwater is known to occur. The animals were monitored for field tick infestation and the comparative performance of the two assays in detecting E. ruminantium infection was also assessed.
Results
The infection rate detected by pCS20 PCR varied between 8.6% and 54.8% over the 162-day study period. Nineteen per cent of the animals in week 1 post-partum tested positive by pCS20 PCR with half of these infections (7/14) detected in the first 3 days after birth, suggesting that transmission other than by tick feeding had played a role. The earliest detectable A. variegatum infestation in the animals occurred in week 16 after birth. Antibodies detected by MAP1-B ELISA also varied, between 11.5% and 90%. Although there is considerable evidence that this assay can detect false positives and due to this and other reasons serology is not a reliable predictor of infection at least for heartwater. In contrast to the pCS20 PCR, the serological assay detected the highest proportion of positive animals in week 1 with a gradual decline in seropositivity with increasing age. The pCS20 PCR detected higher E. ruminantium prevalence in the animals with increasing age and both the Spearman's rank test (rs = -0.1512; P = 0.003) and kappa statistic (-0.091 to 0.223) showed a low degree of agreement between the two assays.
Conclusion
The use of pCS20 PCR supported by transmission studies and clinical data could provide more accurate information on heartwater epidemiology in endemic areas and single-occasion testing of an animal may not reveal its true infection status. The view is supported because both the vector and vertical transmission may play a vital role in the epidemiology of heartwater in young small ruminants; the age range of 4 and 12 weeks corresponds to the period of increased susceptibility to heartwater in traditionally managed small ruminants.
Background
Heartwater is an infectious disease of ruminants caused by a rickettsia, Ehrlichia ruminantium, and transmitted by ixodid ticks of the genus Amblyomma. The disease is endemic in sub-Saharan Africa and on some islands in the Caribbean.
The epidemiology of heartwater in young small ruminants is not adequately understood. In heartwater-endemic areas where extensive husbandry systems exist and tick control is absent or limited, the numbers of Amblyomma ticks are high and animals are subjected to almost continuous tick, and presumably E. ruminantium challenge [1]. Several researchers postulated that the existence of endemic stability for E. ruminantium and tick-borne infections in general may be dependent on infection, by tick transmission, to the very young host during a period of reduced susceptibility to clinical disease [2-4]. It has been reported that newborn calves, lambs and kids possess an inverse age-related resistance to heartwater, which is independent of the immune status of the dam[5,6]; this resistance has been reported to be of short duration, lasting 9 days in lambs [7] and 2 weeks in kids [8]. However, the concept of endemic stability in relation to heartwater in extensively managed small ruminants in The Gambia (local dwarf sheep and goats) is not completely understood and may not be the same as in the case of indigenous cattle. Mortality due to heartwater has been reported frequently in the first two species; and a 12-month risk assessment in The Gambia showed that indigenous small ruminants (local dwarf sheep and dwarf goats) experienced a much lower A. variegatum tick attachment rate of 0.76 ticks/animal than N'Dama cattle (9.36 ticks/animal) (B. Faburay et al., unpublished data). Moreover, evidence has been provided of possible occurrence of vertical transmission of E. ruminantium in calves [9] and that initial transmission of heartwater to calves may not always be by the tick vector [10], findings which could also apply to small ruminants.
Diagnostic tests targeting pCS20 sequences have long been considered specific for E. ruminantium [11,12] and recent advances in molecular diagnostics resulted in the development of a specific and sensitive pCS20 polymerase chain reaction (PCR) assay for detection of all known strains of E. ruminantium in ticks [11,13]. Previous experiments showed that the pCS20 PCR could detect E. ruminantium carrier infections in animals [14]. Preliminary random testing of suspected carrier small ruminants in a heartwater-endemic area (Keneba) in The Gambia using a nested pCS20 PCR detected a 60% (n = 14) infection rate; moreover all samples collected from clinically sick goats maintained on-station at ITC (Kerr Seringe), and confirmed as heartwater cases post mortem, tested positive by the same assay (B. Faburay, unpublished data). In the past, serological tests for detection of antibodies to E. ruminantium suffered from poor specificity due to cross-reactions with other ehrlichial agents [15-17]. Although the MAP1-B ELISA [17] has been reported to detect false-positives in heartwater-free areas attributed to cross-reactions with closely related species [18,19], the assay has higher sensitivity compared to other serological tests to detect E. ruminantium antibodies in ovine and caprine sera [20,21]. This increased sensitivity is attributed to the comparatively longer persistence of MAP1 antibodies in these species [17,20]. In the present study, we monitored the onset (age at first infection) and kinetics of E. ruminantium infection and antibody response in extensively managed newborn lambs and kids at three sites in The Gambia where heartwater is known to occur [22] using pCS20 PCR, and also compared the performance of the pCS20 PCR and indirect MAP1-B ELISA in detecting heartwater infection in small ruminants.
Methods
Study sites
Seventy-seven small ruminants (local dwarf sheep and dwarf goats) were monitored from birth for up to 162 days at three sites in The Gambia: Kerr Seringe (13°43' N, 16°72' W), Keneba (13°20' N, 16°01' W) and Bansang (13°27' W, 14°40' N). The animals at Kerr Seringe and Keneba belonged to the International Trypanotolerance Centre (ITC), whereas those in Bansang were animals of local smallholders. A recent serological study showed presence of MAP1-B specific antibodies at all three sites [22]. In the presence of A. variegatum ticks in these areas [23], we presume that these antibodies are due to exposure to E. ruminantium. Kerr Seringe is located in the western part of The Gambia on the coast, whereas Keneba is about 150 km east of Kerr Seringe. Bansang is located 150 km further east from Keneba. Monitoring of death in extensively managed small ruminants in Kerr Seringe from 1996 to 1999 showed that 17.9% of deaths in local dwarf sheep and 12.5% in local dwarf goats were associated with heartwater; and in Keneba these figures were 36% and 25% for sheep and goats respectively [24]. Although cases of mortality due to hearwater have been observed in sheep and goats in Bansang area, records of these deaths were not kept. E. ruminantium tick infection rates at these sites have been described previously [23].
Animals and husbandry system
The study animals comprised 29 lambs at Kerr Seringe, 21 kids and 13 lambs at Keneba and 10 kids and 4 lambs at Bansang. All newborn animals and their dams were maintained under a traditional husbandry system without acaricide treatment, except for the dams at Kerr Seringe, which had received regular monthly acaricide treatment for about 10 months before the start of this study. Acaricide treatment of all animals was discontinued during the study period. All newborn lambs and kids, together with their dams, were maintained under the traditional husbandry system [25] without acaricide treatment. Under this system, animals were allowed to wander freely around the homestead for feed and food residues and/or graze in the bush during the day. Newborn kids and lambs were mostly tethered until they reached the age of 2 to 3 weeks of age to gain strength to graze with their dams in the bush. At night, depending on the site, the animals were usually penned in sheds or barns.
Sampling and vector dynamics
Sampling and monitoring started in mid-February 2002, following the peak incidence of A. variegatum nymph attachment [26], and ended in mid-July 2002, the beginning of peak abundance of the adult instars. At the study sites, peak activity of Amblyomma nymphs occurs from November to January, which is followed by rapid decline or virtual disappearance of the tick population (both nymphs and adults) until May/June when adult ticks start to appear on animals [27], most frequently on cattle. Whole blood was collected with and without anticoagulant (EDTA) from each newborn animal on the day of birth or at the latest within 3–10 days of birth, and thereafter weekly and fortnightly for testing by pCS20 PCR and MAP1-B ELISA respectively. All newborn animals (0–10 days of age) were examined for ticks at the time of first sampling. Thereafter, selected animals (5 kids and 5 lambs at Keneba, 5 lambs at Kerr Seringe and all 16 neonates at Bansang) were examined weekly for tick infestation by examining the whole body. Post mortem examinations were carried out on all animals that died; and Giemsa-stained brain smears were examined for the presence of E. ruminantium inclusions in brain capillary endothelial cells [28]. The pCS20 PCR is the method of choice for detection of E. ruminantium [29] and analysis by nested pCS20 PCR assay as described below was carried out on the brains of animals, which showed hyperthermia and suspicious clinical symptoms characteristic of heartwater prior to death but were negative microscopically.
