Abstract
A DNA fragment of about 13 kb containing the human tyrosine hydroxylase (TH) promoter was previously isolated from a genomic DNA library and sequenced. The 11 kb from the transcription start of the human TH promoter was successively joined to the green fluorescent protein (GFP) to generate a transgenic mouse model. High levels of GFP expression could be observed in TH-positive cells of the Substantia nigra of embryonic and adult mice. Intriguingly, the sequence of the human TH promoter showed a low degree of homology with the mouse and rat TH promoters. In fact, comparative analysis of the sequences of human, rat, and mouse TH promoters revealed only five small regions of high homology. These five evolutionarily conserved regions were numbered in numeric progression from the 5′ end of human TH promoter. In the present study, a panel of minimal human TH promoters was generated to analyze the transcriptional activity and specificity of gene expression conferred by the five conserved regions (CRs). The series of constructs was termed 250 bp and contained the first–194 bp of the human TH promoter immediately upstream of the transcription start, the first 35 bp the human TH messenger RNA leader, plus one or more of the five CRs. All the constructs were assembled in a self-inactivating form of the latest series of lentiviral vector system based on the human immunodeficiency virus type 1 (HIV-1). Lentiviral-mediated gene transfer was highly efficient for the in vitro transduction of human neuronal progenitor cells (hNPCs). Since a subset of hNPCs express TH following in vitro treatment with a mixture of differentiating agents, it was possible to assess specificity of expression for all the minimal human TH promoters. Overall, the successive addition of the five conserved regions produced a greater degree of specificity in induced TH-positive hNPCs, in particular after the addition of CRI (−8,917, −8,876). However, the human TH minimal promoters did not show any specificity for TH-positive differentiated mouse primary striatal and S. nigra cells, indicating a difference of TH gene regulation between the human and mouse systems. The human TH minimal promoters may provide the opportunity for the selection of TH-positive human embryonic and adult stem cells for brain transplantation experiments in animal models for Parkinson's disease.
A great deal of effort was expended towards better understanding the mechanisms of tyrosine hydroxylase (TH) gene expression. This enzyme catalyzes the hydroxylation of tyrosine in the production of l-dopa (Nagatsu et al., 1964), which is the rate-limiting step in the synthesis of catecholamine neurotransmitters of the central and peripheral nervous systems (Zigmond et al., 1989). Aberrant TH gene expression is associated with psychiatric disorders, such as schizophrenia, bipolar disorder, and side effects caused by alcoholism. More importantly, the degeneration of TH-positive dopaminergic neurons of the Substantia nigra is associated with Parkinson's disease.
In order to elucidate the nature of the machinery that regulates TH gene expression, a 13 kb DNA fragment containing the human TH promoter was isolated from a genomic DNA library and sequenced (Kessler et al., 2003). Approximately 11 kb from the transcription start of the human TH promoter was linked to the green fluorescent protein (GFP) gene and used to generate a transgenic mouse model (Kessler et al., 2003). High levels of GFP expression were detected among TH-positive cells of the S. nigra of both embryonic and adult transgenic mice (Kessler et al., 2003). Interestingly, comparative analysis of the sequences of human, rat, and mouse TH promoters revealed only five small regions of high homology (Kessler et al., 2003). Overall, the degree of homology between the human and mouse TH promoters is in the range of 46.6% (determined with a Clustalx program), whereas the human and rat TH promoters share only a 30% degree of homology (Gandelman et al., 1990; Kim et al., 2003b). This study aims at establishing whether or not these five evolutionary conserved regions play a role in conferring tissue-specificity to the transcriptional activity of the human TH promoter. Towards this end, one series of human TH minimal promoters driving GFP expression was engineered, by adding the five conserved regions to obtain a profile of transcriptional activity in human brain-derived cells.
All the constructs were assembled in a self-inactivating lentiviral vector system based on the human immunodeficiency virus type 1 (HIV-1) (Lois et al., 2002). High titer lentiviral vector stocks can be easily and efficiently produced with a transient over-expression system (Pear et al., 1993; Soneoka et al., 1995; Naldini et al., 1996; Lois et al., 2002). Lentiviral-mediated gene transfer proved very efficient for the genetic manipulation of brain-derived cells both in vitro and in vivo (Romano, 2003; Romano et al., 2003). Intracranial administration of lentiviral vectors could effectively transduce brain cells in a variety of preclinical studies (Romano et al., 2003). Interestingly, some studies already employed viral-mediated gene transfer to analyze the rat TH promoter. The viral vector systems used in these studies were either based on herpes simplex virus type 1 (HSV-1) (Song et al., 1997; Wang et al., 1999, 2004), or on adenovirus (Robert et al., 1997). However, vector systems based on HSV-1 and adenovirus can elicit strong host immune responses and even cause chronic brain inflammations (Dewey et al., 1999; Kielian and Hickey, 1999), as they are highly immunogenic (Romano et al., 2000). In addition, adenoviral and HSV-1-based vectors can be problematic for in vitro applications, as transduced cell populations may become immunogenic once they are re-infused into the host (Romano et al., 2000, 2003). This is due to the presence of leaky viral genes, which may be expressed in the target cells (Romano et al., 2000).
Recombinant TH promoters can be used to isolate dopaminergic neurons either from embryonic stem cells, or from neuronal progenitor cells. In this respect, a group of investigators transfected the rat TH promoter into murine embryonic stem cells, which were then induced to differentiate along the neuronal pathway (Yoshizaki et al., 2004). Interestingly, GFP-expressing neuronal cells were also immunoreactive for TH. GFP-positive cells were selected by fluorescence-activated cell sorting (FACS) and transplanted into the brain of parkinsonian rats. Some of implanted cells survived and innervated the striatum. A partial recovery from parkinsonian behavioral effects was observed in this study (Yoshizaki et al., 2004).
The engineering of a lentiviral-based human TH minimal promoter may also have useful applications for an optimized selection of human TH-expressing stem cells for transplantation experiments in animal models for Parkinson's disease.
