Abstract
Neuregulin (NRG)/ErbB receptor signaling pathways have recently been implicated in the reversal of long-term potentiation at hippocampal glutamatergic synapses. Moreover, polymorphisms in NRG-1 and ErbB-4 genes have been linked to an increased risk for developing schizophrenia. ErbB-4 is highly expressed at glutamatergic synapses where it binds to PSD-95 via its carboxyl terminal T-V-V sequence. Here we investigated the expression, localization and trafficking of ErbB-4 in cultured hippocampal neurons by immunocytochemistry, surface protein biotinylation, and live labeling of native receptors. We show that neuronal ErbB-4 is detected at its highest levels in GABAergic interneurons, as observed in vivo. ErbB-4 immunoreactivity precedes PSD-95 expression, with ErbB-4 cluster initially forming in the absence of, but later associating with, PSD-95-positive puncta. By surface protein biotinylation, the fraction of ErbB-4 receptors on the plasma membrane increases from 30% to 65% between 6 and 16 days in vitro (DIV). Interestingly, 30 minutes of NRG stimulation triggers measurable ErbB-4 receptor internalization at DIV 16, despite increased colocalization with PSD-95. We also investigated the role of TNFα-converting enzyme (TACE)-mediated receptor processing in regulating ErbB-4 surface expression. We found that the cleavage-resistant JM-b isoform accounts for 80% of all ErbB-4 transcripts in cultured hippocampal neurons. Receptor stimulation or treatment with phorbol esters does not induce detectable ErbB-4 processing, indicating that neurons mostly rely on endocytosis of the intact receptor to regulate ErbB-4 surface expression. These results enhance our understanding of the regulation of ErbB-4 – mediated signaling at glutamatergic synapses.
Keywords: ErbB-4, NRG-1, PSD-95, trafficking, GABAergic interneurons, surface protein biotinylation
1. Introduction
ErbB receptors, including the EGF receptor (ErbB-1), ErbB-2, ErbB-3 and ErbB-4, comprise a family of receptor tyrosine kinases involved in numerous signaling processes including the control of cell growth and differentiation. In particular, ErbB2-4 and their cognate neuregulin (NRG) ligands have long been recognized as critical mediators of cell fate, proliferation, migration and differentiation processes in the developing peripheral and central nervous system (for review, see [1,6,10,12,16]. Although NRGs and ErbB receptors continue to be expressed at high levels, until recently their functions in the adult brain were unknown. Work from our laboratory, as well as others, has implicated NRG/ErbB signaling in regulating plasticity of glutamatergic synapses. At CA3-to-CA1 synapses in the hippocampus, activation of ErbB receptor signaling can prevent or revert long-term potentiation (LTP) [21,23]. Depotentiation of LTP in hippocampal neurons is mediated by the internalization of GluR1-containing AMPA receptors without affecting NMDA receptor-evoked postsynaptic currents. Conversely, stimulation of NRG/ErbB signaling in the prefrontal cortex leads to altered surface expression of NMDA receptors at glutamatergic synapses on pyramidal neurons [18]. Consistent with its ability to regulate synaptic plasticity, there is a rapidly growing body of evidence supporting the involvement of the NRG/ErbB pathway, and interactions between ErbB-4 and PSD-95, in the pathogenesis underlying schizophrenia [9,19,20].
By in situ hybridization, ErbB2-4 receptor genes are expressed in the CA1-CA3 regions of the hippocampus, with highest ErbB-4 mRNA levels found in interneurons [17,24,25]. The distribution of ErbB-4 protein in different neuronal populations is less clear; nevertheless, there is general agreement that the highest receptor levels are in GABAergic neurons (see Discussion). ErbB-4 and NMDA receptors colocalize in postsynaptic densities (PSDs) at glutamatergic synapses. ErbB-4 physically interacts via its c-terminal T-V-V sequence with membrane-associated guanylate kinases (MAGUKs) such as PSD-95, SAP-102 and PSD-93 [14,21]. PSD-95 is a major scaffolding component of PSDs, and together with other MAGUKs, plays an important role in organizing the intricate network of receptors, signaling molecules and cytoskeletal adaptor proteins that together mediate synaptic transmission and plasticity (see, for example: [4,15,22]). The association with the PSD positions ErbB-4 as a potentially important modulator of synaptic plasticity, and further supports the notion that NRG/ErbB signaling regulates synaptic plasticity in vivo.
Many growth factor receptors are internalized upon ligand binding to gradually attenuate receptor signaling and to desensitize the cell to excess ligand availability, but also to target activated receptors to other intracellular substrates [37]. Unlike the EGF receptor (ErbB-1), rapid ligand-dependent receptor internalization is notably low for all other ErbB receptors in cancer cell lines expressing ErbB-2 or ErbB-3, or transfected NIH 3T3 cells overexpressing ErbB-4 [3]. Rather, ligand-mediated proteolytic processing has been proposed as a densitization mechanism for ErbB-4 [7,40]. Specifically, it was found that ligand-induced cleavage of the 120 kDa ectodomain by TNF-alpha converting enzyme (TACE; syn. Adam 17) serves to shed the receptor from the surface [31,35,36,40]. Sequence determinants for susceptibility to TACE-dependent ErbB-4 processing reside in a juxtamembrane region that is included in JM-a but that is missing from JM-b transcripts [11]. Interestingly, a recent study reported that JM-a isoforms were upregulated in postmortem brains from schizophrenic individuals [33].
Trafficking of ErbB-4 in neurons has been studied mainly with regard to its NRG-dependent recruitment to lipid rafts [27], a process which is believed to help targeting the ErbB-4 signaling complex to synapses [38]. However, to better understand the emerging role of ErbB receptors, in particular of ErbB-4, as modulators of synaptic plasticity in the adult brain, it is critical to know how receptor availability is regulated in neurons. As our present knowledge about endocytosis of ErbB receptors is largely based on cell lines lacking the functional specializations of mature neurons such as the postsynaptic density, it is unclear if, and how, ErbB-4 receptor processing and endocytosis are regulated in neurons in response to NRG binding. To address this question, we have investigated the surface expression and NRG-stimulated endocytosis of ErbB-4 in dissociated hippocampal neurons by surface protein biotinylation and live cell labeling of endogenous receptors, as well as the role of TACE-mediated ectodomain shedding in regulating surface ErbB-4.
2. Material and Methods
Materials
Human NRG1β1 EGF domain peptide (amino acids 176–246; R&D systems (Minneapolis, MN)) was reconstituted at 5 μM in 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). 4-[(3-bromophenyl)amino]-6-(methylamino)-pyrido[3,4-d]pyrimidine (PD158780), PMA (4α-Phorbol 12-myristate 13-acetate) and the TACE inhibitor TAPI-2 were from Calbiochem (La Jolla, CA). Mouse monoclonal antibodies ab77 and ab72 against the extracellular domain of ErbB-4 [8] and Ab-4 against tubulin were from Lab Vision Corporation (Fremont, CA). Rabbit polyclonal antibody C-18 against the carboxyl terminus of ErbB-4 was from Santa Cruz Biotechnologies (Santa Cruz, CA), mouse monoclonal antibody 7E3-1B8 against PSD-95 was from Affinity Bioreagents (Golden, CO), rabbit polyclonal antibody against GABA was from Sigma (St. Louis, MO), and mouse monoclonal antibody PY-20 against phosphotyrosine was from BD Transductions Laboratories (Lexington, KY). Secondary antibodies conjugated to horseradish peroxidase, Cyanine 3 (Cy3) and Alexa 488 were from Amersham (Piscataway, NJ), Jackson ImmunoResearch (West Grove, PA) and Molecular Probes (Eugene, OR), respectively. A plasmid expressing PSD-95 was a kind gift by D. Bredt.