DNA extraction
Blood with EDTA was introduced into plain glass capillary tubes and centrifuged for 5 minutes at 14 000 × g to separate the buffycoat using Hawksley® HaematoSpin 1400. The tubes were broken and the buffycoats were applied to Whatman® filter paper #3 or #4 and allowed to dry at room temperature. DNA was extracted by the Modified Plowe extraction method [30]. DNA from brain tissues of dead animals was extracted using the protocol for purification of total DNA from animal tissues in DNeasy® Blood and Tissue Handbook (QIAGEN, Hilden Germany).
pCS20 PCR analysis
Previous experiments already showed the specificity of the pCS20 PCR assay for E. ruminantium using AB128 and AB129 primers [12,13]. A nested PCR was carried out as described previously [23]. Briefly, AB128 and AB129 primers [13] were used as internal primers. AB129 was also used as the external reverse primer, while ITM130 (5' TCAATTGCTTAATGAAGCACTAACTCAC 3') was used as the new external forward primer. PCR amplification was carried out in a 25 μl volume comprising 5 μl DNA sample, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.65 mM MgCl2, 400 pmol of each of the deoxynucleoside triphosphates, dATP, dCTP, dGTP and dTTP, 0.4 U of Taq polymerase, 40 pmol of each primer. After a denaturing step of DNA at 94°C for 3 min, the first round of amplification was carried out using the following conditions: 39 cycles of 30 sec denaturation at 94°C, 45 sec annealing at 62°C and 1 min elongation at 72°C and a final extension of 10 min at 72°C. Aliquots of 0.5 μl of PCR product from the first round amplification were transferred as template to a second round of PCR at 84°C (hot start principle). The second round consisted of 25 cycles of the same PCR conditions as in the first round except for the annealing temperature, which was set at 58°C. In each PCR run, positive and negative controls were included. Positive controls were derived from E. ruminantium (Senegal isolate) DNA obtained from cell culture-derived organisms and negative controls were reagent blank samples without DNA. The PCR amplified a 279 bp fragment of open reading frame 2 of the 1,306-bp pCS20 sequence. Amplicons were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light.
Indirect MAP1-B ELISA
Serum was separated by centrifugation from the blood samples collected without anticoagulant, and stored at -20°C until required. The E. ruminantium MAP1-B ELISA was carried out as described previously [17,31]. The assay has been shown to have high sensitivity for detection of E. ruminantium antibodies [32] particularly in small ruminants [18,20,31,33] with a specificity of 98.9% and 99.4% for caprine and ovine sera respectively [17,34]. Each serum sample was tested in duplicate. For sheep, each test included duplicate positive control sera obtained from a heartwater-naïve sheep of the Tesselaar breed experimentally infected with the Senegal isolate of E. ruminantium [35] at the Faculty of Veterinary Medicine, Utrecht, The Netherlands. Duplicate negative control sera were obtained from the same sheep prior to infection. Similar controls were obtained from a Saanen goat and included in duplicate for testing goat sera. Species-specific second step IgG antibodies conjugated with horseradish peroxidase (Nordic Immunology, Tilburg – The Netherlands) were used. Optical densities of the ELISA tests were measured using a Titertek Multiskan® ELISA reader (Titertek, Flow Laboratories Inc.) at 405 nm wavelength. For each plate, the cut-off value was calculated as two times the percentage positivity of the negative control serum relative to the positive control serum [14,17].
Statistical analysis
For analysis of data, the PCR and ELISA test results of the sample animals were grouped according to age: 0 – 10 days, 11 – 21 days, 22 – 49 days, 50 – 77 days, 78 – 98 days, 99 – 126 days, and 127 – 162 days. Correlation analysis was carried out using Spearman's rank correlation coefficient (rs) of Stata® statistical programme to assess the overall level of association between the pCS20 PCR and the MAP1-B ELISA in monitoring the kinetics of E. ruminantium infection in lambs and kids. Proportion of animals positive for E. ruminantium infection determined by both assays was deduced using the same statistical programme. Agreement between the two assays in detection of E. ruminantium infection in animals of the various age categories was assessed by method of determining the kappa statistic:
| kappa = (OP - EP)/(1 - EP), |
where OP = (a + d)/n; the observed proportion agreement between the two tests
| n = (a + b + c + d) |
EP = [{(a + b)/n} × {(a + c)/n}] + [{(c + d)/n} × {(b + d)/n}]; the expected proportion of agreement by chance
Kappa ranges from 1 (complete agreement) to 0 (agreement is equal to that expected by chance), whereas negative values indicate agreement less than is expected by chance. Benchmarks for interpreting kappa values were defined according to Everitt [36]:
>0.81: almost perfect agreement; 0.61 – 0.80: substantial agreement; 0.41 – 0.61:moderate agreement; 0.21 – 0.40: fair agreement; 0 – 0.20: slight agreement; 0: poor agreement
The method of Kaplan-Meier survival estimate was used for survival analysis.
Results
Tick counts
Results of total tick counts carried out weekly on animals at each of the three sample sites are shown in Table 1. The highest level of A. variegatum infestation was recorded in animals at Keneba, with a total of 40 ticks, consisting of 2 nymphs and 38 adult ticks, detected throughout the observation period. Lower levels of A. variegatum infestation were recorded at Kerr Seringe and Bansang, with 1 nymph and 2 female A. variegatum ticks detected respectively. Amongst the young lambs and kids that died of heartwater, A. variegatum ticks were recorded on 1 animal (#1317) at Kerr Keringe and 3 animals, #4333, #4338 and #4340, at Keneba (Table 2 and 3). Generally, tick infestations occurred at the end of the observation period in the early rainy season (mid-June and July); and infestation was first detected on animals at week 16 after birth.
Table 1.
Weekly A. variegatum tick counts on lambs and kids examined at the three sample sites in The Gambia
| Week of observation | Total no. of A. variegatum ticks counted on animals at | ||
| Kerr Seringe (5 lambs) | Keneba (5 lambs, 5 kids) | Bansang (5 lambs, 11 kids) | |
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 16 | 0* | 1N, 1M | 0 |
| 17 | 0 | 0 | 0 |
| 18 | 1N | 7M, 4F | 2F** |
| 19 | 0 | 4M | 0 |
| 20 | 0 | 14M, 4F | 0 |
| 21 | 0 | 0 | 0 |
| 22 | 0 | 0 | 0 |
| 23 | 0 | 1M, 1F | 0 |
* 2 Hyalomma sp. adults and 1 Rhipicephalus sp. adult also recorded
** 1 Hyalomma sp. adult also recorded
Key: N = nymph, M = adult male, F = adult female
Table 2.