MATERIALS AND METHODS
Plasmids
The self-inactivating lentiviral vector system pFUGV was kindly provided by Dr. David Baltimore (California Institute of Technology) and is described elsewhere (Lois et al., 2002). The packaging constructs VSV-G and pCMVΔR9 were kindly provided by Dr. Didier Trono (University of Geneva, Switzerland) (Naldini et al., 1996).
The promoter of the human cytomegalovirus (hCMV) was deleted from the lentiviral vector and replaced with a double stranded oligonucleotide carrying the following restriction sites: 5′-ClaI, SacII, BamHI, EcoRI-3′. This plasmid was termed pGR378. All the plasmids here described are based on a self-inactivating lentiviral vector system.
Plasmid pGR382 contains a 235 bp fragment obtained by SacII and BamHI digestion of the human TH promoter. This 235 bp fragment contains the last 194 bp at the 3′ end of the human TH promoter and the first 35 bp downstream of the transcription start (Kessler et al., 2003). The translated region encoding for the GFP begins 16 bp downstream of the BamHI restriction site, which is at the 3′ end of the 235 bp DNA fragment. The SacII–BamHI fragment of the human TH promoter was ligated into the SacII–BamHI digested and dephosphorylated pGR378 self-inactivating lentiviral-based vector. For the sake of simplicity, all the plasmids that derive from pGR382 are termed 250 bp series. The sequences of and the coordinates of the five conserved regions (CR) are listed in Table 1 (Kessler et al., 2003). The five CR were numbered in Romanic numeric progression from the 5′ end of the human TH promoter (Kessler et al., 2003). Putative binding sites for various transcription factors contained in the five CR are reported in Table 2 (Kessler et al., 2003).
TABLE 1.
Sequences and coordinates of the five evolutionary conserved regions (CR) of the human TH promoter
| Conserved region and coordinates (human TH promoter) |
Sequence |
|---|---|
| CR-I (42 bp) (−8,917; −8,876) | CCAAAGTAATCACATGGCAAACAAGCCCTGTCTAAATATCAC |
| CR-II (42 bp) (−7,248; −7,207) | GTGAGTGACTAATGAGAACTGAATGCCGCTCTTATTGCTTTT |
| CR-III (18 bp) (−5,489; −5,472) | CCCAATTATCCCTAAGTG |
| CR-IV (51 bp) (−5,399; −5,349) | GCTCCATCTGATGGCCTCATTAGGGATAATTGCTCTGGCATTTGGGTCTGA |
| CR-V (60 bp) (−2,423; −2,327) | CGAATTAAAAAGCAATATTTGTATCAGTGGAAGACATTTGCTGAAAGGTTAAATCCACAT |
Conserved regions have been numbered in numeric progression from 5′ end of human TH promoter.
TABLE 2.
List of putative binding sites for transcription factors present in the CR of the human TH promoter
| Conserved region and coordinates (human TH promoter) | List of putative binding sequences for transcription factors per each conserved region |
|---|---|
| CR-I (42 bp) (−8,917; −8,876) | a (unidentified factor); GR; Myogenin; HNF-3; Pit-1; left NurRE 1/2 site |
| CR-II (42 bp) (−7,248; −7,207) | AP-1; Isl-1; GR |
| CR-III (18 bp) (−5,489; −5,472) | HoxA4/A5 or Dfd/Hox1.3 |
| CR-IV (51 bp) (−5,399; −5,349) | TBF-1; CT-1; Isl-1; HoxA4 or Dfd; right NurRE 1/2 site; GR |
| CR-V (60 bp) (−2,423; −2,327) | NHF-3; b (unidentified factor); EcR; Pit-1; AP-3 |
Conserved regions have been numbered in numeric progression from 5′ end of human TH promoter. For a reference of the unidentified factors a and b see Kessler et al. (2003).
The five CR were linked upstream of the SacII restriction site of the minimal human TH promoter sequence of plasmid pGR382 via polymerase chain reaction (PCR), beginning with CR-V, followed in the order by CR-IV, CR-III, CR-II, and CR-I. The list of plasmid based on the series of constructs termed 250 bp is shown in Table 3. Briefly, a series of sense primers and one antisense primer were designed. Each of the sense primer was designed with an overhang containing an EcoRI restriction site, a particular CR and the annealing sequence to the corresponding plasmid template. The sequence of the antisense primer was 5′-gaattcGGCGACCGGTGGATCCGGGCTCCGTCTCCA-3′ (positions 52–22). The lower case letters indicate the EcoRI restriction site contained in the overhang sequence of the antisense primer. The PCR conditions were as follows: an initial denaturation step of the template at 94°C for 1 min, followed by 1 min interval at 48°C to allow for the annealing of the primers to the template and a 5 min incubation at 72°C for polymerase elongation of the primers. This cycle was repeated 35 times. At the end of the 35 cycles, an additional incubation at 72°C for 20 min was included to allow for the completion of the amplification. These PCR conditions were applied for the generation of all the constructs mentioned in this study. The PCR product was isolated with a kit (Qiagen, Santa Clarita, CA). After the isolation, the PCR product was digested with EcoRI and electrophoresed on a 1% (w/v) agarose gel. The correct size product was excised from the gel and purified with a gel extraction kit (Qiagen) following manufacturer's instructions. The final digested PCR product was ligated into EcoRI digested and dephosphorylated pGR378. The inserted PCR fragment was then sequenced to check for orientation and for possible misincorporations.
TABLE 3.
List of human TH minimal promoters assembled in a self-inactivating lentiviral vector system
| Construct | Conserved regions (CR) and series |
Size of minimal promoter |
|---|---|---|
| pGR382 | No conserved region; 250 bp | 235 bp (194 ± 35 ± 6) |
| pGR388 | CR-V; 250 bp | 277 bp (236 ± 35 ± 6) |
| pGR389 | CR-IV-V; 250 bp | 319 bp (278 ± 35 ± 6) |
| pGR392 | CR-III-IV-V; 250 bp | 337 bp (296 ± 35 ± 6) |
| pGR393 | CR-II-III-IV-V; 250 bp | 388 bp (347 ± 35 ± 6) |
| pGR394 | CR-I-II-III-IV-V; 250 bp | 448 bp (407 ± 35 ± 6) |
The reporter gene is green fluorescent protein (GFP). The size of the minimal promoters includes 35 bp of the untranslated messenger RNA leader of the human TH promoter and six extra nucleotides upstream of the start codon of GFP (Kessler et al., 2003).