Cell culture
For post-fixation immunofluorescence and the analysis of ErbB-4 JM-a/b isoform expression, dissociated hippocampal neurons essentially free of glia from embryonic day (E19) Sprague Dawley rats were maintained in serum-free neurobasal medium supplemented with B-27 (Invitrogen, San Diego, CA), as previously described [5,26]. For protein biochemistry experiments and antibody feeding of live neurons, cells were plated on a confluent glial monolayer, as previously described [14].
Semi-quantitative RT-PCR of ErbB-4 JM-a and JM-b splice variants
Total RNA was extracted from rat hippocampal neurons (DIV 6, 16, and 22) and from mouse whole brain using RNAwiz (Ambion, Austin, TX), and reverse transcribed using random hexamers and Superscript II reverse transcriptase (Invitrogen). Primers for the amplification of the sequence encompassing the alternatively spliced exon encoding JM-a were: ErbB-4_JM5’ (rat: 5’-GAAATGTCCAGATGTCCTACAGGG-3’; mouse: 5’-GAAATGTCCAGATGGCCTACAGGG-3’) and ErbB-4_JM3’ (rat: 5’-CTTTTTGATGCTCTTCCTTCTGAC-3’; mouse: 5’-CTTTTTGATGCTCTTTCTTCTGAC-3’) [11]. Target sequences were amplified using Taq Expand (Roche, Indianapolis, IN) at 63°C annealing temperature for 26, 28 and 30 cycles. Aliquots were separated on 2% agarose, transferred to nylon membrane and probed with a [γ-32P]-labeled nested oligonucleotide (5’-AGC AAA CAG TTT CAT ATT TAA GTA CG-3’) against a sequence common to JM-a and JM-b - derived PCR products. Blots were scanned on a Storm 840 phosphorimager (Amersham) and signals were quantified using ImageQuant5.2 software.
Surface protein biotinylation of endogenous ErbB-4
Hippocampal neurons were washed 3 times with progressively colder PBS containing Ca2+ and Mg2+. Surface proteins were labeled with 1 mg/ml NHS-SS-biotin (Pierce, Rockford, IL) in PBS at 4°C for 20 min, and then washed three times with cold 25 mM glycine in PBS. Cells were harvested in l ml of lysis buffer (0.84% Triton X-100, 0.16% SDS in PB solution: 10 mM sodium phosphate, pH 7.4, 5 mM EDTA, 5 mM EGTA, 100 mM NaCl, 1 mM Na3VO4, 10 mM sodium pyrophosphate, 50 mM NaF, and 10 μg/ml aprotinin) at 4°C. The lysates were briefly sonicated, heated at 60°C for 5 min and spun at 20,000 x g at 4°C. Supernatants were incubated with 300 μl of streptavidin beads (UltraLink Plus; Pierce) at 4°C for 2 hr. Aliquots of the slurry were saved to estimate total ErbB-4 protein input. The remaining beads were washed twice in 800 μl of cold PB/Triton, twice more in cold PB/Triton plus 600 mM NaCl, and twice in PB without Triton. SDS samples were generated by heating the beads at 98°C for 3 min in SDS sample buffer and proteins were resolved by PAGE on 6% acrylamide / Tris-glycine gels. ErbB-4 protein was detected by Western blotting using C-18 rabbit polyclonal antibody. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL plus; Amersham), and quantified on a Storm 840 phosphorimager (Amersham) using ImageQuant 5.2 software.
Immunofluorescence of hippocampal neurons
Double-immunofluorescence cytochemistry was performed in cultured neurons fixed for 2 min with methanol at −20°C; non-specific staining was blocked with 5% normal goat serum (NGS) in PBS. Cells were incubated overnight at 4°C with primary antibodies against ErbB-4 and GABA, or ErbB-4 and PSD-95, diluted in PBS containing 0.25% Triton X-100 and 5% NGS, and visualized with secondary antibodies coupled to Alexa-488 or Cy3.
For antibody feeding experiments, live neurons on coverslips were labeled for 30 minutes at 15°C with monoclonal antibody ab77 against the ectodomain of ErbB-4 that was directly conjugated to Alexa 594 using a kit from Molecular Probes. After washing 3 times with MEM to remove the antibody, cells were returned to the incubator and treated for 30 minutes with 5 nM NRG peptide or vehicle. After this incubation, the coverslips were washed with Dulbecco’s-PBS (D-PBS), cells fixed for 20 minutes with 4% paraformaldehyde at room temperature, washed with again, and incubated with Alexa 488 - conjugated secondary antibody to dually label receptors that remain on the cell surface after NRG stimulation. After washing and mounting in Mowiol, cells were imaged on a fluorescent inverted miscroscope (Nikon Diaphot 200) using a 100X lens and a CCD camera (Photometrics, Tucson, AZ). Alexa 594 and 488 immunofluorescence in dendritic areas was recorded and images superimposed to manually quantify the number of surface (labeled yellow) and internalized receptors (labeled red) in 25 μm2 regions of interest (ROIs). To gauge the potential of ab77 to induce NRG-independent receptor autophosphorylation, DIV 8 neurons were stimulated for 30 min in the incubator with 0.4 or 1.0 μg/ml ab77 or ab72. ErbB-4 protein was immunoprecipitated from cell lysates using C-18 rabbit polyclonal antibody, and Western blots were probed with anti-phosphotyrosine antibody PY-20.
Statistics
Student’s t-test was used to assess surface ErbB-4 expression in response to NRG treatment by surface protein biotinylation. Two-way ANOVA, followed by Bonferroni’s post hoc test, was used to compare effects of NRG treatment and age on ErbB-4 surface expression in live antibody feeding assays.
3. Results
ErbB-4 is expressed in cultured hippocampal GABAergic interneurons
Previous evidence from in situ hybridization and immunohistochemistry [17,25] analyses in the hippocampus suggested highest levels of ErbB-4 expression in GABAergic interneurons, based on cell position and morphology. These observations were later corroborated by double-immunofluorescence of the receptor [39]. We double-labeled dissociated hippocampal neurons (DIV 14) with antibodies against GABA and ErbB-4 to test if this expression pattern is maintained in culture. By immunofluorescence, we found that approximately 15% of all cells were positive for GABA in our culture conditions (data not shown). As shown in Figure 1A, most GABA-positive cells were also labeled with the antibody against ErbB-4, while GABA-negative cells rarely expressed ErbB-4. Quantitative analysis revealed that of all cells positive for either GABA or ErbB-4, 88% contained both markers, 10% were only immunoreactive for GABA and 1.5% only for ErbB-4 (Figure 1B). Therefore, the vast majority of cultured hippocampal cells expressing ErbB-4 are GABAergic neurons (>98%).
Figure 1. ErbB-4 is expressed in GABAergic interneurons.