Periods of detection of E. ruminantium DNA and antibodies in lambs at various age levels sampled in Kerr Seringe
| Animal No. | 1Sp. | 2Period DNA detected (PCR) | Period antibodies detected (MAP1-B) | Status | 5AV | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Survived | |||
| 1301 | L | - | - | - | - | + | nd | + | + | + | + | - | - | - | - | Survived | 0 |
| 1302 | L | - | - | - | - | + | nd | + | + | + | + | - | - | - | - | Survived | |
| 1303 | L | - | - | + | - | + | nd | - | + | + | + | - | - | - | - | Survived | 0 |
| 1304 | L | - | - | - | - | - | nd | - | + | + | - | - | - | - | - | Survived | |
| 1305 | L | - | - | + | + | - | nd | + | + | + | + | - | - | - | - | Survived | |
| 1308 | L | - | - | - | + | + | nd | - | + | + | + | + | - | - | - | Survived | |
| 1309 | L | - | - | + | + | + | nd | - | + | + | + | - | - | - | - | Survived | |
| 1311 | L | - | - | + | - | - | nd | - | + | + | + | + | + | + | + | Survived | |
| 1312 | L | - | - | - | - | - | nd | - | + | + | + | + | - | - | - | Survived | |
| 1313 | L | - | - | + | - | + | nd | - | + | + | + | - | - | - | - | Survived | |
| 1314 | L | - | - | - | - | - | nd | - | + | - | - | - | - | - | - | Survived | 0 |
| 1315 | L | - | + | - | - | - | nd | - | + | nd | + | + | - | - | - | Survived | |
| 1316 | L | - | - | - | - | - | nd | + | + | - | - | - | - | - | - | Survived | |
| 1318 | L | - | - | - | nd | + | nd | + | + | + | + | - | - | - | - | Survived | |
| 1319 | L | - | - | - | - | - | nd | - | + | + | + | + | - | - | - | Survived | 0 |
| 1320 | L | - | - | + | + | + | nd | + | + | nd | + | + | - | - | - | Survived | |
| 1322 | L | - | - | - | nd | + | nd | - | + | + | + | + | - | - | - | Survived | |
| 1323 | L | - | - | - | nd | + | nd | - | + | + | + | + | + | + | - | Survived | |
| 1325 | L | - | - | - | nd | - | nd | - | - | - | - | - | - | - | - | Survived | |
| 1327 | L | - | - | - | - | - | nd | - | + | + | + | + | - | - | - | Survived | |
| 1328 | L | - | - | - | nd | - | nd | + | + | + | + | + | - | - | - | Survived | |
| 1329 | L | - | - | - | nd | - | nd | - | - | - | - | + | - | - | - | Survived | |
| 1330 | L | - | - | - | - | nd | - | nd | + | + | + | + | - | - | - | Survived | |
| 1331 | L | - | - | - | - | nd | - | nd | + | + | + | + | - | - | nd | Survived | |
| 13336 | L | nd | + | + | + | 3D(28) 4hw-G | |||||||||||
| 1332 | L | - | - | - | + | + | + | D(42*) | |||||||||
| 1321 | L | - | - | - | + | + | + | D(42*) | |||||||||
| 1306 | L | - | - | - | - | + | + | - | - | D(77*) | |||||||
| 1317 | L | - | - | - | nd | + | + | + | + | D(84) hw-G | 1 N | ||||||
Key: 1Sp. = Species; L = lamb; K = kid; 2Age range per level: 1 (0 – 10 days), 2 (11–21 days), 3 (22 – 49 days), 4 (50 – 77 days), 5 (78 – 98 days), 6 (99 – 126), 7 (127 – 162); 3D = died (numbers in parentheses indicate day of death); 4hw-G heartwater confirmed by Giemsa; hw-PCR: heartwater confirmed by pCS20 PCR;
5AV: A. variegatum tick counts on selected study animals; N = nymph; A = adult; *mortality due to coccidial and/or clostridial enteritis; 6Animal numbers in bold are cases of mortality
Table 3.
Periods of detection of E. ruminantium DNA and antibodies in all lambs and kids at various age levels sampled in Keneba
| Animal No. | Sp. | Week DNA detected (PCR) | Week antibodies detected (MAP1-B) | Status | AV | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| 4308 | K | - | - | - | - | - | nd | + | + | + | + | - | - | - | - | Survived | 1 N |
| 4309 | K | + | - | - | - | - | nd | + | nd | - | - | - | - | - | - | Survived | |
| 4312 | K | + | - | + | - | - | nd | + | + | + | + | + | - | + | - | Survived | 5A |
| 4314 | K | - | - | + | - | - | nd | - | + | + | + | - | - | - | - | Survived | |
| 4315 | K | - | + | + | - | - | nd | + | + | + | + | - | - | + | - | Survived | |
| 4318 | K | + | - | + | - | - | - | - | + | + | - | - | - | - | - | Survived | |
| 4319 | K | + | - | + | - | - | + | - | + | + | - | - | - | - | - | Survived | |
| 4325 | K | + | - | + | + | + | + | + | + | + | - | - | - | - | - | Survived | |
| 4331 | K | - | nd | - | - | - | - | - | + | - | - | - | - | - | - | Survived | |
| 4332 | K | - | nd | + | - | - | - | - | + | + | + | + | - | - | - | Survived | |
| 4335 | K | - | - | - | + | - | + | - | nd | - | + | - | - | - | - | Survived | |
| 4336 | K | - | - | + | + | + | + | - | + | + | - | - | - | - | - | Survived | |
| 4372 | K | nd | - | - | + | + | - | - | nd | + | + | - | + | + | + | Survived | 6A |
| 4376 | K | - | nd | + | - | - | - | - | + | + | + | + | + | + | - | Survived | |
| 4377 | K | - | nd | - | - | - | nd | - | + | + | + | - | - | - | - | Survived | |
| 4378 | K | - | nd | - | - | + | nd | - | + | + | + | + | - | - | - | Survived | |
| 4306 | L | nd | - | + | - | nd | + | + | nd | + | + | + | + | - | + | Survived | 8A |
| 4311 | L | - | - | - | - | - | nd | + | + | + | + | + | - | - | + | Survived | |
| 4316 | L | + | - | + | - | + | nd | + | + | + | - | + | - | - | + | Survived | |
| 4321 | L | + | + | + | + | + | + | - | + | + | + | + | - | + | - | Survived | 7A |
| 4323 | L | + | - | + | + | + | + | - | + | - | - | + | - | - | + | Survived | |
| 4324 | L | + | - | nd | + | + | - | + | + | + | + | + | + | - | - | Survived | |
| 4326 | L | - | nd | - | - | - | - | - | nd | + | - | + | + | - | - | Survived | |
| 4337 | L | + | - | - | - | + | + | - | + | + | + | - | - | + | - | Survived | 4A |
| 4339 | L | + | - | - | - | - | - | D(42) hw-G | |||||||||
| 4375 | K | - | - | - | + | + | + | D (42*) | |||||||||
| 4333 | L | + | + | + | + | - | + | D(56) hw-G | 1 N, 3A | ||||||||
| 4379 | K | - | nd | - | - | + | nd | - | - | day 56* | |||||||
| 4340 | K | - | - | - | + | - | - | - | - | D(63) hw-PCR | 2A | ||||||
| 4338 | L | - | - | + | - | + | + | + | + | D(63) hw-G | 3A | ||||||
| 4320 | K | - | - | - | + | nd | + | + | - | D(91) hw-PCR | |||||||
| 4334 | L | - | - | - | - | + | + | + | + | + | + | D(112) hw-PCR | |||||
| 4305 | L | nd | - | - | - | nd | + | nd | + | + | + | + | - | D(147) hw-G | |||
| 4313 | kid | + | - | - | - | - | + | + | + | + | - | - | - | - | - | day 147* | |
pCS20 PCR and indirect MAP1-B ELISA