Cells and cell lines
Human neuronal progenitor cells (hNPCs) were purchased from Clonexpress, Inc. (Gaithersburg, MD) and grown in DMEM/F12 (Invitrogen, Carlsbad, CA), supplemented with 5% (v/v) fetal bovine serum (FBS) (Invitrogen), 10% (v/v) neuronal cell supplement (Clonexpress, Inc.), 10 ng/ml hEGF (R&D Systems, Minneapolis, MN) and 10 ng/ml bFGF (B&D Biosciences, Palo Alto, CA). The differentiation of human neuronal progenitor cells was carried out 48 h post-lentiviral vector transduction, for an overnight period, at 37°C, in a CO2 incubator, in differentiation medium supplemented with differentiation cocktail. The differentiation medium is comprised of DMEM/F12 (Invitrogen), 20% (w/v) glucose (Sigma, St. Louis, MO), 1% (v/v) insulin-transferrin-selenium mix (Invitrogen), 1.4 mM l-glutamine (Invitrogen). The differentiation cocktail contains: FGFI 10 ng/ml (R&D Systems), dopamine 10 μM (Sigma), TPA 200 nM (Sigma), IBMX 65 μM (Sigma), Forskolin 15 μM (Sigma).
Mouse primary striatal and S. nigra cells were extracted from fetuses 13 days post-coitus as described (Stull et al., 2001). The differentiation of mouse primary striatal cells was carried out 48 h post-lentiviral transduction, for an overnight period, at 37°C, in a CO2 incubator, in differentiation medium supplemented with differentiation cocktail as above.
Fetal human renal carcinoma cell line 293FT was purchased from Invitrogen and was grown in Dulbecco's minimum essential medium (DMEM) (Invitrogen) supplemented with 10% (v/v) heat inactivated FBS (Invitrogen) and 2 mM l-glutamine (Invitrogen).
Human skin carcinoma A431 cell line was purchased from ATCC (Manasas, VA) and grown in DMEM supplemented with 10% (v/v) FBS (Invitrogen) and 2 mM l-glutamine (Invitrogen).
Lentiviral-mediated gene transfer
Lentiviral vector stocks were generated transiently for the gene transduction of target cells as described elsewhere (Soneoka et al., 1995; Naldini et al., 1996; Lois et al., 2002). Transient calcium phosphate DNA transfection of 293FT cell line was carried out as described (Pear et al., 1993; Soneoka et al., 1995). Lentiviral vector stocks were harvested and used for the transduction of target cells as described elsewhere (Soneoka et al., 1995; Naldini et al., 1996; Lois et al., 2002).
Extraction of genomic DNA from hNPCs and DNA dot blot
Genomic DNA was extracted from approximately 5 × 105 hNPCs after lentiviral vector transduction. The extraction of genomic DNA was carried out with standard procedures. Briefly, hNPCs were harvested from tissue culture dishes, washed with PBS and lysed using the hot alkaline lysis system described elsewhere (Reed and Matthaei, 1990). Ten micrograms of genomic DNA were spotted onto a charge modified nylon membrane with a Minifold Microsample Filtration Dot Blot apparatus (Schleicher & Schuell, Keene, NH). The WPRE region was excised from the lentiviral vector plasmid with ClaI digestion (Lois et al., 2002) and radiolabeled with P32γ-ATP following standard procedures. The radiolabeled WPRE DNA fragment was then used as a probe for the DNA dot blot assay (Reed and Matthaei, 1990).
PCR analysis of genomic DNA extracted from human skin carcinoma A431 cell line
Genomic DNA was extracted from approximately 5 × 105 human skin carcinoma A431 cell line after lentiviral vector transduction as above-described. Five micrograms of genomic DNA were used as template for each PCR reaction. Two primers were designed to amplify a 250 bp fragment of the WPRE region of the lentiviral vector system (Lois et al., 2002). The sequence of the sense primer was: 5′-TTTTACGCTATGTGGATACGCTGC-3′. The sequence of the antisense primer was: 5′-AACACCACGGAATTGTCAGTGCCC-3′. The PCR conditions were as above-described.
Antibodies and immunofluorescence
Rabbit antibodies to TH were purchased from Pel-freez Biological (Rogers, AK) and used at a dilution 1:100 (v/v) in PBS. Rhodamine-conjugated secondary antibody to rabbit immunoglobulins was purchased from Jackson ImmunoResearch (West Grove, PA) and used at a dilution 1:50 (v/v) in PBS.
Cells were fixed with 4% (w/v) paraformaldehyde in PBS at room temperature for 10 min. Immunofluorescence assay was carried out as described elsewhere (Du and Iacovitti, 1997; Kessler et al., 2003). Endogenous TH expression was detected with rhodamine-conjugated antibodies. Slides were analyzed on a Nikon-Scanalytic Image System as described elsewhere (Yang et al., 2004). Numbers of green, red, and overlapped green and red cells were counted manually. Three different fields, at a magnification of 20×, were taken per each well in order to score green, red, and yellow cells per each sample.