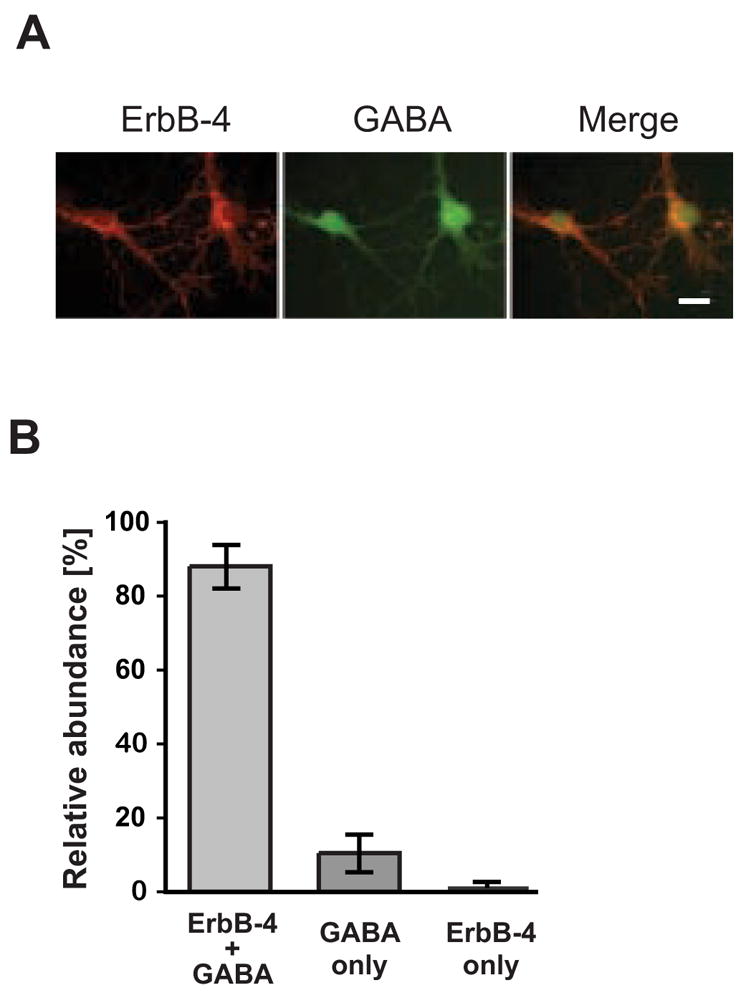
A, Double immunofluorescence labeling of DIV 14 hippocampal neurons with antibodies against ErbB-4 (red) and GABA (green). Scale bar = 20 μm. B, Quantitative analysis of co-expression of ErbB-4 and GABA. The sum of all cells positive for ErbB-4 or GABA was set as 100%. Data represent the mean ±SD of 1004 cells counted in 5 independent experiments.
ErbB-4 cluster associate with PSD-95 puncta during neuronal maturation in vitro
As previously reported, ErbB-4 and PSD-95 physically interact and co-localize at glutamatergic synapses [14,21]. Since interactions with PSD-95 conceivably affect surface expression of ErbB-4 containing receptors, we determined the developmental expression patterns of ErbB-4 and PSD-95 in cultured hippocampal neurons by Western blotting and immunofluorescence. Western blots of whole cell lysates from neurons collected at DIV 5, 9, 12, 16, and 21 were sequentially probed with a polyclonal antibody against ErbB-4, followed by a monoclonal antibody against PSD-95. As shown in Figure 2A, expression of ErbB-4 showed a moderate increase between DIV 5 and 21 (5-fold), while PSD-95 protein levels increased dramatically by 24-fold. The time course of PSD-95 and ErbB-4 increase also differed markedly, with PSD-95 showing a steady accumulation during in vitro development while ErbB-4 mostly increased between DIV 5 and DIV 12 (Figure 2A).
Figure 2. Regulation of ErbB-4 and PSD-95 expression in developing hippocampal neurons.
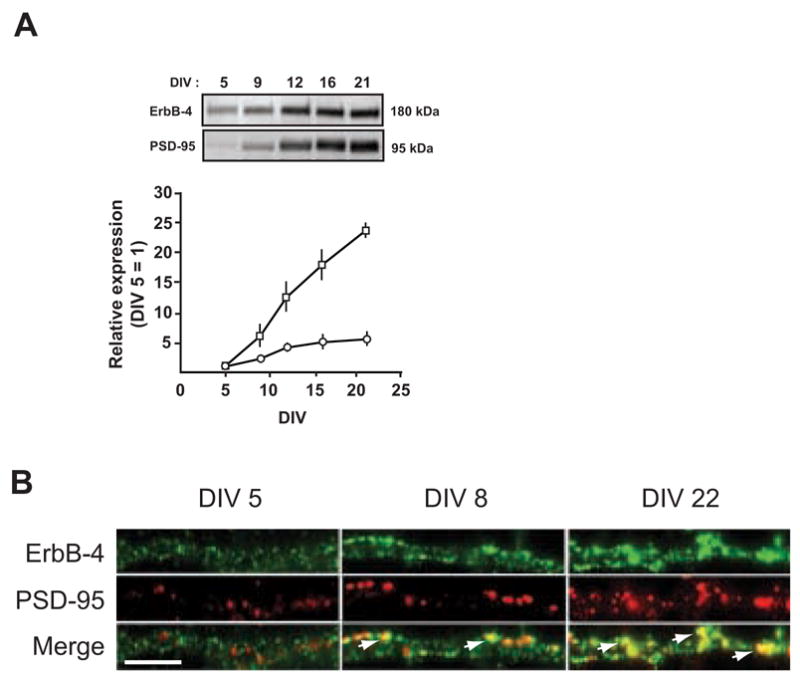
A, (Top) Representative Western blots of whole cell lysates from DIV 5 – 21 neurons, probed with antibodies against ErbB-4 and PSD-95. Equal amounts of protein, as determined by a colorimetric protein assay (Bradford), were loaded in each lane. (Bottom) Quantitative analysis of Western blots. Relative expression levels of ErbB-4 (open circles) and PSD-95 (open rectangles) at DIV 5 were arbitrarily defined as 1. Each data point represents the mean ± SD of three independent experiments. B, Double immunofluorescence of ErbB-4 (green) and PSD-95 (red) in dendritic processes at DIV 5, 8, and 22. Arrows indicate areas of colocalization (yellow) between ErbB-4 and PSD-95. Scale bar = 5 μm.
In order to analyze the subcellular distribution of ErbB-4 and PSD-95 during the maturation of cultured hippocampal neurons, we analyzed their expression using double-immunofluorescence cytochemistry in DIV 5, 8 and 22 cultures. ErbB-4 immunoreactivity was pronounced in both cell bodies (not shown) and in dendritic areas from the earliest time points analyzed, although puncta were more densely packed in DIV 22 neurons (Figure 2B). This observation suggests that hippocampal neurons constitutively express ErbB-4, and that the increase in receptor protein levels seen on Western blots likely results from the extensive arborization of dendritic processes and the increased density of clusters per unit of surface area. Importantly, at all 3 time points most ErbB-4 labeling in dendritic areas was within clusters. In contrast to ErbB-4, PSD-95 immunoreactivity was very sparse in the younger neurons (DIV 5) and became more pronounced as neurons matured. The co-localization of PSD-95 and ErbB-4 immunoreactivity at dendrites was initially low but increased with maturation as indicated by the increase of yellow areas in the overlay (see arrows in Figure 2B). A fraction of ErbB-4-positive puncta did not colocalize with PSD-95, suggesting that these ErbB-4 receptor clusters either reside in different subcellular compartments or that they can associate with different proteins (see Discussion). We conclude that ErbB-4 expression precedes the appearance of PSD-95, and that ErbB-4 clusters initially form in the absence of, but later partially co-localize, with PSD-95-containing puncta.