The pCS20 PCR assay detected E. ruminantium DNA in 57 out of the 77 animals at least once during the study period. Fifteen animals (excluding those that died), 9 in Kerr Seringe, 3 in Keneba and 3 in Bansang, remained negative by this test throughout the study (Table 2, 3 and 4). Data for a number of animals at different age levels were unavailable. Batches of some buffycoat extracts on filter paper (for DNA) stored in the freezer were soaked with water due to a freezer failure rendering some samples unsuitable for further analysis; the batch of samples of level 6 in Kerr Seringe was particularly affected (Table 2). Absence of serological data for some animals at different time periods was mainly due to erasure of ink labels on the serum cryotubes during handling and storage making them unidentifiable. Such samples were excluded from further analysis. The highest number of PCR-positive animals was observed at 78 to 98 days of age. Of the 9 animals (6 kids and 4 lambs) that were sampled immediately after birth (0–3 days) at Keneba, 6 tested positive by PCR; at Bansang, 1 animal was positive, whereas none tested positive at Kerr Kerr Seringe (Table 5). Nineteen per cent of the animals sampled after birth (from day 0 to 10 days), tested positive by PCR (Table 6). The serological assay detected the highest proportion of positive small ruminants (90%) at week 1 of age (0 – 10 days). A decline in the number of serologically positive animals was observed with increasing age (Table 6). The performance of the pCS20 PCR and the MAP1-B ELISA tests in detecting E. ruminantium in lambs and kids of the different age categories are outlined in Tables 2, 3, 4 and 5. All 7 neonates, between 0 and 3 days age range detected positive by PCR, were correspondingly serologically positive (Table 5). Four of these animals (4321, 4323, 4324 and 338) remained intermittently PCR and MAP1-B positive in subsequent weeks (Table 3 and 4) suggesting persistence of infection. E. ruminantium antibodies could not be detected in three PCR-positive neonates (4318, 4319 and 4325) after the first two weeks until the end of the study (Table 3). Assessment of agreement between the two assays by method of determining kappa statistic is shown in Table 7. The kappa coefficient ranged from -0.091, indicating agreement less than is expected by chance, to 0.223, suggesting fair agreement, between the two assays (Table 7). Global comparison of the results by Spearman's rank test showed that the two assays did not always agree (rs = -0.1512; P = 0.003) and data (Table 2, 3, 4 and Table 6) showed that the pCS20 PCR detected higher E. ruminantium infection rates with increasing age in field-exposed small ruminants. For example, a number of animals (#1301, #1302, #1316, #1318, #4325, #2317 and #2318) that tested PCR-positive at 99 to 162 days of age were correspondingly (the same age interval) negative by MAP1-B ELISA. The serology results showed that most neonates tested between 0–3 days of age carried antibodies to MAP1-B antigen of E. ruminantium (Table 5).
Table 4.
Periods of detection of E. ruminantium DNA and antibodies in all lambs and kids at various age levels sampled in Bansang
| Animal No. | Sp. | Week DNA detected (PCR) | Week antibodies detected (MAP1-B) | Status | AV | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| 330 | K | - | - | - | - | + | - | + | - | - | - | nd | + | - | - | Survived | 0 |
| 336 | K | - | - | + | - | + | - | + | nd | + | + | + | nd | + | - | Survived | 0 |
| 338 | K | + | - | - | - | + | + | + | nd | + | - | + | nd | + | - | Survived | 0 |
| 374 | K | - | - | - | - | + | nd | + | + | + | + | - | + | - | - | Survived | 0 |
| 2313 | K | - | - | - | - | + | + | - | nd | nd | nd | nd | nd | - | + | Survived | 0 |
| 2314 | K | - | - | - | - | + | + | + | + | + | + | + | nd | - | - | Survived | 0 |
| 2315 | K | - | - | - | + | + | + | - | nd | nd | nd | nd | nd | + | - | Survived | 0 |
| 2316 | K | - | + | - | + | - | nd | - | nd | nd | nd | - | - | - | - | Survived | 1A |
| 2317 | K | - | - | - | - | nd | + | + | nd | nd | nd | + | - | - | - | Survived | 0 |
| 2318 | K | - | - | - | + | nd | + | + | nd | nd | nd | + | - | - | - | Survived | 0 |
| 2351 | L | - | - | - | - | - | - | - | + | + | + | + | - | - | - | Survived | 0 |
| 2352 | L | - | - | - | - | - | - | - | + | - | + | - | - | - | nd | Survived | 1 A |
| 2353 | L | - | nd | - | - | - | - | nd | - | - | - | nd | - | - | - | Survived | 0 |
| 2354 | L | - | - | - | - | + | + | nd | - | - | - | + | + | - | - | Survived | 0 |
Table 5.
Outcome of pCS20 PCR and MAP1-B ELISA tests on samples collected from animals at 0 – 3 days after birth
| Anim. No. | Species | Site | Day of first sample | PCR | MAPI-B |
| 305 | lamb | Kerr Seringe | 1 | - | + |
| 1306* | lamb | Kerr Seringe | 1 | - | + |
| 1313 | lamb | Kerr Seringe | 0 | - | + |
| 1315 | lamb | Kerr Seringe | 3 | - | + |
| 1318 | lamb | Kerr Seringe | 2 | - | + |
| 4318 | kid | Keneba | 0 | + | + |
| 4319 | kid | Keneba | 0 | + | + |
| 4325 | kid | Keneba | 3 | + | + |
| 4331 | kid | Keneba | 2 | - | + |
| 4332 | kid | Keneba | 1 | - | + |
| 4335 | kid | Keneba | 2 | - | nd |
| 4321 | lamb | Keneba | 0 | + | + |
| 4323 | lamb | Keneba | 3 | + | + |
| 4324 | lamb | Keneba | 1 | + | + |
| 330 | kids | Bansang | 3 | - | - |
| 336 | kids | Bansang | 3 | - | nd |
| 338 | kids | Bansang | 1 | + | nd |
| 374 | kids | Bansang | 0 | - | + |
| 2313 | kids | Bansang | 3 | - | nd |
| 2314 | kids | Bansang | 3 | - | + |
| 2315 | kids | Bansang | 0 | - | nd |
| 2316 | kids | Bansang | 3 | - | nd |
| 2317 | kids | Bansang | 1 | - | nd |
| 2318 | kids | Bansang | 3 | - | nd |
| 2351 | lamb | Bansang | 2 | - | + |
| 2352 | lamb | Bansang | 1 | - | + |
| 2353 | lamb | Bansang | 3 | - | - |
| 2354 | lamb | Bansang | 3 | - | - |
*the only mortality (occurred on day 77) among the group and was due to clostridial enteritis
Table 6.
Prevalence of E. ruminantium DNA and antibodies in lambs and kids according to age as determined by pCS20 PCR and MAP1-B ELISA
| Age group | pCS20 PCR percent % (n) | MAP1-B ELISA percent % (n) | ||||
| Lambs | Kids | Overall | Lambs | Kids | Overall | |
| 0 – 10 days | 16.3 (43) | 23.3 (30) | 19.2 (73) | 88.3 (43) | 90.0 (20) | 90.0 (63) |
| 11 – 21 days | 8.9 (45) | 8.3 (24) | 8.6 (69) | 77.3 (44) | 80.0 (25) | 78.3 (69) |
| 22 – 49 days | 27.3 (44) | 32.3 (31) | 29.3 (75) | 75.6 (45) | 50.0 (26) | 66.2 (71) |
| 50 – 77 days | 20.6 (34) | 30.0 (30) | 25.0 (64) | 65.0 (40) | 33.3 (27) | 52.2 (67) |
| 78 – 98 days | 48.6 (35) | 40.0 (25) | 45.0 (60) | 18.4 (38) | 22.7 (22) | 20.0 (60) |
| 99 – 126 days | 42.9 (14) | 64.7 (17) | 54.8 (31) | 10.8 (37) | 25.9 (27) | 17.2 (64) |
| 127 – 162 days | 34.3 (32) | 48.1 (27) | 40.8 (59) | 14.7 (34) | 7.4 (27) | 11.5 (61) |
Number of samples tested per age group in parentheses
Table 7.