RESULTS
Construction and overall characterization of the lentiviral-based vectors encoding for the human TH minimal promoters carrying the five conserved regions
Only five small sequences of high homology are shared among human, mouse, and rat TH promoters (Kessler et al., 2003). The purpose of this study is to establish whether or not these five evolutionary conserved regions confer tissue-specificity to the human TH promoter. For this reason, one panel of human TH minimal promoters was engineered for the characterization of five evolutionary conserved regions (Materials and Methods). The sequences and the coordinates of the five conserved regions are shown in Table 1 (Kessler et al., 2003). Each of these conserved regions contains a number of putative binding sites for various transcription factors (Table 2) (Kessler et al., 2003). The list of human TH minimal promoters based on a series of constructs termed 250 bp is shown in Table 3. The size of the various minimal promoters ranges from 235 to 448 bp (Table 3). All the various human TH minimal promoters drive the expression of GFP, in the context of a self-inactivating lentiviral vector system (Lois et al., 2002). Lentiviral vector stocks were generated on a transient basis for the gene transduction of human neuronal progenitor cells (hNPCs) (Materials and Methods). Lentiviral-mediated gene transfer allowed for rapid characterization of the various constructs. A DNA dot blot showed that the gene transfer efficiency was comparable for all the constructs in the 250 bp series and for the human CMV promoter (Fig. 1). In this experiment, hNPCs were transduced with lentiviral vectors encoding for the various human TH minimal promoters in the 250 bp series and the human CMV promoter driving the expression of GFP (Materials and Methods). A P32 radiolabeled probe to the WPRE region was used in this experiment to quantify the gene transfer efficiency of the lentiviral-mediated gene transfer for all the constructs. The WPRE region is present in all the lentiviral shuttle vectors (Lois et al., 2002). The efficiency of lentiviral-mediated gene transfer in hNPCs was assessed for plasmid pGR382 by scoring the number of GFP-positive cells versus the total number of cells. The minimal human TH promoter of plasmid pGR382 contains only the first 194 base pairs from the transcription start of the human TH promoter, along with the first 35 bp that are downstream of the transcription start (Table 3). Plasmid pGR382 is referred to as “−194 bp” construct in all the various figures. The transduction efficiency of plasmid pGR382 was in the range of 60% (data not shown), whereas the estimated gene transfer efficiency for the CMV construct was in the range of 80% (data not shown). The apparent 20% difference of transduction efficiency between plasmid pGR382 and the CMV construct does not seem reflect a significant difference in the lentiviral vector titers.
Fig. 1.

DNA dot blot to test gene transfer efficiency in the 250 bp series. The lentiviral vector carrying the CMV-driven GFP was included as control (first lane on left-hand side). Lentiviral transduction was carried out on hNPCs cells as described (Materials and Methods). Genomic DNA was extracted and transferred on a charge modified nylon membrane (Materials and Methods). The dot blot was probed with a P32γ-ATP DNA fragment encoding for the WPRE region of the lentiviral vector system (Materials and Methods). CR, conserved region.
Profile of gene expression and specificity of human TH minimal promoters for TH-expressing cells
Transcriptional activity of the various human TH minimal promoters was simply determined by monitoring GFP expression under an ultraviolet (UV) microscope. This system does not allow for the exact assessment of the levels of GFP expression, as it is not quantitative. On the other hand, it is possible to address in an unequivocal manner the issue of specific gene expression for the various constructs in TH-positive cells. Endogenous TH was detected immunochemically with rhodamine-conjugated antibodies on paraformaldehyde fixed cells. Human progenitor neuronal cells (hNPCs) were differentiated with a previously described mixture of differentiation agents (Du and Iacovitti, 1997), before being fixed with paraformaldehyde (Materials and Methods). The individual staining for GFP, TH and merged picture is shown for each construct (Fig. 2). The number of hNPCs expressing either GFP (green), or human endogenous TH (red), or co-expressing GFP and TH (yellow) was quantified as previously described (Materials and Methods). The analysis of specificity and of GFP expression is reported in Figure 3, parts A and B, respectively.
Fig. 2.


Immunoassay of tyrosine hydroxylase (TH) expression and detection of GFP for the 250 bp series of constructs and for the CMV-driven GFP in differentiated hNPCs (Materials and Methods). The first column of parts A and B shows the merged picture of TH-staining and GFP expression. The second column of parts A and B shows TH-staining (rhodamine). The third column of parts A and B shows the detection of GFP under ultraviolet (UV) light. Row A of part A shows CMV-driven GFP expression. Row B of part A shows the construct of the 250 bp series that contains the first −194 bp from the transcription start of the human TH promoter. Row C of part A shows the construct that contains CR-V upstream of the −194 bp from the transcription start of the human TH promoter. Row D of part A shows the construct that contains CR-IV and CR-V upstream of the −194 bp sequence above mentioned. Row E of part B shows the construct that contains CR-III, CR-IV, CR-V, and the −194 bp sequence. Row F of part B shows the construct that contains CR-II, CR-III, CR-IV, CR-V, and the −194 bp sequence. Row G and part B shows the construct that contains CR-I, CR-II, CR-III, CR-IV, CR-V, and the −194 bp sequence from the transcription start of the human TH promoter.
Fig. 3.

Part A shows the analysis of the specificity in the 250 bp series. Part B shows the number of GFP-positive hNPCs. The number of TH- and GFP-positive cells was determined from Figure 2, as described (Materials and Methods). Each value represents the mean ± standard deviation of three determinants. The percent of specificity is given by the following ratio: yellow cells/(yellow + green cells). The index of basal transactivation is given by the following ratio: yellow cells/(yellow + red cells).
The strongest GFP signal in the 250 bp series was detected for plasmid pGR382 (−194 bp) (Fig. 3, part B), which comprises the first −194 bp from the transcription start of the human TH promoter and the first 35 bp of the promoter that encode for part of the untranslated RNA leader (Materials and Methods). As anticipated, the transduction efficiency of lentiviral-mediated gene transfer was in the range of 60% (data not shown), consequently 60% is the maximum possible overlap between GFP and TH-associated rhodamine expression. The lentiviral vector encoding for CMV-driven GFP showed 80% transduction efficiency (data not shown), therefore, this value represents the maximum possible overlap between GFP and TH-associated rhodamine expression. However, the levels of brightness of the CMV-driven GFP were much higher than those of plasmid pGR382 (Fig. 2, part A). Although this is not a quantitative measure, this observation suggests that the CMV promoter has a stronger transcriptional activity in hNPCs than plasmid pGR382. As expected, most of GFP expression of these two plasmids is ectopic, as revealed by the broad GFP expression in the general cell population (Fig. 3, part B). In the case of plasmid pGR382, 59% of TH-expressing cells also express GFP (ratio of yellow cells over the total number of yellow and red cells), whereas the percentage of TH and GFP co-expressing hNPCs over the total number of GFP-expressing cells is 18% (ratio of yellow cells over the total number of yellow and green cells) (Fig. 3, part A). For the CMV-driven GFP, 80% of TH-expressing cells also express GFP, whereas the ratio of yellow cells over the total number of yellow and green cells is 13%. The baseline for measuring the degree of specificity for all the constructs can be assumed to be in the range of 18–20%. All values that fall below this window cannot be considered specific.