Surface expression of ErbB-4 increases in developing hippocampal cultures
Our developmental analysis of ErbB-4 and PSD-95 expression demonstrated increased co-localization of these proteins in maturing cultured neurons. To determine whether the increased association with PSD-95 is paralleled by an enhancement of surface expression of ErbB-4 receptors, we used biotinylation to measure the relative surface vs. intracellular expression of ErbB-4 during development of hippocampal neurons in vitro. Surface proteins in young (DIV 6) and mature (DIV 16) neurons were biotinylated and whole cell lysates were subsequently separated into surface and internal protein fractions by pulldown with streptavidin-conjugated beads. Western blotting of equivalent fractions of total protein input, as well as internal and surface protein fractions, with an antibody against the ErbB-4 carboxyl terminus indicated that at DIV 6, approximately 30% of ErbB-4 was biotinylated while 70% was not biotinylated and was thus presumed to reside in internal compartments (Figure 3). By contrast, the distribution of ErbB-4 in DIV 16 neurons was practically inverted, with approximately 65% of the total receptor located on the surface. We conclude that ErbB-4 containing receptors become redistributed to the surface in maturing neurons, possibly at least in part due to the concomitant increase in the number of synaptic sites and PSD-95 positive puncta.
Figure 3. Surface expression of ErbB-4 in young and mature hippocampal neurons.
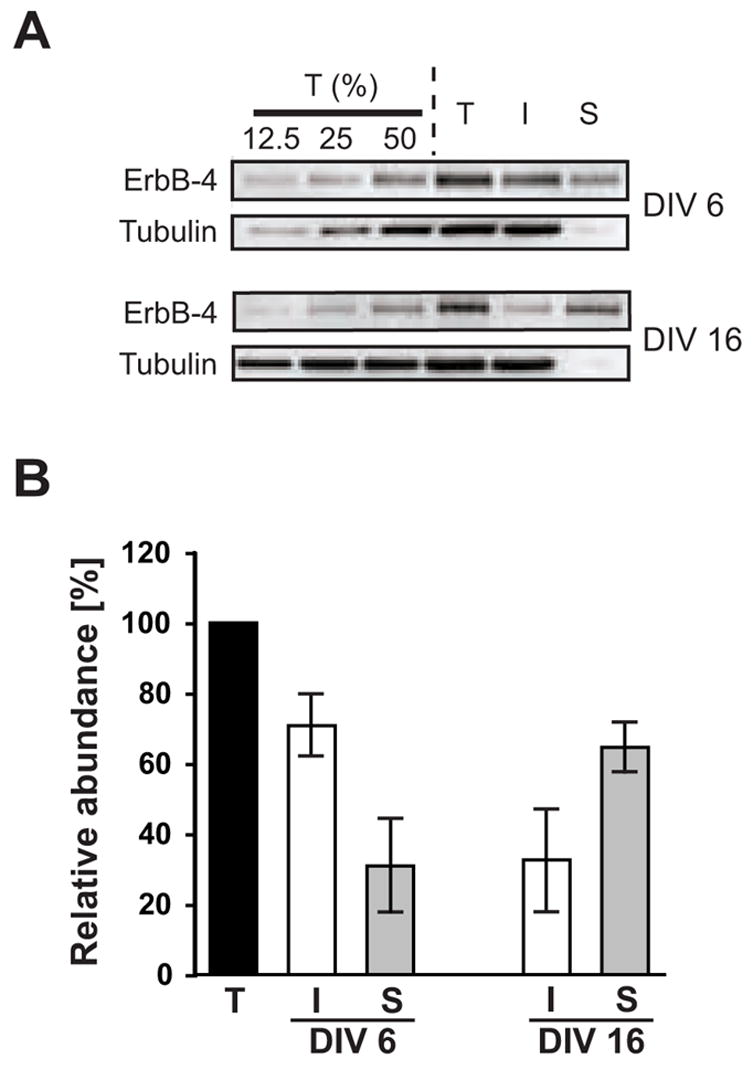
A, Representative Western blots of surface-biotinylated DIV 6 and DIV 16 hippocampal neurons probed with a polyclonal antibody against the carboxyl terminus of ErbB-4. To control for linearity, 12.5%, 25%, and 50% fractions of the total protein input (T) before biotin / streptavidin pulldown were loaded in the first three lanes. I, supernatant after fractionation representing unbiotinylated internal proteins; S, pulldown fraction representing biotinylated surface proteins. To affirm that biotinylation was restricted to surface proteins, blots were re-probed with a monoclonal antibody against a cytosolic marker (tubulin). B, Quantitative analysis of ErbB-4 surface expression. ErbB-4 levels in total input fractions were set as 100%. Bars represent the mean ± S.D. from 7 and 6 independent xperiments for DIV 6 and DIV 16, respectively.
Surface expression of ErbB-4 is attenuated in response to NRG-1 stimulation
Having found that baseline ErbB-4 surface expression increases during development, and knowing that receptor tyrosine kinases attenuate signaling by ligand-induced internalization, we analyzed the extent of NRG-stimulated receptor downregulation using surface protein biotinylation. DIV 6 and DIV 16 cultures were treated for 30 minutes with 5 nM of a peptide encompassing the EGF domain of human NRG-1β1 (hereto forth referred to as NRG peptide) followed by biotinylation as described above. As shown in Figure 4, at DIV 6, there was a 17% (± 2.1% SD) increase in surface ErbB-4 receptor levels after NRG stimulation compared to untreated controls (p<0.05, Student’s t-test). In contrast, surface ErbB-4 was reduced in NRG stimulated DIV 16 neurons compared to mock-treated neurons (21% ± 1.3%; p<0.01). Intriguingly, this finding suggests that ligand binding triggers a modest but significant net receptor exocytosis in young but substantial receptor endocytosis in mature neurons.
Figure 4. Surface expression of ErbB-4 in NRG-stimulated neurons.
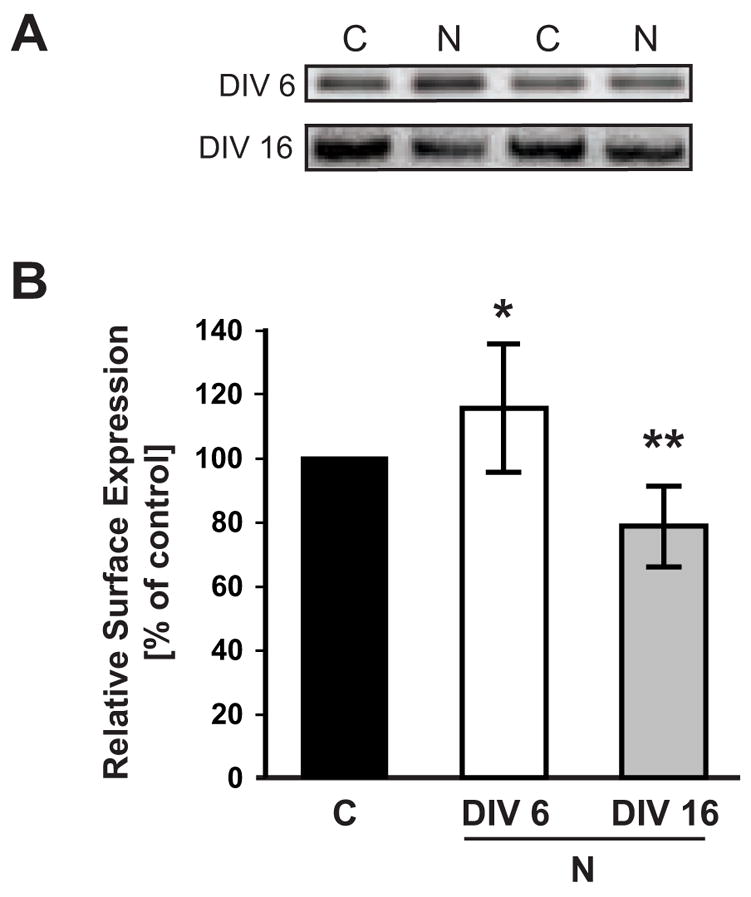
A, Representative Western blots of surface fractions from DIV 6 and DIV 16 neurons treated with 5 nM NRG1 peptide for 30 minutes and biotinylated as described in Figure 3. Two representative examples of surface ErbB-4 expression are shown for NRG-treated cultures (N) and vehicle-treated controls (C). B, Quantitative analysis of ErbB-4 surface expression in response to NRG peptide treatment. Surface ErbB-4 levels in controls were set as 100% (black bar), and compared to surface ErbB-4 levels after NRG treatment at DIV 6 (open bar) and DIV 16 (gray bar). Data represent the mean ± SD from 12 and 10 independent experiments for DIV 6 and DIV 16, respectively. *, p < 0.05; **, p < 0.01.