Number of lambs and kids tested for E. ruminantium (ER) infection by pCS20 PCR and MAP1-B ELISA at the various age levels and the values of kappa statistic
| Age Group | pCS20 PCR | MAP1-B ELISA | Kappa | |
| ER positive | ER negative | |||
| 0 – 10 days | ER positive | 11(a) | 1(b) | 0.015 |
| ER negative | 44(c) | 6(d) | ||
| 11 – 21 days | ER positive | 3 | 1 | -0.015 |
| ER negative | 46 | 8 | ||
| 22 – 49 days | ER positive | 17 | 6 | 0.068 |
| ER negative | 30 | 16 | ||
| 50 – 77 days | ER positive | 6 | 8 | -0.019 |
| ER negative | 24 | 19 | ||
| 78 – 98 days | ER positive | 7 | 16 | 0.223 |
| ER negative | 3 | 28 | ||
| 99 – 126 days | ER positive | 4 | 12 | 0.035 |
| ER negative | 3 | 11 | ||
| 127 – 162 days | ER positive | 3 | 21 | 0.009 |
| ER negative | 4 | 30 | ||
Mortality
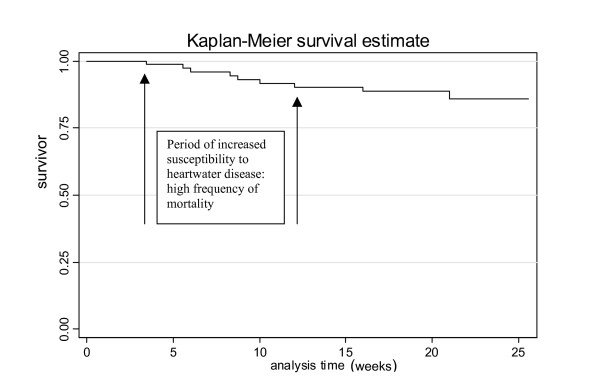
Fifteen animals (10 lambs and 5 kids) died during the course of the study, between 28 and 147 days (Table 2, 3 and 4), representing a crude mortality rate of 19.5% (15/77); nine deaths (Figure 1; Table 2, 3 and 4) were confirmed heartwater-associated by detection of E. ruminantium in brain-crushed smears (6 deaths) or detection of E. ruminantium-specific pCS20 sequences in brain tissue DNA extracts (3 deaths). The 3 deaths confirmed by PCR occurred in Keneba and the lack of Giemsa-stained brain-positive results was attributed to poor storage of the brains prior to shipment to the laboratory for analysis. The three animals, however, showed clinical symptoms (hyperthermia, nervous symptoms) prior to death, and clinical pathological changes (hydropericardium, hydrothorax) at post mortem, which although not pathognomonic but are characteristic for heartwater. Overall case mortality rate for the 3 sites was 11.7% (9/77). At Kerr Seringe, the crude mortality rate was 17.2% (5/29) and case (heartwater) mortality rate was 6.9% (2/29); Keneba showed a crude mortality rate of 29.4% (10/34) and a higher case mortality rate of 20.6% (7/34), which could be associated with the high tick attack rate recorded at this site; in contrast to Bansang, no mortality occurred amongst the animals during the study period (Table 2, 3 and 4). Six animals were negative for E. ruminantium, and death was attributed mainly to coccidial or clostridial enteritis. Of the 9 animals which died of heartwater, 6 were previously positive for E. ruminantium by both PCR and MAP1-B ELISA. Five of the 9 animals that died of heartwater (#4305, #4334, #4338, #4320 and #4340) showed a first positive PCR test after 21 days of age (Table 2, 3 and 4) outside the brief period of inverse age-related resistance. Survival analysis (Figure 1) showed the frequency of mortality due to heartwater among extensively managed small ruminants to be higher in animals aged between 4 and 12 weeks.
Figure 1.
Heartwater mortality data: Kaplan-Meier survival estimate.
Discussion
The present study used pCS20 PCR to monitor the onset (age at first infection) and kinetics of E. ruminantium infection in neonatal lambs and kids maintained under a traditional husbandry system in three major locations of livestock production in The Gambia where heartwater is known to occur. It also compared the performance of the pCS20 PCR and indirect MAP1-B ELISA in detecting E. ruminantium infections in small ruminants. The prevalence of infection detected by pCS20 PCR varied between 8.6% and 54.8% over the 162-day study period. Nineteen per cent (14/73) of the animals in week 1 post-partum (0–10 days of age) were positive by pCS20 PCR. Half of these infections (7/14) were detected in the first 3 days of life (Table 5), suggesting that transmission other than by tick feeding had played a role. In this study, the earliest detectable A. variegatum infestation occurred in week 16 (Table 1). A plausible explanation of this finding is that vertical transmission of E. ruminantium from the dam to the offspring could possibly be occurring in small ruminants maintained under the traditional husbandry system. Vertical transmission was demonstrated to occur in cattle in Zimbabwe under natural field conditions [9], although the mode of transmission, either in utero or with colostrum, remains to be elucidated. Many tick-borne pathogens related to E. ruminantium, such as Anaplasma (Ehrlichia) phagocytophilum [37], E. risticii [38], Anaplasma spp. [39] and Coxiella burnetti [40] can be transmitted in utero. Interestingly, in our study, most of the neonatal infections detected by pCS20 PCR occurred in animals of 0 to 3 days of age and were located in Keneba (67%, 6/9; Table 5) where there is a comparatively high A. variegatum tick abundance [23] and minimal or no tick control was practised. The traditional management system of small ruminants in The Gambia substantially diminishes the likelihood of tick infestation of neonates at or immediately after birth. Parturitions at Keneba and Kerr Seringe occurred in pens, which were regularly cleaned; and at Keneba all births happened precisely between 11 March and 8 April, coinciding with the period of significant decline in Amblyomma nymphs (or near complete disappearance) and least activity of Amblyomma ticks [27]. Furthermore, the period required for transmission of E. ruminantium to occur after attachment of an infected tick to a susceptible host is estimated between 27 and 38 hours for nymphs and between 51 and 75 hours for adults [41], and it is highly unlikely that E. ruminantium transmitted to neonates at birth through tick bite could appear in the blood stream on the very day of or the following day after birth and detected by PCR. Against this background, it is postulated that vertical transmission of E. ruminantium possibly occurs in traditionally managed small ruminants and there is increased likelihood of this occurrence under conditions of medium to high tick challenge in the absence of, or with minimal, tick control. Interestingly, based on the number of PCR-positives, the phenomenon appeared to be more evident in Keneba (Table 5), a site located in the western part of The Gambia characterized by comparatively high rates of E. ruminantium tick infection [23] and tick attachment [22]. Bansang, which is in the eastern part of the country with low rates of tick infection and tick attachment [22,23], showed only 1 positive case. At Kerr Seringe, none of the neonates tested positive (Table 5). The latter site is also located in the western part of the country characterized by high rates of tick infection and tick attachment; and the absence of positive tests in the neonates was attributed their dams being subjected to regular treatment with acaricide as described above. Intensive use of acarides in indigenous livestock results in disruption of endemic stability to tick-borne diseases through disruption of infection by tick transmission [42-45]. It was highly likely that regular acaricide treatment of dams at Kerr Seringe resulted in significant reduction of infection intensity that was required for transmission of infection from dam to offspring to occur. Alternatively, the pCS20 PCR could be detecting E. ruminantium of low pathogenicity or an as yet uncharacterized related organism in these animals, which does not cause disease [46] and is transmitted in utero, neonatally, or by some other route. It should be mentioned that the present study was not designed to demonstrate the occurrence of vertical transmission of E. ruminantium in traditionally managed small ruminants but the findings herein strongly suggested plausibility of the phenomenon and should stimulate further investigation and confirmation through tick feeding experiments. Additionally, it was observed that a significant number of animals tested negative in day-11 to 21-age range by PCR and later became positive in day 22 to 49, with some animals demonstrating intermittent positivity throughout the study period suggesting that the level of E. ruminantium rickettsaemia in the peripheral blood fluctuated and sometimes it was not possible to detect infection [29].