The addition of CR-V caused a steep decline of 72% in GFP-expressing cells, if compared with plasmid pGR382 (Fig. 3, part B). The same trend was observed for the ratio of TH and GFP co-expressing cells over the total number of TH-positive cells, which dropped to 15% (Fig. 3, part A). On the other hand, the ratio between TH and GFP co-expressing cells and the total number of GFP-expressing cells did not vary significantly between the two plasmids (Fig. 3, part A), indicating a lack of specific gene expression.
The addition of CR-IV to CR-V and the first −194 bp from the transcription start of the human TH promoter restored GFP expression almost to the levels of plasmid pGR382 (Fig. 3, part B). Intriguingly, CR-IV contains a half palindromic binding site for Nurr1, which is a transcription factor thought to be involved in TH gene regulation and in development (Zetterstrom et al., 1997; Castillo et al., 1998; Saucedo-Cardenas et al., 1998; Kim et al., 2002). However, the addition of CR-IV did not change the specificity of expression for TH-positive hNPCs. In fact, the ratio of TH and GFP co-expressing cells over the total number of TH-positive cells was increased to 50%, while the ratio of TH and GFP co-expressing cells over the total number of GFP-expressing cells remained at baseline values (Fig. 3, part A).
The addition of CR-III caused a significant decrease of 37.5% in GFP-expressing cells, in comparison with the previous plasmid carrying CR-IV and CR-V (Fig. 3, part B). A slight decline of 10% was observed for the ratio of TH and GFP co-expressing cells over the total number of TH-positive hNPCs, while the ratio of TH and GFP co-expressing cells over the total number of GFP-expressing cells persisted at baseline values (Fig. 3, part A).
The presence of CR-II did not change significantly the number of GFP-expressing cells (Fig. 3, part B) and the degree of specificity for TH-expressing cells (Fig. 3, part A). The ratio of TH and GFP co-expressing cells over the total number of TH-positive cells was 48%, whereas the ratio of TH and GFP co-expressing cells over the total number of GFP-expressing cells was 20%, which is at baseline levels (Fig. 3, part A).
Interestingly, the addition of CR-I decreased by 74% the number of GFP-expressing hNPCs, if compared with the previous construct that contains CR-II, CR-III, CRIV, and CR-V (Fig. 3, part B), while the overall specific gene expression for TH-positive cells was considerably increased (Fig. 3, part A). The ratio of TH and GFP co-expressing cells over the total number of GFP-expressing cells was more than double baseline values, and almost equaled the ratio of TH and GFP co-expressing cells over the total number of TH-positive (index of specificity = 0.47; index of basal transactivation = 0.51) (Fig. 3, part A).
The entire panel of human TH minimal promoters failed to express GFP in human skin carcinoma A431 cell line, in contrast to the lentiviral vector carrying the CMV-driven GFP (data not shown). This cell line was used to demonstrate that the gene expression of the human TH minimal promoters generated in this study was specific for brain-derived cells. The efficiency of lentiviral-mediated gene transfer in human skin carcinoma A431 cell line was confirmed by PCR analysis of genomic DNA for all the constructs (Fig. 4).
Fig. 4.
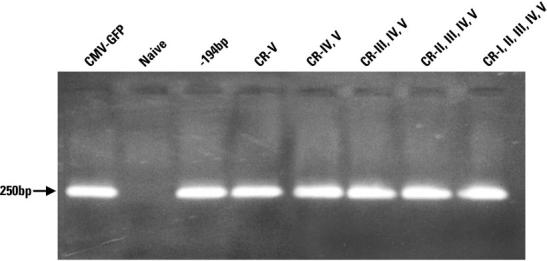
PCR analysis of genomic DNA to test gene transfer efficiency in the 250 bp series for human skin carcinoma A431 cell line. The lentiviral vector carrying the CMV-driven GFP was included as control (first lane on left-hand side). Lentiviral transduction was carried out on A431 cell line as described (Materials and Methods). Genomic DNA was extracted and used as template for PCR reactions (Materials and Methods). The primers were designed to amplify a fragment of the WPRE region of the lentiviral vector system (Materials and Methods). DNA samples were run on 1.5% agarose gel. The size of the band is depicted by an arrow on left-hand side. CR, conserved region.
Expression of human TH minimal promoters in murine primary striatal and S. nigra cells
The construct containing the five conserved regions and the construct that contains only the first −194 bp from the transcription start of the human TH promoter (plasmid pGR382) were both tested for their ability to express GFP in differentiated mouse primary striatal and S. nigra cells (Figs. 5 and 6, respectively). Mouse primary striatal cells are the murine counterpart of hNPCs and require a differentiation mixture to induce TH expression. On the other hand, mouse S. nigra cells intrinsically express TH. The purpose of this experiment was to determine whether or not the five evolutionary conserved regions of the human TH promoter are also functional in the murine system. Interestingly, both plasmids could express GFP in both cell types, however, without any specificity for TH-positive cells, regardless of whether TH expression was induced (mouse primary striatal cells) or intrinsically expressed (mouse S. nigra cells) (Figs. 5 and 6, respectively).
Fig. 5.

This figure shows the immunoassay of TH expression and detection of GFP in differentiated mouse primary striatal cells for the construct that contains all the five conserved regions (lower row) and for the construct that lacks the five conserved regions (upper row).
Fig. 6.

This figure shows the immunoassay of TH expression and detection of GFP in differentiated mouse Substantia nigra cells for the construct that contains all the five conserved regions (lower row) and for the construct that lacks the five conserved regions (upper row).