Next, we used an independent experimental approach utilizing antibody feeding to analyze internalization of endogenous ErbB-4 receptors. To our knowledge, the only available antibodies directed against the extracellular domain of ErbB-4 were mouse monoclonal antibodies ab72 and ab77 [8]. Ab72 does not induce receptor autophosphorylation but competes with NRG for binding to the receptor and was therefore deemed unsuitable to study NRG-stimulated ErbB-4 downregulation. Ab77 in contrast does not interfere with ligand binding, but has been reported to moderately stimulate receptor autophosphorylation and degradation in stably transfected CHO cells [8]. In preliminary experiments, we tested the autophosphorylation potential of ab77 in DIV 8 hippocampal neurons treated for 30 minutes at 37ºC with 0.4 or 1 μg/ml ab77 or ab72. Cells were lysed, immunoprecipitated with an antibody against ErbB-4 and probed for tyrosine phosphorylation. As shown in Figure 5A, we found that ab77 stimulated only low levels of receptor autophosphorylation in neurons compared to 5 or 25 nM of NRG peptide, similar to the very low levels observed for ab72. We then directly conjugated the Alexa 594 fluorophore to ab77 and used this antibody preparation at the lowest concentration that still permitted robust detection of ErbB-4 puncta in dissociated hippocampal neurons (~2.5 μg/ml).
Figure 5. Internalization of activated ErbB-4 in live-labeled neurons.
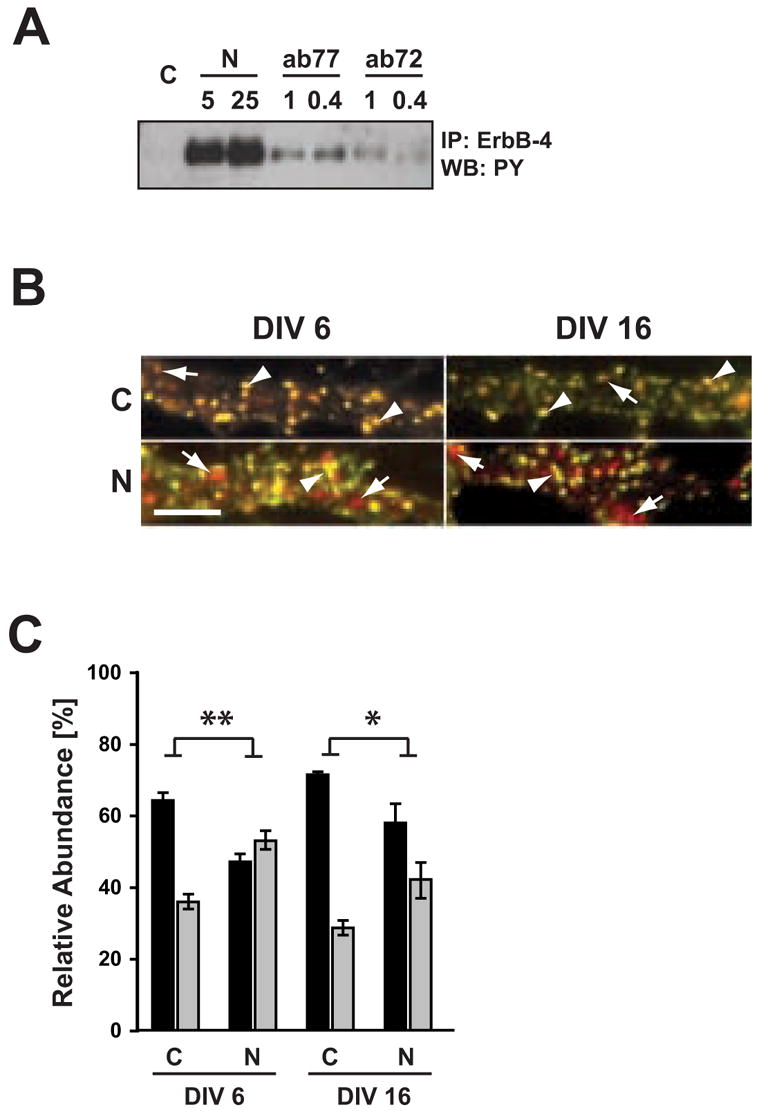
A, Effect of ab77 on ErbB-4 autophosphorylation in dissociated hippocampal neurons. DIV 8 neurons were incubated for 30 minutes at 37ºC with 1 or 0.4 μg/ml of mouse monoclonal antibodies ab77 or ab72, as indicated. ErbB-4 was immunoprecipitated using C-18 polyclonal antibody against the carboxyl terminus, and Western blots were probed for phosphotyrosine. For reference, neurons were stimulated with 5 or 25 nM NRG peptide as indicated. B. Double immunofluorescence of surface and internalized ErbB-4 receptors from representative dendritic areas of hippocampal neurons after NRG stimulation. Surface ErbB-4 receptors were labeled in live DIV 6 and DIV 16 neurons at 15ºC with Alexa 594-conjugated antibody ab77. Following NRG (N) or vehicle (C) treatment at 37ºC, cells were fixed and incubated with an Alexa 488-conjugated secondary antibody against mouse IgG. Yellow puncta (arrowheads) represent surface receptors and red puncta (arrows) represent receptors internalized during the treatment. Scale bar = 5 μm. C, Quantitative analysis of antibody feeding experiments. Data represent relative ErbB-4 abundance at the surface (black bars) and in internal compartments (gray bars). Values represent the mean ± SEM from 3 independent experiments (total number of ROIs analyzed: 99 (C) and 95 (N) for DIV 6; 94 (C) and 90 (N) for DIV 16). *, <0.05; **, <0.01.