Presuming that heartwater infections were the cause of the sero-positive reactions, prevalence of E. ruminantium infection detected by MAP1-B ELISA also varied, between 11.5% and 90%. However, serological assays, generally, unlike PCR, do not show infection status of an animal and only provide information about previous exposure to infection. In contrast to the pCS20 PCR, the serological assay detected the highest proportion of positive animals in the first week of age (0 – 10 days). This was followed by a gradual decline in seropositivity over the 162-day study period with increasing age (Table 2, 3, 4 and Table 6), suggesting widespread presence of maternal antibodies in neonatal lambs and kids born to immune (since they were raised in tick-infested areas exposed to continuous tick challenge) dams maintained under the traditional husbandry system. A similar decline in antibody levels detected by a different serological test was reported over the first 2–3 months of life in traditionally managed calves, lambs and kids in Ghana [47]. The MAP1-B ELISA has high sensitivity for ovine and caprine sera [17,18,20] but is less sensitive for bovine sera, especially in cattle subsequent to the first seroconversion [14,33,47]. However, in the present study, some animals that tested positive by both MAP1-B ELISA and pCS20 PCR later became intermittently negative by MAP1-B ELISA. This suggests the possibility of upregulatory-downregulatory effect on the production of antibodies as reported to occur in cattle during persistent infection [14]. However, persistently high antibody levels have been reported in sheep following immunization [31,48], recovery from experimental heartwater [7], and natural field exposure [21,47]. Thus another possible explanation might be that, if vertical transmission is occurring in small ruminants, the resulting infection in the offspring is atypical in some way and does not result in the persistently high antibody levels seen after experimental or tick-transmitted infection. Immunotolerance to the infectious agent, reported in cattle following pestivirus infection (Bovine Viral Diarrhoea), develops when the virus invades the foetus before development of immune competence resulting in a lifetime of persistence of infection [49]. In this case, the agent is persistently present in the blood without detectable antibodies, which phenomenon, with respect to E. ruminantium infection, could be possibly occurring in some extensively managed small ruminants as well; and it could be a mechanism by which vertically E. ruminantium-infected offspring survive/tolerate the infection. This requires further investigation. Alternatively, the possibility of vertical transmission resulting in time, in mortality, in immunosuppressed animals due to starvation, haemonchosis caused by Haemonchus contortus [50] or trypanosomosis [51-53], all of which are common in small ruminants under the traditional husbandry system, should not be entirely discounted. Indeed, mortality due to confirmed cases of heartwater has been observed in sheep after 3–4 weeks of quarantine in tick/insect proof stables prior to use in heartwater vaccination experiments at ITC. These sheep tested E. ruminantium-positive by nested pCS20 PCR described above suggesting a carrier status which in time, resulted in mortality possibly due to stress factors such as confinement with inadequate nutrition (authors' unpublished information). Furthermore, in this study, results of the pCS20 PCR and MAP1-B ELISA did not always agree (rs = -0.1512; P = 0.003); similarly the kappa statistic determined for the various age categories (Table 7) indicated little agreement between the two assays with agreement ranging between less than is expected to fair, which can be anticipated as both tests target different bio-molecules. For instance, two animals (#2351 and #2352) remained negative throughout the study by pCS20 PCR but tested positive by indirect MAP1-B ELISA; whereas from day 50 – 77 onwards, a number of animals that tested negative by MAP1-B ELISA were positive by pCS20 PCR. On the other hand, a number of animals that tested negative by pCS20 PCR were several times positive by MAP1-B ELISA (Table 2, 3 and 4), which seems to indicate the presence and persistence of maternal antibodies in those animals [47] or that the assay may be detecting antibodies to an uncharacterized closely related ehrlichial organism[19].
This study demonstrated that mortality due to heartwater occurs in young indigenous lambs and kids under a traditional husbandry system in The Gambia as early as 4 weeks after birth. The frequency of mortality was highest in the animals aged between 4 and 12 weeks (Figure 1) suggesting a period of increased risk of/susceptibility to heartwater disease for these animals in this age group. This time interval appears to coincide with the period when the inverse age-related resistance in the newborn has waned. Furthermore, nearly all confirmed cases of heartwater mortality occurred in animals, which tested positive by PCR at 3 weeks of age, or older outside the brief period of inverse age-related resistance suggesting reduced resistance in these animals to any possible latent or new infection. On the other hand, the occurrence of heartwater-associated mortalities in animals prior to week 16, when ticks were first detected, could not be adequately explained. It is postulated that the deaths (8 out of 9) most likely resulted from vertical transmission itself due to possible immunosuppression in the affected animals, or highly unlikely though, that there must have been ticks infesting these animals prior to week 16 which were not detected, and which could have resulted in the positive pCS20 results.
Conclusion
The findings of this study suggest that both the vector and vertical transmission may play a vital role in the epidemiology of heartwater supporting the view of previous studies [1,9]. Although this would require further investigation, the data presented here, coupled with the traditional system of management of neonatal lambs, kids and their dams in The Gambia seem to support the conclusion that vertical transmission may be crucial in the initial establishment of endemic stability to heartwater in indigenous young sheep and goat population exposed to continuous field tick challenge. The study also supports earlier reports that single-occasion testing of a field-exposed animal may not confirm its actual infection status [29] and the use of pCS20 PCR supported by transmission studies and clinical data would help provide more accurate information on the epidemiology of heartwater (E. ruminantium infection) in endemic areas. Additionally, it showed the age range of increased susceptibility to heartwater in traditionally reared small ruminants which information could enhance better targeting of disease control measures especially through vaccination.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
BF performed the serology and PCR tests; participated in the coordination and management of the study; contributed to the conception, design, and acquisition of data; did the analysis of data and wrote the manuscript. DG contributed to the conception and design of the study and critical revision of the manuscript; participated in the management of study. SM contributed to the design, coordination and acquisition of data. LBS contributed to interpretation of data and revising the manuscript. FJ contributed to the design and management of the study and revising the manuscript critically for intellectual content. All authors gave their final approval of the version to be published.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This work was supported by The European Development Fund, contract no. REG/606/1006. We acknowledge the support of Utrecht Scholarship Programme and the ICTTD-2 concerted action project through the INCO-DEV program of the European Commission under contract no. ICA4T-2000-30006. We thank Dr. Cornelis Bekker for his useful comments on the manuscript. The support of Ansumana Ceesay, Saja Kora, Nuha Bojang, Dr. M. Mbake and the late Baba Drammeh is acknowledged. Bonto Faburay holds a Rothamsted International African Fellowship Award.