DISCUSSION
The present study has provided insights into important aspects of how specificity of expression is conferred in the human TH promoter in human brain-derived TH-expressing cells. About 11 kb of the human TH promoter was previously isolated from a genomic DNA library and sequenced (Kessler et al., 2003). Comparative analysis of the sequences of human, mouse, and rat TH promoters led to the identification of only five small evolutionary conserved regions, which, however, do not have the same positions among the promoters of the three species (Kessler et al., 2003). A similar finding was observed in a previous study, which analyzed and compared cell-type specific expression of various DNA elements of the human and rat TH promoters (Gandelman et al., 1990). Indeed, the human and mouse TH promoters share only 46.6% of homology, as determined with a Clustalx program. The low degree of homology between the human and mouse TH promoters was confirmed by a recent report (Kim et al., 2003b). Previous studies have also shown that there is roughly a 30% degree of homology between the human and rat TH promoters (Gandelman et al., 1990; Kim et al., 2003b). Despite the low degree of homology, the entire human TH promoter achieved tissue-specific expression of GFP in transgenic mice (Kessler et al., 2003), indicating that the overall machinery for TH promoter regulation is still preserved in the human and murine systems. However, the present study revealed some differences in TH gene regulation between the human and rodent models.
The functional characterization in our study demonstrated that the five evolutionary conserved regions play a significant role in regulating the specificity of human minimal promoters for human brain-derived TH-expressing cells. The five conserved regions were progressively added to a core sequence of the human TH promoter, which comprised the last 194 bp upstream of the transcription start and the first 35 bp of the untranslated messenger RNA leader of the human TH gene (plasmid pGR382). The entire panel of constructs showed GFP-specific expression for human and mouse brain-derived cells. However, the purpose of this study was to achieve gene expression specific for TH-positive brain derived cells. All the recombinant human TH minimal promoters have in common a core sequence (−194/+35), which contains a scaffold/matrix attachment region (S/MAR) at positions (−186/−16) (Lenatorski and Goc, 2002). The association between nuclear matrix of certain cell types and various DNA binding proteins may constitute a subcellular structure in chromatin organization, which, in turn, may result in a particular tissue-specific gene regulation (van Wijnen et al., 1993). The first intron of the human TH gene contains two other S/MARs (+645/+755 and +835/+945) (Lenatorski and Goc, 2002). Interestingly, the S/ MAR at (+645/+755) also encodes for a microsatellite repeat (TCAT) termed HUMTH01, which acts as a silencer for the human TH gene expression by interacting specifically with the following transcription factors: ZNF191, HBP1, and probably AP-1 (Meloni et al., 1998; Albanese et al., 2001). ZNF191 is a zinc finger protein, while HBP1 is an HMG box transcription factor. A number of studies have already reported the intronic colocalization of S/MARs with either enhancer or silencer regulatory elements in many other systems, besides the human TH promoter (Cockerill and Garrard, 1986; Forrester et al., 1994; Boulikas, 1995). The two S/ MARs at (+645/+755) and (+835/945) were not present in our constructs, as the human TH gene was replaced by the GFP cDNA, with the only exception of the first 35 nucleotides from the transcription start (Kessler et al., 2003). This seems to indicate that the S/MAR at (−186/−16) is per se sufficient to confer specific gene expression at least to human brain-derived cells in the in vitro system. Interestingly, the S/MAR at (−186/−16) contains binding sites for AP-1, OCT-1, and SP-1 transcription factors (van Wijnen et al., 1993; Lenatorski and Goc, 2002). However, the plasmid that contains only the core sequence of the human TH promoter (−194/+35) produced high ectopic GFP expression in brain cell populations. This was revealed by co-staining experiments for TH expression in differentiated human neuronal progenitor cells (hNCPs). Interestingly, the human TH minimal promoter containing all five conserved regions linked to the core sequence showed a significant degree of specificity for TH-positive differentiated hNPCs. This indicates that the five conserved regions play a role probably in concert with S/MAR at (−186/−16) in conferring specific gene expression for human TH-positive brain derived cells. Most likely, the addition of the other two S/MAR encoded in the first intron of the human TH gene might improve the degree of specificity of our human TH minimal promoter for human TH-expressing brain-derived cells. These studies are currently in progress.
As expected, the human TH minimal promoters with or without the five conserved regions produced GFP expression both in differentiated mouse primary striatal and S. nigra cells. However, the five conserved regions did not confer any specificity to the human TH minimal promoters for mouse cells either intrinsically expressing TH (S. nigra), or after induction with differentiation factors (striatum). Therefore, the five evolutionary conserved regions can modulate transcriptional activity of the human TH promoter in a tissue-specific manner only in differentiated human neuronal progenitor cells. Conversely, the 11-kb human TH promoter was specifically expressed in the S. nigra of transgenic mice (Kessler et al., 2003). This finding indicates the presence of other regulatory elements within the recombinant 11 kb human TH promoter, which allow for specific TH-gene expression also in the transgenic mouse model. Indeed, major differences were observed in the past between the genomic organization of human and rat TH promoters, mainly due to the low degree of homology (Gandelman et al., 1990). As anticipated, also the human and the mouse TH promoters share a low degree of homology (Kim et al., 2003b).