To measure relative surface receptor levels, live DIV 6 and 16 neurons were incubated for 30 min at 15ºC with ab77/Alexa 594. The antibody was washed out, and cells were treated for 30 min at 37ºC with 5 nM NRG peptide or vehicle. Following treatment, neurons were again washed, fixed, and incubated under non-permeabilizing conditions with an Alexa 488-conjugated secondary antibody to label receptors that remained at the cell surface throughout the treatment (see Methods). As shown in Figure 5B, images were obtained from dendritic areas and punctate signals from both Alexa 594 (red) and Alexa 488 (green) channels were superimposed to determine the fraction of receptors that were either internalized (red) or remained on the surface (yellow). We found that in vehicle-treated DIV 6 neurons, approximately 35% of red puncta did not co-localize with green puncta, thus representing internalized receptor clusters (see Fig. 5C for quantitative analysis). Hence, in the absence of exogenously added NRG peptide, about one-third of all surface ErbB-4 receptors are internalized within 30 minutes; however, a small contribution of the labeling antibody ab77 to the observed rate of receptor endocytosis cannot be excluded (see above). Importantly, the fraction of internalized receptors noticeably increased in cultures incubated with 5 nM NRG peptide compared to controls (35.8 ± 1.6% SEM controls vs. 53.0 ± 2.2% NRG peptide-treatments), which paralleled a concomitant decrease in surface receptors. The antibody feeding experiments indicate that in young neurons endocytosis of dendritic ErbB-4 receptors is stimulated by the NRG peptide, results that are not immediately apparent to be consistent with data obtained using cell surface biotinylation (see Discussion).
The ligand-independent and -dependent internalization of ErbB-4 receptors was also analyzed in mature DIV16 neuronal cultures. Treatment with 5 nM NRG-peptide increased the fraction of internalized receptors compared to vehicle-treated cultures (42.1 ± 4.7% SEM vs. 28.6 ± 0.9%). The effect of NRG treatment on ErbB-4 internalization was found to be significant (2-way ANOVA; F=29.8, d.f.=1, with p<0.01 for DIV 6 and p<0.05 for DIV 16 [Bonferroni post-test]). These results suggest that ErbB-4 receptors retain substantial endocytic mobility despite the increased co-localization with PSD-95 puncta. However, receptor internalization was significantly attenuated at DIV 16 compared to DIV 6 (2-way ANOVA; F=9.16, d.f.=1, p=0.016), although individually analyzed, neither control nor NRG-treatment were significantly different between DIV 6 and DIV 16 (p>0.05; Bonferroni post-test). This suggests that the association with PSD-95, which accumulates late during development, could stabilize ErbB-4 receptors on the surface. Taken together, both biotinylation and live cell antibody feeding experiments demonstrate that a fraction of ErbB-4 receptors internalizes upon NRG stimulation in mature neurons. However, the effects of NRG-1 stimulation on surface vs. internalized ErbB-4 receptors in cell bodies and dendrites of immature neurons (DIV6) appear to be more complex (see Discussion).
Hippocampal ErbB-4 is not processed upon NRG stimulation
In non-neuronal cell lines, ligand-mediated endocytosis of ErbB2-4 via the clathrin pathway is slow compared to the EGF receptor [3]. An alternative mechanism for the attenuation of ErbB-4 signaling in response to NRG binding is TACE-mediated proteolytic cleavage of receptors containing JM-a exon sequences. We determined the relative expression levels of ErbB-4 JM-a and JM-b transcripts in hippocampal neurons to investigate a possible role of TACE-mediated cleavage in attenuation of NRG-signaling; antibodies specific for the JM variants are not available. Semi-quantitative RT-PCR of total RNA from cultured DIV 6, 16 and 22 hippocampal neurons, as well as from adult mouse brain, was performed using primers flanking the juxtamembrane region of ErbB-4 (Figure 6A). Radioactive hybridization of the resulting PCR products with an oligonucleotide homologous to a sequence shared by both JM-a and JM-b – derived amplicons revealed that 80% of ErbB-4 transcripts represent the JM-b isoform (Figure 6B). This ratio was practically identical for all three developmental time points analyzed and was also found in mouse whole brain.
Figure 6. Cultured hippocampal neurons predominantly express the JM-b isoform and are resistant to NRG or phorbol ester-mediated processing.
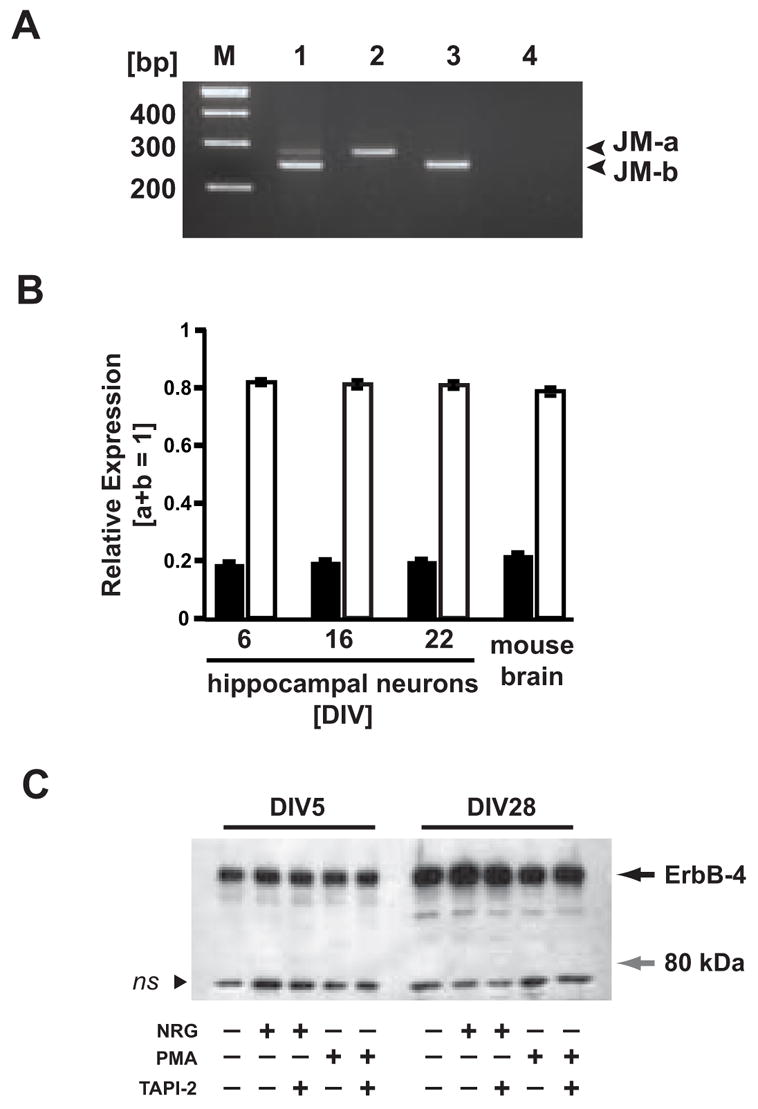
A, Representative RT-PCR of ErbB-4 JM-a/b splice variants. Lengths (in bp) of JM-a and JM-b PCR products are indicated (right). Lane 1, DIV 22 rat hippocampal neurons; lane 2, JM-a plasmid control; lane 3, JM-b plasmid control; lane 4, negative control; M, 100 bp DNA ladder. B, Expression of JM isoforms in hippocampal neurons cultured for different times (DIV 6, 16, 22), and in adult mouse whole brain. Relative expression levels were calculated from phosphorimager scans of RT-PCR products radioactively labeled with a probe common to both PCR products. Bars represent the mean ± SD of data collected from three different PCR cycles (cycles 26, 28, 30). C, Western blot analysis of ErbB-4 processing in NRG and PMA-stimulated neurons. DIV 5 and DIV 28 hippocampal neurons were treated for 30 minutes with 5 nM NRG peptide or 1μM PMA in the presence or absence of the TACE inhibitor TAPI-2 (12.5 μM). Proteins from cell lysates were blotted and probed with the C-18 antibody against the carboxyl terminus of ErbB-4. The black arrow indicates the molecular mass of the full-length ErbB-4 protein (180 kDa). The gray arrow marks the position of the expected 80 kDa fragment resulting from TACE-mediated proteolytic processing. The black arrowhead indicates a nonspecific band (ns) of approximately 70 kDa molecular mass that was present in all samples.