Contributor Information
Bonto Faburay, Email: b.fabureh1@vet.uu.nl.
Dirk Geysen, Email: dgeysen@itg.be.
Susanne Munstermann, Email: smunster@freenet.de.
Lesley Bell-Sakyi, Email: lsakyi@staffmail.ed.ac.uk.
Frans Jongejan, Email: F.Jongejan@vet.uu.nl.
References
- O´Callaghan CJ, G.F. Medley. T.F. Perry. B.D. Perry Investigating the epidemiology of heartwater (Cowdria ruminantium infection) by means of a transmission dynamics model. Parasitology. 1998;117 :49–61. doi: 10.1017/S0031182098002790. [DOI] [PubMed] [Google Scholar]
- Norval RAI, Perry BD, Young AS. Epidemiology of Theileriosis in Africa. Academic Press. London , Academic Press; 1992. [Google Scholar]
- Perry BD, A.S. Young The past and future roles of epidemiology and economics in the control of tick-borne diseases of livestock in Africa: The case of theileriosis. Prev Vet Med. 1995;25 :107–120. doi: 10.1016/0167-5877(95)00546-3. [DOI] [Google Scholar]
- Uilenberg G. Present and future possibilities for the control of cowdriosis and anaplasmosis. Vet Q. 1990;12:39–45. doi: 10.1080/01652176.1990.9694240. [DOI] [PubMed] [Google Scholar]
- Du Plessis JL, Malan L. The non-specific resistance of cattle to heartwater. Onderstepoort J Vet Res. 1987;54 :333–336. [PubMed] [Google Scholar]
- Neitz WO, Alexander RA. The immunization of calves against heartwater. J S Afric Vet Med Assoc. 1941;12 :103–111. [Google Scholar]
- Uilenberg G. Etudes sur la cowdriose a Madagascar. Premiere partie. Rev Elev Méd Vét Pays Trop. 1971;24 :239–249. [PubMed] [Google Scholar]
- Camus E, Barre N. Epidemiology of heartwater in Guadeloupe and in the Caribbean. Onderstepoort J Vet Res. 1987;54 :419–426. [PubMed] [Google Scholar]
- Deem SL, R.A.I. Norval. P.L. Donachie. S.M. Mahan Demonstration of vertical transmission of Cowdria ruminantium, the causative agent of heartwater from cows to their calves. Vet Parasitol. 1996;61:119–132. doi: 10.1016/0304-4017(95)00819-5. [DOI] [PubMed] [Google Scholar]
- Norval RAI, Donachie PL, Meltzer MI, Deem SL, Mahan SM. The relationship between tick (Amblyomma hebraeum) infestation and immunity to heartwater (Cowdria ruminantium infection) in calves in Zimbabwe. Vet Parasitol. 1995;58 :335–352. doi: 10.1016/0304-4017(94)00733-S. [DOI] [PubMed] [Google Scholar]
- Waghela SD, Rurangirwa FR, Mahan SM, Yunker CE, Crawford TB, Barbet AF, Burridge MJ, McGuire TC. A cloned DNA probe identifies Cowdria ruminantium in Amblyomma variegatum ticks. J Clin Microbiol. 1991;29:2571–2577. doi: 10.1128/jcm.29.11.2571-2577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan SM, Waghela SD, McGuire TC, Rurangirwa FR, Wassink LA, Barbet AF. A cloned DNA probe for Cowdria ruminantium hybridised with eight heartwater strains and detects infected sheep. J Clin Microbiol. 1992;30 :981–986. doi: 10.1128/jcm.30.4.981-986.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter TF, Deem SL, Barbet AF, Norval RAI, Simbi BH, Kelly PJ, Mahan SM. Development and evaluation of PCR assay for detection of low levels of Cowdria ruminantium infection in Amblyomma ticks not detected by DNA probe. J Clin Microbiol. 1995;33 :166–172. doi: 10.1128/jcm.33.1.166-172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semu SM, T.F. Peter. D. Mukwedeya. A.F. Barbet. F. Jongejan. S.M. Mahan Antibody responses to MAP 1B and other Cowdria ruminantium antigens are down regulated in cattle challenged with tick-transmitted heartwater. Clin Diagn Lab Immunol. 2001;8 :388–396. doi: 10.1128/CDLI.8.2.388-396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis JL, Bezuidenhout JD, Brett MS, Camus E, Jongejan F, Mahan SM, Martinez D. The sero-diagnosis of heartwater: a comparison of five tests. Rev Elev Méd vét Pays trop. 1993;46 :123–129. [PubMed] [Google Scholar]
- Mahan SM, Tebele N, Mukwedeya D, Semu S, Nyathi CB, Wassink LA, Kelly PJ, Peter T, Barbet AF. An immunoblotting assay for heartwater based on the immunodominant 32-kilodalton protein of Cowdria ruminantium detects false positives in field sera. J Clin Microbiol. 1993;31 :2729–2737. doi: 10.1128/jcm.31.10.2729-2737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet AHM, Van Der Zeijst BAM, Camus E, Mahan SM, Martinez D, Jongejan F. Use of a specific immunogenic region on the Cowdria ruminantium MAP1 protein in a serological assay. J Clin Microbiol. 1995;33 :2405–2410. doi: 10.1128/jcm.33.9.2405-2410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan SM, Semu S, Peter T, Jongejan F. Evaluation of MAP1-B ELISA for cowdriosis with field sera from livestock in Zimbabwe. Ann N Y Acad Sci. 1998;849 :259–261. doi: 10.1111/j.1749-6632.1998.tb11057.x. [DOI] [PubMed] [Google Scholar]
- Kakono O, Hove T, Geysen D, Mahan S. Detection of antibodies to the Ehrlichia ruminantium MAP1-B antigen in goat sera from communal land areas of Zimbabwe by an indirect enzyme-linked immunosorbent assay. Onderstepoort J Vet Res. 2003;70 :243–249. [PubMed] [Google Scholar]
- De Waal DT, Matthee O, Jongejan F. Evaluation of the MAP1b ELISA for the diagnosis of heartwater in South Africa. Ann N Y Acad Sci. 2000;916:622–627. doi: 10.1111/j.1749-6632.2000.tb05348.x. [DOI] [PubMed] [Google Scholar]
- Bell-Sakyi L, Koney EBM, Dogbey O, Sumption KJ, Walker AR, Bath A, Jongejan F. Detection by two enzyme-linked immunosorbent assays of antibodies to Ehrlichia ruminantium in field sera collected from sheep and cattle in Ghana. Clin Diagn Lab Immunol. 2003;10 :917–925. doi: 10.1128/CDLI.10.5.917-925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faburay B, Munstermann S, Geysen D, Bell-Sakyi L, Ceesay A, Bodaan C, Jongejan F. Point seroprevalence survey of Ehrlichia ruminantium infection in small ruminants in The Gambia. Clin Diagn Lab Immunol. 2005;12 :508–512. doi: 10.1128/CDLI.12.4.508-512.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faburay B, Geysen D, Munstermann S, Taoufik A, Postigo M, Jongejan F. Molecular detection of Ehrlichia ruminantium infection in Amblyomma variegatum ticks in The Gambia. Exp Appl Acarol. 2007 doi: 10.1007/s10493-007-9073-2. [DOI] [PubMed] [Google Scholar]
- Faburay B, Munstermann S, Geysen D, Jongejan F. A contribution to the epidemiology of Ehrlichia ruminantium infection (Heartwater) in small ruminants in The Gambia. Animal Health Research Working Paper No. 4, ITC (2004), pp.36. 2004.