It seems reasonable to assume that the low degree of homology between the human and mouse promoters might be in part responsible for some differences in the regulation of TH gene expression. For instance, mouse TH minimal promoters require Nurr1-mediated trans-activation (Iwawaki et al., 2000; Kim et al., 2003a). This is in contrast to our human TH minimal promoters. Intriguingly, no dimeric or heterodimeric NR4A2 (Nurr1) binding sites are present in the 11 kb of the human TH promoter. However, conserved regions one and four of the human TH promoter encode for a half palindromic binding site sequence for NR4A2 (Nurr1). Also this is in contrast to mouse and rat TH promoters, which contain many Nurr1-like consensus-binding sites, in addition to the two half palindromic binding sites for Nurr1 contained in the conserved regions. Since the S. nigra of knockout mice for Nurr1 lacks TH expression, as revealed in two studies (Saucedo-Cardenas et al., 1998; Baffi et al., 1999), Nurr1 expression is thought to be necessary to induce TH expression in dopaminergic neurons. Importantly, one group of investigators showed that Nurr1 had a weak effect on the rat TH promoter in mouse neuroblastoma Neuro2A cell line, although the rat TH promoter encodes for a dimeric Nurr1 binding sequence (Cazorla et al., 2002). In contrast, the homeodomain transcription factor Ptx3 exhibited a considerable interaction on the rat TH promoter, which resulted in an enhancement of transcription in a cell-dependent fashion (Cazorla et al., 2002). Moreover, the co-expression of Ptx3 and Nurr1 showed a synergistic effect in terms of stimulation of the rat TH promoter (Cazorla et al., 2002). Studies are currently in progress to establish the role of Nurr1, in addition to shared consensus sites such as Ptx3, HOXA4, HOXA5, HNF-3β, and other factors that may regulate transcriptional activity and specific gene expression of the human TH promoter (Kessler et al., 2003). As mentioned in the results section, the addition of the first half palindromic Nurr1 binding site of CR-IV increased transcriptional activity of the human TH minimal promoter (Fig. 3, part B), if compared with the plasmid of the same series that contains only CR-V (Fig. 3, part B). However, the specificity for TH-expressing cells was not improved (Fig. 3, part A). Astonishingly, the addition of CR-I had the most profound effect on specific gene expression for TH-positive cells for the human TH minimal promoters (Fig. 3, part A). As expected, the increase of specific gene expression for TH-positive cells was correlated with a diminished number of GFP-expressing cells. Intriguingly, also CR-I contains a half palindromic Nurr1 binding site. Our current priority is to establish whether the presence of the half palindromic Nurr1 binding site of CR-I has real functional significance in regulating the specificity of the human TH promoter, or is simply an epiphenomenon. Interestingly, electro-mobility shift analysis (EMSA) has shown there is no specific interaction at both half palindromic Nurr1 binding sites (unpublished data). Possibly, other sites such as HNF-3b found in CR-I might be responsible for the increased specificity of expression. Indeed, HNF-3β is regulated by sonic hedgehog (Shh), which is one of the two necessary differentiation cues required to signal TH expression in the murine S. nigra (Hynes et al., 2000). The addition of CR-III and CR-II did not have any striking effect in terms of regulation of gene expression for the human TH minimal promoters, but may be required for the overall machinery that confers specificity to the recombinant minimal promoters for human TH-positive brain derived cells.
The possibility that TH gene regulation might be achieved through different mechanisms among species is an appealing one. In this respect, the low degree of homology seems to be a key factor, as it reflects a substantial diverse genomic organization between the human and rodent TH promoters. Interestingly, a recent study has also shown that S/MARs contained in the TH gene might be involved in the differential TH gene regulation among various species (Lenatorski et al., 2003). This study conducted a comparative analysis between bovine and human TH genes in binding nuclear matrix obtained from bovine brain and liver tissues (Lenartowski et al., 2003). In contrast to the human TH gene (Lenartowski and Goc, 2002), the association between bovine TH gene and the nuclear matrix was not tissue specific, although the position of the matrix binding region is conserved in both systems (Lenartowski et al., 2003). This finding indicates that TH gene regulation might indeed be achieved by different mechanisms in the human and bovine models.
In conclusion, the human TH minimal promoter that contains all the five conserved regions may be useful for the selection of TH-expressing neuronal-based cells derived from human stem cells for transplantation studies in animal models of Parkinson's disease (Kessler et al., 2003). Lentiviral-mediated gene transfer of the human TH minimal promoter driving the expression of GFP can easily identify TH-expressing cells in vitro either among human stem cells of various derivation, or adult human neuronal progenitor cells. After marking the cells in vitro with GFP, TH-positive cells may be isolated from the general cell population via cell sorting and subsequently used in brain transplantation experiments in animal models for Parkinson's disease.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: NS24204, NS32519, NS43309.
LITERATURE CITED
- Albanese V, Faucon Biguet N, Kiefer H, Bayard E, Mallet J, Meloni R. Quantitative effects on gene silencing by allelic variation at a tetranucleotide microsatellite. Hum Mol Genet. 2001;10:1785–1792. doi: 10.1093/hmg/10.17.1785. [DOI] [PubMed] [Google Scholar]
- Baffi JS, Palkovits M, Castillo SO, Mezey E, Nikodem VM. Differential expression of tyrosine hydroxylase in catecholaminergic neurons of neonatal wild-type and nurr1-deficient mice. Neuroscience. 1999;93:631–642. doi: 10.1016/s0306-4522(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Boulikas T. Chromatin domains and prediction of MAR sequences. Int Rev Cytol. 1995;162A:279–388. doi: 10.1016/s0074-7696(08)61234-6. [DOI] [PubMed] [Google Scholar]
- Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM. Dopamine biosynthesis is selectively abolished in Substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci. 1998;11:36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- Cazorla P, Smidt MP, O'Malley KL, Burbach JPH. A response element for the homeodomain transcription factor Ptx3 in the tyrosine hydroxylase gene promoter. J Neurochem. 2002;74:1829–1837. doi: 10.1046/j.1471-4159.2000.0741829.x. [DOI] [PubMed] [Google Scholar]