As an alternative approach to analyze TACE-mediated ErbB-4 cleavage, which can be stimulated by activation of the PKC pathway, we utilized Western blots of lysates from DIV 5 and DIV 28 hippocampal neurons treated either with NRG peptide or phorbol esters. Using an antibody against the carboxyl terminus, we were unable to detect the 80 kDa cytoplasmic fragment that is generated from the TACE-mediated processing of ErbB-4 (Figure 6C). The ability to detect this fragment was confirmed by Western blotting of PMA-stimulated HEK293 cells transfected with an expression plasmid for ErbB-4 JM-a (data not shown). Based on our RT-PCR and Western blots results, we conclude that hippocampal ErbB-4 receptors are mostly comprised of the non-cleavable JM-b isoform and that ectodomain shedding does not appreciably contribute to ligand-dependent ErbB-4 receptor trafficking in cultured hippocampal neurons.
4. Discussion
In the past few years, increasing attention has been directed to the functions of the NRG/ ErbB signaling pathway in the adult nervous system. This interest was fueled by two separate but potentially converging lines of research. On the one hand, NRG signaling has been recognized as a potent modulator of synaptic plasticity at glutamatergic synapses of the hippocampus and the prefrontal cortex [18,21,23]. Although the mechanisms by which it regulates synaptic plasticity appear to be somewhat different in these two brain regions, they both have in common that ErbB receptor activation ultimately results in the internalization of ionotropic glutamate receptors (AMPA receptors in the hippocampus and NMDA receptors in the cortex) and a resultant weakening of synaptic strength. On the other hand, based on genetic linkage studies, NRG-1 has emerged as one of the most promising risk factors associated with schizophrenia [20,34]. Interestingly, an impairment of glutamatergic transmission has been noted as a potential contributor to the pathogenesis underlying schizophrenia, making an involvement of the NRG/ErbB pathway in the disease process leading to schizophrenia also biologically plausible [6,9]. Intriguingly, recent studies suggest that changes in ErbB-4 receptor isoform expression and function might contribute to the development of schizophrenia [19,33]. Moreover, recent association studies points to the ErbB-4 gene itself as a risk factor [33]. Because ErbB-4 is likely to be a pivotal point of NRG signaling in the brain, it is imperative to understand how receptor availability at glutamatergic synapses is regulated by its interactions with the NRG ligand and MAGUKs such as PSD-95. In this paper, we present evidence that ErbB-4 containing receptors in developing cultured hippocampal interneurons display substantial endocytic mobility despite their increasing co-localization with postsynaptic scaffolding proteins, as ligand binding induces receptor internalization not only in young but also in mature neurons.
Expression of ErbB-4 in cultured hippocampal neurons
We have carried out a comprehensive quantitative analysis of ErbB-4 expression in cultured hippocampal cells and found that, under our culture conditions, more than 98% of all ErbB-4 positive cells were GABAergic neurons. The higher levels of ErbB-4 expression at GABAergic interneurons in vitro is consistent with the neuronal expression pattern of ErbB-4 in vivo as observed by in situ hybridization [17,25]. Immunohistochemical analyses of brains from distinct species and animals of different ages give more varied results regarding the expression pattern of ErbB-4 protein in neuronal populations. Utilizing immunofluorescence, ErbB-4 expression was readily observed in adult rodent hippocampal GABAergic neurons [17,21,25,39]. Consistent with these results, when immunostaining is performed with biotinyl tyramide amplification, the highest signal is observed in cells that appear to be GABAergic interneurons and lower, but significant, immunostaining is observed in CA1 and CA3 pyramidal neurons and is absent from dentate gyrus granule neurons [17,25]. In human postmortem brain, both inhibitory and excitatory pyramidal neurons were immunostained by independent ErbB-4 antibodies [9,19,20]. At this time it is unclear if the various reported patterns of ErbB-4 expression in distinct neuronal populations represent issues related to the differences in technique sensitivity, species, ages or the cross-reactivity of antibodies utilized. Nevertheless, a point of agreement between all studies using in situ hybridization and immunological approaches both in vivo and in vitro is that GABAergic interneurons express the highest levels of ErbB-4 receptors. Expression of these receptors in GABAergic interneurons has important implications for our understanding of the mechanisms underlying NRG-dependent regulation of synaptic plasticity and the role of this signaling pathway in psychiatric disorders.
Ligand-mediated endocytosis of ErbB-4 containing receptors
We utilized two independent experimental approaches to gauge the extent of ligand-dependent ErbB-4 receptor down regulation in cultured neurons. It is important to note that these approaches differ in the way they assess ErbB-4 surface expression. The protein biotinylation experiments are the most inclusive and measure all punctate and non-punctate surface ErbB-4 receptors in cell bodies and processes accessible during the biotinylation procedure, including receptors that are potentially exocytosed during the NRG treatment. For the same reason, this technique is not suitable to derive internalization rates. In our live antibody feeding experiments we focused our analysis on clustered receptors on dendritic processes. Due to its specific design, this approach measures true ligand-induced internalization of receptors present on the dendritic surface, where PSD-95 is localized, and excludes from the analysis the effects of NRG on ErbB-4 receptor that are exocytosed or present on neuronal somata. Taking these technical considerations into account, we consistently observed a decrease in ErbB-4 surface levels after stimulation with the NRG peptide in DIV 16 neurons, as well as in DIV 6 neurons by antibody feeding. In contrast, the fraction of surface-biotinylated ErbB-4 receptors in DIV 6 neurons was moderately increased after 30 minutes of NRG treatment. Conceivably, this result could reflect differential ErbB-4 trafficking in cell somata and dendrites, or higher rates of receptor exocytosis at DIV 6 than at DIV 16. In young neurons, the relative contribution of the soma to the total cell surface area is much greater than in older neurons in which the extensive arborization of the dendritic tree vastly outweighs the surface area occupied by the cell body. Notwithstanding these considerations, our data are in support of a downregulation of dendritically localized ErbB-4 upon stimulation with NRG.
The extent of ligand-stimulated ErbB-4 internalization we observed in neurons is consistent with previous data obtained from NIH-3T3 cells transfected with chimeric EGF/ErbB-4 receptor constructs [2]. In DIV 16 neurons, we found that approximately 20% of surface ErbB-4 was down-regulated in NRG-stimulated as compared to unstimulated cells in surface protein biotinylation and antibody feeding assays. By comparison, down-regulation of EGF/ErbB-4 receptors in EGF-stimulated NIH-3T3 cells was ~19% after 45 minutes [3]. Of note, fast internalization of NRG-stimulated ErbB-4 receptors was observed in HEK293 cells, although the relative contribution of endocytosis of the intact, as compared to the processed receptor, was not directly investigated [38].