- Jaitner J, Sowe J, Secka-Njie E, Dempfle L. Ownership pattern and management practices of small ruminants in The Gambia - implications for a breeding programme. Small Rum Res. 2001;40:101–108. doi: 10.1016/S0921-4488(00)00221-2. [DOI] [PubMed] [Google Scholar]
- Mattioli RC, Bah M, Reibel R, Jongejan F. Cowdria ruminantium antibodies in acaricide-treated and untreated cattle exposed to Amblyomma variegatum ticks in The Gambia. Exp Appl Acarol. 2000;24 :957–969. doi: 10.1023/A:1010645927535. [DOI] [PubMed] [Google Scholar]
- Mattioli RC, Janneh L, Corr N, Faye JA, Pandey VS, Verhulst A. Seasonal prevalence of ticks and tick transmitted haemoparasites in traditionally managed N'Dama cattle with reference to strategic tick control in The Gambia. Med Vet Entomol. 1997;11:342–348. doi: 10.1111/j.1365-2915.1997.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Purchase HS. A simple and rapid method for demonstrating Rickettsia ruminantium (Cowdry, 1925) in heartwater brains. Vet Rec. 1945;57 :413–414. [Google Scholar]
- Simbi BH, T.F. Peter. M.J. Burridge. Mahan SM. Comparing the detection of exposure to Ehrlichia ruminantium infection on a heartwater-endemic farm by the pCS20 polymerase chain reaction and an indirect MAP1-B enzyme linked immunosorbent assay. Onderstepoort J Vet Res. 2003;70 :231–235. [PubMed] [Google Scholar]
- Geysen D, Delespaux V, Geerts S. PCR-RFLP using Ssu-rDNA amplification as an easy method for species-specific diagnosis of Trypanosoma species in cattle. Vet Parasitol. 2003;110 :171–180. doi: 10.1016/S0304-4017(02)00313-8. [DOI] [PubMed] [Google Scholar]
- Mboloi MM, Bekker CPJ, Kruitwagen C, Greiner M, Jongejan F. Validation of the indirect MAP1-B enzyme-linked immunosorbent assay for diagnosis of experimental Cowdria ruminantium infection in small ruminants. Clin Diagn Lab Immunol. 1999;6 :66–72. doi: 10.1128/cdli.6.1.66-72.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet AHM, Van der Zeijst BAM, Camus E, Mahan SM, Martinez D, Jongejan F. Recombinant expression and use in serology of a specific fragment from Cowdria ruminantium MAP1 protein . Ann N Y Acad Sci. 1996;791:35–45. doi: 10.1111/j.1749-6632.1996.tb53509.x. [DOI] [PubMed] [Google Scholar]
- Peter TF, C.J. O´Callaghan. G.F. Medley. B.D. Perry. S.M. Semu. Mahan SM. Population-based evaluation of the Ehrlichia ruminantium MAP-1B indirect ELISA. Exp Appl Acarol. 2001;25 :881–897. doi: 10.1023/A:1020424718957. [DOI] [PubMed] [Google Scholar]
- Mondry R, Martinez D, Camus E, Liebisch A, Katz JB, Dewald R, Van Vliet AHM, Jongejan F. Validation and comparison of three enzyme-linked immunosorbent assay for the detection of antibodies to Cowdria ruminantium infection. Ann N Y Acad Sci. 1998;846 :262–272. doi: 10.1111/j.1749-6632.1998.tb11058.x. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G, Franssen FFJ, Gueye A, Nieuwenhuijs J. Antigenic differences between stocks of Cowdria ruminantium. Res Vet Sci. 1988;44 :186–189. [PubMed] [Google Scholar]
- Everitt RS. Statistical methods for medical investigations. Oxford Uinversity Press, New York/Edward Arnold, London. 1989.
- Wilson JC, Foggie A, Carmichael MA. Tick-borne fever as a cause of abortion and stillbirths in cattle. Vet Rec. 1964;76:1081–1084. [Google Scholar]
- Dawson JE, Ristic M, Holland CJ, Whitlock RH, Sessions J. Isolation of Ehrlichia risticii, the causative agent of Potomac horse fever, from the fetus of an experimentally infected mare. Vet Rec. 1987;121:232. doi: 10.1136/vr.121.10.232. [DOI] [PubMed] [Google Scholar]
- Zaugg JL. Bovine anaplasmosis: Transplacental transmission as it relates to stage of gestation. Am J Vet Res. 1985;46:570–572. [PubMed] [Google Scholar]
- Fiset P, Wisseman CL, El Bataivi Y. Immunologic evidence of human fetal infection with Coxiella burnetti. Am J Epidemiol. 1975;101:65–69. doi: 10.1093/oxfordjournals.aje.a112072. [DOI] [PubMed] [Google Scholar]
- Bezuidenhout JD. Certain aspects of the transmission of heartwater, the occurrence of the organims in ticks and in vitro culture. DVSc Thesis, University of Pretoria, South Africa. 1988.
- Lawrence JA, Foggin CM, Norval RAI. The effect of war on the control of diseases in livestock in Rhodesia (Zimbabwe) Vet Rec. 1980;107:82–85. doi: 10.1136/vr.107.4.82. [DOI] [PubMed] [Google Scholar]
- Norval RAI. Arguments against intensive dipping. Zimbabwe Vet J. 1983;14 [Google Scholar]
- Bezuidenhout JD. The epidemiology and control of heartwater and other tick-borne diseases of cattle in South Africa. Onderstepoort J Vet Res. 1985;52:211–214. [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- Allsopp METP, Van Strijp MF, Farber E, Josemans AI, Allsop BA. Ehrlichia ruminantium variants which do not cause heartwater found in South Africa. Vet Microbiol. 2007;120:158–166. doi: 10.1016/j.vetmic.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Bell-Sakyi L, Koney EBM, Dogbey O, Walker AR. Ehrlichia ruminantium seroprevalence in domestic ruminants in Ghana. I. Longitudinal survey in the Greater Accra Region. Vet Microbiol. 2004;100 :175–188. doi: 10.1016/j.vetmic.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Du Plessis JL. The application of the indirect fluorescent antibody test to the serology of heartwater. In Tick Biology and Control. Edited by Whitehead GB, Gibson JD. Tick Research Unit, Grahamstown, South Africa. 1981. pp. 47–52.
- Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC. Veterinary Microbiology and Microbial Disease. Blackwell Science Ltd. 9600 Garsington Road, Oxford OX4 2DQ, UK. pp 535. 2002.
- Osaer S, Goossens B. Trypanotolerance in Djallonke sheep and West African Dwarf goats in The Gambia: Importance of trypanosomosis, nutrition, helminth infections and management factors. PhD Thesis, Utrecht University, Utrecht, The Netherlands . 1999.
- Whitelaw DD, Scott JM, Reid HW, Holmes PH, Jennings FW, Urquhart GM. Immunosuppression in bovine trypanosomiasis: studies with loupin-ill vaccine. Res Vet Sci. 1979;26:102–107. [PubMed] [Google Scholar]
- Mattioli RC, Faye JA, Bah M, Jabang B. Experimental Trypanosoma congolense infection on naturally occurring ticks in N'Dama and Gobra zebu cattle. Parasitologia. 1994;36:305–311. [PubMed] [Google Scholar]
- Mattioli RC, Pandey VS, Murray M, Fitzpatrick JL. Immunogenetic influences on tick resistance in African cattle with particular reference to trypanotolerant N'Dama (Bos taurus) and trypanosusceptible Gobra zebu (Bos indicus) cattle. Acta Tropica. 2000;75:263–277. doi: 10.1016/S0001-706X(00)00063-2. [DOI] [PubMed] [Google Scholar]