- Cockerill PN, Garrard WT. Chromosomal loop anchorage sites appear to be evolutionary conserved. FEBS Lett. 1986;204:5–7. doi: 10.1016/0014-5793(86)81377-1. [DOI] [PubMed] [Google Scholar]
- Dewey RA, Morrisey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, Southgate TD, Klatzmann D, Lassmann H, Castro MG, Lowenstein PR. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma cells treated by adenovirus-mediated gene therapy: Implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Du X, Iacovitti L. Protein kinase C activators work in synergy with specific growth factors to initiate tyrosine hydroxylase expression in striatal neurons in culture. J Neurochem. 1997;68:564–569. doi: 10.1046/j.1471-4159.1997.68020564.x. [DOI] [PubMed] [Google Scholar]
- Forrester WC, van Genderen C, Jenuwein T, Grosschedl R. Dependence of enhancer-mediated transcription of the immunoglobulin μ gene on nuclear matrix attachment regions. Science. 1994;265:1221–1225. doi: 10.1126/science.8066460. [DOI] [PubMed] [Google Scholar]
- Gandelman KY, Coker GT, Moffat M, O'Malley KL. Species and regional differences in the expression of cell-type specific elements at the human and rat tyrosine hydroxylase gene loci. J Neurochem. 1990;55:2149–2152. doi: 10.1111/j.1471-4159.1990.tb05811.x. [DOI] [PubMed] [Google Scholar]
- Hynes M, Ye W, Wang K, Stone D, Murone M, Sauvage F, Rosenthal A. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat Neurosci. 2000;3:41–46. doi: 10.1038/71114. [DOI] [PubMed] [Google Scholar]
- Iwawaki T, Kohno K, Kobayashi K. Identification of a potential Nurr1 response element that activates the tyrosine hydroxylase gene reporter in cultured cells. Biochem Bioph Res Commun. 2000;274:590–595. doi: 10.1006/bbrc.2000.3204. [DOI] [PubMed] [Google Scholar]
- Kessler MA, Yang M, Gollomp KL, Jin H, Iacovitti L. The human tyrosine hydroxylase gene promoter. Brian Res Mol Brain Res. 2003;112:8–23. doi: 10.1016/s0169-328x(02)00694-0. [DOI] [PubMed] [Google Scholar]
- Kielian T, Hickey WF. Inflammatory thoughts about glioma gene therapy. Nat Med. 1999;5:1237–1238. doi: 10.1038/15188. [DOI] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ, Lim JK, Anderson T, Isacson O. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem. 2003a;85:622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- Kim TE, Park MJ, Choi EJ, Lee HS, Lee SH, Yoon SH, Oh CK, Lee BJ, Kim SU, Lee YS, Lee MA. Cloning and cell type-specific regulation of the human tyrosine hydroxylase gene promoter. Biochem Biophys Res Commun. 2003b;312:1123–1131. doi: 10.1016/j.bbrc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Lenartowski R, Goc A. Tissue-specific association of the human tyrosine hydroxylase gene with nuclear matrix. Neurosci Lett. 2002;330:151–154. doi: 10.1016/s0304-3940(02)00746-2. [DOI] [PubMed] [Google Scholar]
- Lenartowski R, Grzybowski T, Miscicka-Sliwka D, Wojciechowski W, Goc A. The bovine tyrosine hydroxylase gene associates in vitro with the nuclear matrix by its first intron sequence. Acta Biochim Pol. 2003;50:865–873. [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Meloni R, Albanese V, Ravassard P, Treilhou F, Mallet J. A tetranucleotide polymorphic microsatellite, located in the first intron of the tyrosine hydro-xylase gene, acts as a transcription regulatory element in vitro. Hum Mol Genet. 1998;7:423–428. doi: 10.1093/hmg/7.3.423. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Uderfriend S. Tyrosine hydroxylase, the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retrovirus by transient transfections. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KC, Matthaei KI. Rapid preparation of DNA dot blots from tissue samples, using hot alkaline lysis and filtration onto charge-modified nylon membrane. Nucleic Acids Res. 1990;18:3093. doi: 10.1093/nar/18.10.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert JJ, Geoffroy MC, Finiels F, Mallet J. An adenoviral vector-based system to study neuronal gene expression: Analysis of the rat tyrosine hydroxylase promoter in cultured neurons. J Neurochem. 1997;68:2152–2160. doi: 10.1046/j.1471-4159.1997.68052152.x. [DOI] [PubMed] [Google Scholar]
- Romano G. Gene transfer in experimental medicine. Drug News Perspect. 2003;16:267–276. doi: 10.1358/dnp.2003.16.5.829314. [DOI] [PubMed] [Google Scholar]
- Romano G, Micheli P, Pacilio C, Giordano A. Latest developments in gene transfer technology: Achievements, perspectives, and controversies over therapeutic applications. Stem Cells. 2000;18:19–39. doi: 10.1634/stemcells.18-1-19. [DOI] [PubMed] [Google Scholar]
- Romano G, Claudio PP, Tonini T, Giordano A. Human immunodeficiency virus type 1 (HIV-1) derived vectors: Safety considerations and controversy over therapeutic applications. Eur J Dermatol. 2003;13:424–429. [PubMed] [Google Scholar]
- Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM. Nurr1 is essential for the induction of the dopaminergic phenotype and survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka Y, Cannon P, Ramsdale EE, Griffiths JC, Romano G, Kingsman SM, Kingsman AJ. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak SY, Lang P, Ullrey D, Neve RL, O'Malley KL, Geller AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J Neurochem. 1997;68:1792–1803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- Stull N, Jung JW, Iacovitti L. Induction of a dopaminergic phenotype in cultured striatal neurons by bone morphogenetic proteins. Brain Res Dev Brain Res. 2001;130:91–98. doi: 10.1016/s0165-3806(01)00216-4. [DOI] [PubMed] [Google Scholar]
- Van Wijnen AJ, Bidwell JP, Fey EG, Penman S, Lian JB, Stein JL, Stein G. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry. 1993;32:8397–8402. doi: 10.1021/bi00084a003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu L, Geller AI. Diverse stabilities of expression in the rat brain from different cellular promoters in a helper virus-free herpes simplex virus type 1 vector system. Hum Gene Ther. 1999;10:1763–1771. doi: 10.1089/10430349950017446. [DOI] [PubMed] [Google Scholar]
- Wang X, Kong L, Zhang GR, Sun M, Geller AI. A preproenkephalinneurofilament chimer promoter in a helper virus-free herpes simplex vector enhances long-term expression in the rat striatum. Neurol Dis. 2004;16:596–603. doi: 10.1016/j.nbd.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Yang M, Donaldson AE, Marshall CE, Shen J, Iacovitti L. Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant. 2004;13:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Inaji M, Kouike H, Shimazaki T, Sawamoto K, Ando K, Date I, Kobayashi K, Suhara T, Uchiyama Y, Okano H. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neurosci Lett. 2004;363:33–37. doi: 10.1016/j.neulet.2004.03.074. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]