In non-neuronal cells expressing the JM-a isoform, attenuation of ErbB-4 receptor activation is achieved at least in part by TACE-mediated shedding of the 120 kDa extracellular domain, followed by the release of the intracellular 80 kDa fragment by γ-secretase [11,28,31,35,36]. However, our expression analysis of JM-a/b isoforms in cultured hippocampal neurons revealed that JM-a accounts for only 20% of all ErbB-4 transcripts. Moreover, neither under control conditions nor after stimulation with NRG or phorbol esters did we detect the 80 kDa intracellular fragment in Western blots. As some processing would have been expected to occur solely based on our isoform expression analysis, other mechanisms are likely to contribute to the lack of ErbB-4 processing in neurons, such as targeting of ErbB-4 to lipid rafts [27,40]. As PSD-95 is known to reside in caveolin-like lipid domains [27,29], it is conceivable that the interaction between ErbB-4 and PSD-95 prevents the activated receptor from exiting the lipid rafts, thus stabilizing the receptor in a microdomain that protects it from proteolytic processing [40]. Alternatively, proteins that associate with the receptor and interfere with its signaling, such as a novel histidine acidic phosphatase found at glutamatergic synapses of hippocampal neurons [13], could decrease NRG-stimulated ErbB-4 autophosphorylation and prevent subsequent receptor processing.
Clustering of ErbB-4 by PSD-95
ErbB-4 immunoreactivity in cultured hippocampal neurons at all times displays a punctate pattern both on dendrites as well as in the soma. In contrast, co-localization of ErbB-4 with PSD-95 is infrequent in young neurons and clearly increases as neurons mature, largely attributable to a strong up-regulation of PSD-95 expression and concomitant with the formation of postsynaptic structures. Consequently, there are ErbB-4 positive puncta, mostly in young neurons, that do not co-localize with PSD-95 indicating receptors cluster independently of PSD-95. It is possible that other MAGUKs contribute to ErbB-4 clustering since the carboxyl terminus of the receptor interacts with SAP102 and PSD-93, two other PDZ domain proteins related to PSD-95 [14,21]. SAP102 is expressed during postnatal development and in young hippocampal neurons in culture, and may therefore be involved in clustering ErbB-4 at early stages during neuronal differentiation [30,32]. In fact, we have observed widespread dendritic SAP102 expression in young and mature hippocampal neurons (C. Gonzalez, unpublished observations). Because PSD-93 parallels expression of PSD-95 [32], it could account for ErbB-4 clustering at PSD-95-negative puncta in mature neurons.
An intriguing possibility is that clustering by different postsynaptic density proteins targets ErbB-4 receptors to distinct NR2A or NR2B-containing NMDA receptor subtypes [32], thereby regulating synaptic transmission in a subunit-specific fashion. We tried to directly address the importance of ErbB-4/MAGUK interaction for surface receptor expression in live cell imaging using transfected hippocampal neurons expressing wildtype and MAGUK-binding deficient ErbB-4 fused to pH-sensitive GFP, as previously described [23]. However, wildtype ErbB-4 mediated surface fluorescence was mostly diffuse, indicating that binding to available endogenous MAGUKs was saturated (data not shown). Over-expressing PSD-95 together with fluorescent ErbB-4 on the other hand resulted in severe perturbation of neuronal morphology. We therefore deemed this approach not suitable until conditions for heterologous co-expression of ErbB-4 and PSD-95 have been worked out.
Functional significance of ErbB-4 receptor endocytosis dynamics at glutamatergic synapses
Based on results presented here demonstrating slow NRG-stimulated receptor endocytosis and resistance to receptor processing, we propose that these mechanisms contribute to sustain high-level local ErbB-4 signaling in the vicinity of glutamatergic postsynaptic structures. By maintaining high levels of ErbB-4 at the cell surface, in combination with clustering of receptors by MAGUKs that enhances signaling at the PSD by facilitating receptor dimerization [14,21], they ensure efficient and locally restricted transduction of the incoming NRG signal. Notwithstanding these ideas, it will be interesting to determine the fate of internalized receptors, as data from the EGF receptor and other receptor tyrosine kinases suggest that receptors continue to signal in endosomes [37], raising the intriguing possibility that ErbB-4 may activate additional targets en route from the cell surface to its final lysosomal destination.
Acknowledgments
This work was supported by NICHD, and by a fellowship from NINDS to M.C.-H.
Footnotes
Additional contact information: C. Gonzalez: gonzalca@mail.nih.gov, phone: 301-594-4665, D. Vullhorst: vullhord@mail.nih.gov, phone: 301-451-0942
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–11. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Baulida J, Carpenter G. Heregulin degradation in the absence of rapid receptor-mediated internalization. Exp Cell Res. 1997;232:167–72. doi: 10.1006/excr.1997.3515. [DOI] [PubMed] [Google Scholar]
- 3.Baulida J, Kraus MH, Alimandi M, Di FP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–7. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 4.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–79. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 5.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 6.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–96. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben BN, Leitner O, Ratzkin BJ, Bacus SS, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–9. [PubMed] [Google Scholar]
- 9.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–80. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 10.Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci. 2004;24:9250–60. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–8. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 12.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 13.Fleisig H, El-Din El-Husseini A, Vincent SR. Regulation of ErbB4 phosphorylation and cleavage by a novel histidine acid phosphatase. Neuroscience. 2004;127:91–100. doi: 10.1016/j.neuroscience.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 14.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–80. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 16.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000;22:987–96. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 18.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–84. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA> receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–8. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 20.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- 21.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–55. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 23.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–83. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai C, Feng L. Neuregulin induces proliferation of neural progenitor cells via PLC/PKC pathway. Biochem Biophys Res Commun. 2004;319:603–11. doi: 10.1016/j.bbrc.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 26.Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol. 2004;472:156–72. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- 27.Ma L, Huang YZ, Pitcher GM, Valtschanoff JG, Ma YH, Feng LY, Lu B, Xiong WC, Salter MW, Weinberg RJ, Mei L. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–75. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 29.Perez AS, Bredt DS. The N-terminal PDZ-containing region of postsynaptic density-95 mediates association with caveolar-like lipid domains. Neurosci Lett. 1998;258:121–3. doi: 10.1016/s0304-3940(98)00846-5. [DOI] [PubMed] [Google Scholar]
- 30.Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–52. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275:10379–87. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 32.Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–71. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–8. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 34.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and Susceptibility to Schizophrenia. Am J Hum Genet. 2002;71 doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vecchi M, Baulida J, Carpenter G. Selective cleavage of the heregulin receptor ErbB-4 by protein kinase C activation. J Biol Chem. 1996;271:18989–95. doi: 10.1074/jbc.271.31.18989. [DOI] [PubMed] [Google Scholar]
- 36.Vecchi M, Carpenter G. Constitutive proteolysis of the ErbB-4 receptor tyrosine kinase by a unique, sequential mechanism. J Cell Biol. 1997;139:995–1003. doi: 10.1083/jcb.139.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–8. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- 38.Yang XL, Huang YZ, Xiong WC, Mei L. Neuregulin-induced expression of the acetylcholine receptor requires endocytosis of ErbB receptors. Mol Cell Neurosci. 2005;28:335–46. doi: 10.1016/j.mcn.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–64. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Carpenter G. Heregulin-dependent trafficking and cleavage of ErbB-4. J Biol Chem. 2000;275:34737–43. doi: 10.1074/jbc.M003756200. [DOI] [PubMed] [Google Scholar]


